Summary
Evolutionary arms‐races between plants and insect herbivores have long been proposed to generate key innovations such as plant toxins and detoxification mechanisms that can drive diversification of the interacting species. A novel front‐line of plant defence is the killing of herbivorous insect eggs.
We test whether an egg‐killing plant trait has an evolutionary basis in such a plant–insect arms‐race. Within the crucifer family (Brassicaceae), some species express a hypersensitive response (HR)‐like necrosis underneath butterfly eggs (Pieridae) that leads to eggs desiccating or falling off the plant. We studied the phylogenetic distribution of this trait, its egg‐killing effect on and elicitation by butterflies, by screening 31 Brassicales species, and nine Pieridae species.
We show a clade‐specific induction of strong, egg‐killing HR‐like necrosis mainly in species of the Brassiceae tribe including Brassica crops and close relatives. The necrosis is strongly elicited by pierid butterflies that are specialists of crucifers. Furthermore, HR‐like necrosis is linked to PR1 defence gene expression, accumulation of reactive oxygen species and cell death, eventually leading to egg‐killing.
Our findings suggest that the plants’ egg‐killing trait is a new front on the evolutionary arms‐race between Brassicaceae and pierid butterflies beyond the well‐studied plant toxins that have evolved against their caterpillars.
Keywords: coevolution, counter adaptation, egg deposition, hypersensitive response, induced plant defences, plant toxins, specialist herbivores
Introduction
The biodiversity on Earth is shaped by numerous factors including interorganismal interactions that can result in coevolution of adaptive traits. For example, the coevolutionary interactions between plants and insects as described by Ehrlich & Raven (1964) has driven the diversification of plant defensive metabolites (Swain, 1977; Becerra, 2015). In turn, specialist herbivores have evolved detoxification mechanisms, which allow them to feed on their host plants despite these toxic metabolites (Berenbaum, 1983; Despres et al., 2007); for example, caterpillars of the monarch butterfly (Danaus plexippus) can feed on cardenolide‐containing milkweeds (Cohen, 1985; Malcolm & Brower, 1989), and caterpillars of Pierinae and Plutella xylostella in the Plutellidae can feed on glucosinolate‐containing Brassicaceae (Wittstock et al., 2004; Wheat et al., 2007; Heidel‐Fischer & Vogel, 2015) .
The role of plant defences against herbivore eggs has been understudied, especially in a coevolutionary context between herbivores and plants. The majority of studies on plant–insect interactions have focused on the feeding life stages of herbivorous insects. Yet, in almost half of the ~ 400 000 known herbivorous insects, especially lepidopteran and sawfly species, eggs may be the first life stage to come into contact with the targeted host plant. Indeed, plants can already perceive and respond physiologically to the presence of herbivore eggs before they hatch (Hilker & Fatouros, 2016). Plant defences against insect eggs may have evolved as an important first line of defence, as every insect egg being detected and killed, is one less herbivorous larva or adult insect feeding on the plant in the near future.
Different types of plant defences against insect eggs have been reported in > 30 plant species including gymnosperms and angiosperms (both monocots and eudicots) (Fatouros et al., 2016). In response to insect egg deposition, plants can produce ovicidal substances (Seino et al., 1996), form neoplasms (Doss et al., 2000; Petzold‐Maxwell et al., 2011) or express a hypersensitive response (HR)‐like necrosis beneath the eggs (Shapiro & DeVay, 1987; Balbyshev & Lorenzen, 1997; Petzold‐Maxwell et al., 2011; Fatouros et al., 2014). HR‐like necrosis is an egg‐killing defence leading to eggs desiccating and/or falling off the leaf. It has so far been observed in the plant families Pinaceae (Bittner et al., 2017), Poaceae (Yang et al., 2014), Fabaceae (Garza et al., 2001), Solanaceae (Balbyshev & Lorenzen, 1997; Petzold‐Maxwell et al., 2011) and Brassicaceae (Shapiro & DeVay, 1987; Fatouros et al., 2014; Pashalidou et al., 2015a; Griese et al., 2017). To understand whether egg‐killing traits have evolved as counter‐adaptations to specialist herbivores and their detoxification mechanisms, the phylogenetic occurrence of the HR‐like egg‐killing trait across these plant families and reciprocal insect pest‐clades need to be investigated.
Sequence‐based phylogenetic analysis (Al‐Shehbaz, 2012; Huang et al., 2015; Guo et al., 2017) has established that the Brassicaceae family is split into a core clade containing 3680 species, which is subdivided into three major lineages, and a smaller sister clade containing only the genus Aethionema (61 species) (Beilstein et al., 2006; Beilstein et al., 2008). The model plant Arabidopsis thaliana is a representative of Lineage I and the Brassica crop plants are representatives of Lineage II. Lineage III is a smaller group mostly restricted to Asia and lacking a model or crop species. Cleomaceae is the sister family of the Brassicaceae (Hall et al., 2002). Within the Brassicaceae, defences against feeding herbivores and the genetic basis of this defence have been studied intensively (Xue et al., 1992; Graser et al., 2000; Rask et al., 2000; Windsor et al., 2005). Aliphatic glucosinolates evolved as defensive compounds near or at the origin of the Brassicales clade, and became more diverse and complex with plant species radiation. Although these compounds play an important role in defending the plants against herbivory, many feeding insects have specialized and evolved effective glucosinolate detoxification and/or excretion mechanisms (Winde & Wittstock, 2011; Heidel‐Fischer & Vogel, 2015; Erb & Robert, 2016; Heidel‐Fischer et al., 2019).
The Pieridae butterflies (whites and sulphurs), including approximately 1000 species today (Wahlberg et al., 2014), use host plants belonging to two major plant orders, the Fabales (Fabaceae) and Brassicales (Brassicaceae, Resedaceae, Capparaceae and Cleomaceae), although some species in certain clades also have shifted to Rosales (Rhamnaceae, Rosaceae) or Santalales (Edger et al., 2015). Recent phylogenetic reconstruction of the Pieridae indicates that the ancestral host appears to be fabaceous with multiple independent shifts to other orders. Although the Dismorphiinae and nearly all Coliadinae are Fabales feeders, the sister to the Coliadinae, Pierinae, feed primarily on Brassicales (Braby & Trueman, 2006; Wheat et al., 2007). The latter, thus, represent a single origin of feeding on glucosinolate‐producing plants.
Shortly after the initial evolution of the order Brassicales, some ancestral Pierinae evolved nitrile‐specifier proteins (NSPs) that detoxify glucosinolates. This enabled a host shift from their prior Fabaceae hosts to the Brassicales c. 80 million years (Myr) ago (Edger et al., 2015). Likewise, the evolution of glucosinolate sulfatase in Plutella xylostella (Plutellidae) allowed the caterpillars of this moth to feed on Brassicaceae (Wheat et al., 2007; Heidel‐Fischer & Vogel, 2015). It has been shown that speciation‐rate shifts, as well as genome‐duplication events with gene birth–death dynamics occurred in both Brassicales and Pierinae, usually following a key defence (glucosinolates) or counter‐defence (NSPs) invention in one of the coevolutionary partners (Edger et al., 2015). Defence responses targeting eggs might have added a new layer of traits evolved in response to herbivore specialization. To pinpoint the evolution of transitions and innovations of plant defences to insect eggs, it therefore is necessary also to investigate these trait(s) of interest in a proper phylogenetic context.
Defence responses induced by cabbage white butterfly eggs have been studied mainly in A. thaliana and the black mustard Brassica nigra (Little et al., 2007; Fatouros et al., 2014; Pashalidou et al., 2015b; Firtzlaff et al., 2016; Paniagua Voirol et al., 2020; Stahl et al., 2020). In A. thaliana, Pieris brassicae and P. rapae eggs activate a plant immune response, that resembles pattern‐triggered immunity (PTI) against pathogens. It includes expression of defence genes (e.g. pathogenesis‐related genes (PR1)), accumulation of reactive oxygen species (ROS) and a local cell death response. However, a visible necrosis is rarely expressed and egg‐killing has never been shown (Little et al., 2007; Reymond, 2013; Groux et al. 2020). Egg‐killing resulting from a strong necrosis has been shown for B. nigra in response to Pieris spp. Within B. nigra, there is variation frequency and severity of HR‐like necrosis between accessions (Fatouros et al., 2014; Pashalidou et al., 2015a; Griese, et al., 2017).
The current study explores whether egg‐killing HR‐like necrosis evolved as a specific response to pierid egg deposition in a subset of Brassicaceae. Thus far, to the best of the author’s knowledge, no effort has been made to map the phylogenetic history of any egg defence trait for any plant family. Doing so would be a first necessary step to show an adaptive response to egg deposition. We investigated the phylogenetic occurrence of HR‐like necrosis in the Brassicaceae (mainly lineages I and II) and three species in the Cleomaceae, and also explored the reciprocal phylogenetic co‐occurrence in the Pieridae clade. We tested eggs from four Pieris butterflies (Pierinae) and five relatives: Anthocharis cardamines (Pierinae) feeding on Cardamine spp. (Brassicaceae Lineage I), Aporia crataegi (Pierinae) feeding on Prunus spp. (Rosaceae), Gonopteryx rhamni (Coliadinae) feeding on Rhamnus spp. (Rhamnaceae), Colias spp. (Coliadinae) and Leptidea sinapis (Dismorphinae) both feeding on different species of the Fabaceae. As an outgroup, we used the butterfly Aglais io (Lepidoptera: Nymphalidae) that feeds on Urtica plants (Urticaceae). Additionally, we studied elicitation of the eggs of two moths, Mamestra brassicae (Noctuidae) and Plutella xylostella (Plutellidae), both feeding on Brassicaceae. Besides screening for HR‐like necrosis, we investigated whether important components of plant defences, such as PR1 gene expression, cell death and accumulation of ROS, correlated with the egg‐induced necrosis. We tested the effect of HR‐like on survival of singly‐laid Pieris spp. eggs in different plant species under both field and glasshouse conditions. Finally, we hypothesized the evolution of potential counter‐adaptations to egg‐killing by some pierid butterflies.
Specifically, we addressed the following questions: (1) Is HR‐like necrosis induced in a clade‐specific manner within the Brassicaceae? (2) Are HR‐like necrosis and other defence responses induced by eggs specific to a particular clade of butterfly species (e.g. genus, subfamily or family) and/or specific to species that co‐evolved with the Brassicaceae? And (3) Is the observed necrosis lowering egg survival under glasshouse and field conditions?
Materials and Methods
Plants and insects
For our study, we obtained seeds of thirty‐one species in the Brassicales (28 Brassicaceae and three Cleomaceae), from various sources. For each plant species, between one and 11 accessions were grown (Supporting Information Table S1). Per accession, between three and 17 plants were treated with egg wash to assess elicitation of a HR‐like response by Pieris brassicae. Brassica nigra plants were used to assess elicitation of the HR‐like necrosis by different butterfly species. Finally, egg‐killing was tested for six plant species. In preliminary trials, plant species with unknown developmental times were grown to assess their germination and flowering after sowing. Then, plants were sown in a scheme to ensure that they had reached similar life stages (i.e. vegetative growth) when used for experiments. Therefore, plants were between three and six weeks old when being treated with butterfly eggs or egg wash.
In order to assess the occurrence of HR‐like necrosis across the selected Brassicales species, we used a wash of P. brassicae eggs (see the Egg wash preparation section below). To assess induction of HR‐like necrosis on B. nigra plants, we used egg deposition by different butterfly/moth species and populations (for details, see Methods S1 and Table S2).
Egg wash preparation
Not all butterflies and moths used in this study naturally deposit eggs on all plant species that were selected. In order to be able to test those species and screen a large number of plants efficiently, we developed a method to prepare an egg wash that can be used to mimic oviposition as plant treatment. The development and testing of this method will be submitted elsewhere. We showed that there is no difference in the symptoms induced on B. nigra leaves between eggs and egg wash of P. brassicae (L. Caarls et al., unpublished). For this method, female butterflies of P. brassicae, Pieris rapae and P. napi, and Mamestra brassicae moths were persuaded to lay eggs on paper that was pinned to the underside of a leaf. Wash from Aglais io, Anthocharis cardamines, Aporia crataegi, Colias spp., Gonepteryx rhamni and Leptidea sinapis eggs was made by carefully removing eggs from leaves or inflorescences. Eggs of P. xylostella were collected on parafilm. Collected eggs were counted and washed overnight in MES buffer, and buffer was applied on plant leaves. Concentrations of the egg washes were adjusted based on the size of the eggs used (for details see Methods S2).
Phenotyping of HR‐like necrosis on Brassicales species
Experiments were carried out in a glasshouse compartment (22–27°C, 50–90% RH, 16 h : 8 h, light : dark). For the screening of 31 Brassicales plant species, 5 µl of P. brassicae egg wash was pipetted on a fully mature leaf (the third or fourth leaf from the top) of each plant. Another fully matured leaf (the third or fourth from the top) received pure water containing Tween20 as a control. After four days, leaf disks were harvested from the area where egg wash had been applied using a 1‐cm cork borer and put in a rectangular Petri dish with wet blue filter paper. Pictures were taken using a Dino‐Lite digital microscope (AnMo Electronics Corporation, New Taipei City, Taiwan). These pictures were visually scored for expression of HR‐like necrosis (see below).
Elicitation of HR‐like necrosis by diverse Pieridae species
Female butterflies of P. brassicae (two populations), P. napi and P. rapae (two populations) were allowed to lay between five and 10 eggs on two different B. nigra accessions (SF19 and SF48) (Table S1) (Griese et al., 2017). Aglais io, A. cardamines, Colias sp. and G. rhamni egg wash respectively, were tested on both B. nigra accessions. Aporia crataegi, L. sinapis, P. mannii, M. brassicae and Plutella xylostella wash, were each tested on B. nigra accession SF48. After 4 d, HR‐like necrosis was scored using a scoring system described previously by Griese et al. (2017).
Pathogenesis‐related protein 1 (PR1) gene expression by diverse butterfly and moth species
In order to measure PR1 gene expression, 10 µl egg wash of P. rapae, P. mannii, A. crataegi, A. cardamines, G. rhamni, Colias spp., M. brassicae and P. xylostella were each pipetted on the abaxial leaf side of 20 B. nigra (SF48) plants per butterfly/moth species, except for P. xyllostella where egg wash for only six plants was available. After 24 h, two 6‐mm diameter leaf disks were taken from the egg wash application site and snap frozen in liquid nitrogen. PR1 transcript levels were measured on five biological replicates composed of four pooled individual plants. RNA isolation according to (Oñate‐Sánchez & Vicente‐Carbajosa, 2008), real‐time quantitative PCR analysis and primers are described in detail in Methods S3 and Table S3.
Histochemical staining
Pieris brassicae females were allowed to lay two egg clutches of 5–20 eggs on a single leaf per plant. From every plant, one clutch was used for histochemical staining (hydrogen peroxide (H2O2) or cell death) whereas the other one was used to score the necrotic leaf area. Samples were taken at 48, 72 or 96 h after oviposition by taking a 10‐mm diameter leaf disc around the egg clutch (for details, see Methods S4).
Pieris spp. egg survival on HR‐like expressing plants under glasshouse conditions
Experiments were done under long‐day glasshouse conditions (21 ± 5°C, 45–70% RH, 16 h : 8 h, light : dark) for B. nigra, B. napus, B. oleracea, B. rapa and Crambe hispanica. Pieris brassicae females were manipulated to lay five to 15 separated eggs (i.e. not touching each other) on all lines previously used in the screening of Brassicaceae species. The number of hatching and nonhatching eggs were counted to measure egg survival rates. Previously, P. brassicae egg survival was affected only when eggs were laid singly, not touching each other (Griese et al., 2017). The eggs were left on the plant and four days after oviposition HR‐like necrosis was scored as present or absent. Egg survival on Arabidopsis thaliana, plants were reared under short‐day glasshouse conditions (21 ± 4°C, RH: 70%, 8 h : 16 h, light : dark) to control for fast flowering. Seeds from 36 different Swedish accessions of A. thaliana were obtained from the HapMap population (http://bergelson.uchicago.edu/wp‐content/uploads/2015/04/Justins‐360‐lines.xls). Pieris rapae females were allowed to lay a single egg on one leaf per plant. After five days, survival of eggs was noted by counting the number of hatched caterpillars.
Pieris spp. egg survival assessed by field survey
It has been shown that HR‐like necrosis has weaker effects on egg survival under glasshouse than under natural conditions (Fatouros et al., 2014). A survey was conducted to record survival of P. rapae and P. napi eggs on B. nigra plants from a natural population (for details, see Fatouros et al., 2014, and Methods S5).
Phylogenetic trees of Brassicales and Pieridae species
We used a consensus tree based by two recent studies (Huang et al., 2015; Guo et al., 2017) to place our tested Brassicales species accordingly. Both studies analyzed representatives of the three distinct linages of the core Brassicaceae clade and the first‐branching Aethionema and the outgroup Cleomaceae (For details see Methods S6 and Table S4).
We mapped the HR‐like necrosis induced by the tested butterfly species according to two recent studies: a phylogenomic analysis of Lepidoptera (Kawahara et al., 2019) and phylogenetic analysis of the Papiolionoidea (Wiemers et al., 2020). The first study contained 994 taxa, whereas the second analyzed 496 extant butterfly species in Europe using mitochondrial gene COI and ≤ 11 nuclear gene fragments. The European butterflies used were split in 12 subclades. The Pieridae were considered as a single clade and the Nymphalidae divided into seven subclades (Wiemers et al., 2020).
Statistical analysis
Statistical analyses were done using R (R Core Team, 2016). For the screening of plant accessions, contingency tables and χ 2‐tests were used to determine which plant species/genotypes significantly expressed HR‐like necrosis after egg wash treatment compared to the control treatment. The contingency tables for the χ 2‐tests were constructed with: the number of egg wash‐treated leaves expressing HR‐like necrosis; the number of egg wash‐treated leaves not expressing HR‐like necrosis; the number of control wash‐treated leaves expressing HR‐like necrosis; and the number of control wash‐treated leaves not expressing HR‐like necrosis. With this set‐up, all plant accessions within each plant species were tested independently.
Egg survival was analyzed using binomial generalized linear models (GLMs) in which first all variables (plant species, flowering state, HR expression and all interactions between the factors) were used. Based on Akaike information criterions (AICs), unnecessary variables were removed to obtain a more parsimonious model (plant species, HR expression and interaction). Subsequently, R/emmeans test or Wilcoxon–Mann–Whitney U‐test were performed as post hoc test.
Differences in induction of HR‐like necrosis by different butterflies were tested using binomial GLMs and, to test differences in strength, GLMs with Poisson distribution. Dunn test with Bonferroni–Holm correction was used as post hoc test. For differences in HR‐severity, Kruskal–Wallis tests followed by post hoc Wilcoxon Rank Sum test with Benjamini–Hochberg correction were performed.
Quantification of HR‐like necrosis and histochemical staining for each plant species were compared with a Student's t‐test. Differences in HR‐like necrotic area between plant species were analyzed with ANOVA, followed by a Tukey post hoc test with Benjamini–Hochberg correction. Gene expression of PR1 was analyzed using Kruskal–Wallis test followed by a post hoc Wilcoxon rank sum test with Benjamini–Hochberg correction.
For all of the statistical analyses involving comparison of mean values (egg survival, histochemical staining, gene expression), the choice of parametric or nonparametric methods was made after checking the assumptions of normality (Shapiro–Wilk normality test) and homogeneity of variances (Fligner–Killeen test) on the raw data.
Results
Origin of HR‐like necrosis in the Aethionema and core Brassicaceae
Out of 31 Brassicales plant species used this study, five species responded significantly with a HR‐like necrosis to P. brassicae egg wash. This included species of the tribe Brassiceae and of the genus Aethionema (Fig. 1a). In the tribe Brassiceae, egg wash treatment significantly enhanced expression of HR‐like necrosis in specific accessions of four species: B. napus (in 25–86% of tested plants), B. nigra (63–83%), B. oleracea (20–40%) and C. hispanica (0–86%). HR‐like necrosis of Aethionema arabicum varied among the tested accessions between 0% and 60% (Table S5). There was no significant induction of HR‐like necrosis after egg wash treatment for all other plant species tested compared to control leaves. HR‐like necrosis was expressed at low frequency and low severity in several other species of Lineage II. For example, 30% of wild Lunaria annua plants showed a weak HR‐like necrosis, which was almost significant (P = 0.05; Table S5). Necrosis was expressed rarely in species in lineage I and III (Fig. 1a): only in single plants of some accessions and once in Aethionema carneum.
Fig. 1.
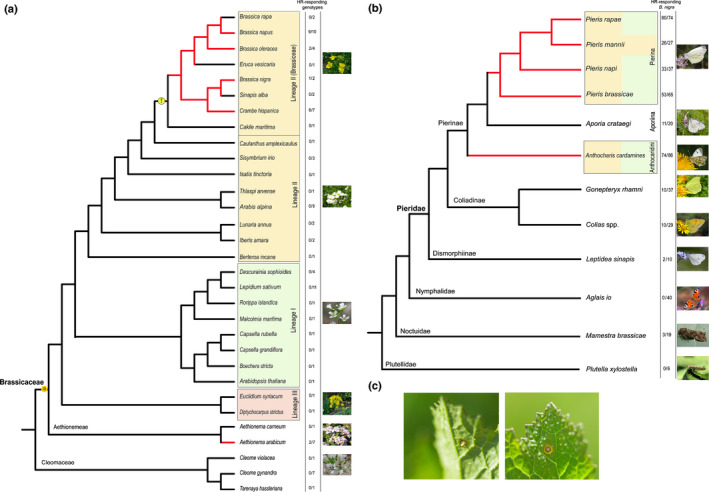
Presence of hypersensitive response (HR)‐like necrosis mapped on phylogenetic trees of Brassicaceae (a) and Pieridae (b). Red lines, at least one genotype of the species expresses HR‐like necrosis induced by egg wash significantly more often compared to control; black lines, no genotype expresses HR‐like necrosis induced by egg wash significantly more often compared to control; (a) Screening of HR‐like necrosis by Pieris brassicae egg wash in 28 Brassicaceae species and three Cleomaceae species based on the published phylogenies (Huang et al., 2015; Guo et al., 2017). Different lineages are coloured in orange (Lineage II/tribe Brassiceae), green (Lineage I) and red (Lineage III). The whole genome duplication (WGD) (α) and genome triplication (T) events are marked on the tree. Numbers in the column represent the number of genotypes expressing HR‐like (left) from the total number of genotypes tested (right) (b) Elicitation of HR‐like necrosis by pierid egg wash or eggs in Brassica nigra leaves by different butterfly and moth species shown on phylogenies based on (Kawahara et al., 2019; Wiemers et al., 2020). Responses of the pierid species were compared to the nymphalid Aglais io, the noctuid moth Mamestra brassicae and the plutellid moth Plutella xylostella. Coloured boxes represent species of the Brassicaceae used as main host plants by the butterflies. Brassicaceae and Lepidoptera (sub)families are written on their nodes where they separate from the rest of the clades. Numbers in column represent the number of B. nigra plants (SF48) expressing HR‐like (left) from the total number of B. nigra plants tested (right). Photos of butterflies and moths taken by Zeynel Cebeci, Charles J. Sharp, Juergen Mangelsdorf (all three creative commons license), Jitte Groothuis, Hans M. Smid, Tibor Bukovinszky, and N. E. Fatouros. (c) HR‐like necrosis induced by a single Pieris sp. egg in B. nigra taken from the under and upper side of the leaf (credits N. E. Fatouros).
Elicitation of HR‐like necrosis by Pierid species adapted to Brassicaceae
The elicitation of HR‐like necrosis in B. nigra by egg deposition or egg wash of nine lepidopteran (eight pierid and one nymphalid species) was tested (Fig. 1b). First, we assessed the HR frequency and severity (scored from 0, no symptoms to 3, strong necrosis) in B. nigra in response to egg deposition or egg wash of four closely related Pieris species, four relatives (A. cardamines, Colias spp., G. rhamni, L. sinapis) and A. io as outgroup. Eggs or egg wash of P. brassicae, P. napi, P. rapae, P. mannii and A. cardamines induced a high fraction of HR‐like necrosis in B. nigra (0.82 ± 0.06; 0.75 ± 0.06; 0.86 ± 0.14, 0.89 ± 0.05, respectively) and all induced with high severity (Table S6). When several populations were available for butterfly species, all populations elicited HR‐like necrosis with similar frequencies (GLM: χ 2 = 1.36, df = 3, P = 0.71) and severity (GLM: χ 2 = 2.60, df = 3, P = 0.46). The fraction and severity of HR‐like elicited by G. rhamni, Colias spp. and L. sinapis were generally lower than HR‐like induced by the eggs of Pieris spp. and A. cardamines (Tables S6, S7). Moreover, the responses induced in plants by the egg wash of these non‐brassicaceous Pieridae in plants appeared to be chlorosis instead of necrosis (Fig. S1). The egg wash of A. io induced no symptoms on B. nigra (Table S6).
HR‐like necrosis severity correlates to PR1 defence gene expression
We then performed an experiment to compare the response induced by egg wash of Pierinae and their relatives including two moths that can feed on Brassicaceae: P. xylostella and M. brassicae. As expected, we observed significant differences in HR‐like frequency between the Pierinae and other butterfly and moth species (GLM: χ2 = 28.3, df = 9, P < 0.001; Table S7) and in HR severity (Kruskal–Wallis: H = 133.37, df = 9, P < 0.001; Fig. 2a). In this experiment, P. rapae and P. mannii induced HR‐like response in all plants tested with high severity (2.88 ± 0.01 and 2.95 ± 0.01; Fig. 2a; Table S7). Anthocharis cardamines also induced high HR severity on 65% of the tested plants (1.55 ± 0.04). The more distantly related Pieridae G. rhamni and Colias spp. induced necrosis in only a few plants with low severity (0.31 ± 0.03 and 0.60 ± 0.04; Table S7), a similar response to that caused by two moth species and the control treatment (Fig. 2a). HR‐like frequency and severity levels induced by A. crataegi were between those induced by Pieris spp. and Anthocharis and the more distantly related species (Fig. 2a; Table S7).
Fig. 2.
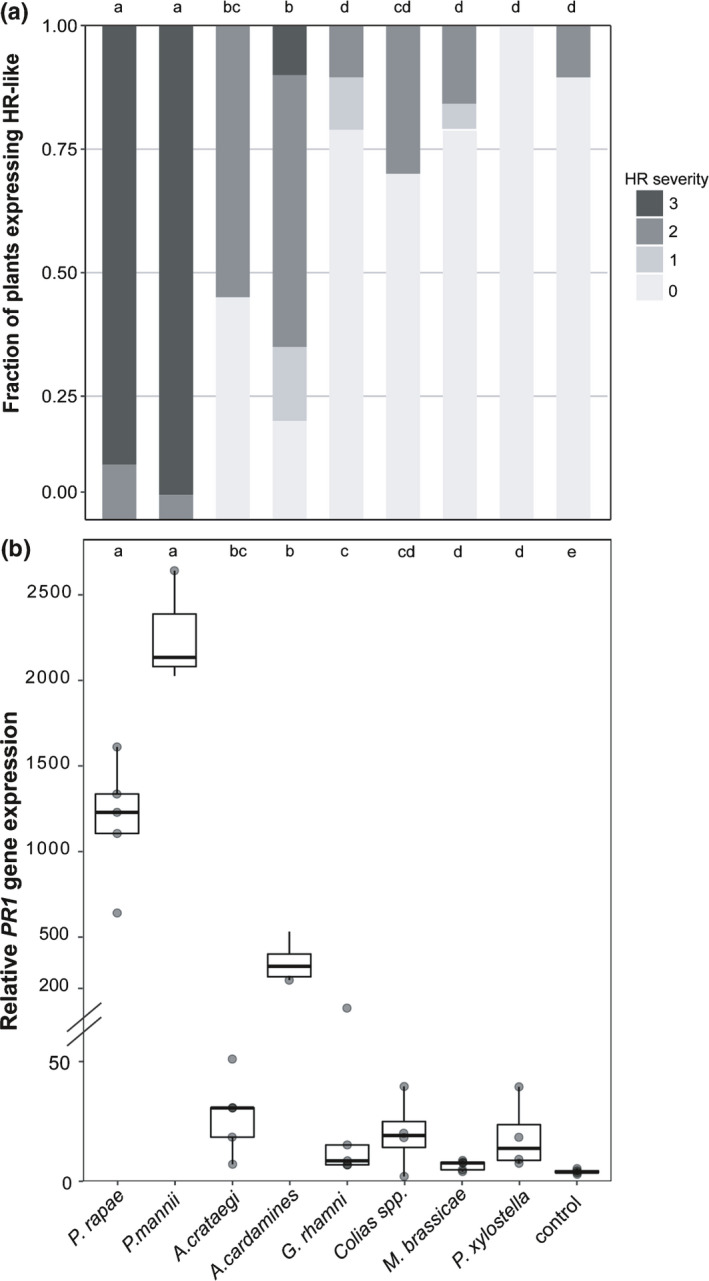
Hypersensitive response (HR)‐like necrosis and pathogenesis‐related (PR1) gene expression induced by egg wash of different butterfly and moth species in Brassica nigra plants. For the experiment, two droplets of 10‐μl egg wash were applied onto one leaf of each plant. (a) Fraction of plants expressing HR‐like necrosis at different severities (0, no response; 3, strong necrosis). A total N = 18–20 plants was tested per butterfly species, whereas Plutella xylostella egg wash was tested on only six plants. (b) Relative expression of PR1 gene upon treatment with egg wash of different butterflies/moths or MES buffer as control. Transcript levels were measured by quantitative real‐time PCR on four to five biological replicates, each composed of four pooled individual plants. The height of the boxes represents the first to the third quartile of the range; the horizontal line within the box is the median; the whiskers indicate the data minimum and maximum; and dots represent outliers. Letters denote differences in HR severity or mean transcript levels between different treatments (Wilcoxon rank sum test, P < 0.05).
Besides a HR‐like necrosis, Pieris spp. egg deposition or their crushed eggs also are known to induce other defence responses in A. thaliana and B. nigra, including PR1 gene expression following egg deposition or egg wash treatment (Little et al., 2007; Fatouros et al., 2015). We tested if egg washes of other butterfly and moth species also induced a defence response in B. nigra by measuring PR1 gene expression. Interestingly, PR1 expression was significantly induced by egg wash of all butterfly and moth species tested, except M. brassicae. Nevertheless, there were significant differences in PR1 induction between the different species (Kruskal–Wallis H = 35.39, df = 8, P < 0.001; Fig. 2b). Expression of PR1 correlated with HR severity and was significantly higher following treatment by egg washes of P. rapae and P. mannii, and also, although less strongly, by egg wash of A. cardamines (Fig. 2b). Plants responding with lower HR severity showed a lower but significant PR1 expression. Notably, egg wash of the Pierinae A. crataegi induced an intermediate PR1 expression between A. cardamines and G. rhamni, correlating to HR‐like severity. Egg wash of Colias spp. induced PR1 expression that although the highest among the non‐Pierinae species, was still about 100‐fold lower than that of P. mannii (Table S8). Interestingly, PR1 expression also was induced by P. xylostella egg wash, which showed no visual symptoms in these plants (Fig. 2a).
HR‐like severity correlates with increased H2O2 and cell death in a subset of Brassicaceae
The screening for HR‐like necrosis across different Brassicaceae revealed interspecific variation in HR frequency (Table S5). In addition, we also observed variation in HR severity between plant species that showed high HR frequency. To quantify the differences observed, we measured the area of necrotic tissue induced by P. brassicae eggs in three species: B. nigra, B. oleracea and C. hispanica. We found that the necrotic area was largest in B. nigra and significantly smaller in the other two species (ANOVA, F = 17.028, df = 2, P < 0.001; Table S9). Previously, P. brassicae eggs on A. thaliana were shown to induce components of plant immunity such as H2O2 and cell death despite the absence of a visible HR‐like necrosis (Little et al., 2007; Gouhier‐Darimont et al., 2013). Therefore, we investigated to what extent the visible stronger or larger HR‐like necrosis correlates with induction of H2O2 and cell death. Plants that showed a small HR‐like necrosis (i.e. B. oleracea and C. hispanica) exhibited a high accumulation of H2O2 and trypan‐blue stained cell death compared to the extension of visible necrosis (Fig. S2). Brassica nigra showed a strong visible necrosis, > 1 mm2 per 10 eggs, exceeding the H2O2 and cell death accumulation (Fig. S2). These results suggest that components of the plant immunity are induced regardless of the variation in HR severity and that B. nigra induces the visible HR‐like response most strongly.
Effect of HR‐like necrosis on Pieris spp. egg survival on different Brassicaceae species
First, we monitored egg survival on wild B. nigra plants of the Pieris spp. most abundant under natural field conditions in the Netherlands (P. napi and P. rapae). Egg survival was 40% lower when eggs induced HR‐like necrosis compared to survival of eggs that did not induce a leaf necrosis (GLM: χ 2 = 11.02, df = 1, P < 0.001; Fig. 3a), confirming previously reported results (Fatouros et al., 2014). Considering the variation in HR severity and HR frequency between plant species, we investigated the effect of HR severity in different species on Pieris egg survival. We tested egg survival on five plant species from the first screening (Fig. 1a) under glasshouse conditions: three species that showed high HR frequency and contrasting HR severity (B. napus, B. nigra and C. hispanica) and two species with low HR frequency (B. rapa and A. thaliana). HR‐like necrosis significantly lowered the survival of singly laid P. brassicae eggs on all three plant species that previously showed high HR frequency (GLM: χ 2 = 38.41, df = 1, P < 0.001; Fig. 3b). On C. hispanica plants egg survival was significantly lower than on B. napus plants (pairwise MWU: P = 0.006; Fig. 3b). Conversely, egg survival was not affected by HR‐like necrosis for B. rapa (GLM: χ 2 = 2.61, df = 1, P = 0.14; Fig. 3c) that generally showed low HR severity. On the different A. thaliana accessions, no visible HR‐like necrosis was observed, and 100% of P. rapae eggs survived (Fig. 3d).
Fig. 3.
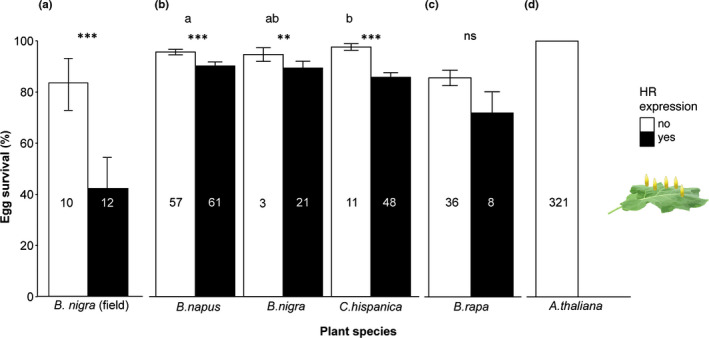
Effect of hypersensitive response (HR)‐like necrosis on survival rates of singly laid Pieris eggs on different plant species. (a) Effect of HR‐like necrosis on egg survival in field conditions. Survey of P. napi and P. rapae eggs on Brassica nigra plants located near the river Rhine in Wageningen (the Netherlands). One to 13 eggs were sampled per plant. (b–d) Effect of HR‐like necrosis on egg survival under glasshouse conditions. Single eggs were separately laid on the leaf without touching each other as shown on the right side. Three experiments were performed with singly laid P. brassicae eggs on different accessions of B. napus, B. nigra, Campe hispanica (b) and B. rapa (c) as well as P. rapae eggs laid on Arabidopsis thaliana (d). Ten single eggs were laid on each plant for experiment (b), five single P. brassicae eggs on each plant for experiment (c) and a single P. rapae egg per plant for (d). If a plant expressed HR‐like necrosis under at least one egg it was counted as HR‐expressing ‘yes’. Numbers in columns represent number of plants tested. Egg survival represents mean ± SE of hatched eggs for each plant. If a plant expressed HR‐like necrosis under at least one egg it was counted as HR‐expressing ‘yes’. Asterisks indicate significant differences in egg survival between plants with or without HR‐like necrosis. Different letters indicate significant differences in egg survival between plant species, without taking HR‐like necrosis into account (GLM; ns, not significant; **, P < 0.01; ***, P < 0.001).
Discussion
Pierid butterflies and their brassicaceous host plants are a fascinating model system of co‐evolutionary interactions, and research so far has explored the evolutionary and genetic basis of these interactions by focusing on the diversifying selection on plant chemical defences (i.e. glucosinolates) and insect nitrile‐specifier protein (NSP) detoxification genes (Edger et al., 2015b; Nallu et al., 2018). Here, we attempt for the first time to map the phylogenetic history of egg induction (i.e. hypersensitive response (HR)‐like necrosis) as a plant defence trait and its reciprocal co‐occurrence in the herbivore clade. We show that a strong HR‐like necrosis induced by Pieris eggs most frequently occurs in one clade within the Brassicaceae. Half of the tested plant species from the Brassiceae tribe in Lineage II express HR‐like necrosis with high frequency in response to P. brassicae egg wash. The visual necrosis was accompanied with increased levels of hydrogen peroxide (H2O2) and cell death in three representative HR + plant species. For the tested Brassica and Crambe spp. (tribe Brassiceae), HR‐like necrosis lowered egg survival both under natural and glasshouse conditions, except for B. rapa that does not express a strong HR‐like necrosis as (e.g.) Crambe hispanica or Brassica nigra. Interestingly, egg survival was generally lower on B. rapa, which could hint to plant defences other than HR‐like necrosis or nonideal circumstances for Pieris eggs. Furthermore, we showed for the first time that only egg wash from species of the subfamily Pierinae that are specialized on the Brassicaceae (i.e. Pieris butterflies and Anthocharis cardamines) elicit a strong HR‐like necrosis and high levels of pathogenesis‐related (PR1) defence gene on B. nigra. Species that are specialized on Fabaceae or Rhamnaceae from the Coliadinae, Colias spp. and Gonopteryx rhamni, and Dismorphiinae, Leptidea sinapis, elicited a weak necrosis or sometimes just a chlorotic response similar to that of Solanum dulcamara to Spodoptera eggs (Geuss et al., 2017). Our results suggest that the elicitation of strong HR‐like necrosis by Pieris eggs may have a single origin in the ancestor of the Brassicaceae (Fig. 1) with variation in the frequency and severity of the trait between species and accessions, whereas the trait is most strongly expressed in the Brassiceae tribe. Moreover, we show that B. nigra plants specifically evolved strong HR‐like necrosis to the eggs of those pierid species that evolved effective glucosinolate detoxification mechanisms.
Evolution of HR‐like necrosis in Brassiceae and Aethionema
Four of eight tested Brassiceae species showed consistent HR‐like necrosis to Pieris egg wash in high frequency and severity in at least one of the genotypes tested. In other plant species in the Brassicaceae, we found no consistent induction of HR‐like necrosis by Pieris egg wash. It is unlikely that the genome triplication event specific to the Brassiceae clade is the only factor involved in the evolution of HR‐like as one Aethionema species responded to Pieris eggs with a strong necrosis. There may be ecological reasons, such as overlap in spatial distribution between butterflies and plant species, that can explain why HR‐like necrosis appears more severe and at higher frequency within the Brassiceae and Aethionema. In fact, besides many of the tested Brassiceae plants, Aethionema are natural host plants for Pierinae species as well. Pieris ergane, Anthocharis gruneri and Euchloe ausonia are specialized on Aethionema species in their southeastern European habitat (Tolman & Lewington, 2009). Because of high abundances of Pierinae species occurring on Aethionemeae, it could be that species of this basal clade of the Brassicaceae retained a severe HR‐like necrosis as an effective trait against eggs of these butterfly species.
In other plant species tested, occasionally a single plant showed a light HR‐like necrosis. These plants might be able to detect insect eggs and respond with a general immune response, as recently shown for A. thaliana (Gouhier‐Darimont et al., 2013, 2019; Stahl et al., 2020). Alternatively, it could be a false‐positive response due to a contamination or a general stress response, as in rare cases, also control wash induced a weak necrosis. In general, not all tested plant species within the Brassiceae tribe within Lineage II expressed HR‐like necrosis though. Variation for the HR‐like necrosis trait between genotypes of one species, as we find here, has been observed before (Pashalidou et al., 2015a; Griese et al., 2017). It is therefore possible that we may have missed HR‐like necrosis expressing plants because of the selection of nonresponsive or less sensitive genotypes for some of the plant species or genus. For example, Sinapis alba did not show HR‐like necrosis (Fig. 1) but previous work on the close relative S. arvensis showed that eggs of P. rapae and P. brassicae strongly induced HR‐like necrosis (Griese et al., 2020). For the model species A. thaliana, a few accessions other than the ones included in this study did show a chlorosis and/or some necrosis to P. brassicae eggs (Reymond, 2013; Groux et al. 2020). For half of the tested species here, only one genotype was tested, increasing the likelihood of selecting only nonresponsive ones (Fig. 1a; Table S1). Some plant species and accessions might have lost the ability to express HR‐like necrosis, or only do so rarely. Those plants may be less frequently used as host plants for pierid butterflies, for example because of a phenological mismatch between the plant species and its potential specialist herbivores. This mismatch can be especially true for species belonging to lineages I and III. For example, in central Europe A. thaliana usually is not attacked by pierid butterflies, as it is rather small and usually completes its life cycle before pierid caterpillars could develop on the plant (Harvey et al., 2007). Notably, A. cardamines was observed to deposit eggs on A. thaliana in North Sweden where both life cycles briefly overlap (Wiklund & Friberg, 2009). Yet, P. rapae eggs did not induce a leaf necrosis lowering Pieris egg survival on Swedish accessions of A. thaliana (Fig. 3d), neither did we observe a visible necrosis on the commonly used genotype Col‐0 in our experiments when using P. brassicae egg wash (Fig. 1; Table S5). The observed variation in HR‐like necrosis between genotypes of the same species suggests that expression of this trait might have negative effects on plant fitness and only evolves with high herbivore pressure. Alternatively, variability in a defence trait might in itself be defensive, as postulated by the moving‐target strategy to counteract the development of efficient plant defensive responses by herbivores (Adler & Karban, 1994). Phenotypic variation in HR‐like necrosis to eggs previously was suggested to be part of such a moving‐target game (Hilker & Fatouros, 2015).
Counter‐adaptations of brassicaceous‐feeding Pierinae species to HR‐like necrosis
Previous work has shown that the NSP glucosinolate detoxification gene was a key innovation in the ancestral Pierinae enabling them to shift host plant from Fabaceae to Brassicaceae (Edger et al., 2015b). We show that strong, egg‐killing HR‐like necrosis linking to high levels of PR1 gene expression in B. nigra seems specific to species of the two independent lineages, Pierini and Anthocharidini, belonging to the Pierinae subfamily that colonized the Brassicales some 50 Myr ago (Wheat et al., 2007). We suggest that this may be a counter‐adaptation of some brassicaceous plants to the nitrile‐specifier genes that evolved in the Pierinae (Edger et al., 2015). Because those nitrile‐specifier genes detoxify glucosinolates and enabled butterflies of those lineages to conquer the Brassicaceae, a new and separate plant defence mechanism might have evolved. Reciprocally, pierid butterflies also may have found ways to counter‐adapt to the egg‐killing HR‐like necrosis. For example, they could do so by clustering eggs, ovipositing on inflorescences and/or shifting to other host plants (Fig. 4). Clustered eggs of P. brassicae were shown to negate the egg‐killing effect of the HR‐like necrosis (Griese et al., 2017). Although the mechanism underlying this is unknown, it has been shown that desiccation can be slowed down by clustering eggs (Clark & Faeth, 1998; Griese et al., 2017). This might be mitigated by the reduced egg surface area exposed to the environment, compared with single eggs. Besides P. brassicae, only the closely‐related P. cheiranthi feeding on Crambe sp. and A. crataegi evolved to oviposit eggs in groups within the Pieridae family. In general, most butterflies deposit eggs singly (Stamp, 1980).
Fig. 4.
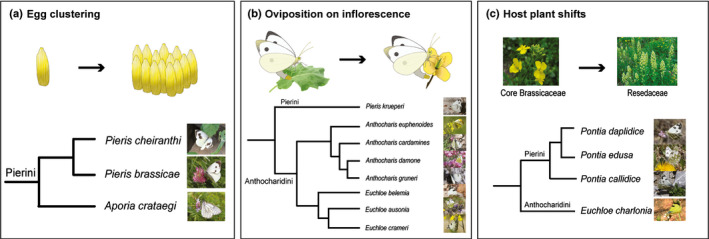
Examples of possible counter‐adaptations to egg‐killing hypersensitive response (HR)‐like necrosis of the Pierinae butterfly clade: egg clustering (a), oviposition on inflorescence (b), and host plant shifts to non‐HR expressing species (c). Phylogenetic relationships are according to Wiemers et al. (2020), and oviposition traits and host plants were retrieved from Tolman (Tolman, 2001). Photo credits: Matt Rowlings and creative common license.
Within the Anthocharidini, the majority of species evolved to oviposit on flower buds instead of leaves (Fig. 4) (Tolman, 2001). Inflorescent organs seem unlikely to develop an HR‐like. When collecting A. cardamines eggs from the inflorescences of Cardamine spp. we did not observe any signs of necrosis (N. E. Fatouros, pers. obs.). A few Euchloe species of the Anthocharidini colonized non‐HR expressing species belonging to Lineage III (E. penia) or Resedaceae (E. charlonia) (Tolman, 2001), which might have enabled them to oviposit on leaves again (Fig. 4). From the Pierini, only P. krueperi seem to have evolved to lay eggs on flower buds. We observed P. napi to lay eggs on inflorescences of flowering B. nigra plants (N. E. Fatouros, N. Bassetti, pers. obs.) but other records are not known so far. It would be interesting to further study the evolution of oviposition on inflorescence in the Pierinae, both on a macro‐ and microevolutionary scale.
After the first shift from Fabaceae to Brassicaceae, some butterfly species have shifted to plants of other families again. The closely related Pontia spp., for example, colonized plants from the Resedaceae and Cleomaceae and A. crataegi the Rosaceae. Within the Pieris spp., many are abundant in nature on species of the Brassiceae clade. Only two butterfly species specialized to plant species outside the Brassiceae: P. ergane feeds on Aethionema spp. and the Southern small white, P. mannii, on Iberis spp. Egg wash of the latter was shown to induce a strong necrosis in B. nigra (Fig. 2). So far, we have not observed that eggs of non‐brassicaceous feeding species (e.g. A. crataegi or G. rhamni) induce HR‐like in their preferred host plants, Prunus spp. or Rhamnus spp., respectively. However, we cannot exclude that plants in those families have developed ways to defend against Pieridae eggs.
Molecular and cellular responses to insect eggs
When the response to Pieris eggs was first described in B. nigra some 30 yr ago (Shapiro & De Vay, 1987), the induction of cell death was only known from biotrophic pathogens, whose spread is limited by the death of cells. It is now clear that cell death is a common phenomenon with many different causes, that can be induced by several different biotic interactors, including insects and nematodes (Balint‐Kurti, 2019). In our study, we found that HR‐like cell death is induced in the Brassiceae tribe by P. brassicae egg wash, and in B. nigra by all Pierinae species tested. To understand if the mechanism of this response is shared between these different plant species, and in response to the different butterfly species, detailed knowledge on the molecular responses to eggs, genes that are involved in the detection and recognition, and elicitors of the response are required. An in‐depth molecular characterization of the Pierinae egg‐induced HR‐like compared to other microbial‐induced HR goes beyond the aim of this study. Nevertheless, we have attempted to start with a description of the molecular response to insect eggs by studying trypan blue‐stained cell death and accumulation of reactive oxygen species (ROS) in three plant species, and PR1 expression in B. nigra towards nine insect species. In this study, ROS accumulation and cell death were induced in all plant species tested, whereas the strong HR‐like necrosis and high PR1 expression was specific to B. nigra and to Pierinae insect species. It is possible that also in other species and accessions in the Brassicaceae that we have not investigated closely, a general immune response lacking a strong cell death is activated by Pieris eggs, as was shown for A. thaliana (Col‐0) (Gouhier‐Darimont et al., 2013, 2019; Stahl et al., 2020; Valsamakis et al. 2020). Our data suggest that the strong HR‐like necrosis is always accompanied by ROS and high PR1 expression. However, because our histochemical stainings involved only three plant species (B. nigra, B. oleracea, C. hispanica), our observations may have been confounded with possible plant interspecific variation in the H2O2 and cell death‐inducing pathways. To understand whether the different species in which P. brassicae egg wash induce cell death share the same or similar mechanisms, requires the identification of genes involved in egg detection and downstream defence response activation in the responsive plant species identified in this study. At the moment, we are undertaking genetic studies to identify putative plant receptors required for perception of Pieris eggs in different Brassica spp.
Elicitor of HR‐like specific to Pierinae eggs
Although induction of strong HR‐like necrosis and high levels of PR1 gene expression in B. nigra was specific to Pieris and Anthocharis species, neither the non‐Pierinae butterflies nor the moth species tested induced a strong HR‐like necrosis on B. nigra (Figs 1, 2; Table S6). This suggests that the elicitor for HR‐like necrosis is one or several molecules found only in Pierinae eggs, rather than a general molecule present in (all) butterfly eggs. The differences in the severity of HR‐like necrosis elicitation between different Pierinae species could either be caused by quantitative differences of these elicitor(s), or by changes in their chemical composition. In A. thaliana, eggs from distantly related insect species were recently shown to release phosphatidylcholines (PCs) that induce a general immune response (i.e. pattern‐triggered immunity) involving salicylic acid and H2O2 accumulation (Little et al., 2007; Gouhier‐Darimont et al., 2013, 2019; Stahl et al., 2020). A lectin receptor kinase, LecRK‐I.8, might be involved in early perception of eggs from two widely divergent species, P. brassicae and Spodoptera littoralis (Gouhier‐Darimont et al., 2019). Interestingly, low PR1 expression was induced by egg wash of Coliadinae butterflies and P. xylostella in B. nigra also in our experiments. These results support a model where a general egg molecule (PCs) is detected by many plants (including A. thaliana and B. nigra) and a Pierinae‐specific egg‐associated molecular pattern (EAMP) may be detected specifically by the Brassiceae tribe. This would be similar to the detection of microbe‐associated molecular patterns (MAMPs) by the plant immune system (van der Burgh & Joosten, 2019). An exciting next step would be the identification of the Pierinae‐specific elicitor(s). Currently, we are analyzing the chemical composition of egg wash from different butterfly species to identify the compounds inducing HR‐like necrosis.
In conclusion, we provide a first attempt to disentangle the evolution of HR‐like in the Brassicales and show that various Brassicaceae plants can mount an HR‐like induced by P. brassicae eggs and that this trait might be under similar selective pressures as plant defences against feeding insects. A coevolutionary arms‐race between eggs from species of the Pierinae and plant species within the Brassiceae clade is likely to have occurred. Plants within this clade make use of necrotic lesions to lower egg survival and in this way might have evolved a new mechanism, possibly co‐opted from pre‐existing plant immunity mechanisms, to combat eggs of specialist herbivores adapted to their host plants’ toxins.
Author contributions
EG, LC, NB, SM, PV, MES and NEF planned and designed the research; ED, LC, NB, SM, PV, GB and NEF performed the experiments and/or analyzed the data; EG, LC, NB, SM, PV, EHP, RG, MES and NEF executed data interpretation; EG, LC, NB and NEF wrote the manuscript with contributions by SM, PV, EHP, RG and MES; and all authors read and drafted the final version. EG and LC contributed equally to this work.
Supporting information
Fig. S1 Leaves from B. nigra treated with egg wash of different butterfly species and controls inducing or not a HR‐like necrosis.
Fig. S2 Quantification of HR necrosis and histochemical staining of reactive oxygen species and cell death in B. nigra, B. oleracea and C. hispanica leaves upon oviposition of P. brassicae egg clutches.
Methods S1 Butterflies and moths.
Methods S2 Preparation of egg wash.
Methods S3 RNA isolation and real‐time qPCR analysis of PR1 genes.
Methods S4 Histochemical staining.
Methods S5 Pieris spp. egg survival assessed by field survey.
Methods S6 Phylogenetic tree construction of Brassicales.
Table S1 Used plant species in screening of HR‐like necrosis by P. brassicae eggs or egg wash.
Table S2 Origin of natural and reared butterfly populations used in the study.
Table S3 Primer sequences and annealing temperature used in PR‐1 real‐time qPCR.
Table S4 Reference numbers of genes used for generating the phylogenetic tree of Brassicales plant species used in this study.
Table S5 Summary of HR‐like necrosis induced by P. brassicae egg wash in different Brassicaceae and Cleomaceae species
Table S6 HR‐like necrosis (score 0–3) expressed by B. nigra plants elicited by different butterfly species.
Table S7 HR‐like necrosis (score 0–3) expressed by B. nigra plants elicited by different butterfly and moth species.
Table S8 Relative expression of PR1 gene upon treatment with egg wash of different butterflies/moths and MES buffer as control.
Table S9 Quantification of HR‐like necrosis in B. nigra, B. oleracea and C. hispanica leaves upon oviposition of P. brassicae egg clutches (10 eggs).
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank the employees of Unifarm (Wageningen University and Research) for rearing and caring of the plants used in the experiment. We are thankful to Pieter Rouweler, André Gidding and Frans van Aggelen for rearing the Dutch Pieris butterflies used in the experiment. Guusje Bonnema (WUR, Plant Breeding), and the Leibniz‐Institut für Pflanzengenetik und Kulturpflanzenforschung (Germany) are thanked for the seeds. Furthermore, we thank Miltos Tsiantis from the Department of Comparative Development and Genetics, Max Planck Institute for Plant Breeding Research for kindly providing C. hirsuta seeds, used as host plants for A. cardamines, and Niclas Backstrom for collecting and shipping L. sinapis eggs and pupae. The authors thank Marcel Dicke and Monika Hilker for reading and commenting on an earlier version of the manuscript. This research has been made possible by funding of the Dutch Research Council (NWO) to NEF (NWO/TTW VIDI 14854 and connected Aspasia).
Data availability
Data available in the Supporting Information.
References
- Adler FR, Karban R. 1994. Defended fortresses or moving targets? Another model of inducible defenses inspired by military metaphors. American Naturalist 144: 813–832. [Google Scholar]
- Al‐Shehbaz IA. 2012. A generic and tribal synopsis of the Brassicaceae (Cruciferae). Taxon 61: 931–954. [Google Scholar]
- Balbyshev NF, Lorenzen JH. 1997. Hypersensitivity and egg drop: a novel mechanism of host plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae). Journal of Economic Entomology 90: 652–657. [Google Scholar]
- Balint‐Kurti P. 2019. The plant hypersensitive response: concepts, control and consequences. Molecular Plant Pathologist 20: 1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra JX. 2015. Macroevolutionary and geographical intensification of chemical defense in plants driven by insect herbivore selection pressure. Current Opinion in Insect Science 8: 15–21. [DOI] [PubMed] [Google Scholar]
- Beilstein MA, Al‐Shehbaz IA, Kellogg EA. 2006. Brassicaceae phylogeny and trichome evolution. American Journal of Botany 93: 607–619. [DOI] [PubMed] [Google Scholar]
- Beilstein MA, Al‐Shehbaz IA, Mathews S, Kellogg EA. 2008. Brassicaceae phylogeny inferred from phytochrome A and ndhF sequence data: tribes and trichomes revisited. American Journal of Botany 95: 1307–1327. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. 1983. Coumarins and caterpillars: a case for coevolution. Evolution 37: 163–179. [DOI] [PubMed] [Google Scholar]
- Bittner N, Trauer‐Kizilelma U, Hilker M. 2017. Early plant defence against insect attack: involvement of reactive oxygen species in plant responses to insect egg deposition. Planta 245: 993–1007. [DOI] [PubMed] [Google Scholar]
- Braby MF, Trueman JW. 2006. Evolution of larval host plant associations and adaptive radiation in pierid butterflies. Journal of Evolutionary Biology 19: 1677–1690. [DOI] [PubMed] [Google Scholar]
- van der Burgh AM, Joosten MHAJ. 2019. Plant immunity: thinking outside and inside the box. Trends in Plant Science 24: 587–601. [DOI] [PubMed] [Google Scholar]
- Clark BR, Faeth SH. 1998. The evolution of egg clustering in butterflies: a test of the egg desiccation hypothesis. Evolutionary Ecology 12: 543–552. [Google Scholar]
- Cohen JA. 1985. Differences and similarities in cardenolide contents of queen and monarch butterflies in Florida and their ecological and evolutionary implications. Journal of Chemical Ecology 11: 85–103. [DOI] [PubMed] [Google Scholar]
- Despres L, David JP, Gallet C. 2007. The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecology & Evolution 22: 298–307. [DOI] [PubMed] [Google Scholar]
- Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy S, Clement SL, Williamson RT, Carney JR, DeVilbiss ED. 2000. Bruchins: insect‐derived plant regulators that stimulate neoplasm formation. Proceedings of the National Academy of Sciences, USA 97: 6218–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edger PP, Heidel‐Fischer HM, Bekaert M, Rota J, Glöckner G, Platts AE, Heckel DG, Der JP, Wafula EK, Tang M et al. 2015. The butterfly plant arms‐race escalated by gene and genome duplications. Proceedings of the National Academy of Sciences, USA 112: 8362–8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich PR, Raven PH. 1964. Butterflies and plants: a study in coevolution. Evolution 18: 586–608. [Google Scholar]
- Erb M, Robert CAM. 2016. Sequestration of plant secondary metabolites by insect herbivores: molecular mechanisms and ecological consequences. Current Opinion in Insect Science 14: 8–11. [DOI] [PubMed] [Google Scholar]
- Fatouros NE, Cusumano A, Danchin EGJ, Colazza S. 2016. Prospects of herbivore egg‐killing plant defenses for sustainable crop protection. Ecology and Evolution 6: 6906–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros NE, Paniagua Voirol LR, Drizou F, Doan QT, Pineda A, Frago E, van Loon JJ. 2015. Role of Large Cabbage White butterfly male‐derived compounds in elicitation of direct and indirect egg‐killing defenses in the black mustard. Frontiers in Plant Sciences 6: 794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros NE, Pineda A, Huigens ME, Broekgaarden C, Shimwela MM, Figueroa IA, Verbaarschot P, Bukovinszky T. 2014. Synergistic effects of direct and indirect defences on herbivore egg survival in a wild crucifer. Proceedings of the Royal Society of London. Series B: Biological Sciences. 281: 20141254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtzlaff V, Oberlander J, Geiselhardt S, Hilker M, Kunze R. 2016. Pre‐exposure of Arabidopsis to the abiotic or biotic environmental stimuli "chilling" or "insect eggs" exhibits different transcriptomic responses to herbivory. Scientific Reports 6: 28544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza R, Vera J, Cardona C, Barcenas N, Singh SP. 2001. Hypersensitive response of beans to Apion godmani (Coleoptera: Curculionidae). Journal of Economic Entomology 94: 958–962. [DOI] [PubMed] [Google Scholar]
- Geuss D, Stelzer S, Lortzing T, Steppuhn A. 2017. Solanum dulcamara's response to eggs of an insect herbivore comprises ovicidal hydrogen peroxide production. Plant, Cell & Environment 40: 2663–2677. [DOI] [PubMed] [Google Scholar]
- Gouhier‐Darimont C, Schmiesing A, Bonnet C, Lassueur S, Reymond P. 2013. Signalling of Arabidopsis thaliana response to Pieris brassicae eggs shares similarities with PAMP‐triggered immunity. Journal of Experimental Botany 64: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouhier‐Darimont C, Stahl E, Glauser G, Reymond P. 2019. The Arabidopsis Lectin Receptor Kinase LecRK‐I.8 is involved in insect egg perception. Frontiers of Plant Sciences 10: 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graser G, Schneider B, Oldham NJ, Gershenzon J. 2000. The methionine chain elongation pathway in the biosynthesis of glucosinolates in Eruca sativa (Brassicaceae). Archives of Biochemistry and Biophysics 378: 411–419. [DOI] [PubMed] [Google Scholar]
- Griese E, Dicke M, Hilker M, Fatouros NE. 2017. Plant response to butterfly eggs: inducibility, severity and success of egg‐killing leaf necrosis depends on plant genotype and egg clustering. Scientific Reports 7: 7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griese E, Pineda A, Pashalidou FG, Iradi EP, Hilker M, Dicke M, Fatouros NE. 2020. Plant responses to butterfly oviposition partly explain preference‐performance relationships on different brassicaceous species. Oecologia 192: 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux R, Stahl E, Gouhier‐Darimont C, Kerdaffrec E, Jimenez‐Sandoval P, Santiago J, Reymond P. 2020. Arabidopsis natural variation in insect egg‐induced cell death reveals a role for LECTIN RECEPTOR KINASE‐I.1. Plant Physiology. doi: 10.1093/plphys/kiaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Liu J, Hao G, Zhang L, Mao K, Wang X, Zhang D, Ma T, Hu Q, Al‐Shehbaz IA et al. 2017. Plastome phylogeny and early diversification of Brassicaceae. BMC Genomics 18: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC, Sytsma KJ, Iltis HH. 2002. Phylogeny of Capparaceae and Brassicaceae based on chloroplast sequence data. American Journal of Botany 89: 1826–1842. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Witjes LMA, Benkirane M, Duyts H, Wagenaar R. 2007. Nutritional suitability and ecological relevance of Arabidopsis thaliana and Brassica oleracea as foodplants for the cabbage butterfly, Pieris rapae . Plant Ecology 189: 117–126. [Google Scholar]
- Heidel‐Fischer HM, Kirsch R, Reichelt M, Ahn S‐J, Wielsch N, Baxter SW, Heckel DG, Vogel H, Kroymann J. 2019. An insect counteradaptation against host plant defenses evolved through concerted neofunctionalization. Molecular Biology and Evolution 36: 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidel‐Fischer HM, Vogel H. 2015. Molecular mechanisms of insect adaptation to plant secondary compounds. Current Opinion in Insect Science 8: 8–14. [DOI] [PubMed] [Google Scholar]
- Hilker M, Fatouros NE. 2015. Plant responses to insect egg deposition. Annual Review of Entomology 60: 493–515. [DOI] [PubMed] [Google Scholar]
- Hilker M, Fatouros NE. 2016. Resisting the onset of herbivore attack: plants perceive and respond to insect eggs. Current Opinion in Plant Biology 32: 9–16. [DOI] [PubMed] [Google Scholar]
- Huang C‐H, Sun R, Hu Y, Zeng L, Zhang N, Cai L, Zhang Q, Koch MA, Al‐Shehbaz I, Edger PP et al. 2015. Resolution of Brassicaceae phylogeny using nuclear genes uncovers nested radiations and supports convergent morphological evolution. Molecular Biology and Evolution 33: 394–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara AY, Plotkin D, Espeland M, Meusemann K, Toussaint EFA, Donath A, Gimnich F, Frandsen PB, Zwick A, Dos Reis M et al. 2019. Phylogenomics reveals the evolutionary timing and pattern of butterflies and moths. Proceedings of the National Academy of Sciences, USA 116: 22657–22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little D, Gouhier‐Darimont C, Bruessow F, Reymond P. 2007. Oviposition by pierid butterflies triggers defense responses in Arabidopsis . Plant Physiology 143: 784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm SB, Brower LP. 1989. Evolutionary and ecological implications of cardenolide sequestration in the monarch butterfly. Experientia 45: 284–295. [Google Scholar]
- Nallu S, Hill JA, Don K, Sahagun C, Zhang W, Meslin C, Snell‐Rood E, Clark NL, Morehouse NI, Bergelson J et al. 2018. The molecular genetic basis of herbivory between butterflies and their host plants. Nature Ecology & Evolution 2: 1418–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñate‐Sánchez L, Vicente‐Carbajosa J. 2008. DNA‐free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Research Notes 1: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniagua Voirol LR, Valsamakis G, Lortzing V, Weinhold A, Johnston PR, Fatouros NE, Kunze R, Hilker M. 2020. Plant responses to insect eggs are not induced by egg‐associated microbes, but by a secretion attached to the eggs. Plant, Cell & Environment 43: 1815–1826. [DOI] [PubMed] [Google Scholar]
- Pashalidou FG, Fatouros NE, Van Loon JJA, Dicke M, Gols R. 2015a. Plant‐mediated effects of butterfly egg deposition on subsequent caterpillar and pupal development, across different species of wild Brassicaceae. Ecological Entomology 40: 444–450. [Google Scholar]
- Pashalidou FG, Frago E, Griese E, Poelman EH, van Loon JJA, Dicke M, Fatouros NE. 2015b. Early herbivore alert matters: plant‐mediated effects of egg deposition on higher trophic levels benefit plant fitness. Ecology Letters 18: 927–936. [DOI] [PubMed] [Google Scholar]
- Petzold‐Maxwell J, Wong S, Arellano C, Gould F. 2011. Host plant direct defence against eggs of its specialist herbivore, Heliothis subflexa . Ecological Entomology 36: 700–708. [Google Scholar]
- R Core Team . 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rask L, Andréasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J. 2000. Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Molecular Biology 42: 93–114. [PubMed] [Google Scholar]
- Reymond P. 2013. Perception, signaling and molecular basis of oviposition‐mediated plant responses. Planta 238: 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Suzuki Y, Sogawa K. 1996. An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, Sogatella furcifera (Horvath) (Homoptera: Delphacidae). Applied Entomology and Zoology 31: 467–473. [Google Scholar]
- Shapiro AM, De Vay JE. 1987. Hypersensitivity reaction of Brassica nigra L. (Cruciferae) kills eggs of Pieris butterflies (Lepidoptera, Pieridae). Oecologia 71: 631–632. [DOI] [PubMed] [Google Scholar]
- Stahl E, Brillatz T, Ferreira Queiroz E, Marcourt L, Schmiesing A, Hilfiker O, Riezman I, Riezman H, Wolfender J‐L, Reymond P. 2020. Phosphatidylcholines from Pieris brassicae eggs activate an immune response in Arabidopsis . eLife 9: e60293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp NE. 1980. Egg deposition patterns in butterflies: why do some species cluster their eggs rather than deposit them singly? American Naturalist 115: 367–380. [Google Scholar]
- Swain T. 1977. Secondary compounds as protective agents. Annual Review of Plant Physiology 28: 479–501. [Google Scholar]
- Tolman T. 2001. Photographic Guide to the Butterflies of Britain & Europe. Oxford, UK: Oxford University Press. [Google Scholar]
- Tolman T, Lewington R. 2009. Collins butterfly guide: The most complete field guide to the butterflies of Britain and Europe. London, UK: Harper Collins. [Google Scholar]
- Valsamakis G, Bittner N, Fatouros Nina E., Kunze R, Hilker M, Lortzing V. 2020. Priming by timing: Arabidopsis thaliana adjusts its priming response to Lepidoptera eggs to the time of larval hatching. Frontiers in Plant Science 11. doi: 10.3389/fpls.2020.619589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg N, Rota J, Braby MF, Pierce NE, Wheat CW. 2014. Revised systematics and higher classification of pierid butterflies (Lepidoptera: Pieridae) based on molecular data. Zoologica Scripta 43: 641–650. [Google Scholar]
- Wheat CW, Vogel H, Wittstock U, Braby MF, Underwood D, Mitchell‐Olds T. 2007. The genetic basis of a plant insect coevolutionary key innovation. Proceedings of the National Academy of Sciences, USA 104: 20427–20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemers M, Chazot N, Wheat CW, Schweiger O, Wahlberg N. 2020. A complete time‐calibrated multi‐gene phylogeny of the European butterflies. ZooKeys 938: 97–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund C, Friberg M. 2009. The evolutionary ecology of generalization: among‐year variation in host plant use and offspring survival in a butterfly. Ecology 90: 3406–3417. [DOI] [PubMed] [Google Scholar]
- Winde I, Wittstock U. 2011. Insect herbivore counteradaptations to the plant glucosinolate–myrosinase system. Phytochemistry 72: 1566–1575. [DOI] [PubMed] [Google Scholar]
- Windsor AJ, Reichelt M, Figuth A, Svatoš A, Kroymann J, Kliebenstein DJ, Gershenzon J, Mitchell‐Olds T. 2005. Geographic and evolutionary diversification of glucosinolates among near relatives of Arabidopsis thaliana (Brassicaceae). Phytochemistry 66: 1321–1333. [DOI] [PubMed] [Google Scholar]
- Wittstock U, Agerbirk N, Stauber EJ, Olsen CE, Hippler M, Mitchell‐Olds T, Gershenzon J, Vogel H. 2004. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proceedings of the National Academy of Sciences, USA 101: 4859–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Lenman M, Falk A, Rask L. 1992. The glucosinolate‐degrading enzyme myrosinase in Brassicaceae is encoded by a gene family. Plant Molecular Biology 18: 387–398. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xu J, Leng Y, Xiong G, Hu J, Zhang G, Huang L, Wang L, Guo L, Li J et al. 2014. Quantitative trait loci identification, fine mapping and gene expression profiling for ovicidal response to whitebacked planthopper (Sogatella furcifera Horvath) in rice (Oryza sativa L.). BMC Plant Biology 14: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Leaves from B. nigra treated with egg wash of different butterfly species and controls inducing or not a HR‐like necrosis.
Fig. S2 Quantification of HR necrosis and histochemical staining of reactive oxygen species and cell death in B. nigra, B. oleracea and C. hispanica leaves upon oviposition of P. brassicae egg clutches.
Methods S1 Butterflies and moths.
Methods S2 Preparation of egg wash.
Methods S3 RNA isolation and real‐time qPCR analysis of PR1 genes.
Methods S4 Histochemical staining.
Methods S5 Pieris spp. egg survival assessed by field survey.
Methods S6 Phylogenetic tree construction of Brassicales.
Table S1 Used plant species in screening of HR‐like necrosis by P. brassicae eggs or egg wash.
Table S2 Origin of natural and reared butterfly populations used in the study.
Table S3 Primer sequences and annealing temperature used in PR‐1 real‐time qPCR.
Table S4 Reference numbers of genes used for generating the phylogenetic tree of Brassicales plant species used in this study.
Table S5 Summary of HR‐like necrosis induced by P. brassicae egg wash in different Brassicaceae and Cleomaceae species
Table S6 HR‐like necrosis (score 0–3) expressed by B. nigra plants elicited by different butterfly species.
Table S7 HR‐like necrosis (score 0–3) expressed by B. nigra plants elicited by different butterfly and moth species.
Table S8 Relative expression of PR1 gene upon treatment with egg wash of different butterflies/moths and MES buffer as control.
Table S9 Quantification of HR‐like necrosis in B. nigra, B. oleracea and C. hispanica leaves upon oviposition of P. brassicae egg clutches (10 eggs).
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
Data available in the Supporting Information.


