Abstract
Objective
The study was undertaken to identify a monogenic cause of early onset, generalized dystonia.
Methods
Methods consisted of genome‐wide linkage analysis, exome and Sanger sequencing, clinical neurological examination, brain magnetic resonance imaging, and protein expression studies in skin fibroblasts from patients.
Results
We identified a heterozygous variant, c.388G>A, p.Gly130Arg, in the eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2) gene, segregating with early onset isolated generalized dystonia in 5 patients of a Taiwanese family. EIF2AK2 sequencing in 191 unrelated patients with unexplained dystonia yielded 2 unrelated Caucasian patients with an identical heterozygous c.388G>A, p.Gly130Arg variant, occurring de novo in one case, another patient carrying a different heterozygous variant, c.413G>C, p.Gly138Ala, and one last patient, born from consanguineous parents, carrying a third, homozygous variant c.95A>C, p.Asn32Thr. These 3 missense variants are absent from gnomAD, and are located in functional domains of the encoded protein. In 3 patients, additional neurological manifestations were present, including intellectual disability and spasticity. EIF2AK2 encodes a kinase (protein kinase R [PKR]) that phosphorylates eukaryotic translation initiation factor 2 alpha (eIF2α), which orchestrates the cellular stress response. Our expression studies showed abnormally enhanced activation of the cellular stress response, monitored by PKR‐mediated phosphorylation of eIF2α, in fibroblasts from patients with EIF2AK2 variants. Intriguingly, PKR can also be regulated by PRKRA (protein interferon‐inducible double‐stranded RNA‐dependent protein kinase activator A), the product of another gene causing monogenic dystonia.
Interpretation
We identified EIF2AK2 variants implicated in early onset generalized dystonia, which can be dominantly or recessively inherited, or occur de novo. Our findings provide direct evidence for a key role of a dysfunctional eIF2α pathway in the pathogenesis of dystonia. ANN NEUROL 2021;89:485–497
Pathogenic variants in various genes are known to cause inherited forms of dystonia. 1 , 2 However, despite recent progress in the identification of new causative genes by next generation sequencing (NGS) methods, the majority of patients are still etiologically unsolved, suggesting that additional causative genes remain to be identified. Eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2), also known as protein kinase R (PKR), is one of the 4 mammalian kinases involved in the integrated cytoprotective reaction to stress conditions (integrated stress response [ISR]). 3 Canonical activation of EIF2AK2 is mainly achieved by double‐stranded RNA (dsRNA) during viral infections. 4 Subsequently, EIF2AK2 activates the ISR by phosphorylating eukaryotic translation initiation factor 2 subunit 1 (EIF2S1; also known as eIF2α). The phosphorylation of EIF2S1 on the Ser51 residue by EIF2AK2 prevents mRNA translation and results in transient suppression of general protein synthesis, and induction of cellular apoptosis. 3 The activity of EIF2AK2 can also be regulated by the protein interferon‐inducible double‐stranded RNA‐dependent protein kinase activator A (PRKRA; also known as PACT), in a dsRNA‐independent manner in response to cellular stress. 5 , 6 , 7 Interestingly, biallelic pathogenic variants in the PRKRA gene cause an autosomal recessive form of early onset generalized dystonia. 8 , 9 Recent experimental evidence suggests that in different inherited forms of dystonia, such as DYT‐TOR1A, DYT‐THAP1, and DYT‐PRKRA, the molecular disease mechanisms converge toward disturbed eIF2α pathway signaling. 10 , 11 , 12 Moreover, rare variants in the ATF4 gene (coding for the downstream effector of eIF2α) have been reported in patients with cervical dystonia. 11 However, a direct genetic involvement of key components of this pathway in dystonia have remained elusive so far. Very recently, de novo EIF2AK1 and EIF2AK2 missense variants were reported in children with developmental delay, leukoencephalopathy, and neurologic regression following febrile illness, with varying combinations of neurological manifestations, including dystonic features in some. 13 Here, we report the identification of EIF2AK2 variants implicated in the development of early onset generalized dystonia, with or without additional neurological disturbances, and inherited as an autosomal dominant or autosomal recessive trait, or occurring de novo.
Subjects and Methods
Patients
The study was approved by the ethical authorities at the participating institutions, and written informed consent was obtained for each participant. Initially, a Chinese Han family from Taiwan with 5 members in 2 consecutive generations affected by early onset, isolated, generalized dystonia (Family A; Fig 1A) was identified. Subsequently, 2 independent series of unrelated patients with early onset dystonia and with whole exome sequencing (WES) data available (n = 50, Centogene, Germany; n = 45, Besta Institute, Italy) were studied. Finally, in 96 unrelated patients with early onset or familial generalized dystonia collected in Lübeck, Germany, the entire coding region and exon–intron boundaries of EIF2AK2 (NM_002759.3) were sequenced by Sanger methods.
FIGURE 1.
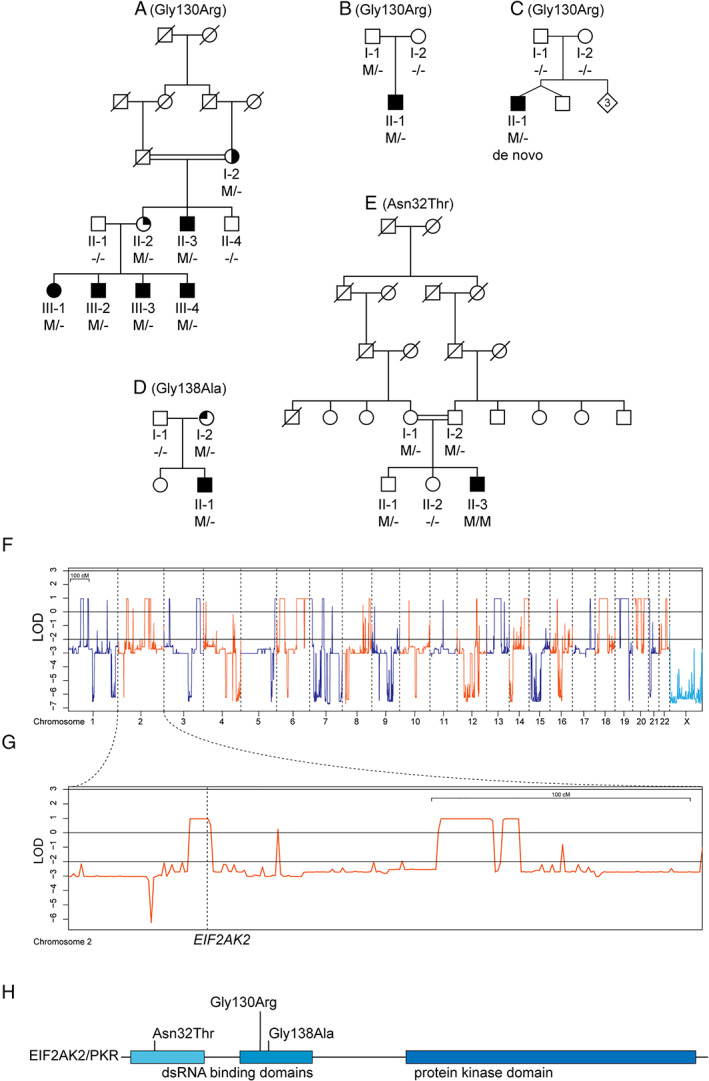
Pedigrees and genetic analyses. (A–E) Pedigrees of the patients with EIF2AK2 variants. Subjects with DNA available are labeled with individual codes; filled symbols denote individuals affected with generalized dystonia; half‐filled symbol indicates right arm and hand dystonia; top‐right quarter‐filled symbol indicates history of mild foot dystonia and asymptomatic upon examination; top‐left quarter‐filled symbol indicates mild bilateral hand tremor; M/M, homozygous carrier of denoted variant; M/−, heterozygous carrier; −/−, wild‐type. (F) Autosomal dominant and X‐linked linkage analysis plots. LOD = logarithm of odds. (G) Linkage analysis plot for chromosome 2 and location of the EIF2AK2 gene. (H) Schematic representation of the EIF2AK2 protein, with functional domains and the relative positions of variants identified in this study. [Color figure can be viewed at www.annalsofneurology.org]
Furthermore, from 3 patients carrying EIF2AK2 variants (A‐III‐4, B‐II‐1, E‐II‐3), skin biopsies were collected and fibroblast cell cultures established for protein expression studies. Fibroblast cell cultures from 3 neurologically normal anonymous donors were used as controls.
Genetic Studies: Family A
Genomic DNA of participating individuals was extracted from peripheral blood using standard protocols. Additionally, in 2 patients (A‐III‐1 and A‐III‐3) mRNA was also isolated and cDNA synthesized using standard protocols. Standard karyotype studies on peripheral blood, and screening of known genes causing dystonia were negative. High‐density genome‐wide genotyping was performed with the Infinium OmniExpress 12v1 Kit (Illumina, San Diego, CA), and genome‐wide copy number variant (CNV) analysis was carried out with Nexus Copy Number (v10, BioDiscovery, El Segundo, CA). Although the pedigree was not large enough to yield genome‐wide significant evidence for linkage, we performed linkage analyses to generate a catalog of genomic regions of interest (regions with logarithm of odds [LOD] score > 0) for filtering of WES variants. Multipoint parametric affected‐only linkage analysis was performed with MERLIN 14 under autosomal dominant, autosomal recessive, and X‐linked dominant or recessive modes of inheritance. In the index case, the exome was captured using Illumina's TruSeq Exome Kit and sequenced with a HiSeq2000 instrument. The reads were aligned to the genome build GRCh37/hg19 with the Burrows–Wheeler Aligner 15 and further processed with Genome Analysis Toolkit Best Practices. 16 Using the exome data, we first searched for variants in genes associated with dystonia. We then searched for variants located within the regions of interest resulting from our linkage analysis. Variants were filtered according to (1) location within linkage regions with LOD > 0; (2) minor allele frequency (MAF) < 0.01% in any public database (dbSNP, ExAC, ESP6500, 1000 Genomes, and gnomAD), as well as in the subpopulation dataset East‐Asia within gnomAD; (3a) exonic nonsynonymous; or (3b) location within ±4 bases from exon–intron boundaries. Retained variants were validated by Sanger sequencing and analyzed for segregation with disease in Family A. In Patients A‐III‐1 and A‐III‐3, we also performed cDNA analysis of EIF2AK2 (protocols and results are available on request).
Additional Genetic Studies
To identify additional patients with EIF2AK2 variants, we consulted 2 independent NGS databases: (1) the Centogene database (Rostock, Germany), containing 50 unrelated patients with early onset isolated forms of generalized dystonia; and (2) the Besta Institute cohort (Milan, Italy), containing 45 unrelated patients with genetically unexplained early onset dystonic disorders.
For samples in the Centogene database, DNA was extracted from ethylenediaminetetraacetic acid (EDTA) blood or from dried blood spots in filter cards (CentoCard) using standard methods. Exome or whole genome sequencing (WGS) was previously performed for diagnostic purposes. For WES, the SureSelect Human All Exon kit (Agilent, Santa Clara, CA) was used for enrichment, then the exome was sequenced using a HiSeq4000 (Illumina) sequencer, with an average coverage targeted to 100x. For WGS, genomic DNA was fragmented by sonication and Illumina adapters ligated for subsequent sequencing on the HiSeqX platform (Illumina) at average coverage depth of 30x. CNV calling is based on the HiSeq Analysis Software's pipeline. Variants were annotated using ANNOVAR 17 and in‐house bioinformatics tools, 18 including CentoFit for prioritization. Samples recruited at the Besta Institute were sequenced at Northwestern University, Chicago, Illinois. Exomes were captured using Illumina's Nextera Rapid Capture according to the manufacturer's recommendations. Indexed and pooled libraries were then sequenced on Illumina's HiSeq3000 (100 base pairs, paired‐end). Bioinformatic analysis of WES data was performed as previously described. 19
In all identified EIF2AK2 variant carriers, variants in other genes associated with dystonia were not found. The EIF2AK2 variants identified by NGS were confirmed using Sanger sequencing (protocols and primers are available upon request). When DNA was available, parents were also tested for the EIF2AK2 variant present in their offspring by Sanger methods.
Cell Culture
Fibroblast cell cultures were expanded in growth medium (Dulbecco modified Eagle medium, 15% fetal calf serum). The day before treatment, fibroblasts were plated at 150,000 cells/well of a 6‐well plate and incubated overnight in growth medium. The next day, cell cultures were transfected with either 2μg/ml (final concentration) polyinosinic acid:polycytidylic acid (poly[I:C]) high molecular weight (HMW) (tlrl‐pic, InvivoGen, San Diego, CA) using Viromer Red transfection reagent (TT100302, OriGene, Rockville, MD) or Viromer Red only (untreated control cell cultures) according to the manufacturers' specifications, and incubated for indicated times at 37°C/5% CO2.
Western Blotting
Western blotting analyses were performed as described previously. 20 , 21 Validated primary antibodies used were rabbit anti–phospho‐eIF2α (Ser51; 119A11; CST, 3597), mouse anti‐eIF2α (sc‐133132, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti‐EIF2AK2 (MAB1980‐SP, R&D Systems, Minneapolis, MN), rabbit recombinant anti‐EIF2AK2 (phospho‐Thr446) antibody (E120, AB32036, Abcam, Cambridge, MA), and mouse anti‐Vinculin (sc‐377103, Santa Cruz Biotechnology). Secondary antibodies used were fluorescently conjugated goat anti‐mouse (IRDye 800) and goat anti‐rabbit (IRDye 680) antibodies (LI‐COR Biosciences, Lincoln, NE) incubated simultaneously. Blots were imaged and analyzed using an Odyssey imaging system (LI‐COR Biosciences).
Statistical Analysis
Two‐way analysis of variance with Tukey multiple comparisons test was performed using Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA), comparing subjects with EIF2AK2 variants and controls. Values represent mean ± standard error (n = 3 biological replicates).
Results
Family A
WES (Patient A‐III‐3) reached an average coverage of 92x of target regions. Variants of interest were not found in known genes causing dystonia. The linkage analysis under an autosomal recessive inheritance model yielded only one region with LOD score > 0 (heterogeneity LOD = 0.968; hg19, chr20:18,152,134–18,967,523); however, WES variants were not found therein. With X‐linked dominant or recessive models, no LOD > 0 regions were obtained. Lastly, the autosomal dominant linkage model yielded 36 genomic regions with LOD > 0 (see Fig 1F), which contained 8 WES variants of interest according to our criteria. After validation by Sanger sequencing, cosegregation analysis left only one variant, c.388G>A, p.Gly130Arg, in heterozygous state in the EIF2AK2 gene, segregating with the disease in Family A. This variant is absent in all population databases, but is predicted as benign by most in silico tools. Furthermore, because the variant is located 2 nucleotides away from an exon–intron boundary, we performed cDNA studies in 2 patients, A‐III‐1 and A‐III‐3, and found no evidence of aberrant EIF2AK2 mRNA splicing (data not shown). Considering the extreme rarity of the p.Gly130Arg missense variant, the rarity of coding variants throughout the entire open reading frame (ORF) of this gene in human databases, the lack of other WES variants of interest, and the known functional links between the proteins encoded by EIF2AK2 and PRKRA (causing DYT‐PRKRA), we considered EIF2AK2 as the candidate disease‐causing gene in Family A.
Additional EIF2AK2 Families
Searching the Centogene WES/WGS databases yielded a total of 3 unrelated probands (B‐II‐1, C‐II‐1, D‐II‐1) with early onset generalized dystonia carrying heterozygous EIF2AK2 missense variants, all absent from gnomAD and other public databases (Table 1). Of note, 2 of these probands carried the same variant as the patients in Family A (c.388G>A,p.Gly130Arg); the first is a German patient of Caucasian origin (B‐II‐1), who inherited the variant from his asymptomatic and neurologically normal father (Family B; see Fig 1B); the other is a boy from Canada with French–Dutch ancestry (C‐II‐1, Family C; see Fig 1C). After testing both his parents by Sanger sequencing for the variant, and for microsatellite markers to confirm paternity, we established the c.388G>A, p.Gly130Arg variant as occurring de novo. Furthermore, one additional Israeli patient of Ashkenazi Jewish and Moroccan origin (D‐II‐1) carried a different heterozygous EIF2AK2 missense variant: c.413G>C, p.Gly138Ala (Family D; see Fig 1D), which was also found in his mother.
TABLE 1.
EIF2AK2 Variants Identified in this Study
| Family | Chromosomal Position | Nucleotide Change | Amino Acid Change | Affected Carriers, n | Presence in gnomAD | Zygosity |
|---|---|---|---|---|---|---|
| A | 2:37368697 | c.388G>A | p.Gly130Arg | 7 | Absent | Heterozygous |
| B | 2:37368697 | c.388G>A | p.Gly130Arg | 1 | Absent | Heterozygous |
| C | 2:37368697 | c.388G>A | p.Gly130Arg | 1 | Absent | Heterozygous, de novo |
| D | 2:37366877 | c.413G>C | p.Gly138Ala | 2 | Absent | Heterozygous |
| E | 2:37374855 | c.95A>C | p.Asn32Thr | 1 | Absent | Homozygous |
Variants are annotated according to genome build GRCh37/hg19 and EIF2AK2 transcript NM_002759.
In the Besta Institute cohort, one Italian patient (E‐II‐3) was identified with early onset generalized dystonia who carried a third EIF2AK2 missense variant, c.95A>C, p.Asn32Thr, in homozygous state (Family E; see Fig 1E). The variant, also absent from gnomAD and all public databases (see Table 1), was detected in heterozygous state in both parents and in one sibling, all asymptomatic and neurologically normal. The parents are consanguineous. Last, Sanger sequencing of the entire EIF2AK2 ORF in the additional 96 dystonia patients from Lübeck detected no further variants with MAF < 1% in gnomAD.
The clinical features in the patients with EIF2AK2 variants identified in this study are reported in Table 2, and videotapes of 5 Taiwanese, the Canadian, and the Italian patient are available (Supplementary Videos). Shared, prominent clinical characteristics include dystonia with onset in infancy or childhood, with subsequent generalization. The anatomical location of the dystonia at onset varied, from the upper to lower extremities. Different patients displayed severe involvement of the axial muscle with dystonic overextension of the back and neck (retrocollis), reminiscent of axial dystonia in DYT‐PRKRA, 9 and neurodegeneration with brain iron accumulation syndromes. 22 Additional neurological or other clinical manifestations were absent in the 5 Taiwanese patients and the German patient, who carried the p.Gly130Arg variant. Their brain magnetic resonance imaging (MRI) scans were unremarkable. Conversely, the Canadian patient with a de novo p.Gly130Arg variant, and the Israeli and the Italian case with different missense variants, displayed prominent, generalized dystonia of early onset, but also varying degrees of additional neurological manifestations and MRI features. These patients are therefore described in more detail.
TABLE 2.
Clinical Findings in Affected Subjects with EIF2AK2 Variants
| Patient | Sex/Ethnicity | EIF2AK2 Variant | AAO/AAE | Site of Onset (dystonia) | Distribution at Last Exam | BFMS | Additional Neurological Signs | Medications | DBS |
|---|---|---|---|---|---|---|---|---|---|
| A‐I‐2 | F/Han Taiwanese | p.Gly130Arg | 17/60 | Right arm and hand | Right arm and hand | nd | None | None | na |
| A‐II‐2 | F/Han Taiwanese | p.Gly130Arg | Child‐hood/51 | Foot | None | nd | None | na | na |
| A‐II‐3 | M/Han Taiwanese | p.Gly130Arg | 18/49 | Trunk | Trunk, legs | 78 | None | T, D, B, C | nd |
| A‐III‐1 | F/Han Taiwanese | p.Gly130Arg | 10/25 | Left toe, leg | Trunk, limbs | 50 | None | T, D, B, C | + |
| A‐III‐2 | M/Han Taiwanese | p.Gly130Arg | 5/24 | Legs, hands | Trunk, limbs, larynx | 114 | None | T, D, B, C, other1 | + |
| A‐III‐3 | M/Han Taiwanese | p.Gly130Arg | 4/21 | Trunk, limbs | Trunk, limbs, larynx | 118 | None | T, D, B, C, other2 | + |
| A‐III‐4 | M/Han Taiwanese | p.Gly130Arg | 10/20 | Feet | Trunk, limbs | 89 | None | D, B, C, other1 | nd |
| B‐II‐1 | M/White German | p.Gly130Arg | 7/14 | Right hand | Trunk, limbs (upper > lower limbs) | 68 | None | nd | |
| C‐II‐1 | M/White Canadian | p.Gly130Arg | 3/6 | Hands | Trunk, limbs | 80 | Spasticity, bilateral Babinski sign, axial hypotonia, mild developmental delay, mild cognitive deficit, abnormal brain MRI | T, B, C, D, L, Bot, other3 | nd |
| D‐I‐2 | F/Ashkenazi and Moroccan | p.Gly138Ala | nk/61 | Cramps in fingers, mild bilateral hand tremor | None | na | None | None | na |
| D‐II‐1 | M/Ashkenazi and Moroccan | p.Gly138Ala | 1/18 | Right leg | Four limbs | 53 | Spasticity, bilateral Babinski sign, pes cavus, abnormal brain MRI | L | nd |
| E‐II‐3 | M/White Italian | p.Asn32Thr | 5/42 | Trunk, neck | Trunk, neck, limbs | 84 | Seizures (neonatal) spasticity, bilateral Babinski sign, reduced vertical gaze, developmental delay, ID, abnormal brain MRI | B, L, Bot | nd |
Variants are annotated according to genome build GRCh37/hg19 and EIF2AK2 transcript NM_00275.
AAE = age at examination; AAO = age at onset; B = baclofen; BFMS = Burke–Fahn–Marsden Dystonia Rating Scale; Bot = botulinum toxin; C = clonazepam; D = diazepam; DBS = deep brain stimulation surgery performed; F = female; ID = intellectual disability; L = L‐dopa; M = male; MRI = magnetic resonance imaging; na = not applicable; nd = not performed; nk = not known; other1 = carisoprodol, carbamazepine, and clozapine; other2 = carbamazepine; other3 = tetrabenazine; T = trihexyphenidyl.
In the Canadian patient (C‐II‐1, Family C; see Fig 1C), family and perinatal history were unremarkable. Bilateral hand tremor was noted at the age of 3.5 years, followed by abnormal posturing (inversion) of his left foot. The dystonic posturing worsened and spread to the ipsilateral arm by the age of 4 years, and to the right body side by 4.5 years, more severe in the leg than the arm, and exacerbated with movement. Mild proximal weakness in the lower extremities and dysarthria were also apparent. When the patient was 5 years old, the dystonia had become generalized, with fluctuating severity. He had frequent falls and difficulty with fine motor skills, due to the tremor and dystonia, and often required a wheelchair to move across longer distances. Interestingly, periodic, stepwise deterioration in his dystonia occurred following certain viral infections and/or surgical interventions. At the age of 5.5 years, following an elective hospitalization for a lumbar puncture and muscle biopsy (under general anesthesia), he never regained the ability to walk, due to increased dystonia and muscle weakness in the legs. At the last examination, at 6.5 years old, he had moderate‐to‐severe generalized dystonia (Burke–Fahn–Marsden Dystonia Rating Scale [BFMS] score = 80.5, disability score = 23/30; see Supplementary Video S6), dysarthria, and dysphagia, and was completely dependent on his parents. He also complained of painful spasms in his left limbs, occurring when he moved or during sleep. He communicated with short sentences, but was difficult to understand. Cognitive assessments revealed a moderate/severe language delay and mild cognitive impairment. There was also moderate axial hypotonia and spasticity in the lower extremities, generalized hyperreflexia, ankle clonus, and bilateral Babinski signs. Cranial nerve examination shows saccadic pursuits and mild tremor of the tongue. There was proximal muscle weakness, more severe in the legs (3/5, with decreased muscle mass) than the arms (4/5). Different oral medications were tried (see Table 2), with only mild or no benefit for the dystonia. Extensive diagnostic workup was initially unremarkable. Brain MRI showed abnormal, nonenhancing, focal, symmetric T2‐hyperintense, T1‐hypointense abnormalities at the bulbomedullary junction (Fig 2A–C). MRI of the spine was unremarkable. A cerebral positron emission tomography (PET) scan revealed a mild global decrease in cerebral activity, particularly in the basal ganglia.
FIGURE 2.
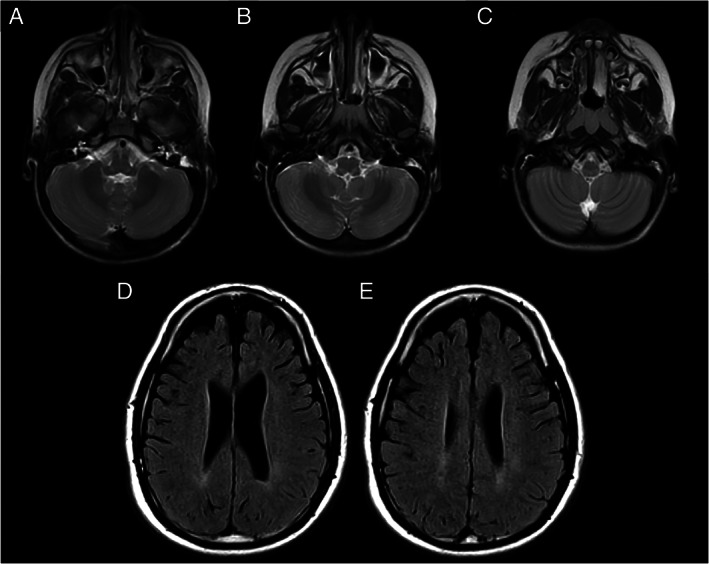
Neuroimaging. (A–C) Brain magnetic resonance imaging (MRI; T2‐axial view) in Patient C‐II‐1 (de novo p.Gly130Arg variant), showing abnormal, focal concentric, symmetric hyperintense signal abnormalities in the posterior brain stem at the bulbomedullary junction. (D, E) Fluid attenuated inversion recovery (FLAIR) MRI images (axial view) in Patient E‐II‐3 (p.Asn32Thr variant), showing bilateral frontal–parietal atrophy and mild hyperintense signal in the posterior periventricular white matter.
The Israeli patient (D‐II‐1) was born at term, following normal pregnancy and delivery, and had normal physical and cognitive development. He started walking at the age of 13 months, with abnormal posturing of the right leg during walking and recurrent falls. The dystonia gradually spread to both legs and then the arms, more prominent on the right side, with no diurnal variation. An L‐dopa trial was discontinued due to gastrointestinal side effects and possible aggravation of the dystonia. At the age of 18 years, he had walking difficulties and could not use his right arm. Neurologic examination showed dystonic postures of the right arm and both legs during walking (BFMS score = 53), normal muscle strength, dystonic tremor in the hands, brisk reflexes, bilateral ankle clonus, bilateral Babinski sign, and pes cavus. Laboratory workup was unremarkable, apart from low serum ceruloplasmin (16mg/dl, normal range = 20–60) and increased urinary copper, in the absence of a Kayser–Fleischer ring, and with an unremarkable liver biopsy. Electromyography/nerve conduction velocity (EMG/NCV) revealed no evidence of polyneuropathy. Brain MRI at the age of 14 years was reported as normal, but hyperintense foci were noted in the left‐frontal white matter (unfortunately, images are not available). His 61‐year‐old mother, known for diabetes mellitus type II and hyperlipidemia, complained of infrequent, transient muscle cramps and numbness of the fingertips, lasting several seconds. Her neurologic examination revealed no neurologic deficits, apart from a minimal postural tremor of the hands.
The Italian patient (E‐II‐3, Family E; see Fig 1E) was born full term after an uncomplicated pregnancy; motor milestones as well as speech were delayed, with independent ambulation at age 3 years, with clumsiness. Generalized tonic epileptic seizures occurred in the first months of life, but were well controlled on antiepileptic drugs. This medication was discontinued at age 3 years, with no recurrence of seizures. The patient also has intellectual disability. At age 5 years, after a febrile illness with high temperature, he developed a slowly progressive generalized dystonia, with a prominent axial component, more severe in the trunk and neck (severe retrocollis). He lost the ability to walk independently in adulthood, and spastic paraparesis developed around age 30 years. Currently, the patient is aged 42 years and his clinical picture is characterized by generalized dystonia with severe axial involvement (BFMS score = 84), spastic paraparesis with bilateral Babinski signs, intellectual disability, spastic/dystonic dysarthria, mild dysphagia, and reduced vertical up‐gaze. Brain MRI at age 30 and 40 years displayed frontal–parietal atrophy and mild hyperintensity in the posterior periventricular white matter on T2‐weighted images (see Fig 2D, E). Oral drugs (L‐dopa, trihexyphenidyl, baclofen, clonazepam) as well as botulinum toxin injections (neck) were marginally beneficial to alleviate dystonia.
Expression Studies
To investigate the effect of the identified EIF2AK2 variants on EIF2AK2 protein function, we analyzed endogenous protein expression levels and phosphorylation status of the EIF2AK2 protein itself, as well as a well‐known downstream target of EIF2AK2 kinase activity, the eIF2α protein, in human skin fibroblast cultures from 3 patients with different EIF2AK2 variants (2 with the heterozygous p.Gly130Arg variant [B‐II‐1, A‐III‐4], 1 with homozygous p.Asn32Thr variant [E‐II‐3]), and 3 unrelated unaffected controls (Fig 3A). We compared the expression levels at steady state (unstimulated cells) as well as in cultures after the EIF2AK2–eIF2α pathway was activated via treatment with the dsRNA molecule poly(I:C) HMW by transfection via Viromer Red compound. This method was chosen to mimic as close as possible the canonical activation of the EIF2AK2‐eIF2α pathway by viral dsRNA, as EIF2AK2 plays a central role in mediating antiviral responses (see Fig 3B, C). 23 Total and Thr446 phosphorylated EIF2AK2, as well as total and Ser51 phosphorylated eIF2α were unchanged at steady state (unstimulated) comparing fibroblasts derived from patients with EIF2AK2 missense variants and unaffected individuals (see Fig 3D, E). Treatment with poly(I:C) HMW elicited a robust activation of the pathway in all cell lines, without significant differences between patients and controls at 4 or 8 hours after treatment (data not shown). Remarkably, after 16 hours, both phosphorylated Thr446 EIF2AK2 and Ser51 eIF2α levels were significantly elevated in all EIF2AK2 patient fibroblasts (phosphorylated Thr446 EIF2AK2: average 5.4 fold ± 1.2 standard deviation [SD]; Ser51 eIF2α: average 3.1 fold ± 0.2 SD) when compared to unaffected control cell lines (phosphorylated Thr446 EIF2AK2: average 0.8 fold ± 0.05 SD; Ser51 eIF2α: average 1.6 fold ± 0.1 SD). These results show that the activation of both EIF2AK2 and eIF2α persists for a longer duration in patient‐derived fibroblasts compared to unaffected control cells.
FIGURE 3.
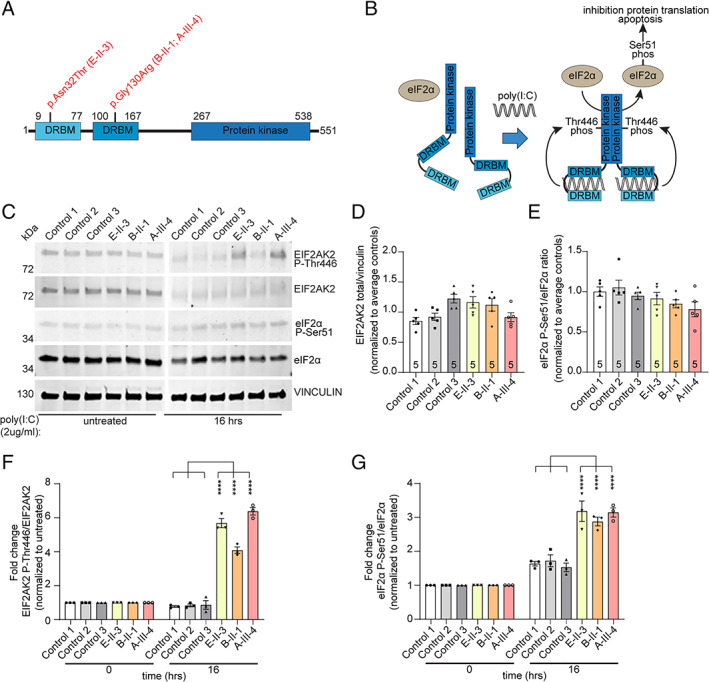
Expression studies. Dystonia‐associated EIF2AK2 variants lead to prolonged phosphorylation of EIF2AK2 Thr446 and downstream target eIF2α‐Ser51 in patient‐derived fibroblasts in response to polyinosinic acid:polycytidylic acid (poly[I:C]) exposure. (A) Schematic representation of the EIF2AK2 protein structure double‐stranded RNA binding motif (DRBM). Locations of the variants detected in the patients are indicated. (B) Schematic diagram showing the downstream effectors of the EIF2AK2 pathway after treatment with poly(I:C). (C) Representative Western blots of protein extracts from control (Control 1, 2, 3) and EIF2AK2 patient (E‐II‐3, B‐II‐1, A‐III‐4) fibroblast cell cultures that were either untreated or treated with poly(I:C) (2μg/ml) for 16 hours. Protein extracts were probed on the same blot for expression of phosphorylated EIF2AK2 (EIF2AK2 P‐Thr446), total EIF2AK2 protein levels (EIF2AK2), phosphorylated eIF2α (eIF2α P‐Ser51), and total eIF2α (eIF2α) protein levels. Vinculin was used as protein loading control. Molecular weights are indicated on the left (kDa). (D) Quantification of total EIF2AK2 levels in untreated cell cultures showing no significant changes between control and patient fibroblast cell cultures (n = 5). (E) Quantification of phosphorylated Ser51 eIF2α protein levels in untreated cell cultures, indicating no changes in EIF2AK2 variant carrying patient cells compared to unrelated control fibroblasts (n = 5). (F) Quantification of the fold change of phosphorylated EIF2AK2 Thr446 over total EIF2AK2 protein levels after poly(I:C) treatment (2μg/ml) for 16 hours normalized to untreated cells. (G) Quantification of the fold change of phosphorylated eIF2αSer51 over total eIF2α protein levels after poly(I:C) treatment (2μg/ml) for 16 hours normalized to untreated cells. Two‐way analysis of variance with Tukey multiple comparisons between controls and subjects with EIF2AK2 variants were performed. Values represent mean ± standard error of the mean (n = 3 biological replicates; ****p < 0.0001). [Color figure can be viewed at www.annalsofneurology.org]
Discussion
Here we report the identification of 3 different EIF2AK2 missense variants, absent from gnomAD and other public databases, that are present in 9 patients from 5 unrelated families with early onset, generalized dystonia. We also show abnormally increased activation of the EIF2AK2 pathway in cell lines from patients carrying 2 of these variants.
The evidence for a causative role in disease development is strong for the c.388G>A, p.Gly130Arg variant, which was prioritized as a result of a genome‐wide unbiased search and segregates with the disease in all patients in the Taiwanese family, as well as being present in 2 unrelated patients of Caucasian ethnicity with very similar or identical phenotypes. Remarkably, in one of these patients the variant occurred de novo. According to the American College of Medical Genetics and Genomics (ACMG) guidelines for interpretation of genetic variants in the clinical setting, 24 the p.Gly130Arg variant would be classified as pathogenic, and the p.Asn32Thr variant as likely pathogenic. The evidence for pathogenicity of the third variant (p.Gly138Ala) remains inconclusive according to these criteria, pending its identification in other patients or functional studies.
It is noteworthy that the p.Gly130Arg variant segregated with disease in heterozygous state in the Taiwanese family, and occurred de novo in the Canadian patient, according to a dominant mechanism of action. Instead, the p.Asn32Thr variant was present in homozygous state in the Italian patient, while heterozygous in his unaffected parents (recessive inheritance). Different modes of inheritance have been reported for other genes causing dystonia (such as DYT‐THAP1 and DYT‐GNAL) 2 or other movement disorders (PDE10A and ADCY5). 25
Of note, the EIF2AK2 variants identified in this study and those reported in the previous publication 13 are not predicted to be functionally damaging by the majority of in silico prediction algorithms. 17 This phenomenon appears to be largely due to the lack of evolutionary conservation of the EIF2AK2 protein sequence. 26 , 27 Likewise, the recurrent p.Pro222Leu missense variant in the PRKRA protein, established as the cause of DYT‐PRKRA, also alters a residue that lacks evolutionary conservation, and that variant is also predicted to be benign by most in silico tools. PRKRA is a functional interactor of PKR, the protein product of EIF2AK2. The phenotype of DYT‐PRKRA and that of EIF2AK2, as we report it here, are also remarkably similar (early onset generalized dystonia, often without additional neurological manifestations). It is therefore tempting to speculate that the PRKRA/EIF2AK2 pathway has acquired special functions in the recent evolution of primates. In keeping with this hypothesis, the EIF2AK2 gene does not display any common coding variants in humans. The most common missense variant (c.575C>G p.Ser192Cys) has an MAF of only 0.000177|50/282502|0.0177% in gnomAD v2.1. These observations collectively suggest an important, human‐specific function of this protein. Independent lines of research have shown that the EIF2AK2 protein is under evolutionary pressure compared to other branches of this family of protein kinases. 27 The accelerated evolution and species‐specificity appear both to be related to the involvement of this protein in the cellular response to viral infections and its coevolution with the infecting viruses. 27
Our functional studies show that EIF2AK2 variants identified in patients with dystonia lead to abnormally persistent activation of the EIF2AK2–eIF2α pathway, whose main function as part of the ISR is inhibition of protein synthesis, and induction of programmed cell death as a first line of defense against numerous types of cellular stress. 28 For efficient recovery and cellular survival after cellular stress, it is also essential that the temporary inhibition of protein synthesis caused by phosphorylated eIF2α is promptly reversed, aided by protein phosphatase 1 complex, leading to synthesis of survival‐related proteins at later time points. 3 As depicted in Figure 3, the activation of EIF2AK2, and eIF2α phosphorylation are abnormally protracted in the patient‐derived fibroblasts as compared to control cells after dsRNA treatment. This might lead to permanent cell abnormalities, as recovery and survival mechanisms may not be induced efficiently in these subjects. Identification of EIF2AK2 variants in our study provides further support for dysregulated eIF2α signaling as a shared pathogenetic theme in dystonia. Previously, other monogenetic causes of dystonia have been identified where the ISR is disturbed (Fig 4).
FIGURE 4.
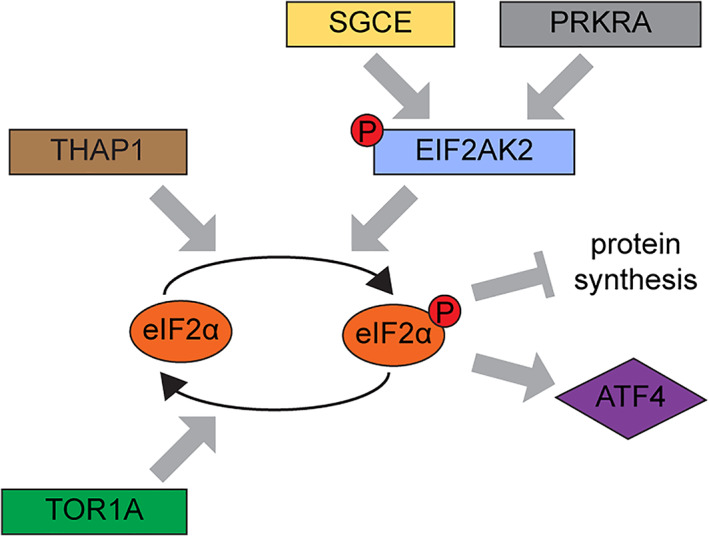
Evidence for dysregulated eIF2α signaling as a shared theme in the pathogenesis of dystonia. Identification of EIF2AK2 variants in this study provides further support for dysregulated eIF2α signaling as a shared pathogenetic theme in dystonia. Evidence for a disturbed integrated stress response (ISR) has been previously reported in other monogenic causes of dystonia, including DYT‐PRKRA, DYT‐TOR1A, DYT‐THAP1, and DYT‐SGCE. Several studies have shown that variants in the PRKRA gene enhanced the susceptibility to endoplasmic reticulum stress leading to heightened EIF2AK2 activation, dysregulation of ISR, and increased apoptosis. 32 , 34 , 35 ISR dysregulation has been reported to play a central role in DYT‐TOR1A. 10 , 11 , 36 THAP1 mutations have been shown to cause dysregulation of the eIF2α signaling pathways in a DYT‐THAP1 mouse model. 12 In a mouse model of DYT‐SGCE, significantly elevated levels of EIF2AK2 transcript are reported, and genes associated with protein translation are among the top downregulated mRNAs. 37 Additionally, rare variants in ATF4—a direct target of eIF2α signaling—have also been reported in cervical dystonia patients. 11 [Color figure can be viewed at www.annalsofneurology.org]
Intriguingly, eIF2α phosphorylation and downstream effectors have also been linked to synaptic plasticity, 29 a pathway that might have important roles in the development of dystonia. 2 However, whether abnormalities in these or other functions of eIF2α signaling are involved in the pathogenesis of dystonia in the patients with EIF2AK2 variants remains to be determined.
All 3 missense variants identified in this study are localized in either the first or second dsRNA binding motif (DRBM) of EIF2AK2 (see Fig 1H). These variants might lead to disturbed dsRNA binding kinetics of the DRBMs, resulting in the extended activation of EIF2AK2 and of the ISR. Alternatively, DRBM1 and DRBM2 are also required for the interaction with the C‐terminal DRBMs of the PRKRA protein. 8 , 31 The variants identified in the dystonia patients might therefore influence the binding kinetics of EIF2AK2 to PRKRA, also resulting in the prolonged activation of the ISR. This would be in line with a previous study showing that in patient‐derived cells carrying the PRKRA dystonia‐causing p.Pro222Leu variant, the affinity of PRKRA–EIF2AK2 interactions is enhanced, thereby leading to intensified EIF2AK2 activation and enhanced cellular death. 32
In the patients described here, the clinical phenotype associated with EIF2AK2 variants appears broad, even in the carriers of the same variant. Childhood onset, severe generalized dystonia was the shared, prominent feature in 9 of our cases. In 6 of these 9, the dystonia remained isolated, without additional neurological manifestations, whereas the other 3 also developed mild intellectual disability, spasticity, and brain MRI alterations. Severe axial involvement was noted in 7 of these 9 cases, being a potential clinical clue. Such a wide clinical spectrum is not surprising, given that other genes causing early onset dystonia are associated with a phenotypic continuum, ranging from pure dystonia to more complex presentations. 2 , 33 Moreover, our observations suggest an incomplete penetrance and variable expressivity for EIF2AK2 variants, as also frequently reported for variants causing other autosomal dominant forms of dystonia, including DYT‐TOR1A and DYT‐THAP1. 1 , 2 Among the subjects who carried the EIF2AK2 p.Gly130Arg variant, one (B‐I‐1) remained asymptomatic and free from dystonic signs or neurological symptoms at examination at the age of 41 years; another (A‐II‐2, 51 years of age) only reported symptoms compatible with foot dystonia in childhood, whereas a third (A‐1‐2, 60 years of age) developed only a segmental form of dystonia (see Table 2). Another subject (D‐I‐2, p.Gly138Ala variant) reported cramps in her hands, and the neurologic examination only revealed a minimal bilateral postural tremor of the hands.
Recently, de novo missense variants in EIF2AK2 have independently been reported as the cause of a complex neurodevelopmental syndrome that appears to share phenotypic and pathogenic mechanisms with central nervous system hypomyelination/vanishing white matter disease. 13 In 8 patients reported with EIF2AK2 de novo missense variants, the phenotype was dominated by developmental delay, language and cognitive impairment, and white matter alterations. Ataxia, dysarthria, hypotonia, parkinsonism, and seizures were also present in some of them, and dystonia (additional details were not provided) was mentioned in 5 cases. 13 All those 8 patients exhibited neurological regression in the setting of febrile illness or infection. This phenotype appears to overlap marginally with that displayed by only some of our cases. Onset of dystonia or deterioration in the context of febrile illness or general anesthesia was also noted in 2 of our patients, and may represent a diagnostic clue to suspect EIF2AK2 mutations in patients with early onset dystonia.
In the same report, it was shown that the EIF2AK2 missense variants encode stable proteins, and the kinase activity of EIF2AK2 is reduced after poly(I:C) treatment in mammalian cell lines and proband‐derived fibroblasts. 13 Although the unaffected stability of the missense EIF2AK2 variants in unstressed cells is in line with our findings, we did not observe reduced kinase activity after poly(I:C) treatment. There may be several explanations for these discrepancies, such as different methodologies, or different EIF2AK2 variants might lead to different functional and clinical outcomes. The variants reported in the previous study and those reported here are not localized at the same amino acid positions, with one exception: at amino acid position 32, the previous study reports an asparagine to serine substitution, whereas in our patient that asparagine is changed into threonine.
One limitation of our study is that, although functional experiments have been performed using endogenous protein expression in patient‐derived fibroblasts, the disease affects brain cells. Although the ISR is ubiquitously present in all organs, we cannot exclude brain tissue‐ or cell‐specific responses that would have been missed in the current study.
Finally, our findings have potential clinical implications for the patients carrying EIF2AK2 variants. First, as EIF2AK2 appears to be hyperactivated in response to cellular stress signals such as viral infections, and neurological deterioration is reported in the context of febrile illness, infections, and general anesthesia, it seems arguable for the patients to limit exposure to viruses and other infective agents, and to conditions of severe metabolic stress. Moreover, development of therapies focused on inhibiting the EIF2AK2 kinase activity as a treatment of dystonia patients carrying the reported variants should be explored. Our data also suggest that caution is warranted when assessing pathogenicity of variants based on DNA or protein sequence conservation, for human genes and pathways under selective evolutionary pressure.
In conclusion, we identify rare EIF2AK2 variants implicated in early onset generalized dystonia, which might be dominantly or recessively inherited, or occur de novo. Our findings provide direct evidence for a key role of a dysfunctional eIF2α pathway in the pathogenesis of dystonia.
Author Contributions
C.‐S.L., C.K., N.E.M., A.M.B.‐A., and V.B. contributed to the conception and design of the study; D.J.S.K., W.M., C.‐S.L., S.O., G.J.B., C.F., V.T., M.C., B.O., L.S.‐D., Y.‐H.W.‐C., C.C.C, H.‐C.C., S.‐L.W., T.‐H.Y., Y.‐H.W., A.E.E., C.P., N.M., M.G.P., A.A.K, J.V., B.L., I.A.M., K.K., M.Q., B.G., K.L., P.B., N.E.M., S.J.L., and A.M.B.‐A. contributed to the acquisition and analysis of data; D.J.S.K., W.M., C.‐S.L., and V.B. contributed to drafting a significant portion of the manuscript or figures.
Potential Conflicts of Interest
C.K. serves as a medical consultant to Centogene on genetic testing reports in the field of movement disorders and dementia, excluding Parkinson disease. K.K., P.B., and A.M.B.‐A. are employees of Centogene, which provides genetic testing for patients with suspected genetic diseases.
Supporting information
SUPPLEMENTARY VIDEO S1 Family A, Patient A‐II‐3, onset age 18 years, videos taken at age 19 years.
SUPPLEMENTARY VIDEO S2 Family A, Patient A‐III‐1, onset age 10 years, video fragments taken at ages 13, 19, and 27 years.
SUPPLEMENTARY VIDEO S3 Family A, Patient A‐III‐2, onset age 5 years, video fragments taken at ages 8, 18, and 24 years.
SUPPLEMENTARY VIDEO S4 Family A, Patient A‐III‐3, onset age 4 years, video fragments taken at age 5 years.
SUPPLEMENTARY VIDEO S5 Family A, Patient A‐III‐4, onset age 10 years, video fragments taken at ages 10 and 20 years.
SUPPLEMENTARY VIDEO S6 Family C, Patient C‐II‐1, onset age 3 years, video fragment taken at age 6 years. First segment documents dysarthria and truncal dystonia. Segments 2 and 3 show generalized dystonia, worse on the left side, with a painful spasm at the end of the video.
SUPPLEMENTARY VIDEO S7 The patient presents spastic/dystonic dysarthria and generalized dystonia with severe axial involvement. He presents retrocollis that is reduced by a geste antagoniste. When he performs movements of prone‐supination of the hands, axial overflow is shown with worsening of retrocollis. The gait is paraparetic and dystonic, with wide base.
Supplementary Videos S1–S5 document the evolution of the dystonia, from early disease stage to advanced phases with severe generalization, in 5 patients from the Taiwanese family.
Acknowledgments
K.L. acknowledges funding from the Damp Foundation for this study. V.B. acknowledges the financial support from the Department of Clinical Genetics, Erasmus MC, University Medical Center Rotterdam, and the Stichting Parkinson Fonds, the Netherlands to his Chair in Genetics of Movement Disorders.
We are indebted to all patients and their relatives.
Demy J. S. Kuipers and Wim Mandemakers are co–first authors.
References
- 1. Lohmann K, Klein C. Dystonia. In: Rosenberg RN, Pascual JM, eds. Rosenberg's molecular and genetic basis of neurological and psychiatric disease. Waltham, MA: Academic Press, 2020:117‐134. [Google Scholar]
- 2. Balint B, Mencacci NE, Valente EM, et al. Dystonia. Nat Rev Dis Primers 2018;4:25. [DOI] [PubMed] [Google Scholar]
- 3. Pakos‐Zebrucka K, Koryga I, Mnich K, et al. The integrated stress response. EMBO Rep 2016;17:1374–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dabo S, Meurs EF. dsRNA‐dependent protein kinase PKR and its role in stress, signaling and HCV infection. Viruses 2012;4:2598–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel RC, Sen GC. PACT, a protein activator of the interferon‐induced protein kinase, PKR. EMBO J 1998;17:4379–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dickerman BK, White CL, Kessler PM, et al. The protein activator of protein kinase R, PACT/RAX, negatively regulates protein kinase R during mouse anterior pituitary development. FEBS J 2015;282:4766–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meyer C, Garzia A, Mazzola M, et al. The TIA1 RNA‐binding protein family regulates EIF2AK2‐mediated stress response and cell cycle progression. Mol Cell 2018;69:622–635. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camargos S, Scholz S, Simon‐Sanchez J, et al. DYT16, a novel young‐onset dystonia‐parkinsonism disorder: identification of a segregating mutation in the stress‐response protein PRKRA. Lancet Neurol 2008;7:207–215. [DOI] [PubMed] [Google Scholar]
- 9. Quadri M, Olgiati S, Sensi M, et al. PRKRA mutation causing early‐onset generalized dystonia‐parkinsonism (DYT16) in an Italian family. Mov Disord 2016;31:765–767. [DOI] [PubMed] [Google Scholar]
- 10. Beauvais G, Rodriguez‐Losada N, Ying L, et al. Exploring the interaction between eIF2alpha dysregulation, acute endoplasmic reticulum stress and DYT1 dystonia in the mammalian brain. Neuroscience 2018;371:455–468. [DOI] [PubMed] [Google Scholar]
- 11. Rittiner JE, Caffall ZF, Hernandez‐Martinez R, et al. Functional genomic analyses of Mendelian and sporadic disease identify impaired eIF2alpha signaling as a generalizable mechanism for dystonia. Neuron 2016;92:1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zakirova Z, Fanutza T, Bonet J, et al. Mutations in THAP1/DYT6 reveal that diverse dystonia genes disrupt similar neuronal pathways and functions. PLoS Genet 2018;14:e1007169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mao D, Reuter CM, Ruzhnikov MRZ, et al. De novo EIF2AK1 and EIF2AK2 variants are associated with developmental delay, leukoencephalopathy, and neurologic decompensation. Am J Hum Genet 2020;106:570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 2002;30:97–101. [DOI] [PubMed] [Google Scholar]
- 15. Li H, Durbin R. Fast and accurate short read alignment with Burrows‐Wheeler transform. Bioinformatics 2009;25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKenna A, Hanna M, Banks E, et al. The genome analysis toolkit: a MapReduce framework for analyzing next‐generation DNA sequencing data. Genome Res 2010;20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high‐throughput sequencing data. Nucleic Acids Res 2010;38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trujillano D, Bertoli‐Avella AM, Kumar Kandaswamy K, et al. Clinical exome sequencing: results from 2819 samples reflecting 1000 families. Eur J Hum Genet 2017;25:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carecchio M, Invernizzi F, Gonzàlez‐Latapi P, et al. Frequency and phenotypic spectrum of KMT2B dystonia in childhood: a single‐center cohort study. Mov Disord 2019;34:1516–1527. [DOI] [PubMed] [Google Scholar]
- 20. Olgiati S, Quadri M, Fang M, et al. DNAJC6 mutations associated with early‐onset Parkinson's disease. Ann Neurol 2016;79:244–256. [DOI] [PubMed] [Google Scholar]
- 21. Quadri M, Mandemakers W, Grochowska MM, et al. LRP10 genetic variants in familial Parkinson's disease and dementia with Lewy bodies: a genome‐wide linkage and sequencing study. Lancet Neurol 2018;17:597–608. [DOI] [PubMed] [Google Scholar]
- 22. Stamelou M, Lai SC, Aggarwal A, et al. Dystonic opisthotonus: a "red flag" for neurodegeneration with brain iron accumulation syndromes? Mov Disord 2013;28:1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci 2013;70:3493–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carecchio M, Mencacci NE. Emerging monogenic complex hyperkinetic disorders. Curr Neurol Neurosci Rep 2017;17:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taniuchi S, Miyake M, Tsugawa K, et al. Integrated stress response of vertebrates is regulated by four eIF2α kinases. Sci Rep 2016;6:32886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rothenburg S, Seo EJ, Gibbs JS, et al. Rapid evolution of protein kinase PKR alters sensitivity to viral inhibitors. Nat Struct Mol Biol 2009;16:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia‐Ortega MB, Lopez GJ, Jimenez G, et al. Clinical and therapeutic potential of protein kinase PKR in cancer and metabolism. Expert Rev Mol Med 2017;19:e9. [DOI] [PubMed] [Google Scholar]
- 29. Trinh MA, Klann E. Translational control by eIF2alpha kinases in long‐lasting synaptic plasticity and long‐term memory. Neurobiol Learn Mem 2013;105:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hughes D, Mallucci GR. The unfolded protein response in neurodegenerative disorders—therapeutic modulation of the PERK pathway. FEBS J 2019;286:342–355. [DOI] [PubMed] [Google Scholar]
- 31. Huang X, Hutchins B, Patel RC. The C‐terminal, third conserved motif of the protein activator PACT plays an essential role in the activation of double‐stranded‐RNA‐dependent protein kinase (PKR). Biochem J 2002;366:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaughn LS, Bragg DC, Sharma N, et al. Altered activation of protein kinase PKR and enhanced apoptosis in dystonia cells carrying a mutation in PKR activator protein PACT. J Biol Chem 2015;290:22543–22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abela L, Kurian MA. Postsynaptic movement disorders: clinical phenotypes, genotypes, and disease mechanisms. J Inherit Metab Dis 2018;41:1077–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burnett SB, Vaughn LS, Strom JM, et al. A truncated PACT protein resulting from a frameshift mutation reported in movement disorder DYT16 triggers caspase activation and apoptosis. J Cell Biochem 2019;120:19004–19018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burnett SB, Vaughn LS, Sharma N, et al. Dystonia 16 (DYT16) mutations in PACT cause dysregulated PKR activation and eIF2α signaling leading to a compromised stress response. Neurobiol Dis 2020;146:105135. [DOI] [PubMed] [Google Scholar]
- 36. Beauvais G, Watson JL, Aguirre JA, et al. Efficient RNA interference‐based knockdown of mutant torsinA reveals reversibility of PERK‐eIF2α pathway dysregulation in DYT1 transgenic rats in vivo. Brain Res 2019;1706:24–31. [DOI] [PubMed] [Google Scholar]
- 37. Xiao J, Vemula SR, Xue Y, et al. Role of major and brain‐specific Sgce isoforms in the pathogenesis of myoclonus‐dystonia syndrome. Neurobiol Dis 2017;98:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY VIDEO S1 Family A, Patient A‐II‐3, onset age 18 years, videos taken at age 19 years.
SUPPLEMENTARY VIDEO S2 Family A, Patient A‐III‐1, onset age 10 years, video fragments taken at ages 13, 19, and 27 years.
SUPPLEMENTARY VIDEO S3 Family A, Patient A‐III‐2, onset age 5 years, video fragments taken at ages 8, 18, and 24 years.
SUPPLEMENTARY VIDEO S4 Family A, Patient A‐III‐3, onset age 4 years, video fragments taken at age 5 years.
SUPPLEMENTARY VIDEO S5 Family A, Patient A‐III‐4, onset age 10 years, video fragments taken at ages 10 and 20 years.
SUPPLEMENTARY VIDEO S6 Family C, Patient C‐II‐1, onset age 3 years, video fragment taken at age 6 years. First segment documents dysarthria and truncal dystonia. Segments 2 and 3 show generalized dystonia, worse on the left side, with a painful spasm at the end of the video.
SUPPLEMENTARY VIDEO S7 The patient presents spastic/dystonic dysarthria and generalized dystonia with severe axial involvement. He presents retrocollis that is reduced by a geste antagoniste. When he performs movements of prone‐supination of the hands, axial overflow is shown with worsening of retrocollis. The gait is paraparetic and dystonic, with wide base.
Supplementary Videos S1–S5 document the evolution of the dystonia, from early disease stage to advanced phases with severe generalization, in 5 patients from the Taiwanese family.


