Abstract
Coagulation predominant-type coagulopathy such as microthrombosis and macrothrombosis is a well-known recognised complication found in COVID-19 infected critically ill patients. In the context of high incidence of thrombotic events in patients with COVID-19, supplementation with anticoagulant therapy has been routinely recommended and shown to reduce mortality. However, the recommended type, dose, duration and timing of anticoagulant has not been determined yet. Spontaneous retroperitoneal haematoma secondary to anticoagulant therapy is one of the well-known but self-limiting conditions. We report a 51-year-old COVID-19 positive woman, who was taking intermediate-intensity heparin therapy for venous thromboembolism prophylaxis and died from complication of retroperitoneal bleeding. Further studies are needed to verify the risk–benefit ratio of anticoagulant therapy in patients with COVID-19. Although anticoagulant deems appropriate to use in patients with COVID-19, clinicians should be cautious about major bleeding complication such as retroperitoneal haemorrhage even when full therapeutic dosage is not used.
Keywords: COVID-19, haematology (drugs and medicines), acute renal failure, pneumomediastinum, haematology (incl blood transfusion)
Background
COVID-19, which is caused by SARS-CoV-2, has caused the pandemic that lasts until now. It is the third pandemic caused by a coronavirus, with its predecessors being severe acute respiratory syndrome in 2002 and Middle East respiratory syndrome in 2012. Despite the name of the virus, it actually causes mortality from the complications involving almost every organ system in a human other than the respiratory system, namely cardiovascular system, nervous system, renal system as well as haematological system. Coagulation problem is one of the established haematological involvements in COVID-19; however, exact mechanism is not yet understood. In the context of high incidence of thrombotic events in patients with COVID-19, supplementation with anticoagulant therapy has been routinely recommended and shown to reduce mortality1 through suppression of microthrombosis and prevention of venous thromboembolism (VTE). However, the recommended type, dose, duration and timing of anticoagulant has not been determined yet. Here, we report a 51-year-old COVID-19 positive woman who was taking intermediate dose heparin therapy for VTE prophylaxis and died from complication of retroperitoneal bleeding after 18 days of hospital admission (28th day of COVID-19 symptoms).
Case presentation
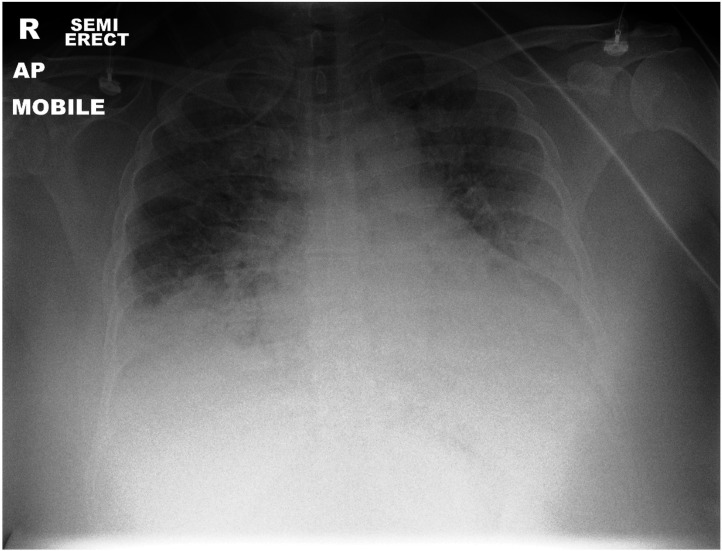
A 51-year-old Vietnamese woman presented to the emergency department with a 10-day history of feeling unwell, non-productive cough, sorethroat and a 1-day history of shortness of breath. She was tested positive for COVID-19 infection on the third day of her symptoms started. She does not have any medical history apart from fatty liver. She does not take any medicine. On presentation, she was afebrile, blood pressure of 150/75 mm Hg, heart rate of 96 per minute, respiratory rate of 35 per minute and oxygen saturation of 85% under 15 L of high flow oxygen mask. Her initial chest radiograph showed extensive bilateral pulmonary infiltrates involving both lower lobes (figure 1). Her initial arterial blood gas showed type 1 respiratory failure: pH 7.4 (normal range:7.35-7.45), PaCO2 4.0 kPa (normal range:4.5-6 kPa), HCO3 24 meq/L (normal range:22-28), base excess 0.4, lactate 3.7 mmol/L (normal value:<2), PaO2 5.1 kPa (with fractional inspired oxygen (FiO2) 0.85) and P/F ratio of 45 mm Hg (normal value:≥400). She was treated for COVID-19 pneumonitis with acute respiratory distress syndrome and admitted to an intensive care unit (ITU). Non-invasive ventilation was started with a setting of positive end-expiratory pressure 10 cmH20, FiO2 65%, inspiratory pressure 30 cmH20, pressure support 9.7 cmH20, peak inspiratory pressure 10.1 cmH20, respiratory rate of 27 per minute, tidal volume 528 mL and minute ventilation 15.2 L/min to maintain oxygen saturation above 90%. She was started on intravenous antibiotics (coamoxiclav 1.2 G three times a day and clarithromycin 500 mg two times a day), intravenous methylprednisolone of 1 mg/kg once a day and enoxaparin 60 mg twice a day subcutaneously. She was also started on variable rate insulin infusion due to high sugar ranging between 11 and 18.5 mmol, which was most likely secondary to steroid-induced hyperglycaemia.
Figure 1.
Chest radiograph showed extensive bilateral pulmonary infiltrates involving both lower lobes.
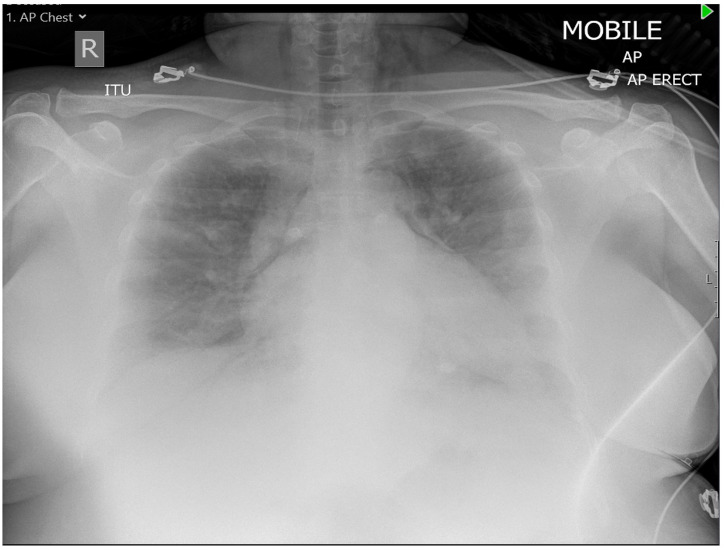
On the third day of ITU, while she was on continuous positive pressure ventilation, she developed chest pain, tachypnoea and hypoxia. Subsequently, chest radiograph (figure 2) confirmed that she had a pneumomediastinum extending to the base of the neck. Echocardiogram showed a normal ejection fraction of 55%–60% with no valvular abnormality. After 10 days of ITU admission (20th day of COVID-19 symptoms), she showed an improvement, in terms of decreased oxygen requirement, and she was stepped down to assisted ventilation unit with 30% optiflow oxygen.
Figure 2.
Chest radiograph showed pneumomediastinum extending to the base of the neck.
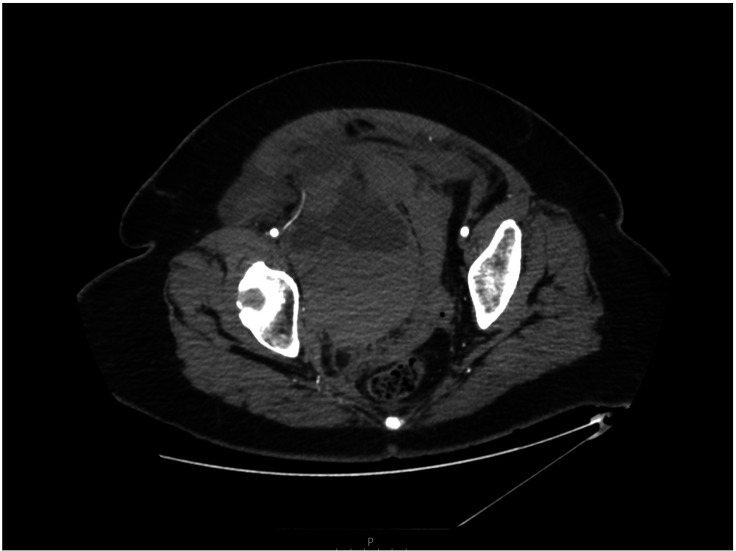
On the 16th day of hospital admission, she had tachycardia and complained of acute abdomen pain. The abdominal examination showed tender and distended abdomen with palpable mass 15×10 cm in the right iliac fossa. Her haemoglobin suddenly dropped 93 g/L with raised lactate of 6.1 mmol/L. Subsequently, CT abdomen was performed, which showed large pelvic haematoma measuring 16×10 cm and compressing the urinary tract system and uterus. The ureter was posteriorly displaced by the haematoma with secondary mild fullness of the right pelvicalyceal system. The haematoma was found to be closely related to the right external iliac artery, extension to the anterior abdominal wall and posterior to the right inferior epigastric artery (figure 3). The repeated CT angiogram abdomen and pelvis did not either show any progression of pelvic haematoma or reveal any bleeding point. The initial treatment included the cessation of anticoagulant and administration of tranexamic acid. She was immediately resuscitated with intravenous fluids and two units of packed red blood cells (PRBCs). Post-transfusion of haemoglobin showed further dropped haemoglobin of 68 g/L, and she received another two units of PRBC, two units of cryoprecipitate and two units of platelets. The gynaecology, general surgery and urology teams were consulted. Multidisciplinary team decision of conservative management was made with due respect to high risk of deterioration and guarded prognosis. Her kidney function started to deteriorate within 24 hours. Her blood investigations throughout the admission are shown in table 1.
Figure 3.
Contrast-enhanced CT of abdomen and pelvis showed voluminous pelvic haematoma in the right retroperitoneal region displacing uterus and urinary bladder.
Table 1.
This table shows blood investigations throughout the admission
| Day 1 | Day 15 | Day 16 | Day 17 | Normal range | |
| Haemoglobin | 142 | 139 | 93 | 68 | 115–160 g/L |
| White cell count | 8.2 | 13.9 | 38 | 31 | 4–11×109/L |
| Neutrophils | 6.4 | 9.0 | 31.6 | 22.9 | 2.0–7.5×109/L |
| Lymphocytes | 1.3 | 3.7 | 7.4 | 7.4 | 1.0–4.5×109/L |
| Platelets | 327 | 434 | 284 | 117 | 150–400×109/L |
| Sodium | 134 | 140 | 128 | 125 | 135–145 mmol/L |
| Potassium | 3.6 | 4.0 | 6.7 | 8.2 | 3.5–5.3 mmol/L |
| Urea | 3.9 | 5.8 | 6.1 | 9.6 | 2.5–6.7 mmol/L |
| Creatinine | 63 | 55 | 212 | 368 | 70–100 μmol/L |
| International normalized ratio | 1.1 | 1.0 | 1.1 | 1.5 | 1 |
| Prothrombin time | 12.5 | 11.6 | 12 | 16.4 | 10–14 s |
| Activated partial thromboplastin time | 25 | 36 | 25 | 81 | 35–45 s |
| D-dimer | 447 | 166 | 158 | 166 | 0–250 ng/mL |
| Ferritin | 580 | 591 | 603 | 563 | 12–200 µg/L |
| Troponin | 188 | 35 | 14 | 359 | <14 ng/L |
| C reactive protein | 156 | 96 | 61 | 75 | <10 mg/L |
Outcome and Follow-up
The patient was readmitted to ITU, and a central line was inserted for haemofiltration due to persistent hyperkalaemia (potassium of 8.2 mmol/L) and oliguria secondary to acute renal failure, which was most likely due to postrenal obstructive uropathy and superimposed by multifactorial factors such as multiple contrast iodine dye, sepsis, disseminated intravascular coagulopathy and multiorgan failure. Soon after central line insertion, cardiac arrest occurred, and cardiopulmonary resuscitation was started immediately. The patient was in pulseless electrical activity, and all reversible causes were evaluated. Hyperkalaemia was corrected with calcium gluconate, insulin and dextrose. After 30 min of resuscitation with intubation, the patient had a return of spontaneous circulation. Later after 20 min while preparing for haemodialysis with maximal vasopressor support, she went into cardiac arrest again and passed away.
Discussion
Coagulopathy is a widely recognised complication in COVID-19, with thrombosis being the prominent type of haematological problem. It is more common in the intensive care unit with some extent found during the recovery period that can happen even after discharge from hospital.2 Although recent studies recommend antithrombotic therapy with heparin in COVID-19 critically ill patients, the risk–benefit ratio is still unknown, and several randomised controlled trials such as HEP-COVID, IMPROVE-COVID and INHIXACOVID19 are still undertaken to study the efficacy and safety of heparin therapy in patients with severe COVID-19 pneumonia. Recently, there are rising numbers of papers reporting major internal bleeding such as retroperitoneal haemorrhage as a complication of COVID-19.3–6 Pelvic haematoma is defined as the presence of blood clot or fluid in the retroperitoneal zone III region, which is bordered with the dome of the bladder as anterior, sacrum as posterior and iliac wings as lateral borders. Spontaneous retroperitoneal haematoma secondary to anticoagulant therapy is one of the well-known but self-limiting conditions.
A recent case in New Jersey demonstrated the retroperitoneal bleeding complication in patient with COVID-19 after receiving therapeutic dose low molecular weight heparin (LMWH) while on antiplatelet in the light of the deranged coagulation profile, which results in haemorrhagic shock.3 A retrospective study of 355 patients with COVID-19 done by Musoke et al7 also convinced that therapeutic anticoagulant is significantly associated with increased risk of bleeding and mortality compared with subtherapeutic (intermediate dose) or prophylactic dose. In the UK, British Thoracic Society and Scottish Intercollegiate Guidelines Network currently suggest use of prophylactic dose LMWH for patients who are managed on a ward and intermediate-dose LMWH (twice daily standard prophylactic dose) for patients on critical care.8 Our patient being admitted to a critical care unit was administered with the recommended VTE prophylaxis treatment (intermediate-dose heparin therapy) accordingly. Retrospectively evaluating our case, the patient was considered to have low bleeding risk in the light of the absence of other comorbidities such as renal disease or bleeding disorder.
A study done by Tang et al9 reported that prolonged PT/APTT, increased D-dimer and low platelet counts are compatible with haemostasis derangement and hallmark of disseminated intravascular coagulation (DIC) in patients with COVID-19. In view of decreasing trend of inflammatory marker (C reactive protein), relatively normal platelet count and coagulation profile on the day 15, before she develops abdominal pain on the next day, we could speculate that her bleeding tendency is not compatible with a state of sepsis-induced DIC, which does not support coagulopathy. Furthermore, according to International Society of Thrombosis and Haemostasis (ISTH) scoring system, our patient’s low score is not suggestive of overt DIC, which is one of the common causes of mortality in patients with COVID-19 other than acute respiratory distress syndrome (ARDS). Nevertheless, this finding is in contradiction to a study done by Tang et al9 from Wuhan, where most of the COVID-19 non-survivors met the ISTH criteria for DIC compared with few survivors. Heparin-induced thrombocytopaenia (HIT) is the another differential to consider the cause of bleeding. HIT is one of the well-known potentially lethal side effect of heparin that can present with more than 50% fall in platelet count with or without thrombosis and bleeding. Our patient’s 4Ts score is low probability for HIT and therefore does not fit into it.
Interestingly, spontaneous retroperitoneal bleeding has been reported in a patient with COVID-19 who is not even on anticoagulant therapy.4 It is debatable to say that bleeding complication in our patient stems merely from a well-known side effect of anticoagulant because anti-factor Xa testing was not done to support it, and plasma heparin levels were unknown. The cause of bleeding still remains unclear. The mechanism of spontaneous pneumomediastinum in our patient could be explained by alveolar rupture due to barotrauma from cough, non-invasive ventilation and fragile ARDS lungs. Likewise, the novel hypothesis of spontaneous retroperitoneal bleeding could also be explained by ruptured of pelvic vessels due to increased intra-abdominal pressure in those patients with severe COVID-19 ARDS who are having cough,4 5 which is one of the common symptoms in patients with COVID-19 and pressure ventilation.5 However, there is no evidence available in the literature about the association between intra-abdominal bleeding and pressure ventilation.
Regards to the management of pelvic retroperitoneal bleeding, a decision for surgical intervention is extremely difficult and risky because the exploration of retroperitoneal haematoma may sometimes turn into unstoppable bleeding. Angiographic embolisation or stent grafting is reported to be effective in some cases of pelvic arterial bleeding,3 but not all of the hospital has that kind of facilities. In the absence of ongoing bleeding and bleeding point on a repeated angiogram, which was done within 24 hours in our case, we could speculate that the clinical course of our patient was rapidly deteriorated by a bleeding complication with low-grade DIC and patient succumbed from hyperkalaemia secondary to acute renal failure. In such a case, urgent haemodialysis should be considered urgently to prevent hyperkalaemic cardiac arrest.
In conclusion, although anticoagulant deems appropriate to use in patients with COVID-19 for VTE prophylaxis, clinicians should be vigilant about major bleeding complication such as retroperitoneal haemorrhage even when full therapeutic dosage is not used. This case report raises concern on the safety of anticoagulant therapy in patients with severe COVID-19 and calls for further studies to provide better evidence and guidance on the safe administration of anticoagulant in COVID-19 infected critically ill patients.
Learning points.
Heparin may be effective as well as harmful in patients with COVID-19.
Although anticoagulant deems appropriate to use in patients with COVID-19, clinicians should be cautious about major bleeding complication such as retroperitoneal haemorrhage even when full therapeutic dosage is not used.
This case report raises concern on the safety of anticoagulant therapy in patients with severe COVID-19 and calls for further studies to provide better evidence and guidance on the safe administration of anticoagulant in COVID-19 infected critically ill patients.
Retroperitoneal bleeding should be kept in mind for acute abdomen in COVID-19 infected patients who are taking anticoagulant, which warrants prompt assessment for diagnosis and intensive management to prevent mortality.
Haemorrhage risk in COVID-19 is as significant as thrombosis risk, especially with the use of anticoagulant, which is commonly used in COVID-19 to prevent thromboembolism events.
Footnotes
Contributors: MHO involves in the management of the patient. She wrote the initial draft of the article. JRN and NPL performed the literature search and wrote the final draft of the article and case description. KMO has provided the appropriate guidance to write and publish the article. MHO revised the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–9. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vadukul P, Sharma DS, Vincent P. Massive pulmonary embolism following recovery from COVID-19 infection: inflammation, thrombosis and the role of extended thromboprophylaxis. BMJ Case Rep 2020;13:e238168. 10.1136/bcr-2020-238168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel I, Akoluk A, Douedi S, et al. Life-Threatening psoas hematoma due to retroperitoneal hemorrhage in a COVID-19 patient on enoxaparin treated with arterial embolization: a case report. J Clin Med Res 2020;12:458–61. 10.14740/jocmr4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdinc B, Raina JS. Spontaneous retroperitoneal bleed coincided with massive acute deep vein thrombosis as initial presentation of COVID-19. Cureus 2020;12:e9772. 10.7759/cureus.9772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conti CB, Henchi S, Coppeta GP, et al. Bleeding in COVID-19 severe pneumonia: the other side of abnormal coagulation pattern? Eur J Intern Med 2020;77:147–9. 10.1016/j.ejim.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pia MI, Islam T. Renal and bleeding complications in critically ill Covid-19 Patient-A case report.
- 7.Musoke N, Lo KB, Albano J, et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res 2020;196:227–30. 10.1016/j.thromres.2020.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez K, Laffan M, Bradbury C. Debate: should the dose or duration of anticoagulants for the prevention of venous thrombosis be increased in patients with COVID-19 while we are awaiting the results of clinical trials? Br J Haematol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–7. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]