Abstract
Background
Despite growing evidence of the presence and clinical relevance of deficits in social cognition in individuals with alcohol use disorder (AUD), less is known about the potential of “natural” recovery with abstinence in this neurocognitive domain. This study investigated the abstinence‐based recovery of neurocognitive social abilities in alcohol‐dependent patients (ADP) using a prospective longitudinal design with follow‐up assessment under controlled conditions of abstinence during alcohol dependence inpatient treatment.
Methods
Seventy‐seven participants (42 ADP and 35 healthy controls [HC]) performed social cognition testing, including facial emotion recognition, perspective taking, and affective responsiveness twice (baseline/T1 and follow‐up/T2) during comparable follow‐up periods. Assessment of social cognition in abstinent ADP was conducted at the beginning (T1; within the first 2 weeks) and at the end (T2; within the last 2 weeks) of long‐term (2 months) abstinence‐oriented alcohol dependence inpatient treatment. Only patients abstinent for >14 days (last heavy drinking day >21 days) at baseline (T1) and who remained abstinent at follow‐up (T2) were included.
Results
ADP, who on average were nearly 2 months abstinent at T1, showed poorer social cognition in all 3 areas (emotion recognition, perspective taking, and affective responsiveness) than HC. There was no difference between groups on the change in performance over time, and group differences (ADP vs. HC) remained significant at T2, indicating persistent social cognition deficits in ADP following controlled abstinence during inpatient treatment.
Conclusions
Our findings indicate no natural recovery of social cognition impairments in ADP during an intermediate to long‐term period of abstinence (2+ months), the usual active treatment phase. Research aimed at developing interventions that focus on the improvement of social cognition deficits (e.g., social cognition training) and determining whether they benefit short‐ and long‐term clinical outcomes in AUD seems warranted.
Keywords: Alcohol Use Disorder, Social Cognition, Recovery, Emotion Recognition, Cognitive Function
This prospective longitudinal study found that deficits in social cognition in alcohol‐dependent patients persist despite alcohol abstinence during inpatient treatment. Novel interventions (e.g., adjunctive personalized neurocognitive rehabilitation therapy) targeting poor social cognition may be needed in addition to standard treatment for alcohol dependence.
Alcohol dependence (AD), such as other severe drug use disorders, previously has been defined as a chronic, relapsing disease of the brain (e.g., Koob and Volkow, 2016). In AD, compromised frontally based networks (frontolimbic, frontocerebellar, and frontostriatal) are associated with a variety of mild‐to‐moderate cognitive deficits (Le Berre et al., 2017; Maillard et al., 2020; Oscar‐Berman et al., 2014; Zahr et al., 2017). Cognitive deficits in AD most prominently involve impairments in memory and executive function/impulsivity (Crowe et al., 2020; Rupp et al., 2006; Stavro et al., 2013), and increasingly have been affirmed to play a critical role in poorer prognosis in this severe illness with high rates of recidivism (Bates et al., 2013; Czapla et al., 2015; Rolland et al., 2019; Rupp et al., 2016; Wilcox et al., 2014). There is now rapidly emerging evidence that cognitive deficits in alcohol use disorder (AUD) include social cognition (Bora and Zorlu, 2017; Le Berre, 2019; Maurage et al., 2019), an important functional domain previously introduced as 1 of 6 neurocognitive core domains for the major/mild neurocognitive disorder diagnosis in DSM‐5 (APA, 2013).
Alcohol‐related deficits in social cognition in AUD (Bora and Zorlu, 2017; Charlet et al., 2014; Freeman et al., 2018; Townshend and Duka, 2003; Valmas et al., 2014) are mainly documented in the major subcategories facial emotion recognition and theory of mind (TOM; Bora and Zorlu, 2017; Maurage et al., 2019). Abilities such as facial emotion recognition (referring to the ability to identify and discriminate between emotional states of others) and TOM (a more complex aspect of social cognition, referring to the ability to infer more complex mental states, i.e., intentions and beliefs of others) are crucial for adaptive social interaction and functioning, and need to be considered in a clinical context (Le Berre, 2019; Le Berre et al., 2017; Maillard et al., 2020; Maurage et al., 2019). Misinterpretations of emotional states of others, for instance, have the potential to contribute to social difficulties associated with alcohol use (Attwood and Munafó, 2014; Maurage and Campanella, 2013; Maurage et al., 2011; Miller et al., 2015; Thoma et al., 2013). Misunderstandings can result in inappropriate reactions, contributing to social problems and conflicts, and thereby raise social stress, which may increase the likelihood of relapse. In line, Kornreich and colleagues (2002) observed that deficits in facial emotion recognition in AD are associated with interpersonal problems (Hoffman et al., 2019). Recently, we found that poorer facial emotion recognition in AD is predictive for less successful treatment with regard to relapse and early dropout of treatment (Rupp et al., 2017), further underpinning the clinical significance of social cognition deficits (Campbell et al., 2018; Foisy et al., 2007; Rolland et al., 2019). Hence, social cognition performance may aid in the early detection of individual risk for poorer treatment prognosis and thus could serve as a clinical biomarker. These deficits need interventions that aim for improvement, such as neurocognitive rehabilitation therapy using a patient‐tailored approach (Campbell et al., 2018; Le Berre, 2019; Maurage et al., 2019; Rolland et al., 2019; Rupp et al., 2017). Inspired by the prospect to potentially improve treatment outcome in AUD with respect to alcohol relapse and psychosocial negative consequences through improving cognitive function, past years witnessed emerging innovative research on cognitive rehabilitation interventions on other cognitive domains with promising results (Bates et al., 2013; Rolland et al., 2019; Rupp et al., 2012; Verdejo‐Garcia et al., 2019). We are not aware of any neurocognitive rehabilitation intervention study focusing specifically on the improvement in social cognition in AUD (or other drug use disorders), yet. However, as a first step, and before developing new neurocognitive rehabilitation treatments to improve clinically relevant social cognition deficits, it appears necessary to first establish whether social cognition deficits are persistent impairments, or whether these deficits show natural recovery with abstinence in AUD (Schulte et al., 2014).
Research on recovery of social cognition in AUD is still in its infancy. We are only aware of 2 longitudinal studies providing inconsistent findings in facial emotion recognition deficits. Using similar observation periods (after detoxification/weeks 3 to 4 to 3 months of abstinence) in alcohol‐dependent patients (ADP) and including healthy controls (HC) in follow‐up assessments, Erol and colleagues (2017) reported improvements, whereas Foisy and colleagues (2007) found persistent deficits. A cross‐sectional study comparing ADP groups with 2 to 3 weeks and >2 months of abstinence observed no group differences in poor emotion recognition (Kornreich et al., 2001), and meta‐analyses found comparable deficits in emotion recognition and TOM in subgroups of <2 and >2 months of abstinence (Bora and Zorlu, 2017).
Cognitive deficits in general may be most pronounced during acute abstinence and may be difficult to distinguish from the subacute pharmacological effects during the first 2 weeks (Schulte et al., 2014). The likelihood of cognitive recovery following sustained abstinence from the intermediate‐term (after detoxification up to 2 months of abstinence) to the long‐term abstinence period (>2 months; Fein et al., 1990) is up to now less clear (Le Berre et al., 2017; Schulte et al., 2014). Although both neuropsychological and neuroimaging studies in AUD evidence that abstinence is associated with improvements (Charlet et al., 2018; Meyerhoff and Durrazo, 2020; Pitel et al., 2009; Schulte et al., 2014), persistent deficits have been reported particularly in verbal memory and executive function (e.g., Brandt et al., 1983; Crowe et al., 2020; Rupp et al., 2006; Stavro et al., 2013). Current knowledge mainly derives from research using a cross‐sectional study design, for example, comparing performance in different abstinent samples (Crowe et al., 2020; Le Berre et al., 2017; Stavro et al., 2013). Of the few longitudinal neurocognitive studies in AUD, many lack a longitudinal design for both, patients and healthy control groups, and thus, practice effects (as a possible explanation for “recovery” effects) cannot be excluded (Schulte et al., 2014). Other factors associated with inconsistent findings and suggested to possibly impact on cognitive recovery in AUD have been discussed with recency and severity of drinking, age (older age, earlier onset of regular drinking), gender differences, and smoking (Charlet et al., 2018; Durazzo and Meyerhoff, 2020; Maillard et al., 2020; Meyerhoff and Durrazo, 2020; Nguyen‐Louie et al., 2017; Pitel et al., 2009), number of prior detoxifications, and a positive family history (Charlet et al., 2018; Loeber et al., 2010; Schulte et al., 2014).
Given the clinical impact of social cognition deficits, and the parallel emerging of new treatments, a better understanding of abstinence‐related recovery or persistence during the usual treatment period in AUD appears of direct clinical relevance. The aim of the present prospective study was to investigate whether deficits in social cognition show recovery following controlled abstinence.
Materials and Methods
Participants
Participants of this study (n = 77) included 42 nonamnesic and nondemented alcohol‐dependent inpatients (ADP) undergoing our long‐term (8‐week) abstinence‐based alcohol dependence inpatient treatment at the Alcohol Dependence Treatment Unit of the Medical University Innsbruck, and 35 HC.
ADPs’ inclusion criteria were as follows: (i) current DSM‐IV diagnosis of alcohol dependence (Structured Clinical Interview DSM‐IV [SCID] disorders; Wittchen et al., 1997), (ii) age between 19 and 65 years, and (iii) a minimum duration of alcohol abstinence of at least >14 days, and last heavy drinking day (male: 60+ g, female: 40+ g) at least of >3 weeks at the time of neuropsychological baseline assessment (T1), which took place within the first 2 weeks of inpatient treatment. According to our study goal, (iv) only abstinent ADP until the follow‐up neuropsychological assessment (NP; T2) at the end of inpatient treatment were included. Exclusion criteria were (i) signs of psychotic symptoms and lifetime psychotic disorders (DSM‐IV, SCID), (ii) low level of premorbid functioning including low formal education (<9 years) and an IQ < 85, (iii) signs of dementia, a neurodegenerative process unrelated to alcohol, a history of traumatic brain injury with neurological symptoms or a loss of consciousness (>5 minutes within the last 15 years), and a Mini‐Mental State Examination (MMSE) score < 24, (iv) severe somatic disorders and physical handicaps, and (v) an insufficient knowledge of the German language.
Thirty‐five HC comparable in age, gender, education, and smoking were recruited in the general population by word of mouth and served as control group (see Table 1). In addition to the exclusion criteria mentioned above, HC had to be free of current or past DSM‐IV mental disorder (SCID), including a history of alcohol or any other drug use disorder (except smoking).
Table 1.
Sociodemographic and Clinical Characteristics
| Characteristics | Alcohol‐dependent patients (ADP) | Healthy controls (HC) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | % | (N) | Mean | SD | % | (N) | ||
| Age, years | 46.4 | 7.7 | 45.3 | 11.4 | NS | ||||
| Gender, female | 31 | (13) | 43 | (15) | NS | ||||
| Education level: without high‐school graduation/with high‐school graduation/university | 81 (34)/14 (6)/5 (2) | 80 (28)/11 (4)/9 (3) | NS | ||||||
| Premorbid intelligence, MWT‐B | 103.4 | 11.4 | 107.4 | 11.4 | NS | ||||
| Mini‐mental state examination, MMSE | 28.2 | 1.1 | 29.5 | 0.7 | <0.001 | ||||
| Days abstinent at neuropsychological assessment (NP) T1 | 54.6 | 31.9 | / | / | |||||
| Days last heavy drinking (HD) at NP T1 | 61.9 | 29.2 | / | / | |||||
| Time between NP T1 and T2, days | 37.4 | 6.4 | 39.9 | 7.5 | NS | ||||
| HD days last year | 224.8 | 113.7 | / | / | |||||
| % HD days, TLFB (last 90 days before treatment entry) | 31.4 | 24.8 | / | / | |||||
| % abstinent days, TLFB (last 90 days before treatment entry) | 67.0 | 25.0 | / | / | |||||
| Drinking amount (g)/drinking day, TLFB (last 90 days before treatment entry)a | 151.8 | 83.8 | / | / | |||||
| Age first sip of alcohol | 13.2 | 4.2 | / | / | |||||
| Age first intoxicationb | 16.7 | 4.9 | / | / | |||||
| Age of regular drinking onset (drinking at least once a week) | 21.4 | 9.0 | |||||||
| Age of regular HD onset (HD at least once a week) | 25.9 | 11.9 | |||||||
| Duration of alcohol dependence, years | 10.7 | 9.0 | / | / | |||||
| Smokers | 91 | (38) | 91 | (32) | NS | ||||
| Drug use (last 3 months): cannabis/stimulants/opioids | 5 (2)/0 (0)/0 (0) | 6 (2)/0 (0)/0 (0) | NS | ||||||
| Comorbidity—substance dependence (SCID/DSM‐IV): | |||||||||
| Alcohol and benzodiazepine (BZD) dependence/alcohol, BZD, and cannabis dependence | 12 (5)/2 (1) | / | |||||||
| Depressive symptoms: | |||||||||
| BDI II at NP T1 | 17.5 | 11.1 | 3.7 | 4.2 | <0.001 | ||||
| BDI II at NP T2c | 9.2 | 9.7 | / | / | |||||
| Psychological distress | |||||||||
| SCL‐90‐R (T score) at NP T1 | 59.2 | 9.0 | 44.3 | 7.2 | <0.001 | ||||
| SCL‐90‐R (T score) at NP T2 | 52.7 | 10.8 | / | / | |||||
| Comorbidity—mental illness (SCID/DSM‐IV): | |||||||||
| Depressive disorder/anxiety disorder | 5 (2)/7 (3) | / | |||||||
NS, p‐value > 0.05; MWT‐B, Mehrfachwahl–Wortschatz test; MMSE, Mini‐Mental State Examination; TLFB, Timeline Followback; SCID, Structured Clinical Interview DSM‐IV disorders; BDI II, Beck Depression Inventory II; SCL‐90‐R, Symptom Checklist‐90‐Revised.
Information for a N = 35 patients (7 patients were already abstinent during this period), and is missing for b1 and c2 patients.
Sociodemographic and Clinical Measures
Medical and psychiatric history, sociodemographic, and drinking characteristics of participants were evaluated using a semi‐structured interview, and the SCID‐I (DSM‐IV). In patients, this was supplemented by medical records, chart reviews, and regular laboratory (blood chemistry, liver function tests, urinalysis) monitoring. Alcohol use in the 90 days before admission was quantified with the Timeline Followback (Sobell and Sobell, 1992). Background basic cognitive measures included the MMSE (German version by Kessler et al., 1990), and the Mehrfachwahl–Wortschatz test (Lehrl, 2005), a multiple‐choice vocabulary test designed to measure premorbid (verbal) intelligence. Depressive symptoms and psychological distress were assessed using the German versions of the Beck Depression Inventory II (BDI II; Hautzinger et al., 2006) and the Symptom Checklist‐90‐Revised (SCL‐90‐R; Franke, 2002). Patients with first‐ or second‐degree relatives with a definite alcohol problem were coded as family history positive (FH+; Family Tree Questionnaire; Mann et al., 1985).
Neurocognitive Measures of Social cognition
We applied 3 social cognition tasks: emotion recognition, perspective taking, and affective responsiveness. All of the stimuli depict basic emotions following the concept from Ekman (Ekman, 1992). Facial stimuli presented in the current tasks were selected from a standardized stimulus set (Gur et al., 2002) and have been validated prior to the study by 30 independent raters. Only stimuli that were correctly identified by over 70% of the raters were included. Similar versions of these tasks have been validated and described in more detail in prior research investigating neural and behavioral differences in healthy women and men as well as other mental disorders (e.g., Derntl et al., 2010, 2012; Peveretou et al., 2020). Most recently and using the same set of tasks and items administered in this study, we have further validated their clinical relevance in ADP (Rupp et al., 2017).
Emotion Recognition
Thirty‐six colored Caucasian facial identities depicting 5 basic emotions (happiness, sadness, anger, fear, and disgust) and neutral expressions were randomly presented (6 stimuli per condition). For emotion recognition, subjects were instructed to choose the correct emotion by selecting from these 6 categories (see Fig. 1 for examples of each task).
Fig. 1.
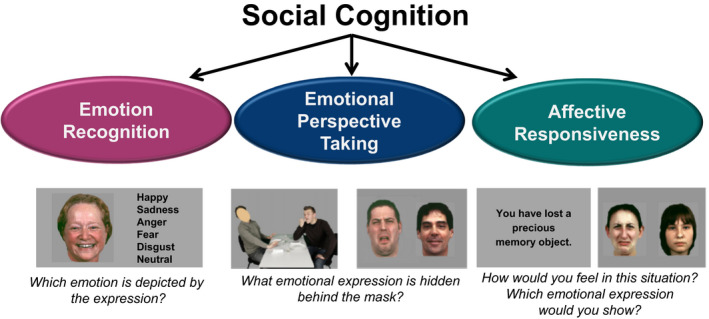
Neurocognitive measures of social cognition.
Perspective Taking
Participants viewed 36 pictures, each presented for 5 seconds, depicting scenes showing 2 Caucasians involved in a social interaction, portraying the same 5 basic emotions, and neutral scenes (6 stimuli per condition). The face of one person was masked, and participants were asked to infer the corresponding emotional expression of the masked face. Responses were made by selecting between 2 different emotional facial expressions or a neutral expression presented after each scene. One option was correct, and the other was selected at random from all other choices.
Affective Responsiveness
Thirty‐six short written sentences describing real‐life situations which are likely to induce basic emotions (the same emotions as described above) and situations that were emotionally neutral (6 stimuli per condition) were presented. Participants were asked to imagine how they would feel if they were experiencing those situations. Stimuli were presented for 5 seconds, and response format was the same as for perspective taking, presenting 2 facial expressions, one showing the correct emotion (neutral expression) and the other was chosen randomly from the other expressions.
The presentation of images and recording of responses was achieved using the Presentation© software package (Neurobehavioral Systems, Inc., Albany, CA).
Procedure
Neuropsychological assessments (NPs) of social cognition abilities in ADP were performed at the beginning of treatment within the first 2 weeks (baseline/T1), and at the end, during the last 2 weeks (NP T2) of our long‐term (8‐week) abstinence‐based alcohol dependence inpatient treatment at the Alcohol Dependence Treatment Unit of the Medical University Innsbruck (see Fig. 2 for a timeline of study procedures). Both assessments included the same task stimuli and sequence (emotion recognition, perspective taking, affective responsiveness). Our standard alcohol dependence treatment (average duration of 7 to 9 weeks), following detoxification conducted in a different unit of our clinic or elsewhere, is a highly structured comprehensive program comprising psychiatric management, cognitive behaviorally oriented individual and group therapy focusing on enhancement of abstinence motivation, relapse prevention, crisis management, relaxation techniques and skill training, and occupational therapy, sports, and activity groups. During treatment, patients were regularly screened for substance and alcohol use by repeated urine and breath alcohol analysis as well as blood laboratory tests (e.g., γ‐glutamyl transferase and carbohydrate‐deficient transferrin). Social cognition abilities were assessed in HC in the same task sequence and an equivalent follow‐up interval (see Table 1). Written informed consent was obtained from participants before participation. This study, as part of a larger study, was approved by the ethics committee of the Medical University Innsbruck.
Fig. 2.
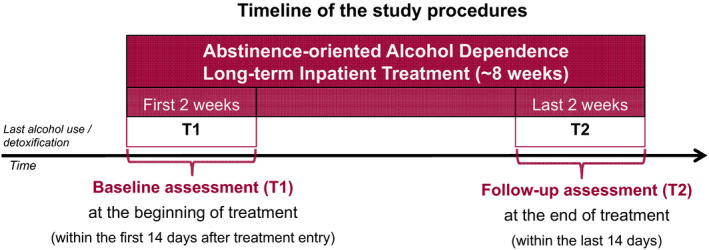
Study procedures.
Statistical Analyses
Comparisons of groups (ADP vs. HC) with respect to sociodemographic and clinical characteristics were evaluated by t‐tests, chi‐square test, and Fisher’s exact test, depending on the variable type. Group differences regarding social cognition measures (emotion recognition, perspective taking, and affective responsiveness) were assessed by repeated‐measures multivariate analysis of variance (MANOVA) and by subsequent repeated‐measures ANOVAs for the individual measures. In these analyses, the variable time (T1 vs. T2) served as the within‐subjects factor and group as the between‐subjects factor. Nonnormally distributed variables of social cognition were subjected to an appropriate transformation prior to these analyses (x→√{highest possible score − x}). Partial eta‐squared (η 2) was calculated as a measure of effect size, making use of Cohen’s convention for small, medium, and large effects (Cohen, 1988). They were reported for group main effects and group × time interactions, as we were interested in differences between ADP and HC, and potential recovery in ADP.
To examine potential gender differences in recovery, gender was added as a between‐subjects factor to the repeated‐measures MANOVA in our complementary analysis.
In addition, to examine potential effects of psychological symptoms, age, abstinence, and other alcohol‐related characteristics on recovery (performance changes) in ADP, we performed correlation analyses (Pearson’s r) of relative changes in social cognition (i.e., the difference in performance between T2 and T1 divided by the performance at T1 for each patient) with relative changes in psychological distress (SCL‐90‐R), and depressive symptoms (BDI II; i.e., the difference between ratings at T2 and T1 divided by the rating at T1), and with age and alcohol‐related characteristics, including duration of alcohol abstinence, duration of last heavy drinking (HD) day, %HD days last 3 months before treatment, drinking amount (g)/drinking day last 3 months, duration of alcohol dependence, and age beginning with a regular HD drinking (at least once a week).
To assess the potential impact of repeated withdrawal from alcohol on recovery of social cognition impairment under controlled abstinence, we compared the performance of ADP according to previous detoxifications using the same criterion as prior research in other cognitive functions (e.g., Loeber et al., 2010) with patients having had less than 2 detoxifications (Lo‐Detox) and those with 2 or more previous detoxifications (Hi‐Detox). Lastly, ADP were compared according to their family history of alcohol (FH+ vs. FH−). Due to unclear “possible” problems in relatives in 4 patients, data analysis was performed with n = 38.
These complementary analyses following examinations of our primary hypotheses mainly served to provide additional information, which may be helpful in guiding future investigations.
Results
Participants
ADP and HC were comparable with regard to age, education, premorbid intelligence, gender, smoking, and follow‐up interval (see Table 1 for sociodemographic and clinical characteristics). Consistent with the extant literature, ADP reported significantly more depressive symptomatology (BDI II), and less psychological well‐being (SCL‐90‐R) than HC at baseline (T1), but showed significant improvement until follow‐up at T2 (all ps < 0.001). In line, only very few ADP had a mental comorbidity, and only 2 participants in each group used a drug (cannabis) within the last 3 months before T1 (last use HC > 1 month, ADP > 10 days). Although groups differed significantly in the MMSE, all scores in both groups were in the “unimpaired” range of this global screening (see in addition our exclusion criteria). Of note, at baseline NP (T1) ADP already showed a period of on average nearly 2 months of abstinence (intermediate duration of abstinence), with a similar period of having had their last HD day (>2 months).
Neurocognitive Measures of Social Cognition
Repeated‐measures MANOVA of social cognition measures revealed a significant main effect of group, indicating an overall deficit of ADP when compared with HC (see Table 2). Results showed a significant main effect of time (p < 0.001), but the main effect of time × group interaction was not significant, indicating that changes in performance from T1 to T2 are not different between ADP and HC.
Table 2.
Social Cognition Performance
| Variables | Alcohol‐dependent patients | Healthy controls | Analysis a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | Group | Time × group | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F‐value | df | p | Partial η 2 | F‐value | df | p | Partial η 2 | |
| Social cognition (%) b | 4.461 | 3, 73 | 0.006 | 0.16 | 0.242 | 3, 73 | 0.876 | 0.01 | ||||||||
| Emotion recognition b | 73.9 | 9.2 | 77.8 | 8.9 | 79.4 | 10.3 | 82.7 | 8.7 | 7.428 | 1, 75 | 0.008 | 0.09 | 0.150 | 1, 75 | 0.699 | 0.00 |
| Perspective taking b | 81.4 | 10.1 | 84.9 | 8.4 | 87.0 | 8.9 | 89.4 | 6.4 | 9.015 | 1, 75 | 0.004 | 0.11 | 0.547 | 1, 75 | 0.462 | 0.01 |
| Affective responsiveness b | 90.5 | 5.7 | 92.5 | 6.9 | 93.7 | 6.0 | 96.1 | 3.2 | 9.522 | 1, 75 | 0.003 | 0.11 | 0.001 | 1, 75 | 0.972 | 0.00 |
MANOVA (printed in italics) or ANOVA.
Significant effect of time (all ps < 0.007).
Analyses of the individual social cognition tasks revealed a significant main effect of group in all social cognition measures, indicating poorer emotion recognition, perspective taking, and affective responsiveness performance of ADP when compared with HC (see Table 2). The main effect of time was significant for all measures (all ps < 0.008), but there was no significant interaction of time × group (see Table 2), indicating no differential effect between ADP and HC. Direct comparisons (see Fig. 3) indicated that ADP showed poorer ability to recognize emotions in faces than HC at the beginning of treatment at T1 (p = 0.014, η 2 = 0.08) and at the end at T2 (p = 0.018, η 2 = 0.07). They also showed poorer perspective‐taking ability at both NP assessments (T1: p = 0.007; η 2 = 0.09; T2: p = 0.015; η 2 = 0.08) and performed worse with regard to affective responsiveness at both NP assessments (T1: p = 0.010, η 2 = 0.08; T2: p = 0.014, η 2 = 0.08).
Fig. 3.
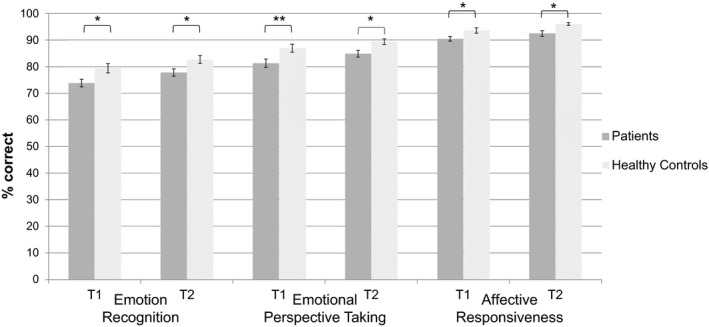
Social cognition performance. Mean accuracy (percent correct) with error bars (standard error) at baseline (T1) and follow‐up assessment (T2) for social cognition measures in alcohol‐dependent patients and HC. *p < 0.05; **p < 0.01.
Complementary Analyses
With regard to potential gender differences, repeated‐measures MANOVA with gender as an additional between‐subjects factor showed no significant main effect of gender (p > 0.7) nor significant interactions of time × gender (p > 0.5) or time × group × gender (p > 0.1).
Additional analyses in ADP revealed no significant correlation between the change in social cognition and improvements in depressive symptoms (BDI II), and psychological well‐being (SCL‐90‐R; all ps > 0.1). In line, group comparisons (ADP vs. HC) excluding ADP with comorbidity (N = 9, SCID) did not alter our findings. Further, there was no significant correlation between the change in social cognition and alcohol‐related characteristics (all ps > 0.2), and age (p > 0.06).
With regard to potential impact of previous detoxification on recovery, repeated‐measures MANOVA of social cognition measures with Hi‐Detox (n = 21) versus Lo‐Detox (n = 21) as between‐subjects factor showed neither a significant main effect of Detox‐group (p > 0.1) nor a significant interaction of time × Detox‐group (p > 0.6). With regard to FH, repeated‐measures MANOVA of social cognition measures with FH+ (n = 24) versus FH− (n = 14) as between‐subjects factor showed no main effect of FH group (p > 0.2) nor a significant time × FH group interaction (p > 0.3), indicating no significant difference in recovery between patients with and without a positive family history of alcohol.
Discussion
This prospective study investigated whether social cognition deficits show “natural” recovery following controlled abstinence in AD. Our findings in AD inpatients indicate persistent deficits in social cognition from the intermediate‐term (after detoxification) to the long‐term abstinence period (>2 months; in our sample up to 6 months), the usually active treatment period.
Specifically, our results show that on average nearly 2‐month abstinent ADP at baseline assessment exhibited poorer facial emotion recognition, perspective taking, and affective responsiveness abilities compared with HC. At follow‐up assessment (at the end of abstinence‐controlled alcohol dependence inpatient treatment), abstinent ADP still performed significantly worse in all assessed social cognition components by comparison with HC. The present study findings, thus, evidence deficits in facial emotion recognition, perspective taking (affective TOM), and affective responsiveness in ADP, and indicate that these social cognition deficits do not recover naturally with controlled abstinence during inpatient treatment.
While there is a clear necessity for longitudinal studies into the characterization of recovery with abstinence in cognitive deficits in AUD in general (Crowe et al., 2020; Schulte et al., 2014), the lack of research in social cognition is even larger (Bora and Zorlu, 2017; Le Berre, 2019; Le Berre et al., 2017; Maurage et al., 2019). Filling this gap and to the best of our knowledge, the present study is the first in AUD prospectively investigating the potential of recovery of several components of social cognition, and the largest of only 2 other longitudinal studies, we are aware of (Erol et al., 2017; Foisy et al., 2007). Contrary to our finding, Erol and colleagues (2017) reported an improvement in facial emotion recognition with 3 months of abstinence approaching performance levels of healthy comparisons. Methodological differences related for instance to the used tasks (only a small number of black‐and‐white photographs in the emotion recognition task) and unknown characteristics of the comparison group by the study of Erol and colleagues (2017) may at least in part account for these inconsistencies. The present findings of persistent deficits in social cognition, including facial emotion recognition, corroborate and extend prior meta‐analytic findings (Bora and Zorlu, 2017), cross‐sectional (Kornreich et al., 2001), and longitudinal research (Foisy et al., 2007) demonstrating persistent facial emotion recognition impairments in AUD.
Our results show performance gains in ADP from baseline to follow‐up assessment. Gains were also observed in HC, and groups (ADP and HC) did not differ in changes in performance, indicating practice effects following prior assessment rather than improved cognitive functioning or recovery, respectively (ADP started lower and ended lower than HC). Insofar, these results corroborate previous suggestions pointing to the necessity of longitudinal study designs with repeated measurements also for the control group in research aiming to characterize cognitive trajectories, such as the potential of recovery of cognitive function in AUD and other drug use disorders (Le Berre et al., 2017; Schulte et al., 2014).
Complementary analyses, aimed to provide additional information in the characterization of abstinence‐based recovery, revealed no significant correlation between changes in psychopathological symptoms and social cognition measures, suggesting that expected improvements in depressive symptoms (BDI II) and psychological well‐being (SCL‐90‐R) in the context of controlled abstinence during inpatient treatment in ADP are not necessarily accompanied by recovery in social cognition. In addition, performing the analysis without the small number of ADP with comorbidity (n = 9, except smoking) revealed the same pattern of findings, further underscoring the relative independence of persistent social cognition deficits in ADP (Bora and Zorlu, 2017; Foisy et al., 2007). Due to the low number of nonsmoking ADP, we abstained from further analysis, but we controlled for smoking in our HC (Durazzo and Meyerhoff, 2020).
Further complementary analyses revealed no differential effect of gender, family history, number of prior detoxifications, and no significant association with alcohol‐related characteristics. These findings suggest that at least during our observation period trajectories of social cognition recovery might not relate to these risk factors in ADP achieving controlled abstinence from the intermediate‐ to long‐term phase (from 2 months up to 6 months of abstinence, and even a slightly longer period regarding last HD day). However, we cannot exclude that vulnerabilities in terms of recovery‐related risk factors (e.g., alcohol‐related history including prior detoxification, family history) may impact social cognition on other stages in the recovery process with abstinence in AUD (e.g., earlier [detoxification] or later stages [long‐term abstinence >6 months]). Moreover, detoxification programs differ between institutions and countries (Loeber et al., 2010), which are usually about 3 weeks of extended inpatient treatment programs comprising much more than medically supervised detoxification. Therefore, “number of prior detoxification,” respectively, low/high prior detoxification categories, may differ between countries.
The present findings may raise the question, whether impaired social cognition rather than being an alcohol‐induced consequence is a pre‐existing risk factor, involved in the development of AUD (Khemiri et al., 2020; Le Berre, 2019; Maurage et al., 2019). Persistent deficits may be genetically influenced risk factors possibly rendering individuals vulnerable for heavy excessive drinking at early age. Our complementary analyses, however, showed no association related to early alcohol use (e.g., poorer recovery in ADP with early age regular HD as a proxy of a potential vulnerability risk factor for development) or to FH (e.g., poorer recovery in ADP with a positive FH, as an indication of a genetically influenced potential endophenotype). Whether cognitive deficits are caused by alcohol intake (and may be more likely amenable to abstinence‐related recovery) represents a vulnerability risk factor, which is present before the onset of the disorder (and possibly less amenable to abstinence‐related recovery), or an interaction of both is still a question under debate in AUD (Le Berre et al., 2017). In this context, promising findings of a recent re‐analysis by Meyerhoff and Durrazo (2020) are of note, indicating that even relapsers with low‐risk drinking levels may show brain (structure/function) recovery comparable with complete abstinence, a nearly unachievable goal for many individuals with AUD. Unfortunately, social cognition measures were not included in this research, and we do not assume that low‐risk drinking level would have led to a recovery not observed in our strictly abstinent APD. Despite all the advantages related to our strict study design in a controlled (inpatient) treatment setting, and including the control for abstinence and other recovery‐related factors (e.g., environmental/lifestyle, dietary factors), our design at the same time bears some limitations, including the particular window of the recovery process observation (postdetoxification alcohol dependence treatment). Hence, the issue of potential impact of alcohol consumption, vulnerability, and risk factors (and their interaction) on social cognition deficits and their recovery should be pursued and clearly needs further longitudinal prospective investigation.
Given the present findings, we have to overrule a previous assumption of our group. Using the same social cognition measures, we previously found that only emotion recognition ability showed predictive utility of poorer treatment outcome (e.g., relapse/treatment drop out) and differentiated between ADP treatment outcome‐related groups (Rupp et al., 2017). We previously assumed that the failure of the other tasks to show a clinical impact possibly might relate to a low sensitivity of these tasks appearing rather easy, which may have led to a ceiling effect in performance. In light of the present findings, indicating that all 3 tasks are sensitive enough to differentiate between abstinent ADP and HC, it seems unlikely that a ceiling effect in performance might have led to this pattern of prior findings, instead rather underlines the particular importance of emotion recognition ability as a clinically relevant neurocognitive risk factor in ADP with regard to treatment outcome (Rupp et al., 2017).
Our present study evidence deficits in ADP in 3 social cognition abilities, respectively, 3 neurocognitive measures previously introduced to tap the core components of empathy separately (Derntl et al., 2010, 2012; Rupp et al., 2017). Due to current diversity in conceptual and operational definitions, and indeed, research may profit by simply using lower‐level constructs, and describe more precisely what is actually being measured (Hall and Schwartz, 2018), we now bypass this term. Accordingly, our study in ADP found deficits with regard to the ability to identify emotions in faces (emotion recognition), the ability to infer an emotional state of another person by taking the social context and people's behavior into account (perspective taking), and poorer ability to put themselves in a certain extrinsic emotional condition (affective responsiveness).
In this respect, and from a clinical perspective the present findings confirm, extend and supplement the current knowledge about social cognition in AUD in important ways, including that these deficits do not recover with controlled abstinence during inpatient alcohol dependence treatment, which does not specifically focus on these deficits. Affective responsiveness is directed on the self, targeting generation and experience of basic emotions. Deficits possibly may indicate poorer emotional experience (self‐awareness, self‐reflection) or poorer ability to simulate emotions vicariously. In contrary, the perspective‐taking task (and the emotion recognition task) takes focus on social interaction with a partner. The observed deficits in perspective taking may indicate that ADP do not benefit from contextual information when interpreting the emotional meaning of a social situation (compared with inferring emotions from facial displays only in the emotion recognition task). The clinical impact of impaired perspective taking (affective TOM) and affective responsiveness in AUD, however, to date still awaits scientifically proven affirmation.
All evidence together, we would like to summarize briefly some clinical implications of current knowledge and future prospects (Campbell et al., 2018; Le Berre, 2019; Le Berre et al., 2017; Maillard et al., 2020; Maurage et al., 2019; Rolland et al., 2019) as follows: Effective alcohol dependence treatment may profit from including social cognition assessment at the beginning of treatment, in order to be able to incorporate individual deficits. The assessment of facial emotion recognition early at the beginning of treatment and by using objective measures (Rupp et al., 2017) might be of particular relevance, in order to identify high‐risk patients for poorer treatment prognosis (relapse/dropout), and to consider these deficits early in the treatment process (e.g., to solve or even prevent any possible miscommunication). In addition, deficits may need alternative, new interventions that aim to improve these deficits, for instance, neurocognitive rehabilitation therapy. We are not aware of any intervention study specifically aiming to improve social cognition in AUD yet. From current knowledge, interventions aimed to improve social cognition in AUD may focus on emotion recognition ability, given its clinical relevance with regard to priority treatment outcome. Future research to develop interventions focusing on the improvement of social cognition deficits in AUD may benefit from promising prior research in other disorders (e.g., Grant et al., 2017), and by using a personalized training approach, similar as previously demonstrated by our group as an add‐on intervention to treatment as usual in other neurocognitive domains (attention, executive function, memory) and with generalizing psychological (well‐being) and alcohol‐related (craving) effects (Rupp et al., 2012).
Despite the relevant findings, some limitations of this study must be noted. Although to the best of our knowledge even the largest of the few longitudinal studies on social cognition in AUD (Erol et al., 2017; Foisy et al., 2007), our sample size is still small. Further, our study focused on the potential of abstinence‐based recovery. This study was not aimed to investigate etiology or causal relation, and we abstained from analyses on factors possibly impacting on general heterogeneity of social cognition deficits in AUD. This is also due to the fact that according to our study goal, importantly, we have included a rather selected patient sample, namely treatment‐seeking ADP achieving controlled long‐term abstinence in alcohol dependence inpatient treatment. Our findings, thus, might not generalize, for instance, to non–treatment‐seeking populations, and the general clinical population in AUD treatment, including patients dropping out or relapsing during/before alcohol dependence treatment (e.g., earlier during/after detoxification treatment). Our present findings, however, may derive from a clinical AUD population with even less poor social cognition abilities. Social cognition deficits may lead to relapse‐related social dysfunction (Hoffman et al., 2019; Kornreich et al., 2002; Maurage et al., 2011, 2019), may hamper individuals to seek treatment related to impaired perception and understanding of the repercussion of alcohol on an individuals' social sphere (Le Berre, 2019), and have been associated with relapse and treatment dropout (Foisy et al., 2007; Rupp et al., 2017). Although the interpretation of results cannot be extrapolated to other abstinence periods (earlier/later) and to other AUD populations than those investigated in this study, it is possible that with our strict study design according to our primary study goal, we might have missed a clinical population with AUD with even poorer social cognition abilities, at least with regard to emotion recognition ability.
To conclude, there is a lack of research on neurocognitive recovery in AUD in general and on social cognition in particular. As an effort to fill this gap, the present study found persistent social cognition deficits in ADP, which do not recover with controlled abstinence during inpatient alcohol dependence treatment. Future research to develop interventions focusing on the improvement of social cognition deficits (e.g., neurocognitive social cognition rehabilitation) and to determine the possible benefit of enhancement in clinical short‐ and long‐term outcomes seems warranted.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
We thank all participants and the multidisciplinary clinical treatment team for their support during study conduction at the Alcohol Dependence Treatment Unit of the Medical University Innsbruck (Division of Psychiatry I, Department of Psychiatry, Psychotherapy and Psychosomatics).
[Corrections added on February 09, 2021, after online publication: the article title “Do Social Cognition Deficits Recover in Alcohol‐Dependent Patients With Abstinence?” has been modified to “Do Social Cognition Deficits Recover with Abstinence in Alcohol‐Dependent Patients?”]
References
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing, Washington DC. [Google Scholar]
- Attwood AS, Munafó MR (2014) Effects of acute alcohol consumption on the processing of emotion in faces: implications for understanding alcohol‐related aggression. J Psychopharmacol 28:719–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Buckman JF, Nguyen TT (2013) A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol Rev 23:27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Zorlu N (2017) Social cognition in alcohol use disorder: a meta‐analysis. Addiction 112:40–48. [DOI] [PubMed] [Google Scholar]
- Brandt J, Butters N, Ryan C, Bayog R (1983) Cognitive loss and recovery in long‐term alcohol abusers. Arch Gen Psych 40:435–442. [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Lawrence AJ, Perry CJ (2018) New steps for treating alcohol use disorder. Psychopharmacology 235:1759–1773. [DOI] [PubMed] [Google Scholar]
- Charlet K, Rosenthal A, Lohoff FW, Heinz A, Beck A (2018) Imaging resilience and recovery in alcohol dependence. Addiction 113:1933–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet K, Schlagenhauf F, Richter A, Naundorf K, Dornhof L, Weinfurtner CE, König F, Walaszek B, Schubert F, Müller CA, Gutwinski S, Seissinger A, Schmitz L, Walter H, Beck A, Gallinat J, Kiefer F, Heinz A (2014) Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addict Biol 19:439–451. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates, Mahwah NJ. [Google Scholar]
- Crowe SF, Cammisuli DM, Stranks EK (2020) Widespread cognitive deficits in alcoholism persistent following prolonged abstinence: an updated meta‐analysis of studies that used standardised neuropsychological assessment tools. Arch Clin Neuropsychol 35:31–45. [DOI] [PubMed] [Google Scholar]
- Czapla M, Simon JJ, Richter B, Kluge M, Friederich HC, Herpertz S, Mann K, Herpertz SC, Loeber S (2015) The impact of cognitive impairment and impulsivity on relapse of alcohol‐dependent patients: implications for psychotherapeutic treatment. Addict Biol 21:873–884. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Eickhoff S, Kellermann T, Falkenberg DI, Schneider F, Habel U (2010) Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology 35:67–82. [DOI] [PubMed] [Google Scholar]
- Derntl B, Seidel EM, Schneider F, Habel U (2012) How specific are emotional deficits? A comparison of empathic abilities in schizophrenia, bipolar and depressed patients. Schizophr Res 142:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ (2020) Cigarette smoking history is associated with poorer recovery in multiple neurocognitive domains following treatment for an alcohol use disorder. Alcohol 85:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P (1992) An argument for basic emotions. Cogn Emot 6:169–200. [Google Scholar]
- Erol A, Kirdok AA, Zorlu N, Polat S, Mete L (2017) Empathy, and its relationship with cognitive and emotional function in alcohol dependency. Nordic J Psychiatry 71:205–209. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L (1990) Cognitive impairments in abstinent alcoholics. West J Med 152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Foisy ML, Kornreich C, Fobe A, D'Hondt L, Pelc I, Hanak C, Verbanck P, Philippot P (2007) Impaired emotional facial expression recognition in alcohol dependence: do these deficits persist with midterm abstinence? Alcohol Clin Exp Res 31:404–410. [DOI] [PubMed] [Google Scholar]
- Franke GH (2002) SCL‐90‐R: Die Symptom‐Checkliste von LR Derogatis. 2nd ed. Beltz Test, Göttingen. [Google Scholar]
- Freeman CR, Wiers CE, Sloan ME, Zehra A, Ramirez V, Wang GJ, Volkow ND (2018) Emotion recognition biases in alcohol use disorder. Alcohol Clin Exp Res 42:1541–1547. [DOI] [PubMed] [Google Scholar]
- Grant N, Lawrence M, Preti A, Wykes T, Cella M (2017) Social cognition interventions for people with schizophrenia: a systematic review focussing on methodological quality and intervention modality. Clin Psychol Rev 56:55–64. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE (2002) A method for obtaining 3‐dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods 115:137–143. [DOI] [PubMed] [Google Scholar]
- Hall JA, Schwartz R (2019) Empathy present and future. J Soc Psychol 159:225–243. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Keller F, Kuhner C (2006) Beck Depressions‐Inventar (BDI II). Harcourt Test Services, Frankfurt am Main. [Google Scholar]
- Hoffman LA, Lewis B, Nixon SJ (2019) Neurophysiological and interpersonal correlates of emotional face processing in alcohol use disorder. Alcohol Clin Exp Res 43:1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J, Denzler P, Markowitsch HJ (1990) Mini‐Mental‐Status‐Test (MMST). Deutsche Fassung. Hogrefe Testzentrale, Göttingen. [Google Scholar]
- Khemiri L, Franck J, Jayaram‐Lindström N (2020) Effect of alcohol use disorder family history on cognitive function. Psychol Med 14:1–13. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Hess U, Noël X, Streel E, Le Bon O, Dan B, Pelc I, Verbanck P (2001) Deficits in recognition of emotional facial expression are still present in alcoholics after mid‐ to long‐term abstinence. J Stud Alcohol 62:533–542. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, Hess U, Noël X, Pelc I, Verbanck P (2002) Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol Alcohol 37:394–400. [DOI] [PubMed] [Google Scholar]
- Le Berre AP (2019) Emotional processing and social cognition in alcohol use disorder. Neuropsychology 33:808–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Fama R, Sullivan EV (2017) Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res 41:1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S (2005) Mehrfachwahl‐Wortschatz‐Intelligenztest MWT‐B. 5th ed. Spitta, Balingen. [Google Scholar]
- Loeber S, Duka T, Welzel Marquez H, Nakovics H, Heinz A, Mann K, Flor H (2010) Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol 45:541–547. [DOI] [PubMed] [Google Scholar]
- Maillard A, Cabé N, Viader F, Pitel AL (2020) Neuropsychological deficits in alcohol use disorder: impact on treatment, in Cognition and Addiction, Chapter 8 (VerdejoGarcia A ed), pp. 103–128. Academic Press, London. [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D (1985) Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend 15:61–67. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S (2013) Experimental and clinical usefulness of crossmodal paradigms in psychiatry: an illustration from emotional processing in alcohol‐dependence. Front Hum Neurosci 7:394. 10.3389/fnhum.2013.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Grynberg D, Noël X, Joassin F, Hanak C, Verbanck P, Luminet O, de Timary P, Campanella S, Philippot P (2011) The “Reading the Mind in the Eyes” test as a new way to explore complex emotions decoding in alcohol dependence. Psych Res 190:375–378. [DOI] [PubMed] [Google Scholar]
- Maurage P, Rolland B, D'Hondt F (2019) Socio‐emotional deficits in severe alcohol use disorders, in Neuroscience of Alcohol, Chapter 39 (Preedy VR ed), pp. 373–381. Academic Press, London. [Google Scholar]
- Meyerhoff DJ, Durrazo TC (2020) Not all is lost for relapsers: relapsers with low WHO risk drinking levels and complete abstainers have comparable regional gray matter volumes. Alcohol Clin Exp Res 44:1479–1487. [Google Scholar]
- Miller MA, Bershad AK, de Wit H (2015) Drug effects on responses to emotional facial expressions: recent findings. Behav Pharmacol 26:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen‐Louie TT, Matt GE, Jacobus J, Li I, Cota C, Castro N, Tapert SF (2017) Earlier alcohol use onset predicts poorer neuropsychological functioning in young adults. Alcohol Clin Exp Res 41:2082–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar‐Berman M, Valmas MM, Sawyer KS, Mosher Ruiz S, Luhar RB, Gravitz ZR (2014) Profiles of impaired, spared, and recovered neuropsychological processes in alcoholism. Handb Clin Neurol 125:183–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peveretou F, Radke S, Derntl B, Habel U (2020) A short empathy paradigm to assess empathic deficits in schizophrenia. Behav Sci 10(2):41. 10.3390/bs10020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Rivier J, Beauneiux H, Vabret F, Desgranges B, Eustache F (2009) Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6‐month period. Alcohol Clin Exp Res 33:490–498. [DOI] [PubMed] [Google Scholar]
- Rolland B, D'Hondt F, Montègue S, Brion M, Peyron E, D'Aviau de Ternay J, de Timary P, Nourredine M, Maurage P (2019) A patient‐tailored evidence‐based approach for developing early neuropsychological training programs in addiction settings. Neuropsychol Rev 29:103–115. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Beck JK, Heinz A, Kemmler G, Manz S, Tempel K, Fleischhacker WW (2016) Impulsivity and alcohol dependence treatment completion: Is there a neurocognitive risk factor at treatment entry? Alcohol Clin Exp Res 40:152–160. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Derntl B, Osthaus F, Kemmler G, Fleischhacker WW (2017) Impact of social cognition on alcohol dependence treatment outcome: poorer facial emotion recognition predicts relapse/dropout. Alcohol Clin Exp Res 41:2197–2206. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Drexler A, Hausmann A, Hinterhuber H, Kurz M (2006) Executive function and memory in relation to olfactory deficits in alcohol‐dependent patients. Alcohol Clin Exp Res 30:1355–1362. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Kemmler G, Kurz M, Hinterhuber H, Fleischhacker WW (2012) Cognitive remediation therapy during treatment for alcohol dependence. J Stud Alcohol Drugs 73:625–634. [DOI] [PubMed] [Google Scholar]
- Schulte MH, Cousijn J, den Uyl TE, Goudriaan AE, van den Brink W, Veltman DJ, Schilt T, Wiers RW (2014) Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin Psychol Rev 34:531–550. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline followback: a technique for assessing self‐reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods (Litten RZ, Allen JP eds), pp. 41–72. Humana Press, Totowa, NJ. [Google Scholar]
- Stavro K, Pelletier J, Potvin S (2013) Widespread and sustained cognitive deficits in alcoholism: a meta‐analysis. Addict Biol 18:203–213. [DOI] [PubMed] [Google Scholar]
- Thoma P, Friedmann C, Suchan B (2013) Empathy and social problem solving in alcohol dependence, mood disorders and selected personality disorders. Neurosci Biobehav Rev 37:448–470. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T (2003) Mixed emotions: alcoholics' impairments in the recognition of specific emotional facial expressions. Neuropsychologia 41:773–782. [DOI] [PubMed] [Google Scholar]
- Valmas MM, Mosher Ruiz S, Gansler DA, Sawyer KS, Oscar‐Berman M (2014) Social cognition deficits and associations with drinking history in alcoholic men and women. Alcohol Clin Exp Res 38:2998–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo‐Garcia A, Lorenzetti V, Manning V, Piercy H, Bruno R, Hester R, Pennington D, Tolomeo S, Arunogiri S, Bates ME, Bowden‐Jones H, Campanella S, Daughters SB, Kouimtsidis C, Lubman DI, Meyerhoff DJ, Ralph A, Rezapour T, Tavakoli H, Zare‐Bidoky M, Zilverstand A, Steele D, Moeller SJ, Paulus M, Baldacchino A, Ekhtiari H (2019) A roadmap for integrating neuroscience into addiction treatment: a consensus of the neuroscience interest group of the international society of addiction medicine. Front Psychiatry 10:877. 10.3389/fpsyt.2019.00877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA (2014) Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev Neurosci 25:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M (1997) SKID I. Strukturiertes Klinisches Interview für DSM‐IV. Achse I: Psychische Störungen. Hogrefe, Göttingen. [Google Scholar]
- Zahr NM, Pfefferbaum A, Sullivan EV (2017) Perspectives on fronto‐fugal circuitry from human imaging of alcohol use disorders. Neuropharmacology 122:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]


