Abstract
BACKGROUND
Aedes albopictus is a highly invasive mosquito and has become a potential vector of dengue, chikungunya and Zika viruses. Insecticide‐based mosquito interventions are the main tools for vector‐borne disease control. However, mosquito resistance to insecticides is a major threat to effective prevention and control. Five Ae. albopictus populations across Hainan Province, China were investigated for susceptibility to multiple insecticide and resistance mechanisms.
RESULTS
Larval bioassays indicated that resistance to pyrethroids was common in all larval populations. Adult bioassays revealed all populations were either resistant or highly resistant to at least four of the six synthetic insecticides (deltamethrin, permethrin, cyfluthrin, propoxur, malathion, and DDT) tested. Pre‐exposure of mosquitoes to the synergistic agent piperonyl butoxide (PBO) increased mosquito mortality by 2.4–43.3% in bioassays to DDT, malathion, and permethrin and rendered mosquito sensitive to deltamethrin, cyfluthrin, and propoxur. The frequency of knockdown resistance (kdr) mutations (F1534S and F1534C) ranged from 69.8% to 89.3% and from 38.1% to 87.0% in field‐resistant and sensitive populations, respectively. F1534S mutation was significantly associated with pyrethroid resistance. No mutation was detected in the acetylcholinesterase (ace‐1) gene in the two examined populations.
CONCLUSION
This study provides evidence of widespread resistance to multiple insecticides in Ae. albopictus in Hainan Province, China. Both kdr mutations and metabolic detoxification were potential causes of insecticide resistance for Ae. albopictus. Our findings highlight the need for insecticide resistance management and mosquito control measures that do not entirely depend on synthetic insecticides. © 2020 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Aedes albopictus, insecticide resistance, knockdown resistance (kdr), piperonyl butoxide, Hainan Province
WHO standard insecticide susceptibility tests found multiple insecticide resistance in major dengue vector Aedes albopictus across Hainan Province, China.
© 2020 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
1. BACKGROUND
Aedes albopictus (Skuse) is a potential vector of at least 20 arboviruses with public health importance in tropical and subtropical regions, including yellow fever virus, dengue virus (DENV), chikungunya virus (CHIKV), and Zika virus (ZIKV). 1 , 2 , 3 , 4 , 5 , 6 , 7 It is listed among the most dangerous invasive mosquito species globally. 8 Furthermore, Ae. albopictus was responsible for the vast majority of dengue and chikungunya outbreaks in China during the past two decades. 9 , 10 , 11 , 12 The species is widely distributed in China from the southern tropical area of Hainan Province to the temperate climate Liaoning Province in the north. 13 , 14
So far, there is no effective drug or vaccine for most Ae. albopictus transmitted diseases except the vaccine for yellow fever, therefore mosquito control is the most important method for preventing Aedes mosquito‐borne infectious diseases. 1 , 2 , 3 , 4 Adult Aedes mosquito control depends largely on outdoor and indoor insecticide spraying, while larval control relies on either chemical (e.g. temephos) or biological (e.g. bti) insecticides and community engagement for habitat management. 15 , 16 , 17 In China, the primary tool for mosquito control is the use of chemical insecticides. 18 , 19 However, Ae. albopictus resistance to all four main classes of insecticides (carbamates, organochlorines, organophosphates, and pyrethroids) has been detected in the Americas, Africa, and Asia in recent years. 15 , 20 , 21
Hainan Province, located in southern China, is a dengue fever epidemic area. Dengue fever outbreaks have occurred several times since the late 1970s. The 1979–1982 dengue pandemic on the island caused a total of 604 854 cases and 475 deaths. 22 , 23 The most recent Dengue outbreaks occurred on this island in 2019. 24 As an international tourist destination and now a free‐trade area, mosquito‐borne infectious disease risk will likely grow due to the increasing influx of goods and human travel. This threat is underscored by the presence of dengue fever throughout the neighboring southeastern countries. 25 Furthermore, the tropical climate of the island makes it particularly suitable for the development and survival of vector mosquitoes. Ae. albopictus is widely distributed on the island, and the species is now almost the sole vector of dengue fever in Hainan. 26 , 27 Previously, Ae. aegypti was the major dengue vector from the 1970s to the 1990s, but Ae. aegypti has not been found on the island during the past 10 years. 23 , 28 , 29
In the past 40 years, Hainan Province has used a large quantity of insecticides to help control vector‐borne diseases, including malaria and dengue. 23 However, there is limited information available on the current status of insecticide resistance in Ae. albopictus. A previous study in Haikou city in Hainan indicated that Ae. albopictus had developed resistance to deltamethrin, permethrin, and beta‐cypermethrin. 22 Thus, monitoring the development of insecticide resistance in Aedes mosquitoes has become a local key task for dengue control. 7
Due to massive agricultural and public health use of insecticides, systematic studies on Ae. albopictus resistance to insecticides are critically needed for the prevention and control of mosquito‐borne diseases. In the present study, we studied the insecticide resistance profiles of larval and adult Ae. albopictus in five areas across Hainan Province during 2017–2018. We implemented synergist and molecular assays to identify putative resistance mechanisms in Ae. albopictus adults.
2. MATERIALS AND METHODS
2.1. Ethics statement
No permits were required for the described field studies. Mosquito collections in breeding sites were consented orally by the owners at each location. For the insecticide usage survey, oral consent was obtained from all participating households after explanation of the study aim and design. This study did not involve collection of any human‐related samples or personal information such as participants' names, addresses, phone numbers, and GPS location of their homes etc.
2.2. Study sites and mosquito collections
Immature Ae. albopictus mosquitoes were collected from five study sites in Hainan Province, southern China between 2017 and 2018 (Fig. 1 and Table 1). The five sites represent different ecological settings on the island (Table 1). Annual mean temperature ranges from 23.1 to 25.9 °C, and annual precipitation ranges from 1300 to 2300 mm (Table 1).
Figure 1.
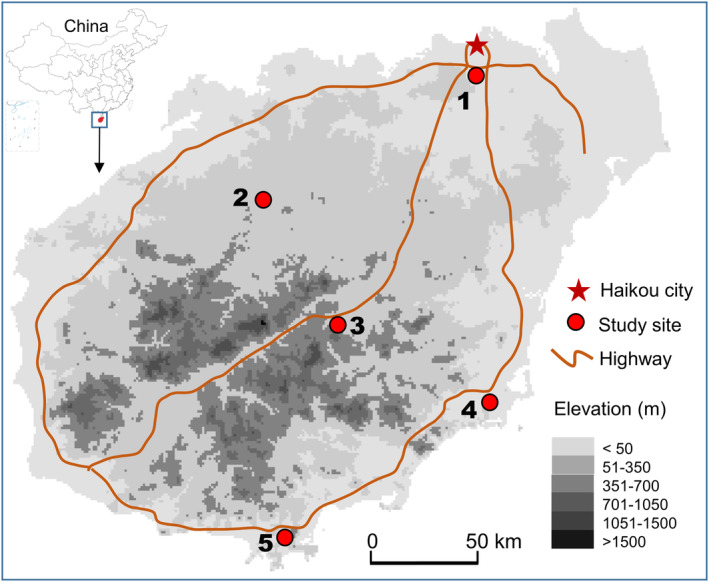
Map of study area in Hainan Province, China.
Table 1.
Description of Aedes albopictus mosquito population collection sites in Hainan Province, China
| Study area | Landscape | Coordinates | Altitude (m) | |
|---|---|---|---|---|
| Latitude (N) | Longitude (E) | |||
| Haikou | Densely populated port city | 110°20′29″ | 20°04′39″ | 4 |
| Wanning | Plain, agriculture area | 110°23′15″ | 18°48′10″ | 13 |
| Sanya | Tourist destination | 109°30′28″ | 18°17′32″ | 26 |
| Qiongzhong | Heavily forested area | 109°50′47″ | 19°03′03″ | 267 |
| Danzhou | Major agricultural area | 109°33′35″ | 19°31′30″ | 184 |
Mosquito larvae were collected from at least 50 habitats in each study site, which included both urban and suburban areas. Larvae were brought back to the insectary located at Hainan Medical University. Larvae were reared at 27 ± 2 °C, 70% ± 10% relative humidity (RH) and a 12:12 h light:dark period, and fed with yeast. Emerged adults were fed with 10% sugar solution at 27 ± 2 °C, 70% ± 10% RH.
2.3. Insecticide resistance bioassays
2.3.1. Larval bioassays
Eight insecticides were used to examine larval mortality. Seven of the insecticides were obtained from the Chinese Center for Disease Control and Prevention (China CDC). Three of them were pyrethroid insecticides: deltamethrin (technical grade 95.95%), permethrin (99%), and beta‐cypermethrin (92.00%). The other chemical insecticides included were propoxur (95.56%), malathion (95%), temephos (87.40%), and DDT (94.00%). Lastly, one microbial larvicide was evaluated: Bacillus thuringiensis israelensis (Bti) (7000 ITU/mg, Wuhan Nature's Favour Bioengineering Co., Ltd, Wuhan, China). All chemical insecticides were diluted in acetone, and Bti was diluted in water to the required dosage following WHO guidelines. 30 We included adulticides for larval resistance tests because in China pyrethroid and other adulticides have been widely used for agricultural pest control and for public health use such as control mosquitoes inside rainwater drain basins. The residue of these insecticides in the water may causes larval resistance after long‐term use.
The larvae of F1‐2 generations were used for larval bioassays after species identification. In each test, 25 third‐ and fourth‐instar larvae were incubated in a 100 mL solution consisting of 99 mL of distilled water and 1 mL of insecticide solution at the specified concentration using the standard 250 mL plastic cup. 30 Control treatments for the chemical insecticides were incubated in 99 mL of distilled water and 1 mL of acetone. Control treatments for Bti were incubated in 100 mL of water only. Three to five replicates were tested for each concentration. Each bioassay comprised one control group. Five to nine concentrations, providing a range of mortalities between 0% and 100%, were used to determine the 50% mortality lethal concentration (LC50). Mosquitoes of the Foshan strain, which have been reared in the laboratory since 1981 without insecticide exposure, were used as a control. Larval mortality was recorded after 24 h of exposure. Bioassays were conducted at 27 ± 2 °C, 70% ± 10% RH, and a 12: 12 h light: dark period.
2.3.2. Adult resistance bioassays
The adults from F1‐2 of field‐collected Ae. albopictus larvae were tested using the standard WHO insecticide susceptibility tube test. 31 , 32 The mosquitoes were subjected to insecticide susceptibility tests against pyrethroids (0.15% cyfluthrin, 0.03% deltamethrin, and 0.25% permethrin), organochlorine (4% DDT), organophosphate (0.8% malathion), and carbamate (0.1% propoxur). The susceptible Foshan strain was used as a control. For each population of the field mosquitoes and each insecticide, eight replicates (four each in urban and suburban) of 20 nonblood‐fed 3–5 day‐old female mosquitoes for each replicate were subjected to WHO tube bioassays. Data analysis used pooled values of the eight replicates at each site. For control populations, two replicates were used for each insecticide. WHO test and control papers were supplied by the School of Biological Sciences, Universiti Sains Malaysia (Penang, Malaysia). Mosquitoes were exposed to test papers for 1 h. The knockdown number of females was recorded every 10 min during the 60 min exposure period. Mortality was scored after the 24 h recovery period. After the bioassay, both survivors and dead mosquitoes were stored individually in 95% alcohol at −80 °C for subsequent DNA analysis.
2.3.3. Synergist assay with piperonyl butoxide
To investigate the role of oxidase‐specific metabolic resistance mechanisms, synergist assays with piperonyl butoxide (PBO) were performed together with the six insecticides tested above: deltamethrin, permethrin, cyfluthrin, malathion, propoxur, and DDT. Nonblood‐fed adult mosquitoes which were 3–5‐days old were pre‐exposed to 4% PBO test paper for 1 h, followed by exposure to insecticide‐treated test papers for 1 h. There were three replicates for each insecticide, with 25 mosquitos per replicate. Mortality was recorded after the 24 h recovery period. Two replicates of 1 h PBO exposure were performed without subsequent exposure to an insecticide for the controls.
2.3.4. Molecular assays
Genomic DNA was extracted using the method published by Chang et al. 33 One adult mosquito leg for each mosquito was used for DNA extraction. Extracted DNA was stored at −20 °C or used immediately for PCR. The primers and PCR conditions used for amplification and sequencing of the voltage‐gated sodium channel (VGSC) gene and the acetylcholinesterase (ace‐1) gene of Ae. albopictus were according to the protocol from Su et al and Kasai et al. 13 , 34 PCR products were purified and directly sequenced in both directions with the same primers. PCR products were purified with ExoSAP‐IT (USB, Cleveland, OH, USA) according to the manufacturer's protocol and directly sequenced by Retrogen, Inc. (San Diego, CA, USA). The sequences were analyzed using BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) and Codon Code (http://www.codoncode.com/).
2.3.5. Community survey of insecticide usage
Questionnaire surveys were conducted in the study communities to determine insecticide usage for public health and agricultural pest control. Questionnaire surveys were conducted from September to October of 2019 in all study sites. Each survey site included urban, suburban, and rural areas with a total of 30 questionnaires. Oral consent was obtained from all participants after explanation of the study purpose to them.
2.3.6. Statistical analysis
Resistance status of adult mosquito was classified according to WHO criteria: resistant for <90% mortality, probable resistance for 90–98% mortality, and susceptible for >98% mortality. 31 In the larval bioassay, the median lethal concentration (LC50), the 90% lethal concentration (LC90), and the corresponding 95% confidence intervals were calculated based on the recorded data using Schoofs and Willhite's probit analysis program. 35 The degree of resistance intensity was determined by the resistance ratio (RR50), calculated as the ratio of LC50 value for the field population over the LC50 value for the susceptible laboratory colony. By WHO definition, RR50 < 5 is considered susceptible, 5 < RR50 < 10 is moderately resistant, and RR >10 is highly resistant. 31 For the adult bioassays, χ2‐tests were used to examine the association between kdr mutations and the resistance phenotype. Here, mosquitoes dead after the 24 h recovery period were classified as susceptible and surviving mosquitoes as resistant. For each population and each insecticide, the mortalities with mosquitoes exposed to PBO + insecticide were compared with those exposed with insecticide alone using t‐tests.
3. RESULTS
3.1. Larval Ae. albopictus developed resistance to pyrethroid insecticides
Larval bioassays revealed that Ae. albopictus have developed different levels of resistance (RR50 ≥ 5) to all pyrethroid insecticides at nearly all study sites, but they were all susceptible (RR50 < 5) to all nonpyrethroid insecticides (Table 2 and Fig. 2). All larval populations were highly resistant to deltamethrin (LC50 = 0.17–0.47 mg/L; RR50 range 17–47), and most of them were moderately or highly resistant to permethrin (LC50 = 0.36–1.86 mg/L, RR50 range 7–37). The highest RR50 was 53 for beta‐cypermethrin (LC50 = 1.06 mg/L) in the Haikou population, and the lowest RR50 was 3 for beta‐cypermethrin (LC50 = 0.06 mg/L) in the Danzhou population (Fig. 2 and Table 2). In addition, all of the populations tested were susceptible to Propoxur (LC50 = 0.89–4.78 mg/L, RR50 range 0.8 to 4.2), malathion (LC50 = 2.40–5.97 mg/L, RR50 range 0.7–2.4), temephos (LC50 = 0.07–0.16 mg/L, RR50 range 1.8–4.0), DDT (LC50 = 1.92–4.96 mg/L, RR50 range 0.8–2.1), and Bti (LC50 = 0.55–0.99 mg/L, RR50 range 0.7–1.2). (Table 2 and Fig. 2).
Table 2.
Insecticide concentrations that caused 50% mortality (LC50) of Aedes albopictus in different populations
| Insecticide | Haikou | Wanning | Sanya | Qiongzhong | Danzhou | Susceptible strain |
|---|---|---|---|---|---|---|
| Deltamethrin | 0.35 (0.25, 0.48) | 0.47 (0.38, 0.56) | 0.26 (0.20, 0.31) | 0.27 (0.15, 0.45) | 0.17 (0.09, 0.31) | 0.01 (0.01, 0.01) |
| Beta‐cypermethrin | 1.06 (0.50, 2.48) | 0.15 (0.09, 0.27) | 0.17 (0.15, 0.19) | 0.09 (0.06, 0.14) | 0.06 (0.05, 0.06) | 0.02 (0.02, 0.03) |
| Permethrin | 1.86 (1.28, 2.57) | 1.24 (0.37, 2.78) | 0.51 (0.26, 0.94) | 0.86 (0.48, 1.35) | 0.36 (0.28, 0.44) | 0.05 (0.03, 0.06) |
| Malathion | 3.22 (2.90, 3.55) | 2.81 (2.21, 3.49) | 5.97 (4.45, 8.18) | 2.83 (1.97, 3.98) | 2.40 (1.88, 2.96) | 3.37 (2.70, 4.18) |
| Temephos | 0.12 (0.11, 0.13) | 0.15 (0.14, 0.17) | 0.13 (0.12, 0.14) | 0.07 (0.06, 0.07) | 0.16 (0.14, 0.17) | 0.04 (0.04, 0.04) |
| Propoxur | 4.78 (4.46, 5.12) | 2.65 (2.43, 2.88) | 3.83 (3.58, 4.09) | 0.89 (0.71, 1.10) | 1.48 (1.22, 1.78) | 1.14 (1.07, 1.23) |
| DDT | 3.72 (2.62, 5.44) | 3.42 (2.94, 3.98) | 1.92 (1.11, 3.41) | 4.96 (4.19, 5.89) | 3.46 (3.07, 3.89) | 2.41 (1.82, 3.28) |
| Bti | 0.55 (0.43, 0.68) | 0.60 (0.53, 0.70) | 0.82 (0.52, 1.92) | 0.99 (0.86, 1.20) | 0.95 (0.89, 1.02) | 0.81 (0.66, 1.03) |
LC50 lethal concentration that kills 50% of the population (mg/L), values are LC50 (95% CI).
Bti: 7000 ITU/mg.
Figure 2.
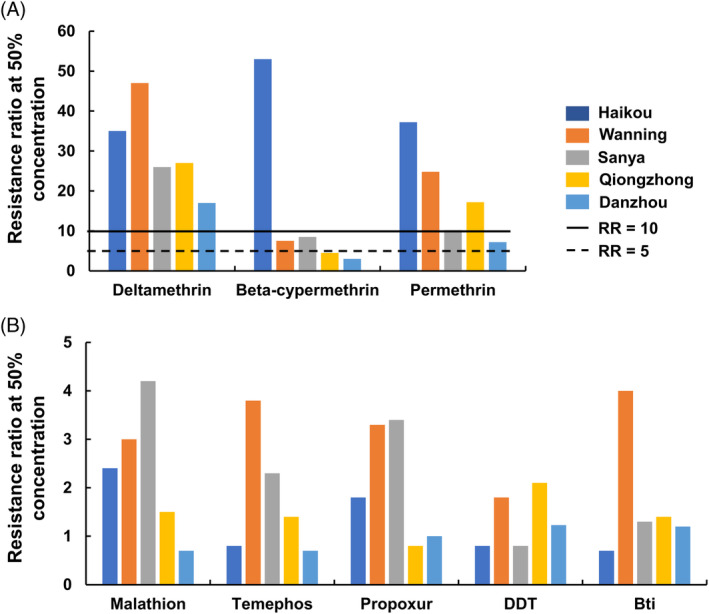
The resistance of Aedes albopictus larvae to currently used insecticides (resistance ratio at 50% concentration). (A) Insecticide pyrethroids (cyfluthrin, deltamethrin, and permethrin); (B) organophosphate (malathion, temephos), organochlorine (DDT), carbamate (propoxur), and Bti. RR50, resistance ratio, LC50 test population/LC50 laboratory‐susceptible strain.
3.2. Adult Ae. albopictus developed resistance to all the insecticides used
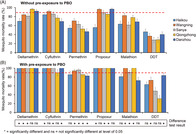
WHO tube bioassays revealed that nearly all Ae. albopictus populations were resistant (<90% mortality) to the six synthetic insecticides tested, as indicated by the low mortality rate after the 24 h recovery period (Fig. 3(a)). Although mortality rates varied, mosquitoes were highly resistant to DDT (mortality range 28–46%), permethrin (mortality range 47–71%), and malathion (mortality range 61–82%) at all study sites (Fig. 3(a)). Mosquito resistance levels ranged from highly resistant to suspected resistant to cyfluthrin (mortality range 69–91%), propoxur (mortality range 56–96%), and deltamethrin (mortality range 70–96%) (Fig. 3(a)).
Figure 3.
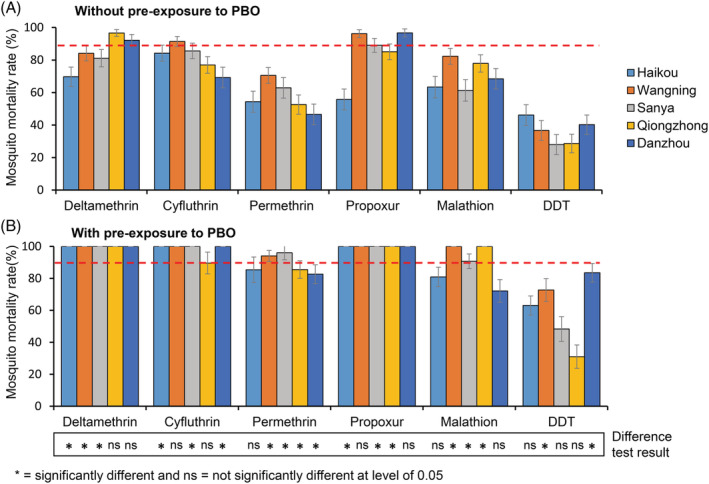
Mortality rates of adult Aedes albopictus after exposure to insecticides without (A) and with (B) pre‐exposure to PBO. Error bars represent standard error of the mean. The bottom panel indicated significant level of difference in mortalities for the same insecticide between PBO and non‐PBO pre‐exposed populations from the same study site.
3.3. Synergist PBO moderately increased mosquito mortality
Pre‐exposure of Ae. albopictus mosquitoes to the PBO synergist before exposure to insecticides led to the full recovery of Ae. albopictus susceptibility to deltamethrin, propoxur, and cyfluthrin (except Wanning population, mortality 89%) and partial recovery of susceptibility to malathion (mortality range 72–100%) and permethrin (mortality range 82–96%) (Fig. 3(b)). However, Ae. albopictus mortality was still low (31–83%) against DDT after PBO treatment (Fig. 3(b)). The effect of pre‐exposure to PBO on mosquito mortality varied substantially for a given insecticide among different sites and at a given site among different insecticides (Fig. 3, bottom panel), i.e. increase in mosquito mortality was observed overall, but showed strong heterogeneity (Fig. 3, bottom panel).
3.4. kdr mutations were associated with resistance phenotypes in adult mosquitoes
A total of 479 Ae. albopictus mosquitoes (234 resistant and 245 sensitive) were examined for kdr mutations. We detected F1534S and F1534C mutations in the VGSC gene. Overall, the total kdr mutation frequency was significantly higher in the resistant mosquitoes (mean 78.6%, range 69.8–89.3%) than that in the susceptible mosquitoes (mean 46.5%, range 38.1–50.9%) in four of the five populations (χ2‐test, P < 0.05; Table 3). The odds ratio for the F1534S mutation ranged from 2.4 to 10.8 (but was 0.8 in Haikou) in resistant mosquitoes compared to susceptible mosquitoes, indicating the association between pyrethroid phenotypic resistance and the kdr mutation (Table 3). The F1534C mutation was not associated with pyrethroid resistance in any of the five populations (Table 2). The F1534L mutation was not detected at any study site. Genotyping of the ace‐1 gene in Haikou and Danzhou populations (n = 120), the two populations that exhibited the highest resistance to malathion, did not reveal any mutations at codon 119.
Table 3.
Kdr mutation frequency relationship to pyrethroid resistance in different Aedes albopictus populations
| Study site | Phenotype | Sample size | Allele frequency (%) | Odds ratio (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Wildtype | F1534C | F1534S | F1534C | F1534S | |||
| Haikou | Resistant | 61 | 13.1 | 26.2 | 60.7 | 1.1 (0.6–2.1) NS | 0.9 (0.5–1.6)NS |
| Susceptible | 50 | 13.0 | 24.0 | 63.0 | |||
| Qiongzhong | Resistant | 14 | 10.7 | 3.6 | 85.7 | 1.5 (0.1–25.3) NS | 10.8 (3.2–37.0)*** |
| Susceptible | 21 | 61.9 | 2.4 | 35.7 | |||
| Sanya | Resistant | 36 | 16.7 | 26.4 | 56.9 | 1.5 (0.7–3.2) NS | 3.3 (1.7–6.4)** |
| Susceptible | 42 | 52.4 | 19.0 | 28.6 | |||
| Wanning | Resistant | 48 | 30.2 | 4.2 | 65.6 | 0.6 (0.2–2.3) NS | 2.4 (1.4–4.2)** |
| Susceptible | 56 | 49.1 | 6.4 | 44.5 | |||
| Danzhou | Resistant | 75 | 28.0 | 2.0 | 70.0 | NA | 2.4 (1.5–3.8)*** |
| Susceptible | 76 | 50.7 | 0.0 | 49.3 | |||
P < 0.01.
P < 0.001.
NS, no significance.
3.5. Local residents used all types of insecticides
Questionnaire surveys of local residents in the study area revealed that six classes of insecticides, i.e. pyrethroids, organophosphates, carbamates, microbial insecticide, nitromethyl, and nicotine insecticide, were used in Hainan Province (Table 4). Pyrethroids were the most commonly used insecticide for public health and agricultural pest control, with 73.3–100.0% of the surveyed households reporting using different types of pyrethroid insecticides for mosquito control (Table 4). Organophosphates were primarily used for agricultural pest control, and carbamates were used only in the Haikou site for mosquito control among a small proportion of surveyed households (Table 4).
Table 4.
Summary of public health and agricultural use of pesticides based on questionnaire survey
| Percentage of households (%) | |||||||
|---|---|---|---|---|---|---|---|
| Site | Purpose | Environment | Insecticide | Never | Weekly | Biweekly | Monthly |
| Sanya | Public health | Indoor* | Pyrethroids | 3.3 | 96.7 | 0.0 | 0.0 |
| Agricultural | Outdoor | Pyrethroids | 80.0 | 13.3 | 0.0 | 6.7 | |
| Organophosphate | 66.7 | 6.7 | 13.3 | 13.3 | |||
| Microbial insecticide | 90.0 | 3.3 | 3.3 | 3.3 | |||
| Wanning | Public health | Indoor* | Pyrethroids | 10.0 | 36.7 | 33.3 | 20.0 |
| Agricultural | Outdoor | Pyrethroids | 93.3 | 0.0 | 6.7 | 0.0 | |
| Organophosphate | 56.7 | 0.0 | 33.3 | 10.0 | |||
| Nitromethyl | 86.7 | 0.0 | 6.7 | 6.7 | |||
| Haikou | Public health | Indoor* | Pyrethroids | 26.7 | 43.3 | 13.3 | 16.7 |
| Carbamate | 93.3 | 0.0 | 3.3 | 3.3 | |||
| Agricultural | Outdoor | Pyrethroids | 86.7 | 0.0 | 6.7 | 6.7 | |
| Organophosphate | 73.3 | 0.0 | 16.7 | 10.0 | |||
| Danzhou | Public health | Indoor* | Pyrethroids | 26.7 | 40.0 | 13.3 | 20.0 |
| Agricultural | Outdoor | Organophosphate | 63.3 | 0.0 | 20.0 | 16.7 | |
| Nicotine insecticide | 86.7 | 0.0 | 10.0 | 3.3 | |||
| Qiongzhong | Public health | Indoor* | Pyrethroids | 0.0 | 100.0 | 0.0 | 0.0 |
| Agricultural | Outdoor | Pyrethroids | 56.7 | 0.0 | 3.3 | 40.0 | |
| Organophosphate | 66.7 | 0.0 | 0.0 | 33.3 | |||
Total number of households surveyed was 30 per site.
Pyrethroid‐based mosquito repellents.
4. DISCUSSION
This study presents the first resistance profile of Ae. albopictus across Hainan Province. The results indicate that the majority of adult Ae. albopictus populations in Hainan are resistant or highly resistant to the four classes of synthetic insecticides, especially the most commonly used pyrethroids. The occurrence of widespread insecticide resistance in Ae. albopictus adults potentially compromises the efficacy of current intervention measures in controlling Ae. albopictus populations on the island. While adult Ae. albopictus resistance to different insecticides has been reported in other places, 15 Ae. albopictus adults in Hainan showed high resistance to all insecticides at all study sites. In Hainan province, all types of insecticides have been frequently used for mosquito control, especially for epidemic controls. These insecticides have also been widely used for agricultural pest controls, which can lead to resistance. Resistance management strategies are urgently needed to help mitigate failures in future vector control interventions.
The reemergence of dengue fever has been reported in many countries and often has been caused by invading Ae. albopictus. 36 Dengue epidemics were reported in Hainan island in the 1980s and 1990s after approximately 30 years of no reported local transmission. In 2019, Dengue fever outbreak occurred in Hainan. The high insecticide resistance in the primary vector Ae. albopictus adds another dimension of risk in dengue outbreak control for the near future. Currently, larval control is a key measure to control the mosquito population in Hainan. In Hainan, pyrethoids are widely used for agricultural pests and mosquito adult control but not larval control. However, we found that Ae. albopictus larvae are resistant to pyrethroid insecticides, which is consistent with other studies in China. 19 , 37 This finding is likely caused by the residues left in the habitats from adult mosquito spraying or agricultural pest controls. However, we cannot rule out inherited resistance from adult mosquitoes, which needs further investigation. Ae. albopictus larvae remained sensitive to some other insecticides, including organophosphorus (malathion, temephos), propoxur, and the microbial larvicide Bti. However, it has been reported that Aedes larvae have developed high resistance to temephos in South‐East Asia and South America. 13 , 38 , 39 , 40 Therefore, Aedes larval resistance to insecticides must be closely monitored and integrated strategies should be developed to combat resistance.
Due to widespread multiple insecticide resistance in the dengue vector Ae. albopictus in Hainan Province, new strategies should be developed to enhance the efficacy of currently available insecticides and to slow down the development of insecticide resistance. The main strategies for insecticide resistance management, as recommended by WHO, include rotations, mosaics, and mixtures of insecticides among which there is no cross‐resistance. 41 For larval control, community‐involved combinations of ecological, biological, and social strategies have been demonstrated to reduce Aedes mosquito abundance, 42 , 43 i.e. combining larval source reduction with larvicides, especially microbial larvicides, may prevent further development of insecticide resistance in Ae. albopictus. Using PBO‐insecticides for adult mosquito control, which combines PBO with insecticides and is widely used in the USA, is another potential option to slow down insecticide resistance. 44 However, to our knowledge PBO insecticide has not been tested in China. Our study indicated that pre‐exposure to PBO could potentially fully recover the efficacy of some insecticides. In addition, field studies have indicated that insecticide‐treated mosquito nets impregnated with PBO can significantly enhance the efficacy of standard net and reduces malaria infections. 45 , 46 Therefore, adding PBO to insecticides may be an effective formulation for resistance management, and this strategy warrants further examination for Ae. albopictus control.
The kdr mutation in the VGSC genes is one indicator of mosquito resistance to pyrethroid insecticides. 47 , 48 In malaria vector Anopheles mosquitoes, previous studies have found strong associations between kdr mutation frequency and phenotypic resistance status. 49 , 50 However, this association does not necessarily hold true for all mosquito species. For example, the kdr mutation was not found in An. funestus 51 nor in some populations of An. sinensis, 52 even when WHO tube test mortality was very low (i.e. very high observed insecticide resistance). The kdr mutation, specifically the F1534 mutation, has been found in Ae. albopictus in China and elsewhere. 21 , 22 , 53 Some studies found F1534 mutations to be associated with phenotypic resistance, 54 , 55 , 56 but a separate study did not find this relationship. 57 Our results indicate that the F1534S mutation is associated with pyrethroid phenotypic resistance in Ae. albopictus in Hainan Province. However, kdr mutation frequency was high in both resistant and susceptible individuals, indicating that the F1534S mutation is not completely predictive of resistance status. 54 Similarly, although with very low mortality, resistant Ae. albopictus did not show higher F1534C mutations in comparison with susceptible mosquitoes, indicating that F1534C is also not likely to be a good indicator of resistance, which is consistent with other studies reported from China. 54 It is possible that there are regional differences in kdr mutations as shown in An. sinensis, 54 , 56 but this hypothesis requires further investigation. In this study, no F1534L mutations were detected, which is different from what has been reported from nearby Guangdong province. 13 This finding may be due to the geographic variation influenced by either environmental conditions or insecticide usage.
Ace‐1 mutations have been found to confer insensitivity to organophosphates and carbamates in mosquito populations. 49 Our study did not detect any G119 mutations in ace‐1 in Ae. albopictus populations, which suggests either that Ae. albopictus in Hainan lacks mutations at the target sites for these insecticides or that the mutation frequency was extremely low. Other studies also found that resistance to ace‐1 agonists is common in Aedes, but that ace‐1 mutations do not occur widely. 13 , 21 , 58 , 59 , 60 Interestingly, exposure to the PBO synergist increased Ae. albopictus sensitivity to pyrethroids, DDT, and malathion, which suggests that metabolic resistance may play an important role in resistance. However, in high permethrin‐, DDT‐, and malathion‐resistant Ae. albopictus populations, PBO exposure only partially recovered insecticide efficacy and mosquito mortality rate, which suggests that other mechanisms may also contribute to insecticide resistance in these populations. 61 , 62
Although, in general, Ae. albopictus populations were resistant to insecticides, it is worth noting that resistance levels varied between study sites and between different insecticides. For example, Ae. albopictus populations were highly resistant to permethrin and DDT in all study sites although with variations in mortality, but resistance to deltamethrin in Qiongzhong was very low. These differences may be due to the differences in the usages of specific insecticide in local residents, as some insecticides may be more frequently used in certain areas but not in others. For example, in Danzhou, farmers used organophosphate twice a month. However, farmers in Qiongzhong used the same type of insecticide monthly. In Qiongzhong, all the surveyed households used pyrethroid for household pest controls, while only about half of the residents in Haikou did the same. These differences in insecticide usage in different sites may have led to variation in resistance levels in different Ae. albopictus populations. Consequently, the mechanisms of the resistance need to be further investigated.
5. CONCLUSION
Aedes mosquito resistance to insecticides is influenced by a complex interplay of factors, including environmental factors and intervention strategies. The development of insecticide resistance to multiple insecticides which has occurred in Hainan will complicate the current vector control strategies. Nonetheless, the findings from this study can help in guiding the selection of insecticides for Aedes mosquito control. First, high pyrethroid resistance calls for the use of alternative insecticides. Second, PBO insecticides may be an option to mitigate the negative impact of pyrethroid resistance. Finally, to achieve effective vector management, a public health response beyond routine larviciding or focal spraying is essential. Targeted vector management can make a difference in terms of reducing vector abundance and controlling mosquito‐borne infectious disease outbreaks.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Figure S1. Chromatograms and alignments of the kdr genotypes detected in Aedes albopictus from Hainan, China. The position at codon 1534 of the para‐type sodium channel gene is indicated by a rectangle. (A) Six types of genotypes detected. (B) Codon 1534 of the para‐type voltage‐gated sodium channel gene is indicated by a red box. Different peak colours distinguish the four bases T (red), C (blue), A (green) and G (black). K = G/T; Y = T/C; S = G/C
Table S1. Mutations at codon 1534 of the voltage‐gated sodium channel gene for pyrethroid‐resistant and ‐susceptible Aedes albopictus populations from Hainan Province, China
ACKNOWLEDGEMENTS
We wish to thank Faxing Fu, Lirong Wu, Mengyuan Lin, and Haoze Yang for their assistance with mosquito collection and resistance bioassay, and Qiang Zhang, Peilin Guo, Yitao Li, Tuquan Zheng, Xiaofang Lin, and Na Li for assistance with the insecticide use survey. This study was supported by the National Natural Science Foundation of China (81660345, 82060379 and 31760318), the Talent Introduction Fund of Hainan Medical University (2016011), the Guizhou Science and Technology Foundation ([2019]1441 and [2018]5779‐17), the Jiangsu Natural Science Foundation (BK20180994), the Postdoctoral Fellowship of China (2018 M632382), and a China Scholarship Council fellowship (201808460069).
Contributor Information
Guzhen Cui, Email: cuiguzhen@gmc.edu.cn.
Guiyun Yan, Email: guiyuny@uci.edu.
REFERENCES
- 1. Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM et al., The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus . Elife 4:e08347 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gratz NG, Critical review of the vector status of Aedes albopictus . Med Vet Entomol 18:215–227 (2014). [DOI] [PubMed] [Google Scholar]
- 3. Bonizzoni M, Gasperi G, Chen X and James AA, The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol 29:460–468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lwande OW, Obanda V, Lindström A, Ahlm C, Evander M, Näslund J et al., Globe‐trotting Aedes aegypti and Aedes albopictus: risk factors for arbovirus pandemics. Vector Borne Zoonotic Dis 20:71–81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization , Ten threats to global health in 2019, Retrieved November 2019 (2019).
- 6. Mousson L, Zouache K, Arias‐Goeta C, Raquin V, Mavingui P and Failloux AB, The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus . PLoS Neglected Trop Dis 6:e1989 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao C, Zhu C, Kai W, Liu G, Liu Q, Lin L et al., Genotypes of knockdown resistance gene and their distribution in Aedes albopictus in Haikou, China, in 2018. Chin J Vector Biol Control 30:7–11 (2019). [Google Scholar]
- 8.Global Invasive Species Database, 100 of the World's Worst Invasive Alien Species (2021). Available: http://www.iucngisd.org/gisd/100_worst.php. [2 July 2020].
- 9. Li Y, Kamara F, Zhou G, Puthiyakunnon S, Li C, Liu Y et al., Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Neglected Trop Dis 8:e3301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peng H, Lai H, Zhang Q, Xu B, Zhang H, Liu W et al., A local outbreak of dengue caused by an imported case in Dongguan China. BMC Public Health 12:83 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu D, Wu J, Zhang Q, Zhong H, Ke C, Deng X et al., Chikungunya outbreak in Guangdong Province, China, 2010. Emerg Infect Dis 18:493–495 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei Y, Wang J, Song Z, He Y, Zheng Z, Fan P et al., Patterns of spatial genetic structures in Aedes albopictus (Diptera: Culicidae) populations in China. Parasit Vectors 12:552 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su X, Guo Y, Deng J, Xu J, Zhou G, Zhou T et al., Fast emerging insecticide resistance in Aedes albopictus in Guangzhou,China: alarm to the dengue epidemic. PLoS Neglected Trop Dis 13:e0007665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng X, Zhong D, He Y and Zhou G, Seasonality modeling of the distribution of Aedes albopictus in China based on climatic and environmental suitability. Infect Dis Poverty 8:98 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I et al., Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Neglected Trop Dis 11:e0005625 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roiz D, Wilson AL, Scott TW, Fonseca DM, Jourdain F, Müller P et al., Integrated Aedes management for the control of Aedes‐borne diseases. PLoS Neglected Trop Dis 12:e0006845 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marcombe S, Chonephetsarath S, Thammavong P and Brey PT, Alternative insecticides for larval control of the dengue vector Aedes aegypti in Lao PDR: insecticide resistance and semi‐field trial study. Parasit Vectors 11:616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu S, Cui F, Yan S and Qiao C, Investigation of organophosphate and pyrethroid resistance in vector mosquitoes in China. Chin J Vector Biol Control 22:184–189 (2011). [Google Scholar]
- 19. Zhao C, Zhu C, Jia Q, Yan D, Liu G, Wu H et al., Resistance of Aedes albopictus to commonly used insecticides in different areas of China, 2017‐2018. Chin J Vector Biol Control 30:7–11 (2020). [Google Scholar]
- 20. Corbel V, Fonseca DM, Weetman D, Pinto J, Achee NL, Chandre F et al., International workshop on insecticide resistance in vectors of arboviruses, December 2016, Rio de Janeiro, Brazil. Parasites Vectors 10:278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Xu J, Zhong D, Zhang H, Yang W, Zhou G et al., Evidence for multiple‐insecticide resistance in urban Aedes albopictus populations in southern China. Parasites Vectors 11:4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen H, Li K, Wang X, Yang X, Lin Y, Cai F et al., First identification of kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus populations from Haikou City, Hainan Island, China. Infect Dis Poverty 5:31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei S, Lin S and Yang C, The 50‐year history of the development of health in Hainan Province, Haikou: Southern Press 396–7 (2007). [in Chinese]
- 24.The People's Government of Hainan Province (2019). Available: http://www.hnntv.cn/st/jrrd/2019-09-10/350737.html [10 September 2019]
- 25. Zhong S, Xie W, Chen J, Fu F, Peng Y, Wu L et al., Knowledge, attitude and practice concerning control of mosquito vectors in residents of Haikou. China Trop Med 19:731–737 (2019). [Google Scholar]
- 26. Liu Q, Dengue fever in China: new epidemical trend, challenges and strategies for prevention and control. Chin J Vector Biol Control 31:1–6 (2020). [Google Scholar]
- 27. Gong D and Zhou H, Progress in dengue fever important vector Aedes albopictus in China. Chin J Vector Biol Control 20:607–610 (2009). [Google Scholar]
- 28. He C, Zhao W, Wang S, Zeng L, Li S and Ou T, Analysis of mosquito density and seasonality in urban areas of Hainan province, China in 2012. Chin J Vector Biol Control 25:15–17 (2014). [Google Scholar]
- 29. Su A, Pei Z, Fu J, Li J, Feng F, Zhu Q et al., Analysis of distribution and population density changes of Aedes egypti the transmission vector of dengue fever in Haikou City. China Trop Med 06:1394–1395 (2005). [Google Scholar]
- 30. World Health Organization , Guidelines for Laboratory and Field Testing of Mosquito Larvicides. WHO, Geneva: (2005). [Google Scholar]
- 31. World Health Organization , Monitoring and Managing Insecticide Resistance in Aedes Mosquito Populations: Interim Guidance for Entomologists. WHO, Geneva: (2016). [Google Scholar]
- 32. World Health Organization , Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. WHO, Geneva: (2016). [Google Scholar]
- 33. Chang X, Zhong D, Li X, Huang Y, Zhu G, Wei X et al., Analysis of population genetic structure of Anopheles sinensis based on mitochondrial DNA cytochrome oxidase subunit I gene fragment. Nan Fang Yi Ke Da Xue Xue Bao 35:234–238, 247 (2015). [PubMed] [Google Scholar]
- 34. Kasai S, Ng LC, Lam‐Phua SG, Tang CS, Itokawa K, Komagata O et al., First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus . Jpn J Infect Dis 64:217–221 (2011). [PubMed] [Google Scholar]
- 35. Finney D, Probit Analysis. Cambridge University Press, Cambridge: (1971). [Google Scholar]
- 36. Rezza G, Aedes albopictus and the reemergence of dengue. BMC Public Health 12:72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y, Liu X, Li C, Su T, Jin J, Guo Y et al., A survey of insecticide resistance in Aedes albopictus (Diptera: Culicidae) during a 2014 dengue fever outbreak in Guangzhou, China. J Econ Entomol 110:239–244 (2017). [DOI] [PubMed] [Google Scholar]
- 38. Saeung M, Ngoen‐Klan R, Thanispong K, Muenworn V, Bangs MJ and Chareonviriyaphap T, Susceptibility of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to Temephos in Thailand and surrounding countries. J Med Entomol 57:1207–1220 (2020). [DOI] [PubMed] [Google Scholar]
- 39. Valle D, Bellinato DF, Viana‐Medeiros PF, Lima JBP and Martins Junior AJ, Resistance to temephos and deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Mem Inst Oswaldo Cruz 114:e180544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elia‐Amira NMR, Chen CD, Lau KW, Lee HL, Low VL, Norma‐Rashid Y et al., Organophosphate and organochlorine resistance in larval stage of Aedes albopictus (Diptera: Culicidae) in Sabah, Malaysia. J Econ Entomol 111:2488–2492 (2018). [DOI] [PubMed] [Google Scholar]
- 41. Dusfour I, Vontas J, David JP, Weetman D, Fonseca DM, Corbel V et al., Management of insecticide resistance in the major Aedes vectors of arboviruses: advances and challenges. PLoS Neglected Trop Dis 13:e0007615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quintero J, Brochero H, Manrique‐Saide P, Barrera‐Pérez M, Basso C, Romero S et al., Ecological, biological and social dimensions of dengue vector breeding in five urban settings of Latin America: a multi‐country study. BMC Infect Dis 14:38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arunachalam N, Tana S, Espino F, Kittayapong P, Abeyewickreme W, Wai KT et al., Eco‐bio‐social determinants of dengue vector breeding: a multicountry study in urban and periurban Asia. Bull World Health Organ 88:173–184 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dadzie SK, Chabi J, Asafu‐Adjaye A, Owusu‐Akrofi O, Baffoe‐Wilmot A, Malm K et al., Evaluation of piperonyl butoxide in enhancing the efficacy of pyrethroid insecticides against resistant Anopheles gambiae s.l. in Ghana. Malar J 16:342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oumbouke WA, Rowland M, Koffi AA, Alou LPA, Camara S and N'Guessan R, Evaluation of an alpha‐cypermethrin + PBO mixture long‐lasting insecticidal net VEERALIN® LN against pyrethroid resistant Anopheles gambiae s.s.: an experimental hut trial in M'bé, central Côte d'Ivoire. Parasit Vectors 12:544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gleave K, Lissenden N, Richardson M, Choi L and Ranson H, Piperonyl butoxide (PBO) combined with pyrethroids in insecticide‐treated nets to prevent malaria in Africa. Cochrane Database Syst Rev 11:CD012776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soderlund DM and Knipple DC, The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem Mol Biol 33:563–577 (2003). [DOI] [PubMed] [Google Scholar]
- 48. Fang Y, Shi W, Wu J, Li Y, Xue J, Zhang Y et al., Resistance to pyrethroid and organophosphate insecticides, and the geographical distribution and polymorphisms of target‐site mutations in voltage‐gated sodium channel and acetylcholinesterase 1 genes in Anopheles sinensis populations in Shanghai, China. Parasites Vectors 12:396 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu N, Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol 60:537–559 (2015). [DOI] [PubMed] [Google Scholar]
- 50. Hemming‐Schroeder E, Strahl S, Yang E, Nguyen A, Lo E, Zhong D et al., Emerging pyrethroid resistance among Anopheles arabiensis in Kenya. Am J Trop Med Hyg 98:704–709 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coetzee M and Koekemoer LL, Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus . Annu Rev Entomol 58:393–412 (2013). [DOI] [PubMed] [Google Scholar]
- 52. Zhong D, Chang X, Zhou G, He Z, Fu F, Yan Z et al., Relationship between knockdown resistance, metabolic detoxification and organismal resistance to pyrethroids in Anopheles sinensis . PLoS One 8:e55475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu H, Liu L, Cheng P, Yang L, Chen J, Lu Y et al., Bionomics and insecticide resistance of Aedes albopictus in Shandong, a high latitude and high‐risk dengue transmission area in China. Parasites Vectors 13:11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao J, Chen H, Shi H, Peng H and Ma Y, Correlation between adult pyrethroid resistance and knockdown resistance (kdr) mutations in Aedes albopictus (Diptera: Culicidae) field populations in China. Infect Dis Poverty 7:86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu J, Bonizzoni M, Zhong D, Zhou G, Cai S, Li Y et al., Multi‐country survey revealed prevalent and novel F1534S mutation in voltage‐gated sodium channel (VGSC) Gene in Aedes albopictus . PLoS Neglected Trop Dis 10:e0004696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rath A, Mohanty I and Hazra RK, Insecticide susceptibility status of invasive Aedes albopictus across dengue endemic districts of Odisha, India. Pest Manag Sci 74:1431–1440 (2018). [DOI] [PubMed] [Google Scholar]
- 57. Kushwah RB, Mallick PK, Ravikumar H, Dev V, Kapoor N, Adak TP et al., Status of DDT and pyrethroid resistance in Indian Aedes albopictus and absence of knockdown resistance (kdr) mutation. J Vector Borne Dis 52:95–98 (2015). [PubMed] [Google Scholar]
- 58. Grigoraki L, Lagnel J, Kioulos I, Kampouraki A, Morou E, Labbe P et al., Transcriptome profiling and genetic study reveal amplified carboxylesterase genes implicated in temephos resistance, in the Asian tiger mosquito Aedes albopictus . PLoS Neglected Trop Dis 9:e0003771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grisales N, Poupardin R, Gomez S, Fonseca‐Gonzalez I, Ranson H and Lenhart A, Temephos resistance in Aedes aegypti in Colombia compromises dengue vector control. PLoS Neglected Trop Dis 7:e2438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marcombe S, Poupardin R, Darriet F, Reynaud S, Bonnet J, Strode C et al., Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: a case study in Martinique Island (French West Indies). BMC Genomics 10:494 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kamgang B, Yougang AP, Tchoupo M, Riveron JM and Wondji C, Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaoundé, the capital city of Cameroon. Parasites Vectors 10:469 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chang X, Zhong D, Fang Q, Hartsel J, Zhou G, Shi L et al., Multiple resistances and complex mechanisms of Anopheles sinensis mosquito: a major obstacle to mosquito‐borne diseases control and elimination in China. PLoS Neglected Trop Dis 8:e2889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Chromatograms and alignments of the kdr genotypes detected in Aedes albopictus from Hainan, China. The position at codon 1534 of the para‐type sodium channel gene is indicated by a rectangle. (A) Six types of genotypes detected. (B) Codon 1534 of the para‐type voltage‐gated sodium channel gene is indicated by a red box. Different peak colours distinguish the four bases T (red), C (blue), A (green) and G (black). K = G/T; Y = T/C; S = G/C
Table S1. Mutations at codon 1534 of the voltage‐gated sodium channel gene for pyrethroid‐resistant and ‐susceptible Aedes albopictus populations from Hainan Province, China


