Abstract
The emergence of SARS-CoV in 2003 and SARS-CoV-2 in 2019 highlights the need to develop universal vaccination strategies against the broader Sarbecovirus subgenus. Using chimeric spike designs, we demonstrate protection against challenge from SARS-CoV, SARS-CoV-2, SARS-CoV-2 B.1.351, bat CoV (Bt-CoV) RsSHC014, and a heterologous Bt-CoV WIV-1 in vulnerable aged mice. Chimeric spike mRNAs induced high levels of broadly protective neutralizing antibodies against high-risk Sarbecoviruses. In contrast, SARS-CoV-2 mRNA vaccination not only showed a marked reduction in neutralizing titers against heterologous Sarbecoviruses, but SARS-CoV and WIV-1 challenge in mice resulted in breakthrough infection. Chimeric spike mRNA vaccines efficiently neutralized D614G, UK B.1.1.7., mink cluster five, and the South African B.1.351 variant of concern. Thus, multiplexed-chimeric spikes can prevent SARS-like zoonotic coronavirus infections with pandemic potential.
Keywords: SARS-CoV-2, SARS-like virus, Sarbecovirus, mRNA vaccine, universal coronavirus vaccine
Sentence:
Chimerized RBD, NTD, and S2 spike mRNA-LNPs protect mice against epidemic, zoonotic, and pandemic SARS-like viruses
Introduction
A novel severe acute respiratory syndrome coronavirus (SARS-CoV) emerged in 2003 and caused more than 8,000 infections and ~800 deaths worldwide (1). Less than a decade later, the Middle East Respiratory Syndrome (MERS-CoV) coronavirus emerged in Saudi Arabia in 2012 (2), with multiple outbreaks that have resulted in at least ~2,600 cases and 900 deaths (3). In December 2019, another novel human SARS-like virus from the genus Betacoronavirus and subgenus Sarbecovirus emerged in Wuhan China, designated SARS-CoV-2, causing the COVID-19 pandemic (4, 5).
Bats are known reservoirs of SARS-like coronaviruses (CoVs) and harbor high-risk “pre-emergent” SARS-like variant strains, such as WIV-1-CoV and RsSHC014-CoV, which are able to use human ACE2 receptors for entry, replicate efficiently in primary airway epithelial cells, and in mice, and may escape existing countermeasures (6–12) Given the high pandemic potential of zoonotic and epidemic Sarbecoviruses (12), the development of countermeasures, such as broadly effective vaccines, antibodies and drugs is a global health priority (13–16).
Sarbecovirus spike proteins have immunogenic domains: the receptor binding domain (RBD), the N-terminal domain (NTD), and the subunit 2 (S2) (17–20). RBD, NTD, and S2 are a target for neutralizing antibodies elicited in the context of natural SARS-CoV-2 and MERS-CoV infections (17, 20–24). In fact, passive immunization with SARS-CoV-2 NTD-specific antibodies protect naïve mice from challenge, demonstrating that the NTD is a target of protective immunity (18, 24, 25). However, it remains unclear if vaccine-elicited neutralizing antibodies can protect against in vivo challenge with heterologous epidemic and bat coronaviruses. Here, we generated nucleoside-modified mRNA-lipid nanoparticle (LNP) vaccines expressing chimeric spikes containing admixtures of RBD and NTD domains from zoonotic, epidemic, and pandemic CoVs and examined their efficacy against homologous and heterologous Sarbecovirus challenge in aged mice.
Results
Design and expression of chimeric spike constructs to cover pandemic and zoonotic SARS-related coronaviruses
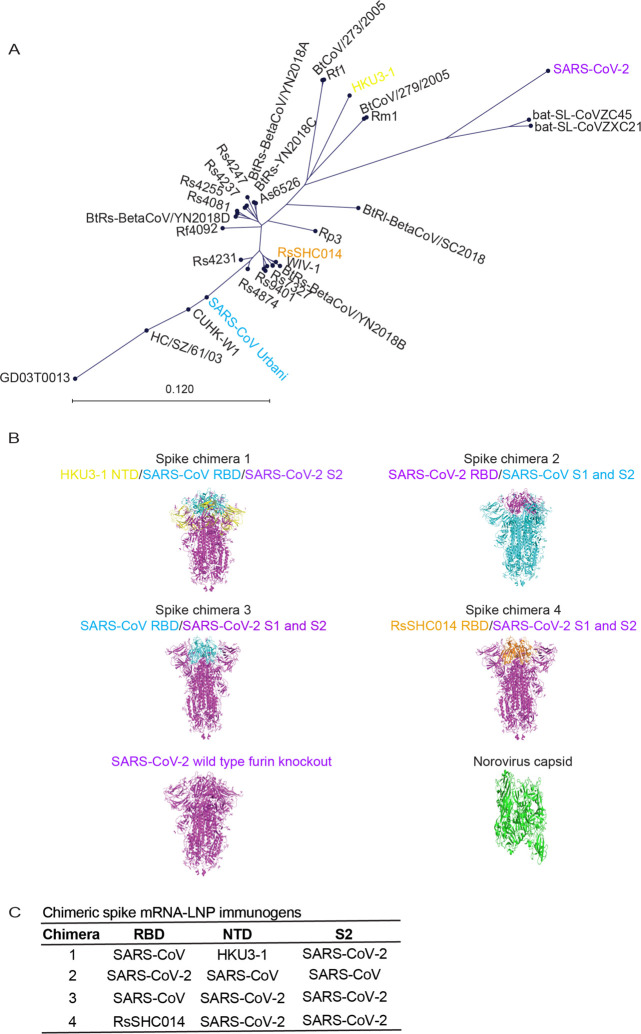
Sarbecoviruses exhibit considerable genetic diversity (Fig. 1A) and SARS-like bat CoVs (Bt-CoVs) are recognized threats to human health (8, 12). Harnessing the modular structure of CoV spikes (26), we designed chimeric spikes by admixture of divergent clade I-III Sarbecovirus NTD, RBD, and S2 domains into “bivalent” and “trivalent” vaccine immunogens that have the potential to elicit broad protective antibody responses against distant strains (e.g., Sarbecovirus). The approach is designed to maximize immune breadth in monovalent and multiplexed formulations. We designed four sets of chimeric spike constructs that contained admixtures of the RBD and/or NTD, and S2 neutralizing domains from various Sarbecoviruses. Chimera 1 included the NTD from clade II Bt-CoV Hong Kong University 3–1 (HKU3–1), the clade I SARS-CoV RBD, and the clade III SARS-CoV-2 S2 (Fig. 1B). Chimera 2 included SARS-CoV-2 RBD and SARS-CoV NTD and S2 domains (16). Chimera 3 included the SARS-CoV RBD, and SARS-CoV-2 NTD and S2, while chimera 4 included the RsSHC014 RBD, and SARS-CoV-2 NTD and S2. We also generated a monovalent SARS-CoV-2 spike furin knock out (KO) vaccine, partially phenocopying the Moderna and Pfizer mRNA vaccines in human use, and a negative control norovirus GII capsid vaccine (Fig. 1B, 1C). We generated these chimeric spikes and control spikes as lipid nanoparticle-encapsulated, nucleoside-modified mRNA vaccines with LNP adjuvants (mRNA-LNP) as described previously (27). This mRNA LNP stimulate robust T follicular helper cell activity, germinal center B cell responses, and durable long-lived plasma cells and memory B cell responses (20, 28). We verified their chimeric spike expression in HEK cells (Fig. S1B). To confirm that scrambled coronavirus spikes are biologically functional, we also designed and recovered several high titer recombinant live viruses of RsSHC014/SARS-CoV-2 S1, NTD, RBD and S2 domain chimeras that included deletions in non-essential, accessory ORF7&8 and that encoded nanoluciferase (Fig. S1C).
Figure 1. Genetic design of chimeric Sarbecovirus spike vaccines.
(A) Genetic diversity of pandemic and bat zoonotic coronaviruses. SARS-CoV is shown in light blue, RsSHC014 is shown in purple, and SARS-CoV-2 is shown in red. (B) Spike chimera 1 includes the NTD from HKU3–1, the RBD from SARS-CoV, and the rest of the spike from SARS-CoV-2. Spike chimera 2 includes the RBD from SARS-CoV-2 and the NTD and S2 from SARS-CoV. Spike chimera 3 includes the RBD from SARS-CoV and the NTD and S2 SARS-CoV-2. Spike chimera 4 includes the RBD from RsSHC014 and the rest of the spike from SARS-CoV-2. SARS-CoV-2 furin KO spike vaccine and is the norovirus capsid vaccine. (C) Table summary of chimeric spike constructs.
Immunogenicity of mRNAs expressing chimeric spike constructs against coronaviruses
We next sought to determine if simultaneous immunization with mRNA-LNP expressing the chimeric spikes of diverse Sarbecoviruses was a feasible strategy to elicit broad binding and neutralizing antibodies. We immunized aged mice with the chimeric spikes formulated to induce type-specific and/or cross-reactive responses against multiple divergent clade I-III Sarbecoviruses, a SARS-CoV-2 furin KO spike, and a GII.4 norovirus capsid negative control. Group 1 was primed and boosted with chimeric spikes 1, 2, 3, and 4 (Fig. S1A). Group 2 was primed with chimeric spikes 1 and 2 and boosted with chimeric spikes 3 and 4 (Fig. S1A). Group 3 was primed and boosted with chimeric spike 4 (Fig. S1A). Group 4 was primed and boosted with the monovalent SARS-CoV-2 furin knockout spike (Fig. S1A). Finally, group 5 was primed and boosted with a norovirus capsid GII.4 Sydney 2011 strain (Fig. S1A). We then examined the binding antibody responses by ELISA against a diverse panel of CoV spike proteins that included epidemic, pandemic, and zoonotic coronaviruses.
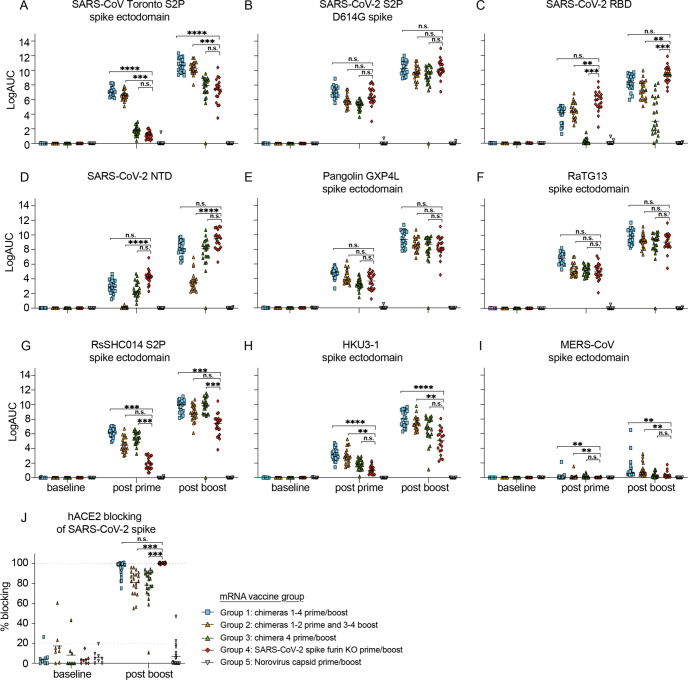
Mice in groups 1 and 2 generated the highest magnitude responses to SARS-CoV Toronto Canada isolate (Tor2), RsSHC014, and HKU3–1 spike compared to group 4 (Fig 2A, 2G, and 2H). While mice in group 2 generated lower magnitude binding responses to both SARS-CoV-2 RBD (Fig. 2C) and SARS-CoV-2 NTD (Fig 2D), mice in group 1 generated similar magnitude binding antibodies to SARS-CoV-2 D614G compared to mice immunized with the SARS-CoV-2 furin KO spike mRNA-LNP (Fig 2B). Mice in groups 1 and 2 generated similar magnitude binding antibody responses against SARS-CoV-2 D614G, Pangolin GXP4L, and RaTG13 spikes (Fig. 2B, 2E, and 2F) compared to mice from group 4. Mice in group 1 and group 4 elicited high magnitude levels of hACE2 blocking responses, as compared to groups 2 and 3 (Fig. 2J). As binding antibody responses post boost mirrored the trend of the post prime responses, it is likely that the second dose is boosting immunity to the vaccine antigens in the prime (Fig. 2). Finally, we did not observe cross-binding antibodies against common-cold CoV spike antigens from HCoV-HKU1, HCoV-NL63, and HCoV-229E in most of the vaccine groups (Fig. S2A–2D), but we did observe low binding levels against more distant group 2C MERS-CoV (Fig. 2I) and other Betacoronaviruses like group 2A HCoV-OC43 in vaccine groups 1 and 2 (Fig. S2B). These results suggest that chimeric spike mRNA vaccines elicit broader and higher magnitude binding responses against pandemic and bat SARS-like viruses compared to monovalent SARS-CoV-2 spike mRNA-LNP vaccines.
Figure 2. Human pathogenic coronavirus spike binding and hACE2-blocking responses in chimeric and monovalent SARS-CoV-2 spike-vaccinated mice.
Serum antibody ELISA binding responses were measured in the five different vaccination groups. Pre-immunization, post prime, and post-boost binding responses were evaluated against Sarbecoviruses, MERS-CoV, and common-cold CoV antigens including: (A) SARS-CoV Toronto Canada (Tor2) S2P, (B) SARS-CoV-2 S2P D614G, (C) SARS-CoV-2 RBD, (D) SARS-CoV-2 NTD, (E) Pangolin GXP4L spike, (F) RaTG13 spike, (G) RsSHC014 S2P spike, (H) HKU3–1 spike, (I) MERS-CoV spike, (J) hACE2 blocking responses against SARS-CoV-2 spike in the distinct immunization groups. Blue squares represent mice from group 1, orange triangles represent mice from group 2, green triangles represent mice from group 3, red rhombuses represent mice from group 4, and upside-down triangle represent mice from group 5. Statistical significance for the binding and blocking responses is reported from a Kruskal-Wallis test after Dunnett’s multiple comparison correction. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Neutralizing antibody responses against live Sarbecoviruses and variants of concern
We then examined the neutralizing antibody responses against SARS-CoV, Bt-CoV RsSHC014, Bt-CoV WIV-1, and SARS-CoV-2 and variants of concern using live viruses as previously described (Fig 3A–3D) (29). Group 4 SARS-CoV-2 S mRNA vaccinated animals mounted a robust response against SARS-CoV-2, however responses against SARS-CoV, RsSHC014, and WIV-1 were 18-, >500- or 116-fold more resistant, respectively (Fig 3A–3D and Fig. S3G–H). In contrast, aged mice in group 2 showed a 42- and 2-fold increase in neutralizing titer against SARS-CoV and WIV1, and less than 1-fold decrease against RsSHC014 relative to SARS-CoV-2 neutralizing titers (Fig 3A–3D and Fig. S3C–D). Mice in group 3 elicited 3- and 7-fold higher neutralizing titers against SARS-CoV and RsSHC014 yet showed a 3-fold reduction in WIV-1 neutralizing titers relative to SARS-CoV-2 (Fig 3A–3D and Fig. S3E–F). Finally, mice in group 1 generated the most balanced and highest neutralizing titers that were 13- and 1.2-fold higher against SARS-CoV and WIV-1 and less than 1-fold lower against RsSHC014 relative to the SARS-CoV-2 neutralizing titers (Fig 3A–3D and Fig. S3A–B). The serum of mice from groups 1 and 4 neutralized the dominant D614G variant with similar potency as the wild type D614 non-predominant variant, and both groups had similar neutralizing antibody responses against the U.K. B.1.1.7 and the mink cluster 5 variants as compared to the D614G variant (Fig. 3E 3F). Despite the significant but small reduction in neutralizing activity against the B.1.351 variant of concern (VOC), we did not observe a complete ablation in neutralizing activity in either group. Mice from groups 1 and 2 elicited lower binding and neutralizing responses to SARS-CoV-2 compared to group 4 perhaps reflecting a lower amount of mRNA vaccine incorporated into multiplexed formulations, whereas the monomorphic vaccines may drive a more focused B cell responses to SARS-CoV-2 whereas chimeric spike antigens lead to more breadth against distant Sarbecoviruses. Thus, both monovalent SARS-CoV-2 vaccines and multiplexed chimeric spikes elicit neutralizing antibodies against newly emerged SARS-CoV-2 variants and multiplexed chimeric spike vaccines outperform the monovalent SARS-CoV-2 vaccines in terms of breadth of potency against multiclade Sarbecoviruses.
Figure 3. Live Sarbecovirus neutralizing antibody responses in vaccinated mice.
Neutralizing antibody responses in mice from the five different vaccination groups were measured using nanoluciferase-expressing recombinant viruses. (A) SARS-CoV neutralizing antibody responses from baseline and post boost in the distinct vaccine groups. (B) SARS-CoV-2 neutralizing antibody responses from baseline and post boost. (C) RsSHC014 neutralizing antibody responses from baseline and post boost. (D) WIV-1 neutralizing antibody responses from baseline and post boost. (E) The neutralization activity in groups 1 and 4 against SARS-CoV-2 D614G, South African B.1.351, U.K. B.1.1.7, and mink variants (F) Neutralization comparison of SARS-CoV-2 D614G vs. South African B.1.351, vs. U.K. B1.1.7, and mink variants. Statistical significance for the live-virus neutralizing antibody responses is reported from a Kruskal-Wallis test after Dunnett’s multiple comparison correction. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
In vivo protection against heterologous Sarbecovirus challenge
To assess the ability of the mRNA-LNP vaccines to mediate protection against previously epidemic SARS-CoV, pandemic SARS-CoV-2, and Bt-CoVs, we challenged the different groups and observed the mice for signs of clinical disease. Mice from group 1 or group 2 were completely protected from weight loss, lower, and upper airway virus replication as measured by infectious virus plaque assays following 2003 SARS-CoV mouse-adapted (MA15) challenge (Fig. 4A, 4B and 4C). Similarly, these two vaccine groups were also protected against SARS-CoV-2 mouse-adapted (MA10) challenge. In contrast, group 3 showed some protection against SARS-CoV MA15 induced weight loss, but not against viral replication in the lung or nasal turbinates. Group 3 was fully protected against SARS-CoV-2 MA10 challenge. In contrast, group 5 vaccinated mice developed severe disease including mortality in both SARS-CoV MA15 and SARS-CoV-2 MA10 infections (Fig. S5B, S5C). Monovalent SARS-CoV-2 mRNA vaccines were highly efficacious against SARS-CoV-2 MA10 challenge but failed to protect against SARS-CoV MA15-induced weight loss, and replication in the lower and upper respiratory tract (Fig. 4A, 4B, and 4C), suggesting that SARS-CoV-2 mRNA-LNP vaccines are not likely to protect against future SARS-CoV emergence events. Mice from groups 1–4 were completely protected from weight loss and lower airway SARS-CoV-2 MA10 replication (Fig. 4D, 4E, and 4F). Using both a Bt-CoV RsSHC014 full-length virus and a more virulent RsSHC014-MA15 chimera in mice, we also demonstrated protection in groups 1–3 against RsSHC014 replication in the lung and nasal turbinates (Fig. S4) but not in mice that received the SARS-CoV-2 mRNA vaccine. Group 5 control mice challenged with RsSHC014-MA15 developed disease including mortality (Fig. S5D). Group 3 mice, which received a SARS-CoV-2 NTD/RsSHC014 RBD/S2, was fully protected against both SARS-CoV-2 and RsSHC014 challenge whereas group 4 was not, demonstrating that a single NTD and RBD chimeric spike can protect against more than one virus compared to a monomorphic spike.
Figure 4. In vivo protection against Sarbecovirus challenge after mRNA-LNP vaccination.
(A) Percent starting weight from the different vaccine groups of mice challenged with SARS-CoV MA15. (B) SARS-CoV MA15 lung viral titers in mice from the distinct vaccine groups. (C) SARS-CoV MA15 nasal turbinate titers. (D) Percent starting weight from the different vaccine groups of mice challenged with SARS-CoV-2 MA10. (E) SARS-CoV-2 MA10 lung viral titers in mice from the distinct vaccine groups. (F) SARS-CoV-2 MA10 nasal turbinate titers. (G) Percent starting weight from the different vaccine groups of mice challenged with WIV-1. (H) WIV-1 lung viral titers in mice from the distinct vaccine groups. (I) WIV-1 nasal turbinate titers. (J) Percent starting weight from the different vaccine groups of mice challenged with SARS-CoV-2 B.1.351. (K) SARS-CoV-2 B.1.351 lung viral titers in mice from the distinct vaccine groups. (L) SARS-CoV-2 B.1.351 nasal turbinate titers. Figure legend at the bottom right depicts the vaccines utilized in the different groups. Statistical significance for weight loss is reported from a two-way ANOVA after Dunnett’s multiple comparison correction. For lung and nasal turbinate titers, statistical significance is reported from a one-way ANOVA after Tukey’s multiple comparison correction. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
We then performed a heterologous challenge experiment with the bat pre-emergent WIV-1-CoV (9). Consistent with the protection observed against SARS-CoV, mice from groups 1 and 2 were fully protected against heterologous WIV-1 challenge whereas mice that received the SARS-CoV-2 mRNA vaccine breakthrough replication in the lung (Fig. 5G, 5H, and 5I). We also challenged with a virulent form of SARS-CoV-2 VOC B.1.351, which contains deletions in the NTD and mutations in the RBD, and observed full protection in vaccine groups 1, 2, and 4 compared to controls, whereas breakthrough replication was observed in group 3, further underlining the importance of the NTD in vaccine-mediated protection (Fig. 5J, 5K, and 5L) and its inclusion in universal vaccination strategies. Moreover, the SARS-CoV-2 mRNA vaccine protected against SARS-CoV-2 B.1.351 challenge in aged mice despite a reduction in the neutralizing activity against this VOC.
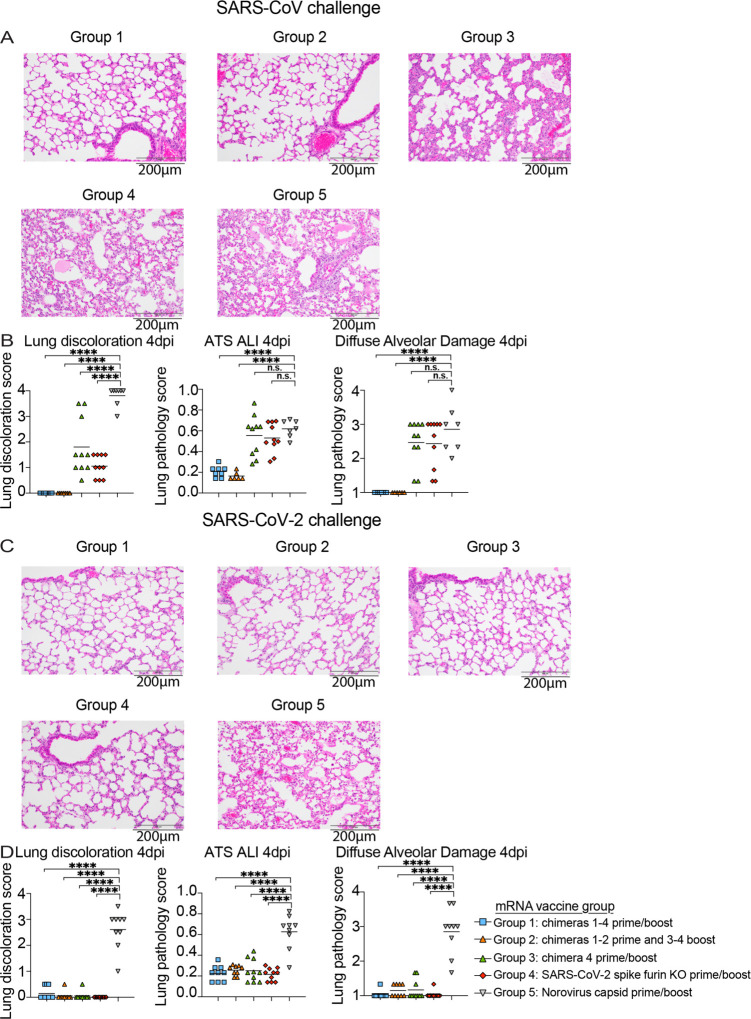
Figure 5. Lung pathology in vaccinated mice after SARS-CoV and SARS-CoV-2 challenge.
(A) Hematoxylin and eosin 4 days post infection lung analysis of SARS-CoV MA15 challenged mice from the different groups: group 1: chimeras 1–4 prime and boost, group 2: chimeras 1–2 prime and 3–4, group 3: chimera 4 prime and boost, SARS-CoV-2 furin KO prime and boost, and norovirus capsid prime and boost. (B) Lung pathology quantitation in SARS-CoV MA15 challenged mice from the different groups. Macroscopic lung discoloration score, microscopic acute lung injury (ALI) score, and diffuse alveolar damage (DAD) in day 4 post infection lung tissues are shown. (C) Hematoxylin and eosin 4 days post infection lung analysis of SARS-CoV-2 MA10 challenged mice from the different groups. (D) Lung pathology measurements in SARS-CoV-2 MA10 challenged mice from the different groups. Macroscopic lung discoloration score, microscopic acute lung injury (ALI) score, and diffuse alveolar damage (DAD) in day 4 post infection lung tissues are shown. Statistical significance is reported from a one-way ANOVA after Dunnet’s multiple comparison correction. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Lung pathology and cytokines in mRNA-LNP vaccinated mice challenged with epidemic and pandemic coronaviruses
Lung discoloration is the gross manifestation of various processes of acute lung damage, including congestion, edema, hyperemia, inflammation, and protein exudation. We used this macroscopic scoring scheme to visually score mouse lungs at the time of harvest. To quantify the pathological features of acute lung injury (ALI) in mice, we used a tool from the American Thoracic Society (ATS - Matute-Bello lung pathology score). With a complementary histological quantitation tool, we similarly scored lung tissue sections for diffuse alveolar damage (DAD), the pathological hallmark of ALI (cellular sloughing, necrosis, hyaline membranes, etc.) (30, 31) and found these data were consistent with those from the lung discoloration scores. We observed significant lung pathology by both the Matute-Bello and DAD scoring tools in groups 4 and 5 vaccinated animals, consistent with the weight loss and lung titer after heterologous SARS-CoV MA15 challenge. In contrast, multiplexed chimeric spike vaccine formulations in groups 1 and 2 provided complete protection from lung pathology after SARS-CoV MA15 challenge (Fig. 5A and 5B). Mice immunized with the SARS-CoV-2 mRNA vaccine that showed breakthrough infection with SARS-CoV MA15 developed similar lung inflammation as control vaccinated animals, potentially suggesting that future outbreaks of SARS-CoV may cause disease even in individuals vaccinated with SARS-CoV-2. As eosinophilic infiltrates have been observed in vaccinated, 2003 SARS-CoV challenged mice previously (32), we analyzed lung tissues in protected vs. infected animals with SARS-CoV MA15 for eosinophilic infiltrates by immunohistochemistry (Fig. S6). Groups 1 and 2 contained rare, scattered eosinophils in the interstitium. Group 3 showed bronchus-associated lymphoid tissue. In contrast, group 4 and group 5 contained frequent perivascular cuffs with prevalent eosinophils. In contrast to the heterologous SARS-CoV MA15 challenge, all groups challenged with SARS-CoV-2 MA10 were protected against lung pathology compared to the norovirus capsid-immunized control group, supporting the hypothesis that the SARS-CoV-2 NTD present in the chimeric spike from group 3 is sufficient for protection (Fig 5C and 5D).
We measured lung proinflammatory cytokines and chemokines in the different vaccination groups. Groups 1 and 2 had baseline levels of macrophage activating cytokines and chemokines including, IL-6, CCL2, IL-1α, G-SCF, and CCL4, compared to group 5 following SARS-CoV MA15 challenge (Fig. S7A). In contrast, group 3 and group 4 showed high and indistinguishable levels of IL-6, CCL2, IL-1α, G-SCF, and CCL4 compared to group 5 mice following SARS-CoV MA15 challenge. Following SARS-CoV-2 MA10 challenge, group 4 and group 1 showed the lowest levels of IL-6, and G-SCF relative to group 5 controls (Fig. S7B), and we only observed significant reductions in CCL2, IL-1α, CCL4 lung levels in groups 3 and 4 compared to the group 5 control despite full protection from both weight loss and lower airway viral replication.
Discussion
The Moderna and Pfizer/BioNTech SARS-CoV-2 mRNA-LNP vaccines are safe and efficacious against SARS-CoV-2 infections in large Phase 3 efficacy human clinical trials (33–35), but there is a growing concern that VOCs like South African B1.351, which is 5–6 fold more resistant to vaccine-elicited polyclonal neutralizing antibodies (36). We sought to replicate this platform to establish the breadth of existing SARS-CoV-2 mRNA vaccines, but also to formulate chimeric vaccines that specifically target distant Sarbecovirus strains. A caveat of including multiple chimeric spikes in a single shot is the potential formation of heterotrimers not present in the intended vaccine formulation. While it remains unknown if our chimeric mRNA-LNP vaccines generate heterotrimers in vivo, the observation that NTD and RBD chimeric spikes can protect against more than one virus is important. Chimera 4, which contains the RsSHC014 RBD and SARS-CoV-2 NTD and S2, elicited binding and neutralizing antibodies and mice were fully protected from Bt-CoV RsSHC014 and SARS-CoV-2 challenge, whereas SARS-CoV-2 full length did not fully protect against RsSHC014, suggesting that CoV spikes vaccines can be designed to maximize their display of protective epitopes. The lack of protection against WIV-1, SARS-CoV, and only partial protection against RsSHC014 challenge in SARS-CoV-2 immunized mice underlines the need for the development of universal vaccination strategies that can achieve broader coverage against pre-emergent bat SARS-CoV-like and SARS-CoV-2-like viruses. While other strategies exist, including multiplexing mosaic Sarbecovirus RBDs (37), RBDs on nanoparticles (38), chimeric spike mRNA-LNP vaccination can achieve broad protection using existing manufacturing technologies, and are portable to other high-risk emerging coronaviruses like group 2C MERS-CoV-related strains.
As previously reported with RNA recombinant viruses, our chimeric spike live viruses containing SARS-CoV-2 antigenic domains reaffirm the known interchangeability and functional plasticity of CoV spike glycoprotein structural motifs (26, 39, 40). Our demonstration of cross-protection against multiple Sarbecovirus strains in mice, coupled with the absence of immune pathology, lends support to the notion that universal vaccines against group 2B CoVs is likely achievable. Moving forward, it will be important to determine if other combinations of chimeric mRNA-LNP vaccines from other SARS-like viruses are protective, elicit broad T cell responses, prevent the rapid emergence of escape viruses, and elicit protective responses in nonhuman primate models of Sarbecovirus pathogenesis.
Supplementary Material
ACKNOWLEDGEMENTS
Funding:
David R. Martinez is currently supported by a Burroughs Wellcome Fund Postdoctoral Enrichment Program Award and a Hanna H. Gray Fellowship from the Howard Hugues Medical Institute and was supported by an NIH NIAID T32 AI007151 and an NIAID F32 AI152296. This research was also supported by funding from the Chan Zuckerberg Initiative awarded to R.S.B. This project was supported by the North Carolina Policy Collaboratory at the University of North Carolina at Chapel Hill with funding from the North Carolina Coronavirus Relief Fund established and appropriated by the North Carolina General Assembly. This project was funded in part by the National Institute of Allergy and Infectious Diseases, NIH, U.S. Department of Health and Human Services award U01 AI149644, U54 CA260543, AI157155 and AI110700 to R.S.B., AI124429 and a BioNTech SRA to D.W., and E.A.V., as well as an animal models contract from the NIH (HHSN272201700036I). Animal histopathology services were performed by the Animal Histopathology & Laboratory Medicine Core at the University of North Carolina, which is supported in part by an NCI Center Core Support Grant (5P30CA016086-41) to the UNC Lineberger Comprehensive Cancer Center. We thank B. L. Mui and Y.K. Tam from Acuitas Therapeutics, Vancouver, BC V6T 1Z3, Canada, for supplying the LNPs.
Footnotes
Competing interests: The University of North Carolina at Chapel Hill has filed provisional patents for which D.R.M. and R.S.B are co-inventors (U.S. Provisional Application No. 63/106,247 filed on October 27th, 2020) for the chimeric vaccine constructs and their applications described in this study.
Data and materials availability: The amino acid sequences of the chimeric spike constructs are included in table S1.
REFERENCES AND NOTES
- 1.Cherry J. D., Krogstad P., SARS: The First Pandemic of the 21st Century. Pediatric Research 56, 1–5 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaki A. M., van Boheemen S., Bestebroer T. M., Osterhaus A. D., Fouchier R. A., Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367, 1814–1820 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Paules C. I., Marston H. D., Fauci A. S., Coronavirus Infections-More Than Just the Common Cold. Jama 323, 707–708 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Zhou P. et al. , A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5, 536–544 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P. et al. , Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 556, 255–258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards C. E. et al. , Swine acute diarrhea syndrome coronavirus replication in primary human cells reveals potential susceptibility to infection. Proc Natl Acad Sci U S A 117, 26915–26925 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menachery V. D. et al. , A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med 21, 1508–1513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menachery V. D. et al. , SARS-like WIV1-CoV poised for human emergence. Proc Natl Acad Sci U S A 113, 3048–3053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W. et al. , Bats Are Natural Reservoirs of SARS-Like Coronaviruses. Science 310, 676–679 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Ge X. Y. et al. , Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503, 535–538 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu B. et al. , Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog 13, e1006698 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koff W. C., Berkley S. F., A universal coronavirus vaccine. Science 371, 759–759 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Sheahan T. P. et al. , Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rappazzo C. G. et al. , Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody. Science 371, 823–829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez D. R. et al. , A broadly neutralizing antibody protects against SARS-CoV, pre-emergent bat CoVs, and SARS-CoV-2 variants in mice. bioRxiv, (2021). [Google Scholar]
- 17.Premkumar L. et al. , The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 5, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suryadevara N. et al. , Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan J. et al. , A human SARS-CoV neutralizing antibody against epitope on S2 protein. Biochem Biophys Res Commun 333, 186–193 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou P. et al. , A protective broadly cross-reactive human antibody defines a conserved site of vulnerability on beta-coronavirus spikes. bioRxiv, (2021). [Google Scholar]
- 21.Liu L. et al. , Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 584, 450–456 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Dai L. et al. , A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell 182, 722–733.e711 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D. et al. , The functions of SARS-CoV-2 neutralizing and infection-enhancing antibodies in vitro and in mice and nonhuman primates. bioRxiv, (2021). [Google Scholar]
- 24.Voss W. N. et al. , Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitopes. Science, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCallum M. et al. , N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker M. M. et al. , Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proc Natl Acad Sci U S A 105, 19944–19949 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laczkó D. et al. , A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 53, 724–732.e727 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lederer K. et al. , SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 53, 1281–1295.e1285 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou Y. J. et al. , SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheahan T. P. et al. , Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nature Communications 11, 222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt M. E. et al. , Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog 14, e1006810 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolles M. et al. , A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol 85, 12201–12215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson L. A. et al. , An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. New England Journal of Medicine 383, 1920–1931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh E. E. et al. , Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine 383, 2439–2450 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baden L. R. et al. , Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu K. et al. , Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine — Preliminary Report. New England Journal of Medicine, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen A. A. et al. , Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science, eabf6840 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders K. O. et al. , SARS-CoV-2 vaccination induces neutralizing antibodies against pandemic and pre-emergent SARS-related coronaviruses in monkeys. bioRxiv, (2021). [Google Scholar]
- 39.Banner L. R., Keck J. G., Lai M. M., A clustering of RNA recombination sites adjacent to a hypervariable region of the peplomer gene of murine coronavirus. Virology 175, 548–555 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keck J. G., Soe L. H., Makino S., Stohlman S. A., Lai M. M., RNA recombination of murine coronaviruses: recombination between fusion-positive mouse hepatitis virus A59 and fusion-negative mouse hepatitis virus 2. J Virol 62, 1989–1998 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freyn A. W. et al. , A Multi-Targeting, Nucleoside-Modified mRNA Influenza Virus Vaccine Provides Broad Protection in Mice. Mol Ther 28, 1569–1584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leist S. R. et al. , A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice. Cell 183, 1070–1085.e1012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts A. et al. , A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog 3, e5 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.