Introduction
Proteins are the true machines of life. Protein enzymes carry out virtually all complex chemical transformations in living organisms, such as nucleic acid synthesis and replication, posttranslational modifications, carbohydrate and lipid metabolism, hormone biosynthesis, proteolysis, and many more. Proteins provide functional context to cells, tissues, and organisms in the form of receptors, signaling cascades, channels, and transporters. Proteins are also major structural components of the cytoskeleton and extracellular matrices. The pathobiology of virtually all diseases can be understood as the malfunctioning of any one or more of this myriad of actions that proteins perform in a spatially and temporally highly orchestrated manner. By contrast, many diseases, especially cancers, continue to be viewed in a nucleic acid-based genomic and mutation-centric manner, but is this good enough?
Proteomics is the global study of proteins in an organism, tissue, cell, or subcellular compartment that captures both quantity and context [1]. Which proteins are up- or down-regulated in a disease? What types of protein modifications occur? What binds to what and why? Who signals to whom? We now know that, under physiologic conditions, the quantitative correlation between mRNA transcript abundance and corresponding, actually present protein product is poor at best and even frequently anti-correlated. Even worse, dynamic oscillations or other time-dependent (e.g., circadian) changes at protein level are often not present at mRNA level [2]. Why is that so? Synthesis, half-life, and degradation dynamics between mRNAs and proteins are regulated differently, and that can uncouple mRNA and protein worlds. Given all this, we posit that it is time to develop Proteome-Based Diagnostics as the “next frontier in Precision Medicine.”
Precision Pathology – the central science in precision medicine
As a thought experiment for why proteomics “sees” more than nucleic acid-centered approaches, let’s consider two identical twins (Fig. 1 A): their somatic genomes are the same (and genomic diagnostics will not be able to distinguish between the two individuals), yet the individual fingerprint of each twin is unique and different. As criminology teaches us, “macromolecular phenotyping” by fingerprinting will indeed distinguish the two identical twins. By analogy, let’s imagine two cancer cells with essentially the same genomic alterations. Even though sequencing would find them identical, the biochemical signaling states (“functional proteomic states”) of each cancer may be vastly different and thus their sensitivity and response to molecularly targeted drugs would also expected to be different. The key insight is that we need alternative, biochemically sound, and functional ways to interrogate the activity states of cancers.
Figure 1.
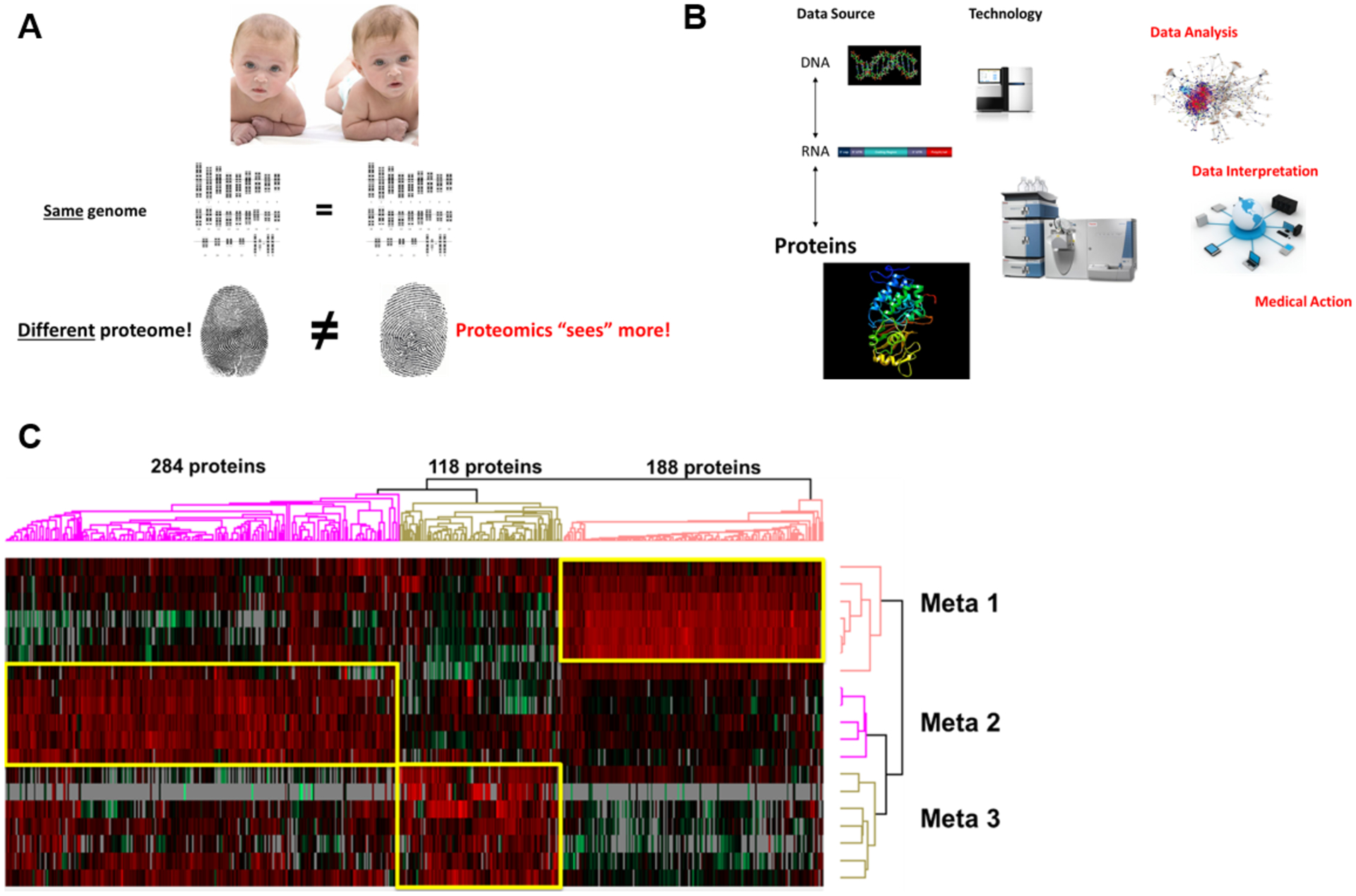
(A) The identical twin thought experiment to illustrate why proteomics “sees” more than nucleic acid-only approaches. (B) An integrated multi-omic diagnostic approach to human disease requires proteomics. (C) Example of Proteome-Based Diagnostics and proteomic classification of 3 types of colon cancer metastases (Meta 1–3) that are characterized by unique sets of upregulated proteins.
Modern medicine is essentially composed of two processes: making a correct diagnosis (i.e., determining precisely which disease entity a patient has) and choosing an optimal therapy (i.e., determining how to treat this disease). Historically, pathology has been a primarily diagnostic discipline and thus concerned mostly with the former. More than a century of histomorphologic and microscopic description of cancers, immunohistochemical typing, and even DNA/RNA-based molecular diagnostics can be understood in this context. Over the last few years, however, the role of pathology has been rapidly expanding into the realms of therapy decision making, patient response monitoring, and resistance detection. Pathologists have been characterized as the “Physician’s Physician”, and Sir William Osler’s famous quote seems more fitting than ever in this age of diagnostic empowerment: “As is your Pathology, so is your Medicine.” Virtually no new therapeutics are now approved without a closely tied “companion” diagnostic assay that measures almost always either the direct target protein (e.g., PD-L1 for checkpoint inhibitors) or proteins related to specific targeted pathways (e.g., a kinase cascade pathway). Thus precision medicine is in urgent need of expanding its methodological toolbox to lead the way and merge diagnostic and therapeutic concepts. We argue that Proteome-Based Pathology is a major step in this direction.
Advances in proteomics
The Cancer Genome Atlas (TCGA), coordinated by the US National Cancer Institute (NCI), has been instrumental for our genomic understanding across many cancer types. A new and transformative initiative, the Clinical Proteomic Tumor Analysis Consortium (CPTAC), is currently underway and aims to catalogue the proteomic changes of numerous cancer types [3]. Additionally, CPTAC is a platform for the development of proteomic technologies (both deep proteomic profiling and targeted quantitative protein assays) and computational approaches to analyze high-dimensional proteomic and integrated proteogenomic data. Furthermore, the Human Proteome Organization (HUPO) has recently launched the Pathology Pillar of the Human Proteome Project (https://www.hupo.org/Pathology-Pillar).
Mass spectrometry-based proteomics [4] has seen dramatic improvements in instrumentation over the past 5 years (sensitivity and robustness) that make installation of such systems in a clinical environment feasible [5]. This includes liquid chromatography front-ended as well as MALDI imaging-based instruments [6]. These instruments are much more powerful and sophisticated than the simpler small molecule analytical mass specs that have entered the clinical diagnostic labs over a decade ago for monitoring drugs of abuse, immunosuppressants, or vitamin D. A significant advantage over single- or multiplex antibody-dependent detection methods is that direct protein mass spectrometry detects analytes without cognate antibody requirement and is thus unbiased (Table 1) [7]. An exciting recent development is the scaling down of mass spectrometric sample input to ever smaller amounts (including single cells or even organelles) [8]. This will open enormous opportunities for understanding biochemical plasticity and diversity within cancers, their microenvironment, and between primary site cancers and metastases.
Table 1.
Types of protein-directed diagnostic approaches and their dependence on an antigen-antibody interaction
| In liquids (whole blood, serum, plasma, etc.) | In tissues | Dependence on availability of specific antibodies | Features |
|---|---|---|---|
|
|
No |
|
|
|
Yes |
|
|
|
No |
|
ALT, alanine transaminase; GGT, gamma-glutamyltransferase; AChE, acetylcolinesterase; LC-MS, liquid chromatography-mass spectrometry; MALDI-MS, matrix-assisted laser desorption/ionisation-mass spectrometry
One of the most attractive features of proteome-based diagnostics is access to activation states of cell signaling by means of measuring both total proteomes and post-translationally modified proteomes (foremost phosphorylated proteins as a measure of on or off activity state, but also glycosylation [9], methylation, acetylation, ubiquitinylation, etc.). It is important to remember that none of these features are ever accessible from genomic or transcriptomic analyses!
Proteomics is moving towards functional proteome-based pathology. Imagine a clinical microbiology lab that were to only speciate microorganisms cultured from patients but were not to determine antibiotic susceptibility/resistance patterns based on functional growth assays? Unfortunately, this is essentially where current cancer diagnostics is stuck, lacking the ability to test drug response in predictive functional assays. Here proteomics may come to the rescue. Picture a scenario where a patient’s cancer is first biopsied at time of diagnosis. The biopsy is characterized with conventional genomic and transcriptomic profiling (to identify coding mutations, indels, copy number changes, putative gene fusions, etc.). In addition, the biopsy is also characterized with proteome-based technologies [10] to classify cancers and metastases into proteomic subtypes (Figs. 1 B and C), to assess which signaling pathways are on or off (and to what extent), which genomically predicted mutations or fusions actually make it into the protein realm and are indeed present, and which neoproteins/neoepitopes are expressed in a tumor. Based on such integrated proteogenomic analysis, quantitative predictions are made about the most sensitive molecular pathway targets that can be manipulated with pharmacologic agents (small molecules, antibodies, CAR T cells, etc.) and intrinsic resistance or evasion mechanisms already present within the cancer.
Another powerful application of proteome-based diagnostics is the vast realm of diseases with poorly understood etiologies and pathophysiologies. For example, there are more than one hundred autoimmune diseases that are inadequately defined molecularly. Proteome-base approaches can further differentiate individual patients within a class of autoimmune disease, such as systemic lupus erythematosus [11]. Proteomics is capable of cataloguing the entire autoantigen-ome (the totality of proteins that can become antigenic) of individual patients by analyzing both tissue and circulating serum antibodies. Patterns and time evolution of antigens and antibody signatures may be diagnostic and also predict outcome and help discover new targets for therapeutic development [12]. An excellent example of proteomics as part of an interventional clinical trial companion diagnostic is urinary proteome-based chronic kidney disease classifier CKD273 [13].
Proteome-Based Pathology: a prolific future in precision medicine
We are certain that a focus on proteomics and proteome-based diagnostics will have enormous impact on diagnostics, risk prediction [14–16], disease monitoring, and therapy selection in the immediate future. This will require long overdue modifications to our routine clinical practice (more fresh and frozen tissue biopsies, rather than the century-old paraffin wax embedding). There is also an education and training need: physicians must be educated about the possibilities (and limitations) of proteomics in medicine and how it complements with other molecular diagnostics. The technology is ready. Proteome-Based Diagnostics promises an unprecedented quantitative, biochemical, functional, and dynamic look at human disease. We believe that it will become the cornerstone of a new generation of companion diagnostics and that it will be a decisive element of clinical trials and drug development of the next decade.
Funding Statement
MHR acknowledges NCI R21 CA231109, NCI R21 CA251992, and funding from a Cycle for Survival Equinox innovation grant. This research was funded in part through the MSKCC NIH/NCI Cancer Center Support Grant P30 CA008748. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of Interest
VBR declares no conflicts of interest related to this study. JYW is founder and equity holder of Curandis. MHR is member of the Scientific Advisory Boards of Proscia, Trans-Hit, and Universal DX. None of these companies had any influence on support, design, execution, data analysis, or any other aspect of this paper. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Doll S, Gnad F, Mann M. The Case for Proteomics and Phospho-Proteomics in Personalized Cancer Medicine. Proteomics Clinical applications. 2019. Mar;13(2):e1800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang B, Wang J, Wang X, et al. Proteogenomic characterization of human colon and rectal cancer. Nature. 2014. Sep 18;513(7518):382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Paper about a nice example of the CPTAC consortium highlighting that mRNA and proteome worlds are often not correlated
- 3.Rodriguez H, Pennington SR. Revolutionizing Precision Oncology through Collaborative Proteogenomics and Data Sharing. Cell. 2018. Apr 19;173(3):535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aebersold R, Mann M. Mass-spectrometric exploration of proteome structure and function. Nature. 2016. Sep 15;537(7620):347–55. [DOI] [PubMed] [Google Scholar]
- 5.Poulos RC, Hains PG, Shah R, et al. Strategies to enable large-scale proteomics for reproducible research. Nature communications. 2020. Jul 30;11(1):3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan DJ, Spraggins JM, Caprioli RM. Protein identification strategies in MALDI imaging mass spectrometry: a brief review. Current opinion in chemical biology. 2019. Feb;48:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omenn GS, Lane L, Overall CM, et al. Research on The Human Proteome Reaches a Major Milestone: >90% of Predicted Human Proteins Now Credibly Detected, according to the HUPO Human Proteome Project. Journal of proteome research. 2020. Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budnik B, Levy E, Harmange G, et al. SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome biology. 2018. Oct 22;19(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Paper describing progress towards single cell proteomics
- 9.Rho JH, Roehrl MH, Wang JY. Glycoproteomic analysis of human lung adenocarcinomas using glycoarrays and tandem mass spectrometry: differential expression and glycosylation patterns of vimentin and fetuin A isoforms. The protein journal. 2009. May;28(3–4):148–60. [DOI] [PubMed] [Google Scholar]
- 10.Gillette MA, Satpathy S, Cao S, et al. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell. 2020. Jul 9;182(1):200–225.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Seminal paper describing proteogenomics and therapeutic vulnerabilities of lung adenocarcinoma
- 11.Rho JH, Zhang W, Murali M, et al. Human proteins with affinity for dermatan sulfate have the propensity to become autoantigens. The American journal of pathology. 2011. May;178(5):2177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Paper describing the concept of the autoantigen-ome and use of proteomics in autoimmunity
- 12.Yang Q, Bavi P, Wang JY, et al. Immuno-proteomic discovery of tumor tissue autoantigens identifies olfactomedin 4, CD11b, and integrin alpha-2 as markers of colorectal cancer with liver metastases. Journal of proteomics. 2017. Sep 25;168:53–65. [DOI] [PubMed] [Google Scholar]
- 13.Tofte N, Lindhardt M, Adamova K, et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinology. 2020. Apr;8(4):301–312. [DOI] [PubMed] [Google Scholar]; **Nice example of the use of a proteomic assay in the context of an interventional clinical trial
- 14.Tanaka A, Wang JY, Shia J, et al. Maspin as a Prognostic Marker for Early Stage Colorectal Cancer With Microsatellite Instability [Original Research]. Frontiers in Oncology. 2020. 2020-June-10;10(945):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Example of proteomics for prognostic outcome prediction in colorectal cancer
- 15.Tanaka A, Zhou Y, Ogawa M, et al. STAT1 as a potential prognosis marker for poor outcomes of early stage colorectal cancer with microsatellite instability. PLoS One. 2020;15(4):e0229252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka A, Zhou Y, Shia J, et al. Prolyl 4-hydroxylase alpha 1 protein expression risk-stratifies early stage colorectal cancer. Oncotarget. 2020. Feb 25;11(8):813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]


