Abstract
Purpose of Review
In this article, the current use and limitations of existing retinal tamponades are discussed. Potential novel developments that address those limitations are subsequently highlighted, along with areas of future improvements.
Recent Findings
While retinal tamponades have existed for decades and improved the treatment of retinal detachments, many problems still exist with their use, including inadequate tamponade of the inferior retina, toxicity from retained heavy liquids, glaucoma, and keratopathy, among others. New advancements in the components of heavy liquids and vitreous substitutes aim to mitigate those issues.
Summary
Existing retinal tamponades, including perflurocarbon heavy liquids, fluorinated gases, and silicone oil, have specific limitations that cause potentially avoidable morbidity. New developments, such as heavy silicone oil, novel vitreous gels, and future avenues of approach, such as potentially reabsorbing heavy liquids may help increase our ability to treat retinal detachments with fewer complications.
Keywords: Retinal Detachment, Vitreous, Tamponade, Perfluorocarbon Liquid, Silicone Oil, Heavy Fluid
Introduction
Retinal detachments are a leading cause of emergent permanent blindness worldwide, with a geographically varying incidence ranging from 6 to 18 individuals per 100,000 in the population.1 Given the relatively rapid onset of this disease, its repair has been a source of investigation for over a century.2,3 With improvements in surgical techniques and instrumentation, the primary reattachment rate has increased from less than 1 percent without intervention to approximately 90 percent in the present day.4,5,6 However, significant morbidity often remains, particularly with inferior retinal detachments, which are prone to re-detachments and carry a relatively increased risk of eventual blindness.7 The current surgical paradigm of reattachment involves the use of intraoperative heavy liquid tamponades, and post-operative gases or oils, the latter of which function in effect as longer term vitreous substitutes.8,9 While effective, each one of these agents carries its own list of drawbacks, which ultimately leads to many of the subsequent complications seen during and after retinal detachment repair. The purpose of this paper is to explain the current use of these tools, identify their disadvantages, and explore potential solutions to those issues, many of which are currently being developed.
Existing Commonly Used Tamponades
In the context of retinal detachment repair, the ultimate purpose of a retinal tamponade is to provide surface tension over an existing break, thus rendering it unable to convey fluid into the subretinal space and permitting the retina to reapproximate its native position.10 This tamponade must be maintained until a more permanent treatment, usually laser photocoagulation around the edges of the existing break, can take hold and provide a longer-lasting seal around this area, after which the tamponade can be removed. Considering that the adhesion strength of laser photocoagulation starts occurring in earnest at around 18 hours after application and continues to increase until its maximum adherence at around 5 days, any theoretical post-operative tamponade should at least remain in place for that range of time.11,12 Hence, after clearance of the native vitreous via vitrectomy, an oft-used method for reattaching retinal detachments is first to place an intraoperative heavy liquid tamponade that can stabilize the posterior retina, then to drain subretinal fluid, then to place laser photocoagulation around existing breaks, then to remove the existing heavy liquid, and finally to place a longer term tamponade into the eye which can passively disappear via diffusion into the bloodstream (gases) or actively must be removed via a second surgery (oils). Thus, when conceptualizing tamponades, it is helpful view them as those that are used predominantly while within the operating room, and those that chosen for their effect predominantly while outside of the operating room.
Intraoperative Use
By far, the most commonly used intraoperative tamponade in the US is n-perfluoro-octane. Since its class of chemicals were introduced into the retina space over 3 decades ago, it has become a necessary surgical tool, particularly for addressing complex retinal detachments.13 Chemically, n-perfluoro-octane is an analogue of the highly flammable straight chain alkane octane, but critically, with all hydrogen atoms replaced with those of fluorine, thus making the molecule incredibly stable at biological conditions and without a flash point. It is immiscible in water (permitting surface tension across retinal breaks if necessary even under fluidic conditions), has a density higher than that of water (permitting posterior localization under fluidic conditions in which the retina is detached), and is both optically clear and with a refractive index near that of water (thus allowing for unencumbered visualization during surgery). Due to its increased density relative to water, n-perfluoro-octane is euphemistically referred to as part of a class of fluorinated “heavy liquids”, which include other agents such as perfluorodecalin, among others. These qualities make it ideal for stabilizing the posterior pole in situations where it would be virtually impossible to achieve surgical success.14,15,16 However, because the chemical is so stable, it has no mechanism of passive evacuation outside the vitreous cavity and thus must be actively removed at some point prior to the end of the case.
Post-operative/Clinic Use
In the US, the most frequently used tamponades designed to remain within the eye post-operatively are gases and oils, mainly due to their high surface tension and relative immiscibility, particularly against water. However, due to their low densities, both are buoyant in water and, when placed, float in the vitreous cavity.9
The most commonly used gases are perfluoropropane (C3F8) and sulfur hexafluoride (SF6), with some physicians using n-perfluoroethane (C2F6)8. Notably, like n-perfluoro-octane, both perfluoropropane and n-perfluoroethane are analogues of their highly combustible linear alkane namesakes (propane and ethane), but with critical stability once again lent by the substitution of fluorine atoms for those of hydrogen. Similarly, sulfur hexafluoride is fully fluorinated. The differences between each gas are its native expansile quality (due to endogenous air-derived blood gases, particularly nitrogen, initially diffusing into a pure form of the gas) and its latency within the vitreous cavity after placement. Specifically, pure sulfur hexafluoride will expand approximately 2 times its initial volume over 1 to 2 days, pure n-perfluoroethane will expand approximately 3 times its initial volume over 1 to 2 days, and pure perfluoropropane will expand approximately 4 times over 3 to 4 days.18 It is instructive to note that, as expected, a pure air bubble is non-expansile as the partial pressures of its components are equal to those found in blood and thus, no diffusion gradient exists for the initial inflow of blood gases. Due to these large increases in volumes, pure gases are typically used in extremely low volumes, usually via non-operating room clinic-based procedures such as pneumatic retinopexy (Figure 1). However, after surgical vitrectomy, ideally the entire vitreous cavity requires filling; thus, a diluted form of each gas with air is often used to abate any volumetric expansion. Due to this dilution, each gas becomes isoexpansile and over time is spontaneously reabsorbed, albeit at different rates, via passive diffusion through the blood stream due to constant ocular blood flow and respiration. The intraocular latency of isoexpansile sulfur hexafluoride (20%) is around 2 weeks, that of isoexpansile n-perfluoroethane (16%) is 4–5 weeks, and that of isoexpansile perfluoropropane (14%) is around 8 weeks. Thus, for a patient that requires a temporary post-operative tamponade that has the capacity to reabsorb without any additional surgery, particularly one in which a superior retinal detachment was the primary source of pathology, fluorinated gases are the current ideal choice.
Figure 1.
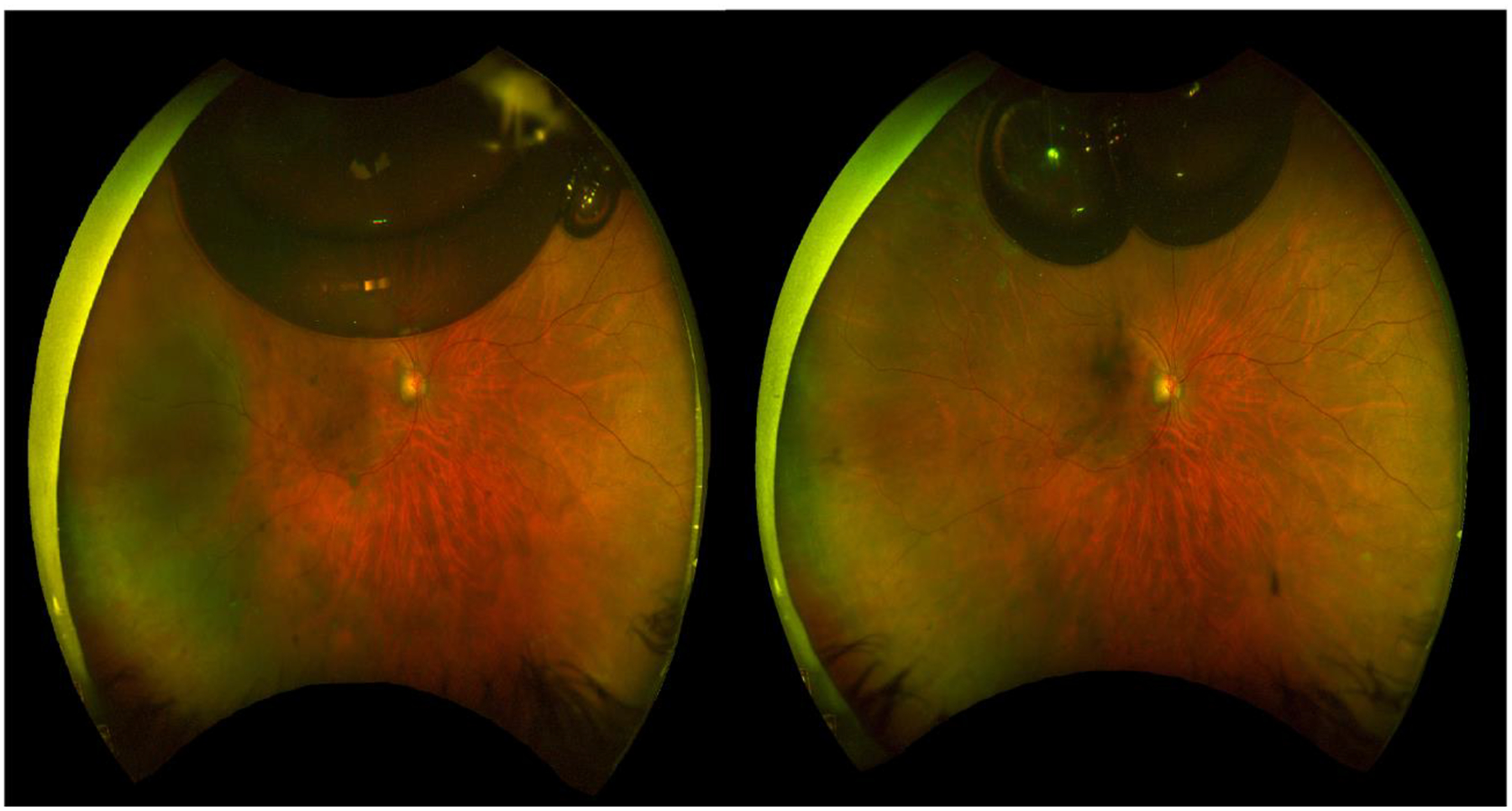
Wide-field fundus photography of a 60 year old man presenting with a primary rhegmatogenous retinal detachment 1 week (left) and 2 weeks (right) after pneumatic retinopexy with pure perfluoropropane. Note the temporal fluid (left) which later resolves, as well as the placed endolaser (right). Note the buoyancy of the gas bubble and its subsequent positioning in the superior aspect of the globe.
On the other hand, silicone oils are typically chosen when an even longer term tamponade is required. More generally, the term “silicone oil” refers to a set of hydrophobic repeating monomeric or polymeric molecules joined via silicone-oxygen bonds and collectively are part of a group termed “organosiloxanes.” Depending on the chain size, these molecules retain most of their hydrophobic properties but can vary by viscosity.18 Due to their hydrophobicity and generally elevated viscosities relative to water, these compounds are colloquially termed “oils”. By far, the most commonly used silicone oil is poly-dimethyl-siloxane (PDMS), specifically the forms with viscosities of 1000 and 5000 centistokes.19,20 This variance of viscosities allows for the surgeon to choose efficiency during placement and removal (typically easier with lower viscosity forms) versus reducing the risk of shear forces causing subsequent oil emulsification (typically lower with higher viscosity forms21). At present, silicone oils are the most tolerated long term retinal tamponade, and thus are widely used for a number of complex pathologies requiring such tamponade, including giant retinal tears, inferior retinal detachments, and retinal detachments due to proliferative vitreoretinopathy.22,23 However, like existing heavy liquids (though unlike gases), silicone oils are incapable of spontaneous resorption and thus, must be surgically removed at some later date.24
Problems with Current Agents
While it is universally acknowledged that the introduction of fluorinated heavy liquids, fluorinated gases, and silicone oils have substantially advanced our ability to treat retinal detachments, a number of important drawbacks still exist with each type of tamponade, rendering them less than ideal in very clinically meaningful ways.
Inferior Retinal Detachments
By far, the most important problem that exists with current technologies is the inability to adequately treat detachments due to inferior breaks, which some studies indicate account for over 40 percent of all detachments.25 In an analysis of pneumatic retinopexy, in which a pure form of fluorinated gas is placed in the clinic to allow for immediate tamponade, the most decisive prognostic factor of retinal re-detachment was inferior pathology.7 Moreover, studies highlighting the risk factors for the development of proliferative vitreoretinopathy and its associated complex pathology often include detachments that involve the inferior retina.26,27 Given the buoyancy of currently used post-operative gases and oils, it is often very difficult to tamponade the inferior retina, which requires a vitreous cavity that is fully filled with the agent in order to satisfactorily make contact with the inferior part of the eye. Practically, this ineffective tamponade prevents the surface tension of the surgically placed gas or oil to become apposed to the inferior retinal break, which in turn allows for vitreous fluid to maintain access to the subretinal space. Hence, a potential inferior retinal detachment is allowed to persist well before any applied laser can form its needed adhesions to the retinal pigment epithelium and permanently seal the retinal tear.28 Furthermore, isoexpansile gases by their very nature theoretically begin to reabsorb almost immediately after placement (as they are designed to not expand) and this reabsorption occurs inferiorly. Thus, in a normal head-up standing or sitting position, the area that has the longest time under tamponade, even as the gas bubble itself is diminishing in volume, is the superior retina. In order to obviate this problem, patients are routinely asked to position in very uncomfortable, sometimes impossible positions (e.g. face-down) for extended periods of time. As a result, even after surgical intervention in which gas or oil is placed, many studies have shown that the most significant risk factor for retinal re-detachment was the initial presence of inferior retinal breaks.29
Common Complications associated with Heavy Liquids
A major complication with the intraoperative use of fluorinated heavy liquids is the possibility of its retention in the eye at the conclusion of the case (Figure 2). Due to the innate properties of these liquids (immiscibility, optical clarity, stability, high density, high surface tension), it is often challenging to remove the entirety of the fluid, particularly under situations of decreased visualization or complex pathology. Some long term follow up studies have placed the rate of heavy liquid retention anywhere from less than 1 percent to as high as 11 percent of all cases in which it is used.30,31 Even more problematic is that small volumes of these fluids can subsequently move into the subretinal space and due to patient positioning, can easily localize under the macula itself. This complication can cause persistent problems with vision and, in some cases, require further surgery to remove.32
Figure 2.
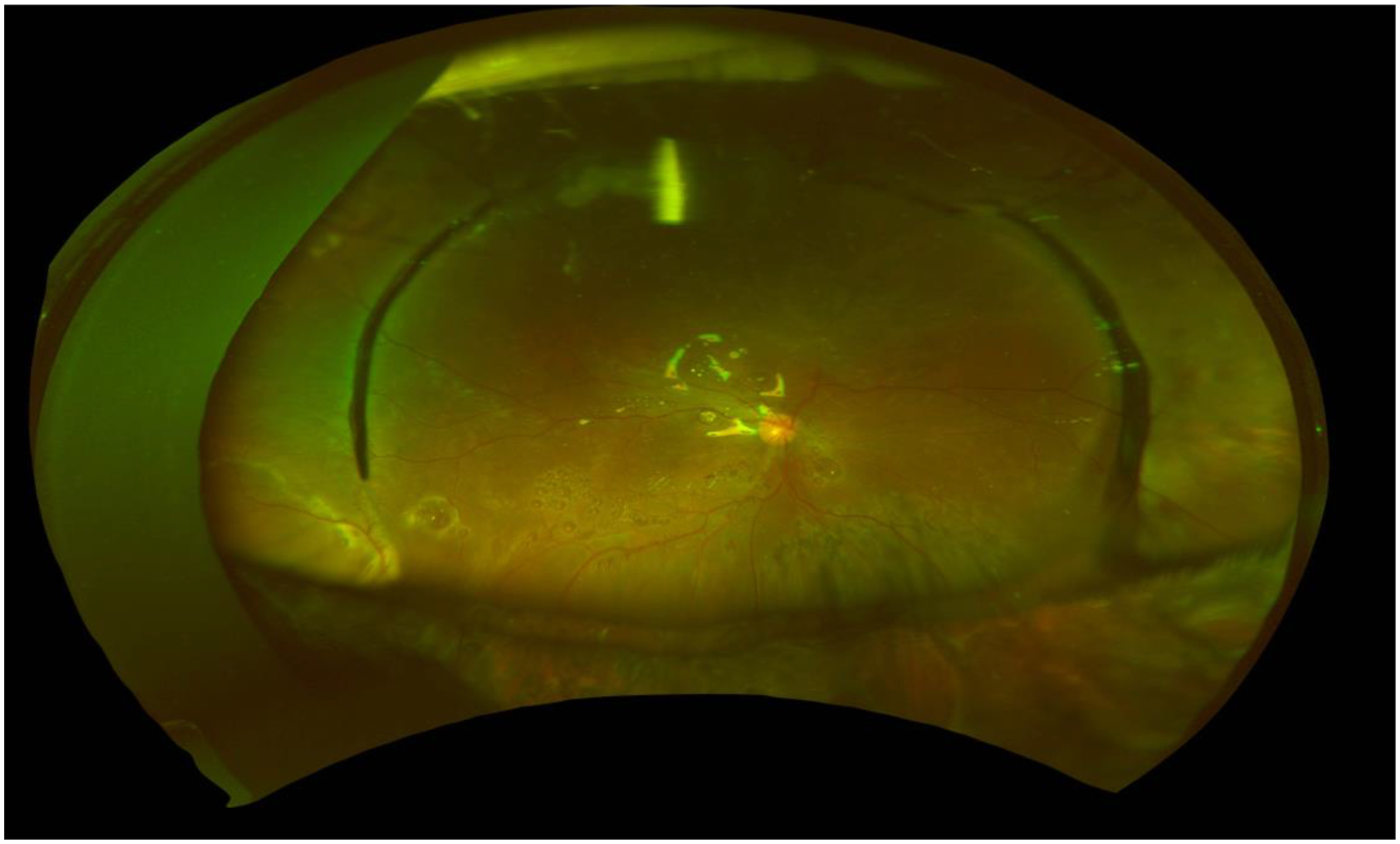
Wide-field fundus photography of a 23 year old man 7 months after complex retinal detachment repair with encircling scleral buckle, and silicone oil. Multiple areas of subretinal n-perfluoro-octane can be readily seen throughout the posterior funds and beneath the macula. The patient later had a second surgery to remove the oil and some of the subretinal heavy liquid.
In addition to the physical effects of retention, fluorinated heavy liquids can have a toxic effect on the retina if permitted to remain within the vitreous cavity for an extended period of time. In animal studies, it has been noted that retained n-perfluro-octane for longer than a week can lead to histological changes that include photoreceptor disruption – particularly of the outer segments – and accumulation of macrophages in the vitreous cavity, with more profound changes over longer time periods.33,34 Notably, these histologic changes all occurred predominantly in the inferior areas of the retina in which the heavy liquid had persistent contact. In addition, studies that have analyzed humans appear to show analogous findings to those of animal trials, with macrophages laden with intracellular vacuoles containing the heavy liquid, a generalized inflammatory response, and findings indicative of mechanical compression in areas in contact with the agent.35,36 Even anterior chamber complications, such as corneal toxicity, endothelial cell loss, and secondary glaucoma can occur with prolonged contact with the fluid.37,38 As a result, despite its inherent ability to tamponade the inferior retina, the potential for long-term use of these existing liquids postoperatively is limited at best, and expedited removal is often needed to prevent subsequent complications.
Common Complications associated with Fluorinated Gases
Outside of their less than ideal tamponade of the inferior retina, the major complications associated with gases include migration into unwanted areas of the eye, increased intraocular pressure, and cataract formation.39 Gas migration can occur into the anterior chamber, which can cause difficulties in visualization of the retina and potential toxicity to the cornea due to interruptions in endothelial cell nutrition.40,41 Notably, this complication can occur even in phakic patients with no evidence of phacodonesis or zonular laxity.42 Increased intraocular pressure has been reported in almost 60 percent of eyes, and both open angle (due to expansion of the gas) and closed angle (due to anterior displacement of the lens-iris diaphragm with iridocorneal touching by the posteriorly placed gas exerting forward pressure) forms can occur.43.44 However, most sources indicate that the elevated intraocular pressures are transient and tend to resolve as the gas reabsorbs.45 Finally, cataract formation is common after vitrectomy itself – over 50 percent of patients require cataract surgery 6 months following the procedure – and its risk is increased with the existence of gas which permits elevated retrolental oxygen levels that result in the formation of lens opacities.46 This cataract formation risk is increased the longer the gas remains in the eye.47 Yet despite the above, gases are tolerated relatively well given that their use is transient by design, and any prolonged amount of the agent will eventually reabsorb spontaneously.
Common Complications associated with Silicone Oils
While silicone oil is the most used long-term post-operative tamponade and is often chosen for the treatment of inferior retinal detachments and complex retinal pathology, its use is frequently associated with a number of complications. These complications include oil emulsification, glaucoma, cataract, keratopathy, and even oil migration into the retina and optic nerve.48,49 In particular, the presence of emulsified oil droplets in the anterior chamber can complicate visualization of the posterior pole during postoperative care. Additionally, despite its ability to remain in the eye for longer periods of time relative to gases, its similarly buoyant nature when within water makes inferior tamponade difficult. As a result, some sources note anatomic failure rates as high as 30 percent, though it must be acknowledged that silicone oil is typically used only in the most challenging of cases.18 Furthermore, the use of silicone oil necessitates a second surgery for removal, as spontaneous reabsorption is not an option, and prolonged maintenance of the agent within the vitreous cavity increases the risk for any of the above complications to occur.
Potential Innovations
The widespread challenges that exist with the tamponades currently in use has precipitated a fairly extensive push for new approaches that attempt to fix some of those problems. Table 1 is an abridged list of various easily compared technologies that have been developed. In general, novel approaches address current limitations by either sealing retinal breaks quickly (thus potentially reducing the need for post-operative tamponades in the first place), manufacturing some type of post-operative tamponade that reduces issues with buoyancy (thus providing some form of long-term inferior tamponade), or creating a post-operative vitreous analog that can provide circumferential tamponade but also potentially remain in the eye and summarily self-degrade (thus avoiding a second surgery for removal).
Table 1.
Current technologies
| Chemical name | Specific gravity (g/cm3) at 25°C |
|---|---|
| Semi-Fluorinated Alkanes | |
| Perfluorohexylhexane (F6H6) | 1.42 |
| Perfluorohexyloctane (F6H8) | 1.35 |
| Perfluorohexylethane (O62) | 1.62 |
| Fully Fluorinated Hydrocarbons | |
| Perfluorooctane (nPFO) | 1.77 |
| Perfluorodecalin | 1.92 |
| Heavy Silicone Oils | |
| Densiron 68 | 1.06 |
| Oxane HD | 1.02 |
Sealing Retinal Breaks
An interesting approach to the limitations described herein is to avoid any extra tamponade altogether through the use of glue-like substances that are directly applied to individual retinal breaks.50 The procedure involves first removing the vitreous via vitrectomy, then removing subretinal fluid from around all breaks, then the replacement of fluid with air within the vitreous cavity, then placement of endolaser spots around the breaks, and finally application of a glue-like substance that seals the break while the laser is given time to adhere strongly. Many types of sealant have been considered, including cyanoacrylate, viscoelastic (sodium chondroitin and sodium hyaluronate), Seprafilm Adhesion Barrier (Sanofi, Bridgewater, NJ, USA) (sodium hyaluronate and carboxymethylcellulose patch), and fibrin glue (TISSEEL Kit, Baxter AG, Vienna, Austria), the latter two with very little evidence of long term toxicity.51 In particular, fibrin glue has previously been used to surgically treat optic pits, and during these procedures appeared to stay in place for 1–2 weeks without any known toxicity.52 Thus, for simple uncomplicated retinal detachments requiring vitrectomy, a glue-like substance can potentially remain in place with enough time for laser based strong adhesions to occur without need for any additional longer-term tamponade.50
Heavy Oils and Partially Fluorinated Hydrocarbons
One relatively obvious avenue of improvement would be to create silicone oils that have a higher density than water, thus allowing it to localize inferiorly and provide long-term tamponade in that area while avoiding the known toxicities of currently used retained heavy liquids. Given that the addition of fluorine atoms to organic substances often creates an elevated specific gravity, some approaches naturally explored the use of directly fluorinated silicone oils as a tamponade. However, long term use of these substances result in some of the same toxicities that retained fluorinated heavy liquids demonstrate.53 Hence, attention was then turned to potentially moderating the problems of fluorinated heavy liquids by making them less heavy (thus reducing potential mechanical trauma) and less hydrophobic, as it was thought that slightly more hydrophilicity might permit a better metabolic profile to the treated retina (which typically exists in a naturally water based environment). Thus, semi-fluorinated alkanes were created; however, these too demonstrated individual retinal toxicity on par with other fluorinated liquids.54,55
As a result of these findings, research turned to a combination of silicone oils and semi-fluorinated, termed “Heavy Silicone Oils” with much better success. In particular, Densiron 68 (a combination of 5000 centistoke silicone oil and the semi-fluorinated alkane F6H8) and Oxane HD (5700 centistoke silicone oil and the semi-fluorinated alkene RMN-3) have been shown to have promise in potentially treating inferior detachments.56 Interestingly, in these cases, complex pathology such as proliferative vitreoretinopathy tends to occur in the superior hemi-field of the retina, as the heavy oil preferentially fills the inferior part of the cavity first, and inflammatory cytokines congregate in the hydrophilic aqueous above.18 Thus, some approaches suggest placement of standard silicone oil first (which due to its native buoyancy would cause proliferation to occur inferiorly) with subsequent removal and treatment with heavy silicone oil, thus mitigating overall proliferative vitreoretinopathy and re-detachment risk.57
Vitreous Substitutes
Perhaps one of the more long-standing developments is in research attempting to best approximate the structure and function of native vitreous as a postoperative tamponade. Theoretically, these substances should be able to fully fill the vitreous cavity, providing surface tension throughout the entire internal surface area of the vitreous cavity, and not suffer from the same long-term toxic effects of the other agents currently in use. Over the years, many different substances have been tried, including hydrogels, silicone gels, collagen derivatives, and even modified cellulose.58,59,60,61,62 Major limitations of these substances tend to include inflammation, premature degradation, and an inability to surgically place externally formed matrices given the shearing that occurs within small gauge vitrectomy.63,64,65 Newer substances attempt to solve this problem by forming cross-linked bonds after placement within the vitreous cavity itself. Often this is accomplished via an exposure to photic energy, to thermal energy, or simply a reaction that occurs over time.66,67 Recently, a rapidly gelling thermosensitive co-polymer has been shown to be nontoxic in both rabbit and non-human primate models, with a refractive index approximating that of native vitreous and with the capacity to stably biodegrade after 3 months, thus making it and advancements like it potentially useful for future study and potential treatment.68
Future Paths
Considering that all of the above meritorious developments are not tools for intraoperative use in stabilizing the retina, a potential avenue for further improvement not currently being explored is via the creation of an intraoperative tamponade that could spontaneously reabsorb over time. One idealized concept of a tamponade would be a substance that is biologically nontoxic and unifies certain functions of all three canonical agents discussed herein, namely fluorinated heavy liquids (immiscibility, high surface tension in water, optical clarity, elevated specific gravity for inferior tamponade), silicone oil (tolerated longer-term presence in the vitreous cavity), and fluorinated gases (buoyancy for superior tamponade with spontaneous reabsorption) without having substantially elevated intraocular pressure increases. At the very least, such an agent could be used intraoperatively while, importantly, avoiding the complication of retained heavy liquid that routinely plagues their current use. In addition, much like gas is currently used for superior retinal detachments in pneumatic retinopexy, the ability to spontaneously reabsorb could permit this theoretical agent to potentially be used at bedside for inferior detachments in an “inverse pneumatic” fashion.
Notably, many of the above described advances could be used in combination with one another for synergistic effect. In particular, one could easily envision a hypothetical clinical scenario in which a spontaneously reabsorbing heavy liquid is surgically used for intraoperative retinal stabilization and, if impossible to remove during the case, permitted to remain in small quantities without fear of persistence and its subsequent toxicity. Thereafter, a novel, optically clear, biologically safe vitreous substitute could be placed and function as a longer-term tamponade, subsequently self-degrading after some period of time.
Conclusion
The introduction of retinal tamponades in the 20th century heralded an era in which retinal detachments became a condition that could be addressed with great success. However, despite those advancements, re-detachments continue to occur in non-trivial numbers, with permanent blindness still lurking as an ever-present danger with each surgery despite best efforts. In order to address these important clinical problems, new developments are attempting to remove some of the limitations that exist with current tamponades, and potentially move the field into new paradigms of treatment.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Avnish Deobhakta and Richard Rosen each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Supported by a grant from the New York Eye and Ear Foundation.
References
- 1.Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94(6):678–684. [DOI] [PubMed] [Google Scholar]
- 2.Ohm J On the treatment of retinal detachment by surgical evacuation of subretinal fluid and injection of air into the vitreous. Albrecht Von Graefes Arch Für Ophthalmol. 1911;79(3):442–450. [Google Scholar]
- 3.Gonin J The treatment of detached retina by searing the retinal tears. Arch Ophthalmol. 1930;4(5):621–625. [Google Scholar]
- 4.Murtagh PJ, Stephenson KA, Rhatigan M, McElnea EM, Connell PP, Keegan DJ. Rhegmatogenous retinal detachments: primary reattachment rates and visual outcomes over a 4-year period. Ir J Med Sci. 2020. February;189(1):355–363. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Cheung N, Jia L, Zhang H, Liu N. SURGICAL OUTCOMES OF 25-GAUGE PARS PLANA VITRECTOMY USING AIR AS AN INTERNAL TAMPONADE FOR PRIMARY RHEGMATOGENOUS RETINAL DETACHMENT. Retina 2020. January 7. [DOI] [PubMed] [Google Scholar]
- 6.Wang JC, Ryan EH, Ryan C, Kakulavarapu S, Mardis PJ, Rodriguez M, Stefater JA, Forbes NJ, Gupta O, Capone A Jr, Emerson GG, Joseph DP, Eliott D, Yonekawa Y; Primary Retinal Detachment Outcomes (PRO) Study Group. FACTORS ASSOCIATED WITH THE USE OF 360-DEGREE LASER RETINOPEXY DURING PRIMARY VITRECTOMY WITH OR WITHOUT SCLERAL BUCKLE FOR RHEGMATOGENOUS RETINAL DETACHMENT AND IMPACT ON SURGICAL OUTCOMES (PRO STUDY REPORT NUMBER 4). Retina. 2019. December 23. [DOI] [PubMed] [Google Scholar]
- 7.Goldman DR, Shah CP, Heier JS. Expanded criteria for pneumatic retinopexy and potential cost savings. Ophthalmology. 2014. January;121(1):318–26. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed S, Lai TY. Intraocular gas in vitreoretinal surgery. HK J Ophthalmol. 2010;14(1):8–13. [Google Scholar]
- 9.Petersen J The physical and surgical aspects of silicone oil in the vitreous cavity. Graefes Arch Clin Exp Ophthalmol. 1987;225(6):452–456. [DOI] [PubMed] [Google Scholar]
- 10.Regillo CD, Tornambe PE. Primary retinal detachment repair. In: Regillo CD, Brown GC, Flynn HW Jr, editors. Vitreoretinal Disease: The Essentials. 1st ed. New York: Thieme; 1998:631–646. [Google Scholar]
- 11.Zauberman H Tensile strength of chorioretinal lesions produced by photocoagulation, diathermy, and cryopexy. Br J Ophthalmol 1969;53:749–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon YH, Marmor MF. Rapid enhancement of retinal adhesion by laser photocoagulation. Ophthalmology 1988;95:1385–138. [DOI] [PubMed] [Google Scholar]
- 13.Chang S Low viscosity liquid fluorochemicals in vitreous surgery. Am J Ophthalmol. 1987. January 15;103(1):38–43. [DOI] [PubMed] [Google Scholar]
- 14.Kreiger AE, Lewis H Management of Giant Retinal Tears without Scleral Buckling: Use of Radical Dissection of the Vitreous Base and Perfluoro-octane and Intraocular Tamponade (1992) Ophthalmology, 99 (4), pp. 491–497. [DOI] [PubMed] [Google Scholar]
- 15.Scott IU, Murray TG, Flynn HW Jr, Feuer WJ, Schiffman JC Outcomes and complications associated with giant retinal tear management using perfluoro-n-octane (2002) Ophthalmology, 109 (10), pp. 1828–1833. [DOI] [PubMed] [Google Scholar]
- 16.Shunmugam M, Ang GS, Lois N. Giant retinal tears. Surv Ophthalmol. 2014. Mar-Apr;59(2):192–216. [DOI] [PubMed] [Google Scholar]
- 17.Kreissig I The perfluorocarbon gases. In: Practical Guide to Minimal Surgery for Retinal Detachment. Vol 2, 1st ed. Stuttgart: Thieme; 2000:129–132. [Google Scholar]
- 18.Russo A, Morescalchi F, Donati S, Gambicorti E, Azzolini C, Costagliola C, Semeraro F. Heavy and standard silicone oil: intraocular inflammation. Int Ophthalmol. 2018. April;38(2):855–867. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan PM, Luff AJ, Aylward GW (1997) Results of primary retinal reattachment surgery: a prospective audit. Eye Lond Engl 11(Pt 6):869–871. [DOI] [PubMed] [Google Scholar]
- 20.Scott IU, Flynn HW Jr, Murray TG, Smiddy WE, Davis JL, Feuer WJ. Outcomes of complex retinal detachment repair using 1000-vs 5000-centistoke silicone oil. Arch Ophthalmol. 2005;123(4):473–478. [DOI] [PubMed] [Google Scholar]
- 21.Douglas JF, et al. Viscosity. In: Fluid Mechanics. 5th Ed. Harlow: England; 2005:11–14. [Google Scholar]
- 22.Stilma JS, Koster R, Zivojnović R (1986) Radical vitrectomy and silicone-oil injection in the treatment of proliferative vitreoretinopathy following retinal detachment. Doc Ophthalmol Adv Ophthalmol 64:109–116. [DOI] [PubMed] [Google Scholar]
- 23.Azen SP, Scott IU, Flynn HW et al. (1998) Silicone oil in the repair of complex retinal detachments. A prospective observational multicenter study. Ophthalmology 105:1587–1597. [DOI] [PubMed] [Google Scholar]
- 24.Lai WW, Yusof W, Lo A, Wong IY, Wong D. Long-term intraocular tamponade with silicone oil. In: Narendran V, Kothar AR, editors. Principles and Practice of Vitreoretinal Surgery. 1st ed. New Delhi: JP Medical Ltd; 2014:145–150. [Google Scholar]
- 25.Shunmugam M, Shah AN, Hysi PG, Williamson TH. The pattern and distribution of retinal breaks in eyes with rhegmatogenous retinal detachment. Am J Ophthalmol. 2014. January;157(1):221–226.e1. [DOI] [PubMed] [Google Scholar]
- 26.Pastor JC, de la Rúa ER, Martín F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res. 2002. January;21(1):127–44. [DOI] [PubMed] [Google Scholar]
- 27.Idrees S, Sridhar J, Kuriyan AE. Proliferative Vitreoretinopathy: A Review. Int Ophthalmol Clin. 2019. Winter;59(1):221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiraki N, Sakimoto S, Sakaguchi H, Nishida K, Nishida K, Kamei M. Vitrectomy without prone positioning for rhegmatogenous retinal detachments in eyes with inferior retinal breaks. PLoS One. 2018. January 26;13(1):e0191531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu El-Asrar AM, Al-Kwikbi HF, Kangave D. Prognostic factors after primary vitrectomy and perfluorocarbon liquids for bullous rhegmatogenous retinal detachment. Eur J Ophthalmol. 2009. Jan-Feb;19(1):107–17. [DOI] [PubMed] [Google Scholar]
- 30.Ghoraba HH, Ghoraba HH, Heikal MA, Elgouhary SM, Mansour HO, Abdelhafez MA, Zaky AG. Submacular perfluorocarbon liquid: long-term follow-up. Int Ophthalmol. 2020. January 24. [DOI] [PubMed] [Google Scholar]
- 31.Winter M, Winter C, Wiechens B (1999) Quantification of intraocular retained perfluorodecalin after macroscopic complete removal. Graef Arch Clin Exp Ophthalmol 237:153–156. [DOI] [PubMed] [Google Scholar]
- 32.Shulman M, Sepah YJ, Chang S, Abrams GW, Do DV, Nguyen QD (2013) Management of retained subretinal perfluorocarbon liquid. Ophthalmic Surg Lasers Imaging Retina 44(6):577–583. [DOI] [PubMed] [Google Scholar]
- 33.Chang S, Sparrow JR, Iwamoto T, Gershbein A, Ross R, Ortiz R (1991) Experimental studies of tolerance to intravitreal perfluoro-n-octane liquid. Retina 11(4):367–374. [PubMed] [Google Scholar]
- 34.Eckardt C, Nicolai U, Winter M, Knop E (1991) Experimental intraocular tolerance to liquid perfluorooctane and perfluoropolyether. Retina 11(4):375–384. [PubMed] [Google Scholar]
- 35.Elsing SH, Fekrat S, Green WR, Chang S, Wajer SD, Haller JA. Clinicopathologic findings in eyes with retained perfluoro-n-octane liquid. Ophthalmology 2001;108:45–48. [DOI] [PubMed] [Google Scholar]
- 36.Singh J, Ramaesh K, Wharton SB, Cormack G, Chawla HB. Perfluorodecalin-induced intravitreal inflammation. Retina (Philadelphia, Pa) 2001;21:247–251. [DOI] [PubMed] [Google Scholar]
- 37.Wilbanks GA, Apel AJ, Jolly SS, Devenyi RG, Rootman DS. Perfluorodecalin corneal toxicity: five case reports. Cornea 1996;15:329–334. [DOI] [PubMed] [Google Scholar]
- 38.Foster RE, Smiddy WS, Alfonso EC, Parrish RK. Secondary glaucoma associated with perfluorophenanthrene. Am J Ophthalmol 1994; 118: 253. [DOI] [PubMed] [Google Scholar]
- 39.Kanclerz P, Grzybowski A. Complications Associated with the Use of Expandable Gases in Vitrectomy. J Ophthalmol. 2018. November 18;2018:8606494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Horn DL, Edelhauser HF, Aaberg TM, Pederson HJ. Invest Ophthalmol. 1972. December; 11(12):1028–36. [PubMed] [Google Scholar]
- 41.Tan CSH, Wee K, Zaw M-D, Lim TH Anterior chamber gas bubble following pneumatic retinopexy in a young, phakic patient. Clinical and Experimental Ophthalmology. 2011;39(3):276–277. [DOI] [PubMed] [Google Scholar]
- 42.Taher RM, Haimovici R. Anterior chamber gas entrapment after phakic pneumatic retinopexy. Retina. 2001;21(6):681–2. [DOI] [PubMed] [Google Scholar]
- 43.Han DP, Lewis H, Lambrou FH Jr, Mieler WF, Hartz A. Ophthalmology. 1989. September; 96(9):1357–62. [DOI] [PubMed] [Google Scholar]
- 44.Chang S, Lincoff HA, Coleman DJ, Fuchs W, Farber ME. Perfluorocarbon gases in vitreous surgery. Ophthalmology. 1985. May;92(5):651–6. [DOI] [PubMed] [Google Scholar]
- 45.Chen CJ. Glaucoma after macular hole surgery. Ophthalmology. 1998. January;105(1):94–9; discussion 99–100. [DOI] [PubMed] [Google Scholar]
- 46.Modi A, Giridhar A, Gopalakrishnan M. SULFURHEXAFLUORIDE (SF6) VERSUS PERFLUOROPROPANE (C3F8) GAS AS TAMPONADE IN MACULAR HOLE SURGERY. Retina. 2017. February;37(2):283–290. [DOI] [PubMed] [Google Scholar]
- 47.Yee KMP, Tan S, Lesnik Oberstein SY, et al. Incidence of cataract surgery after vitrectomy for vitreous opacities. Ophthalmology Retina. 2017;1(2):154–157. [DOI] [PubMed] [Google Scholar]
- 48.Ichhpujani P, Jindal A, Jay Katz L (2009) Silicone oil induced glaucoma: a review. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Für Klin Exp Ophthalmol 247:1585–1593. [DOI] [PubMed] [Google Scholar]
- 49.a Cour M, Lux A, Heegaard S (2010) Visual loss under silicone oil. Klin Monatsblätter Für Augenheilkd 227:181–184. [DOI] [PubMed] [Google Scholar]
- 50.Tyagi M, Basu S. Glue-assisted retinopexy for rhegmatogenous retinal detachments (GuARD): A novel surgical technique for closing retinal breaks. Indian J Ophthalmol. 2019. May;67(5):677–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haruta M, Arai M, Sueda J, Hirose T, Yamakawa R. Patching retinal breaks with Seprafilm for treating retinal detachments in humans: 9 years of follow-up. Eye. 2017;31:776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georgalas I, Petrou P, Koutsandrea C, Papaconstadinou D, Ladas I, Gotzaridis E. Optic disc pit maculopathy treated with vitrectomy, internal limiting membrane peeling, and gas tamponade: a report of two cases. Eur J Ophthalmol. 2009. Mar-Apr;19(2):324–6. [DOI] [PubMed] [Google Scholar]
- 53.Doi M, Refojo MF (1995) Histopathology of rabbit eyes with silicone-fluorosilicone copolymer oil as six months internal retinal tamponade. Exp Eye Res 61:469–478 [DOI] [PubMed] [Google Scholar]
- 54.Schatz B, El-Shabrawi Y, Haas A, Langmann G (2004) Adverse side effects with perfluorohexyloctane as a long-term tamponade agent in complicated vitreoretinal surgery. Retina Phila Pa 24:567–573. [DOI] [PubMed] [Google Scholar]
- 55.Georgalas I, Ladas I, Tservakis I, Taliantzis S, Gotzaridis E, Papaconstantinou D, Koutsandrea C. Perfluorocarbon liquids in vitreoretinal surgery: a review of applications and toxicity. Cutan Ocul Toxicol. 2011. December;30(4):251–62. [DOI] [PubMed] [Google Scholar]
- 56.Levasseur SD, Schendel S, Machuck RW, Dhanda D. High-density silicone oil Densiron-68 as an intraocular tamponade for primary inferior retinal detachments. Retina. 2013. March;33(3):627–33 [DOI] [PubMed] [Google Scholar]
- 57.Caporossi T, Franco F, Finocchio L, Barca F, Giansanti F, Tartaro R, Virgili G, Rizzo S. Densiron 68 heavy silicone oil in the management of inferior retinal detachment recurrence: analysis on functional and anatomical outcomes and complications. Int J Ophthalmol. 2019. April 18;12(4):615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peyman GA, Conway MD, Karacorlu M, et al. Evaluation of Silicone Gel as a Long-term Vitreous Substitute in Non-human Primates. Ophthalmic Surg. 1992;23:811–817. [PubMed] [Google Scholar]
- 59.Liang CP, Peyman GA, Serracarbassa P, Calixto N, Chow AA, Rao P. An Evaluation of Methylated Collagen as a Substitute for Vitreous and Aqueous Humor. Int Ophthalmol. 1998;22:13–18. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa M, Tanaka M, Miyata T. Evaluation of Collagen Gel and Hyaluronic Acid as Vitreous Substitutes. Ophthalmic Res. 1997;29:409–420. [DOI] [PubMed] [Google Scholar]
- 61.Chirila TV, Sharp C, Moore SR, et al. Synthetic Hydrogel as an Artificial Vitreous Body. A One-Year Animal Study of Its Effects on the Retina. Cells Mater. 1995;5:83–96. [Google Scholar]
- 62.Fernandez-Vigo J, Refojo MF, Verstraeten T. Evaluation of a Viscoelastic Solution of Hydroxypropyl Methylcellulose as a Potential Vitreous Substitute. Retina. 1990;10:148–152. [DOI] [PubMed] [Google Scholar]
- 63.Crafoord S, Andreasson S & Ghosh F Experimental vitreous tamponade using polyalkylimide hydrogel. Graefes Arch Clin Exp Ophthalmol 249, 1167 (2011). [DOI] [PubMed] [Google Scholar]
- 64.De Jong C, Bali E, Libert J, et al. ADCON-L hydrogel as a vitreous substitute: preliminary results. Bull Soc Belge Ophtalmol. 2000:71–75. [PubMed] [Google Scholar]
- 65.Maruoka S, Matsuura T, Kawasaki K, Okamoto M, Yoshiaki H, Kodama M, Sugiyama M, Annaka M. Biocompatibility of polyvinylalcohol gel as a vitreous substitute. Curr Eye Res. 2006. Jul-Aug;31(7–8):599–606. [DOI] [PubMed] [Google Scholar]
- 66.Katagiri Y, Iwasaki T, Ishikawa T, Yamakawa N, Suzuki H, Usui M. Application of thermo-setting gel as artificial vitreous. Jpn J Ophthalmol. 2005. Nov-Dec;49(6):491–496. [DOI] [PubMed] [Google Scholar]
- 67.Hayashi K, Okamoto F, Hoshi S, et al. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nat Biomed Eng. 2017;1:0044. [Google Scholar]
- 68.Liu Z, Liow SS, Lai SL et al. Retinal-detachment repair and vitreous-like-body reformation via a thermogelling polymer endotamponade. Nat Biomed Eng 3, 598–610 (2019). [DOI] [PubMed] [Google Scholar]


