Abstract
Purpose of review:
Alcohol use continues to rise globally. We review the current literature on the effect of alcohol on bone health, homeostasis and fracture repair to highlight what has been learned in people and animal models of alcohol consumption.
Recent findings:
Recently, forkhead box O (FoxO) has been found to be upregulated and activated in mesenchymal stem cells (MSC) exposed to alcohol. FoxO has also been found to modulate Wnt/β-catenin signaling, which is necessary for MSC differentiation. Recent evidence suggests alcohol activates FoxO signaling, which may be dysregulating Wnt/β-catenin signaling in MSCs cultured in alcohol.
Summary:
This review highlights the negative health effects learned from people and chronic and episodic binge alcohol consumption animal models. Studies using chronic alcohol exposure or alcohol exposure then bone fracture repair model have explored several different cellular and molecular signaling pathways important for bone homeostasis and fracture repair, and offer potential for future experiments to explore additional signaling pathways that may be dysregulated by alcohol exposure.
Keywords: alcohol, fracture, mesenchymal stem cells, Wnt/β-catenin signaling, oxidative stress, FoxO
Introduction
Despite the knowledge and understanding behind alcohol consumption and its negative health effects, alcohol consumption continues to increase globally(1*). In fact, alcohol consumption is the number one substance use disorder, with one study showing that the total volume of alcohol consumed on average increased 70% from 1990 to 2017(1*). Alcohol consumption is known to affect many different organs and body systems. One organ system that is particularly affected by alcohol consumption is the skeletal system. The effects of alcohol on the skeletal system occur through both direct and indirect mechanisms, with the consensus that the overall effect of alcohol consumption is a negative effect on bone health and homeostasis. Therefore, the health effects of alcohol abuse on bone health are still an area for continuing research.
There are many alcohol exposure regimes and animal models employed to study the effect of alcohol on bone health and homeostasis. One model system that provides valuable insight into the complex mechanism involved in primary bone formation, remodeling and homeostasis is bone fracture repair. Bone fracture repair recapitulates early bone formation and offers a unique system to assess many aspects of bone growth, development and homeostasis in a short time window. When considering that alcohol consumption and incidences of traumatic injury, specifically orthopaedic injuries, are highly correlated, studying the effects of alcohol on bone fracture repair is one valuable tool to uncovering the negative health consequences on bone health and homeostasis. This review focuses on the negative health effects of alcohol on bone health and homeostasis by analyzing different models of alcohol consumption, but in large part focuses on what has been learned from bone fracture repair models.
Alcohol and bone
Osteoporosis is a major health problem worldwide that continues to increase in prevalence every year. The World Health Organization estimates the number of osteopenia and osteoporosis patients to be greater than 200 million people worldwide and is only second to cardiovascular disease as a global healthcare problem(2). Many risk factors contribute to the etiology of osteoporosis, however, excessive alcohol use is one established risk factor that continues to rise worldwide. While there is a debate whether a health benefit exists for light-to-moderate alcohol consumption, research examining the effects of alcohol on bone generally arrive on the consensus that heavy or chronic alcohol use is detrimental to overall bone health and homeostasis.
Chronic alcohol consumption has been described to affect multiple organs, body systems and molecular pathways. One mechanism by which chronic alcohol use resulted in skeletal abnormalities was noted in rats, where two research groups found that chronic alcohol disturbed vitamin D metabolism(3, 4). Turner, et al. showed that rats receiving 38% of their overall caloric intake from alcohol over a 10-month period showed reduced serum magnesium levels, increased 25-hydroxyvitamin D3, and decreased 1,25-dihydroxyvitamin D3. Alcohol consumption also led to reduced overall tibial lengths with increased medullary area and reduced trabecular bone. Another study by Mercer et al. found that vitamin D supplementation protected against chronic alcohol-induced bone loss(5). Interestingly, the effect of alcohol on bone loss was related to the amount of drinking in this study. These researchers found that rodents receiving 10% of their total calories from alcohol had no effect on any of the measured bone parameters, whereas rodents that received 36% of calories from alcohol saw marked reductions in bone mineral density and bone strength. Combined, these studies suggest an alcohol-related increase in bone resorption that was not compensated by an increase in bone formation. Indeed, these findings are supported by several studies that show alcohol consumption increases osteoclastogenesis and osteoclast activity in rodents and humans(5–12). The disruption in bone homeostasis could account for bone loss by shifting the balance towards bone resorption. One mechanism that has been examined in rodents found that alcohol directly modulates gene expression in osteoblasts to induce expression of receptor activator of nuclear factor-κB ligand (RANKL), resulting in increased osteoclastogenesis(13). Chen et al. found chronic alcohol exposed rodents had decreased tibial trabecular bone and bone mineral density, but elevated RANKL within the bone marrow. In the same study, RANKL mRNA expression was also found to be elevated in primary osteoblasts co-cultured in alcohol. Interestingly, the effect of alcohol on RANKL mRNA expression is restored to control levels when primary osteoblasts or rodents are treated with 17β-estradiol. These findings suggest that estrogen can antagonize the effect of alcohol on bone homeostasis. One additional study found that binge alcohol decreased trabecular bone mineral density and compression strength in vertebral bone, while concurrently increasing RANKL expression in adult animals, supporting the hypothesis that alcohol exposure exacerbates bone loss through increased bone resorption(6) While evidence in rodents and humans support the finding that chronic alcohol exposure leads to increased resorption, one study in alcohol-consuming Rhesus Macaques found a decrease in bone resorption and bone remodeling(14). Gaddani et al. found that serum levels of bone resorption biomarker carboxy-terminal collagen crosslinks (CTx) were reduced in chronic binge alcohol exposed Rhesus Macaques compared to control Rhesus Macaques. In addition, cortical bone porosity and osteon density were decreased in chronic alcohol exposed animals compared to control animals. The differences between these findings and findings from rodents and human studies could be related to drinking pattern or blood alcohol concentrations and levels of consumptions. In fact, light to moderate alcohol consumption in humans shows a similar effect on reduced bone remodeling(15). However, these findings highlight an important limitation to using rodent systems; the apparent lack of Haversian remodeling of cortical bone in small rodents(16–18). However, bone remodeling is not completely absent in rodents; Woven and lamellar bone created during bone fracture repair are remodeled into mature cortical bone, making a fracture repair model an option to study the effect of alcohol on bone remodeling activity in rodents.
Alcohol-induced osteoporosis through increased osteoclast number and activity is not the only mechanism described in the literature. It is well documented that alcohol has a direct effect on bone anabolism as well. When compared to non-drinkers, chronic alcohol users have increased incidence of osteopenia accompanied with decreased serum bone Gla-protein (more commonly known as osteocalcin)(19, 20). Osteocalcin functions to bind and concentrate calcium within bone tissue. Osteocalcin is secreted solely by osteoblasts and is considered a biomarker for active bone formation; Levels of osteocalcin within patient serum has been shown to positively correlate with increased bone volume and bone formation rate(21). A recent study showed ethanol consumption biomarker phosphatidylethanol (PEth) levels correlated with decreased osteocalcin in a study that included human immunodeficiency virus positive individuals(22). These findings suggest chronic alcohol users have decreased bone volume and bone formation rates. Interestingly, when chronic alcohol users abstained for 7 days they saw an increase in osteocalcin, suggesting the effect of alcohol use on serum osteocalcin is reversible and that inhibition of osteoblastic activity is one of the main mechanisms responsible for bone loss. These findings were later confirmed in another study that followed patients for 6 months. In this cohort, patients who continued to misuse alcohol showed a worsening of serum osteocalcin and increased osteopenia, whereas patients who abstained had improvements in both parameters(23). Recent evidence suggests alcohol inhibits expression of other integral bone structural components. Pedersen et al. reported the effect of chronic alcohol exposure on several bone structural and matrix proteins(24). In this study rodents were fed on an alcohol diet for 3 months before researchers preformed RNA-Seq on mRNA isolated from the femur. Researchers found that all six bone collagens and matrix proteins osteonectin, sialoprotein, and decorin expression were decreased with alcohol exposure. The findings suggest alcohol may have a direct effect on osteoblasts.
When examining the direct effect of alcohol on osteoblasts, several studies found that even moderate quantities of alcohol inhibited in vitro osteoblast proliferation and induced cellular senescence(25–29). One study found alcohol activated p21 and p53 in rodent osteoblasts, leading to activation of senescence-associated β-galactosidase(29). These findings suggest that alcohol consumption may contribute to loss of bone mineral density by inhibiting osteoblast proliferation, while cellular senescence suggests a loss of osteoblast activity. Indeed, two human studies found that chronic alcohol users with reduced bone formation showed signs of inhibited osteoblastic activity without differences in resorption when compared to non-alcohol users(20, 30). Similar findings were also seen in rodents exposed to alcohol(31). Fanti et al. showed that the effect of alcohol on bone formation rate was dose dependent and the increasing alcohol dose concentration did not affect observed osteoclast numbers. These findings were supported by Chavassieux et al., who reported that primary osteoblasts cultured ex vivo in alcohol had inhibited alkaline phosphatase activity(28), one osteoblastic enzyme necessary for mineralization and a marker of osteoblast activity.
While most studies focus on the chronic mode of alcohol consumption and acute exposure of cell cultures, there is evidence that binge or episodic excessive alcohol consumption leads to similar effects. Using a rodent model of binge alcohol, one group found tibial bone mineral density was reduced by 25% and accompanied with decreased serum osteocalcin, and that 30 days of alcohol abstinence reversed these pathologies(32). Interestingly, the loss of vertebral trabecular bone related to binge alcohol exposure was not reversed following a 30-day abstinence, suggesting binge alcohol has both short- and long-term consequences for bone homeostasis. Binge alcohol has also been shown to exacerbate bone loss in ovariectomized rodents in a model of post-menopausal osteoporosis(33). In these experiments, the researchers found that animals who received a binge alcohol paradigm following ovariectomy had more severe trabecular and cortical bone loss than the individual conditions. Interestingly, Kidder and Turner found that both a chronic low and high dose alcohol diet did not enhance bone loss in ovariectomized animals(34), suggesting that binge versus chronic alcohol consumption may have differential effects on bone loss in ovariectomized rodents.
Several potential treatment modalities have been described in the literature to prevent the loss of bone associated with binge alcohol drinking. Intermittent parathyroid hormone has been shown to modulate bone homeostasis through bone formation and resorption(35, 36), and importantly, acute and chronic alcohol consumption has been shown to reduce serum parathyroid levels in pregnant, non-pregnant rodents, and humans(37–40). To this end, Callaci et al. found that intermittent parathyroid hormone mitigated the effect of binge alcohol on bone loss following ovariectomy(33). Another adjuvant therapy described focused on are restoring vitamin D deficiencies that are common among alcohol abusers. As mentioned earlier, chronic alcohol use has been shown to reduce systemic vitamin D in both human and animal models. Using a binge alcohol paradigm, Wezeman et al. found that exogenous vitamin D prevented alcohol-induced tibial and vertebral bone loss(41). One family of adjuvants used frequently to prevent bone loss in osteoporotic patients are bisphosphonates. Several studies show that bisphosphonates can mitigate the effects of binge and chronic alcohol consumption in animal models, likely by mitigating the effect of alcohol on bone resorption(11, 42). Taken together, these findings suggest that the main mechanism of alcohol-induced bone loss is primarily through perturbing bone homeostasis resulting in a decrease in bone density over time.
Alcohol and fracture
Alcohol use is associated with an increased risk of bone fracture(43–46). In addition to bone fragility-related fractures(47), alcohol use is also associated with an increased risk of traumatic injury occurrence(48, 49). Indeed, nearly 50% of orthopaedic trauma patients have elevated blood alcohol at the time of injury(50, 51). The method of injury is also significant when comparing alcohol abusers and non-abusers. Alcohol abusers sustained twice as many fractures compared to alcohol non-abusers when falling at ground level and 3 times as many fractures when falling from higher levels(52). Alcohol abusers are also at a higher risk for developing fracture nonunion(53). Bone fractures can progress to nonunion, when the bone fails to develop an ossified fracture callus that completely bridges both ends of the fracture site. Long bones have a greater propensity of developing a fracture nonunion than fractures at other skeletal sites (54, 55*). The prevalence of fracture nonunion in long bones is estimated to be 5–10% of cases, requiring additional surgeries, prolonged patient recovery time and substantial increase in healthcare costs and morbidity(55*,56–58). One skeletal long bone in particular, the tibia, is prone to developing facture nonunion, with nonunion rates as high as 19%(59). One factor that may be contributing to the development of a facture nonunion in patients who abuse alcohol is bone fracture healing time. It has been shown that individuals who abuse alcohol also experience significantly longer bone fracture healing times compared to non-abusers(52). When comparing a population of adult male patients who sustained a tibial shaft fracture, those who abused alcohol took an average of 42 days longer for their fractures to heal. Interestingly, the difference in fracture healing time was only observed for a certain pattern of fracture sustained. There were no differences between alcohol abusers and non-abusers for oblique tibial fractures, but male patients who abused alcohol had a longer healing time for transverse tibial fractures when compared to non-abusers.
In line with observations in human alcohol abusers, chronic alcohol consumption models in rodents also demonstrate evidence of inhibition of fracture healing. Chakkalakal et al. found that animals receiving 36% of their calories from alcohol for 6 weeks before and 6 weeks after a surgically administered fibula fracture had reduced fracture callus bone strength, stiffness and rigidity compared to pair-fed animals(60, 61). Interestingly, Chakkalakal et al. found that animals receiving alcohol before fracture and abstinence after injury had normal fracture callus biomechanical properties. Similar observations were found in another study investigating tibia fractures, but this study also found that even uninjured tibias also exhibited reduced stiffness and strength compared to pair-fed controls(62). Both studies found that chronic alcohol consumption led to reduced bone mass in the fracture when compared to pair-fed animals. These findings were supported by histological and radiographic evidence in a study by Elmali et al., who found that animals who received alcohol had lower fracture healing scores and bone mineral density, respectively(63).
In rodents, binge alcohol exposure also significantly delays fracture healing and reduces biomechanical strength of the fracture callus(63–66). Upon histological examination, several studies show that alcohol significantly inhibits normal fracture callus formation(63, 66). In one study, Lauing et al. showed that alcohol-treated animals had reduced fracture callus size, reduced cartilaginous area within the fracture callus, and displayed decreased fracture callus biomechanical strength(66). Histological analysis in similar experiments by Roper, et al. found that animals receiving alcohol had significantly reduced fracture callus cartilaginous callus area and significantly reduced fracture callus hypertrophic chondrocyte area(67). These findings suggest that alcohol is likely detrimental to mesenchymal stem cell activity during homeostasis and at the site of fracture repair.
Effects of alcohol on mesenchymal stem cell activity and differentiation
Mesenchymal stem cells (MSCs) are multipotent cells that give rise to osteoblasts (bone producing cell) and chondrocytes(cartilage producing cell) lineages, which are necessary for fracture callus formation and repair. MSCs migrate from local periosteal tissue to the fracture site to initiate fracture healing(68–70). It has been reported that fracture callus tissue produces stromal cell-derived factor-1 and osteopontin to recruit MSCs to the fracture site following injury (71, 72) A recent study showed that rodents exposed to alcohol have reduced osteopontin expression within the fracture callus(73). In the same study, MSCs cultured ex vivo in alcohol were found to have reduced osteopontin-mediated migration and reduced integrin β1 receptor expression, one receptor reported to play a role in MSC osteopontin-mediated migration(74, 75). CD44 protein expression, another known osteopontin receptor, was unaffected by alcohol exposure, suggesting MSCs utilize integrin β1 receptor for osteopontin-mediated migration. These results suggest that one effect of alcohol on MSCs may be the inhibition of MSC recruitment to the site of fracture injury. Relatedly, Obermeyer et al. showed that intravenous administered MSCs were capable of migrating to the fracture site(76). In this study, animals binged with alcohol had improved fracture callus biomechanics and normalized histological and microcomputed tomography after receiving intravenous MSCs. These findings suggest that endogenous MSC activity may be negatively affected following binge alcohol exposure. This hypothesis is supported by another study that examined the effect of alcohol on ex vivo cultured MSCs and found that alcohol induced MSC cellular senescence(27). In this study, Chen et al. treated MSCs with several concentrations of alcohol and examined the osteogenic potential after culturing the MSCs for 21 days later. Alcohol exposed MSCs had reduced mineralization and osteogenic marker mRNA expression when compared to control MSCs.
As covered earlier, several studies have shown that alcohol inhibits osteoblast activity and proliferation. Relatedly, it has also been reported that alcohol not only inhibits MSC proliferation and expansion, but impairs multipotentiality(77). To examine the effect of alcohol on MSC expansion and proliferation, Huff et al. placed rodents on liquid diets containing 36% alcohol for several weeks and found that animals on the alcohol diet had reduced MSC doublings/day and increased doubling time of ex vivo cultured MSCs(77). When compared to control MSCs, alcohol exposed rodent MSCs had a lasting effect on proliferation over several passage and perturbed capacity of MSCs to differentiate to osteoblast and adipocyte lineages.
Chronic alcohol exposure of rodents and non-human primates is associated with reduced bone density and increased bone marrow adiposity(78–81). In support of these findings, Chen et al. showed alcohol exposure enhances adipogenesis(82). In this study researchers co-cultured a well described MSC-like cell line, C3H10T1/2, in alcohol and adipogenic media and found that alcohol significantly elevated peroxisome proliferator-activated receptor gamma (PPARγ), triglycerides, and activating protein 2 (AP2, a fatty acid carrier protein). There is also growing evidence to suggest alcohol induces transdifferentiation of osteoblasts towards adipocytes. One research group found that human MSCs co-cultured with alcohol and osteogenic media showed upregulated expression of PPARγ, master regulator of adipogenesis, and contained increased number of lipid droplets, suggesting alcohol skewed osteogenic differentiation towards adipogenesis(83). In a similar experiments, Chen et al. found that MSCs treated with alcohol and osteogenic media had a significant suppression of Wnt/β-catenin signaling and increased PPARγ compared to control MSCs(84). The MSCs treated with alcohol in these experiments also had reduced mineralization and osteogenesis markers compared to control MSCs. Along these lines, Huang et al. used small interfering RNAs to inhibit PPARγ expression in MSCs treated with alcohol and found that knockdown of PPARγ rescued mineralization and osteogenic marker expression(85). These findings suggest a reciprocal relationship between Wnt/β-catenin signaling and PPARγ, redirecting MSC differentiation from osteogenesis towards adipogenesis. Taken together, these experiments suggest that alcohol’s effect on cellular lineage commitment are due to dysregulated cellular signaling pathways that regulate MSC differentiation potential.
Effects of alcohol on signaling during bone homeostasis and fracture repair
Bone development, homeostasis and fracture repair are all regulated in part through Canonical Wnt signaling(86). Wnt receptor activation leads to stabilization of β-catenin and translocation into the nucleus where β-catenin promotes transcription of genes involved in bone formation and regeneration. Studies show that animals exposed to alcohol have dysregulated β-catenin within the fracture callus(66, 87). In one study, Lauing et al. showed that the downstream effector of the Canonical Wnt signaling pathway, β-catenin, was hyperphosphorylated in the fracture callus of rodents exposed to alcohol, targeting β-catenin to the proteasome for degradation(87). Wnt/β-catenin signaling is tightly regulated during fracture callus formation. It has been reported that alterations in β-catenin levels or changes in post-translational modification can have positive or negative effects on fracture healing(88). One such protein that modifies β-catenin post-translationally is glycogen synthase kinase 3 beta (GSK-3β). In a model of binge alcohol exposure, it was found that rodents had higher levels of active GSK-3β (phospho-Y216) in the fracture callus of rodents who received alcohol compared to controls(87). Active GSK-3β phosphorylates β-catenin, which destabilizing β-catenin and designates β-catenin for degradation(89). To this end, Lauing et al. showed exogenous activation of the Wnt/β-catenin signaling pathway using GSK-3β inhibitor lithium chloride post-fracture mitigated the negative effect of alcohol on fracture callus size and histological score(66). A recent study by Chen et al. showed MSCs and bone tissue from animals exposed to alcohol had increased expression and active levels of phosphatase and tensin homolog (PTEN)(90). Active PTEN dephosphorylates phosphatidylinositol-3,4,5-triphosphate (PIP3) back to phosphatidylinositol 4,5-bisphosphate (PIP2), which is important for recruitment of phosphoinositide-dependent kinase-1 (PDK1) and subsequent phosphorylation and activation of AKT(91). Chen et al. found that alcohol-induced activated PTEN blocks AKT activation, which is necessary for phosphorylation and inhibition of GSK-3β. PTEN-specific inhibitors restored osteogenic marker expression and improved rodent bone parameters when co-administered with alcohol, suggesting a possible therapeutic approach in the prevention of alcohol-induced osteopenia. Two additional studies describe alternative mechanisms in which alcohol may contribute to alterations in bone homeostasis through inhibition of Wnt/β-catenin signaling. González-Reimers et al. found serum sclerostin, an inhibitor of Wnt/β-catenin signaling secreted by osteocytes that inhibits osteoblast function, differentiation and survival, was significantly higher in alcoholic patients when compared to control patients(92). Alcoholic patients in this study also had decreased markers of bone formation and increased markers for bone resorption. The second study by Chen et al. found femurs from chronically alcohol-fed rodents had increased Wnt signaling antagonist dickkopf-related protein 1 (DKK1) when compared to control animals(82). Co-administration of an antioxidant attenuated the effects of alcohol on bone mineral density and osteogenic markers, and restored DKK1 to control levels in animals treated with alcohol.
The phosphatidylinositide 3-kinase (PI3K)-AKT-Mammalian (mechanistic) target of rapamycin (mTOR) signaling pathway has been postulated to be involved in bone homeostasis and some evidence suggests PI3K-AKT-mTOR signaling pathway may be involved in MSC lineage differentiation(93). To this end, Liu et al. performed experiments to determine the effect of alcohol on PI3K-AKT-mTOR signaling in MSCs undergoing ex vivo osteogenic differentiation(94). In this study, researchers recapitulated previous findings that showed chronic alcohol exposure skewed differentiating MSCs cultured in osteogenic media away from osteogenesis and towards adipogenesis. Liu et al. reported that MSCs cultured in high dose alcohol and MSCs isolated from animals exposed to chronic high dose alcohol had activated PI3K-AKT-mTOR signaling and downregulated Runt-related transcription factor 2 (RUNX2, key transcription factor in osteoblast differentiation) and upregulated of PPARγ. Co-administration of rapamycin, a mTOR-specific inhibitor, with chronic high dose alcohol prevented the upregulation of PPARγ and rescued RUNX2 expression and MSC osteogenic differentiation, resulting in an attenuation of alcohol-induced bone loss and increased bone marrow adiposity. Similar findings were observed in a rodent model of alcohol-induced osteonecrosis(95), where Yang et al. and colleagues found that mTOR modulation rescued osteogenic marker expression and protected animals from alcohol-induced osteonecrosis.
Alcohol use leads to increases in systemic and intracellular reactive oxidative stress (ROS)(96). Furthermore, researchers have shown that MSCs and osteoblasts treated with alcohol directly produce ROS(27, 97, 98). To this end, several groups have reported on the effect of antioxidant treatments, e.g. N-acetylcysteine (NAC), in models of chronic alcohol exposure and bone fracture experiments combined with alcohol exposure(65, 67, 81, 82, 97, 99, 100). In a series of in vitro experiments in 2008, Chen et al. found NAC co-administered with alcohol blocked alcohol-induced osteoclastogenesis and RANKL expression in osteoblasts(97). As previously reported by Chen et al. in 2006, extracellular signal-regulated kinase (ERK) and downstream signal transducers and activators of transcription 3 (STAT3) were activated in response to alcohol exposure(13), but were attenuated when co-administered with NAC(97). These findings were mirrored in chronic alcohol experiments, where Chen et al. in 2010 showed NAC co-administered with alcohol prevented alcohol-mediated increases in osteoclast numbers, decreases in osteoblast numbers, and bone mineral density(100). Lastly, NAC co-administration also ameliorated senescence-associated p21 expression in the femurs of alcohol exposed animals(80). These findings suggest alcohol-induced ROS plays a major role in regulating bone homeostasis. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) enzymes have been shown to be upregulated in osteoblasts from animals exposed to alcohol, of which the primary subtypes expressed in response to alcohol were NOX1, NOX2, and NOX4, and alcohol-induced NOX1/2/4 upregulation is reversed when co-administered with NAC(97, 100). NOX enzymes are membrane-bound proteins that produce superoxides, which can be further converted in ROS. Interestingly, alcohol co-administration with NAC or a NOX-specific inhibitor prevents alcohol-induced ERK/STAT3 activation in osteoblasts, RANKL expression, and bone loss(97, 100). Experiments involving the use of animals with a loss-of-function (LOF) of NOX2 showed that NOX2 activity is necessary for alcohol-induced bone loss(9). NOX2 LOF animals exposed to alcohol were protected from alcohol-induced increases in serum CTx, suggesting NOX2 function is necessary for alcohol-induced bone resorption. However, NOX2 LOF animals saw no protection from alcohol-induced decreases in serum alkaline phosphatase and osteocalcin, suggesting that alcohol inhibition of osteoblasts occurs independently of functional NOX2(9). In the same study, Mercer et al. treated an osteoblast cell line with a NOX2-specific inhibitor and found that NOX2 was solely responsible for the production of ROS after alcohol exposure and increased RANKL expression. Recent studies investigating whether knockout of whole-body NOX4(101) or MSC-specific NOX4(24) were protected against the deleterious effects of alcohol on bone formation revealed the effect of alcohol on osteoblast functioned independently of NOX4. While NOX4 knockout animals had increased cortical thickness and density, further studies are needed to determine the role of NOX enzymes in alcohol-induced bone loss. Taken together, these findings illustrate one alcohol-mediated signaling cascade where alcohol exposure upregulates NOX proteins, leading to ROS generation that activates ERK/STAT3 signaling and subsequent RANKL expression. Similar effects have been observed in bone fracture models. One study performed by Duryee et al. found NAC reduced tissue levels of oxidative stress, RANKL expression, and osteoclast activity while improving fracture callus biomechanical strength(99). In a study of binge alcohol exposure, rodents treated with NAC following an alcohol binge had an improvement in fracture callus size, biomechanical properties and histological appearance compared to animals receiving alcohol binge alone(67). Combined, these findings suggest the physiological response to alcohol and alcohol metabolism have profound effects on bone health and homeostasis that may benefit from antioxidant therapies.
There are several cellular mechanisms capable of responding to oxidative stress. One intracellular molecular responder is the Forkhead box O (FoxO) family of transcription factors. In a study in 2016, Roper et al. found that the fracture callus of alcohol exposed rodents had elevated levels of total and active FoxO1 phosphorylated at S207 concurrent with reduced levels of inactive FoxO phosphorylated at S253(67). In the same study, the authors found that the FoxO signaling perturbed by alcohol exposure was restored to levels of saline treated rodents by NAC treatment following fracture(67). The fracture callus is produced by the differentiation of MSCs into osteoblasts and chondrocytes, and evidence from fracture studies suggest that alcohol is disrupting cellular processes in early fracture callus development(67). To this end, a recent study by Sharieh et al. examined the effect of alcohol on ex vivo MSC differentiation and FoxO1/3 signaling(102*). In this study, researchers found MSCs cultured in alcohol had increased FoxO1/3 expression and activity when compared to control MSCs. Past studies found MSCs cultured in alcohol had reduced multipotentiality(77). Similarly, Sharieh et al. found that primary MSCs co-cultured with alcohol and osteogenic or chondrogenic media had reduced marker expression for MSC osteochondral lineage differentiation(102*). The effect of alcohol on osteogenic and chondrogenic lineage marker expression was mitigated when FoxO1/3 expression was knocked down using both pharmacologic and genetic approaches in MSCs cultured in alcohol(102*). These findings suggest that the activation of FoxO signaling by alcohol impairs MSCs osteochondral differentiation. Canonical Wnt/β-catenin signaling is necessary for the commitment of MSCs into osteoblasts and chondrocyte cell lineages, which initiate repair and produce the fracture callus(53–56). Further studies are necessary to determine whether the inhibition of cartilaginous fracture callus formation in animals exposed to alcohol is the result of inhibition of fracture site MSC differentiation (Figure 1). Interestingly, FoxO is known to modulate Wnt/β-catenin signaling by sequestering β-catenin from the Wnt signaling pathway(103*,104,105*). Almeida et al. found that an osteoblast cell line treated with an oxidative stressor, hydrogen peroxide, increased FoxO and β-catenin association, which increased expression of FoxO-mediated transcription and decreased osteoblast differentiation. In a separate study, Almeida et al. found that rodents lacking FoxO1/3/4 in osteoprogenitor cells have increased bone mass, bone formation, and increased Wnt signaling(106). In addition, osteoprogenitor-specific FoxO knockout animals had reduced bone marrow adiposity, suggesting osteoprogenitor cells were skewed away from adipogenesis towards osteogenesis.
Figure 1:
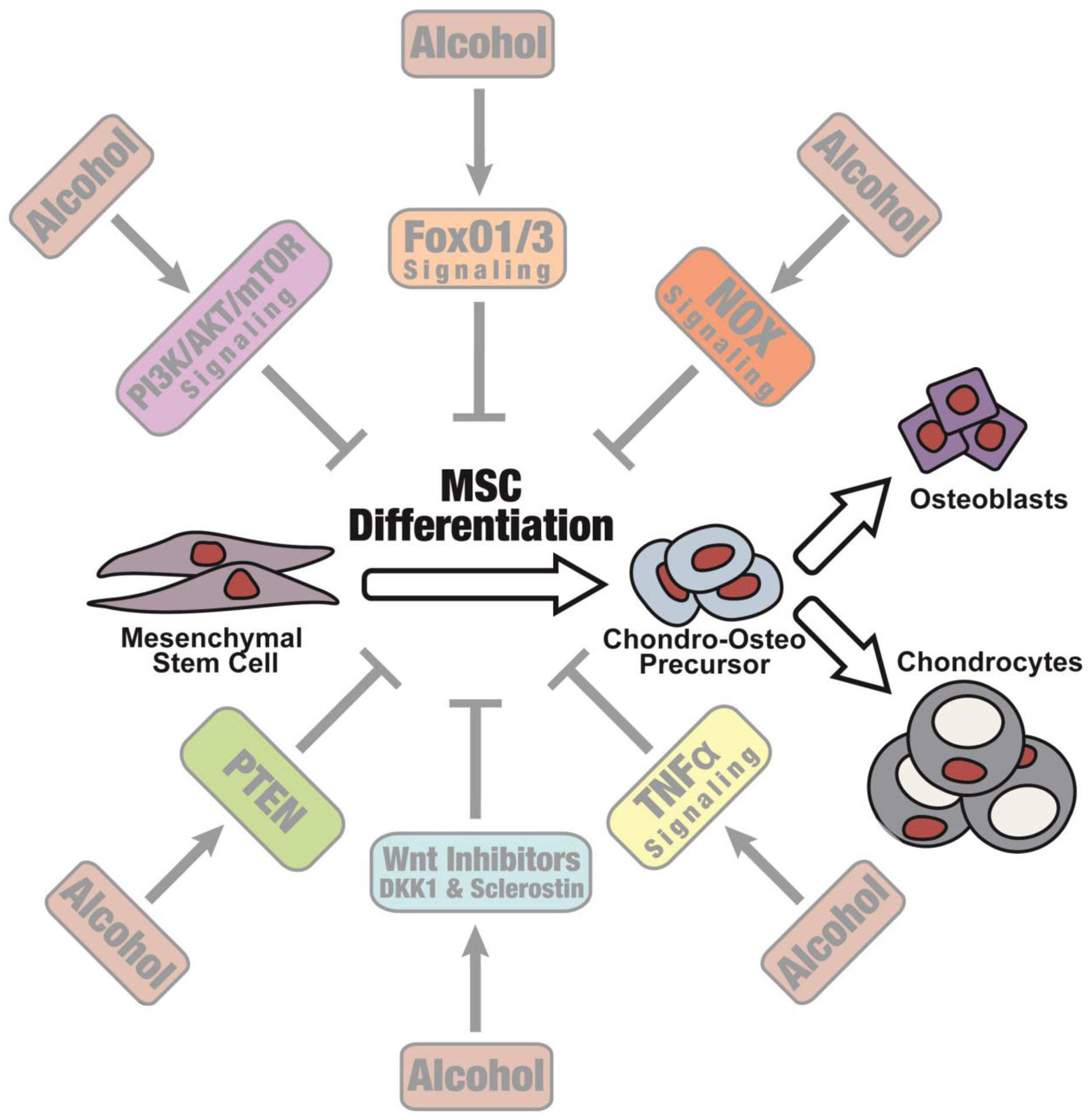
Hypothesized effect of alcohol inhibition in mesenchymal stem cells at the fracture site.m
Another important signaling pathway in bone growth and homeostasis is the transforming growth factor beta (TGF-β) superfamily, including the bone morphogenetic protein (BMP) signaling pathway. BMP-2 activity is not required for normal bone formation and homeostasis, but experiments using BMP-2-deficient animals found that BMP-2 is required for the initiation of fracture healing(107). In a study of binge alcohol exposure, Bratton et al. found that animals receiving chronic binge alcohol before sustaining a tibial fracture had increased BMP-2 and BMP receptor 2 within the fracture callus(108). BMP-2 is also known to bind and activate BMP receptor 1a, which is necessary for osteoblast extracellular matrix deposition(109). In the same study researchers found that BMP receptor 1a was found to be decreased in animals receiving alcohol when compared to control animals. BMP-2 signaling can be modulated by endogenous BMP-2 antagonist, chordin. A previous study by Kloen et al. found BMP-2 inhibitors like chordin are increased in fracture nonunion tissue(110). Bratton, et al. found that chordin levels were increased in the fracture callus of animals exposed to alcohol. Taken together, these data suggest alcohol exposure perturbs BMP-2 signaling within the fracture callus and may predispose a fracture towards nonunion. Another component of the TGF-β superfamily that is important for fracture repair is TGF-β1(111). TGF-β1 has been shown to function as a chemoattractant in MSC migration and to promote proliferation of osteoblasts and chondrocytes(112), which are necessary for proper fracture callus formation. In a study of binge alcohol exposure, Driver et al. found that animals exposed to alcohol had decreased levels of TGF-β1 in fracture callus tissue(113). These findings offer further evidence that alcohol dysregulates TGF-β and BMP signaling in fracture callus of alcohol exposed animals.
In the early inflammatory phase of bone fracture repair, several cytokines and chemokines are generated from surrounding and injured tissue to attract immune and stem cells to initiate fracture repair as part of the normal physiological response. Tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL-1β) are two pro-inflammatory cytokines that have been long shown to have negative effects on normal bone formation(114, 115). Indeed, TNFα and IL-1β antagonism has been shown to improve bone formation(116). In 2003, Perrien et al. reported that chronic alcohol exposure in a bone fracture model led to elevated levels of TNFα and IL-1β in fracture tissue(117). Interestingly, chronic alcohol exposed animals receiving TNFα and IL-1β antagonists on the day of bone fracture had improved bone bridging and improvements in bone mineralization(118). Later experiments targeting only TNFα signaling revealed the TNFα was the major signaling axis in alcohol inhibition of a distraction osteogenesis fracture repair model. Wahl et al. utilized a systemically administered soluble TNF receptor 1 (TNFR1) derivative to adsorb excess TNFα generated in chronic alcohol exposed animals(119). Alcohol exposed animals receiving the soluble TNFR1 derivative had improved distraction osteogenesis bridging and histological evidence of new bone formation as compared to control animals. Experiments utilizing transgenic TNFR1 knockout animals confirmed that the increased TNFα generated in alcohol exposed animals inhibited distraction osteogenesis through TNFR1(120). Similar findings were observed in experiments measuring the effect of alcohol on bone formation. In these experiments, Shankar et al. found soluble TNFR1 derivative abolished the negative effect of alcohol feeding on bone formation and reduced expression of adipogenic marker expression in bone tissue(81). Yu et al. found that one potential source of elevated TNFα generation was generated from MSCs(121). Cultured MSCs from alcohol-induced osteonecrosis patients expressed TNFα nearly 2-times that of healthy MSCs over a 21-day period. Alcohol-induced osteonecrosis patient MSCs also had reduced osteogenic potential, with decreased osteogenic marker expression when undergoing osteogenic differentiation as compared to control MSCs. In the same study, researchers treated healthy MSCs with exogenous TNFα and found that TNFα directly inhibited osteogenesis. Whereas healthy MSCs treated with a well-known TNFα inhibitor, Adalimumab, improved osteogenesis. Microarray analysis revealed miR-31 was upregulated in alcohol-induced osteonecrosis MSCs patients and TNFα-treated MSCs. Importantly, miR-31 mimic transfections inhibited two important osteogenic transcription factors, RUNX2 and special AT-rich sequence-binding protein 2 (SATB2). These findings suggest TNFα signaling works to inhibit osteogenesis by upregulation of miR-31, leading to downregulation of RUNX2 and SATB2.
Conclusion
Much work has been done to study the effect of alcohol on bone and bone fracture. The studies reviewed in this article illustrate the need for further research and suggest areas of research needed to fill gaps within the current literature. One aspect of this research that appears consistent is that alcohol’s effect on bone remodeling, formation and fracture healing is likely mediated through the suppression of MSC-mediated bone formation activities and activation of osteoclast-mediated bone resorption.
Acknowledgements:
This work was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R01AA016138, R21AA021225, R21AA025551, F31AA019613, F31AA021308, F31AA028147 and T32AA013257, the National Institute of General Medical Sciences under award number T32GM008750, and multiple AO North America Resident Research grants (AONAR).
List of Abbreviations
- AP2
Activating Protein 2
- BMP
Bone Morphogenetic Protein
- CTx
Carboxy-terminal collagen crosslinks
- DKK1
Dickkopf-related protein 1
- ERK
Extracellular signal-regulated kinase
- FoxO
Forkhead box O
- GSK-3β
Glycogen synthase kinase 3 beta
- IL-1β
Interleukin 1 beta
- LOF
Loss-of-functionm
- TOR
Mammalian (mechanistic) target of rapamycin
- MSC
Mesenchymal stem cell
- MSCs
Mesenchymal stem cells
- NAC
N-acetylcysteine
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NOX
NADPH Oxidase
- PI3K
Phosphatidylinositide 3-kinase
- PEth
Phosphatidylethanol
- PDK1
Phosphoinositide-dependent kinase-1
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PIP3
Phosphatidylinositol-3,4,5-triphosphate
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PTEN
Phosphatase and tensin homolog
- RANKL
Nuclear factor-κB ligand
- RUNX2
Runt-related transcription factor 2
- ROS
Reactive Oxygen Species
- SATB2
Special AT-rich sequence-binding protein 2
- STAT3
Signal transducers and activators of transcription 3
- TGF-β
Transforming growth factor beta
- TNFα
Tumor necrosis factor alpha
- TNFR1
TNF receptor 1
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: Jonathan M. Eby, Farah Sharieh, and John J. Callaci declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as: * Of importance
- 1.*.Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet (London, England). 2019;393(10190):2493–502. [DOI] [PubMed] [Google Scholar]; This study forecasts that the amount of alcohol consumed per adult per year will continue to rise globally.
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17(12):1726–33. [DOI] [PubMed] [Google Scholar]
- 3.Turner RT, Aloia RC, Segel LD, Hannon KS, Bell NH. Chronic alcohol treatment results in disturbed vitamin D metabolism and skeletal abnormalities in rats. Alcoholism, clinical and experimental research. 1988;12(1):159–62. [DOI] [PubMed] [Google Scholar]
- 4.Shankar K, Liu X, Singhal R, Chen JR, Nagarajan S, Badger TM, et al. Chronic ethanol consumption leads to disruption of vitamin D3 homeostasis associated with induction of renal 1,25 dihydroxyvitamin D3–24-hydroxylase (CYP24A1). Endocrinology. 2008;149(4):1748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercer KE, Wynne RA, Lazarenko OP, Lumpkin CK, Hogue WR, Suva LJ, et al. Vitamin D supplementation protects against bone loss associated with chronic alcohol administration in female mice. The Journal of pharmacology and experimental therapeutics. 2012;343(2):401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaci JJ, Himes R, Lauing K, Wezeman FH, Brownson K. Binge alcohol-induced bone damage is accompanied by differential expression of bone remodeling-related genes in rat vertebral bone. Calcified tissue international. 2009;84(6):474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung RC, Gray C, Boyde A, Jones SJ. Effects of ethanol on bone cells in vitro resulting in increased resorption. Bone. 1995;16(1):143–7. [PubMed] [Google Scholar]
- 8.Ronis MJ, Mercer K, Chen JR. Effects of nutrition and alcohol consumption on bone loss. Current osteoporosis reports. 2011;9(2):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercer KE, Sims CR, Yang CS, Wynne RA, Moutos C, Hogue WR, et al. Loss of functional NADPH oxidase 2 protects against alcohol-induced bone resorption in female p47phox−/− mice. Alcoholism, clinical and experimental research. 2014;38(3):672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diez A, Puig J, Serrano S, Marinoso ML, Bosch J, Marrugat J, et al. Alcohol-induced bone disease in the absence of severe chronic liver damage. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1994;9(6):825–31. [DOI] [PubMed] [Google Scholar]
- 11.Callaci JJ, Juknelis D, Patwardhan A, Sartori M, Frost N, Wezeman FH. The effects of binge alcohol exposure on bone resorption and biomechanical and structural properties are offset by concurrent bisphosphonate treatment. Alcoholism, clinical and experimental research. 2004;28(1):182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner RT, Kidder LS, Kennedy A, Evans GL, Sibonga JD. Moderate alcohol consumption suppresses bone turnover in adult female rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(3):589–94. [DOI] [PubMed] [Google Scholar]
- 13.Chen JR, Haley RL, Hidestrand M, Shankar K, Liu X, Lumpkin CK, et al. Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-kappaB ligand in osteoblasts. The Journal of pharmacology and experimental therapeutics. 2006;319(3):1182–90. [DOI] [PubMed] [Google Scholar]
- 14.Gaddini GW, Grant KA, Woodall A, Stull C, Maddalozzo GF, Zhang B, et al. Twelve months of voluntary heavy alcohol consumption in male rhesus macaques suppresses intracortical bone remodeling. Bone. 2015;71:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sripanyakorn S, Jugdaohsingh R, Mander A, Davidson SL, Thompson RP, Powell JJ. Moderate ingestion of alcohol is associated with acute ethanol-induced suppression of circulating CTX in a PTH-independent fashion. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(8):1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68(10):1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaddini GW, Turner RT, Grant KA, Iwaniec UT. Alcohol: A Simple Nutrient with Complex Actions on Bone in the Adult Skeleton. Alcoholism, clinical and experimental research. 2016;40(4):657–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58(5):424–30. [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Calvin JL, Garcia-Sanchez A, Bellot V, Munoz-Torres M, Raya-Alvarez E, Salvatierra-Rios D. Mineral metabolism, osteoblastic function and bone mass in chronic alcoholism. Alcohol and alcoholism (Oxford, Oxfordshire). 1993;28(5):571–9. [PubMed] [Google Scholar]
- 20.Diamond T, Stiel D, Lunzer M, Wilkinson M, Posen S. Ethanol reduces bone formation and may cause osteoporosis. The American journal of medicine. 1989;86(3):282–8. [DOI] [PubMed] [Google Scholar]
- 21.Brown JP, Delmas PD, Malaval L, Edouard C, Chapuy MC, Meunier PJ. Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet (London, England). 1984;1(8386):1091–3. [DOI] [PubMed] [Google Scholar]
- 22.Watt J, Schuon J, Davis J, Ferguson TF, Welsh DA, Molina PE, et al. Reduced Serum Osteocalcin in High-Risk Alcohol Using People Living With HIV Does Not Correlate With Systemic Oxidative Stress or Inflammation: Data From the New Orleans Alcohol Use in HIV Study. Alcoholism, clinical and experimental research. 2019;43(11):2374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvisa-Negrin J, Gonzalez-Reimers E, Santolaria-Fernandez F, Garcia-Valdecasas-Campelo E, Valls MR, Pelazas-Gonzalez R, et al. Osteopenia in alcoholics: effect of alcohol abstinence. Alcohol and alcoholism (Oxford, Oxfordshire). 2009;44(5):468–75. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen KB, Osborn ML, Robertson AC, Williams AE, Watt J, Denys A, et al. Chronic Ethanol Feeding in Mice Decreases Expression of Genes for Major Structural Bone Proteins in a Nox4-Independent Manner. The Journal of pharmacology and experimental therapeutics. 2020;373(3):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein RF, Fausti KA, Carlos AS. Ethanol inhibits human osteoblastic cell proliferation. Alcoholism, clinical and experimental research. 1996;20(3):572–8. [DOI] [PubMed] [Google Scholar]
- 26.Dyer SA, Buckendahl P, Sampson HW. Alcohol consumption inhibits osteoblastic cell proliferation and activity in vivo. Alcohol (Fayetteville, NY). 1998;16(4):337–41. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Li M, Yan J, Liu T, Pan G, Yang H, et al. Alcohol Induces Cellular Senescence and Impairs Osteogenic Potential in Bone Marrow-Derived Mesenchymal Stem Cells. Alcohol and alcoholism (Oxford, Oxfordshire). 2017;52(3):289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavassieux P, Serre CM, Vergnaud P, Delmas PD, Meunier PJ. In vitro evaluation of dose-effects of ethanol on human osteoblastic cells. Bone Miner. 1993;22(2):95–103. [DOI] [PubMed] [Google Scholar]
- 29.Chen JR, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJ. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereas estradiol attenuates the effects of ethanol in osteoblasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(2):221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Vernejoul MC, Bielakoff J, Herve M, Gueris J, Hott M, Modrowski D, et al. Evidence for defective osteoblastic function. A role for alcohol and tobacco consumption in osteoporosis in middle-aged men. Clinical orthopaedics and related research. 1983(179):107–15. [PubMed] [Google Scholar]
- 31.Fanti P, Monier-Faugere MC, Geng Z, Cohen D, Malluche HH. Moderately high consumption of ethanol suppresses bone resorption in ovariectomized but not in sexually intact adult female rats. Alcoholism, clinical and experimental research. 1997;21(6):1150–4. [DOI] [PubMed] [Google Scholar]
- 32.Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol (Fayetteville, NY). 2008;42(8):649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callaci JJ, Juknelis D, Patwardhan A, Wezeman FH. Binge alcohol treatment increases vertebral bone loss following ovariectomy: compensation by intermittent parathyroid hormone. Alcoholism, clinical and experimental research. 2006;30(4):665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidder LS, Turner RT. Dietary ethanol does not accelerate bone loss in ovariectomized rats. Alcoholism, clinical and experimental research. 1998;22(9):2159–64. [PubMed] [Google Scholar]
- 35.Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1992;7(1):65–72. [DOI] [PubMed] [Google Scholar]
- 36.Poole KE, Reeve J. Parathyroid hormone - a bone anabolic and catabolic agent. Current opinion in pharmacology. 2005;5(6):612–7. [DOI] [PubMed] [Google Scholar]
- 37.Keiver K, Weinberg J. Effect of duration of alcohol consumption on calcium and bone metabolism during pregnancy in the rat. Alcoholism, clinical and experimental research. 2003;27(9):1507–19. [DOI] [PubMed] [Google Scholar]
- 38.Duggal S, Simpson ME, Keiver K. Effect of chronic ethanol consumption on the response of parathyroid hormone to hypocalcemia in the pregnant rat. Alcoholism, clinical and experimental research. 2007;31(1):104–12. [DOI] [PubMed] [Google Scholar]
- 39.Diez A, Serrano S, Cucurull J, Mariñoso L, Bosch J, Puig J, et al. Acute effects of ethanol on mineral metabolism and trabecular bone in Sprague-Dawley rats. Calcified tissue international. 1997;61(2):168–71. [DOI] [PubMed] [Google Scholar]
- 40.Perry HM 3rd, Horowitz M, Fleming S, Kaiser FE, Patrick P, Morley JE, et al. Effect of recent alcohol intake on parathyroid hormone and mineral metabolism in men. Alcoholism, clinical and experimental research. 1998;22(6):1369–75. [PubMed] [Google Scholar]
- 41.Wezeman FH, Juknelis D, Himes R, Callaci JJ. Vitamin D and ibandronate prevent cancellous bone loss associated with binge alcohol treatment in male rats. Bone. 2007;41(4):639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wezeman FH, Emanuele MA, Moskal SF, Steiner J, Lapaglia N. Alendronate administration and skeletal response during chronic alcohol intake in the adolescent male rat. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15(10):2033–41. [DOI] [PubMed] [Google Scholar]
- 43.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, et al. Alcohol intake as a risk factor for fracture. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16(7):737–42. [DOI] [PubMed] [Google Scholar]
- 44.Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA Jr., et al. Association between alcohol consumption and both osteoporotic fracture and bone density. The American journal of medicine. 2008;121(5):406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santori C, Ceccanti M, Diacinti D, Attilia ML, Toppo L, D’Erasmo E, et al. Skeletal turnover, bone mineral density, and fractures in male chronic abusers of alcohol. J Endocrinol Invest. 2008;31(4):321–6. [DOI] [PubMed] [Google Scholar]
- 46.Turner RT. Skeletal response to alcohol. Alcoholism, clinical and experimental research. 2000;24(11):1693–701. [PubMed] [Google Scholar]
- 47.Wark JD. Osteoporotic fractures: background and prevention strategies. Maturitas. 1996;23(2):193–207. [DOI] [PubMed] [Google Scholar]
- 48.Black DM, Cooper C. Epidemiology of fractures and assessment of fracture risk. Clinics in laboratory medicine. 2000;20(3):439–53. [PubMed] [Google Scholar]
- 49.Johnston JJ, McGovern SJ. Alcohol related falls: an interesting pattern of injuries. Emerg Med J. 2004;21(2):185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. Journal of orthopaedic trauma. 1996;10(1):21–7. [DOI] [PubMed] [Google Scholar]
- 51.Savola O, Niemela O, Hillbom M. Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol and alcoholism (Oxford, Oxfordshire). 2005;40(4):269–73. [DOI] [PubMed] [Google Scholar]
- 52.Nyquist F, Berglund M, Nilsson BE, Obrant KJ. Nature and healing of tibial shaft fractures in alcohol abusers. Alcohol and alcoholism (Oxford, Oxfordshire). 1997;32(1):91–5. [DOI] [PubMed] [Google Scholar]
- 53.Duckworth AD, Bennet SJ, Aderinto J, Keating JF. Fixation of intracapsular fractures of the femoral neck in young patients: risk factors for failure. J Bone Joint Surg Br. 2011;93(6):811–6. [DOI] [PubMed] [Google Scholar]
- 54.Ekegren CL, Edwards ER, de Steiger R, Gabbe BJ. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int J Environ Res Public Health. 2018;15(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.*.Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, et al. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA surgery. 2016;151(11):e162775. [DOI] [PubMed] [Google Scholar]; This study found that tibial fractures have a greater propensity to developing a fracture nonunion.
- 56.Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45 Suppl 2:S3–7. [DOI] [PubMed] [Google Scholar]
- 57.Tay WH, de Steiger R, Richardson M, Gruen R, Balogh ZJ. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury. 2014;45(10):1653–8. [DOI] [PubMed] [Google Scholar]
- 58.Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury. 2007;38 Suppl 2:S3–9. [DOI] [PubMed] [Google Scholar]
- 59.Hak DJ. Management of aseptic tibial nonunion. The Journal of the American Academy of Orthopaedic Surgeons. 2011;19(9):563–73. [DOI] [PubMed] [Google Scholar]
- 60.Chakkalakal DA, Novak JR, Fritz ED, Mollner TJ, McVicker DL, Garvin KL, et al. Inhibition of bone repair in a rat model for chronic and excessive alcohol consumption. Alcohol (Fayetteville, NY). 2005;36(3):201–14. [DOI] [PubMed] [Google Scholar]
- 61.Chakkalakal DA, Novak JR, Fritz ED, Mollner TJ, McVicker DL, Lybarger DL, et al. Chronic ethanol consumption results in deficient bone repair in rats. Alcohol and alcoholism (Oxford, Oxfordshire). 2002;37(1):13–20. [DOI] [PubMed] [Google Scholar]
- 62.Nyquist F, Halvorsen V, Madsen JE, Nordsletten L, Obrant KJ. Ethanol and its effects on fracture healing and bone mass in male rats. Acta orthopaedica Scandinavica. 1999;70(2):212–6. [DOI] [PubMed] [Google Scholar]
- 63.Elmali N, Ertem K, Ozen S, Inan M, Baysal T, Guner G, et al. Fracture healing and bone mass in rats fed on liquid diet containing ethanol. Alcoholism, clinical and experimental research. 2002;26(4):509–13. [PubMed] [Google Scholar]
- 64.Janicke-Lorenz J, Lorenz R. Alcoholism and fracture healing. A radiological study in the rat. Archives of orthopaedic and traumatic surgery Archiv fur orthopadische und Unfall-Chirurgie. 1984;103(4):286–9. [DOI] [PubMed] [Google Scholar]
- 65.Volkmer DL, Sears B, Lauing KL, Nauer RK, Roper PM, Yong S, et al. Antioxidant therapy attenuates deficient bone fracture repair associated with binge alcohol exposure. Journal of orthopaedic trauma. 2011;25(8):516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lauing KL, Roper PM, Nauer RK, Callaci JJ. Acute alcohol exposure impairs fracture healing and deregulates beta-catenin signaling in the fracture callus. Alcoholism, clinical and experimental research. 2012;36(12):2095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roper PM, Abbasnia P, Vuchkovska A, Natoli RM, Callaci JJ. Alcohol-related deficient fracture healing is associated with activation of FoxO transcription factors in mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2016;34(12):2106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Granero-Molto F, Weis JA, Miga MI, Landis B, Myers TJ, O’Rear L, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem cells (Dayton, Ohio). 2009;27(8):1887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taguchi K, Ogawa R, Migita M, Hanawa H, Ito H, Orimo H. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochemical and biophysical research communications. 2005;331(1):31–6. [DOI] [PubMed] [Google Scholar]
- 70.Shirley D, Marsh D, Jordan G, McQuaid S, Li G. Systemic recruitment of osteoblastic cells in fracture healing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23(5):1013–21. [DOI] [PubMed] [Google Scholar]
- 71.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60(3):813–23. [DOI] [PubMed] [Google Scholar]
- 72.Raheja LF, Genetos DC, Yellowley CE. Hypoxic osteocytes recruit human MSCs through an OPN/CD44-mediated pathway. Biochemical and biophysical research communications. 2008;366(4):1061–6. [DOI] [PubMed] [Google Scholar]
- 73.Natoli RM, Yu H, Meislin MC, Abbasnia P, Roper P, Vuchkovska A, et al. Alcohol exposure decreases osteopontin expression during fracture healing and osteopontin-mediated mesenchymal stem cell migration in vitro. Journal of orthopaedic surgery and research. 2018;13(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou C, Luo Q, Qin J, Shi Y, Yang L, Ju B, et al. Osteopontin promotes mesenchymal stem cell migration and lessens cell stiffness via integrin beta1, FAK, and ERK pathways. Cell biochemistry and biophysics. 2013;65(3):455–62. [DOI] [PubMed] [Google Scholar]
- 75.Gong Z, Wezeman FH. Inhibitory effect of alcohol on osteogenic differentiation in human bone marrow-derived mesenchymal stem cells. Alcoholism, clinical and experimental research. 2004;28(3):468–79. [DOI] [PubMed] [Google Scholar]
- 76.Obermeyer TS, Yonick D, Lauing K, Stock SR, Nauer R, Strotman P, et al. Mesenchymal stem cells facilitate fracture repair in an alcohol-induced impaired healing model. Journal of orthopaedic trauma. 2012;26(12):712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huff NK, Spencer ND, Gimble JM, Bagby GJ, Nelson S, Lopez MJ. Impaired expansion and multipotentiality of adult stromal cells in a rat chronic alcohol abuse model. Alcohol (Fayetteville, NY). 2011;45(4):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maddalozzo GF, Turner RT, Edwards CH, Howe KS, Widrick JJ, Rosen CJ, et al. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(9):1529–38. [DOI] [PubMed] [Google Scholar]
- 79.Kahler-Quesada AM, Grant KA, Walter NAR, Newman N, Allen MR, Burr DB, et al. Voluntary Chronic Heavy Alcohol Consumption in Male Rhesus Macaques Suppresses Cancellous Bone Formation and Increases Bone Marrow Adiposity. Alcoholism, clinical and experimental research. 2019;43(12):2494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alund AW, Mercer KE, Pulliam CF, Suva LJ, Chen JR, Badger TM, et al. Partial Protection by Dietary Antioxidants Against Ethanol-Induced Osteopenia and Changes in Bone Morphology in Female Mice. Alcoholism, clinical and experimental research. 2017;41(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shankar K, Hidestrand M, Liu X, Chen JR, Haley R, Perrien DS, et al. Chronic ethanol consumption inhibits postlactational anabolic bone rebuilding in female rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23(3):338–49. [DOI] [PubMed] [Google Scholar]
- 82.Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ. A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(5):1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wezeman FH, Gong Z. Adipogenic effect of alcohol on human bone marrow-derived mesenchymal stem cells. Alcoholism, clinical and experimental research. 2004;28(7):1091–101. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y, Chen L, Yin Q, Gao H, Dong P, Zhang X, et al. Reciprocal interferences of TNF-alpha and Wnt1/beta-catenin signaling axes shift bone marrow-derived stem cells towards osteoblast lineage after ethanol exposure. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;32(3):755–65. [DOI] [PubMed] [Google Scholar]
- 85.Huang Q, Zhang H, Pei FX, Chen ZY, Wang GL, Shen B, et al. Use of small interfering ribonucleic acids to inhibit the adipogenic effect of alcohol on human bone marrow-derived mesenchymal cells. Int Orthop. 2010;34(7):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature medicine. 2013;19(2):179–92. [DOI] [PubMed] [Google Scholar]
- 87.Lauing KL, Sundaramurthy S, Nauer RK, Callaci JJ. Exogenous activation of Wnt/beta-catenin signaling attenuates binge alcohol-induced deficient bone fracture healing. Alcohol and alcoholism (Oxford, Oxfordshire). 2014;49(4):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, et al. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS medicine. 2007;4(7):e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends in biochemical sciences. 2010;35(3):161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen YX, Zhu DY, Gao J, Xu ZL, Tao SC, Yin WJ, et al. Diminished membrane recruitment of Akt is instrumental in alcohol-associated osteopenia via the PTEN/Akt/GSK-3beta/beta-catenin axis. The FEBS journal. 2019;286(6):1101–19. [DOI] [PubMed] [Google Scholar]
- 91.Carnero A, Paramio JM. The PTEN/PI3K/AKT Pathway in vivo, Cancer Mouse Models. Frontiers in oncology. 2014;4:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.González-Reimers E, Martín-González C, de la Vega-Prieto MJ, Pelazas-González R, Fernández-Rodríguez C, López-Prieto J, et al. Serum sclerostin in alcoholics: a pilot study. Alcohol and alcoholism (Oxford, Oxfordshire). 2013;48(3):278–82. [DOI] [PubMed] [Google Scholar]
- 93.Chen C, Akiyama K, Wang D, Xu X, Li B, Moshaverinia A, et al. mTOR inhibition rescues osteopenia in mice with systemic sclerosis. The Journal of experimental medicine. 2015;212(1):73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y, Kou X, Chen C, Yu W, Su Y, Kim Y, et al. Chronic High Dose Alcohol Induces Osteopenia via Activation of mTOR Signaling in Bone Marrow Mesenchymal Stem Cells. Stem cells (Dayton, Ohio). 2016;34(8):2157–68. [DOI] [PubMed] [Google Scholar]
- 95.Yang Q, Yin W, Chen Y, Zhu D, Yin J, Zhang C, et al. Betaine alleviates alcohol-induced osteonecrosis of the femoral head via mTOR signaling pathway regulation. Biomed Pharmacother. 2019;120:109486. [DOI] [PubMed] [Google Scholar]
- 96.Aytacoglu BN, Calikoglu M, Tamer L, Coskun B, Sucu N, Kose N, et al. Alcohol-induced lung damage and increased oxidative stress. Respiration. 2006;73(1):100–4. [DOI] [PubMed] [Google Scholar]
- 97.Chen JR, Shankar K, Nagarajan S, Badger TM, Ronis MJ. Protective effects of estradiol on ethanol-induced bone loss involve inhibition of reactive oxygen species generation in osteoblasts and downstream activation of the extracellular signal-regulated kinase/signal transducer and activator of transcription 3/receptor activator of nuclear factor-kappaB ligand signaling cascade. The Journal of pharmacology and experimental therapeutics. 2008;324(1):50–9. [DOI] [PubMed] [Google Scholar]
- 98.Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem cells and development. 2015;24(10):1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duryee MJ, Dusad A, Hunter CD, Kharbanda KK, Bruenjes JD, Easterling KC, et al. NAcetyl Cysteine Treatment Restores Early Phase Fracture Healing in Ethanol-Fed Rats. Alcoholism, clinical and experimental research. 2018;42(7):1206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Lumpkin CK, Badger TM, et al. Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. The Journal of pharmacology and experimental therapeutics. 2011;336(3):734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watt J, Alund AW, Pulliam CF, Mercer KE, Suva LJ, Chen JR, et al. NOX4 Deletion in Male Mice Exacerbates the Effect of Ethanol on Trabecular Bone and Osteoblastogenesis. The Journal of pharmacology and experimental therapeutics. 2018;366(1):46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.*.Sharieh F, Eby JM, Roper PM, Callaci JJ. Ethanol inhibits mesenchymal stem cell osteochondral lineage differentiation due in part to an activation of FoxO-specific signaling. Alcoholism, clinical and experimental research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that alcohol activates FoxO1/3 signaling in MSCs, leading to osteochondral lineage differentiation inhibition.
- 103.*.Ma X, Su P, Yin C, Lin X, Wang X, Gao Y, et al. The Roles of FoxO Transcription Factors in Regulation of Bone Cells Function. International journal of molecular sciences. 2020;21(3). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a comprehensive review that covers the importance of FoxO transcription factors in bone formation and homeostasis.
- 104.Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. The Journal of biological chemistry. 2007;282(37):27298–305. [DOI] [PubMed] [Google Scholar]
- 105.*.Kim HN, Iyer S, Ring R, Almeida M. The Role of FoxOs in Bone Health and Disease. Current topics in developmental biology. 2018;127:149–63. [DOI] [PubMed] [Google Scholar]; This is a comprehensive review that covers the importance of FoxO transcription factors in bone health and disease.
- 106.Iyer S, Ambrogini E, Bartell SM, Han L, Roberson PK, de Cabo R, et al. FOXOs attenuate bone formation by suppressing Wnt signaling. The Journal of clinical investigation. 2013;123(8):3409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38(12):1424–9. [DOI] [PubMed] [Google Scholar]
- 108.Bratton A, Eisenberg J, Vuchkovska A, Roper P, Callaci JJ. Effects of Episodic Alcohol Exposure on BMP2 Signaling During Tibia Fracture Healing. Journal of orthopaedic trauma. 2018;32(6):288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, et al. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. The Journal of biological chemistry. 2004;279(26):27560–6. [DOI] [PubMed] [Google Scholar]
- 110.Kloen P, Lauzier D, Hamdy RC. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone. 2012;51(1):59–68. [DOI] [PubMed] [Google Scholar]
- 111.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17(3):513–20. [DOI] [PubMed] [Google Scholar]
- 112.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. The Journal of bone and joint surgery American volume. 2002;84(6):1032–44. [DOI] [PubMed] [Google Scholar]
- 113.Driver J, Weber CE, Callaci JJ, Kothari AN, Zapf MA, Roper PM, et al. Alcohol inhibits osteopontin-dependent transforming growth factor-beta1 expression in human mesenchymal stem cells. The Journal of biological chemistry. 2015;290(16):9959–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen L, Dewhirst FE, Hauschka PV, Stashenko P. Interleukin-1 beta stimulates bone resorption and inhibits bone formation in vivo. Lymphokine Cytokine Res. 1991;10(1–2):15–21. [PubMed] [Google Scholar]
- 115.Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319(6053):516–8. [DOI] [PubMed] [Google Scholar]
- 116.Kimble RB, Matayoshi AB, Vannice JL, Kung VT, Williams C, Pacifici R. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 1995;136(7):3054–61. [DOI] [PubMed] [Google Scholar]
- 117.Perrien DS, Liu Z, Wahl EC, Bunn RC, Skinner RA, Aronson J, et al. Chronic ethanol exposure is associated with a local increase in TNF-alpha and decreased proliferation in the rat distraction gap. Cytokine. 2003;23(6):179–89. [DOI] [PubMed] [Google Scholar]
- 118.Perrien DS, Wahl EC, Hogue WR, Feige U, Aronson J, Ronis MJ, et al. IL-1 and TNF antagonists prevent inhibition of fracture healing by ethanol in rats. Toxicological sciences : an official journal of the Society of Toxicology. 2004;82(2):656–60. [DOI] [PubMed] [Google Scholar]
- 119.Wahl EC, Aronson J, Liu L, Liu Z, Perrien DS, Skinner RA, et al. Chronic ethanol exposure inhibits distraction osteogenesis in a mouse model: role of the TNF signaling axis. Toxicology and applied pharmacology. 2007;220(3):302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wahl EC, Aronson J, Liu L, Skinner RA, Ronis MJ, Lumpkin CK, Jr. Distraction osteogenesis in TNF receptor 1 deficient mice is protected from chronic ethanol exposure. Alcohol (Fayetteville, NY). 2012;46(2):133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu L, Xu Y, Qu H, Yu Y, Li W, Zhao Y, et al. Decrease of MiR-31 induced by TNF-α inhibitor activates SATB2/RUNX2 pathway and promotes osteogenic differentiation in ethanol-induced osteonecrosis. Journal of cellular physiology. 2019;234(4):4314–26. [DOI] [PubMed] [Google Scholar]


