Abstract
Backtracking, the reverse motion of the transcriptase enzyme on the nucleic acid template, is a universal regulatory feature of transcription in cellular organisms but its role in viruses is not established. Here we present evidence that backtracking extends into the viral realm, where backtracking by the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) may aid viral transcription and replication. Structures of SARS-CoV-2 RdRp bound to the essential nsp13 helicase and RNA suggested the helicase facilitates backtracking. We use cryo-electron microscopy, RNA-protein crosslinking, and unbiased molecular dynamics simulations to characterize SARS-CoV-2 RdRp backtracking. The results establish that the single-stranded 3’-segment of the product-RNA generated by backtracking extrudes through the RdRp NTP-entry tunnel, that a mismatched nucleotide at the product-RNA 3’-end frays and enters the NTP-entry tunnel to initiate backtracking, and that nsp13 stimulates RdRp backtracking. Backtracking may aid proofreading, a crucial process for SARS-CoV-2 resistance against antivirals.
Keywords: backtracking, coronavirus, cryo-electron microscopy, molecular dynamics, RNA-dependent RNA polymerase
Classification: Biological Sciences, Biophysics and Computational Biology
Introduction
SARS-CoV-2 is the causative agent of the current COVID-19 pandemic (1, 2). The SARS-CoV-2 genome is replicated and transcribed by its RNA-dependent RNA polymerase holoenzyme [holo-RdRp, subunit composition nsp7/nsp82/nsp12 (3, 4)] in a replication-transcription complex (RTC), which is the target for antivirals such as remdesivir [Rdv; (5)]. The holo-RdRp is thought to coordinate with many co-factors to carry out its function (6, 7). Some of these co-factors, such as the nsp13 helicase (8) and the nsp10/nsp14 proofreading assembly (9, 10), are also essential for viral replication and are antiviral targets (11–13).
We recently reported the first views of the SARS-CoV-2 RTC in complex with the nsp13 helicase [cryo-electron microscopy structures at a nominal resolution of 3.5 Å; (14)]. The overall architecture of the nsp13-RTC places the nucleic acid binding site of nsp13 directly in the path of the downstream template-strand RNA (t-RNA), and cryo-electron microscopy (cryo-EM) difference maps revealed the 5’-single-stranded t-RNA overhang engaged with nsp13 before entering the RdRp active site (14). The nsp13 helicase translocates on single-stranded nucleic acid in the 5’->3’ direction (15–22). Thus, this structural arrangement presents a conundrum: The RdRp translocates in the 3’->5’ direction on the t-RNA strand, while nsp13 translocates on the same strand in the opposite direction. Translocation of each enzyme opposes each other, and if the helicase prevails it is expected to push the RdRp backward on the t-RNA (14). This reversible backward sliding, termed backtracking, is a well-studied feature of the cellular DNA-dependent RNA polymerases [DdRps; (23–30)].
Backtracking by the cellular DdRps plays important roles in transcription regulation, including the control of DdRp pausing during transcription elongation, termination, DNA repair, and transcription fidelity (25). In backtracking, the DdRp and associated transcription bubble move backwards on the DNA, while the RNA transcript reverse threads through the complex to maintain the register of the RNA-DNA hybrid (23–30). This movement generates a single-stranded 3’-segment of the RNA transcript which is extruded out the secondary or NTP-entry tunnel that branches off from the primary DdRp active-site cleft around the conserved bridge helix (27–31).
Although evolutionarily unrelated to the DdRps, a secondary channel, formed by the RdRp motif F β-hairpin loop and proposed to serve as an NTP-entry tunnel, branches off from the main SARS-CoV-2 RdRp active site channel (32). This NTP-entry tunnel is well positioned to receive the single-stranded 3’-segment of backtracked RNA, a structural architecture analogous to the DdRps (14). We envisaged that translocation by the helicase could mediate backtracking of the RdRp, an otherwise energetically unfavorable process, enabling the key viral functions such as proofreading (9, 10, 12, 33) and template switching during subgenomic transcription (7, 34). Here we outline the structural basis for SARS-CoV-2 RTC backtracking and describe the role of nsp13 in stimulating backtracking.
Results
SARS-CoV-2 RdRp backtracked complexes for cryo-electron microscopy.
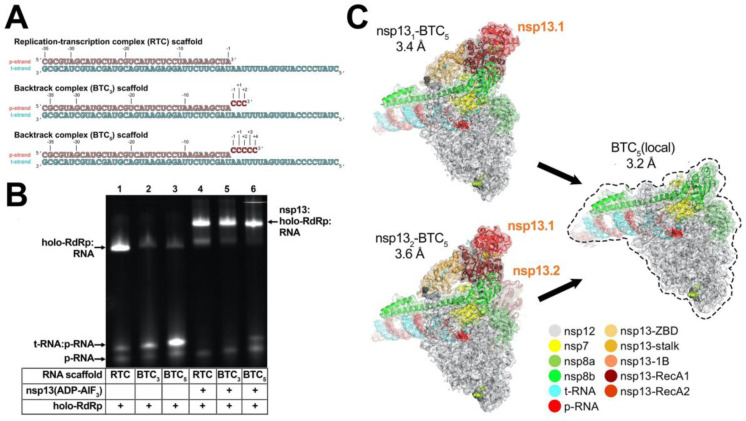
Previously, DdRp backtracked complexes (BTCs) were generated for structural studies by direct incubation of the DdRp with DNA-RNA scaffolds containing mismatched nucleotides at the RNA 3’-end (27, 28, 30); these BTC-scaffolds bind with the downstream Watson-Crick base pairs of the RNA-DNA hybrid positioned in the DdRp active site and the single-stranded 3’-segment of mismatched RNA extruding out the DdRp NTP-entry tunnel. To study RdRp BTCs, we therefore designed and tested RNA scaffolds based on our original SARS-CoV-2 RTC-scaffold but with three or five mismatched cytosine nucleotides added to the product-RNA (p-RNA) 3’-end (BTC3- and BTC5-scaffolds; Fig. 1A).
Fig. 1. SARS-CoV-2 backtrack complex.
A. RNA scaffolds: (top) replication-transcription complex (RTC) scaffold (14); (bottom) backtrack complex scaffolds (BTC3 and BTC5).
B. A native gel electrophoretic mobility shift assay reveals that holo-RdRp requires nsp13(ADP-AlF3) to bind the BTC scaffolds efficiently.
C. Cryo-EM structures of SARS-CoV-2 BTCs. Shown is the transparent cryo-EM density [local-resolution filtered; (47)] with the refined models superimposed (Table S1). The models and density are colored according to the key.
Native electrophoretic mobility shift assays revealed that although the holo-RdRp (nsp7/nsp82/nsp12) bound the RTC-scaffold as observed previously [Fig. 1B, lane 1; SI Appendix, Fig. S1A; (14)], nsp13 was required for efficient binding to the BTC-scaffolds (Fig. 1B). Stable nsp13-holo-RdRp complexes with BTC-scaffolds were also observed by native mass-spectrometry (Fig. S1B and C).
To determine the structural organization of the SARS-CoV-2 BTC, we assembled nsp13(ADP-AlF3) and holo-RdRp with the BTC5-scaffold (Fig. 1A; hereafter called BTC5) and analyzed the samples by single-particle cryo-EM. The sample comprised two major classes: nsp131-BTC5 (3.4 Å nominal resolution), and nsp132-BTC5 (3.6 Å; Fig. 1C; SI Appendix, Fig. S2 and S3). To eliminate structural heterogeneity in the nsp13 subunits and obtain a higher-resolution view of the BTC, the particles from both classes were combined and locally refined inside a mask applied around the holo-RdRp and RNA (excluding the nsp13 subunits), leading to the BTC5(local) combined map (3.2 Å; Fig. 1C; SI Appendix, Fig. S2 and S3, Table S1).
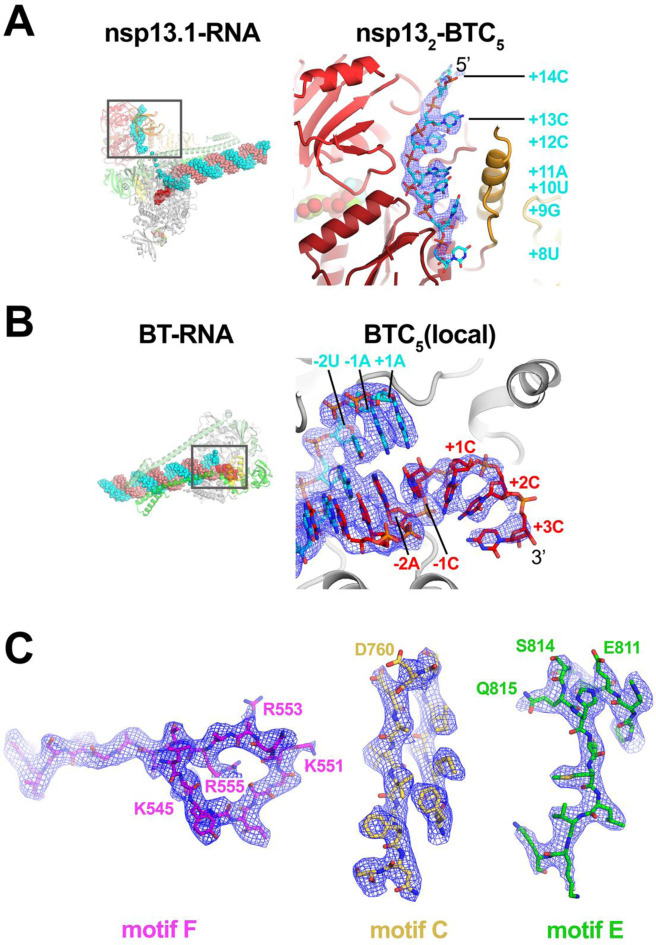
The cryo-EM maps (Fig. 1C and 2) revealed two significant differences with the nsp13-RTC structures (14): 1) The single-stranded downstream template-RNA (t-RNA) engaged with nsp13.1 was resolved (Fig. 2A), and 2) a single-stranded p-RNA 3’-segment was extruded into the RdRp NTP-entry tunnel (Fig. 2B).
Fig. 2. Cryo-EM density maps.
A. (left) Overall view of nsp131-BTC5. The boxed region is magnified on the right. (right) Magnified view of the t-RNA segment (+14–5’-CCCAUGU-3’-+8) enclosed in the nsp13.1 helicase subunit. The cryo-EM density map (from the nsp132-BTC structure) for the RNA is shown (blue mesh).
B. (left) Overall view of the BTC structure. The boxed region is magnified on the right.
(right) Magnified view of the region around the RdRp active site, showing the t-RNA (cyan) and p-RNA (red) with the backtracked RNA segment. The cryo-EM density map for the RNA [from BTC5(local)] is shown (blue mesh).
C. BTC5(local) cryo-EM density maps around nsp12 conserved motifs F, C, and E. Selected residues are labeled.
Nsp13 binds the downstream single-stranded t-RNA.
In the nsp131-BTC5 and nsp132-BTC5 cryo-EM maps, the single-stranded 5’-segment of the t-RNA was engaged with nsp13.1. This region of the cryo-EM density was well-resolved (Fig. 2A), allowing identification of the t-RNA segment engaged within the helicase as +14 to +8 (numbering defined in Figure 1A),5‘CCCAUGU3‘. The five-nucleotide segment connecting the t-RNA between the helicase and the RdRp (+7 to +3) was disordered and not modeled.
Fig. 5. Comparison of active-site proximal RNA in the RTC and BTC structures, and from simulations of a mismatched nucleotide at the p-RNA 3’-end.
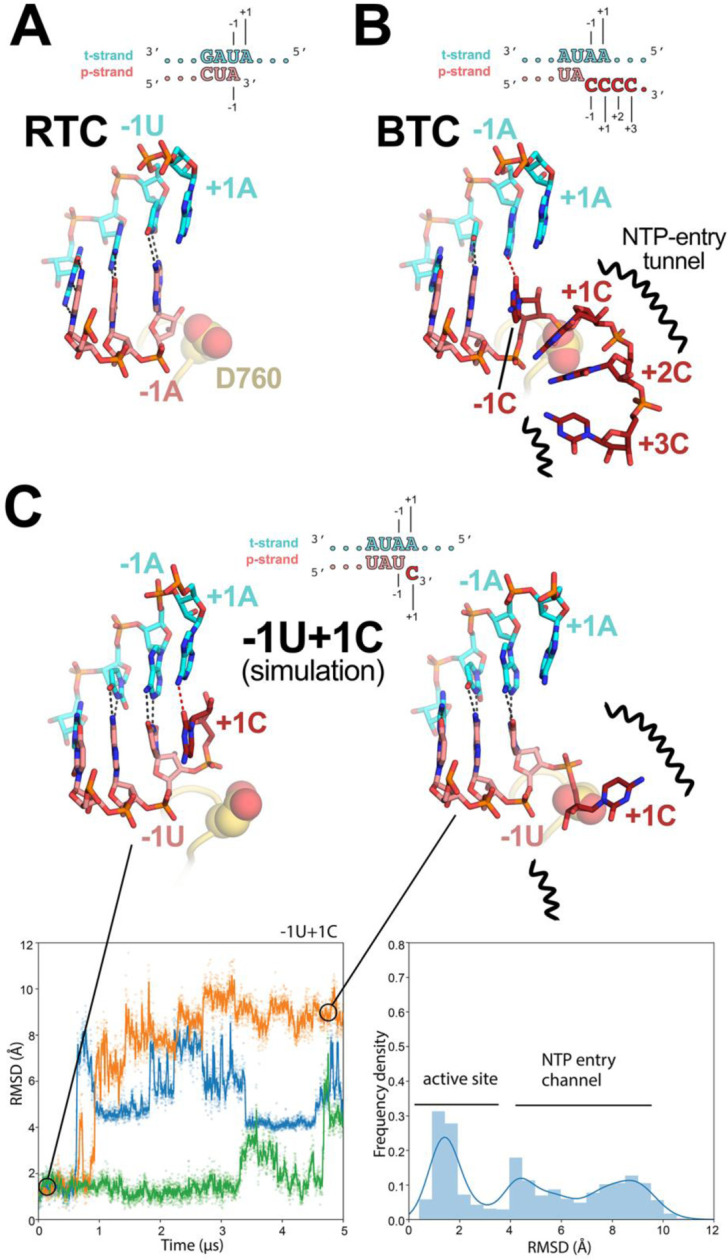
A-B, Comparison of the active-site proximal RNA in the RTC [A; PDB ID: 6XEZ; (14)], BTC5(local) (B), and from selected snapshots of molecular dynamics simulations of a −1U+1C complex (C). The schematics denote the nucleotides shown in the context of the RTC- (A) and BTC5-scaffolds (B; full scaffold sequences shown in Figure 1A) or generated from the BTC5-scaffold for the simulations (C). Carbon atoms of the t-RNA are colored cyan, p-RNA are colored salmon except in the case of mismatched C’s at the 3’-end, which are colored dark red. Watson-Crick base pairing hydrogen-bonds are denoted as dark gray dashed lines, other hydrogen-bonds as red dashed lines. Nsp12 motif C is shown as a yellow-orange backbone ribbon, and the side-chain of D760 is shown as atomic spheres.
A. The RTC is in a post-translocated state, with the A-U base pair at the p-RNA 3’-end in the −1 position (14).
B. The BTC5(local) RNA is translocated compared to the RTC; the base pair corresponding to A-U at the 3’-end of the RTC RNA in the −1 position is in the −2 position of the BTC RNA. A C-A mismatch occupies the BTC −1 site. The +1, +2 and +3 mismatched C’s trail into the RdRp NTP-entry tunnel (denoted by black squiggly lines). The +4C (present in the BTC5-scaffold; Figure 1A) is exposed to solvent, disordered and not modelled.
C. Molecular dynamics simulations of the nsp132-BTC−1U+1C complex. The complex was simulated with 3 replicates. RMSD values plotted as a function of time represent the heavy-atom RMSD of the +1C of the p-RNA compared with the starting configuration (see Methods). The RMSD histograms (plotted on the right) are an aggregate of all three replicates. Two structures taken from one of the simulations are shown, one showing the +1C of the p-RNA in the active site (t = 0 μs) and the other showing the +1C frayed into the NTP-entry tunnel (t = 4.5 μs).
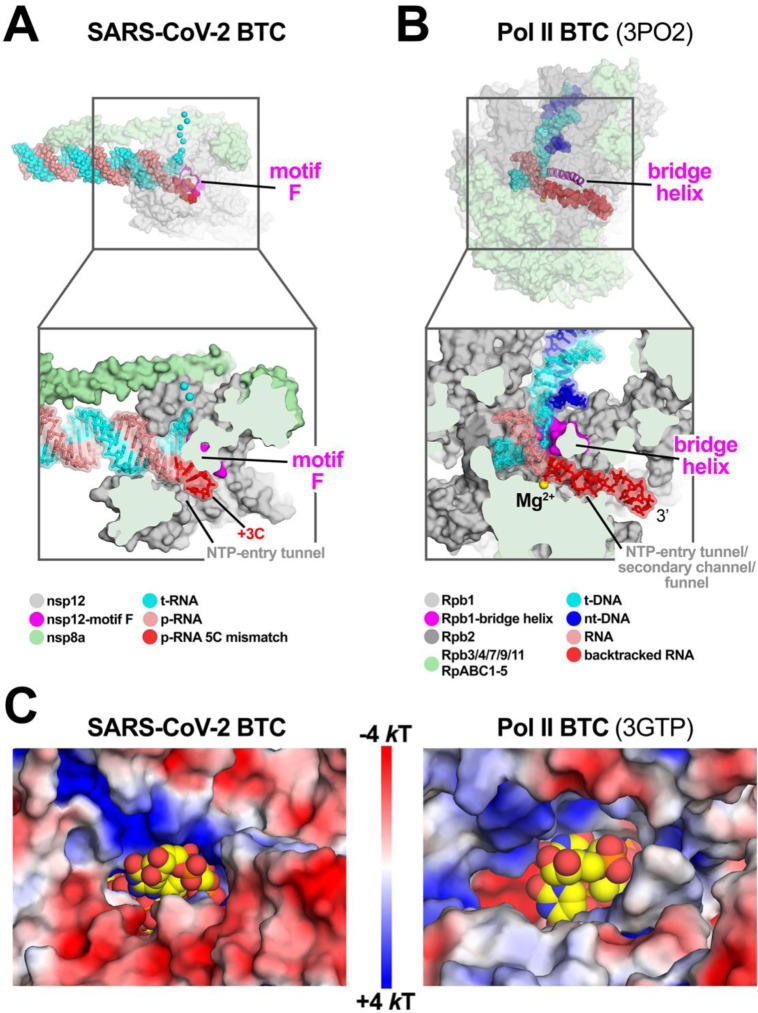
Fig. 3. SARS-CoV-2 RdRp and DdRp BTCs.
A, B. SARS-CoV-2 RdRp (A) and DdRp (B) BTCs.
(top) Proteins are shown as transparent molecular surfaces, nucleic acids as atomic spheres. The boxed regions are magnified on the bottom.
(bottom) Magnified, cross-sectional view. Proteins are shown as molecular surfaces, nucleic acids in stick format with transparent molecular surface.
A. The SARS-CoV-2 BTC5(local). Nsp8a and nsp12 are shown (nsp7 and nsp8b are removed for clarity). Nsp12 motif F is shown as a magenta backbone ribbon (top). Backtracked RNA (+1C to +3C of the BTC5-scaffold; Figure 1A) extrude out the NTP-entry tunnel.
B. A DdRp (Saccharomyces cerevisiae Pol II) BTC [PDB ID: 3PO2; (29)]. The bridge helix is shown as a magenta backbone ribbon. The backtracked RNA extrudes out the NTP-entry tunnel/secondary channel/funnel.
C. Views from the outside into the NTP-entry tunnels of the SARS-CoV-2 (left) and an S. cerevisiae DdRp [PDB ID: 3GTP; (27) BTC. Protein surfaces are colored by the electrostatic surface potential [calculated using APBS; (48)]. Backtracked RNA is shown as atomic spheres with yellow carbon atoms.
The SARS-CoV-2 RdRp NTP-entry tunnel accommodates the backtracked RNA.
The cryo-EM maps also resolved a single-stranded p-RNA 3’-segment of the BTC5-scaffold extruding into the RdRp NTP-entry tunnel (Fig. 2B), confirming the formation of a BTC (Fig. 3A). The overall architecture of the SARS-CoV-2 BTC is analogous to DdRp BTCs [Fig. 3; (14)]. The DdRp bridge helix [BH; (35)] separates the DdRp active site cleft into a channel for the downstream template DNA (over the top of the BH; Fig. 3B) and the NTP-entry tunnel (underneath the BH; Fig. 3B). Similarly, the viral RdRp motif F [SI Appendix, Fig. S4A; (32)] serves as the strand separating structural element for the backtracked RNA (Fig. 3A). The downstream t-RNA passes over the top of motif F, while the backtracked RNA extrudes out the NTP-entry tunnel underneath motif F (Fig. 3A).
The RdRp NTP-entry tunnel provides a steric and electrostatic environment conducive to channeling the backtracked RNA out of the active site without specific polar protein-RNA interactions that could hinder the RNA movement (Fig. 3C and 4). Comparing the electrostatic surface potential of the NTP-entry tunnels of the SARS-CoV-2 RdRp with eukaryotic and bacterial DdRps reveals a similar overall electrostatic surface environment that may facilitate backtracked RNA entry (Fig. 3C; SI Appendix, Fig. S4B), including a ‘track’ of conserved positively-charged Arg and Lys residues of motif F (SARS-CoV-2 nsp12 K545, K551, R553, and R555; Fig. 4; SI Appendix, Fig. S4A). Conserved residues of RdRp motifs C and E complete the active-site/NTP-entry tunnel environment surrounding the backtracked RNA (Fig. 4; SI Appendix, Fig. S4A).
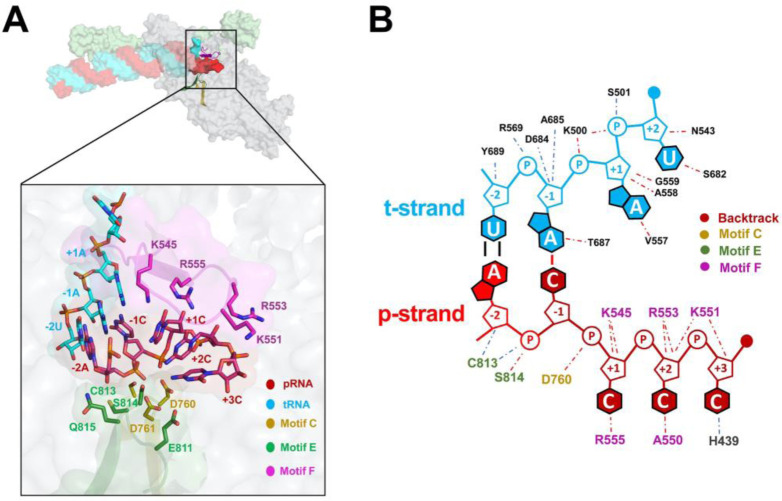
Fig. 4. Protein-RNA interactions in the BTC.
A. (top) Overall view of BTC5(local). Proteins are shown as transparent molecular surfaces, nucleic acids as atomic spheres. Nsp8a and nsp12 are shown (nsp7 and nsp8b are removed for clarity). Nsp12 motifs C, E, and F are shown as backbone ribbons (colored according to the key on the bottom. The boxed region is magnified below.
(bottom) RNA is shown from −2 to +3. Proteins are shown as transparent molecular surfaces. RdRp motifs C, E, and F are shown as transparent backbone ribbons (colored according to the key) with side chains of residues that approach the backtracked RNA (≤ 4.5 Å) shown.
B. Schematic illustrating the same protein-RNA interactions as (A). Drawn using Nucplot (49).
In the nsp13-RTCs, the RTC-scaffold (Fig. 1A) is bound in a post-translocated state (14); the 3’ p-RNA A is base-paired to the t-RNA U at the −1 site near the catalytic nsp12-D760 (Fig. 5A). The next t-RNA base (A at +1) is positioned to receive the incoming nucleoside-triphosphate (NTP) substrate, but the site for the incoming NTP substrate is empty (Fig. 5A). By contrast, the BTC structures were translocated by one base pair compared to the RTCs; the base pair corresponding to the A-U Watson-Crick base pair at the 3’-end of the p-RNA (located in the −1 site of the RTCs) was in the −2 position of the BTCs (Fig. 1A, 4, and 5B). The −1 position of the BTC was occupied by the first C-A mismatch; the p-RNA −1C made a non-Watson-Crick hydrogen bond with the opposing t-RNA A (Fig. 4 and 5B). The next three mismatched p-RNA nucleotides (+1C, +2C, +3C) trailed into the NTP entry tunnel (Fig. 4 and 5B). The 3’-nucleotide of the BTC5-scaffold p-RNA (+4C; Fig. 1A) was solvent-exposed at the outward-facing end of the NTP-entry tunnel, lacked density and was therefore not modelled (Fig. 2B). The trajectory of the backtracked nucleotides at positions +1/+2 was sharply bent due to spatial constraints of motif F residues (Fig. 4A).
Nsp13 stimulates backtracking.
The SARS-CoV-2 wild-type holo-RdRp required the nsp13 helicase to bind the BTC-scaffolds efficiently (Fig. 1B). However, we observed that the holo-RdRp containing nsp12 with a single amino acid substitution (D760A) did not require nsp13 to bind the BTC-scaffolds (SI Appendix; Fig. S1A, lane 4). Nsp12-D760 is a conserved residue of the RdRp motif C that chelates a crucial Mg2+ ion in catalytic complexes [SI Appendix; Fig. S4A; (32)], but in RdRp structures lacking substrate (including the BTC structures), the Mg2+ ions are absent (14, 36, 37). The catalytic Asp residues of the DdRps typically chelate the Mg2+ ion even in the absence of substrate (31, 38), and this Mg2+ is retained in DdRp backtracked structures (27–30). Our RdRp BTC structures suggest that in the absence of a Mg2+ ion, D760 presents an electrostatic barrier to the phosphate backbone of the backtracked RNA (Fig. 5B), explaining the requirement for the helicase to surmount this barrier and why removal of D760 stabilizes binding to the BTC-scaffolds.
To generate the SARS-CoV-2 BTCs for structural studies, we used the BTC5-scaffold with five mismatched C’s at the p-RNA 3’-end (Fig. 1A). To study the formation of SARS-CoV-2 BTCs from an RTC-scaffold (fully Watson-Crick base paired p-RNA 3’-end), we analyzed UV-induced crosslinking from 4-thio-U incorporated penultimate to the p-RNA 3’-end [RTC(4-thio-U)-scaffold; Si Appendix, Fig. S5A; (39)]. Crosslinking was absolutely dependent on the presence of 4-thio-U in the RNA, establishing specificity (SI Appendix; Fig. S5B). RTCs assembled with wild-type nsp12 and the RTC(4-thio-U)-scaffold gave little to no protein-RNA crosslinking upon UV exposure (SI Appendix; Fig. S5A, lane 2). These conditions favor a post-translocated RTC (14, 36, 37) with the 4-thio-U sequestered in the RNA-RNA hybrid and thus not available for protein-RNA crosslinking. Crosslinking of the p-RNA to nsp12 was substantially increased by the addition of nsp13 (with 2 mM ATP, which is present in all the lanes; SI Appendix; Fig. S5A, lane 3). Under these conditions, we propose that the translocation activity of nsp13 backtracked a fraction of the complexes, freeing the 4-thio-U from the RNA-RNA hybrid for crosslinking to nsp12. Replacing wild-type nsp12 with nsp12-D760A (nsp12*; SI Appendix, Fig. S5A, lanes 6–8), which is more prone to backtracking (SI Appendix; Fig. S1A), increased the protein-RNA crosslinking under all conditions, with the maximal crosslinking occurring under the conditions expected to favor backtracking the most (SI Appendix; Fig. S5A, lane 7). These results affirm the view that nsp13 facilitates backtracking of the SARS-CoV-2 RdRp.
A mismatched nucleotide at the p-RNA 3’-end spontaneously frays and enters into the RdRp NTP-entry tunnel.
The SARS-CoV-2 RTC is a highly processive and rapid replicase/transciptase, capable of replicating an ~1 kb RNA template at an average rate of ~170 nt/s (40). However, studies of other viral RdRps suggest that misincorporation slows the overall elongation rate and may induce backtracking (41–43). We used molecular dynamics simulations to explore the fate of a mismatched nucleotide incorporated at the p-RNA 3’-end. Starting with the nsp132-BTC5 structure, the −1C was mutated to U, and the +2 to +4 C’s were removed. The resulting pre-translocated p-RNA had a matched −1U and a mismatched +1C (−1U+1C; Fig. 5C). In three 5 μs simulations we observed the 3’-mismatched +1C alternating between two positions, either remaining in the vicinity of the active site (RMSD < 3.5 Å) or fraying away from the p-RNA:t-RNA hybrid towards or into the NTP-entry tunnel (RMSD > 3.5 Å; Fig. 5C). Based on analysis of the aggregated −1U+1C simulations, the mismatched +1C spent about 40% of the time near the active site and about 60% of the time frayed towards or in the NTP-entry tunnel. In control simulations with a fully matched p-RNA 3’-end (−1U+1U), the matched +1U at the p-RNA 3’-end did not fray and spent 100% of the time in the active site pocket (SI Appendix; Fig. S6).
Discussion
Our results establish that the SARS-CoV-2 RTC backtracks, that backtracking is facilitated by the nsp13 helicase, and that the resulting single-stranded 3’-segment of the p-RNA extrudes out the RdRp NTP-entry tunnel in a manner analogous to the evolutionarily unrelated cellular DdRps (Fig. 3). Thus, a secondary tunnel to accommodate backtracked RNA, facilitating fidelity and possibly other functions (Fig. 6), appears to be a crucial feature of transcriptase enzymes that evolved independently.
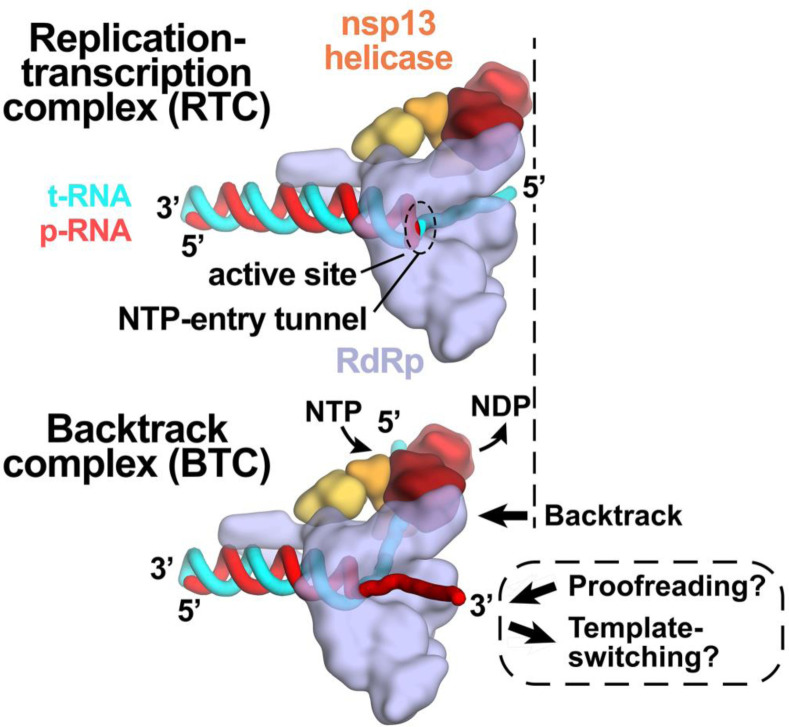
Fig. 6. Role of backtracking in proofreading and template-switching during sub-genomic transcription.
Schematic illustrating the proposed model for backtracking of the SARS-CoV-2 RTC and its potential role in proofreading and template-switching during sub-genomic transcription. The structural models are shown as cartoons (holo-RdRp, light blue; nsp13 helicase, orange shades; RNA strands, colored tubes as indicated).
(top) In the RTC, the elongating RdRp moves from left-to-right. The RdRp active site holds the p-RNA 3’-end. The NTP-entry tunnel provides access from solution to the RdRp active site. The downstream (5’) single-stranded t-RNA is not engaged with nsp13.
(bottom) In the BTC, nsp13 translocates on the downstream (5’) single-stranded t-RNA, pushing the RdRp backwards (right-to-left) on the RNA. This causes the p-RNA to reverse-thread through the complex, with the resulting single-stranded 3’-fragment extruding out the NTP-entry tunnel. The exposure of the p-RNA 3’-end could facilitate proofreading (9, 10, 12, 50) and also template-switching during sub-genomic transcription (7, 34).
Backtracking of Φ6 and poliovirus RdRps has been reported based on analysis of single-molecule observations (41–43). The nsp13 helicase facilitates efficient backtracking of the SARS-CoV-2 RTC (SI Appendix; Fig. S5). We note that in bacteria, the UvrD helicase has been shown to induce DdRp backtracking, suggesting that a role for helicases in backtracking may be widespread (44).
Our results are consistent with the view that a matched nucleotide at the pre-translocated p-RNA 3’-end remains base paired to the t-RNA (Fig. 5; SI Appendix, Fig. S6), facilitating translocation and subsequent NTP addition and thus rapid elongation (at a maximum elongation rate of ~170 nt/s, a translocation event would occur approximately every 6 msec, on average, explaining why translocation was not observed in our 5 μs simulations; Fig. 5; SI Appendix, Fig. S6). However, upon misincorporation, the pre-translocated, mismatched nucleotide at the p-RNA 3’-end spends more than half the time frayed from the t-RNA and towards or in the NTP-entry tunnel (Fig. 5C), a configuration that is likely recalcitrant to translocation and subsequent elongation. The favorable environment of the NTP-entry tunnel (Fig. 3 and 4) may further encourage backtracking. The resulting inhibition of translocation may enable the tight engagement of the nsp13.1 helicase with the downstream single-stranded t-RNA (Fig. 2A), allowing the 5’->3’ translocation activity of the helicase to more robustly backtrack the complex (SI Appendix; Fig. S5).
Our findings have implications for the processes of subgenomic transcription and proofreading in SARS-CoV-2 [Fig. 6; (14)]. Generation of mRNAs for the viral structural proteins begins with transcription initiation at the 3’-poly(A) tail of the (+)-strand RNA genome. The process, called sub-genomic transcription, ultimately generates a nested set of transcripts that are both 5’- and 3’-co-terminal with the viral genome and involves a remarkable template-switch from the 3’-portion of the genome to the 5’-leader (7, 34). The template-switching event is thought to involve base-pairing between the 3’-end of the nascent transcript and a complementary sequence (the Transcription Regulatory Sequence, or TRS) near the (+)-strand 5’-leader (45). Backtracking could extrude the 3’-end of the nascent transcript out the NTP-entry tunnel, making it available for base pairing to the 5’-TRS (Fig. 6). Our results establishing that the SARS-CoV-2 RTC can backtrack validates a key prediction of this model for the mechanism of template-switching during sub-genomic transcription (14).
Nucleotide analogs that function by being incorporated into product RNA by viral RdRps are important antiviral therapeutics (46). Notably, their incorporation may induce backtracking by the RdRp (43). Rdv, a nucleotide analog, is the only FDA-approved drug for COVID-19 treatment (5). Our results support a model in which RdRp misincorporation or incorporation of nucleotide analogs can pause the RdRp, allowing nsp13 to engage with the downstream single-stranded t-RNA to induce backtracking (14). The resulting exposure of the p-RNA 3’-end out the NTP-entry tunnel (Fig. 3A and 6) could provide access for the SARS-CoV-2 proofreading machinery [nsp10/14; (9, 12)] to degrade the p-RNA 3’-end, thus removing the misincorporation or analog. This proofreading activity, which is unique to the nidovirus order to which CoVs belong (10), is a major determinant for the resistance of CoVs against many nucleotide analog inhibitors (13). Thus, understanding RdRp backtracking and its potential role in CoV proofreading can facilitate the development of therapeutics.
Materials and Methods
Detailed descriptions of SARS-CoV-2 nsp12, 7, 8, and 13 protein purification, assembly of the RTC complexes, Native EMSAs, native mass-spectrometry, cross-linking, specimen preparation for cryo-EM, cryo-EM data acquisition and processing, model building and refinement, and molecular dynamics simulations are provided in the SI Appendix.
Supplementary Material
Significance Statement.
The COVID-19 pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The SARS-CoV-2 genome is replicated and transcribed by its RNA-dependent RNA polymerase (RdRp), which is the target for antivirals such as remdesivir. We use a combination of approaches to show that backtracking (backwards motion of the RdRp on the template RNA) is a feature of SARS-CoV-2 replication/transcription. Backtracking may play a critical role in proofreading, a crucial process for SARS-CoV-2 resistance against many antivirals.
Acknowledgments
We thank M. Ebrahim, J. Sotiris, and H. Ng at The Rockefeller University Evelyn Gruss Lipper Cryo-electron Microscopy Resource Center for help with cryo-EM. Some of the work reported here was conducted at the Simons Electron Microscopy Center (SEMC) and the National Resource for Automated Molecular Microscopy (NRAMM) and National Center for CryoEM Access and Training (NCCAT) located at the NYSBC, supported by grants from the NIH National Institute of General Medical Sciences (P41 GM103310), NYSTAR, the Simons Foundation (SF349247), the NIH Common Fund Transformative High Resolution Cryo-Electron Microscopy program (U24 GM129539) and NY State Assembly Majority. This work was supported by the Pels Family Center for Biochemistry and Structural Biology (The Rockefeller University), and NIH grants P41 GM109824 and P41 GM103314 to B.T.C, R35 GM118130 to S.A.D, and R01 GM114450 to E.A.C.
Footnotes
Data deposition: The cryo-EM density maps have been deposited in the EMDataBank: EMD-23007 (nsp131-BTC5), EMD-23008 (nsp132-BTC5), EMD-23009 [BTC5(local)]. Atomic coordinates have been deposited in the Protein Data Bank: 7KRN (nsp131-BTC5), 7KRO (nsp132-BTC5), 7KRP [BTC5(local)] and will be released upon peer-review publication. Please contact Elizabeth Campbell, if you require the data before. The molecular dynamics trajectories described in this work are available at https://www.deshawresearch.com/downloads/download_trajectory_sarscov2.cgi/.
Competing Interest Statement: The authors declare no conflict of interest.
References
- 1.Wu F., et al. , A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., et al. , A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subissi L., et al. , One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proceedings of the National Academy of Sciences of the United States of America 111, E3900–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirchdoerfer R. N., Ward A. B., Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nature Communications 10, 2342–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U. S. F. and D. Administration, Remdesivir-EUA-Letter-Of-Authorization.pdf. undefined (2020).
- 6.Snijder E. J., Decroly E., Ziebuhr J., The Nonstructural Proteins Directing Coronavirus RNA Synthesis and Processing. Adv Virus Res 96, 59–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sola I., Almazán F., Zúñiga S., Enjuanes L., Continuous and Discontinuous RNA Synthesis in Coronaviruses. Ann Rev Virol 2, 265–288 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann K. C., Snijder E. J., Posthuma C. C., Gorbalenya A. E., What we know but do not understand about nidovirus helicases. Virus Res 202, 12–32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minskaia E., et al. , Discovery of an RNA virus 3’->5’ exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc National Acad Sci 103, 5108–5113 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorbalenya A. E., Enjuanes L., Ziebuhr J., Snijder E. J., Nidovirales: Evolving the largest RNA virus genome. Virus Res 117, 17–37 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R., et al. , The nsp1, nsp13, and M proteins contribute to the hepatotropism of murine coronavirus JHM.WU. J Virol 89, 3598–609 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denison M. R., Graham R. L., Donaldson E. F., Eckerle L. D., Baric R. S., Coronaviruses: An RNA proofreading machine regulates replication fidelity and diversity. Rna Biol 8, 270–279 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith E. C., Blanc H., Surdel M. C., Vignuzzi M., Denison M. R., Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. Plos Pathog 9, e1003565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J., et al. , Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. Cell 182, 1560–1573.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adedeji A. O., et al. , Mechanism of Nucleic Acid Unwinding by SARS-CoV Helicase. Plos One 7, e36521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bautista E. M., Faaberg K. S., Mickelson D., McGruder E. D., Functional Properties of the Predicted Helicase of Porcine Reproductive and Respiratory Syndrome Virus. Virology 298, 258–270 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov K. A., Ziebuhr J., Human Coronavirus 229E Nonstructural Protein 13: Characterization of Duplex-Unwinding, Nucleoside Triphosphatase, and RNA 5′-Triphosphatase Activities. J Virol 78, 7833–7838 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee N.-R., et al. , Cooperative translocation enhances the unwinding of duplex DNA by SARS coronavirus helicase nsP13. Nucleic Acids Res 38, 7626–36 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mickolajczyk K. J., et al. , Force-dependent stimulation of RNA unwinding by SARS-CoV-2 nsp13 helicase. Biophys J (2020) 10.1016/j.bpj.2020.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seybert A., van Dinten L. C., Snijder E. J., Ziebuhr J., Biochemical Characterization of the Equine Arteritis Virus Helicase Suggests a Close Functional Relationship between Arterivirus and Coronavirus Helicases. J Virol 74, 9586–9593 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SEYBERT A., HEGYI A., SIDDELL S. G., ZIEBUHR J., The human coronavirus 229E superfamily 1 helicase has RNA and DNA duplex-unwinding activities with 5′-to-3′ polarity. Rna 6, 1056–1068 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanner J. A., et al. , The Severe Acute Respiratory Syndrome (SARS) Coronavirus NTPase/Helicase Belongs to a Distinct Class of 5′ to 3′ Viral Helicases. J Biol Chem 278, 39578–39582 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komissarova N., Kashlev M., RNA Polymerase Switches between Inactivated and Activated States By Translocating Back and Forth along the DNA and the RNA. J Biol Chem 272, 15329–15338 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Komissarova N., Kashlev M., Transcriptional arrest: Escherichia coli RNA polymerase translocates backward, leaving the 3’ end of the RNA intact and extruded. Proc National Acad Sci 94, 1755–1760 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nudler E., RNA polymerase backtracking in gene regulation and genome instability. Cell 149, 1438–1445 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nudler E., Mustaev A., Lukhtanov E., Goldfarb A., The RNA-DNA hybrid maintains the register of transcription by preventing backtracking of RNA polymerase. Cell 89, 33–41 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Wang D., et al. , Structural basis of transcription: backtracked RNA polymerase II at 3.4 angstrom resolution. Science 324, 1203–1206 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekine S., Murayama Y., Svetlov V., Nudler E., Yokoyama S., The ratcheted and ratchetable structural states of RNA polymerase underlie multiple transcriptional functions. Molecular Cell 57, 408–421 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Cheung A. C. M., Cramer P., Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature 471, 249–253 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Abdelkareem M., et al. , Structural Basis of Transcription: RNA Polymerase Backtracking and Its Reactivation. Mol Cell 75, 298–309.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G., et al. , Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 A resolution. Cell 98, 811–824 (1999). [DOI] [PubMed] [Google Scholar]
- 32.te Velthuis A. J. W., Common and unique features of viral RNA-dependent polymerases. Cell Mol Life Sci Cmls 71, 4403–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agostini M. L., et al. , Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. Mbio 9, e00221–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawicki S. G., Sawicki D. L., Advances in Experimental Medicine and Biology. Adv Exp Med Biol 440, 215–219 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Lane W. J., Darst S. A., Molecular Evolution of Multisubunit RNA Polymerases: Structural Analysis. Journal of Molecular Biology 395, 686–704 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q., et al. , Structural Basis for RNA Replication by the SARS-CoV-2 Polymerase. Cell (2020) 10.1016/j.cell.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillen H. S., et al. , Structure of replicating SARS-CoV-2 polymerase. Nature, 1–6 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Cramer P., et al. , Architecture of RNA polymerase II and implications for the transcription mechanism. Science 288, 640–649 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Sontheimer E. J., Site-specific RNA crosslinking with 4-thiouridine. Mol Biol Rep 20, 35–44 (1994). [DOI] [PubMed] [Google Scholar]
- 40.Seifert M., et al. , Signatures and mechanisms of efficacious therapeutic ribonucleotides against SARS-CoV-2 revealed by analysis of its replicase using magnetic tweezers. Biorxiv, 2020.08.06.240325 (2020). [Google Scholar]
- 41.Dulin D., et al. , Backtracking behavior in viral RNA-dependent RNA polymerase provides the basis for a second initiation site. Nucleic Acids Res 43, 10421–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dulin D., et al. , Elongation-Competent Pauses Govern the Fidelity of a Viral RNA-Dependent RNA Polymerase. Cell Reports 10, 983–992 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Dulin D., et al. , Signatures of Nucleotide Analog Incorporation by an RNA-Dependent RNA Polymerase Revealed Using High-Throughput Magnetic Tweezers. Cell Reports 21, 1063–1076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epshtein V., et al. , UvrD facilitates DNA repair by pulling RNA polymerase backwards. Nature 505, 372–377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasternak A. O., van den Born E., Spaan W. J. M., Snijder E. J., Sequence requirements for RNA strand transfer during nidovirus discontinuous subgenomic RNA synthesis. Embo J 20, 7220–7228 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clercq E. D., Li G., Approved Antiviral Drugs over the Past 50 Years. Clin Microbiol Rev 29, 695–747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardone G., Heymann J. B., Steven A. C., One number does not fit all: mapping local variations in resolution in cryo-EM reconstructions. Journal of structural biology 184, 226–236 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jurrus E., et al. , Improvements to the APBS biomolecular solvation software suite. Protein Sci 27, 112–128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luscombe N. M., Laskowski R. A., Thornton J. M., NUCPLOT: A Program to Generate Schematic Diagrams of Protein-Nucleic Acid Interactions. Nucleic Acids Res 25, 4940–4945 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith E. C., Sexton N. R., Denison M. R., Thinking Outside the Triangle: Replication Fidelity of the Largest RNA Viruses. Ann Rev Virol 1, 111–132 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.