Abstract
Novel noncombustible tobacco products offer adult smokers (ASs) alternatives to combustible cigarettes lower on the continuum of risk; however, the abuse potential of such products has not been well studied. The objective of this study was to evaluate the abuse potential of 2 chewable tobacco‐derived nicotine containing products, VERVE Chews Blue Mint (test 1) and Green Mint (test 2), in ASs compared with own‐brand cigarettes (CIGS) and nicotine polacrilex gum (GUM) using subjective measures and nicotine pharmacokinetics. ASs used the test products during a 5‐day at‐home trial prior to completing an in‐clinic 4‐period randomized crossover study. During the study ASs used test products, CIGS, and GUM once on separate days. Responses to Tobacco/Nicotine Withdrawal and Direct Effects of Product questionnaires were documented, and blood samples were collected to assess nicotine pharmacokinetics during each product use. Nicotine pharmacokinetic parameters (Cmax and AUC) were statistically significantly lower with use of test products compared with CIGS and statistically significantly higher compared with GUM. No appreciable differences were noted between the 2 flavors for any of the end points measured. Reductions in maximum urge to smoke and maximum responses to the question “Is the Product ‘Pleasant’ Right Now?” for the test products were statistically significantly lower than CIGS but comparable to GUM. Similar results were observed for responses to other items in the 2 questionnaires. The test products, under the conditions of this study, carry lower abuse potential than own‐brand cigarettes and similar to nicotine polacrilex gum.
Keywords: abuse liability, nicotine, novel tobacco products, pharmacodynamics, pharmacokinetics
Cigarette smoking is a leading cause of preventable death in the United States, contributing to nearly half a million premature deaths annually. 1 , 2 Despite overwhelming scientific evidence demonstrating negative health consequences, >37 million Americans continue to smoke. 3 Although quitting all tobacco products is optimum for lowering the health impact from tobacco products, adult smokers (ASs) unable or unwilling to quit could benefit by switching completely from combustible products like cigarettes to lower‐risk noncombustible tobacco products. Analysis of data from Population Assessment of Tobacco and Health Wave 1 indicates that more than half of ASs (∼55%) are indeed interested in switching to lower‐risk tobacco products.
A growing body of evidence indicates that noncombustible products like heat‐not‐burn tobacco products, e‐vapor products, snus, and traditional smokeless tobacco products have the potential to reduce the risk from smoking‐related diseases. 4 , 5 , 6 , 7 , 8 Many in public health 9 , 10 , 11 have recognized the existence of a continuum of risk among tobacco products, with conventional, combustible cigarettes at the highest end of that spectrum and noncombustible products on the lower end.
In the current study, we assessed nicotine pharmacokinetics and subjective responses from 2 chewable tobacco‐derived nicotine containing products—VERVE Chews, Blue Mint (test 1) and Green Mint (test 2). Each test product contains ∼1.5 mg of tobacco‐derived nicotine that meets specifications recognized by the U.S. Pharmacopeia. The purpose of this study was to develop scientific evidence to address the abuse potential of new tobacco products. In the context of pharmaceutical products, the terms “abuse liability” and “abuse potential” are often used synonymously and include chemical, pharmacological, and pharmacokinetic characteristics of a drug. Although some elements of abuse liability regarding pharmaceutical products such as opioids differ from that of tobacco products, clinical studies have used similar methodologies to evaluate the abuse potential of tobacco products and medicinal nicotine and have included various measures of subjective effects, craving reduction, and nicotine exposure. 12 , 13 , 14 Our approach to addressing abuse potential focuses on the rate and extent of nicotine uptake and subjective effects relative to ASs’ own‐brand cigarettes (CIGS) and nicotine polacrilex gum (GUM).
Methods
Subjects
Study subjects were male and female ASs, aged 21 to 65 years, who self‐reported having smoked 10 to 20 machine‐manufactured combustible cigarettes per day, on average, for at least 1 year. Health evaluations included standard physical and oral examinations with measurements of vital signs and an electrocardiogram, medical history including concomitant medications, and clinical laboratory assessments. Primary exclusion criteria included any clinically significant medical condition that would preclude the subject from participating in the study, including women who were pregnant or lactating, dentition that prevented using test products or GUM, and allergies or intolerance to mint‐flavoring agents. Subjects were also excluded if they had attempted to quit smoking in the 3 months prior to participation, were postponing a quit attempt to participate, used any tobacco or nicotine‐containing product (including test products) other than combustible cigarettes within 30 days of check‐in, or had >5 lifetime uses of test products. Enrolled subjects were provided quit assist information (QuitAssist website containing resources that could be used for smoking cessation) at screen and end‐of‐study visits.
Study Products
Chemical analyses of test products demonstrated that, other than nicotine, levels of harmful and potentially harmful constituents were absent or substantially lowered compared with cigarettes and smokeless tobacco products and comparable to that observed for GUM. 15 Each test product contains ∼1.5 mg tobacco‐derived nicotine. Subjects’ own‐brand cigarettes and Nicorette Fresh Mint 2‐mg nicotine polacrilex gum (GlaxoSmithKline Consumer Healthcare, L.P., Warren, New Jersey) functioned as high‐ and low‐abuse potential comparators, respectively. Test products and GUM were provided free of charge, whereas subjects provided their own cigarettes.
Study Design
This study used a randomized, open‐label, 4‐period crossover design and occurred at a single research center (Celerion, Lincoln, Nebraska).
Ethics Approval
The Chesapeake Institutional Review Board (Columbia, Maryland) reviewed and approved this study. The investigator and all research staff conducted the study in accordance with the ethical standards in the Declaration of Helsinki, applicable sections of the US Code of Federal Regulations, and ICH E6 Guideline for Good Clinical Practice. All participants provided written informed consent.
Product Trial Period
Subjects participated in a 5‐day at‐home product trial period prior to the in‐clinic product evaluations to become familiar with test products and to determine whether they would be willing to use them during the study. Subjects received 2 packages of each of test 1 and test 2 products (12 pieces per package) and instructions to use them ad libitum, with no limit on the number per day or duration per use. Subjects documented their use behavior during the product trial period, including number of CIGS smoked per day and the number and average duration of test products used each day.
Study Product Evaluation Period
Subjects who remained eligible and interested in continued participation following the product trial period were randomized into 4 product use sequence groups based on sex and age (above and below median).
Subjects remained in the clinic from the time of check‐in until completion of the study events 4 days later. Products were used once each day in the morning with ∼24 hours between product uses. Subjects chewed 1 piece of test product for up to 30 minutes, took 10 puffs (no limit on puff duration) from 1 cigarette at 30‐second intervals, or chewed 1 piece of GUM for up to 30 minutes according to the product's instructions (“chew and park” method). Research staff documented adverse events and the need for concomitant medications throughout the study.
Nicotine Measurement and Pharmacokinetics
Serial samples to measure plasma nicotine concentrations were collected ∼5 minutes prior to and 2, 5, 10, 12, 15, 20, 30, 60, 120, and 180 minutes following the start of each product use. Plasma nicotine concentrations were analyzed by liquid chromatography‐tandem mass spectometry using validated analytical methods (Celerion, Lincoln, Nebraska) in accordance with applicable FDA Good Laboratory Practice regulations (Title 21 CFR Part 58). The plasma samples were extracted with solid‐phase extraction using a polymeric solvent. The sample extracts were injected into an isocratic chromatography system utilizing a polar organic solvent to achieve reversed‐phase separation. The internal standard was d4‐nicotine. Detection was achieved on an AB SCIEX AB 5500 triple quadrapole instrument in which positive ions are detected in multiple reaction monitoring mode. The transitions monitored were 163.2‐130.1 and 166‐132 for nicotine and d3‐nicotine, respectively. The lower limit of quantification was 0.200 ng/mL. Intrabatch precision was 2.5%‐8.5%, intrabatch accuracy was −11.0% to 13.0%, interbatch precision was 3.3%‐9.7%, and interbatch accuracy was 4.5%‐7.5%. The lower limit of quantification for nicotine was 0.200 ng/mL.
Subjective Measures
Subjective measures were assessed using the Modified Cigarette Evaluation Questionnaire (mCEQ): Tobacco/Nicotine Withdrawal Questionnaire, Direct Effects of Product, and Use the Product Again questionnaires. All questionnaires were administered using the Cantab Connect platform from Cambridge Cognition (Cambridge, UK), which displayed 1 question at a time with numerical checkboxes or a visual analog scale (VAS). Subjects received training on the check‐in day on the use of the device and questionnaires. The mCEQ was further modified for use with test products (ie, “cigarettes” replaced by “chews”) and was completed on the clinic check‐in day. Each item was answered on a scale of 1 (“Not at All”) to 7 (“Extremely”). The items on the Direct Effects of Product represent subjective measures with demonstrated sensitivity for detecting between‐product differences in a clinical laboratory setting. 16 The Tobacco/Nicotine Withdrawal Questionnaire was adapted from the Minnesota Nicotine Withdrawal Scale. 17 Subjects responded to each item of these questionnaires with a standard 100‐mm VAS anchored with “Not at All” and “Extremely.” Subjects completed the Direct Effects of Product and Tobacco/Nicotine Withdrawal Questionnaires about 5, 15, 30, and 60 minutes following the start of each product use and provided a baseline value for the Tobacco/Nicotine Withdrawal Questionnaire about 10 minutes before the starting the use of each product. The Use the Product Again Questionnaire (“If given the opportunity, I would want to use this product again”) was administered immediately after each product use and 180 minutes following the start of each product use and was adapted from abuse‐deterrent formulation drug trials measure “I would want to take this drug again.”18 A 100‐mm bipolar VAS was used, with “Definitely Would Not” (−50) at the left anchor, “Don't Care” (0) at the midpoint and “Definitely Would” (+50) at the right anchor.
Data Analyses
Phoenix WinNonlin bersion 6.3 (Pharsight, Princeton, New Jersey) was used to calculate the pharmacokinetic parameters, and the statistical summarization and analysis were performed using SAS version 9.3 (SAS, Cary, North Carolina). The plasma concentrations were adjusted for baseline levels, using 1‐compartment pharmacokinetics. Cmax, AUC0‐180, and Tmax were calculated from the individual baseline‐adjusted plasma nicotine concentrations using a noncompartmental approach. A linear mixed model for analysis of variance was performed on the natural log‐transformed Cmax and AUC parameters. The model included sequence, study product, and period as fixed effects and subject nested within sequence as a random effect.
“Urge to Smoke” from the Tobacco/Nicotine Withdrawal Questionnaire and “Is the Product Pleasant Right Now” from the Direct Effects of Product Questionnaire served as the primary subjective measure variables for statistical analysis. The maximum reduction in the “Urge to Smoke” item (defined as the maximum difference between the baseline and postbaseline VAS scores) and the maximum score for “Pleasant” were calculated for each subject under each product condition and served as the basis of comparison in the analysis. The model included sequence, study product, and period as fixed effects and subject nested within sequence as a random effect, and the number of cigarettes smoked per day reported at screening as a covariate. The other items on the Direct Effects of Product and Tobacco/Nicotine Withdrawal Questionnaires were summarized descriptively. Responses to the Use the Product Again Questionnaire were summarized using frequency counts of positive scores (response from “Don't Care” to “Definitely Would”), negative scores (response from “Don't Care” to “Definitely Would Not”), or neutral (response of “Don't Care”).
Results
Subjects
Fifty‐seven subjects were screened for the study, 30 participated in the product trial period, and 28 were randomized. Two subjects discontinued their participation for personal reasons early after being randomized.
The study population (Table 1) was predominantly Caucasian (96%) and male (64%), with an average age ± standard deviation (SD) of 42.3 ± 13.3 years, smoked an average of 16.8 ± 3.6 cigarettes per day, for an average of 23.5 ± 15.4 years, and 6 subjects (21%) reported smoking a menthol cigarette.
Table 1.
Demographics
| n = 28 | ||
|---|---|---|
| Sex, n (%) | Female | 10 (36%) |
| Male | 18 (64%) | |
| Race, n (%) | Black or African American | 1 (4%) |
| White | 27 (96%) | |
| Other | 0 (0%) | |
| Ethnicity, n (%) | Hispanic or Latino | 1 (4%) |
| Not Hispanic or Latino | 27 (96%) | |
| Age, years | Mean | 42.3 |
| SD | 13.3 | |
| BMI, kg/m² | Mean | 27.8 |
| SD | 4.1 | |
| Cigarettes per day | Mean | 16.8 |
| SD | 3.6 | |
| Years of smoking | Mean | 23.5 |
| SD | 15.4 | |
| Own brand cigarettes, n (%) | Menthol | 6 (21%) |
| Nonmenthol | 22 (79%) |
Study Product Use
The self‐reported daily use of test products during the product trial period ranged from 1 to 7 pieces (median, 1‐2 pieces) of either flavor each day, with a use duration ranging from 3 to 30 minutes (median, 12‐18 minutes). During the study product evaluation period, 27 subjects completed use of test products, and 26 subjects completed use of the CIGS and GUM.
Nicotine Pharmacokinetics
The pharmacokinetic profiles were essentially superimposable for the 2 test products (Figure 1). Plasma nicotine concentrations following use of test products increased slowly and were markedly lower than that for the own‐brand cigarettess. Further, the peak nicotine concentrations from test products occurred at approximately the same time as for GUM, but considerably later than the CIGS.
Figure 1.
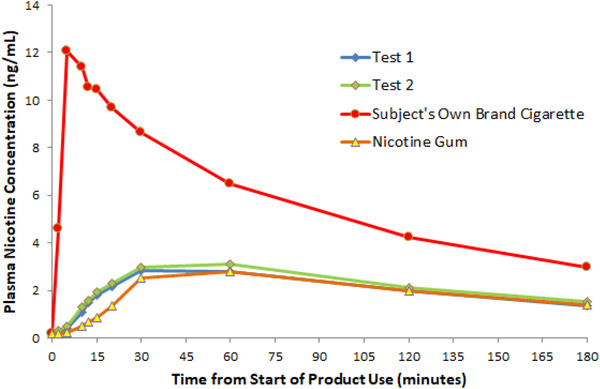
Mean plasma nicotine concentration‐time profiles.
As shown in Table 2A, the maximum plasma nicotine concentration, Cmax, with test products (test 1, 2.73 ng/mL; test 2, 2.90 ng/mL) was statistically significantly lower than the corresponding concentrations with CIGS (12.11 ng/mL, P < .0001 for all comparisons). Likewise, overall exposure (AUC0‐180), as shown in Table 2B, was also statistically significantly lower with use of test products (327.11 and 348.98 ng·min/mL) compared with CIGS (946.29 ng·min/mL, P < .0001 for all comparisons). The Cmax and AUC were statistically significantly higher for test products compared with GUM (2.04 ng/mL and 246.30 ng·min/mL, respectively; P < .05 for all comparisons).
Table 2.
Nicotine Pharmacokinetic Parameters
| A. Cmax(0‐180) (ng/mL) | ||||
|---|---|---|---|---|
| Mean ± SD | Geo. LS Mean | Geo. LS Mean Ratio (95%CI), Test/Cigarette (%) | Geo. LS Mean Ratio (95%CI), Test/Gum (%) | |
| Own‐brand cigarette (n = 26) | 13.56 ± 7.1747 | 12.11 | ||
| Nicotine polacrilex gum (n = 26) | 2.781 ± 1.8058 | 2.04 | ||
| Test 1 (n = 27) | 2.894 ± 0.95257 | 2.73 | 22.57 (17.78‐28.65) a | 133.72 (105.38‐169.69) b |
| Test 2 (n = 27) | 3.133 ± 1.2021 | 2.90 | 23.99 (18.91‐30.45) a | 142.14 (111.99‐180.40) b |
| B. AUC0‐180 (ng·min/mL) | ||||
|---|---|---|---|---|
| Mean ± SD | Geo. LS Mean | Geo. LS Mean Ratio (95%CI), Test/Cigarette (%) | Geo. LS Mean Ratio (95%CI), Test/Gum (%) | |
| Own‐brand cigarette (n = 26) | 1017 ± 303.53 | 946.29 | ||
| Nicotine polacrilex gum (n = 26) | 332.0 ± 205.17 | 246.30 | ||
| Test 1 (n = 27) | 349.1 ± 121.81 | 327.11 | 34.57 (28.12‐42.49) a | 132.81 (108.07‐163.21) b |
| Test 2 (n = 27) | 380.0 ± 155.44 | 348.98 | 36.88 (30.01‐45.32) a | 141.69 (115.28‐174.15) b |
Values are presented as arithmetic mean ± SD, geometric least‐squares mean and geometric least‐squares mean ratio (%) with sample size (n) and the 95% confidence interval (CI) for the geometric mean ratio between the test products and own‐brand cigarette and nicotine polacrilex gum.
P < .0001.
P < .05.
Subjective Measures
Modified Cigarette Evaluation Questionnaire
Average factor scores on the mCEQ following 5 days of at‐home test products use ranged from 1.54 to 2.8 of a possible 7 points, with most scores falling into the “very little” (2) to “a little” (3) range on the scales.
Tobacco/Nicotine Withdrawal
The urge to smoke decreased rapidly within 5 minutes of the start of product use (Figure 2). Maximum mean reductions in smoking urge with use of test products were experienced within 15 to 30 minutes and remained steady through 60 minutes. Peak responses to smoking occurred at 15 minutes, and the urge increased toward baseline thereafter. The shape of the response curve for GUM was generally similar to test products, although the urge to smoke continued to decrease modestly from 5 to 60 minutes.
Figure 2.
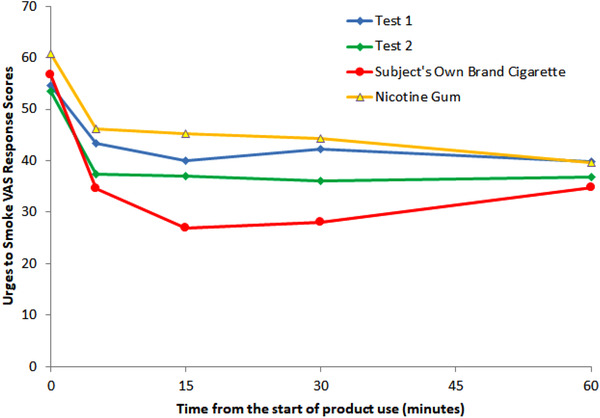
The change in “Urge to Smoke” VAP response based on the Tobacco/Nicotine Withdrawal Questionnaire.
The maximum reduction from baseline for the Urge to Smoke (Table S1) was statistically significantly smaller with use of test 1 (21.48 ± 22.13) and test 2 (24.19 ± 22.87) compared with smoking (40.35 ± 32.37). The maximum reduction for GUM (26.46 ± 30.81) was not statistically significantly different from test products.
For the remaining items on the Tobacco/Nicotine Withdrawal Questionnaire (Table S2), the maximal effects were consistently lower for test products than for the smoking condition and generally similar to GUM. In addition, the responses tended to be slightly higher for test 2, but the differences between the test products were relatively small.
Direct Effects of Product
Table 3 illustrates the maximum VAS scores recorded during the 60 minutes following product use. Compared with the smoking condition, maximum VAS scores for the “Is the Product ‘Pleasant’ Right Now” question were statistically significantly lower (Table S1) with the use of test 1 (42.07 ± 25.58) and test 2 (38.33 ± 29.22) test products compared with cigarettes (74.31 ± 25.31). The response for GUM (44.92 ± 26.16) was not statistically significantly different compared with test products.
Table 3.
Maximum Direct Effects of Product Scores
| Question | Test 1 (n = 27) | Test 2 (n = 27) | Own‐Brand Cigarette (n = 26) | Nicotine Polacrilex Gum (n = 26) |
|---|---|---|---|---|
| Is the Product “Satisfying” Right Now? | 41.41 ± 27.81 | 41.63 ± 30.43 | 74.69 ± 25.75 | 46.19 ± 25.08 |
| Is the Product Making You Feel “Calm” Right Now? | 39.15 ± 27.12 | 44.56 ± 27.82 | 73.19 ± 25.22 | 46.85 ± 26.81 |
| Is the Product Helping You “Concentrate” Right Now? | 32.04 ± 25.48 | 29.70 ± 24.44 | 59.15 ± 30.78 | 35.96 ± 24.51 |
| Is the Product Making You Feel More “Awake” Right Now? | 34.74 ± 24.81 | 35.70 ± 27.13 | 53.23 ± 29.35 | 39.42 ± 25.46 |
| Is the Product Making You Feel “Sick” Right Now? | 28.70 ± 28.11 | 20.93 ± 23.09 | 16.00 ± 21.22 | 18.31 ± 23.87 |
| Is the Product Reducing Your “Hunger” for Food Right Now? | 29.11 ± 22.62 | 25.93 ± 23.49 | 40.65 ± 27.01 | 29.46 ± 24.06 |
| Would You Like “More” of the Product Right Now? | 26.22 ± 25.10 | 32.33 ± 26.94 | 69.04 ± 26.60 | 33.31 ± 26.96 |
Values are presented as mean ± SD.
Intent to Use the Product Again
Responses to the Use the Product Again Questionnaire for each product were fairly consistent between times (Table S3). Use of the test products resulted in positive responses (VAS score > 0) in 52% to 63% of the subjects, which was considerably lower than smoking (81%‐92%) and generally slightly higher than GUM (38%‐50%).
Safety
All products were well tolerated as used during the study. No serious adverse events were reported, and no subjects were discontinued because of adverse events. Three adverse events were reported by 2 of 30 subjects during the product trial period, whereas 15 events were reported by 10 of 28 subjects during the study product evaluation period. All events were considered mild in severity by the principal investigator (PI). Two headache events (1 following use of test 2 and one following GUM), 2 dizziness events (both following smoking), and 1 nausea event (following smoking) experienced during the study product evaluation period were considered by the PI to be possibly related to study product, and all others were considered not related. Presyncope was the most frequent adverse event reported during blood draw, experienced by 5 subjects, 4 of which followed smoking and 1 followed use of GUM.
Discussion
In the current study, we assessed nicotine uptake and responses to subjective measures to gain an understanding of the relative abuse potential of the test products compared with CIGS and 2‐mg GUM. Our results suggest that nicotine uptake and subjective responses from the test products are more analogous to GUM and significantly lower than cigarettes. Overall, our assessment demonstrates that the abuse potential of the test products is lower than cigarettes and similar to GUM. In addition, nicotine uptake and subjective responses were similar for the 2 flavor variants of the test products, suggesting that flavor does not likely impact abuse potential of the product.
In this study, we compared the nicotine pharmacokinetics of test products relative to CIGS and GUM. Many researchers believe that the speed and efficiency of nicotine delivery affects the reinforcing efficacy of tobacco/nicotine products, which may influence subsequent use behavior. 12 , 18 We collected venous blood samples at multiple points, which allowed for an examination of the rate and extent of nicotine delivery following controlled single use of test product, cigarettes, and GUM. The relative differences in the pharmacokinetics results seen in this study are consistent with the oral versus pulmonary routes of uptake. Cigarette smoking resulted in a higher Cmax, shorter Tmax, and larger AUC than both GUM and the test product use. The pharmacokinetic parameters for test product were ∼30%‐40% higher compared with GUM. These differences may be attributed to the source of nicotine, tobacco‐derived nicotine in test product versus nicotine bound to an ion‐exchange resin (polacrilex). Another likely reason could be the “chew and park” instructions for using GUM, which may not have resulted in sufficient chewing to extract as much nicotine relative to the test product.
We enrolled only established ASs into this study, as this is the intended audience that would benefit by switching completely from cigarettes to the test products. Use of the subjects’ CIGS and GUM were intended to serve as high and low abuse potential comparators, respectively. Similar to approaches used previously, 19 we used a lead‐in period that gave all subjects the same opportunity to become familiar with the characteristics of the products prior to formal evaluation, which to some extent mimics the early experimentation period that consumers go through when trying a tobacco product for the first time. The subjective measures used in this study are reported to be related to the positive and negative reinforcing effects of the products. 12 , 13 , 16 , 20 , 21 Responses to the subjective effect items generally aligned with nicotine pharmacokinetics, with CIGS resulting in the highest ratings and similar ratings for the test products and GUM. Responses to the urge to smoke question followed an expected pattern inverse to the rise and fall of nicotine in the blood, and a significant reduction from the baseline urge to smoke was observed with use of each of the products. The relative differences in the subjective responses observed in this study are consistent with the routes of product administration. The gradual absorption of nicotine from the orally administered products resulted in a delayed pharmacologic effect. Smoking CIGS alleviated withdrawal symptoms to a greater extent than test product and GUM. The peak urge to smoke response and overall shape of the urge to smoke response‐time curves with the use of the test products were generally similar to GUM, and the responses to the other withdrawal items following use of these products were also comparable overall. The test products trended similar to cigarettes regarding suppression of urge to smoke, and GUM had a lower response. Despite the 25% lower level of nicotine in the test products (1.5 mg) compared with GUM (2.0 mg), The test products were able to suppress the urge to smoke to a slightly greater extent. Sufficient, sustained suppression of withdrawal symptoms and the urge to smoke after a period of withdrawal or cessation is an important component for a tobacco or other nicotine‐containing product to be adopted as an alternative to cigarettes. 12 These observations suggest that the test product may be considered a suitable alternative to smoking for some ASs. We have observed such behavior in a separate 6‐week ambulatory study in which ∼23% of ASs not planning to quit switched completely to the test product. 22
Our findings align with a report 23 on another novel oral tobacco‐derived nicotine‐containing product (test 1, containing 1.5 mg nicotine), which is similar to test products except having a different chewable polymer matrix. Koszowski et al 23 evaluated nicotine delivery, satisfaction and urge suppression with the use of VERVE Discs in 13 daily smokers following periods of abstinence and nonabstinence from smoking. Plasma nicotine concentrations increased from baseline by 1.4 and 2.7 ng/mL, respectively, 5 minutes following a 15‐minute use period. Despite the small degree of nicotine boost and “low” to “moderate” product‐liking scores, subjects experienced significant reductions in cigarette craving, as measured by the Questionnaire for Smoking Urge factor scores following the period of abstinence. In another study, Buzzell et al 24 observed lower ratings of satisfaction and consumer acceptability compared with traditional smokeless tobacco with the use of VERVE Discs.
We observed that responses to the Direct Effect of Product questions were significantly lower for the test products and nicotine polacrilex gum compared with own‐brand cigarettes. Further, when asked about future use of each product, subjects overwhelmingly (>80%) responded with an intent to smoke their cigarettes again. A substantial proportion (∼50%‐60%) expressed intent to use the test products, whereas relatively fewer subject expressed interest in the nicotine polacrilex gum (∼30%‐50%). These results are consistent with other reports in which ASs with an established preference for their own cigarettes typically responded more favorably to their own product than to other tobacco products. 19 , 20 , 23
Although the test products are not intended for a cessation indication because of the similarity with route of administration (oral ingestion), form (chewable), and amount of nicotine, we compare our results to extensive literature regarding the abuse potential of nicotine replacement therapy. Overall our findings also align with extensive literature regarding the abuse potential of nicotine replacement therapy (NRT), which includes pharmacokinetic 25 , 26 and pharmacodynamic evaluations, 27 as well as human abuse potential studies with measures of drug identification, drug liking, subjective effects, and physiological effects. 28 , 29 , 30 , 31 In addition, numerous clinical trials 18 , 32 , 33 and observational studies 34 , 35 have examined use behavior and dependence associated with oral NRT use. The use of nicotine, either as NRT or oral tobacco‐derived nicotine products, appears to have relatively low abuse potential compared with more traditional forms of tobacco products, such as cigarettes and smokeless tobacco products. The results of our study combined with the literature on oral NRTs, which are similar in form and composition to the test products, suggest that the test products likely have low abuse potential.
Results of this study also demonstrate no differences between the flavor variants of the test products on the nicotine delivery or subjective responses. We observed similar ratings on the Direct Effects of Product Questionnaire, similar craving/withdrawal relief, and nearly identical PK curves between test 1 and test 2 product varieties. These findings are comparable to published literature reports, which demonstrated no change in abuse potential of GUM with improvement in flavor. 29
The study results should be considered in the context of some limitations inherent with such investigations. The sample size of the study is relatively small (n = 27); however, typical sample sizes for studies examining nicotine pharmacokinetics and subjective effects are often within this range. 19 , 23 , 25 Furthermore, the study was conducted in a confined clinic environment, and the subjective responses may not reflect real‐world observations. However, this inherent limitation is essential to gather robust and reliable subjective responses to questionnaires administered under the supervision of clinic staff. Another limitation relates to characterization of study end points from a single use of the test products. Additional characterization of subjective responses under real‐world settings, for example, an actual use study under ambulatory settings, may further inform the abuse potential of such novel products. Despite some of these limitations, this study provides useful data regarding the nicotine uptake and subjective effects of the test products.
This study also had several strengths. First, we enrolled only established ASs, who represent the individuals that would benefit by switching completely from cigarettes to the test products. All subjects self‐reported they were naive to the test products prior to screening, which minimized the potential presence of previously established bias toward or against the test products. We then implemented a lead‐in period, which gave all subjects the same level of familiarity with the characteristics of the test products prior to formal evaluation, which to some extent, mimicked the early stage of use that tobacco users go through when trying a tobacco product for the first time. Second, we included subjects’ CIGS and GUM as positive (high abuse potential) and negative (low abuse potential) control comparators, respectively. Inclusion of these comparators demonstrated sensitivity of the laboratory‐based model in that we observed the expected differences between CIGS and GUM. Inclusion of these comparators also allowed us to benchmark the relative abuse potential of the test products. Finally, we collected nicotine blood plasma levels and subjective effects at multiple times following product use. This rigorous data collection procedure allowed us to characterize the full‐time course of product effects, including the peak subjective and pattern of subjective effects relative to nicotine blood plasma concentrations.
In conclusion, the results of this study demonstrate that the abuse potential of the test products is likely lower than with cigarettes and similar to nicotine polacrilex gum. Furthermore, no significant impact of the flavor variants was observed for the study end points.
Conflicts of Interest
J.L., J.W., A.V., J.E., and M.S. are employees of Altria Client Services LLC. D.G. was an employee of Celerion, Inc., who was contracted by Altria Client Services LLC to perform the study and analyze the study data.
Funding
There was no external funding for this article.
Author Contributions
J.L., A.V., and M.S. were responsible for developing the study design. J.L. was primarily responsible for managing the study conduct along with D.G. from Celerion Inc. J.W. was the primary statistician and managed the statistical analysis. M.S. was primarily involved in synthesizing, integrating the results, and writing the article along with help from the coauthors.
Data Accessibility Statement
The authors will respond to reasonable requests for data reported in this article.
Supporting information
Supporting Information
Acknowledgments
The authors acknowledge the efforts of the staff at Celerion Inc., for the recruitment of study participants, study conduct, and data analysis.
References
- 1. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking‐50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [PubMed] [Google Scholar]
- 2. USSGR . The Health Consequences of Smoking‐50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: USSGR; 2014. [Google Scholar]
- 3. Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(2):53‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colilla SA. An epidemiologic review of smokeless tobacco health effects and harm reduction potential. Regul Toxicol Pharmacol. 2010;56(2):197‐211. [DOI] [PubMed] [Google Scholar]
- 5. Fairchild AL, Lee JS, Bayer R, Curran J. E‐cigarettes and the harm‐reduction continuum. N Engl J Med. 2018;378(3):216‐219. [DOI] [PubMed] [Google Scholar]
- 6. Gartner C, Hall W. The potential role of snus in tobacco harm reduction. Addiction. 2009;104(9):1586‐1587. [DOI] [PubMed] [Google Scholar]
- 7. Haziza C, de La Bourdonnaye G, Donelli A, et al. Reduction in exposure to selected harmful and potentially harmful constituents approaching those observed upon smoking abstinence in smokers switching to the menthol tobacco heating system 2.2 for three months (part 1). Nicotine Tob Res. 2020;22(4):539‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Timberlake DS, Nikitin D, Johnson NJ, Altekruse SF. A longitudinal study of smokeless tobacco use and mortality in the United States. Int J Cancer. 2017;141(2):264‐270. [DOI] [PubMed] [Google Scholar]
- 9. Abrams DB, Glasser AM, Pearson JL, Villanti AC, Collins LK, Niaura RS. Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu Rev Public Health. 2018;39:193‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nutt DJ, Phillips LD, Balfour D, et al. Estimating the harms of nicotine‐containing products using the MCDA approach. Eur Addict Res. 2014;20(5):218‐225. [DOI] [PubMed] [Google Scholar]
- 11. Zeller M, Hatsukami D, Strategic Dialogue on Tobacco Harm Reduction Group Collaborators. The strategic dialogue on tobacco harm reduction: a vision and blueprint for action in the US. Tob Control. 2009;18(4):324‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carter LP, Stitzer ML, Henningfield JE, O'Connor RJ, Cummings KM, Hatsukami DK. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3241‐3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotlyar M, Mendoza‐Baumgart MI, Li ZZ, et al. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tob Control. 2007;16(2):138‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl). 2000;149(3):198‐202. [DOI] [PubMed] [Google Scholar]
- 15. Danielson TM, Brown AP, Jin X, Wilkinson CT, Pithawalla Y, McKinney WJ. Evaluation of novel, oral tobacco‐derived nicotine products for HPHC. Paper presented at: 72nd Tobacco Science Research Conference 2018; 2018; Memphis, TN. [Google Scholar]
- 16. Hanson K, O'Connor R, Hatsukami D. Measures for assessing subjective effects of potential reduced‐exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3209‐3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hughes JR, Hatsukami DK, Skoog KP. Physical dependence on nicotine in gum. A placebo substitution trial. JAMA. 1986;255(23):3277‐3279. [PubMed] [Google Scholar]
- 18. Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61(5):743‐750. [DOI] [PubMed] [Google Scholar]
- 19. Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology (Berl). 2017;234(17):2643‐2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cobb CO, Weaver MF, Eissenberg T. Evaluating the acute effects of oral, non‐combustible potential reduced exposure products marketed to smokers. Tob Control. 2010;19(5):367‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU‐brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7‐16. [DOI] [PubMed] [Google Scholar]
- 22. Vansickel AR, Apkarian L, Schendel J, Largo E. Cigarette smoking behavior among adult cigarette smokers using VERVE® discs or chews during 6‐weeks of at‐home use. Poster presented at: Society for Research on Nicotine and Tobacco Meeting; 2019; San Francisco, CA.
- 23. Koszowski B, Viray LC, Stanfill SB, et al. Nicotine delivery and pharmacologic response from verve, an oral nicotine delivery product. Pharmacol Biochem Behav. 2015;136:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buzzell GA, Das B, Cruz‐Cano R, et al. Using electrophysiological measures to assess the consumer acceptability of smokeless tobacco products. Nicotine Tob Res. 2016;18(9):1853‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blank MD, Eissenberg. Evaluating oral noncombustible potential‐reduced exposure products for smokers. Nicotine Tob Res. 2010;12(4):336‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borland R, Li L, Mortimer K, McNeil A, King B, O'Connor RJ. The acceptability of nicotine containing products as alternatives to cigarettes: findings from two pilot studies. Harm Reduct J. 2011;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benowitz NL. Toxicity of nicotine: implications with regard to nicotine replacement therapy. Progress Clin Biol Res. 1988;261:187‐217. [PubMed] [Google Scholar]
- 28. Heishman SJ, Snyder FR, Henningfield JE. Performance, subjective, and physiological effects of nicotine in non‐smokers. Drug Alcohol Depend. 1993;34(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 29. Houtsmuller EJ, Fant RV, Eissenberg TE, Henningfield JE, Stitzer ML. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol Biochem Behav. 2002;72(3):559‐568. [DOI] [PubMed] [Google Scholar]
- 30. Houtsmuller EJ, Henningfield JE, Stitzer ML. Subjective effects of the nicotine lozenge: assessment of abuse liability. Psychopharmacology (Berl). 2003;167(1):20‐27. [DOI] [PubMed] [Google Scholar]
- 31. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289‐294. [DOI] [PubMed] [Google Scholar]
- 32. Etter JF, Huguelet P, Perneger TV, Cornuz J. Nicotine gum treatment before smoking cessation: a randomized trial. Arch Intern Med. 2009;169(11):1028‐1034. [DOI] [PubMed] [Google Scholar]
- 33. Nemeth‐Coslett R, Benowitz NL, Robinson N, Henningfield JE. Nicotine gum: chew rate, subjective effects and plasma nicotine. Pharmacol Biochem Behav. 1988;29(4):747‐751. [DOI] [PubMed] [Google Scholar]
- 34. Hughes JR, Pillitteri JL, Callas PW, Callahan R, Kenny M. Misuse of and dependence on over‐the‐counter nicotine gum in a volunteer sample. Nicotine Tob Res. 2004;6(1):79‐84. [DOI] [PubMed] [Google Scholar]
- 35. Shiffman S, Hughes JR, Di Marino ME, Sweeney CT. Patterns of over‐the‐counter nicotine gum use: persistent use and concurrent smoking. Addiction. 2003;98(12):1747‐1753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The authors will respond to reasonable requests for data reported in this article.


