Abstract
The organophosphorus insecticide chlorpyrifos (CPF) is suspected to cause developmental neurotoxicity in children leading to long term effects. Developmental exposure of rat pups to CPF at low levels disrupts degradation of the brain endocannabinoids through the inhibition of fatty acid amide hydrolase (FAAH) and decreases the reactivity of juvenile rats in an emergence test. In this study, we further investigated the effects of developmental CPF exposure on behavior but also included exposure to PF-04457845, a specific inhibitor of FAAH, for comparison of behavior altered by FAAH inhibition with behavior altered by CPF. Ten day old rat pups were exposed orally either to 0.5, 0.75, or 1.0 mg/kg CPF or 0.02 mg/kg PF-04457845 daily for 7 days. In an open field (day 23), the high CPF and PF-04457845 groups exhibited increased motor activity but no differences in the time spent in the field’s center. In an elevated plus maze (day 29), all treatment groups had increased open arm activity but ethological behaviors associated with anxiety were not altered. Behaviors in the maze associated with increased general activity and exploratory drive were increased. Social interactions (day 36) were measured and all treatment groups exhibited increased levels of play behavior. The similarities in behavior between PF-04457845 and CPF suggest that enhanced endocannabinoid signaling during the exposure period plays a role in the persistent alteration of behavior observed following developmental CPF exposure.
Keywords: chlorpyrifos, developmental, endocannabinoid, fatty acid amide hydrolase, social play
INTRODUCTION
The organophosphorus (OP) insecticide chlorpyrifos (CPF) is the most commonly used OP insecticide (Grube et al., 2011). Concern has been raised about the negative impact of CPF exposure on the developing nervous system in children. While CPF is no longer registered for use in households in the United States, it is still widely used agriculturally and opportunities exist for children of agricultural communities to be exposed to CPF as well as other OPs (Arcury et al., 2007; Koch et al., 2002). In fact, developmental exposure to OP insecticides, such as CFP, is suspected to cause negative effects in children such as decreased motor skills, decreased cognitive abilities, and increased signs of attention deficit disorder and attention deficit/hyperactivity disorder (ADHD) (Bouchard et al., 2011; Butler-Dawson et al., 2016; Engel et al., 2011; Gunier et al., 2017; Marks et al., 2010; Ruckart et al., 2004; van Wendel de Joode et al., 2016).
At higher levels, OPs exert their toxicity through actions on the cholinergic system via the inhibition of cholinesterase (ChE) leading to accumulation of acetylcholine in the brain and hyperstimulation of the cholinergic system. However, long term negative effects have been observed with multiple OPs at exposure levels that induce only minimal levels of brain ChE inhibition and do not produce characteristic signs of cholinergic hyperactivity (Slotkin et al., 2007; Slotkin et al., 2006; Timofeeva et al., 2008a; Timofeeva et al., 2008b). This has led to the hypothesis that the cholinergic system may not be the primary target especially with respect to a real world exposure scenario which would involve very low environmental levels of an OP. This non-cholinergic target is currently unknown.
Previous work in adult animals has shown that the endocannabinoid system is susceptible to the effects of OP exposure. Acute exposure of adult mice to high levels of CPF resulted in significant inhibition of fatty acid amide hydrolase (FAAH) and monoacyglycerol lipase (MAGL) which lead to the accumulation of their respective substrates, anandamide (AEA) and 2-arachiodonoylglycerol (2-AG) which are the two most common endocannabinoids present in brain (Nomura et al., 2008; Nomura and Casida, 2011; Quistad et al., 2006; Quistad et al., 2001; Quistad et al., 2002). These previous studies reported that FAAH was a more sensitive target for inhibition following high level CPF exposure than was ChE suggesting that FAAH inhibition may be important during developmental exposure to OP insecticides. Our studies extended this work and reported that the endocannabinoid system in developing animals is also susceptible to CPF exposure and found that FAAH inhibition and AEA accumulation occurs at exposure levels that do not alter brain ChE activity (Carr et al., 2013; Carr et al., 2011; Carr et al., 2014). The effect on endocannabinoid metabolism in the absence of effects on ChE activity suggests that the endocannabinoid system may be a non-cholinergic target. It is known that the endocannabinoid system plays a pivotal role in brain development since altering endocannabinoid signaling during brain development via exposure to exogenous cannabinoids results in long term neurological effects (for review see Campolongo et al., 2011; Higuera-Matas et al., 2015). Thus, it is possible that altering the normal breakdown of endocannabinoids during brain development could also lead to long term negative effects including alteration of behavior.
Recently, we reported that exposure of preweanling rat pups to low levels of CPF results in decreased anxiety-like behavior in male and female rats tested during the early postweanling period (Carr et al., 2017). Previous studies have reported the effects of developmental exposure on anxiety utilizing the elevated-plus maze (Aldridge et al., 2005; Braquenier et al., 2010; Icenogle et al., 2004; Ricceri et al., 2006) and on social-anxiety utilizing social behavior measurements (De Felice et al., 2014; Ricceri et al., 2003; Venerosi et al., 2006; Venerosi et al., 2008; Venerosi et al., 2010; Venerosi et al., 2015). However, we are the first to report alteration of anxiety-like behavior at dosages that do not inhibit brain ChE. The behavioral task we measured was the emergence test which measures an animal’s conflict between exploring a novel environment and avoiding a brightly illuminated open space. However, a single test of anxiety does not give a true measure of an animal’s emotional state and testing with multiple behavioral tasks is necessary to fully characterize this state.
The overall objective of this project is to further investigate the effects of developmental low level exposure to CPF on anxiety-like behavior by administering additional behavioral tests. These included the open field, the elevated plus maze, and a social interaction test; all of which involve aspects of anxiety-related behavior (for review, see Ramos, 2008; Cryan and Sweeney, 2011; Steimer, 2011). In addition to anxiety, the social interaction test also measures investigative, solicitation, and play behaviors. However, the experiment includes two additional caveats. First, as initially reported by Hogg (1996), aversive test conditions, such as elevated light levels, can increase the sensitivity of rodents to potential anxiolytics. It has been demonstrated that during disruption of endocannabinoid metabolism (i.e., inhibited FAAH activity) anxiolytic effects become detectable when the testing conditions are made more aversive by non-habituation and increasing the illumination of the test arena (Haller et al., 2009; Naidu et al., 2007). Campolongo et al. (2012) reported altered novel object recognition under high (but not low) aversive conditions in rats whose endocannabinoid tone was pharmacologically enhanced with AM404, an endocannabinoid transport inhibitor. Therefore, for all behavior tests, the apparatus was highly illuminated to make it more aversive and no habituation to the apparatus or testing room occurred. Secondly, in addition to the three dosages of CPF used previously in our emergence test, we have included an additional treatment group that were exposed to a specific inhibitor of FAAH (PF-04457845) (Ahn et al., 2011) using a similar treatment paradigm as that used with CPF. This allowed us to determine if the behavioral effects observed following developmental CPF exposure were similar to those induced by the inhibition of FAAH during development.
MATERIALS AND METHODS
Chemicals.
Chlorpyrifos was a generous gift from DowElanco Chemical Company (Indianapolis, IN). PF-04457845 was purchased from MedChem Express (Monmouth Junction, NJ). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Animal Treatment.
Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility with temperature control (22 ± 2EC) and with lights on between 0700 and 1900 for a 12-h dark-light cycle. Tap water and LabDiet rodent chow were freely available during the experimentation. The procedures used in this project were approved by the Mississippi State University Institutional Animal Care and Use Committee. From a breeding colony of adult male and female Sprague Dawley rats (Hsd:SD; Envigo, Indianapolis, IN), male and female rat pups were obtained and the day of birth was considered as postnatal day 0 (PND 0). On PND 10, rat pups within the same litter were assigned to different treatment groups. There was always a representative control animal of the same sex present in each litter to match the CPF treated animals. This project used rats from 27 litters.
Oral gavage (per os) administration of CPF occurred daily from PND10-16 at a volume of 0.5 ml/kg body weight. This time frame corresponds to the postnatal age in humans in which significant brain maturation occurs (Andersen, 2003; Counotte et al., 2011; Tau and Peterson, 2010). The dosages of CPF were designed to span the range between no inhibition of brain ChE and low inhibition of brain ChE (Carr et al., 2013; Carr et al., 2017; Carr et al., 2011; Carr et al., 2014). However, it is unclear whether how these dosages relate to the actual exposure levels in children. It is likely that these exposure levels are much higher that would be expected in occur in children.
The CPF dosages selected for treatment were well below the oral repeated NOEL for signs of toxicity (4.5 mg/kg) for postnatal rats but span the oral repeated No Observed Effect Level (NOEL) for inhibition of brain ChE activity (0.75 mg/kg) as reported by Zheng et al. (2000). The treatment groups include (1) Control (corn oil); (2) Low CPF (0.5 mg/kg); (3) Medium CPF (0.75 mg/kg); High CPF (1 mg/kg); and PF-04457845 (0.02 mg/kg). The dosage of PF-04457845 was selected based on a preliminary dose response study (3 replications) and the dosage was designed to yield inhibition of FAAH activity similar to that obtained with CPF exposure. However, the dose response curve for in vivo inhibition of FAAH by PF-04457845 is very steep (Ahn et al., 2011) and, as the experiment progressed, it became evident that the selected dosage was exerting slightly higher inhibition than intended.
For dosing, the solution was delivered to the back of the throat using a 25 μl tuberculin syringe equipped with a 1-inch 24-gauge straight intubation needle (Popper and Sons, Inc., New Hyde Park, NY). Each day, body weights were recorded and weight gain was calculated as the difference between the body weights on PND11-16 and the original body weight at initiation of treatment on PND10. Following the treatment period, handling of the rats continued throughout experimentation to allow marking of each animal for identification.
Impact on Endocannabinoid Levels and Metabolism.
We have previously determined the effect of CPF used in this study on endocannabinoid levels and metabolism (Buntyn et al., 2017; Carr et al., 2013; Carr et al., 2017; Carr et al., 2011; Carr et al., 2014) but have not determined these parameters in developing rats exposed to PF-04457845. Therefore, an additional cohort of rats was treated with either control (corn oil) or PF-04457845 (0.02 mg/kg) as described above. Following sacrifice at 12 h after the last administration on PND16, brains were rapidly removed and dissected to obtain the forebrain (excluding the medulla and cerebellum). The forebrain was divided into right and left hemispheres and frozen on a stainless steel plate on top of dry ice, and maintained at −80°C until assay. The right hemisphere was used to measure the activities of ChE, FAAH, and MAGL as previously described (Carr et al., 2011). Protein concentrations of tissue extracts were quantified with the Folin phenol reagent using bovine serum albumin as a standard (Lowry et al., 1951). Specific activities were calculated as nmoles (ChE) or pmoles (FAAH or MAGL) product produced min−1 mg protein−1 (n=11-17). The left hemisphere was used to determine the levels of the endocannabinoids AEA and 2-AG, and the levels of the non-cannabinoid N-acylethanolamides palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) as previously described (Carr et al., 2013) with some modifications (Carr et al., 2017). The amounts are normalized on the brain wet weight and expressed as pmoles/g brain (AEA, OEA, and PEA) or nmoles/g brain (2-AG) (n=15-22). Additional unpublished values obtained from the forebrain of control and CPF treated animals using the same exposure paradigm and methods described above were available. These unpublished data along with the data obtained from the PF-04457845 cohort were combined with raw data previously published in Carr et el. (2013; 2014; 2017) and Buntyn et al. (2017) for statistical analysis. Inclusion of all these data in a single analysis provided a direct comparison of each parameter for all dosage groups.
Behavioral Testing.
Juveniles/adolescent rats were assessed for anxiety and locomotor activity in the open field (PND23), for anxiety-like behavior in the elevated plus maze (PND29), and for any alterations in social behavior (PND35-36). As described in the Introduction, the rats were not habituated to the behavior apparatuses or testing room prior to testing and each test was conducted under high illumination. On the day of testing, rats were individually transported to the testing room immediately prior to initiation of testing. The order and time period (6 days) between tests was employed to reduce the interaction between tests (McIlwain et al., 2001; Paylor et al., 2006).
Open field (PND23).
The open field was a clear plexiglass open-field (28 × 28 × 46 cm) with a black plexiglass barrier present between the experimenter and the apparatus. Illumination was ~700 lux. The rats were placed in the rear left corner of the open field and allowed to explore the box for 20 min. Movements were recorded using Limelight Software (Actimetrics, Wilmette, IL). The software divided the open field arena into 16 squares of 7 × 7 cm. The central four squares were defined as the center zone, in which animals’ activity was regarded as a measure of emotionality (Prut and Belzung, 2003). The software recorded (a) total number of crosses across squares, (b) total distance traveled (cm), (c) crosses into the center zone, and (d) time spent in the center zone (sec) (n=42-46). Between each subject, the chambers were thoroughly cleaned and wiped down with 70% ethanol. Due to a computer malfunction, the open field data for 16 animals was not collected and unavailable for inclusion in the statistical analysis.
Elevated plus maze (PND29).
The elevated plus maze was constructed of black-painted wood and consisted of four arms (50×10 cm), two open and two enclosed (40 cm wall), intersecting at a 10 × 10 cm central square. The arms were perpendicular distances to each other to form a 90° angle and elevated to a height of 50 cm above the floor. Illumination was ~600 lux in the open arms and ~100 lux in the closed arms. The rats were placed in the central square of the maze and allowed to explore the maze for 5 min. Each test session was recorded using a remotely operated Canon EOS Rebel camera.
The videos were viewed by two independent observers who were blind to treatment. The observers scored the following behavioral parameters: (1) number of entries into the open arms, (2) number of entries into the closed arms, (3) time spent in the open arms (sec), (4) time spent in the closed arms (sec), and (5) time spent in the central square (sec). From these data, the (6) percent open arm entries and (7) percent open arm time were calculated. Also measured were: (8) number of crosses from closed arm to closed arm and (9) number of crosses from open arm to open arm were recorded. An arm entry was defined as when all four limbs of the rat were within the arm. In addition, the following ethological oriented parameters were scored: (10) number of stretch-attend postures (defined as exploration of the open arm with the front part of the body, while the hind region remains in the closed arm), (11) number of entry attempts (attempt at entry into open arms from the central square region by placing both front paws into the open arm followed by retraction), and (12) number of head-dips (protruding the head over the ledge of an open arm and downwards towards the floor regardless of the location of the animals body) (n=44-50). Between each subject, the maze was thoroughly cleaned and wiped down with 70% ethanol. One rat from the 0.5 mg/kg CPF treatment groups immediately entered the open arm upon being placed in the maze and remained in the same position for the duration of the test. This animal was removed from analysis. Due to a recording malfunction, the elevated plus maze data for 8 animals was not collected and unavailable for inclusion in the statistical analysis.
Social behavior (PND35-36).
The behavioral arena was a clear plexiglass rat cage filled with clean litter. Illumination was ~600 lux. Each test session was recorded using a remotely operated Canon EOS Rebel camera. Following a 24 hour isolation period, two rats of the same treatment, sex, age and size but from different litters were placed into different corners of the behavioral apparatus. The rats remained in the arena together for 10 min. The videos were viewed by two observers who were blind to treatment. The observers scored behavioral parameters as previously described (Himmler et al., 2013; Ku et al., 2016; Vanderschuren et al., 1997; Varlinskaya and Spear, 2002). These include: (1) time to first interaction, (2) frequency of grooming, (3) frequency of social exploration (body or anogenital sniffing or licking), (4) frequency of crawling over and under, (5) frequency of chasing, (6) frequency of pouncing/nape attacks (playful attacks directed at the nape of the neck), (7) frequency of play fighting (boxing and wrestling), (8) frequency of pinning (one animal lying with its back on the floor with the other animal standing over it), and (9) the time spent play fighting (sec) (n=20-22). When scoring these parameters, the behavior of each individual rat was not recorded and the score obtained is a combined measure of the occurrence of the behavior in both rats regardless of which rat performed/initiated the behavior. After each test, the litter from the cage was emptied, cleaned with 70% ethanol, dried, and refilled with fresh litter.
Western Blotting for the endocannabinoid receptors:
Immediately following behavioral testing, brains were extracted and frozen on dry ice. Brains were also collected from a matching cohort of non-behavioral animals. Frozen brains were sliced at 500 micron increments, and the brain regions of interest were collected by punching. These brain regions included the agranular insular cortex, amygdala, hippocampus, prelimbic cortex, and nucleus accumbens. The collected tissue was lysed in RIPA lysis buffer containing 0.01% phenylmethylsulfonyl fluoride (PMSF), 0.01% sodium orthovanadate, and 0.01% protease inhibitor cocktail. Total protein concentration was measured using the Bradford protein assay. The protein extracts containing same amount of protein were resolved by 6-20% polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride (PVDF) membrane. The proteins of interest were detected using rabbit-anti-rat primary antibodies against CB1 at a 1:1000 dilution, pCB1 at a 1:300 dilution, CB2 at a 1:300 dilution, or α-tubulin at a 1:300 dilution (Santa Cruz Biotechnology, Santa Cruz, CA) followed by a goat-anti-rabbit secondary horseradish peroxidase antibody at a 1:500dilution (Sigma Aldrich, St. Louis, MO). PVDF membranes were developed using 4-CN and DAB in conjunction and imaged using ChemiDoc XRS+ System with Image Lab Software (Bio-Rad). The bands were then quantified using ImageJ software (National Institutes of Health). CB1, pCB1, and CB2 levels were normalized based on the α-tubulin levels (n=8).
Statistical Analysis.
Statistical analysis was performed using SAS statistical package (SAS Institute Inc., Cary, NC). Weight gain was subject to sphericity testing by analysis of variance (ANOVA) using the general linear model with a repeated measures paradigm and found to violate the assumption of sphericity. Therefore, analysis was conducted using the Mixed procedure (Littell et al., 1996) with a repeat measures paradigm using a Huynh-Feldt covariance structure (Huynh and Feldt, 1970). The analysis identified significant differences in the main effects (sex, treatment, and day) and all possible interactions.
Enzyme specific activities, endocannabinoid levels, N-acylethanolamides levels, receptor levels, and behavioral data were analyzed by ANOVA using the Mixed procedure to determine significant differences in sex, treatment, and sex × treatment interactions. Prior to analysis, the distribution of the conditional residuals for all data was evaluated for each parameter to determine the appropriateness of the statistical model. Any parameter not found to be sufficiently normally distributed were subjected to Blom’s transformation in SAS using the PROC RANK procedure with the NORMAL option (Altman, 1990; Blom, 1958) prior to analysis. All analyses included litter and sex × treatment × litter as random effects. Mean separation was performed by least-square means. The criterion for significance was set at p < 0.05.
RESULTS
Following repeated developmental exposure to either CPF or PF-04457845, there were no signs of overt toxicity or cholinergic hyperstimulation. With respect to weight gain, there was no significant overall effect of treatment or sex but there was a significant overall effect of day (p<0.0001) indicating significant growth over the period treatment. Weight gain following treatment is presented in Figure 1. The lack of effect on weight gain by the dosages of CPF used in this study is in agreement with our previous observations (Carr et al., 2017; Carr et al., 2011; Carr et al., 2014).
Figure 1.
Body weight gain of rat pups exposed daily from postnatal day 10 through 16 to either corn oil (control) 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF) or 0.02 mg/kg PF-04457845, a specific inhibitor of FAAH. Values are expressed as weight gain ± SE (n=44-50).
With respect to the effects of exposure on the activities of ChE, MAGL, and FAAH, there was no significant overall effect of sex and no significant sex × treatment interaction with any enzyme. Therefore, male and female data were pooled for analysis for each enzyme and results are presented in Table 1. There was a significant effect of treatment on ChE activity (F(4,113) = 22.97, p < 0.0001), MAGL activity (F(4,121) = 3.8, p = 0.0061), and FAAH activity (F(4,122) = 83.84, p < 0.0001). For ChE and MAGL, significant inhibition was only observed in the 1.0 mg/kg CPF treatment group. For FAAH, significant inhibition was present in all four treatment groups. The impact of this enzyme inhibition on the levels of the endocannabinoids AEA and 2-AG and the non-cannabinoid N-acylethanolamides PEA and OEA was also determined. There was no significant overall effect of sex and no significant sex × treatment interaction with either AEA, 2-AG, PEA, or OEA. There was a significant effect of treatment on the levels of 2-AG (F(4,110) = 4.63, p = 0.0017), AEA (F(1,145) = 19.39, p < 0.0001), OEA (F(4,105) = 28.35, p < 0.0001), and PEA (F(4,94) = 17.65, p < 0.0001). A significant increase (28%) in the levels of 2-AG was observed in the 1.0 mg/kg CPF treatment group with no effects observed with the lower CPF treatments or with PF-04457845. AEA, OEA and PEA levels were significantly increased in all four treatment groups.
Table 1.
Enzyme specific activities and endocannabinoid and N-acylethanolamine levels from the forebrain of rat pups exposed developmentally to either chlorpyrifos (CPF) or the FAAH inhibitor PF-044578451.
| Control2 | 0.5 mg/kg CPF |
0.75 mg/kg CPF |
1.0 mg/kg CPF |
0.02 mg/kg PF-04457845 |
|
|---|---|---|---|---|---|
| Enzymes3 | |||||
| AChE | 82.85 ± 1.01 | 82.81 ± 1.15 | 79.12 ± 1.22 | 64.11 ± 2.34* −23% |
85.18 ± 2.00 |
| MAGL | 348.83 ± 6.57 | 337.03 ± 10.01 | 339.57 ± 7.71 | 307.94 ± 13.41* −12% |
373.20 ± 8.60 |
| FAAH | 20.27 ± 0.53 | 15.03 ± 0.45* −26% |
13.45 ± 0.44* −34% |
8.74 ± 1.05* −57% |
5.029 ± 0.56* −75% |
| Endocannabinoids4 | |||||
| 2-AG | 12.25 ± 0.39 | 11.89 ± 0.97 | 13.26 ± 0.69 | 15.67 ± 0.73* +28% |
13.86 ± 0.70 |
| AEA | 25.16 ± 1.07 | 32.21 ± 1.53* +28% |
38.86 ± 2.61* +55% |
42.23 ± 3.23* +68% |
45.24 ± 3.33* +80% |
| N-acylethanolamines5 | |||||
| OEA | 14.67 ± 0.75 | 21.26 ± 1.57* +45% |
25.91 ± 1.50* +77% |
29.96 ± 2.59* +104% |
35.41 ± 3.32* +141% |
| PEA | 20.36 ± 1.14 | 31.31 ± 2.46* +54% |
44.59 ± 2.03* +119% |
47.55 ± 2.89* +133% |
66.35 ± 12.69* +226% |
Rat pups underwent daily oral exposure from PND10-16 and sampled at 12hrs following the last administration of chemical on PND16. Data presented is the result of analysis of new data combined with data previously published in Carr et al., 2011; 2013; 2017 and Buntyn et al. 2017.
Values are expressed as mean ± SE (n = 8-64). Asterisks (*) indicate statistical significance (p ≤ 0.05) relative from the respective control. Percent change from control levels are presented below each significantly different value.
Specific activity units are expressed as nmoles min−1 mg protein−1 for acetylcholinesterase (AChE) and pmoles min−1 mg protein−1 for monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH).
Endocannabinoid values are expressed as nmoles/ g tissue for 2-arachidonylglycerol (2-AG) and pmoles/ g tissue for arachidonoylethanolamide (AEA).
N-acylethanolamine values are expressed as pmoles/ g tissue for both oleoylethanolamide (OEA) and palmitoylethanolamide (PEA).
Open field (PND23).
With respect to locomotor activity in the open field, there was a significant overall effect of treatment for both total number of grid crosses (F(4,186) = 3.78, p = 0.0056) and distance traveled (F(4,186) = 5.1, p = 0.0006). There was no significant overall effect of sex and no significant sex × treatment interaction. Therefore, male and female data for each parameter were pooled for post hoc analysis. There was a significant increase in the number of grid crosses (Figure 2A) in the 1.0 mg/kg CPF treatment group (p=0.0095) and the PF-04457845 treatment group (p=0.0046) but no effects in the 0.5 or 0.75 mg/kg CPF treatment groups. There was a significant increase in the distance traveled (Figure 2B) in the 1.0 mg/kg CPF treatment group (p=0.0049) and the PF-04457845 treatment group (p=0.0015) but no effects in the 0.5 or 0.75 mg/kg CPF treatment groups. There were no significant effects on the number of crosses into the center zone (Figure 2C) or the time spent in the center zone (Figure 2D).
Figure 2.
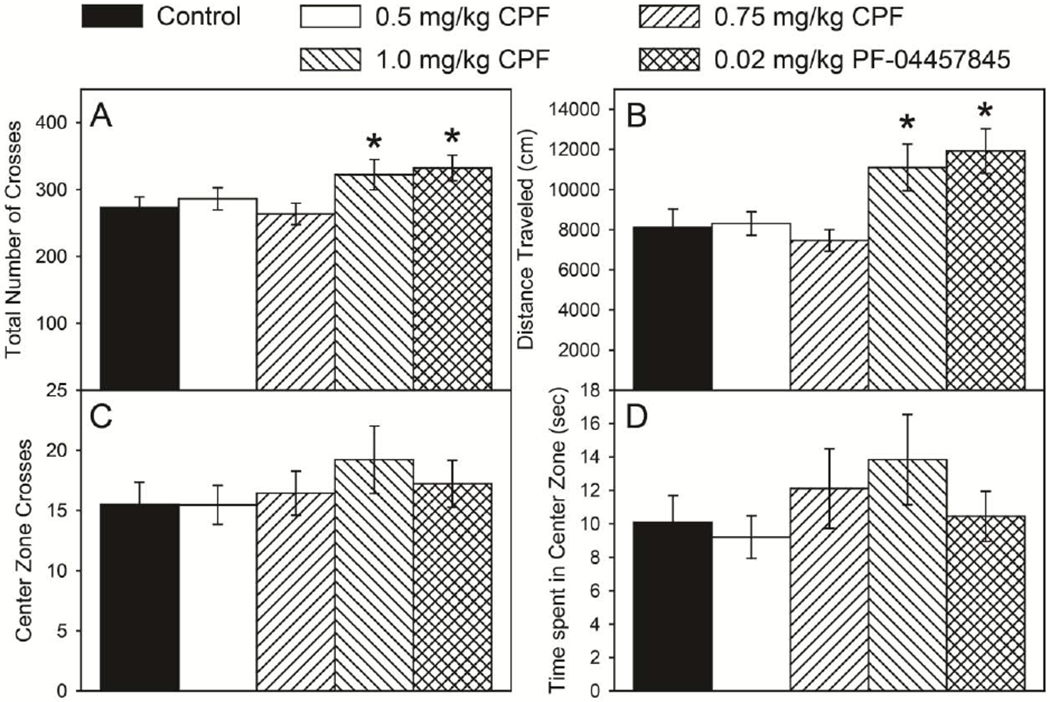
Total number of crosses (A), distance traveled (B), crossings in the center zone (C), and time spent in center zone (D) of rats tested in an open field as described in the Materials and methods. Behavior was measured on postnatal day 23 following daily exposure from postnatal day 10 through 16 to either corn oil (control) or 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF) or 0.02 mg/kg PF-04457845, a specific inhibitor of FAAH. Values are expressed as mean ± SE (n=42-46). Bars indicated with an asterisk (*) are statistically significant (p ≤ 0.05) from control.
Elevated plus maze (PND29).
With respect to performance in the elevated plus maze on PND29, there was no significant overall effect of sex and no significant sex × treatment interaction in any parameter. Therefore, male and female data for each parameter were pooled for post hoc analysis when treatment alone was significant (numerical data presented in Table 1). There was a significant overall effect of treatment for the number of entries into the open arms (F(4,201) = 5.63, p = 0.0003), the amount of time spent in the open arms (F(4,201) = 5.85, p = 0.0002), percent open arm entries (F(4,201) = 2.57, p = 0.0394), and percent open arm time (F(4,201) = 4.94, p = 0.0008). All three CPF treatment groups and the PF-04457845 treatment group had significantly higher numbers of entries into the open arms (Figure 3A) and spent significantly more time in the open arms (Figure 3B) as compared to the control group. When calculated as a percent of the total, all three CPF treatment groups and the PF-04457845 treatment group exhibited a significantly higher percent of open arm entries (Figure 3C) and percent time spent in the open arm (Figure 3D). These data suggest that all treatment groups had reduced levels of anxiety-like behavior possibly bordering on anxiolytic behavior.
Figure 3.
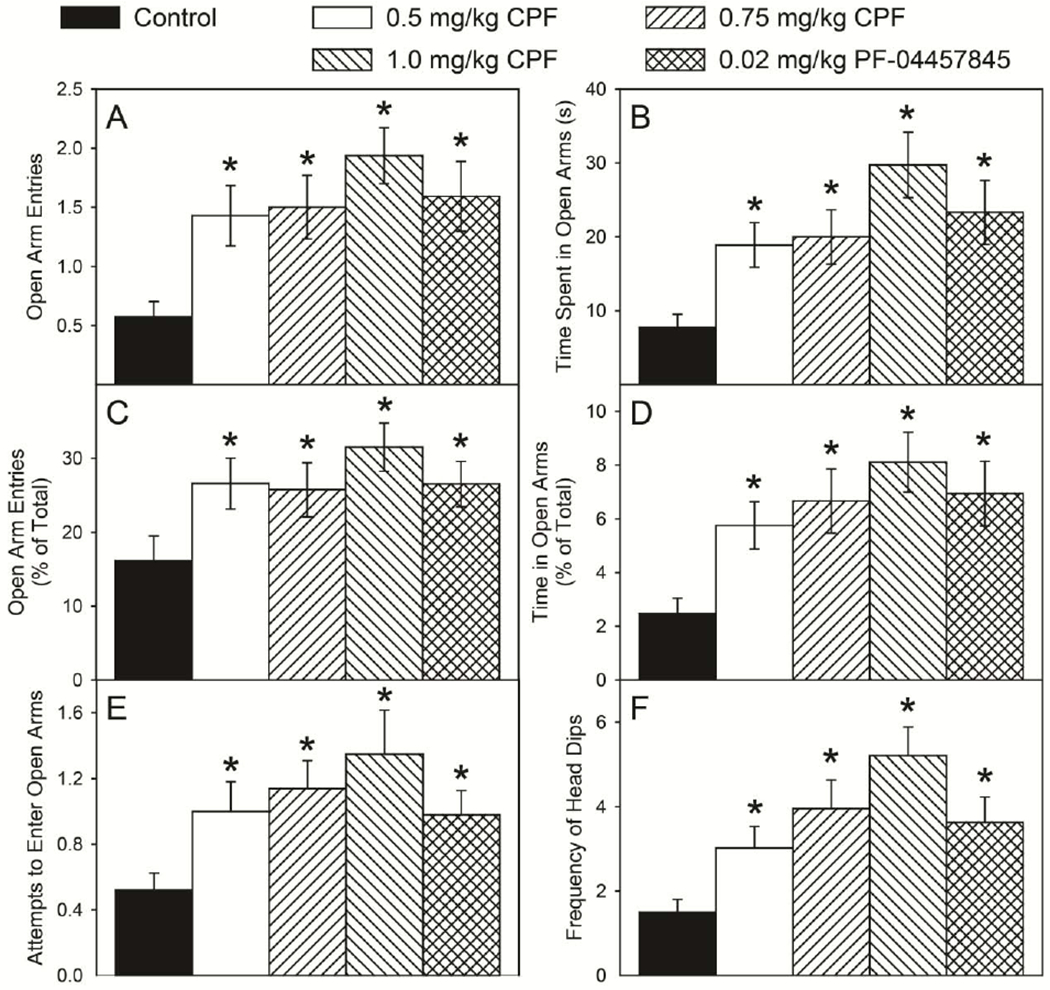
Open arm entries (A), time spent in open arms (B), open arm time (% of total) (C), and time in the open arms (% of total) (D), attempts to enter open arms (E) and frequency of head dips (F) of rats tested in an elevated plus maze as described in the Materials and methods. Behavior was measured on postnatal day 29 following daily exposure from postnatal day 10 through 16 to either corn oil (control) or 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF) or 0.02 mg/kg PF-04457845, a specific inhibitor of FAAH. Values are expressed as mean ± SE (n=44-50). Bars indicated with an asterisk (*) are statistically significant (p ≤ 0.05) from control.
However, there was also a significant overall effect of treatment for the amount of time spent in the closed arms (F(4,201) = 7.68, p < 0.0001), the number of entries into the closed arms (F(4,201) = 5.93, p = 0.0002), the total number of arm entries (F(4,201) = 6.83, p < 0.0001), and the number of central crosses (F(4,201) = 5.53, p=0.0003). As compared to controls, all three CPF treatment groups and the PF-04457845 treatment group spent significantly less time in the closed arms, made significantly higher numbers of entries into the closed arms, made significantly higher number of total arm entries, and made higher number of central crosses (Table 1). For the higher number central crosses, the increase was due to an increase in both the number of crosses from closed arm to closed arm (F(4,201) = 3.96, p = 0.0041) and the number of crosses from open arm to open arm (F(4,201) = 3.56, p = 0.0079) (Table 1). While not detected in the open field, the higher number of total arm entries and central crosses suggest that the rats in the treated groups had a higher overall activity levels compared to controls.
Stretch-attend postures, entry attempts, and head-dips in the open arms are ethologically relevant behaviors that are considered measures of the animal’s assessment of risk. There were no significant differences in the number of stretch-attend postures between treatment groups (Table 1) but there was a a significant overall effect of treatment for the number of attempts to enter the open arms (F(4,201) = 3.01, p < 0.0195) and the number of head-dips made from the open arms (F(4,201) = 5.54, p < 0.0003). All three CPF treatment groups and the PF-04457845 treatment group made significantly more attempts to enter the open arms (Figure 3E) and significantly more head-dips (Figure 3F) than did controls. These data are consistent with the higher overall activity levels observed in the treatment groups.
Social Interactions (PND35).
With respect to social interactions, there was no significant overall effect of sex and no significant sex × treatment interaction in any parameter. Therefore, male and female data for each parameter were pooled for post hoc analysis when treatment alone was significant. There was no significant effect for the time to first interaction, the frequency of grooming, the frequency of social interactions, or the frequency of pouncing/nape attacks. The frequency of pinning was over twice as high in the treatment groups as compared to control but due to high variability, it was not statistically significant. At the level of significance of p ≤ 0.1, there was a difference in the frequency of chasing, (F(4,82) = 2.05, p = 0.0951). All treatment groups exhibited a higher frequency of chasing with the 0.5 mg/kg CPF treatment group (p ≤ 0.0132) and the specific FAAH inhibitor treatment group (p ≤ 0.0184) being significantly different from controls while the 0.75 mg/kg CPF treatment group (p ≤ 0.0971) and the 1.0 mg/kg CPF treatment group (p ≤ 0.0968) trending towards significance (Figure 4A). However, there was a significant overall effect of treatment for the frequency of crawling over and under, (F(4,82) = 3.82, p = 0.0067), the frequency of play fighting (boxing and wrestling), (F(4,82) = 6.26, p = 0.0002), and the time spent playing (F(4,82) = 9.28, p = 0.0001). All treatment groups exhibited a significantly greater frequency of crawling over and under (Figure 4B) and of play fighting (Figure 4C) than did controls. In addition, once play fighting was initiated, all treatment groups spent significantly more time engaged in play fighting (Figure 4D) as compared to the controls.
Figure 4.
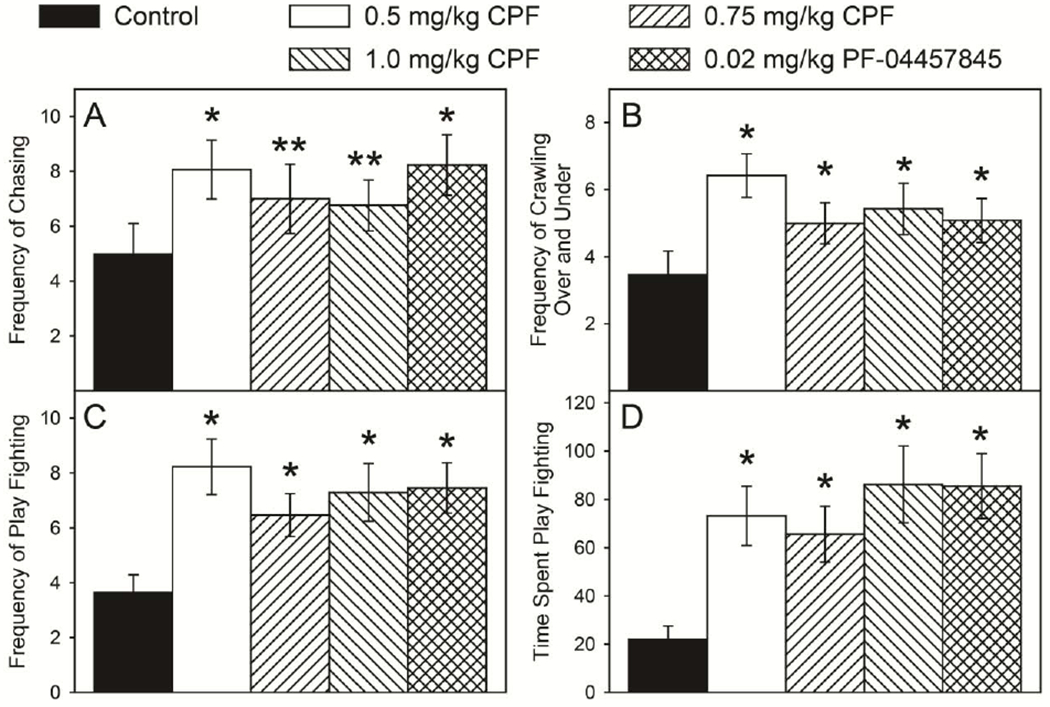
Frequency of chasing (A), frequency of crawling over and under (B), frequency of play fighting (C), and time spent play fighting (D) of rats tested in a social interaction test as described in the Materials and methods. Behavior was measured on postnatal day 35 following daily exposure from postnatal day 10 through 16 to either corn oil (control) or 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF) or 0.02 mg/kg PF-04457845, a specific inhibitor of FAAH. Values are expressed as mean ± SE (n=20-22). Bars indicated with a double asterisk (**) are statistically significant (p ≤ 0.1) and bars indicated with an asterisk (*) are statistically significant (p ≤ 0.05) from control.
CB1 Receptor Phosphorylation.
Immediately following completion of social play, the levels of the CB1 and CB2 receptors and the level of phosphorylation of the CB1 receptor were determined in selected brain regions. There were no changes in the levels of the CB1 or CB2 receptors either between control and treatment rats or between social play and non-behavioral rats in any of the five brain regions studied (data not shown). There was an increased level of CB1 receptor phosphorylation in rats who been engaged in social play as compared to non-behavioral rats in all the five brain regions. This was expected as it has previously been reported (Trezza et al., 2012) but there were no effects of CPF on CB1 phosphorylation (data not shown).
DISCUSSION
The biochemical data indicate that exposure to exposure to dosages of CPF (0.5 and 0.75 mg/kg CPF) that do not inhibit ChE do not inhibit MAGL or result in an increase of its substrate 2-AG. Likewise, exposure to PF-04457845 does not inhibit MAGL or alter 2-AG levels. The three dosages of CPF inhibited FAAH in a dose dependent manner and resulted in an increase in the levels of AEA in a dose dependent manner. Exposure to 0.02 mg/kg PF-04457845 resulted in a higher inhibition of FAAH and accumulation of AEA. This specific inhibition of FAAH and accumulation of AEA agrees with the current thought that FAAH is the primary enzyme responsible for the hydrolysis of AEA. FAAH is also responsible for the hydrolysis of the non-cannabinoid N-acylethanolamides palmitoylethanolamide (PEA) and oleoylethanolamide (OEA). All dosages of CPF and PF-04457845 led to the accumulation of both of these compounds. OEA and PEA are lipids structurally related to AEA but, unlike AEA, they do not activate CB1 receptors. Instead, they are primarily agonists at the nuclear peroxisome proliferator-activated receptor alpha (PPARα) (Fu et al., 2003; LoVerme et al., 2005). While AEA is an agonist to the CB1 receptor, it has also been reported to bind to the PPARα receptor (Sun et al., 2007). Historically, the PPARα was considered to mainly regulate fatty acid metabolism. However, many recent studies have demonstrated that PPARα activation has an influence on many neurological functions (Campolongo et al., 2009; D’Agostino et al., 2015; Guida et al., 2017; Jiang et al., 2017; Luchicchi et al., 2010; Mazzola et al., 2009a; Scheggi et al., 2016) but it not clear if these compounds play a role in brain maturation.
Exposure to the highest dosage of CPF increased locomotor activity in the open field. This finding is in agreement with a previous report using the same dosage (1 mg/kg) administered at a similar developmental age (Ricceri et al., 2003). As we have reported previously (Carr et al., 2013; Carr et al., 2017), exposure to this level of CPF results in significant inhibition of ChE. Since there was no inhibition of ChE or change in locomotor activity observed with the two lower dosages of CPF, it could be hypothesized that this increased level of activity was a lingering effect of the ChE inhibition. However, exposure to the specific FAAH inhibitor PF-04457845 also increased locomotor activity. This increase is in contrast to previous reports where acute exposure of adults to the FAAH inhibitor URB597 did not alter motor activity (Hill et al., 2007; Mazzola et al., 2009a, b) and repeated gestational/neonatal exposure to URB597 did not alter motor activity in the adult offspring (Wu et al., 2014). It is also in contrast to the previous report of decreased locomotor activity in adult animals exposed to URB597 during adolescence (Macri et al., 2012). Thus, it appears that the timing of exposure during development can result in different effects with respect to locomotor activity. Our data do suggest that inhibition of FAAH, at levels of 60% or higher, during the preweanling juvenile period can result in increased locomotor activity when measured during the preadolescent period. However, this exposure scenario does not appear to decrease anxiety levels since it did not result in more time spent in the center zone of the open field which is considered as a marker of reduced anxiety.
In the elevated plus maze, the increased number of open arm entries and greater time spent in the open arms in rats treated with both CPF and the specific inhibitor of FAAH suggest reduced anxiety-like behavior. Our previous reports also suggested this for CPF (Carr et al., 2017). However, in the open field, the highest dosage of CPF and PF-04457845 increased locomotor activity. In the EPM, all four treatment groups also appeared to exhibit increased activity levels as indicated by the increased total number of closed arm entries and number of central crosses. Thus, the increased open arm activity could be merely the result of increased spontaneous activity. It is difficult to simply declare decreased anxiety levels because of this difficulty in dissociating increased spontaneous activity from anxiolytic behavior. The ethological risk assessment behaviors have been proposed to be sensitive measures related to anxiety on the elevated plus maze (Rodgers and Cole, 1993; Rodgers et al., 1999). Of these behaviors, the treated rats exhibited increased episodes of head dipping. In a factor analysis study on the elevated plus maze, increased head dipping loaded positively on the reverse of anxiety factor (Cruz et al., 1994) suggesting that the treated rats in our study had a lower level of anxiety. However, it has been suggested that head dipping may be a direct measure of exploration and may be influenced more by changes in exploratory drive than by anxiety (Brown et al., 1999; File, 2001; Weiss et al., 1998). An increased exploratory drive could explain the increased locomotor activity and concurrent open arm activity that we observed. Stretch attend postures, another ethological behavior associated with anxiety (Cruz et al., 1994), were not statistically different between control and treated rats. Stretch attend postures can be considered a measure of risk assessment; that is, a behavior designed to gather information of potentially threatening situation (Blanchard and Blanchard, 1989; Grewal et al., 1997; Molewijk et al., 1995). The similarities in stretch attend postures in the different treatment groups imply that all of the animals, both control and treated, perceived the situation similarly and possibly were experiencing similar levels of anxiousness. However, it could be that increased exploratory drive in the treated rats made them less concerned about the potentially threatening situation and more willing to actively take risks and enter the open arms.
The literature contains multiple reports of developmental CPF exposure resulting in altered social behaviors in mice. Many of these involve either gestational exposure or combined gestational-postnatal exposure followed by the assessment of adult social behavior (De Felice et al., 2015; De Felice et al., 2014; Venerosi et al., 2006; Venerosi et al., 2015). Several studies did utilize an exposure period (PND11-14) similar to the one used in this study but assessed social behavior in adults rather than adolescents (Venerosi et al., 2008; Venerosi et al., 2010). In our study, the most significant effects on social behavior were altered social play parameters. In rats, social play emerges during the late preweanling period and its frequency continues to increase to its peak during adolescence (PND28-40) after which it declines with the onset of sexual maturity (Pellis and Pellis, 1997; Vanderschuren and Trezza, 2014). Thus, age differences in the time of measuring of social behavior make direct comparison of our results to other studies difficult. More importantly, mice, unlike rats, do not engage in complex social play except in a very rudimentary form (Bell, 2018; Pellis and Pasztor, 1999; Ricceri et al., 2007) which also makes comparisons difficult. However, one study did utilize a similar exposure period (PND11-14) to ours and measured social interactions during a similar period (PND45) (Ricceri et al., 2003). Similar to our study, this study reported no effects on non-social behavior (i.e., grooming) or investigative behaviors (i.e., social investigation) but did observe increased solicitation behaviors (i.e., chasing and crawling over/under). This suggests some effects are similar across these species.
Previous work has demonstrated that increasing anandamide levels either by administration of either the FAAH inhibitor URB597 or the endocannabinoid uptake blocker VDM11 enhances social play in adolescent rats (Manduca et al., 2014; Trezza et al., 2012; Trezza and Vanderschuren, 2008a, b, 2009). However, in all of these studies, social behavior was measured immediately following a single administration of each compound which would have resulted in accumulation of endocannabinoid levels during the period of behavioral testing. Our previous data involving juvenile CPFs exposure (PND10-16) indicates that the FAAH activity and endocannabinoid levels return to levels similar to that of controls with a few days following cessation of exposure (Carr et al., 2013). This suggests that the endocannabinoid levels in the brain would be at normal levels during the time that we conducted behavioral testing in this study. However, this does not imply that the exposure did not have a lasting effect. In fact, there are a few reports on the persistent effects of developmental effect of FAAH inhibition. Repeated adolescent exposure to the FAAH inhibitor URB597 resulted in altered levels of the CB1 receptor in adult rats (Marco et al., 2009) and decreased locomotor activity and decreased performance in a food reward progressive operant behavior in adult mice (Macri et al., 2012). Gestational/perinatal exposure to URB597 did not alter locomotion, anxiety, or sensorimotor gating in adult mice but did increase depressive-type behaviors, decrease spontaneous alternation, and decreased place preference for a passive reward (Wu et al., 2014). While none of these studies involved exposure during the developmental period utilized in the present study, a consensus in these studies was that inhibition of FAAH during development can induce subtle changes in behavior during latter life. The changes in adolescent behavior following inhibition of FAAH during juvenile ages observed in the present study support this premise.
Of interest, Wu et al., (2014) suggested that gestational/perinatal inhibition of FAAH altered the function of the reward circuits in adults as evidenced by the decreased conditioned place preference for cocaine. Reward is mediated by the dopaminergic, endocannabinoid, and opioid systems. The roles of these systems in social play have been well described (Trezza and Vanderschuren, 2008a, b, 2009). Social play is generally enhanced by increased activity in the opioid, endocannabinoid, or dopaminergic systems. The activity of the dopaminergic system modulates the effect of endocannabinoids on social play. Opioid and endocannabinoid neurotransmission also interact in the modulation of social play suggesting that the effect of each neurotransmitter system on social play is regulated by other neurotransmitter systems (Trezza and Vanderschuren, 2008b). In fact, the endocannabinoid system plays an important role in the maturation of multiple neurotransmitter systems including the opioid and dopaminergic system as evidenced by the findings where developmental exposure to exogenous cannabinoids altered the maturation of multiple neurotransmitter systems (Fernandez-Ruiz et al., 2004).Thus, any alteration in the endocannabinoid signaling during development may lead to alterations in the functions of other neurotransmitter systems that play a role in reward. This being said, the increased levels of social play in adolescents observed after juvenile CPF and PF-04457845 treatment could be the consequence of altered maturation of the functional circuits of the opioid or dopaminergic systems or even another neurotransmitter system. However, the molecular mechanisms responsible for this altered behavior are still unclear.
In conclusion, we have demonstrated previously that the developmental exposure to low levels of CPF inhibits endocannabinoid metabolism resulting in accumulation of endocannabinoids and disrupts anxiety-like behavior in preadolescent rats (Carr et al., 2017). In this study, we utilized additional behavioral tests to attempt to confirm this effect on anxiety since a single behavioral test does not provide a clear picture of anxiety related behavior. However, we report here that developmental exposure to CPF does not alter anxiety but rather increases exploratory behavior and increases social play. These behavioral changes occurred even though the testing occurred under aversive conditions which should have suppressed these behaviors. We also demonstrate that exposure to PF-04457845 significantly inhibits FAAH and results in accumulation of AEA, OEA, and PEA shortly after exposure which is similar to what is observed following CPF exposure. Identification of the mechanisms responsible for this altered behavior will be a focus in future studies. One important finding of this study is that a similar pattern of adolescent behavioral alterations was induced as a result of treatment of juveniles with either CPF or PF-04457845. This strongly supports the hypothesis that one mechanism responsible for many of the persistent effects associated with developmental CPF exposure is the inhibition of brain FAAH that occurs during the exposure.
Table 2.
Parameters obtained in the Elevated Plus Maze in Adolescent Rats exposed Developmentally to either Chlorpyrifos (CPF) or the FAAH Inhibitor PF-04457845.
| Control | 0.5 mg/kg CPF |
0.75 mg/kg CPF |
1.0 mg/kg CPF |
0.02 mg/kg PF-04457845 |
|
|---|---|---|---|---|---|
| Open Arm Entries | 0.57 ± 0.13 | 1.43 ± 0.25* p = 0.0076 |
1.50 ± 0.27* p = 0.0046 |
1.94 ± 0.24* p ≤ 0.0001 |
1.59 ± 0.30* p = 0.0011 |
| Open Arm Time | 7.72 ± 1.81 | 24.52 ± 6.35* p = 0.0122 |
19.98 ± 3.66* p = 0.0111 |
29.72 ± 4.43* p ≤ 0.0001 |
23.31 ± 4.31* p = 0.0008 |
| % Open Arm Entries | 16.16 ± 3.36 | 26.59 ± 3.43* p = 0.0354 |
25.75 ± 3.65* p = 0.0399 |
31.51 ± 3.27* p = 0.0023 |
26.53 ± 3.06* p = 0.0364 |
| % Open Arm Time | 2.47 ± 0.57 | 5.76 ± 0.88* p = 0.0122 |
6.66 ± 1.19* p = 0.0035 |
8.11 ± 1.12* p ≤ 0.0001 |
6.94 ± 1.20* p = 0.0010 |
| Closed Arm Time | 288.23 ± 2.26 | 273.49 ± 3.86* p = 0.0025 |
274.39 ± 3.83* p = 0.0126 |
258.81 ± 5.37* p = 0.0001 |
267.78 ± 4.90* p = 0.0001 |
| Closed Arm Entries | 1.55 ± 0.16 | 2.43 ± 0.32* p = 0.0372 |
3.09 ± 0.39* p = 0.0005 |
3.13 ± 0.32* p ≤ 0.0001 |
3.06 ± 0.37* p = 0.0005 |
| Total Number of Entries | 2.13 ± 0.26 | 3.86 ± 0.53* p = 0.0094 |
4.59 ± 0.56* p = 0.0004 |
5.06 ± 0.49* p ≤ 0.0001 |
4.65 ± 0.58* p = 0.0003 |
| Central Crosses | 0.38 ± 0.15 | 1.18 ± 0.24* p = 0.0132 |
1.95 ± 0.39* p = 0.0001 |
1.60 ± 0.29 p = 0.0002 |
1.84 ± 0.39* p = 0.0003 |
| Closed Arm Crosses | 0.32 ± 0.14 | 0.65 ± 0.15** p = 0.0813 |
1.34 ± 0.34* p = 0.0048 |
1.00 ± 0.22* p = 0.0023 |
1.39 ± 0.31* p = 0.0006 |
| Open Arm Crosses | 0.06 ± 0.04 | 0.52 ± 0.13* p = 0.0064 |
0.61 ± 0.18* p = 0.0035 |
0.60 ± 0.13* p = 0.0010 |
0.45 ± 0.17* p = 0.0479 |
| Stretch-attend Postures | 0.94 ± 0.17 | 1.28 ± 0.15 NS |
1.70 ± 0.24 NS |
1.26 ± 0.18 NS |
1.35 ± 0.24 NS |
| Attempts | 0.52 ± 0.10 | 0.98 ± 0.18* p = 0.0560 |
1.14 ± 0.17* p = 0.0034 |
1.35 ± 0.27* p = 0.0032 |
0.98 ± 0.15* p = 0.0262 |
| Head-Dipping | 1.50 ± 0.30 | 3.02 ± 0.51* p = 0.0706 |
3.95 ± 0.68* p = 0.0058 |
5.20 ± 0.68* p ≤ 0.0001 |
3.63 ± 0.60* p = 0.0057 |
Values are expressed as mean ± SE (n = 44-50).
Data presented as means ± SEM. Asterisks indicate significantly different from controls (p value presented under mean). NS = not significant from controls.
Highlights.
Chlorpyrifos (CPF) is an organophosphorus insecticide and suspected developmental neurotoxicant.
Repeated exposure of juvenile rats to CPF alters the adolescent pattern of behavior including increased exploratory behavior and increased social play behaviors.
Similar biochemical effects and adolescent behavioral alterations were induced as a result of treatment of juveniles with either CPF or the specific fatty acid amide hydrolase (FAAH) inhibitor PF-04457845.
Our data suggest that the inhibition of brain FAAH that occurs during the exposure is one mechanism responsible for many of the persistent effects associated with developmental CPF exposure.
ACKNOWLEDGMENTS
The authors acknowledge the statistical expertise of Dr. Robert Wills. Research was supported by the Mississippi Agricultural and Forestry Experiment Station (MAFES), the College of Veterinary Medicine, Mississippi State University, and NIH R15ES023162. LC-MS/MS analyses was supported by R15ES015348-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT, Young T, Wren P, Zhang Y, Swaney S, Van Becelaere K, Blankman JL, Nomura DK, Bhattachar SN, Stiff C, Nomanbhoy TK, Weerapana E, Johnson DS, Cravatt BF, 2011. Mechanistic and pharmacological characterization of PF-04457845: a highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. Journal of Pharmacology and Experimental Therapeutics 338(1), 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA, 2005. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environmental Health Perspectives 113, 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG, 1990. Practical Statistics for Medical Research. Chapman & Hall/CRC, London. [Google Scholar]
- Andersen SL, 2003. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews 27(1-2), 3–18. [DOI] [PubMed] [Google Scholar]
- Arcury TA, Grzywacz JG, Barr DB, Tapia J, Chen H, Quandt SA, 2007. Pesticide urinary metabolite levels of children in eastern North Carolina farmworker households. Environmental Health Perspectives 115, 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, 2018. Comparing postnatal development of gonadal hormones and associated social behaviors in rats, mice, and humans. Endocrinology 159, 2596–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom G, 1958. Statistical Estimates and Transformed Beta Variables. John Wiley and Sons, Inc., New York, NY. [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B, 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environmental Health Perspectives 119(8), 1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquenier JB, Quertemont E, Tirelli E, Plumier JC, 2010. Anxiety in adult female mice following perinatal exposure to chlorpyrifos. Neurotoxicology & Teratology 32(2), 234–239. [DOI] [PubMed] [Google Scholar]
- Brown RE, Corey SC, Moore AK, 1999. Differences in measures of exploration and fear in MHC-Congenic C57BL/6J and B6-H-2K mice. Behavior Genetics 29, 263–271. [Google Scholar]
- Buntyn RW, Alugubelly N, Hybart RL, Mohammed AN, Nail CA, Parker GC, Ross MK, Carr RL, 2017. Inhibition of endocannabinoid-metabolizing enzymes in peripheral tissues following developmental chlorpyrifos exposure in rats. International Journal of Toxicology 36(5), 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Dawson J, Galvin K, Thorne PS, Rohlman DS, 2016. Organophosphorus pesticide exposure and neurobehavioral performance in Latino children living in an orchard community. NeuroToxicology 53, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Ratano P, Manduca A, Scattoni ML, Palmery M, Trezza V, Cuomo V, 2012. The endocannabinoid transport inhibitor AM404 differentially modulates recognition memory in rats depending on environmental aversiveness. Frontiers in Behavioral Neuroscience 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, Fu J, McGaugh JL, Piomelli D, 2009. Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proceedings of the National Academy of Sciences of the United States of America 106(19), 8027–8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Trezza V, Ratano P, Palmery M, Cuomo V, 2011. Developmental consequences of perinatal cannabis exposure: Behavioral and neuroendocrine effects in adult rodents. Psychopharmacology 214(1), 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Adams AL, Kepler DR, Ward AB, Ross MK, 2013. Induction of endocannabinoid levels in juvenile rat brain following developmental chlorpyrifos exposure. Toxicological Sciences 135(1), 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Armstrong NH, Buchanan AT, Eells JB, Mohammed AN, Ross MK, Nail CA, 2017. Decreased anxiety in juvenile rats following exposure to low levels of chlorpyrifos during development. NeuroToxicology 59, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Borazjani A, Ross MK, 2011. Effect of developmental chlorpyrifos exposure, on endocannabinoid metabolizing enzymes, in the brain of juvenile rats. Toxicological Sciences 122(1), 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Graves CA, Mangum LC, Nail CA, Ross MK, 2014. Low level chlorpyrifos exposure increases anandamide accumulation in juvenile rat brain in the absence of brain cholinesterase inhibition. NeuroToxicology 43, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Smit AB, Pattij T, Spijker S, 2011. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Developmental Cognitive Neuroscience 1, 430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG, 1994. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacology, Biochemistry and Behavior 49, 171–176. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Sweeney FF, 2011. The age of anxiety: role of animal models of anxiolytic action in drug discovery. British journal of pharmacology 164, 1129–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino G, Cristiano C, Lyons DJ, Citraro R, Russo E, Avagliano C, Russo R, Raso GM, Meli R, De Sarro G, Heisler LK, Calignano A, 2015. Peroxisome proliferator-activated receptor alpha plays a crucial role in behavioral repetition and cognitive flexibility in mice. Molecular Metabolism 4(7), 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice A, Scattoni ML, Ricceri L, Calamandrei G, 2015. Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLOS ONE 10, e0121663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice A, Venerosi A, Ricceri L, Sabbioni M, Scattoni ML, Chiarotti F, Calamandrei G, 2014. Sex-dimorphic effects of gestational exposure to the organophosphate insecticide chlorpyrifos on social investigation in mice. Neurotoxicology and Teratology 46, 32–39. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur I, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS, 2011. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environmental Health Perspectives 119, 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Gomez M, Hernandez M, de Miguel R, Ramos JA, 2004. Cannabinoids and gene expression during brain development. Neurotoxicity Research 6(5), 389–401. [DOI] [PubMed] [Google Scholar]
- File SE, 2001. Factors controlling measures of anxiety and responses to novelty in the mouse. Behavioural Brain Research 125(1–2), 151–157. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, LoVerme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D, 2003. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 425(6953), 90–93. [DOI] [PubMed] [Google Scholar]
- Grube A, Donaldson D, Kiely T, Wu L, 2011. Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates, EPA 733-R-11-001, U.S. Environmental Protection Agency, Washington, DC. pp. 1–41. [Google Scholar]
- Guida F, Boccella S, Iannotta M, De Gregorio D, Giordano C, Belardo C, Romano R, Palazzo E, Scafuro MA, Serra N, de Novellis V, Rossi F, Maione S, Luongo L, 2017. Palmitoylethanolamide reduces neuropsychiatric behaviors by restoring cortical electrophysiological activity in a mouse model of mild traumatic brain injury. Frontiers in Pharmacology 8, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Bradman A, Harley KG, Kogut K, Eskenazi B, 2017. Prenatal residential proximity to agricultural pesticide use and IQ in 7-year-old children. Environmental Health Perspectives 125, 057002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S, 2009. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology 204(4), 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Matas A, Ucha M, Ambrosio E, 2015. Long-term consequences of perinatal and adolescent cannabinoid exposure on neural and psychological processes. Neuroscience and Biobehavioral Reviews 55, 119–146. [DOI] [PubMed] [Google Scholar]
- Hill MN, Karacabeyli ES, Gorzalka BB, 2007. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology 32, 350–357. [DOI] [PubMed] [Google Scholar]
- Himmler BT, Pellis VC, Pellis SM, 2013. Peering into the dynamics of social interactions: measuring play fighting in rats. Journal of Visualized Experiments(71), e4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S, 1996. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacology, Biochemistry, and Behavior 54, 21–30. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS, 1970. Conditions under Which Mean Square Ratios in Repeated Measurements Designs Have Exact F-Distributions. Journal of the American Statistical Association 65(332), 1582–1589. [Google Scholar]
- Icenogle LM, Christopher NC, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, Slotkin TA, Levin ED, 2004. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicology & Teratology 26(1), 95–101. [DOI] [PubMed] [Google Scholar]
- Jiang B, Wang YJ, Wang H, Song L, Huang C, Zhu Q, Wu F, Zhang W, 2017. Antidepressant-like effects of fenofibrate in mice via the hippocampal brain-derived neurotrophic factor signalling pathway. British Journal of Pharmacology 174(2), 177–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch D, Lu C, Fisker-Andersen J, Jolley L, Fenske RA, 2002. Temporal association of children’s pesticide exposure and agricultural spraying: Report of a longitudinal biological monitoring study. Environmental Health Perspectives 110(8), 829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku KM, Weir RK, Silverman JL, Berman RF, Bauman MD, 2016. Behavioral phenotyping of juvenile Long-Evans and Sprague-Dawley rats: Implications for preclinical models of autism spectrum disorders. PLOS ONE 11(6), e0158150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, 1996. Sas System for Mixed Models. SAS Institute, Inc, Cary, NC. [Google Scholar]
- LoVerme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D, 2005. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Molecular Pharmacology 67(1), 15–19. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ, 1951. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193(1), 265–275. [PubMed] [Google Scholar]
- Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL, Yasar S, Goldberg SR, Pistis M, 2010. Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-α nuclear receptors. Addiction Biology 15(3), 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Ceci C, Canese R, Laviola G, 2012. Prenatal stress and peripubertal stimulation of the endocannabinoid system differentially regulate emotional responses and brain metabolism in mice. PLOS ONE 7(7), e41821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca A, Servadio M, Campolongo P, Palmery M, Trabace L, Vanderschuren LJ, Cuomo V, Trezza V, 2014. Strain- and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behavior in rats. European Neuropsychopharmacology 24(8), 1337–1348. [DOI] [PubMed] [Google Scholar]
- Marco EM, Rubino T, Adriani W, Viveros MP, Parolaro D, Laviola G, 2009. Long-term consequences of URB597 administration during adolescence on cannabinoid CB1 receptor binding in brain areas. Brain Research 1257, 25–31. [DOI] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B, 2010. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environmental Health Perspectives 118(12), 1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, Drago F, Cadet JL, Goldberg SR, Yasar S, 2009a. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-α nuclear receptors. Learning & Memory 16(5), 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, Drago F, Cadet JL, Goldberg SR, Yasar S, 2009b. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learn Mem 16(5), 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R, 2001. The use of behavioral test batteries: Effects of training history. Physiology & Behavior 73(5), 705–717. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH, 2007. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology 192(1), 61–70. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Blankman JL, Simon GM, Fujioka K, Issa RS, Ward AM, Cravatt BF, Casida JE, 2008. Activation of the endocannabinoid system by organophosphorus nerve agents. Nature Chemical Biology 4(6), 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Casida JE, 2011. Activity-based protein profiling of organophosphorus and thiocarbamate pesticides reveals multiple serine hydrolase targets in mouse brain. Journal of Agricultural and Food Chemistry 59(7), 2808–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S, 2006. The use of behavioral test batteries. II: Effect of test interval. Physiol Behav 87(1), 95–102. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pasztor TJ, 1999. The developmental onset of a rudimentary form of play fighting in C57 mice. Developmental Psychobiology 34(3), 175–182. [PubMed] [Google Scholar]
- Pellis SM, Pellis VC, 1997. The prejuvenile onset of play fighting in laboratory rats (Rattus norvegicus). Developmental Psychobiology 31(3), 193–205. [PubMed] [Google Scholar]
- Prut L, Belzung C, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology 463(1-3), 3–33. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE, 2006. Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice. Toxicology & Applied Pharmacology 211(1), 78–83. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE, 2001. Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicology and Applied Pharmacology 173(1), 48–55. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Segall Y, Nomura DK, Casida JE, 2002. Selective inhibitors of fatty acid amide hydrolase relative to neuropathy target esterase and acetylcholinesterase: toxicological implications. Toxicology and Applied Pharmacology 179(1), 57–63. [DOI] [PubMed] [Google Scholar]
- Ramos A, 2008. Animal models of anxiety: do I need multiple tests? Trends in Pharmacological Sciences 29, 493–498. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Markina N, Valanzano A, Fortuna S, Cometa MF, Meneguz A, Calamandrei G, 2003. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicology and Applied Pharmacology 191(3), 189–201. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Moles A, Crawley J, 2007. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behavioural Brain Research 176(1), 40–52. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, Calamandrei G, 2006. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicological Sciences 93(1), 105–113. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC, 1993. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiology & Behavior 54, 729–736. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF, 1999. Corticosterone response to the plus-maze: High correlation with risk assessment in rats and mice. Physiology & Behavior 68, 47–53. [DOI] [PubMed] [Google Scholar]
- Ruckart PZ, Kakolewski K, Bove FJ, Kaye WE, 2004. Long-term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio. Environmental Health Perspectives 112, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggi S, Melis M, De Felice M, Aroni S, Muntoni AL, Pelliccia T, Gambarana C, De Montis MG, Pistis M, 2016. PPARα modulation of mesolimbic dopamine transmission rescues depression-related behaviors. Neuropharmacology 110(Pt A), 251–259. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F, 2007. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: Similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environmental Health Perspectives 115(6), 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ, 2006. Organophosphate insecticides target the serotonergic system in developing rat brain regions: Disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environmental Health Perspectives 114(10), 1542–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T, 2011. Animal models of anxiety disorders in rats and mice: some conceptual issues. Dialogues in clinical neuroscience 13, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, Kendall DA, Bennett AJ, 2007. Cannabinoid activation of PPARα: A novel neuroprotective mechanism. British Journal of Pharmacology 152(5), 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS, 2010. Normal development of brain circuits. Neuropsychopharmacology 35, 147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED, 2008a. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicology & Teratology 30(1), 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, Wells C, Perraut C, Seidler FJ, Slotkin TA, Levin ED, 2008b. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Research Bulletin 77(6), 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Manduca A, Petrosino S, Van Kerkhof LW, Pasterkamp RJ, Zhou Y, Campolongo P, Cuomo V, Di Marzo V, Vanderschuren LJ, 2012. Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. Journal of Neuroscience 32(43), 14899–14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ, 2008a. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology (Berl) 197(2), 217–227. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ, 2008b. Cannabinoid and opioid modulation of social play behavior in adolescent rats: Differential behavioral mechanisms. European Neuropsychopharmacology 18(7), 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ, 2009. Divergent effects of anandamide transporter inhibitors with different target selectivity on social play behavior in adolescent rats. Journal of Pharmacology and Experimental Therapeutics 328(1), 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wendel de Joode B, Mora AM, Lindh CH, Hernandez-Bonilla D, Cordoba L, Wesseling C, Hoppin JA, Mergler D, 2016. Pesticide exposure and neurodevelopment in children aged 6-9 years from Talamanca, Costa Rica. Cortex 85, 137–150. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM, 1997. The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral Reviews 21(3), 309–326. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Trezza V, 2014. What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. Current Topics in Behavioral Neurosciences 16, 189–212. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, 2002. Acute effects of ethanol on social behavior of adolescent and adult rats: Role of familiarity of the test situation. Alcoholism, Clinical and Experimental Research 26(10), 1502–1511. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Calamandrei G, Ricceri L, 2006. A social recognition test for female mice reveals behavioral effects of developmental chlorpyrifos exposure. Neurotoxicology & Teratology 28(4), 466–471. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Cutuli D, Colonnello V, Cardona D, Ricceri L, Calamandrei G, 2008. Neonatal exposure to chlorpyrifos affects maternal responses and maternal aggression of female mice in adulthood. Neurotoxicology and Teratology 30(6), 468–474. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Rungi A, Sanghez V, Calamandrei G, 2010. Gestational exposure to the organophosphate chlorpyrifos alters social-emotional behaviour and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology 208(1), 99–107. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Tait S, Stecca L, Chiarotti F, De Felice A, Cometa MF, Volpe MT, Calamandrei G, Ricceri L, 2015. Effects of maternal chlorpyrifos diet on social investigation and brain neuroendocrine markers in the offspring - a mouse study. Environmental Health 14, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SM, Wadsworth G, Fletcher A, Dourish CT, 1998. Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neuroscience and Biobehavioral Reviews 23, 265–271. [DOI] [PubMed] [Google Scholar]
- Wu CS, Morgan D, Jew CP, Haskins C, Andrews MJ, Leishman E, Spencer CM, Czyzyk T, Bradshaw H, Mackie K, Lu HC, 2014. Long-term consequences of perinatal fatty acid amino hydrolase inhibition. British Journal of Pharmacology 171(6), 1420–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]


