Abstract
Background
Photoageing describes complex cutaneous changes which occur following chronic exposure to solar ultraviolet radiation (UVR). Amongst White Northern Europeans, facial photoageing appears as distinct clinical phenotypes: ‘hypertrophic’ photoageing (HP) and ‘atrophic’ photoageing (AP). Deep, coarse wrinkles predominate in individuals with HP, whereas those with AP have relatively smooth, unwrinkled skin with pronounced telangiectasia. AP individuals have an increased propensity for developing keratinocyte cancers.
Objectives
To investigate whether histological differences underlie these distinct phenotypes of facial photoageing.
Methods
Facial skin biopsies were obtained from participants with AP (10 M, 10 F; mean age: 78.7 years) or HP (10 M, 10 F; mean age: 74.5 years) and were assessed histologically and by immunohistochemistry.
Results
Demographic characterization revealed 95% of AP subjects, as compared to 35% with HP, were Fitzpatrick skin type I/II; of these, 50% had a history of one or more keratinocyte cancers. There was no history of keratinocyte cancers in the HP cohort. Analysis of UVR‐induced mitochondrial DNA damage confirmed that all volunteers had received similar lifetime cumulative doses of sun exposure. Histologically, male AP had a significantly thicker epidermis than did AP females or those of either sex with HP. HP facial skin exhibited severe solar elastosis, whereas in AP facial skin, solar elastosis was apparent only in females. Loss of papillary dermal fibrillin‐rich microfibrils occurred in all HP and AP female subjects, but not in AP males. Furthermore, male AP had a significant reduction in collagen VII at the dermal–epidermal junction than did AP females or those of either sex with HP.
Conclusions
This study provides further evidence that AP and HP represent distinct clinical and histological entities. Knowledge of these two phenotypes is clinically relevant due to the increased prevalence of keratinocyte cancers in those – particularly males – with the AP phenotype.
Introduction
Photoageing describes complex cutaneous changes consequent on chronic exposure to solar ultraviolet radiation (UVR), known as photodamage, superimposed on a background of intrinsic skin ageing. It occurs in habitually sun‐exposed areas of the body such as face, neck and arms 1 and manifests clinically as wrinkles, lentigines, telangiectasia, mottled pigmentation, roughened texture, sallow complexion, laxity and decreased elasticity. Histologically, photoageing affects both epidermis and dermis; epidermal changes include thinning of the spinous layer and flattening of the dermal–epidermal junction (DEJ) caused by loss of rete ridges. 2 In the dermis, the most pronounced histological feature is accumulation of abnormally deposited amorphous elastin – termed solar elastosis 3 – with disintegration of the well‐organized elastic fibre network containing fibrillin‐rich microfibrils (FRMs). 4 Mature dermal collagen fibres become fragmented 5 and there is severe reduction in collagen VII‐containing anchoring fibrils at the DEJ, 6 further contributing to the weakened structural integrity of the tissue.
The degree of photodamage is significantly affected by an individual's ethnicity and Fitzpatrick skin type (FST). Thus, fair‐skinned individuals of Northern European descent (FST I‐III) are more prone to photoageing than individuals with skin of colour (FST IV‐VI). 7 It is accepted that amongst individuals of White Northern European descent, severe facial photoageing may result in two clinical phenotypes: ‘hypertrophic’ photoageing (HP) characterized by deep wrinkles and a leathery appearance or ‘atrophic’ photoageing (AP) characterized by telangiectasia, a shiny – almost translucent – unwrinkled appearance, and development of a variety of benign and malignant cutaneous neoplasms. 8 , 9 In this study, we provide detailed assessment of the histological features that characterize these differential facial phenotypes that arise as a consequence of exposure to chronic UVR by comparing the facial skin of individuals with severe AP to those with severe HP.
Materials and methods
Participant information and skin biopsy procurement
Volunteers were recruited to the study following clinical assessment (conducted by JA) and were deemed suitable to participate if they were White Northern European (FST I‐III) and >50 years of age. Basic demographic information was collected, and participants were asked to self‐declare their ethnicity (Table S1, Supporting Information). The clinical manifestations of AP and HP were captured using clinical photography (VISIA® Skin Analysis System, Canfield Scientific, Parsippany, NJ, USA). Skin biopsies were obtained from 20 volunteers with severe AP (grade 6 or above 8 ; males n = 10; female n = 10; mean age ± SEM; 78.7 ± 2.0 years) and 20 volunteers with severe HP (grade 6 or above 1 ; males n = 10; females n = 10; mean age 74.5 ± 2.1 years). Local ethical approval was obtained from the Greater Manchester Central NHS Research Committee (13/NW/0723). Written informed consent was obtained, and the study adhered to Declaration of Helsinki principles. Biopsies were taken from two sites: photoexposed face (4‐mm punch biopsy taken from the right upper zygomatic arch of the face, immediately lateral to the outer canthus of the eye) and photoprotected buttock (6‐mm punch biopsy). Each biopsy was obtained under 1% lignocaine anaesthesia; biopsies were snap‐frozen in liquid nitrogen and stored at −80°C.
For further experimental details, see Appendix S1 (Supporting Information).
Results
Clinical and demographic features of the AP and HP cohorts
Individuals with AP had characteristic telangiectasia and a smooth, unwrinkled facial appearance; in contrast, those with HP had deep furrows and wrinkles and a leathery appearance to the skin (Fig. 1). FST differed between cohorts with 95% of AP being FST I or II and 5% FST III, as compared to 35% of HP being FST I or II. Analysis of skin cancer occurrence revealed that 50% (n = 10/20) of AP participants had a history of one or more facial keratinocyte cancers compared with none of those with HP (n = 0/20; see Table 1). To confirm that both cohorts had been exposed to similar levels of solar UVR, facial skin was assayed for the occurrence of mitochondrial DNA damage. 10 , 11 Similar levels of UVR‐induced mitochondrial DNA damage were detected, irrespective of photoageing phenotype (P = 0.1391; Fig. S1, Supporting Information); thus, we conclude that all subjects had received similar lifetime cumulative doses of UVR.
Figure 1.
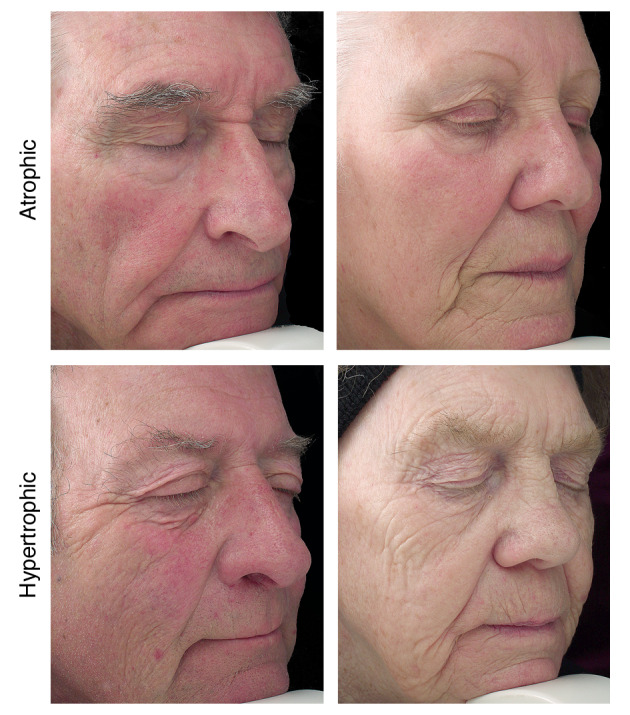
Clinical photographs of subjects with atrophic photoageing and hypertrophic photoageing. Individuals with Fitzpatrick skin phototypes I and II tend towards the atrophic photoageing (AP) phenotype with sparse fine wrinkles and focal depigmentation/dysplastic changes such as freckles and naevi. Furthermore, telangiectasia is common in individuals presenting clinically with the AP. In contrast, individuals with skin phototype III tend to show a hypertrophic photoageing (HP) phenotype and present clinically with responses such as tanning, deep wrinkles, coarseness, a leathery appearance of the skin and lentigines.
Table 1.
Subject demographics
| Atrophic | Hypertrophic | |||
|---|---|---|---|---|
| Males (n = 10) | Females (n = 10) | Males (n = 10) | Females (n = 10) | |
| Age [mean (SD)] | 80.2 (9.0) years | 77.2 (9.2) years | 71.3 (9.4) years | 77.7 (9.6) years |
| BMI [mean (SD)] | 26.5 (3.8) | 28.7 (11.6) | 24.1 (2.7) | 24.8 (5.2) |
| Fitzpatrick skin type [n (%)] | ||||
| I | 5 (50) | 5 (50) | 1 (10) | 4 (40) |
| II | 4 (40) | 5 (50) | 1 (10) | 1 (10) |
| III | 1 (10) | 0 (0) | 8 (80) | 5 (50) |
| IV | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| At least one sunburn before the age of 21 [Yes n (%)] | 7 (70) | 7 (70) | 3 (30) | 5 (50) |
| At least one facial keratinocyte cancer [Yes n (%)] | 5 (50) | 5 (50) | 0 (0) | 0 (0) |
Histological characterization of AP and HP
Analysis of facial skin identified that epidermal thickness was significantly reduced in HP skin as compared to AP skin (P < 0.001). Furthermore, in those with AP, epidermal thickness was significantly reduced in females as compared to males (P = 0.0076), whereas no such difference was identified for HP (P = 0.9298; Fig. 2a). Individuals with AP show some clinicopathological similarities with the condition dermatoporosis, described by Saurat as analogous to osteoporosis. 12 One factor common to both conditions is skin fragility which for dermatoporosis is characterized by marked reduction in CD44, a major cell surface receptor of hyaluronic acid. Facial skin showed significant differences in the intensity of CD44 expression between AP and HP (P = 0.004), with AP males displaying a significant reduction in CD44 expression as compared to AP females (P = 0.0057; Fig. 2b).
Figure 2.
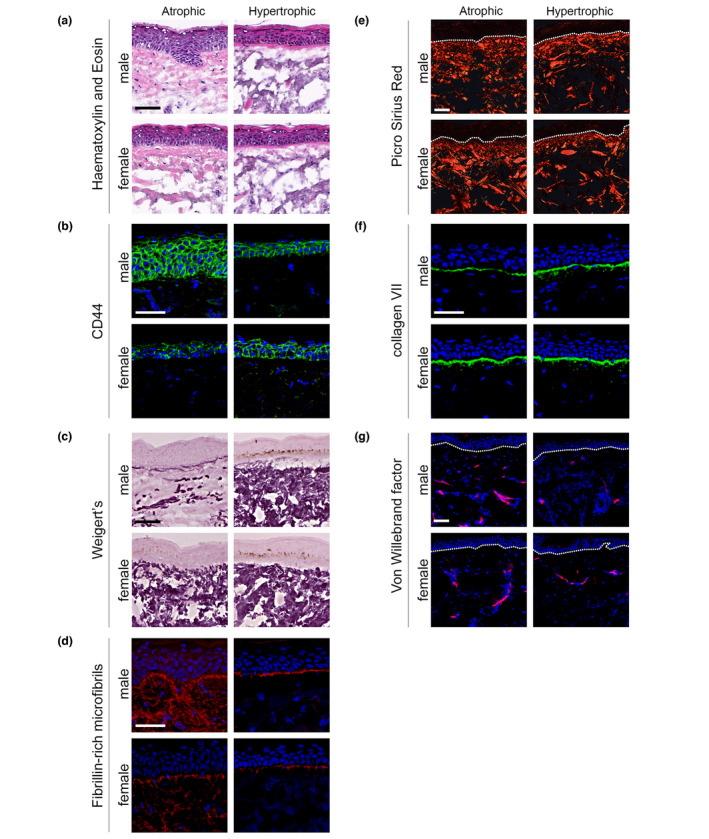
Differential histological features are present in atrophic photoageing and hypertrophic photoageing. All data refer to facial skin and all volunteers, regardless of their phenotypic status, exhibited characteristic flattening of the dermal–epidermal junction (DEJ). Epidermal thickness was maintained in male AP skin, but was significantly thinned in AP females and all HP facial skin (a). Gender differences were apparent within the AP cohort for CD44, a major cell surface receptor of hyaluronic acid; AP females had significantly reduced CD44 abundance as compared to AP males. In contrast, no gender differences were identified for HP (b). Weigert's resorcin fuchsin staining identified severe solar elastosis in AP females and in both males and females with HP. Solar elastosis was not detected in AP males; however, the amount elastic fibres was significantly depleted (c). Immunofluorescence staining identified that in all HP there was a marked loss of fibrillin‐rich microfibrils (FRMs) at the DEJ. In AP facial skin, a reduction in FRM at the DEJ was observed in females, whilst FRM morphology was preserved in AP males (d). Picrosirius red staining for organized fibrillar collagens identified no significant difference within the papillary dermis for either AP or HP cohorts (e). The distribution and intensity of collagen VII immunofluorescence at the DEJ were significantly reduced for male AP as compared to female AP and HP (f). Immunofluorescence staining for von Willebrand factor identified more vascular structures in AP than HP (g). Scale bars: 50 µm.
Weigert's resorcin fuchsin was used for histological assessment of solar elastosis, as it identifies the entirety of the elastic fibre network (FRM, elaunin fibres and elastic fibres‐proper). Facial skin showed significant differences in the deposition of elastotic material between the two phenotypes (P = 0.0011). In all cases, HP facial skin exhibited severe solar elastosis, characterized by extensive deposition of amorphous, abnormally thickened, curled and fragmented material in the papillary and reticular dermis (Fig. 2c). In AP facial skin, solar elastosis was readily apparent in females but absent in males (P = 0.0174). Loss of papillary dermal FRM is also a distinguishing feature of photoageing and significant differences in the deposition of papillary dermal FRM were identified between AP and HP (P = 0.0004). All cases of HP facial skin exhibited a marked loss of FRM at the DEJ (Fig. 2d). In AP facial skin, a reduction in FRM at the DEJ was observed in females, whilst FRM abundance was preserved in males (P = 0.0232). Reductions in fibrillar collagens are a further histological consequence of chronic photodamage; picrosirius red staining allows assessment of not only quantity of fibrillar collagens, but also their organization. This identified no difference in the collagenous dermal matrix as a function of phenotypic status (Fig. 2e). Collagen VII and laminin‐332 are essential components of the DEJ, acting to anchor epidermis to dermis. Both collagen VII and laminin‐332 are decreased in ageing, 13 and loss of collagen VII and the Lama3 subunit of laminin‐332 has been implicated in cutaneous squamous cell carcinoma (SCC) invasion. 14 The distribution and intensity of Lama3 were assessed across the cohorts by immunofluorescence. Lama3 was localized to the DEJ and was similarly abundant in all samples, irrespective of photoageing phenotype (Fig. S3, Supporting Information). In contrast, there were significant differences in the distribution and intensity of collagen VII between AP and HP (P = 0.0201). In AP facial skin, males displayed a significant reduction in collagen VII compared with females (P = 0.0011; Fig. 2f). Telangiectasis is a characteristic feature of individuals with AP and immunofluorescent staining for von Willebrand factor (vWF) identified significantly more vascular structures in AP facial skin compared with HP (P < 0.0001; Fig. 2g).
Discussion
We have established that clinical phenotypes of facial photoageing have distinct histological characteristics (Table 2). Overall, AP males had a histological phenotype separable from that of AP females and either gender with HP; in this instance, the epidermis maintained its thickness, there was no solar elastosis, papillary dermal FRMs were preserved and there was a significant reduction in collagen VII at the DEJ. This distinct pattern of cutaneous architecture and composition for AP males also appears to align with mechanisms that may increase their risk of developing keratinocyte cancers. 15 , 16 The global incidence of keratinocyte cancers has increased rapidly over the past half‐century, 17 , 18 and there are several reports of an association with AP. 8 , 9 Patients treated for facial basal cell carcinomas (BCCs) rarely present with deep coarse wrinkling. 19 Similarly, individuals with FSTs I/II are more likely to develop BCC or SCC than those who always tan (FST IV) 19 , 20 – also findings that are consistent with our AP cohort.
Table 2.
Properties of facial and buttock skin from atrophic photoageing and hypertrophic photoageing cohorts
| Parameter | Facial skin | Buttock skin | ||||
|---|---|---|---|---|---|---|
| AP males | AP females | HP males | HP females | AP | HP | |
| Epidermal thickness (µm) | 48.8 ± 3.9 | 35.8 ± 3.7 | 22.7 ± 2.3 | 21.2 ± 2.4 | 35.4 ± 2.9 | 27.2 ± 2.6 |
| Dermal–epidermal junction convolution | 1.28 ± 0.13 | 1.31 ± 0.09 | 1.52 ± 0.13 | 1.36 ± 0.15 | 1.52 ± 0.08 | 1.41 ± 0.08 |
| Solar elastosis abundance | 1.15 ± 0.36 | 3.10 ± 0.23 | 3.45 ± 0.21 | 3.20 ± 0.33 | 0.55 ± 0.15 | 0.62 ± 0.15 |
| Fibrillar collagen abundance (%) | 57.4 ± 2.6 | 52.7 ± 2.3 | 55.8 ± 2.2 | 56.4 ± 2.7 | 67.4 ± 1.7 | 67.2 ± 1.9 |
| Fibrillar collagen organization (%) | 55.5 ± 3.3 | 57.1 ± 6.4 | 57.7 ± 4.0 | 57.6 ± 6.5 | 66.5 ± 1.9 | 68.1 ± 2.3 |
| CD44 (a.u.) | 116.6 ± 5.4 | 90.8 ± 6.7 | 102.7 ± 4.4 | 106.8 ± 6.0 | 100.7 ± 4.0 | 104.2 ± 3.4 |
| Fibrillin‐rich microfibrils (a.u.) | 2.90 ± 0.1 | 2.00 ± 0.2 | 1.40 ± 0.3 | 2.10 ± 0.2 | 2.80 ± 0.1 | 3.00 ± 0.1 |
| Blood vessels (count) | 318.7 ± 30.0 | 359.7 ± 41.7 | 197.8 ± 20.1 | 184.9 ± 31.7 | 245.3 ± 31.2 | 187.2 ± 25.5 |
| Collagen VII (AUC; a.u.) | 20.8 ± 1.5 | 35.3 ± 1.4 | 33.4 ± 2.8 | 35.9 ± 3.7 | 35.2 ± 3.1 | 34.4 ± 2.3 |
| Lama3 (a.u.) | 20 793 ± 4798 | 15 363 ± 1340 | 17 328 ± 3593 | 15 548 ± 2191 | 15 714 ± 2528 | 15 641 ± 2633 |
All data values are presented as mean ± SEM.
AP, atrophic photoageing; a.u., arbitrary units; AUC, area under curve; HP, hypertrophic photoageing.
The presence of wrinkles themselves could be considered an important factor in protecting against the development of keratinocyte cancers. Our cohort of HP individuals, who present clinically with deep facial wrinkles and were less cancer‐prone, displayed severe solar elastosis and a marked reduction in FRM within the papillary dermis. In contrast, our smooth‐skinned, cancer‐prone, male AP cohort exhibited significant depletion of dermal elastic fibres, little or no solar elastosis, and remarkably well‐preserved FRM architecture. The composition and organization of the dermis may therefore play an important role in the progression of keratinocyte cancers; feasibly, accumulation of solar elastotic material arises as a consequence of the loss of the FRM scaffold for elastic fibres which of itself is in part, a protective response to damage from solar UVR. 21 Furthermore, keratinocyte cancers need to be able to expand into the dermis; this growth and expansion might be impaired by amorphous elastotic material. 9 , 22 The key changes that occur in the skin to create a pro‐oncogenic environment remain to be elucidated; however, aberrant intercellular signalling, increased fibroblast proliferation, extracellular matrix (ECM) remodelling and expression of CD44 have all been implicated to date. 23
Epidermal atrophy is a known sequela of chronological ageing which is probably accelerated by cumulative sun exposure. 24 The clinical appearance of AP skin suggests, erroneously, that it is ‘thin’ 9 due to its translucency, shininess and pronounced telangiectasia. The increased number of dilated blood vessels in AP 25 may be an important contributory factor to tumour growth. This in turn could alter the cytokine milieu, affecting immunoregulatory pathways and thus facilitating tumour development. 26 , 27 Furthermore, AP males had a significant reduction in collagen VII in the dermis compared with AP females or either gender with HP. Loss of collagen VII promotes skin tumour migration and invasion through a mechanism that involves disorganized keratinocyte differentiation. 28 Thus, this pathway may also be relevant to the development of keratinocyte cancers in males with AP. Females with AP appear to represent an intermediate phenotype – histologically their dermal ECM more closely resembles that seen in HP. However, despite this, AP females are more likely to develop keratinocyte cancers than their HP counterparts. Thus, features such as telangiectasia, increased dermal vascularization and lighter skin phototype may all play a contributing role to this more pro‐oncogenic environment.
These distinct differences in clinical appearance and histology lead us to propose a hypothetical model of how lifelong exposure to UVR induces disparate mechanisms for AP and HP (Fig. 3). For both phenotypes, ECM damage occurs via the induction of proteases, such as matrix metalloproteinases (MMPs), by UVR. 29 However, in the case of HP, the partial degradation of FRMs at the DEJ may also liberate reactive oxygen species (ROS) 30 leading to atypical transforming growth factor (TGF)‐β signalling 21 , 29 and further activation of MMPs. Post‐transcriptional mechanisms within dermal fibroblasts allow the accumulation of tropoelastin that is unable to form structurally competent elastic fibres. In addition, disorganized mature elastin accumulates within the dermis as a consequence of the loss of the FRM scaffold 21 ; together, these two mechanisms drive the formation of solar elastosis. In contrast, AP males maintain their epidermal thickness, which may help to protect these superficial FRM at the DEJ from direct photo‐degradation. 31 Alternatively, it is conceivable that FRMs in AP skin are biochemically distinct to those found in HP in that they are not as susceptible to UV degradation due to differences in their UV chromophore content. 31 Hence, the cascade of events that ultimately results in solar elastosis is not triggered in AP males.
Figure 3.
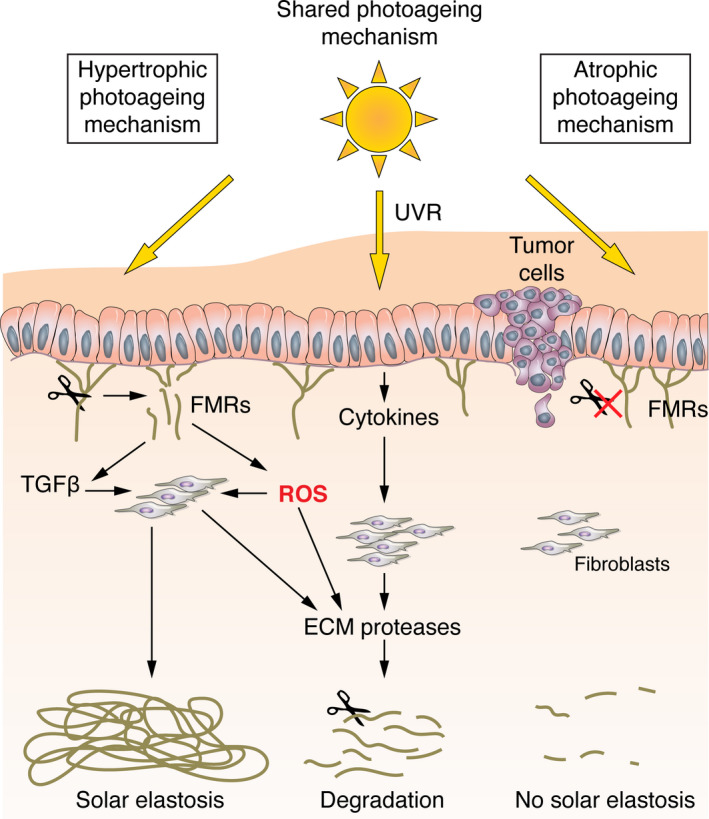
Hypothetical model depicting how lifelong exposure to ultraviolet radiation (UVR) may produce disparate mechanisms for atrophic and hypertrophic photoageing. In both atrophic photoageing (AP) and hypertrophic photoageing (HP), degradation of dermal components occurs via a cell‐mediated process driven by UVR‐induced expression of extracellular matrix (ECM) proteases. In HP, UVR may also interact directly with fibrillin‐rich microfibrils (FRMs) causing their degradation. This leads to the further upregulation of ECM proteases, the release of ROS and atypical TGF‐β signalling. Post‐transcriptional mechanisms within dermal fibroblasts allow the deposition of tropoelastin. In addition, disorganized mature elastin accumulates as a consequence of the loss of the FRM scaffold, and together, these two mechanisms drive the formation of solar elastosis. In contrast, AP males maintain epidermal thickness which may help to protect superficial FRM from direct photo‐degradation. FRMs in AP skin may also be biochemically distinct to those found in HP in that they are not as susceptible to UV degradation due to differences in their UV chromophore content. Thus, the cascade of events that ultimately results in solar elastosis is not triggered in AP males and this in turn is associated with an increased susceptibility to keratinocyte cancers.
Taken together, we present novel data that provide insight into the observed polarity of clinical phenotypes of chronically sun‐exposed skin. This study identifies several histological factors including FRM preservation, absence of solar elastosis, loss of collagen VII and neovascularization that increase the chances of developing keratinocyte cancers in AP, particularly in men. In contrast, it appears that coarse wrinkling may be a ‘photoprotective mechanism’ and somewhat beneficial to an individual in the sense that there are fewer keratinocyte cancers. Our subjects represent extreme, i.e., severe examples of the two photoageing phenotypes; thus, it is likely that the histological findings also represent the most extreme differences. Knowledge of these two phenotypes of photoageing is of clinical relevance due to the increased prevalence of keratinocyte cancers in ageing populations with the AP phenotype. This is an aspect of skin ageing and carcinogenesis that warrants further exploration.
Supporting information
Fig. S1. mtDNA damage detection reveals that all participants were exposed to similar levels of UVR.
Fig. S2. Histological features of buttock skin from AP and HP cohorts.
Fig. S3. Lama3 expression is not differentially regulated across different photoageing phenotypes.
Table S1. Summary of ancestral heritage for subjects within each cohort.
Appendix S1. Supplementary materials and methods.
Acknowledgements
The healthy volunteers in this manuscript have given written informed consent to publication of their clinical photographs.
Conflict of interest Dr. Langton, Dr. Ayer, Dr. Rashdan, Dr. Naidoo, Dr. Caley and Dr. Birch‐Machin have nothing to disclose. Dr. T Griffiths reports grants and personal fees from Walgreens Boots Alliance, during the conduct of the study. Dr. O'Toole reports grants and other from Kamari Pharma, Sanofi, Palvella Therapeutics and Mayne Pharma, outside the submitted work. Professor C Griffiths reports grants and personal fees from Walgreens Boots Alliance, during the conduct of the study. Professor Watson reports grants from Walgreens Boots Alliance, during the conduct of the study.
Funding sources This work was supported by a programme grant from Walgreens Boots Alliance, Nottingham, UK. CEMG and REBW are funded in part by the National Institute of Health Research (NIHR) Manchester Biomedical Research Centre.
References
- 1. Griffiths CEM, Wang TS, Hamilton TA, Voorhees JJ, Ellis CN. A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol 1992; 128: 347–351. [PubMed] [Google Scholar]
- 2. Allan AK. The area of the dermo‐epidermal junction in human skin. Anato Rec 1958; 131: 717–725. [Google Scholar]
- 3. Kligman AM. Early destructive effect of sunlight on human skin. JAMA 1969; 210: 2377–2380. [PubMed] [Google Scholar]
- 4. Watson REB, Griffiths CEM, Craven NM, Shuttleworth CA, Kielty CM. Fibrillin‐rich microfibrils are reduced in photoaged skin. Distribution at the dermal‐epidermal junction. J Invest Dermatol 1999; 112: 782–787. [DOI] [PubMed] [Google Scholar]
- 5. El‐Domyati M, Attia S, Saleh F et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002; 11: 398–405. [DOI] [PubMed] [Google Scholar]
- 6. Craven NM, Watson REB, Jones CJ, Shuttleworth CA, Kielty CM, Griffiths CEM. Clinical features of photodamaged human skin are associated with a reduction in collagen VII. Br J Dermatol 1997; 137: 344–350. [PubMed] [Google Scholar]
- 7. Vierkotter A, Krutmann J. Environmental influences on skin aging and ethnic‐specific manifestations. Dermatoendocrinol 2012; 4: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayer J, Ahmed A, Duncan‐Parry E et al. A photonumeric scale for the assessment of atrophic facial photodamage. Br J Dermatol 2018; 178: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 9. Sachs DL, Varani J, Chubb H et al. Atrophic and hypertrophic photoaging: clinical, histologic, and molecular features of 2 distinct phenotypes of photoaged skin. J Am Acad Dermatol 2019; 81: 480–488. [DOI] [PubMed] [Google Scholar]
- 10. Bösch C, Lietz G, Birch‐Machin MA. Effects of polyphenols on mitochondrial DNA damage in skin fibroblasts. Br J Dermatol 2014; 170: e9. [Google Scholar]
- 11. Bösch C, Sadeghpour A, Rappolt M, Birch‐Machin MA. Differential protective effects of flavonols towards mitochondrial stress. Br J Dermatol 2016; 174: e70. [Google Scholar]
- 12. Saurat JH. Dermatoporosis. The functional side of skin aging. Dermatology 2007; 215: 271–272. [DOI] [PubMed] [Google Scholar]
- 13. Langton AK, Halai P, Griffiths CEM, Sherratt MJ, Watson REB. The impact of intrinsic ageing on the protein composition of the dermal‐epidermal junction. Mech Ageing Dev 2016; 156: 14–16. [DOI] [PubMed] [Google Scholar]
- 14. Caley M, Martins V, Moore K et al. Loss of laminin alpha 3 drives SCC invasion via ROCK signalling. J Invest Dermatol 2016; 136: S241. [Google Scholar]
- 15. Hemminki K, Zhang H, Czene K. Time trends and familial risks in squamous cell carcinoma of the skin. Arch Dermatol 2003; 139: 885–889. [DOI] [PubMed] [Google Scholar]
- 16. Wassberg C, Thorn M, Johansson AM, Bergstrom R, Berne B, Ringborg U. Increasing incidence rates of squamous cell carcinoma of the skin in Sweden. Acta Derm Venereol 2001; 81: 268–272. [DOI] [PubMed] [Google Scholar]
- 17. Hussain SK, Sundquist J, Hemminki K. Incidence trends of squamous cell and rare skin cancers in the Swedish national cancer registry point to calendar year and age‐dependent increases. J Invest Dermatol 2010; 130: 1323–1328. [DOI] [PubMed] [Google Scholar]
- 18. Trakatelli M, Ulrich C, del Marmol V, Euvrard S, Stockfleth E, Abeni D. Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol 2007; 156(Suppl 3): 1–7. [DOI] [PubMed] [Google Scholar]
- 19. Brooke RC, Newbold SA, Telfer NR, Griffiths CEM. Discordance between facial wrinkling and the presence of basal cell carcinoma. Arch Dermatol 2001; 137: 751–754. [PubMed] [Google Scholar]
- 20. English DR, Armstrong BK, Kricker A. Reproducibility of reported measurements of sun exposure in a case‐control study. Cancer Epidemiol Biomarkers Prev 1998; 7: 857–863. [PubMed] [Google Scholar]
- 21. Watson REB, Gibbs NK, Griffiths CEM, Sherratt MJ. Damage to skin extracellular matrix induced by UV exposure. Antioxid Redox Signal 2014; 21: 1063–1077. [DOI] [PubMed] [Google Scholar]
- 22. Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014; 15: 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kretzschmar K, Weber C, Driskell RR, Calonje E, Watt FM. Compartmentalized epidermal activation of beta‐catenin differentially affects lineage reprogramming and underlies tumor heterogeneity. Cell Rep 2016; 14: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Contet‐Audonneau JL, Jeanmaire C, Pauly G. A histological study of human wrinkle structures: comparison between sun‐exposed areas of the face, with or without wrinkles, and sun‐protected areas. Br J Dermatol 1999; 140: 1038–1047. [DOI] [PubMed] [Google Scholar]
- 25. Helfrich YR, Maier LE, Cui Y et al. Clinical, histologic, and molecular analysis of differences between erythematotelangiectatic rosacea and telangiectatic photoaging. JAMA Dermatol 2015; 151: 825–836. [DOI] [PubMed] [Google Scholar]
- 26. Carmeliet P. Angiogenesis in health and disease. Nat Med 2003; 9: 653–660. [DOI] [PubMed] [Google Scholar]
- 27. Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol 1992; 3: 65–71. [PubMed] [Google Scholar]
- 28. Martins VL, Vyas JJ, Chen M et al. Increased invasive behaviour in cutaneous squamous cell carcinoma with loss of basement‐membrane type VII collagen. J Cell Sci 2009; 122: 1788–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rittie L, Fisher GJ. UV‐light‐induced signal cascades and skin aging. Ageing Res Rev 2002; 1: 705–720. [DOI] [PubMed] [Google Scholar]
- 30. Sander CS, Chang H, Salzmann S et al. Photoaging is associated with protein oxidation in human skin in vivo . J Invest Dermatol 2002; 118: 618–625. [DOI] [PubMed] [Google Scholar]
- 31. Hibbert SA, Watson REB, Gibbs NK et al. A potential role for endogenous proteins as sacrificial sunscreens and antioxidants in human tissues. Redox Biol 2015; 5: 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. mtDNA damage detection reveals that all participants were exposed to similar levels of UVR.
Fig. S2. Histological features of buttock skin from AP and HP cohorts.
Fig. S3. Lama3 expression is not differentially regulated across different photoageing phenotypes.
Table S1. Summary of ancestral heritage for subjects within each cohort.
Appendix S1. Supplementary materials and methods.


