Abstract
Between October 2015 and August 2016, Zimbabwe conducted the Zimbabwe Population-Based HIV Impact Assessment (ZIMPHIA) cross-sectional survey to determine progress toward epidemic control. Of 25,131 eligible adults aged 15–64 years, 20,577 (81.8%) consented to face-to-face questionnaire and biomarker testing in this nationally representative household survey. Home-based rapid HIV testing was performed using Determine, First Response, and STAT-PAK as the tiebreaker. HIV-positive tests were confirmed in a laboratory using Geenius HIV-1/2; viral load (VL) was measured using Roche TaqMan and BioMerieux NucliSENS. Recency of infection was tested using Sedia HIV-1 Limiting Antigen (LAg)-Avidity. Presence of antiretroviral (ARV) drugs was detected using high performance liquid chromatography/mass spectrometry (HPLC/MS). The recent infection testing algorithm included LAg-avidity enzyme immunoassay [normalized optical density (ODn ≤1.5), VL ≥1,000 copies/mL, and absence of ARV drugs]. Weighted annual HIV incidence was compared with United Nations Joint Programme on HIV/AIDS (UNAIDS) Spectrum models estimates. Overall, 26 of 2,901 HIV-seropositive individuals had a recent infection (men, 8; women, 18). Overall weighted annual incidence among persons aged 15–64 years was 0.42% [95% confidence interval (CI): 0.25–0.59] and was 0.44% (95% CI: 0.25–0.62) for those aged 15–49 years, similar to 2016 Spectrum model estimate (0.54%, 95% CI: 0.49–0.66) for this age group. Among persons aged 15–49 years, HIV prevalence was 13.35% (95% CI: 12.71–14.02), estimated HIV-positive individuals were 968,951 (95% CI: 911,473–1,026,430), of these, 41,911 (95% CI: 37,412–44,787) were annual-new infections, and this was similar to 2016 Spectrum estimates. The observed HIV incidence in ZIMPHIA 2015–2016 validated the 2016 Spectrum estimates and Zimbabwe’s progress toward epidemic control.
Keywords: comparison, incidence, Spectrum
Introduction
Reducing HIV incidence, the rate of new infections occurring in a susceptible population during a specified period, has become a global priority to end the HIV epidemic by 2030.1,2 Global targets aim to reduce HIV incidence by 75% by the end of 2020.2–4 These targets for reducing new infections are supported by the accelerated scale-up of antiretroviral treatment (ART) programs and biomedical and behavioral prevention strategies. The United Nations Joint Programme on HIV/AIDS (UNAIDS) first reported major declines in high HIV disease burden in Eastern and Southern Africa in 2015.2 The decline in HIV prevalence reported for Zimbabwe, Kenya, and Malawi was attributed, among other factors, to prevention strategies, such as increasing condom use and reducing sexual partners.3
Across the world, in the early 1990s, countries used HIV prevalence among young people as a proxy measure for incidence because HIV infection occurs primarily through sexual transmission.5,6 The Futures Group under contract from UNAIDS developed mathematical models for estimating HIV incidence, including the Spectrum model, that have been validated in some settings.7–9 Program data, HIV prevalence data from antenatal clinic surveillance, and nationally representative survey data are used to calibrate the model and to provide estimates of prevalence, number of new infections, and program impact projections.7 The UNAIDS Spectrum estimates indicate that globally the number of new HIV infections decreased between 2000 and 2014.2–4 In sub-Saharan Africa, where 70% of the 37.2 million are people living with HIV (PLHIV), new HIV infections decreased by 41%.3 On the basis of mathematically modeled HIV incidence, UNAIDS further suggested that Eastern and Southern Africa experienced the largest declines in new infections between 2000 and 2014, while these decreases are now marginal or almost static in the rest of the world as of 2015.3
Laboratory assays and recent infection testing algorithms (RITA) can estimate HIV incidence directly, and estimates are similar to actual incidence measured in prospective cohort studies.10 Although these laboratory assays are inexpensive, a large sample size is required when incidence is low. Recent advances in laboratory assays have made it possible to measure HIV incidence using samples collected in nationally representative population-based surveys that use cross-sectional methodology. The limiting antigen-avidity enzyme immunoassay (LAg EIA assay) was first applied to samples collected in the Swaziland HIV Incidence Measurement Survey (SHIMS; 2010–2011).11 This survey estimated HIV incidence using three methods: prospectively observed seroconversions, LAg plus HIV RNA [viral load (VL)]-based estimates, and nucleic acid amplification testing (NAAT).11,12 The LAg assay and NAAT estimated incidence at 2.6%, which was comparable to the prospectively observed rate (2.4%).12 The LAg assay is now widely accepted and used to estimate HIV-1 incidence in population-based surveys, and subsequent studies using LAg assays have been conducted in Swaziland and South Africa to measure HIV incidence among pregnant women and in other large-scale surveys.11,13
Estimated HIV prevalence rates in Zimbabwe suggest a sharp decrease in the HIV epidemic.14 On the basis of Zimbabwe Demographic Health Surveys conducted in 2005–2006 and 2015–2016 data, we observed HIV prevalence declined from 18.1% to 13.8% between 2005 and 2015.15,16 In a 2010 mathematical modeling study, Gregson et al. suggested that exposure to HIV prevention programs and high morbidity and mortality rates within communities resulted in more awareness that led to behavioral change and subsequent reductions in HIV incidence.17 Halperin et al. highlighted the adoption of safe sexual practices as a main driver of reductions in HIV incidence.14 Using data from antenatal women in Harare, Hargrove et al. developed a simple susceptible-infected model to fit age-stratified pooled prevalence, incidence, and mortality.18 They estimated that HIV incidence had peaked at 5.5% in 1991 and declined to 1.0% in 2010 among women attending antenatal services in Harare.18
Zimbabwe is one of the top five Sub-Saharan with high HIV diseases burden, mainly from heterosexual transmission.19 UNAIDS has supported Zimbabwe to generate HIV incidence estimates using the Spectrum model since 2004. To confirm these model-based HIV incidence estimates (especially the 2016 Spectrum model estimates) and to evaluate the impact of large-scale investments in HIV prevention, care, and treatment in the last decade, the government of Zimbabwe conducted the first Zimbabwe Population-Based HIV Impact Assessment (ZIMPHIA) from October 2015 to August 2016. ZIMPHIA assessed HIV incidence at the national level; provided critical information on the utilization of past prevention, care, and treatment programs; and captured current data on HIV-related factors at the population level, including among individuals who do not know their HIV status.
In this report, we estimate HIV incidence from ZIMPHIA data and describe population-level demographic factors. We also compare ZIMPHIA HIV incidence estimates with the 2016 HIV incidence estimates derived from the UNAIDS Spectrum model.
Materials and Methods
ZIMPHIA 2015–2016 was a nationally representative cross-sectional population-based household survey using a two-stage cluster sample design. In the first stage, 500 enumeration areas were included in the sample using probability proportional to size. In stage two, an average of 30 households in each enumeration area was randomly selected using an equal probability method. The survey was powered to detect HIV incidence in adults (aged 15–64 years) at a national level with standard error of ≤24.9%. Full methods were published in the final report.20
Before administering individual interviews, study staff obtained written informed consent (recorded electronically) from individuals aged ≥16 years. Parents or guardians provided written permission before unemancipated 15-year-olds were approached. After completing the individual interview, participants provided written consent before participating in the biomarker component of the survey. All individuals were considered eligible for participation if they slept at the household the night before; could speak English, Ndebele, or Shona; and had the visual, auditory, and mental capacities to consent to the survey.
Laboratory testing
Home-based HIV testing was performed using the national serial rapid test algorithm beginning with the Alere Determine HIV-1/2 test (Abbott Laboratories, Lake Forest, IL) as the screening test. Reactive samples on Determine were confirmed using First Response HIV 1–2-0 Card Test (Premier Medical Corp Ltd, Mumbai, India). Samples testing nonreactive on First Response underwent further testing on the HIV 1/2 STAT-PAK Assay (Chembio, Medford, NY) as the tiebreaker test. Samples that tested reactive by two of the three rapid tests were classified as HIV positive and were further confirmed using Geenius HIV-1/2 Confirmatory Assay (Bio-Rad Laboratories, Hercules, CA) in a satellite laboratory. All HIV-positive samples were tested for RNA VL levels using COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0 (Roche Diagnostics Corp, Basel, Switzerland) for plasma specimens or NucliSENS EasyQ HIV-1 v2.0 assay (BioMérieux, France) for dried blood spot specimens at the central laboratory. Recency testing was performed on all HIV-positive samples using Sedia HIV-1 LAg-Avidity EIA (Sedia Biosciences, Portland, OR) for both plasma and dried blood spot (DBS) samples (separate kits).21,22
A qualitative, high-performance, liquid chromatography tandem mass spectrometry assay was developed and validated for the assessment of efavirenz, lopinavir, and nevirapine from dried blood spots at the Division of Clinical Pharmacology, University of Cape Town. The samples were processed with a protein precipitation extraction method. Deuterated internal standards were used for each analyte. The extraction procedure was followed by liquid chromatographic separation using a Phenomenex Kinetex EVO C18 (1.7 μm, 2.1 ×·50 mm, 100 Å; Torrance, CA) analytical column. An AB Sciex API 4000 (Framingham, MA) mass spectrometer at unit resolution in the multiple reaction monitoring mode was used to monitor the transition of the protonated precursor ions m/z 705.6, 316.0, 629.6, and 267.1 to the product ions m/z 168.2, 243.9, 447.3, and 226.0 for efavirenz, lopinavir, and nevirapine, respectively. Electrospray ionization was used for ion production. The assay was validated over the range of 0.02–5.0 μg/mL, and 0.02 μg/mL was used as the cutoff concentration (Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Personal Communication).
Incidence outcome
On the basis of recent unpublished results, the absence of selected antiretroviral (ARVs) drugs was added to RITA to avoid misclassifying as recently infected participants with a long-term infection who were receiving ART but did not have VL suppression. Samples were therefore categorized as recent based on an algorithm including LAg (≤1.5 normalized optical density units), HIV RNA VL (≥1,000 copies/mL), and the absence of the selected ARV agents. Annualized incidence estimates were based on methods described below.
Ethical considerations
The study received ethical approval from the Medical Research Council of Zimbabwe, the Research Council of Zimbabwe, the Centers for Disease Control and Prevention (CDC) Institutional Review Board (IRB), Columbia University IRB, and WESTAT IRB.
Statistical analysis
Previous estimates of HIV incidence were based on Microsoft Excel spreadsheets that were collectively known as Assay-Based Incidence Estimation tools. These calculators were initially developed by the United States based CDC and are now jointly updated and maintained in collaboration with UNAIDS, the World Health Organization (WHO), the Consortium for the Evaluation and Performance of HIV Incidence, and South African Centre for Epidemiological Modelling and Analysis at Stellenbosch University and are available online (www.incidence-estimation.org/page/spreadsheet-tools-forbiomarker-incidence-surveys).23 These calculators have now been superseded by the R inctools package.20
In this analysis, we used SAS script to obtain incidence estimates using the formula recommended by the WHO Incidence Working Group and CEPHIA (Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Personal Communication). The initial results from the laboratory classify individuals as negative or positive for the HIV assay test. In the first step, individual blood weights are applied to HIV status and recency status variables to derive the following inputs as required in the code: R, the number of individuals testing recent, N′ the adjusted number of individuals testing HIV negative, and Q the number of HIV-positive people tested.
The annual risk of infection is the probability of becoming infected within a period of 1 year. Formally, the adjusted annual risk of HIV infection (JT) is calculated from:
| (1) |
Alternatively, the adjusted instantaneous incidence rate (IT) is given by:
| (2) |
where R is the number of recent cases among P testing HIV positive, N is the number testing HIV negative, and T is the time (1 year in our case) over which the mean duration of recent infection (MDRI) (ΩT) and the false recency rate (ε) are defined. The unadjusted values (jT and iT) are found by setting ε = 0 in Equations (1) and (2) to give:
| (3) |
| (4) |
The following LAg assay parameters were used: MDRI [130 days, 95% confidence interval (CI): 118–142 days], T as cutoff time for the assay set at 1 year, and the proportion of false-recent individuals set to zero on the basis of the algorithm that includes LAg assay, VL, and ART testing.
The annual incidence rate (Ia) was calculated using the equation:
| (5) |
The number of individuals with a recent infection can be derived by multiplying the Ia by the number of people at risk in the country.
We focused our analysis on all adults aged 15–64 years and stratified results into two age groups (15–49 year group and 15–24 year group) because of the global strategies targeting these age groups.
To mitigate inaccurate estimates arising from differential response across demographic groups, we used the chi-square automatic interaction detection (CHAID), a decision tree technique, based on adjusted significance testing. The technique was developed in South Africa and was published in 1980 by Gordon V. Kass, who had completed a PhD thesis on this topic. CHAID adjustment was used for nonresponse to add a robust approach to weighting, as described in the ZIMPHIA Final Report.19
UNAIDS Spectrum incidence estimates
The Spectrum mathematical models use HIV surveillance, program data, and indicator inputs from population and special survey data to predict annual HIV incidence, prevalence, and program gaps and coverages. Each model provides estimates for previous years and future projections. These outputs cannot be compared with other versions because each version has features and calibrations that are specific to the version. In this regard, outputs across the different years can only be compared for each model. The UNAIDS has supported Zimbabwe Estimates team to prepare annual estimates and projections since 2004. In this report, we compare the ZIMPHIA survey data with the 2016 HIV estimates that were generated using the 2016 Spectrum model, version 5.43.24
Results
Between October 2015 and August 2016, the heads of household from 11,717 of 13,828 (84%) selected, occupied households in Zimbabwe agreed to participate in the survey. A total of 25,131 potentially eligible adults (14,033 men and 11,098 women) from these participating households were approached. Of these, using weighted percentages, 89% agreed to participate in the individual interview, and 92% and 90% of eligible women and men, respectively, provided blood samples for biomarker analysis. A total of 20,577 adults (aged 15–64 years) consented to both a face-to-face interview and HIV testing, resulting in an unweighted response rate of 82% among all eligible adults. Over one third of the population consisted of adolescents and young adults aged 15–24 years (36.6%). Most study participants (64%) resided in rural areas, and 25% reported that they had never tested for HIV (Table 1). The overall HIV prevalence for adults aged 15–64 years was 14.08% (95% CI: 13.5–14.7) and was 12.0% (95% CI: 11.2–12.8) among men and 16.0% (95% CI: 15.3–16.7) among women. In the 15–24 year group, HIV prevalence among women was 5.9% (95% CI: 5.0–6.7), which was nearly twice that of men (3.0%, 95% CI: 2.4–3.7).
Table 1.
Demographic Characteristics, Testing History, and HIV-Related Test Results of Adults (Aged 15–64 Years) in Zimbabwe, Zimbabwe Population-Based HIV Impact Assessment 2015–2016
|
Total |
Women |
Men |
||||
|---|---|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | |
| Age, years | ||||||
| 15–24 | 7,100 (36.6) | 36.4–36.6 | 3,930 (35.2) | 35.1–35.4 | 3,170 (38.8) | 37.3–38.8 |
| 25–34 | 5,072 (27.5) | 27.4–27.5 | 3,151 (28.5) | 28.4–28.6 | 1,921 (26.3) | 26.2–26.4 |
| 35–49 | 5,285 (24.7) | 24.6–24.7 | 3,136 (23.9) | 23.8–23.9 | 2,149 (25.5) | 25.4–25.6 |
| 50–64 | 3,116 (11.0) | 11.1–11.6 | 1,961 (12.4) | 12.1–12.7 | 1,155 (10.2) | 9.9–10.5 |
| 15–49 | 17,457 (89) | 88–89 | 10,217 (88.8) | 87–89 | 7,240 (90) | 90–90 |
| 15–64 | 20,577 (100) | 12,182 (100) | 8,395 (100) | |||
| Residence | ||||||
| Rural | 14,503 (64.4) | 62.8–65.9 | 8,260 (62.9) | 61.3–64.5 | 6,243 (66.0) | 64.2–67.7 |
| Urban | 6,068 (35.6) | 34.1–37.2 | 3,918 (37.1) | 35.5–38.7 | 2,150 (34.0) | 32.28–35.8 |
| Prior HIV testinga | ||||||
| Never tested | 4,933 (25.4) | 24.7–26.1 | 2,134 (18.5) | 17.8–19.2 | 2,799 (32.9) | 31.7–34.1 |
| Ever tested | 15,611 (74.64 | 73.9–75.4 | 10,035 (81.5) | 80.8–82.2 | 5,576 (67.1) | 65.9–68.3 |
| HIV prevalence | ||||||
| 15–24 | 344 (4.4) | 3.9–5.1 | 246 (5.8) | 5.0–6.7 | 98 (3.0) | 2.4–3.7 |
| 25–34 | 849 (14.0) | 13.0–15.2 | 642 (17.9) | 16.6–19.3 | 207 (9.4) | 8.0–10.9 |
| 35–49 | 1,532 (25.8) | 24.5–27.2 | 968 (28.1) | 26.6–29.7 | 564 (23.5) | 21.7–25.4 |
| 50–64 | 654 (19.8) | 18.1–21.5 | 370 (17.1) | 15.4–19.0 | 284 (23.3) | 20.5–26.3 |
| 15–49 | 2,725 (13.4) | 12.7–14.0 | 1,856 (15.8) | 15.1–16.6 | 869 (10.7) | 9.9–11.5 |
| 15–64 | 3,379 (14.1) | 13.5–14.7 | 2,226 (16.0) | 15.3–16.0 | 1,153 (12.0) | 11.2–12.8 |
All percentages are weighted.
Does not add up to 20,577.
CI, confidence interval; ZIMPHIA, Zimbabwe Population-Based HIV Impact Assessment.
Of the of 2,901 HIV-positive individuals tested for evidence of recent infection, 26 (18 women, 8 men) were identified as recently infected via RITA, which included testing for VL and for the presence of ARVs to improve positive predictive value (Table 2). Most individuals with a recent HIV infection (n = 22, 84.6%) reported previously testing negative for HIV. The incidence estimates among those who had previously ever tested for HIV was 0.48% (95% CI: 0.27–0.69) and was 0.25% (95% CI: 0.01–0.49) for participants without prior HIV testing.
Table 2.
HIV Incidence Estimates by Key Characteristics (Zimbabwe Population-Based HIV Impact Assessment, 2015–2016)
|
Total |
Women |
Men |
||||
|---|---|---|---|---|---|---|
| % Annual incidence (n)a | 95% CI | % Annual incidence (n)a | 95% CI | % Annual incidence (n)a | 95% CI | |
| Age, years | ||||||
| 15–24 | 0.30 (7.25) | 0.08–0.52 | 0.46 (6.02) | 0.09–0.82 | 0.14 (1.58) | 0.00–0.37b |
| 25–34 | 0.72 (11.28) | 0.28–1.16 | 0.95 (8.81) | 0.30–1.61 | 0.48 (2.95) | 0.00–1.05b |
| 35–49 | 0.32 (4.53) | 0.00–0.66 | 0.27 (2.14) | 0.00–0.68b | 0.38 (2.23) | 0.00–0.91b |
| 50–64 | 0.24 (2.15) | 0.000–0.58 | — (0) | — | 0.59 (1.86) | 0.00–1.43b |
| 15–49 | 0.44 (23.53) | 0.25–0.62 | 0.57 (17.50) | 0.29–0.85 | 0.30 (6.92) | 0.07–0.53 |
| 15–64 | 0.42 (26.19) | 0.25–0.59 | 0.50 (18.27) | 0.25–0.75 | 0.33 (8.58) | 0.10–0.55 |
| Residence | ||||||
| Urban | 0.41 (7.80) | 0.11–0.73 | 0.62 (7.27) | 0.15–1.10 | 0.19 (1.29) | 0.00–0.56b |
| Rural | 0.42 (18.37) | 0.22–0.60 | 0.43 (10.66) | 0.17–0.69 | 0.40 (7.75) | 0.11–0.68 |
| Prior HIV testing | ||||||
| Never tested | 0.25 (4.18) | 0.01–0.49 | 0.25 (1.83) | 0.00–0.62b | 0.25 (2.35) | 0.00–0.57b |
| Ever tested | 0.48 (22.16) | 0.27–0.69 | 0.57 (16.53) | 0.28–0.86 | 0.37 (6.22) | 0.06–0.68 |
Note MDRI = 130 days (95% CI: 118–142 days), PFR = 0.00, time cutoff (T) = 1 year were used to calculate incidence.
All numbers in parentheses represent the weighted number of individuals with evidence of a recent HIV infection.
The small population size resulted in negative values of CI—these were set to zero.
MDRI, mean duration of recent infection; PFR, proportional false recent.
The weighted annual HIV incidence was 0.42% (95% CI: 0.25–0.59) among all adults aged 15–64 years and was 0.44% (95% CI: 0.25–0.62) for adults aged 15–49 years. In the 15–24 year group, HIV incidence was 0.14% (95% CI: 0.00–0.37) among men and was 0.46% (95% CI: 0.09–0.82) among women. By residence, HIV incidence among women was 0.62% (95% CI: 0.15–1.10) in urban areas compared with 0.43% (95% CI: 0.17–0.69) in rural areas. For men, incidence was 0.19% (95% CI: 0.00–0.56) in urban areas compared with 0.40% (95% CI: 0.11–0.68) in rural settings.
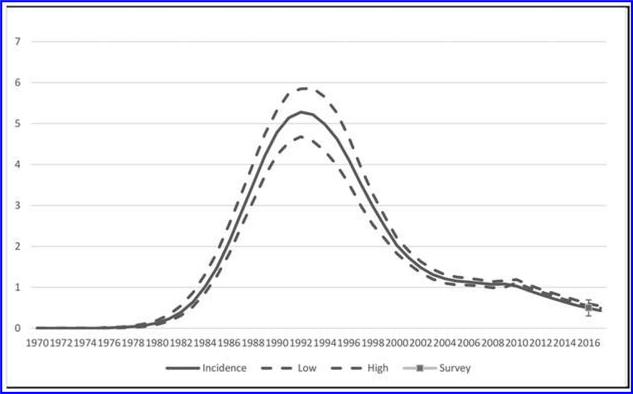
The UNAIDS Spectrum models show a steady decline in HIV incidence from 4.66% in 1993, to 1.03% in 2010, to 0.66% in 2015, to 0.54% (95% CI: 0.49–0.66) in 2016 (Fig. 1).20 The sharpest decline in HIV incidence was between 1994 and 2005. The ZIMPHIA incidence estimate based on LAg, VL, and absence of selected ARV agents was 0.44% (95% CI: 0.25–0.62), and the estimate based on LAg and VL was 0.48% (95% CI: 0.29–0.66); both estimates were similar to the 2016 Spectrum estimate (0.54%, 95% CI: 0.49–0.66).
FIG. 1.
Comparison of HIV incidence among all adults aged 15–49 years obtained in ZIMPHIA (VL+ART) with 2016 Spectrum estimates. ART, antiretroviral treatment; VL, viral load; ZIMPHIA, Zimbabwe Population-Based HIV Impact Assessment.
Other key indicators derived from ZIMPHIA also were comparable to estimates derived using the 2016 Spectrum model for adults aged 15–49 years (Table 3).
Table 3.
Comparison of Observed HIV in Zimbabwe Population-Based HIV Impact Assessment with Spectrum Estimates Among Adults Aged 15–49 Years
| HIV prevalence | 95% CI | Total number | 95% CI | No. of new infections | 95% CI | |
|---|---|---|---|---|---|---|
| ZIMPHIA | 13.4 | 12.7–14.0 | 968,951 | 911,473–1,026,430 | 41,911 | 37,412–44,787 |
| Spectrum 2016 | 14.2 | 12.5–15.5 | 1,038,219 | 921,796–1,158,764 | 40,507 | 36,521–44,301 |
The disease burden (total PLHIV) and the number of new infections estimated by both ZIMPHIA and Spectrum were similar (overlap in 95% CI). The numerically higher estimate obtained in ZIMPHIA despite a lower incidence is due to differences in the base population denominators that were applied.
Discussion
The first nationally representative Zimbabwe Population-Based HIV Impact Assessment (ZIMPHIA) survey found that annual HIV incidence was 0.44% among adults aged 15–49 years in 2015–2016, similar to the 2016 UNAIDS Spectrum incidence estimate (0.54%). The Spectrum estimates for HIV prevalence, number of PLHIV, and those with new infections were also comparable to the ZIMPHIA point estimates.
Although the primary objective of the survey was to obtain population-based estimates of HIV incidence and VL suppression, this survey also provided an opportunity to validate the UNAIDS Spectrum estimates. The 2016 Spectrum HIV incidence estimates are not disaggregated by demographic data such as age group or by programmatic disaggregation such as by HIV testing status, that is, ever tested was done in ZIMPHIA. However, the small sample size of recently infected individuals in ZIMPHIA did not support exploration of sexual risk behaviors (e.g., age at first sex, paid sex, multiple sexual partners, or condom use in high-risk sex) and other sociodemographic characteristics. Although limited, this analysis of ZIMPHIA data is useful in identifying the potential drivers of new infections in Zimbabwe.
The ZIMPHIA survey provided the first national profile of incidence estimates disaggregated by demographic and programmatic variables. The HIV incidence point estimate was nearly twice as high among women compared with men in the 15–49 year age group; although these estimates were not significantly different, they do suggest the need for gender-sensitive interventions. The point estimate for HIV incidence of 0.95% among women aged 25–34 years is notable and raises the question of whether this is driven by multiple concurrent partners while in a marital union, as observed in other studies.19 In the 15–24 year group, the point estimate among young women (0.46%) was higher than that of young men (0.14%); as above, there was an overlap in the CI suggesting that these were not different. Regardless, of this, the trend suggests age-specific interventions may be warranted.
The 2012 Zimbabwe census showed that 67% of the population resided in rural areas; however, internal migration is unquantified, with unofficial reports from urban councils suggesting that an influx from rural to urban areas resulted from economic challenges in 2006–2010.25 Overall, ZIMPHIA showed similar incidence in urban and rural settings.
The ZIMPHIA incidence estimate is conservative because the proportional false recent (PFR) of RITA was set to zero. If we had used a PFR that was above zero, this would have resulted in a further lowering of incidence. In our case, adding ARV screening lowered incidence from 0.48% to 0.44% in the 15–49 year age group. However, SHIMS 201111 and Huerga et al.26 show that ARV adjustment may not always be needed: the non-ARV adjusted LAg incidence estimate (2.5%) basically matched both the NAAT-based estimate (2.6%) and the prospectively observed HIV incidence (2.4%) in SHIMS.11 The utility of ARV adjustments in RITA depend on the state of ARV use in the population, which is expanding, and may be important in subsequent surveys.
The small sample of recent infections did not allow us to conduct detailed explorations of associations with sexual behaviors. While a second wave of PHIAs is currently ongoing, there was no attempt to increase the adult sample size as it was considered to be sufficiently powered to detect the change in HIV incidence at the national level and allow for trend analysis between the two surveys. The additional costs were therefore considered to be unwarranted. In addition to providing a national HIV incidence estimate, the PHIA results are now being used in subsequent Spectrum estimates. PHIA results on prevalence by age, sex, and region have been used in subsequent Spectrum applications to improve the estimates.
Conclusion
The observed incidence in ZIMPHIA 2015–2016 was comparable to and validated the estimates obtained using the Spectrum model. The overall downward trend in HIV incidence as demonstrated by Spectrum may reflect success in the scale-up of prevention, care, and treatment services. Similarities in other key indicators observed in the survey and the Spectrum estimates support the utility of population-based surveys and modeled estimates in tracking the HIV epidemic. It is recommended that a similar national population level survey to measure HIV incidence and associated indicators be repeated and compared with SPECTRUM outputs to track the HIV epidemic.
Acknowledgment
We would like to acknowledge Meade Morgan for statistical analysis support in earlier phases of article development.
Funding Information
This project was supported by funding from the President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention under grant GH001226 and #U2GGH000994 to ICAP, Columbia University, New York, NY.
Footnotes
Author Disclosure Statement
B.P. as inventor of the Limiting Antigen Avidity Assay method receives a portion of the royalties received by the CDC from commercialization of the assay. All other authors declare no conflicts of interest.
Disclaimer
The findings and conclusions in this presentation/report are those of the authors and do not necessarily represent the views of the CDC/the Agency for Toxic Substances and Disease Registry.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS): HIV Prevention 2020 Road Map—Accelerating HIV prevention to reduce new infections by 75%. November 2017. Available at: https://www.unaids.org/sites/default/files/media_asset/hiv-prevention-2020-road-map_en.pdf accessed November 17, 2017.
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS). 2014 Fast-track: ending the AIDS epidemic by 2030. Geneva. Available at www.unaids.org/sites/default/files accessed November 21, 2016. [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS). Progress report on the Global Plan 2016. Available at www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf accessed October 13, 2017.
- 4.Joint United Nations Programme on HIV/AIDS (UNAIDS). Data book 2017. Available at www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf accessed Janurary 19, 2019.
- 5.Mahy M, Garcia-Calleja JM, Marsh KA: Trends in HIV prevalence among young people in generalized epidemics: Implications for monitoring the HIV epidemic. Sex Transm Infect 2012;88(Suppl_2):i65–i75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallett TB, Stover J, Mishra V, Ghys PD, Gregson S, Boerma T: Estimates of HIV incidence from household-based prevalence surveys. AIDS 2010;24:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joint United Nations Programme on HIV/AIDS (UNAIDS; 2019. Available at www.unaids.org/en/dataanalysis/datatools/spectrum-epp accessed Janurary 19, 2019.
- 8.Mahy M, Brown T, Stover J, et al. : Producing HIV estimates: From global advocacy to country planning and impact measurement. Glob Health Action 2017;10(sup1): 1291169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silhol R, Gregson S, Nyamukapa C, et al. : Empirical validation of the UNAIDS Spectrum model for subnational HIV estimates: Case-study of children and adults in Manicaland, Zimbabwe. AIDS 2017;31 Suppl 1:S41–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joint United Nations Programme on HIV/AIDS (UNAIDS and World Health Organization. 2013. When and How to Use Assays for Recent Infection to Estimate HIV Incidence at a Population Level. Available at: www.aidsdatahub.org/when-and-how-to-use-assays-for-recent-infection-to-estimate-hiv-incidence-at-a-population-level-unaids-and-who accessed September 20, 2017.
- 11.Justman J, Reed JB, Bicego G, et al. : Swaziland HIV Incidence Measurement Survey (SHIMS): A prospective national cohort study. Lancet HIV 2017;4:e83–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parekh BS, Ou C-Y, Fonjungo PN, et al. : Diagnosis of human immunodeficiency virus infection. Clin Microbiol Rev 2018;32:e00064–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shisana O, Rehle T, Simbayi LC, et al. : South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. HSRC Press, Cape Town, 2014. [Google Scholar]
- 14.Halperin DT, Mugurungi O, Hallett TB, et al. : A surprising prevention success: Why did the HIV epidemic decline in Zimbabwe? PLoS Med 2011;8:e1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International. Zimbabwe Demographic and Health Survey 2005/6. ZIMSTAT and ICF International, Calverton, MD, 2006. [Google Scholar]
- 16.Zimbabwe National Statistics Agency and ICF International. Zimbabwe Demographic and Health Survey 2015: Final Report. Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International, Rockville, MD, 2016. [Google Scholar]
- 17.Gregson S, Gonese E, Hallett TB, et al. : HIV decline in Zimbabwe due to reductions in risky sex? Evidence from a comprehensive epidemiological review. Int J Epidemiol 2010;39:1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hargrove JW, Humphrey JH, Mahomva A, et al. : Declining HIV prevalence and incidence in perinatal women in Harare, Zimbabwe. Epidemics 2011;3:88–94. [DOI] [PubMed] [Google Scholar]
- 19.Muchini B, Benedikt C, Gregson S, et al. : Local perceptions of the forms, timing and causes of behavior change in response to the AIDS epidemic in Zimbabwe. AIDS Behav 2011;15:487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health and Child Care (MOHCC), Zimbabwe. Zimbabwe Population-based HIV Impact Assessment (ZIMPHIA) 2015–2016: Final Report. Harare: MOHCC; August 2019. Available at https://phia.icap.columbia.edu/countries/zimbabwe/ accessed August 2019. [Google Scholar]
- 21.Duong YT, Qiu M, De AK, et al. : Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: Potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One 2012;7:e33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duong YT, Kassanjee R, Welte A, et al. : Recalibration of the limiting antigen avidity EIA to determine mean duration of recent infection in divergent HIV-1 subtypes. PLoS One 2015;10:e0114947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.South African Centre for Epidemiological Modelling and Analysis (SACEMA) online resource for calculation of incidence. Available at www.incidence-estimation.org/page/spreadsheet-tools-for-biomarker-incidence-surveys accessed January, 2019.
- 24.Ministry of Health and Child Care Zimbabwe. Zimbabwe National HIV Estimates 2016. Harare, 2016. [Google Scholar]
- 25.Zimbabwe National Statistical Agency (ZIMSTATS) in Zimbabwe National Statistical Agency Population Census 2012, Harare, Zimbabwe. [Google Scholar]
- 26.Huerga H, Shiferie F, Grebe E, et al. A comparison of self-report and antiretroviral detection to inform estimates of antiretroviral therapy coverage, viral load suppression and HIV incidence in Kwazulu-Natal, South Africa. BMC Infect Dis 2017;17:653. [DOI] [PMC free article] [PubMed] [Google Scholar]