Abstract
Background
We showed excellent adherence and satisfaction with our telehealth care (TC) approach for COPD. Here, the results of a consecutive randomized controlled trial are presented.
Methods
Patients were randomly assigned to TC or standard care (SC). During TC, patients answered six daily questions online, and focused on the early recognition of exacerbations, in addition to SC.
Results
The mean increase in COPD assessment test (CAT) was 1.8 vs. 3.6 points/year in the TC and SC groups, respectively (P = 0.0015). Satisfaction with care (VAS) at baseline was 8.2; at the end of SC, 8.5 (P = 0.062); and after TC, 8.8 (P < 0.001). We detected significantly more moderate exacerbations during TC.
Conclusion
Whilst receiving TC, the slope of the CAT increase – an indicator of the naturally progressive course of COPD – was reduced by 50%. Satisfaction with care increased with TC. The higher number of detected moderate exacerbations probably indicates a higher diagnostic sensitivity than without TC.
Keywords: COPD, COPD assessment test, COPD exacerbation, telehealth
Introduction
Telehealth care (TC) could be a relevant component of integrated care for chronic obstructive pulmonary disease (COPD). In brief, TC was described by McLean [1] as electronic transfer of information from the patient over a distance to healthcare professionals, who give personalized feedback and advice to the patient. By the current state, there are still conflicting results considering the improvement of health‐related quality of life (HRQOL) or reduction in acute exacerbations of COPD (AECOPD) by TC [1, 2, 3, 4]. In addition, there exists a wide variability of different interventions in TC, such as monitoring of physiological parameters and vital signs or self‐reported symptoms [5]. It remains unclear which modalities should best be used to improve the patient’s health status or to detect exacerbations [6, 7].
AECOPD is characterized by symptoms, which often occur already days before the event [8]. For early detection of such exacerbation symptoms, disease‐specific questions can be used. In a pilot study, 6 questions were successfully used for the early detection of AECOPD. Further, the feasibility of the same TC procedure as used in this study was shown. Patients appreciated TC and satisfaction with care improved [9]. An extended analysis of this pilot study investigated the change in the COPD assessment test (CAT) score. The slope of the CAT increase was positively correlated with the risk of future exacerbations [10].
The primary aim of the current study was to investigate the impact of a TC procedure on the course of COPD and HRQOL as assessed by the slope of individual CAT changes over the study periods.
Materials and methods
Trial design
This was a multicentre randomized controlled crossover trial, involving 6 centres in Switzerland and Germany. Inclusion period was from April 2016 to September 2018. Last patient last visit was September 2019.
Patients
The continuous screening of all COPD patients took place in daily clinical practice. Each patient participated in the study for 12 months with a crossover after 6 months (Figure S1). Inclusion criteria were a diagnosis of COPD and age ≥ 40 years. The only exclusion criteria were inability to provide informed consent or to follow trial procedures. Trial language was German. All patients provided written informed consent. The study was approved by the Swiss Institutional Review Board (Swissethics, EKSG 15/184, BASEC Nr. 2015‐00065). Trial registration number at ClinicalTrials.gov is NCT04485832.
General procedure
After screening and inclusion, a first consultation was arranged to collect baseline characteristics, perform randomization and give instructions to the participants. A user account on a web‐based online healthcare platform was installed (‘Evita’ by Swisscom) on the patient’s own technical device. For the study, an adapted plug‐in was created. In the following 6 months, patients starting with the intervention phase daily answered six questions, which were focused on the detection of AECOPD, and completed the CAT weekly. Patients starting with the control phase only filled the CAT questionnaire weekly. After 6 months, patients presented for the second visit, where the crossover took place. The last visit took place after 12 months. During the whole year, all patients had consultations for their COPD treatment as usual. Same access for both groups to equal clinical care according to local practice was granted.
TC intervention
The daily asked ‘yes’ or ‘no’ questions focused on the recognition of AECOPD (Figure S2). Whilst being in the intervention phase, patients had to answer them every morning. One reminder short message was automatically sent in the late morning in the case of missing entry. From Monday to Friday, the study team analysed all answers in the late morning. An overview (‘cockpit’) showed all patients and for each day of the past week one small box. The different colours of the boxes indicated whether (i) the patient had not answered the questions (grey), (ii) had answered zero or one question with ‘yes’ (green), (iii) had answered two or more questions with ‘yes’, but had a green box on the previous day (yellow), or (iv) had answered two or more questions with ‘yes’ and already had a yellow or red box the previous day (red) (Figure S2). The study team reacted according to a prespecified action plan. A yellow box was a first warning sign, but no action was carried out. If the following day the box turned to red (=two ‘yes’ answers on two consecutive days), this indicated a possible AECOPD. The patient was then contacted by phone by the study team for further evaluation. From that moment on, the physician performing the phone call assumed responsibility for all further actions taken. This could mean to instruct the patient to administer self‐medication, to consult the general practitioner (GP) or to visit the ambulatory or the emergency department (ED) for clinical judgement.
HRQOL measurement
The CAT was filled weekly by the patients in both phases. For the study, the German version of the questionnaire was used.
End‐points
The primary end‐point was the group‐specific slope difference in weekly CAT over time. Satisfaction with care was assessed by a visual analogue scale from 0 to 10. Additional end‐points were the number of exacerbations and hospitalizations (as well as the days in hospital) due to AECOPD and the treatment costs per patient and year. Data were collected through hospital records, contacting the patient’s general practitioner and the rehabilitation clinics and asking the patients themselves. Treatment costs included the exact expenses for hospital and rehabilitation stays as well as estimated costs for planned and unplanned outpatient visits, medication and telephone contacts.
Statistical considerations
The evolution of the CAT over time was analysed using linear mixed‐effects models. The fixed‐effects terms included time, group (intervention vs. control) and the sequential period in the crossover design. The patient ID was defined as a random‐effects term. The secondary and exploratory end‐points were analysed using generalized linear mixed models (including logistic and Poisson regression) and Student's paired t‐tests. All parameter estimates were reported together with their 95% confidence intervals. All analyses were done using the R statistical software with the extension package lme4.
Sample size calculation
The trial was designed to detect a clinically relevant within‐patient difference in 2 CAT score points in 1 year. In order to detect a statistically significant difference in the primary end‐point in a within‐patient crossover design with a power of 80% and a significance level of 5% (two‐sided), and considering an expected dropout rate of 5% and a learning effect explaining 25% of the effect size in the patients starting with the intervention phase, 175 patients should be included in the study.
Results
In total, 150 of 168 patients (89%) completed the trial. The baseline characteristics of all 168 included patients are summarized in Table 1.
Table 1.
Baseline characteristics
| Number of patients included | 168 |
| Median age, years (IQR) | 67 (61–73) |
| Male gender | 65% |
| Smoking status | Current: 19% |
| Former: 79% | |
| Never: 2% | |
| Smoking history: median pack years (IQR) | 48 (30–60) |
| Body mass index (kg/m2), median (IQR) | 26.0 (23.0–29.1) |
| GOLD stage, % of patients | |
| 1 | 8.70% |
| 2 | 40.50% |
| 3 | 32.90% |
| 4 | 17.90% |
| GOLD risk class, % of patients | |
| A | 4.00% |
| B | 51.40% |
| C | 8.70% |
| D | 35.80% |
| Mean FEV1, % predicted (range) | 51 (16–115) |
| Mean SGRQ score (range) | 42 (5–83) |
| Mean mMRC score (range) | 2 (0–4) |
| 6MWD in metres, median (IQR) | 418 (360–506) |
| BODE index, median (IQR) | 3 (1–4) |
| Pharmacotherapy for COPD, % of patients | |
| SAMA | 13% |
| SABA | 39% |
| LAMA | 86% |
| LABA | 87% |
| ICS | 54% |
| Systemic corticosteroids | 8% |
| Mucolytics | 10% |
| LTOT, % of patients | 26% |
| CPAP, % of patients | 7% |
| NIV, % of patients | 9% |
| Supervised rehabilitation in the past, % of patients | 31% |
| Volume reduction therapy, % of patients | 7% |
| Comorbidities, % of patients | |
| Arterial hypertension | 44% |
| Coronary artery disease | 18% |
| Congestive heart failure | 6% |
| Myocardial infarction | 9% |
| Pulmonary hypertension | 3% |
| Malignancy | 9% |
| Diabetes | 10% |
| Renal failure | 8% |
| Psychiatric disease | 9% |
| Influenza vaccination applied, % of patients | 71% |
| Pneumococcal vaccination applied, % of patients | 44% |
| Charlson comorbidity index, median (IQR) | 1 (1–2) |
CPAP, continuous positive airway pressure; FEV1, forced expiratory volume in 1 s; GOLD, Global Initiative for Obstructive Lung Disease; ICS, inhalative corticosteroids; IQR, interquartile range; LABA, long‐acting beta agonists; LAMA, long‐acting muscarinic antagonists; LTOT, long‐term oxygen therapy; NIV, noninvasive ventilation; SABA, short‐acting beta agonists; SAMA, short‐acting muscarinic antagonists.
Primary end‐point – CAT
The overall mean CAT score was 15.6 points. The mean CAT increase was 1.8 (standard error 0.55) vs. 3.6 (standard error 0.56) points/year in the TC and SC groups, respectively (P = 0.0015; Figure 1).
Fig. 1.
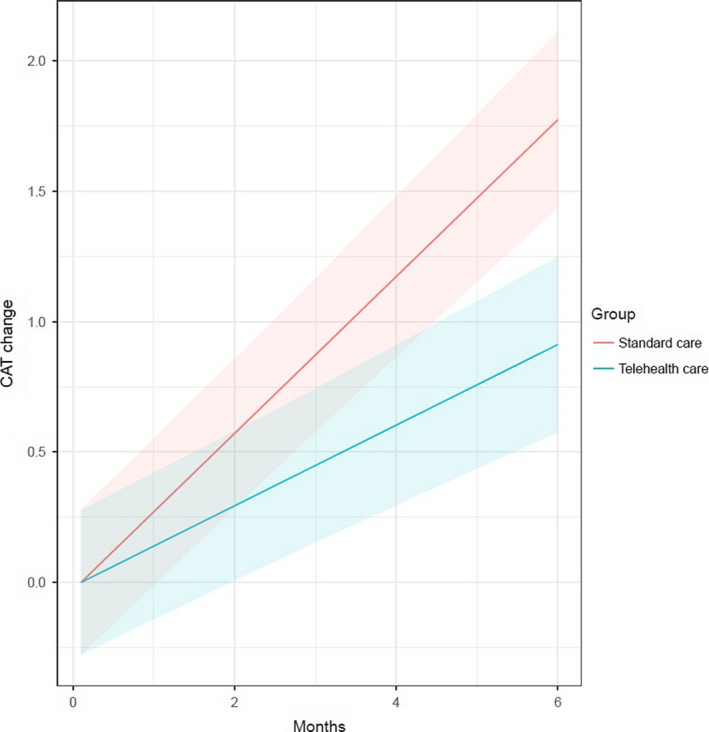
Mean CAT increase over time.
Secondary end‐point
Satisfaction with care on a visual analogue scale (ranging from 0 to 10) at baseline was 8.2; at the end of SC, 8.5 (mean of differences SC‐baseline = 0.26; 95% CI: −0.01 −0.53; P = 0.062); and after TC, 8.8 (mean of differences TC‐baseline = 0.64; 95% CI: 0.37–0.9; P < 0.001).
Exploratory end‐points
The results of exploratory end‐points are summarized in Table 2.
Table 2.
Exploratory end‐points
| TC | SC | P | |
|---|---|---|---|
| ED visit rate due to AECOPD |
0.47/year [95% CI: 0.37–0.59] |
0.62/year [95% CI: 0.5–0.75] |
0.140 |
| Hospitalization rate due to AECOPD |
0.17/year [95% CI: 0.11–0.25] |
0.17/year [95% CI: 0.11–0.25] |
0.618 |
| Number of AECOPD (total) | 125 | 99 | 0.060 |
| Number of mild AECOPD | 17 | 15 | 0.759 |
| Number of moderate AECOPD | 99 | 72 | 0.028 |
| Number of severe AECOPD | 9 | 12 | 0.524 |
| Days in hospital due to AECOPD (total) | 187 | 282 | 0.497 |
| COPD‐related costs (total) | 2812 USD/py | 4609 USD/py | 0.364 |
| Mortality due to COPD (number) | 0 | 0 | – |
| Mortality overall (number) | 0 | 5 | – |
| Data completeness | 88.2% | na | – |
| Number of phone contacts (total) | 311 | na | – |
| Duration of telephone contacts (mean, per py) | 9.47 min | na | – |
ED, emergency department min, minutes; na, not applicable; py, patient‐year; USD, US dollars.
Discussion
Whilst receiving telehealth care, the slope of the CAT increase – an indicator of the naturally progressive course of COPD – was significantly reduced by 50% (−1.8 points/year) in comparison with standard care. Satisfaction with care was already high before the intervention and increased further with TC compared to SC. With TC, we detected a higher number of moderate AECOPD. TC was associated with a trend towards fewer days in hospital and lower total COPD‐related costs compared with SC.
Strength of this study is the multicentre, randomized controlled crossover design, so that every patient experiences both SC and TC, serving as his own control, minimizing selection bias and improving generalizability. The latter has also been supported by keeping inclusion criteria as open as possible and exclusion criteria at a minimum. By this, in fact, nearly all COPD patients were eligible for the study. To the best of the authors’ knowledge, the association between CAT values and telehealth interventions has not been investigated in such a manner until now.
The demonstrated bisection of the CAT increase over time reflects a highly relevant modification of the disease course of COPD. Looking at the above‐mentioned exploratory end‐points, one mechanism of how our TC procedure could have influenced disease course might be the better detection and consequently timely treatment of significantly more moderate exacerbations, which are underdiagnosed according to the literature [2]. Recently, Tupper et al [11] could not find a change in CAT score after 6 months of their TC approach. One difference to our study is that they measured CAT only at baseline and after the study, whereas we provide a weekly CAT and calculated the slope, resulting in a much higher resolution of values. Further, our TC approach systematically assessed patients on a daily basis, whereas the ‘reaction interval’ in the mentioned study was variable. This seems to be a critical factor for the success of telehealth interventions for COPD looking at other trials in that area, where we can observe that studies with longer reaction intervals tended to show negative results more frequently [12, 13]. In our view, another critical point besides reaction interval and mode of intervention is the choice of the primary end‐point. Many authors chose emergency department visits or hospitalizations and failed to show positive effects of their telehealth interventions [12, 13, 14, 15, 16, 17]. The main reason could be the fact that these events are rather rare, taking the whole COPD population into account, leading to the problem of underpowered studies. We made the same observation in our feasibility trial [9] and in this study (explorative end‐points).
Satisfaction with care significantly increased with TC, which we had already observed in a feasibility trial [9]. Our experience and a possible explanation were that patients appreciated the closer contact to the care team and the easier availability of support, for example by the possibility to make comments and ask questions using the TC platform. The increase in satisfaction with care by TC interventions has also been described by others [18, 19, 20].
Regarding total days in hospital due to COPD and total COPD‐related costs (including all costs related to the TC intervention), we could not find a statistically significant difference between TC and SC, but the actual numbers suggest that there might be a benefit by using TC. However, the current study was not powered to evaluate this hypothesis appropriately.
Limitations
Regarding important exploratory end‐points like discussed above, we had insufficient statistical power to show significant differences. Achieving this would have required considerably more included patients.
Conclusion
Whilst receiving TC, the slope of the CAT increase – an indicator of the naturally progressive course of COPD – was significantly reduced by 50% (−1.8 points/year). Satisfaction with care – already high before the intervention – increased further with TC. With TC, we detected a higher number of moderate AECOPD, probably indicating a higher diagnostic sensitivity than without TC. This could be achieved with manageable effort and by trend lower total COPD‐related costs.
Conflict of interest statement
The study was funded by Swisscom Health AG, SWICA Krankenversicherung AG, PneumRx GmbH, AstraZeneca AG and Boehringer Ingelheim Schweiz GmbH. None of these had any influence on design, conduct, interpretation or writing of the manuscript. Prof. Kohler reports personal fees from Bayer, Boehringer, Novartis, Astra and Mundipharma, all outside the submitted work, and grants and personal fees from Roche and GSK. Prof. Tamm reports grants from Vifor AG and Schwabe Pharma AG, all outside the submitted work. Prof. Stolz reports grants from AstraZeneca AG, Curetis AG and Boston Scientific and personal fees from AstraZeneca AG, Novartis AG, GSK AG, Roche AG, Zambon, Pfizer, Schwabe Pharma AG and Vifor AG, all outside the submitted work.
Author Contribution
F. Rassouli: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); supervision (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). A. Germann: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); project administration (supporting); writing – original draft (supporting); writing – review and editing (supporting). F. Baty: Data curation (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). M. Kohler: Conceptualization (supporting); data curation (supporting); investigation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). D. Stolz: Conceptualization (supporting); data curation (supporting); investigation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). R. Thurnheer: Conceptualization (supporting); data curation (supporting); investigation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). T. Brack: Conceptualization (supporting); data curation (supporting); investigation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). C. Kähler: Conceptualization (supporting); data curation (supporting); investigation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). S. Widmer: Conceptualization (supporting); data curation (supporting); investigation (supporting); methodology (supporting); project administration (supporting). U. Tschirren: Data curation (supporting); investigation (supporting); project administration (supporting). N. A. Sievi: Data curation (supporting); investigation (supporting); project administration (supporting). M. Tamm: Conceptualization (supporting); data curation (supporting); investigation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). M. H. Brutsche: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (supporting); methodology (supporting); project administration (supporting); writing – original draft (supporting); writing – review and editing (supporting).
Supporting information
Figure S1. Randomized crossover design (CAT: COPD assessment test; eCRF: electronic case report form).
Figure S2. Left upper part: patient view of the e‐health platform. By pressing “COPD study”, patients were transferred to the questionnaire. Lower part: screenshot of daily online questions to be answered by the patients (“yes” or “no”). Right upper part: “cockpit” of the study team with color‐coded alerts in the right column under “Status today” (red = AECOPD suspected, need for telephone call; yellow = more symptoms than usually, but for < 24 h; green = not more symptoms than usually; gray = questions not answered). Under “Weekly status”, the alerts of the last 7 days are displayed. Patients could make comments and ask questions. Adapted and translated from our pilot study [9] with permission from S. Karger AG, Basel, Switzerland.
Acknowledgements
We would like to thank all participating patients and the study personnel in the six participating hospitals.
Rassouli F, Germann A, Baty F, Kohler M, Stolz D, Thurnheer R, Brack T, Kähler C, Widmer S, Tschirren U, Sievi NA, Tamm M, Brutsche MH (Cantonal Hospital St. Gallen, St. Gallen; University Hospital Zurich, Zurich; University Hospital Basel, Basel; Cantonal Hospital Münsterlingen, Münsterlingen; Cantonal Hospital Glarus, Glarus, Switzerland; and Waldburg‐Zeil‐Kliniken, Wangen, Germany). Telehealth mitigates COPD disease progression compared to standard of care: a randomized controlled crossover trial (Brief Report). J Intern Med 2021; 289: 404–410. 10.1111/joim.13230
References
- 1. McLean S, Nurmatov U, Liu JLY, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease: Cochrane Review and meta‐analysis. Br J Gen Pract 2012; 62: e739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract 2014; 68: 369–78. [DOI] [PubMed] [Google Scholar]
- 3. Gregersen TL, Green A, Frausing E, Ringbæk T, Brøndum E, Ulrik CS. Do telemedical interventions improve quality of life in patients with COPD? A systematic review. Int J Chron Obstruct Pulmon Dis 2016; 11: 809–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekeland AG, Bowes A, Flottorp S. Effectiveness of telemedicine: a systematic review of reviews. Int J Med Inform 2010; 79: 736–71. [DOI] [PubMed] [Google Scholar]
- 5. Bourbeau J, Farias R, Li PZ, et al. The Quebec Respiratory Health Education Network: integrating a model of self‐management education in COPD primary care. Chron Respir Dis 2018; 15: 103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alcazar B, de Lucas P, Soriano JB, et al. The evaluation of a remote support program on quality of life and evolution of disease in COPD patients with frequent exacerbations. BMC Pulm Med 2016; 16: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKinstry B. The use of remote monitoring technologies in managing chronic obstructive pulmonary disease. QJM 2013; 106: 883–5. [DOI] [PubMed] [Google Scholar]
- 8. Camargo CA Jr, Ginde AA, Clark S, Cartwright CP, Falsey AR, Niewoehner DE. Viral pathogens in acute exacerbations of chronic obstructive pulmonary disease. Intern Emerg Med 2008; 3: 355–9. [DOI] [PubMed] [Google Scholar]
- 9. Rassouli F, Pfister M, Widmer S, Baty F, Burger B, Brutsche MH. Telehealthcare for chronic obstructive pulmonary disease in Switzerland is feasible and appreciated by patients. Respiration 2016; 92: 107–13. [DOI] [PubMed] [Google Scholar]
- 10. Rassouli F et al. Longitudinal change of COPD assessment test (CAT) in a telehealthcare cohort is associated with exacerbation risk. Int J Chron Obstruct Pulmon Dis 2017; 12: 3103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tupper OD, Gregersen TL, Ringbaek T, et al. Effect of tele‐health care on quality of life in patients with severe COPD: a randomized clinical trial. Int J Chron Obstruct Pulmon Dis 2018; 13: 2657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berkhof FF, van den Berg JWK, Uil SM, Kerstjens HAM. Telemedicine, the effect of nurse‐initiated telephone follow up, on health status and health‐care utilization in COPD patients: a randomized trial. Respirology 2015; 20: 279–85. [DOI] [PubMed] [Google Scholar]
- 13. Ringbaek T, Green A, Laursen LC, Frausing E, Brøndum E, Ulrik CS. Effect of tele health care on exacerbations and hospital admissions in patients with chronic obstructive pulmonary disease: a randomized clinical trial. Int J Chron Obstruct Pulmon Dis 2015; 10: 1801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinnock H, Hanley J, McCloughan L, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ 2013; 347: f6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tabak M, VanderValk P, Hermens H, Vollenbroek‐Hutten M, Brusse‐Keizer M. A telehealth program for self‐management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J Chron Obstruct Pulmon Dis 2014; 9: 935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McDowell JE, McClean S, FitzGibbon F, Tate S. A randomised clinical trial of the effectiveness of home‐based health care with telemonitoring in patients with COPD. J Telemed Telecare 2015; 21: 80–7. [DOI] [PubMed] [Google Scholar]
- 17. Chatwin M, Hawkins G, Panicchia L, et al. Randomised crossover trial of telemonitoring in chronic respiratory patients (TeleCRAFT trial). Thorax 2016; 71: 305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitzsimmons DA, Thompson J, Bentley CL, Mountain GA. Comparison of patient perceptions of Telehealth‐supported and specialist nursing interventions for early stage COPD: a qualitative study. BMC Health Serv Res 2016; 16: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talboom‐Kamp EP, Verdijk NA, Blom CMG, et al. e‐Vita: design of an innovative approach to COPD disease management in primary care through eHealth application. BMC Pulm Med 2016; 16: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoaas H, Andreassen HK, Lien LA, Hjalmarsen A, Zanaboni P. Adherence and factors affecting satisfaction in long‐term telerehabilitation for patients with chronic obstructive pulmonary disease: a mixed methods study. BMC Med Inform Decis Mak 2016; 16: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Randomized crossover design (CAT: COPD assessment test; eCRF: electronic case report form).
Figure S2. Left upper part: patient view of the e‐health platform. By pressing “COPD study”, patients were transferred to the questionnaire. Lower part: screenshot of daily online questions to be answered by the patients (“yes” or “no”). Right upper part: “cockpit” of the study team with color‐coded alerts in the right column under “Status today” (red = AECOPD suspected, need for telephone call; yellow = more symptoms than usually, but for < 24 h; green = not more symptoms than usually; gray = questions not answered). Under “Weekly status”, the alerts of the last 7 days are displayed. Patients could make comments and ask questions. Adapted and translated from our pilot study [9] with permission from S. Karger AG, Basel, Switzerland.


