Abstract
COVID-19 has varied cardiovascular manifestations including microvascular and macrovascular thrombi leading to multiorgan system injury and failure. This case describes a patient presenting with acute hypoxaemic respiratory failure from COVID-19 who abruptly developed a large thrombus in the right coronary artery leading to myocardial infarction. This case report reviews the ECG, heart catheterisation images prepercutaneous and postpercutaneous coronary intervention, critical care management and outcome in the context of the height of the COVID-19 pandemic in the Virginia area. A brief review of relevant literature regarding cardiovascular complications of COVID-19 is also provided. Unfortunately, the patient ultimately passed after 2 weeks of inability to wean off the ventilator.
Keywords: interventional cardiology, cardiovascular medicine, COVID-19, pneumonia (respiratory medicine)
Background
SARS-CoV-2 causes COVID-19 which has spread across the globe leading to more than two million deaths at the time of this writing. Pneumonia appears to be the most common presentation, but COVID-19 can also lead to acute respiratory distress syndrome, arrythmias, myocarditis, shock and thromboembolic complications such as pulmonary embolism and acute stroke. Acute coronary syndrome has also been seen in this population, and here we present a case of acute inferior/posterior ST segment elevation myocardial infarction (STEMI). Early recognition of these patients is paramount in order to improve patient morbidity and mortality as well as to minimise exposure to medical staff.
Case presentation
A 60-year-old Korean man with no significant medical history besides chronic lumbar radiculopathy status postlaminectomy in 2013 presented to our tertiary care facility with acute onset of lethargy and confusion. The patient described subjective fever, shortness of breath, cough, vague left-sided chest pain and intermittent diarrhoea for several days. Family revealed he had experienced fatigue for 7 days associated with anorexia and new-onset of apneic episodes when sleeping. He had no family history of heart disease and was not taking any medications. He had a remote history of tobacco use but quit 30 years ago. He drank 1–2 beers per week and denied any illicit drug use. He reported no allergies and no sick contacts. On arrival to the emergency room, the patient was lethargic and confused with a temperature of 37.2°C and hypoxic to 60% on room air. His exam revealed increased work of breathing and decreased breath sounds with bilateral crackles. Admission chest radiograph revealed extensive bilateral airspace disease (figure 1). Initial 12-lead ECG (figure 2) revealed sinus tachycardia with no ST segment deviation.
Figure 1.
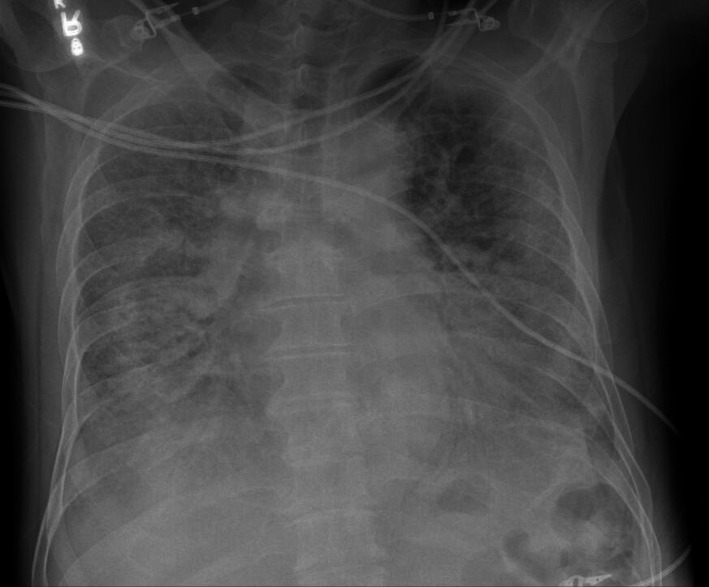
Admission chest radiograph: portable anterior–posterior chest radiograph showing extensive bilateral pulmonary infiltrates highly suggestive of COVID-19.
Figure 2.
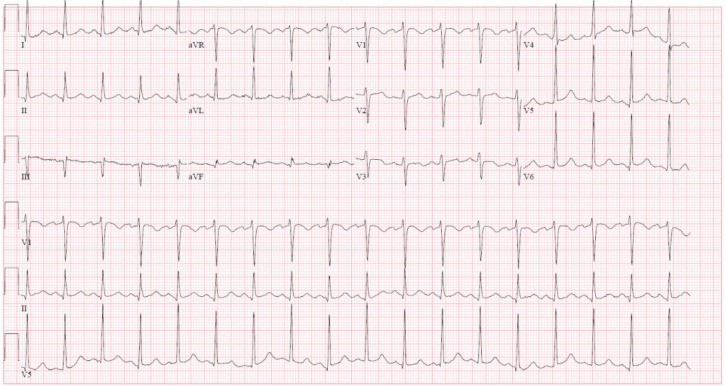
Admission ECG: sinus tachycardia with borderline left ventricular hypertrophy.
Due to refractory hypoxaemia, he was initially placed on high flow nasal cannula and subsequently self-proned. Overnight, the patient became restless with severe, typical angina. Repeat ECG (figure 3) showed an acute inferior/posterior STEMI. The patient was started on a heparin infusion, high-dose aspirin and high intensity statin. Due to increased oxygenation requirements, he was electively intubated in the cardiac intensive care unit (CICU) and taken to the cardiac catheterisation lab.
Figure 3.
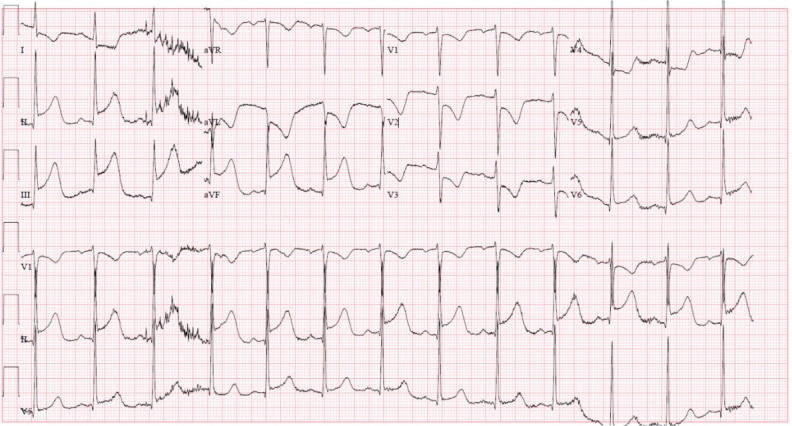
Intensive care unit ECG: new ST segment elevations in leads II, III and augmented vector foot (aVF) with reciprocal changes in leads I, augmented vector left (aVL) and V1 through V4, in addition to T wave inversions in aVL, and V2–3, reflecting an acute inferior/posterior ST segment elevation myocardial infarction.
Investigations
Initial laboratory studies were significant for white cell count of 11.94×109/L with manual absolute lymphocyte count of 0.60×109/L, platelet count of 433×109/L, aspartate transaminase 91 and alanine aminotransferase 82 U/L, total bilirubin 1.6 mg/dL, alkaline phosphatase 109 U/L. Additional laboratory studies revealed lactic acid 2.8 mmol/L, troponin 0.45 ng/mL, B-natriuretic peptide 68 pg/mL, ferritin 3966 ng/mL, C reactive protein 12.4 mg/dL, sedimentation rate 19 mm/hour, D-dimer 2.22 ug/mL, procalcitonin 0.07 ng/mL, negative alcohol level and negative drug screen. Rapid influenza A and B swab were negative. Haemoglobin A1c was 5.9%. Lipid panel showed cholesterol 204 mg/dL, high density lipoprotein 58 mg/dL, low density lipoprotein 98 mg/dL and triglycerides 241 mg/dL. Troponin peaked at 394 ng/mL. Respiratory viral panel resulted positive for SARS-CoV-2.
Differential diagnosis
The leading aetiology of his hypoxia was COVID-19 pneumonia. The supporting evidence for this diagnosis included his profound hypoxia on presentation, prodromal symptoms and bilateral airspace disease on his admission chest X-ray. In addition, this patient presented in March 2020 which corresponded to the peak of pandemic cases in the Virginia area. Bacterial pneumonia was also considered but his procalcitonin levels were low and his viral respiratory panel showed SARS-CoV-2 positivity. Acute heart failure exacerbation was considered but the patient was euvolemic with no known cardiac history. Pulmonary malignancy was also considered given his remote smoking history but his acute presentation and chest X-ray were less suggestive. Lastly, pulmonary embolus remained on the differential but seemed less likely given his positive respiratory viral panel. It is possible that this patient had a concomitant pulmonary embolus, but the patient was hypoxic and deemed to be too unstable for a CT scan of the chest on presentation. His acute coronary syndrome was later diagnosed due to positive ECG findings, troponin levels and confirmed thrombus on angiography.
Treatment
Angiography revealed an acute thrombotic occlusion of the distal right coronary artery. Aspiration thrombectomy yielded large casts of thrombus (figure 4). A 4.5×16 mm Synergy drug-eluting stent was deployed (figure 5). Left main and circumflex coronary arteries were without disease. Left anterior descending had moderate disease. The left ventriculogram revealed an ejection fraction of 55% with inferobasilar hypokinesis and a left ventricular end diastolic volume of 16 mm Hg. The patient was treated with 25 µg/kg/min bolus of intravenous tirofiban and kept on a continuous infusion at 0.15 µg/kg/min for twelve hours. Patient was started on aspirin 81 mg daily, ticagrelor 90 mg two times per day and atorvastatin 80 mg daily and transferred back to the CICU for further management.
Figure 4.
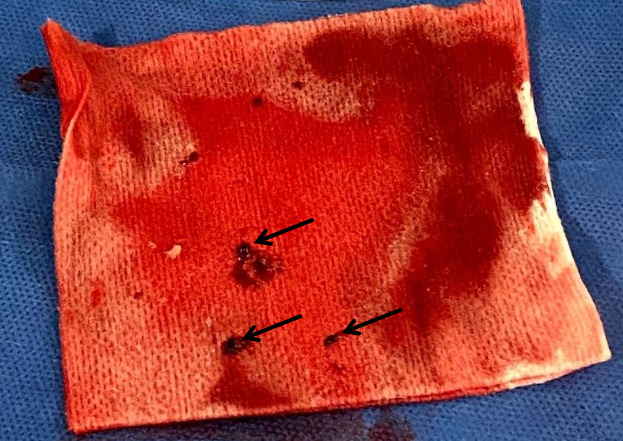
Thrombus: postprocedure photo of thrombus (black arrows) removed from right coronary artery with atherectomy.
Figure 5.
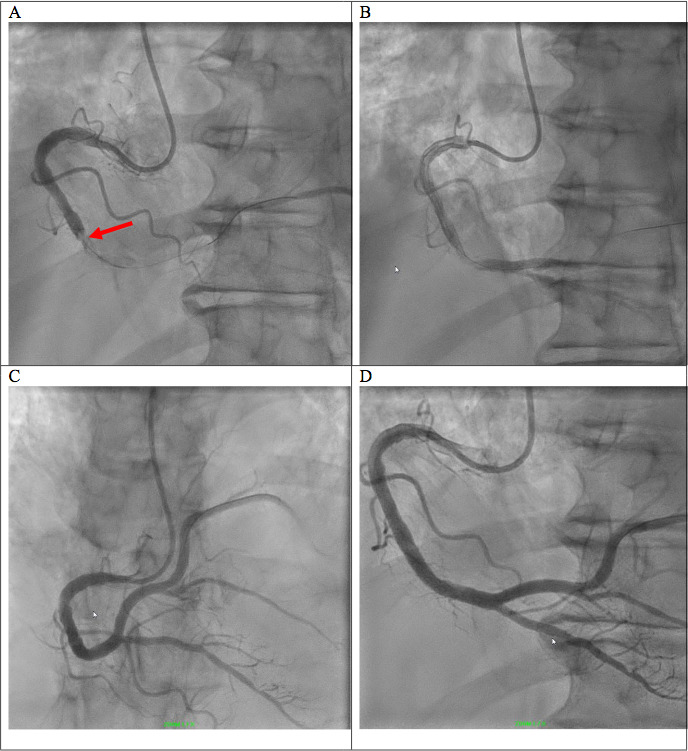
Heart catheterisation with angiograms: preatherectomy (A) and postatherectomy (B) angiogram of right-sided circulation, in the left anterior oblique view 20° cranial 2°, with initial complete occlusion of the distal segment (red arrow) then partial restoration of flow after thrombus removal. Poststent deployment angiogram in the right anterior oblique view 1° cranial 36° (C) and in the left anterior oblique view 20° cranial 2° (D).
Outcome and follow-up
The patient was extubated 1-day postprocedure but became profoundly hypoxic several hours later, ultimately requiring reintubation. The patient was treated according to the ARDSNet protocol. Intermittent 16-hour proning trials appeared to improve his oxygenation; however, he became profoundly hypoxic with supination. He was treated with dexamethasone, therapeutic dose enoxaparin and a course of emergency use remdesivir. He was managed in the CICU for more than 2 weeks, and he was ultimately compassionately extubated after family discussions. The patient died shortly after extubation.
Discussion
As the COVID-19 pandemic sweeps across the globe, our understanding of its myriad manifestations, pathophysiology and potential therapeutic options is concomitantly evolving. Although COVID-19 was initially thought to be a primarily respiratory illness, more recent evidence suggests it causes cardiac, vascular and systemic derangements.1 One possible mechanism driving these poor outcomes is the hypercoagulability of the disease with respect to microvascular or macrovascular thrombi affecting multiple organ systems. Studies from both China and the USA suggest markers such as D-dimer may be associated with increased mortality in hospitalised patients with COVID-19, with follow-up studies showing complications related to thrombosis in the lungs and brain as well as clotting of renal replacement and extracorporeal membrane oxygenation circuits.2–4 COVID-19 has significantly disrupted management of acute cardiovascular disease. Data from China showed that an elevation in troponin, with or without previous underlying cardiovascular conditions, was associated with increased mortality.5 6 Primary cardiac manifestations of COVID-19 were also examined in an Italian study where 85% of patients presenting with STEMI were eventually found to be COVID-19 positive. Interestingly, up to 40% of patients had no culprit lesion identified on angiogram.7 Recently, a case series from New York City on COVID-19 patients with STEMI revealed that all of these patients also presented with an elevated D-dimer with 27% requiring percutaneous coronary intervention.8 Synergising this data suggests that the increased thrombotic risk of COVID-19 could manifest as acute coronary thrombosis and STEMI. Management of these patients initially presented logistical challenges with respect to prompt intervention, although this has improved as protocols and procedures have evolved.9 In our case report, the patient presented with acute STEMI while in the intensive care unit. Thus, the patient received dual antiplatelet therapy, high-dose statin, heparin infusion and prompt percutaneous coronary intervention, per clinical guidelines for management of acute coronary syndrome. Postintervention management remains an active area of fertile investigation, as the potential interactions of antiviral and antibody medications used to treat systemic COVID-19 may interfere with common antiplatelet therapies and anticoagulation.10
Patient’s perspective.
Family was contacted and declined to provide their perspective.
Learning points.
COVID-19, initially thought to be a primarily respiratory illness, can have a myriad of presentations due to its hypercoagulability.
Although most patients with COVID-19 present with non-ST segment elevation myocardial infarction (STEMI) and myocarditis, it is important to recognise STEMI as a possible thrombotic complication of COVID-19 in order to provide prompt treatment, including percutaneous coronary intervention.
STEMI can present during the hospital course in COVID-19, not only on admission, as exemplified by our case report. Given the high fatality with STEMI, physicians should remain on high alert not to miss this diagnosis in COVID-19 patients even after admission.
More research is needed on management of anticoagulation and antiplatelet agents in the setting of COVID-19, especially in the setting of STEMI.
Footnotes
Twitter: @lenny_genovese
LG and DR contributed equally.
Contributors: The corresponding author, LG, and DR were jointly responsible for the majority of the writing of this manuscript. BT was the operator for the procedure and intimately involved in postprocedural care. He reviewed and edited the manuscript. SS was the primary cardiologist involved in the care of this patient postprocedure while in the hospital. He reviewed and was involved in the internal editing process for this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA 2020;323:jama.2020.6775 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J, Tacquard C, Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–98. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefanini GG, Montorfano M, Trabattoni D, et al. St-Elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation 2020;141:2113–6. 10.1161/CIRCULATIONAHA.120.047525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangalore S, Sharma A, Slotwiner A. St-Segment elevation in patients with COVID-19: a case series. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from the ACC's interventional Council and ScaI. J Am Coll Cardiol 2020;75:2372–5. 10.1016/j.jacc.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikdeli B, Madhavan MV, Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


