Abstract
Aim
To investigate the characteristics and severity of dysarthria in children and adults with ataxia telangiectasia.
Method
All children and adults with ataxia telangiectasia who visited our multidisciplinary outpatient clinic for ataxia telangiectasia were asked to participate in this study, which took place in March 2019. To evaluate dysarthria, we used the Radboud Dysarthria Assessment in adults (older than 18y) and the paediatric Radboud Dysarthria Assessment in children (5–18y), including the observational tasks ‘conversation’ and ‘reading’, and the speech‐related maximum performance tasks ‘repetition rate’, ‘phonation time’, ‘fundamental frequency range’, and ‘phonation volume’. Speech intelligibility was measured using the Intelligibility in Context Scale.
Results
Twenty‐two individuals (15 children [5–17y], seven adults [19–47y]; 14 males and eight females; mean age 19y, SD 15y 2mo) participated. Dysarthria was present in all participants and characterized by ataxic components in adults and similar uncontrolled movements in children. In most participants, speech was mildly to mildly/severely affected. Almost all participants had an abnormal score for at least one maximum performance task.
Interpretation
Dysarthria in ataxia telangiectasia is characterized by uncontrolled, ataxic, and involuntary movements, resulting in monotonous, unstable, slow, hypernasal, and chanted speech.
What this paper adds
Dysarthria in ataxia telangiectasia is characterized by uncontrolled, ataxic, and involuntary movements.
Dysarthria in ataxia telangiectasia results in monotonous, unstable, slow, hypernasal, and chanted speech.
Dysarthria in ataxia telangiectasia can be assessed using the Radboud Dysarthria Assessment and the paediatric Radboud Dysarthria Assessment.
What this paper adds
Dysarthria in ataxia telangiectasia is characterized by uncontrolled, ataxic, and involuntary movements.
Dysarthria in ataxia telangiectasia results in monotonous, unstable, slow, hypernasal, and chanted speech.
Dysarthria in ataxia telangiectasia can be assessed using the Radboud Dysarthria Assessment and the paediatric Radboud Dysarthria Assessment.
Abbreviations
- ATM
Ataxia telangiectasia mutated
- FFR
Fundamental frequency range
- ICS
Intelligibility in Context Scale
- MPT
Maximum phonation time
- MPV
Maximum phonation volume
- MRR
Maximum repetition rate
- p‐RDA
Paediatric Radboud Dysarthria Assessment
- RDA
Radboud Dysarthria Assessment
Ataxia telangiectasia is a rare, autosomal recessively inherited, complex, and progressive multi‐system disorder, caused by pathogenic variants in the ataxia telangiectasia mutated (ATM) gene, which encodes the ATM kinase enzyme. 1 ATM kinase plays a pivotal role in numerous cellular processes such as repair of double‐strand DNA breaks, cell growth and apoptosis, mitochondrial energy metabolism, cell cycle control, and oxidative stress responses. 1 , 2
Classic ataxia telangiectasia is associated with the absence of ATM kinase activity. Individuals with ataxia telangiectasia and a classic phenotype have an early childhood‐onset cerebellar ataxia, extrapyramidal movement disorders, and peripheral neuropathy. Furthermore, the disease is characterized by the presence of oculocutaneous telangiectasias, which are prominent dilated blood vessels on the eye sclerae and sun‐exposed skin, abnormal immunological and respiratory functions, and an increased risk of developing malignancies. 3 Individuals with classic ataxia telangiectasia generally do not survive beyond the age of 30 years owing to mortality from malignancies and respiratory failure. 4 There is no cure for ataxia telangiectasia.
Besides classic ataxia telangiectasia, there is a milder clinical phenotype which is designated ‘variant ataxia telangiectasia’. Individuals with this have residual ATM kinase activity, 5 which is associated with a later onset (80% have their first symptoms by age of 10y 6 ), fewer systemic symptoms, and milder neurological impairment. 7 In addition, individuals with variant ataxia telangiectasia have much longer lifespans compared with individuals with the classic form. 4 , 7
As part of the complex cerebellar and extrapyramidal movement disorders in ataxia telangiectasia, dysarthria is a common symptom. 8 , 9 Although the literature describes the occurrence of dysarthria in children and adults with ataxia telangiectasia, little attention has been given to the precise description of its characteristics and to the resulting communication limitations in these individuals. Ten years ago, we studied the characteristics of cognition and speech in eight children with ataxia telangiectasia, and showed that dysarthria was present in all participants. 9 All children had a mixture of the ataxic and hyperkinetic types of dysarthria. In most of the children, speech was moderately to severely affected, and they were hampered by decreased speech intelligibility. The speech‐related activities and functional capabilities in these children were not studied. Because dysarthria has a profound impact on children’s development, social interactions, and quality of life, knowledge about dysarthria in children and adults with ataxia telangiectasia is essential to optimize speech and language therapy, and by doing so to improve quality of life.
Standardized and validated assessment tools to examine dysarthria in general, let alone in children and adults with ataxia telangiectasia, are lacking. For adults, the need for such a tool is covered by the Radboud Dysarthria Assessment (RDA), which was finished and released in 2014. 10 , 11 Since speech problems in children differ from those found in adults, a paediatric version of this assessment, the paediatric RDA (p‐RDA), was finished and released in 2019. 12
The aim of the present study was to systematically investigate the characteristics and severity of dysarthria in children and adults with ataxia telangiectasia, using the RDA and the p‐RDA. In this way, we aimed to get more insights into the motor abnormalities that underlie dysarthria in individuals with ataxia telangiectasia, as well as their functional consequences, to improve advice for treatment and training by speech language pathologists.
METHOD
Study design
This prospective observational cohort study was performed between 18th and 22nd March 2019 at the outpatient clinic of the Radboud University Medical Center in Nijmegen, the Netherlands.
This study was part of the baseline measurements from an interventional study that we conducted in children and adults with ataxia telangiectasia (see ClinicalTrials.gov, identifier NCT03962114), in which dysarthria served as an outcome measure to investigate the effect of the intervention.
Participants
All known 19 children and 15 adults (n=34) with (a genetically confirmed diagnosis of) ataxia telangiectasia who visited our multidisciplinary outpatient ataxia telangiectasia clinic were considered potential candidates and were asked to participate in this study.
On the basis of the conditions of the study instruments (see below), individuals who did not understand and speak the Dutch or French language, and children below the age of 5 years, had to be excluded from participation. Written informed consent was obtained from all participants or their parents/legal guardians, according to the tenets of the Declaration of Helsinki (1983 revision). The study was approved by the Regional Committee on Research involving Human Subjects Arnhem‐Nijmegen (NL68197.091.18).
Instruments
To evaluate dysarthria, we used the RDA in adults (aged older than 18y) and the p‐RDA in children (aged 5–18y). The RDA is a reliable and valid assessment instrument 11 , 12 that includes two observational tasks: a short conversation about any topic and a standardized text to read out loud. Furthermore, four speech‐related maximum performance tasks are part of the RDA: maximum repetition rate (MRR), maximum phonation time (MPT), fundamental frequency range (FFR), and maximum phonation volume (MPV). On the basis of all conducted speech performances, the type and severity of dysarthria can be determined. The type of dysarthria is classified as spastic, ataxic, hypokinetic, hyperkinetic, and/or flaccid, using protocols for the classification of dysarthria on the basis of the five aspects of speech production, respiration, phonation articulation, nasal resonance, and prosody.
The severity of dysarthria on a function level was scored using the following scale: no dysarthria (0), minimal dysarthria (1), mild dysarthria (2), mild/severe dysarthria (3), severe dysarthria (4), and very severe dysarthria/anarthria (5). The scale describing severity on activity level was defined as effective communication (0), effective despite minimal imperfections (1), occasional repetitions are required (2), frequent repetitions are required (3), communication possible with help from a well‐known person (4), and no oral communication possible (5). The score of the activity level was derived from the conversation and reading tasks.
The basic structure of the p‐RDA is similar to the adult version (two observational tasks and four maximum performance tasks). Adjustments of the p‐RDA compared with the RDA are easier reading tasks and a list of words and sentences to imitate. Furthermore, the classification of dysarthria in the p‐RDA does not match with the neuro‐anatomical‐based classification for adults. 13 Considering the differences in the pathophysiology of dysarthria between children and adults, differences in speech characteristics exist. 14 Dysarthria in adults generally involves a speech system that once was intact, whereas childhood dysarthria usually involves a developing motor, cognitive, and linguistic system. In addition, the neural basis and, often, the cause of dysarthria in children and adults differ, so an adult‐based neurobehavioral classification system is not valid for children. 12 Therefore, the p‐RDA uses a description to indicate the characteristics that can be heard in the speech of children. With these characteristics it is possible to describe the dysarthria, indicating the most dominant components (i.e. flaccid, strained, uncontrolled, and involuntary).
To get insight into everyday communicative functioning of individuals with ataxia telangiectasia, the intelligibility of speech was measured using the Intelligibility in Context Scale (ICS). 15 This seven‐item questionnaire rates the degree to which the participant’s speech is understood by different communication partners (parents/life partners, immediate family, extended family, friends, acquaintances, teachers/colleagues, strangers) on a five‐point scale (1, never; 2, rarely; 3, sometimes; 4, usually; 5, always).
Procedure
All speech assessments from the RDA and p‐RDA were performed in the native language of the participant by one of the speech language pathologists (EMM and MHJCvG). To optimize analysis and scoring, all sessions were recorded by video/audio, using a Canon Legria FS200 camera (Canon, Tokyo, Japan) and a Tascam DR‐05 audio recorder (Teac Corporation, Tokyo, Japan). All assessments were performed under protocolled conditions according to preparation (optimal body position and a room without ambient noise), camera position (in front at eye level), and position of external microphone (30cm distance from the participant’s mouth). All recordings were individually scored by an experienced speech language pathologist (MHJCvG), to determine severity and characteristics of dysarthria.
To measure the maximum performance tasks, participants had to perform several assignments. For assessment of the MRR, participants were asked to repeat the trisyllabic sequence ‘pataka’ as fast as possible. MRR was analysed with Praat (Praat: doing phonetics by computer. Version 6.0.21, Edition 2018) and expressed in syllables per second. For measuring MPT, participants were requested to produce an ‘a’ for as long as possible after maximal inhalation. MPT was analysed with Praat and expressed in seconds.
The FFR was determined by asking participants to produce an ‘a’ from the lowest possible to the highest possible pitch and vice versa. FFR was analysed with Praat and expressed in hertz. FFR was converted from hertz to semitones using the following formula: semitones=39.87×log(F/50). 16
To assess the MPV, participants were asked to shout ‘Hello!’ as loudly as possible. MPV was measured with a decibel‐meter (Voltcraft SL‐100; Voltcraft, Hirschau, Germany) at 30cm distance from the mouth, and expressed in decibels.
For all maximum performance tasks, a centile score was determined on the basis of the raw scores. Maximum performance tasks were considered as abnormal below the fifth centile.
To measure speech intelligibility, we asked the parents (or caregivers) of children and the parents or life partners of adults with ataxia telangiectasia to fill out the ICS questionnaire. The questionnaire was sent by e‐mail via the online database Castor‐Electronic Data Capture. For all participants, a total ICS score was measured. The total score was defined as the mean of the seven completed items. 15
To present our results, we used descriptive statistics. Statistical analysis was performed using IBM SPSS statistics 25.0 for Windows (IBM SPSS Inc., Chicago, IL, USA). For ordinal data, medians and ranges are given; for nominal data, means and standard deviations (SD) are presented.
RESULTS
Fifteen children and seven adults (total n=22) with ataxia telangiectasia were included in the study: 14 males and eight females (mean age 19y, SD 15y 2mo). Sixteen participants had the classic ataxia telangiectasia phenotype (mean age 11y 2mo, SD 5y 8mo) and six had variant ataxia telangiectasia (mean age 39y 10mo, SD 11y 9mo).
Twenty participants were native Dutch speakers. Two participants lived in Belgium, and for one of them the native language was French. One child had Turkish nationality but had lived in the Netherlands for several years and was raised bilingually (Turkish and Dutch). Participant characteristics are summarized in Table 1. Twelve other individuals with ataxia telangiectasia did not participate in the present study for the following reasons: person or parents refused to participate (n=6); despite several attempts we could not reach individuals (n=2); or children were too young to be assessed for dysarthria (n=4).
Table 1.
Participants’ characteristics
| Total, n=22 | 5–18y, n=15 | >18y, n=7 | |
|---|---|---|---|
| Age, mean (SD), y:mo | 19 (15:2) | 10:2 (4:7) | 38 (11:3) |
| Sex (n) | |||
| Male | 14 | 11 | 3 |
| Female | 8 | 4 | 4 |
| Phenotype (n) | |||
| Classic | 16 | 14 | 2 |
| Variant | 6 | 1 | 5 |
| Native language (n) | |||
| Dutch | 21 | 14 | 7 |
| French | 1 | 1 | 0 |
| Loss of autonomous walking (n) | |||
| Yes | 15 | 10 | 5 |
| No | 7 | 5 | 2 |
Dysarthria: prevalence, severity on function and activity level, and characteristics
Dysarthria was present in all 22 participants (Table 2). In most of them, speech was mildly to mildly/severely affected on the functional level. Within the paediatric sample, a wider range of severity scores was found on the functional level (minimal dysarthria to severe dysarthria). Despite dysarthria, oral communication was possible in all participants. Nevertheless, regarding severity scores on the activity level, most participants (16 out of 22) needed to repeat their words to be understood. Thereby, children needed to repeat their words more frequently than adults (12 out of 15 children; 3 out of 7 adults).
Table 2.
Overview of dysarthria
| Patient | Characteristics | Dysarthria | Maximum performance tasks | ICS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y), sex | Ataxia telangiectasia phenotype | Predominant feature | Activity | Function | MRR | MPT | FFR (H–L) | MPV | Mean | ||||||
| 1 | 5, M | C | U+I | 2 | 2 | a | 4.94 | <c5 | 13.4 | c5–25 | 105.6 | c50 | 5 | ||
| 2 | 5, M | C | U | 2 | 2 | a | 5.73 | <c5 | 11.43 | c5–25 | 91 | <c5 | 4 | ||
| 3 | 6, M | C | U | 1 | 1 | a | 2.71 | <c5 | 28.41 | c75–90 | 97.3 | c5 | 3.86 | ||
| 4 | 6, M | C | U | 1 | 1 | 4.93 | c5–25 | 8.73 | c5–25 | 15.66 | c5–c25 | 113 | c75–90 | 3.14 | |
| 5 | 7, M | C | U | 2 | 3 | 2.68 | <c5 | 4.05 | <c5 | 8.09 | <c5 | 101.1 | c5–25 | 3.86 | |
| 6 | 8, F | C | U+S | 3 | 3 | a | a | a | 94.1 | <c5 | 4 | ||||
| 7 | 8, M | C | U | 2 | 3 | 3.44 | <c5 | 2.89 | <c5 | 11.04 | <c5 | 96.7 | <c5 | 3.86 | |
| 8 | 8, F | C | U | 3 | 3 | 2.8 | <c5 | 6.64 | c5 | 12.88 | c5–25 | 92.5 | <c5 | 3.71 | |
| 9 | 11, M | C | U | 2 | 2 | 3.96 | <c5 | 2.37 | <c5 | 7.55 | <c5 | 95.4 | <c5 | 4.29 | |
| 10 | 12, M | C | U | 2 | 3 | 2.59 | <c5 | 3.76 | <c5 | 9.36 | <c5 | 89.8 | <c5 | 3.57 | |
| 11 | 13, F | C | U | 2 | 3 | 2.42 | <c5 | 4.81 | <c5 | 17.58 | c5–25 | 89.2 | <c5 | 3.43 | |
| 12 | 15, M | C | U | 3 | 4 | a | a | a | a | 3.57 | |||||
| 13 | 16, M | C | U | 2 | 3 | 4.2 | <c5 | 2.5 | <c5 | 9.6 | <c5 | 101.9 | c5–c25 | 3.43 | |
| 14 | 16, F | V | U | 2 | 2 | 4.93 | <c5 | 5.17 | <c5 | 5.36 | <c5 | 79.9 | <c5 | b | |
| 15 | 17, M | C | U | 1 | 2 | 5.24 | <c5 | 15.11 | c50 | 24.14 | c50 | 85.9 | c25 | 4.43 | |
| Median: | 2 | 2.47 | Mean: | 3.71, SD: 1.08 | 5.34, SD: 3,88 | 13.42, SD: 4.52 | 95,.24, SD: 9.3 | 3.86, SD: 0.47 | |||||||
| 16 | 19, M | C | A | 2 | 3 | 3.26 | <c5 | 6.07 | <c5 | 12 | <c5 | 88.2 | <c5 | b | |
| 17 | 24, M | C | A | 2 | 3 | 3.46 | <c5 | 4.22 | <c5 | 12.37 | <c5 | 93.6 | <c5 | 3.71 | |
| 18 | 43, M | V | A | 1 | 3 | 3.6 | <c5 | 3.59 | <c5 | 12.85 | <c5 | 91.5 | <c5 | 4.86 | |
| 19 | 43, F | V | A | 2 | 3 | 2.89 | <c5 | a | 18.05 | c5 | 104.2 | c90 | 3.71 | ||
| 20 | 44, F | V | A | 1 | 2 | 4.39 | <c5 | 10.62 | c5–c25 | 14.46 | <c5 | 101.2 | c50 | 4.29 | |
| 21 | 46, F | V | A | 1 | 3 | 2.97 | <c5 | 14.16 | c5–c25 | 18.38 | c5–c25 | 102.5 | c50–90 | 4.14 | |
| 22 | 47, F | V | A | 1 | 2 | a | 5.31 | <c5 | 12.09 | <c5 | 95.6 | <c5 | 3 | ||
| Median: | 1.48 | 2.71 | Mean: | 3.43, SD: 2.61 | 7.33, SD: 4.55 | 14.31, SD: 2.61 | 96.66, SD: 6.21 | 3.93, SD: 0.63 | |||||||
Activity: severity on activity level: effective communication (0), effective despite minimal imperfections (1), occasional repetitions are required (2), frequent repetitions are required (3), communication possible with help from a well‐known person (4), no oral communication possible (5). Function: severity on function level: no dysarthria (0), minimal dysarthria (1), mild dysarthria (2), mild/severe dysarthria (3) severe dysarthria (4), very severe dysarthria/anarthria (5). Total ICS score: never (1), rarely (2), sometimes (3), usually (4), always (5).
Missing values for maximum performance tasks owing to difficulties for patients to perform the task.
Missing values for mean ICS, owing to not completing the questionnaire. <c5 indicates an abnormal score, ≥c5 indicates a normal score. ICS, Intelligibility in Context Scale; MMR, maximum repetition rate (syllables per second); MPT, maximum phonation time (seconds); FFR, Fundamental frequency range (semitones); H–L, high to low; MPV, maximum phonation volume (decibel); C, classic phenotype; V, variant phenotype; U, uncontrolled; I, involuntary movements; S, strained; A, ataxic; c, centile.
All children showed predominantly uncontrolled dysarthric components, resulting in a monotonous, unstable, slow, hypernasal, and chanted speech (Video S1, online supporting information). In two children this was combined with strained components or involuntary movements. In all adults, ataxic dysarthria was found with some strained components as part of the ataxia.
Maximum performance tasks
The results for each maximum performance task are presented in Table 2. We intended to measure maximum performance tasks in all 22 participants. Unfortunately, we did not succeed in measuring all tasks in all participants owing to difficulties for participants in performing the tasks, or as a result of coughing, laughing, and breathing: sometimes the assessment demanded too much cooperation of children (especially young children).
Intelligibility
We received a completed ICS questionnaire from 20 participants. The total ICS for all participants was 3.88 (n=20, range 3.14–5). Children had a total ICS score of 3.83 (n=14, range 3.14–5). This indicated that the children in our study population were sometimes to usually understood. The total ICS score in adults was 4 (n=5, range 3–4.85), suggesting that adults in our study population were usually understood.
Intelligibility compared with severity on activity level
Intelligibility scores measured with the ICS compared with the severity levels of the activity domain are shown in Figure 1. In both adults and children, ICS was scored higher than the severity on activity domain in most cases.
Figure 1.
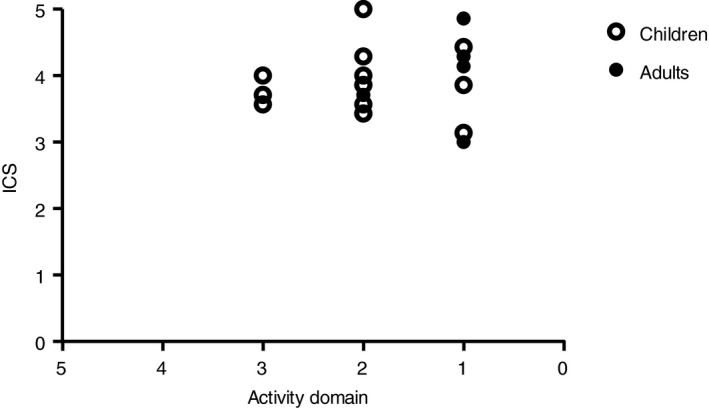
Total Intelligibility in Context Scale (ICS) compared with severity on activity level. Total ICS score: never (1), rarely (2), sometimes (3), usually (4), always (5), understood. Severity on activity level: effective communication (0), effective despite minimal imperfections (1), occasional repetitions are required (2), frequent repetitions are required (3).
DISCUSSION
In this study, different aspects of dysarthria in children and adults with ataxia telangiectasia were investigated. With the exception of a descriptive study in a small number of children with ataxia telangiectasia, 9 this is the first study describing dysarthria in detail in this population as far as we are aware.
Dysarthria: prevalence, severity on function and activity level, and characteristics
Dysarthria was present in all participants. On function level, most had mild/severe speech impairment and none were anarthric. Despite the fact that the median score on the severity of function scale was 2.47 in children and 2.71 in adults, a larger distribution in function level was seen among children (1–4 in children and 2–3 in adults). The larger distribution on function level in children can probably be explained by several factors. As classic ataxia telangiectasia arises in childhood, children with ataxia telangiectasia may have a milder speech impairment than adults and, owing to the progressive course of classic ataxia telangiectasia, children invariably show more severe speech impairments as they grow older. Additionally, the variation in development of the motor and linguistic system in children may contribute to the wider range in severity on function level.
In almost all participants, severity on function level was higher than severity on activity level. This means that individuals with ataxia telangiectasia have a relatively good intelligibility compared with their severity on function level. An explanation for the better score on activity level is that many participants lower their speech rate to compensate for better intelligibility.
Dysarthria was characterized by uncontrolled movements in all children and ataxic components in all adults (that is, in comparable forms of expression). Characteristics of uncontrolled or ataxic speech were monotonous, unstable, slow, hypernasal, and chanted speech. These findings are in line with the clinical description of people with ataxia telangiectasia 3 and previous study findings. 9 Additionally, one child showed involuntary movements which fits in with extrapyramidal movement disorders in ataxia telangiectasia. Another child had strained components of dysarthria, which can be related to a compensation mechanism to suppress involuntary movements.
Maximum performance tasks
In only one participant (participant 4) were all maximum performance tasks normal (i.e. all scores were above the fifth centile). Among the other participants, at least one of the maximum performance tasks was abnormal.
Maximum performance tasks attempt to measure the upper limits of speech performance. 17 , 18 Although maximum performance tasks differ from normal speech production, they provide useful information on motor speech abilities underlying dysarthria (e.g. articulatory coordination, breath control, speaking rate, speech fluency, articulatory accuracy, and temporal variability). 19 These measurements contribute to quantifying the speech motor capacities, which contributes to differential diagnostic decisions and allows for assessment of therapeutic progress. 19 , 20 MRR is widely used to assess motor coordination and control of articulation. 19 MRR was abnormal in all participants except for one child. This finding corresponds with our clinical experience that individuals with ataxia telangiectasia have slow spontaneous speech due to their neuromotor problems. MPT produces information about the control of air flow under different conditions of articulation or phonation. 19 Lack of breath support can result in short phrases and possible rushes of speech, hypernasality, and problems with articulation. 21 Children tend to have a shorter MPT and show more variation than adults. 22 MPT was abnormal in 14 out of 19 participants (10 children and four adults). FFR is applied to evaluate the melodic range of speech. Restricted FFR is related to monotone speech and decreased intonation which is compared with poor intelligibility. 23 FFR was abnormal in 11 out of 20 participants (six children, five adults). MPV is essentially the subdivision of lung volume which can be used to support a maximum sustained phonation. Phonation volume varies with vital capacity. 22 Previous research shows that vital capacity is decreased in adolescents with ataxia telangiectasia. 24 A decline of phonation volume may not be detected in spontaneous speech because only a small part of the full range is used in normal conversation. 11 MPV was decreased in 13 out of 22 participants (nine children, four adults). Despite the fact that more than half of the participants had a reduced MPV, our experience is that the loudness during spontaneous speech is normal. However, coordination problems can result in outliers in speech volume, indicating that even when the overall volume in speech is quite normal, some abnormalities can exist owing to these outliers.
Intelligibility
All participants were intelligible for various communication partners. Adults achieved better intelligibility than children. A possible explanation for this finding was the difference in phenotype between adults (most of them have variant ataxia telangiectasia) and children (only 2 out of 16 have variant ataxia telangiectasia) in this study. As described earlier, differences in phenotype explain variations in severity of neurological symptoms.
Furthermore, speech errors can reduce intelligibility of younger children, as a result of the fact that the linguistic system is still developing in childhood. For example, some phonological components may still be present in children. 25 In addition, adults may apply better compensation mechanisms (reduction of speech rate) to make themselves more intelligible, as they are more experienced and more developed than children and therefore know better which adaptions are helpful for being more intelligible. 26 Intelligibility compared with the function on activity domain showed a higher total ICS score than severity on activity level. This finding may be the result of the fact that parents or someone close to the participant is more familiar with the participant’s speech. Parents of children with a speech disorder provided relatively higher ratings of the intelligibility of the child than people who are unfamiliar with the child’s speech. 27
Strengths and limitations
Our study adds an objective measure of dysarthria in adults and children with ataxia telangiectasia to the current literature. Further strengths of the present study include the use of validated assessment tools and questionnaire (RDA, p‐RDA, and ICS). Another strength is that, by measuring maximum performance tasks, information about the possibilities of speech in individuals with ataxia telangiectasia is provided, since participants are fully challenged. This can contribute to optimizing speech language therapy in this population. Limitations of the present study can be found in the small sample size and the unequal distribution of classic and variant ataxia telangiectasia between the children and adults. However, given the rarity of ataxia telangiectasia and the differences in disease course between classic and variant ataxia telangiectasia (more adults have variant ataxia telangiectasia, because of longer lifespans), these factors are inevitable.
Clinical implications
Dysarthria in children and adults with ataxia telangiectasia is characterized by uncontrolled, ataxic, and involuntary movements; therefore, the primary advice to improve speech is positioning the individual in a stable sitting position. 28 Attention to breath control and speaking at the beginning of an exhalation is the next step in therapy. 21 Finally, speech intelligibility often improves by slowing down the rate of speech.
Besides providing information on the type and severity of dysarthria in individuals with ataxia telangiectasia, this study illustrates the availability and potential applicability of two recently developed clinical instruments to assess the characteristics and severity of dysarthria in children and adults in general. These instruments may provide useful tools for monitoring the effects of speech therapy of individuals with ataxia telangiectasia, as well as objective outcome measures in clinical research, especially in future therapeutic intervention studies.
Supporting information
Video S1: Two children and one adult performing tasks from the RDA and p‐RDA.
Acknowledgements
We are grateful to the participants and their families who participated in this study. Furthermore, we thank the Twan Foundation (Veenendaal, the Netherlands) and the A‐T Children’s Project (Coconut Creek, FL, USA) for their support. The authors have stated that they had no interests that might be perceived as posing conflict or bias.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Savitsky K, Bar‐Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI‐3 kinase. Science 1995; 268: 1749–53. [DOI] [PubMed] [Google Scholar]
- 2. Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013; 14: 197–210. [PubMed] [Google Scholar]
- 3. Boder E, Sedgwick RP. Ataxia‐telangiectasia; a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics 1958; 21: 526–54. [PubMed] [Google Scholar]
- 4. van Os NJ, Jansen AF, van Deuren M, et al. Ataxia‐telangiectasia: immunodeficiency and survival. Clin Immunol 2017; 178: 45–55. [DOI] [PubMed] [Google Scholar]
- 5. Taylor A, Lam Z, Last J, Byrd P. Ataxia telangiectasia: more variation at clinical and cellular levels. Clin Genet 2015; 87: 199–208. [DOI] [PubMed] [Google Scholar]
- 6. Schon K, van Os NJ, Oscroft N, et al. Genotype, extrapyramidal features, and severity of variant ataxia‐telangiectasia. Ann Neurol 2019; 85: 170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verhagen MM, Last JI, Hogervorst FB, et al. Presence of ATM protein and residual kinase activity correlates with the phenotype in ataxia‐telangiectasia: a genotype‐phenotype study. Hum Mutat 2012; 33: 561–71. [DOI] [PubMed] [Google Scholar]
- 8. Nissenkorn A, Levi YB, Vilozni D, et al. Neurologic presentation in children with ataxia‐telangiectasia: is small head circumference a hallmark of the disease? J Pediatr 2011; 159: 466–71.e1. [DOI] [PubMed] [Google Scholar]
- 9. Vinck A, Verhagen MM, Gerven Mv, et al. Cognitive and speech‐language performance in children with ataxia telangiectasia. Dev Neurorehabil 2011; 14: 315–22. [DOI] [PubMed] [Google Scholar]
- 10. Knuijt S, Kalf JG, Gerven MV, et al. Nederlandstalig Dysartrieonderzoek – volwassenen. Houten: Bohn Stafleu van Loghum, 2014. [Google Scholar]
- 11. Knuijt S, Kalf JG, van Engelen BGM, de Swart BJM, Geurts ACH. The Radboud Dysarthria Assessment: development and clinimetric evaluation. Folia Phoniatr Logop 2017; 69: 143–53. [DOI] [PubMed] [Google Scholar]
- 12. Ruessink M, Engel‐Hoek LL, de Swart B, Spek B, van Gerven M, Kalf H.Validation of the pediatric Radboud Dysarthria Assessment. Forthcoming. [DOI] [PMC free article] [PubMed]
- 13. Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res 1969; 12: 246–69. [DOI] [PubMed] [Google Scholar]
- 14. Liégeois FJ, Morgan AT. Neural bases of childhood speech disorders: lateralization and plasticity for speech functions during development. Neurosci Biobehav Rev 2012; 36: 439–58. [DOI] [PubMed] [Google Scholar]
- 15. McLeod S, Harrison LJ, McCormack J. The Intelligibility in Context Scale: validity and reliability of a subjective rating measure. J Speech Lang Hear Res 2012; 55: 648–56. [DOI] [PubMed] [Google Scholar]
- 16. Rietveld T, Van Heuven VJ. Algemene Fonetiek (3rd completely revised edition). Bussum: Coutinho, 2009. [Google Scholar]
- 17. Wit J, Maassen B, Gabreels F, Thoonen G. Maximum performance tests in children with developmental spastic dysarthria. J Speech Lang Hear Res 1993; 36: 452–9. [DOI] [PubMed] [Google Scholar]
- 18. Knuijt S, Kalf J, Van Engelen B, Geurts A, de Swart B. Reference values of maximum performance tests of speech production. Int J Speech Lang Pathol 2019; 21: 56–64. [DOI] [PubMed] [Google Scholar]
- 19. Thoonen G, Maassen B, Wit J, Gabreels F, Schreuder R. The integrated use of maximum performance tasks in differential diagnostic evaluations among children with motor speech disorders. Clin Linguist Phon 1996; 10: 311–36. [Google Scholar]
- 20. Rvachew S, Hodge M, Ohberg A. Obtaining and interpreting maximum performance tasks from children: a tutorial. J Speech Lang Path Audiol 2005; 29: 146. [Google Scholar]
- 21. Pennington L, Smallman C, Farrier F. Intensive dysarthria therapy for older children with cerebral palsy: findings from six cases. Child Lang Teach Ther 2006; 22: 255–73. [Google Scholar]
- 22. Kent RD, Kent JF, Rosenbek JC. Maximum performance tests of speech production. J Speech Hear Disord 1987; 52: 367–87. [DOI] [PubMed] [Google Scholar]
- 23. Kent RD, Rosenbek JC. Prosodic disturbance and neurologic lesion. Brain Lang 1982; 15: 259–91. [DOI] [PubMed] [Google Scholar]
- 24. McGrath‐Morrow S, Lefton‐Greif M, Rosquist K, et al. Pulmonary function in adolescents with ataxia telangiectasia. Pediatr Pulmonol 2008; 43: 59–66. [DOI] [PubMed] [Google Scholar]
- 25. Allison KM, Hustad KC. Impact of sentence length and phonetic complexity on intelligibility of 5‐year‐old children with cerebral palsy. Int J Speech Lang Pathol 2014; 16: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perkell J, Matthies M, Lane H, et al. Speech motor control: acoustic goals, saturation effects, auditory feedback and internal models. Speech Commun 1997; 22: 227–50. [Google Scholar]
- 27. Van Doornik A, Gerrits E, McLeod S, Terband H. Impact of communication partner familiarity and speech accuracy on parents’ ratings of their child for the Intelligibility in Context Scale: Dutch. Int J Speech Lang Pathol 2018; 20: 350–60. [DOI] [PubMed] [Google Scholar]
- 28. Ward R, Leitão S, Strauss G. An evaluation of the effectiveness of PROMPT therapy in improving speech production accuracy in six children with cerebral palsy. Int J Speech Lang Pathol 2014; 16: 355–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1: Two children and one adult performing tasks from the RDA and p‐RDA.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


