Abstract
A simple, rapid, and sensitive LC-MS/MS method for determining concentrations of the anticancer alkaloid vincristine in micro volumes of mouse plasma was developed and validated in positive ion mode. Separation of vincristine and the internal standard [2H3]-vincristine was achieved on an Accucore aQ column with a gradient mobile phase delivered at a flow rate of 0.4 mL/min and a run time of 2.2 min. Calibration curves were linear (r2 > 0.99, n = 8) up to 250 ng/mL, with a lower limit of quantitation of 2.5 ng/mL. The matrix effect and extraction recovery for vincristine were ranging 108–110% and 88.4–107%, respectively. The intra-day and inter-day precision of quality controls tested at 3 different concentrations were always less than 15%, and accuracy ranged from 91.7 to 107%. The method was successfully applied to evaluate the pharmacokinetic profile of vincristine in wild-type and CYP3A-deficient mice in support of a project to provide mechanistic insight into drug-drug interactions and to identify sources of inter-individual pharmacokinetic variability associated with vincristine-induced peripheral neuropathy.
Keywords: Vincristine, UHPLC-MS/MS, Pharmacokinetics, Mouse plasma
1. Introduction
Vincristine is an effective chemotherapeutic Vinca alkaloid used in the treatment of various hematological cancers, in particular pediatric acute lymphoblastic leukemia [1,2]. However, the clinical use of vincristine is hampered by the onset of severe peripheral neuropathy [3–5], the associated mechanism of which remains to this day incompletely understood. The disposition properties of vincristine are characterized by extensive differences in clearance between patients receiving the same therapeutic regimen [6], and this high degree of inter-individual pharmacokinetic variability may have important toxicological ramification [7,8]. It has been speculated that a critical determinant of this variability is associated with differential expression of polymorphic drug-metabolizing enzymes and/or transporters at sites of elimination. Evidence from in vitro studies has indicated that vincristine undergoes cytochrome P-450 3A (CYP3A)-mediated metabolism [9,10], with CYP3A5 contributing to 75% of the intrinsic clearance of vincristine [11–13]. Interestingly, the incidence of vincristine-induced peripheral neuropathy appears to be lower in patients who functionally express CYP3A5 [14,15], and is increased in patients concurrently treated for fungal infections [16] with azole antifungals that potently inhibit CYP3A-mediated metabolism, such as ketoconazole [17]. Even though no pharmacokinetic data are available, the assumed mechanism for this interaction is through inhibition of CYP3A, leading to decreased vincristine clearance and increased side effects [4]. This thesis would be consistent with the observation that co-administration of weaker CYP3A-inhibiting azoles, such as fluconazole, do not increase the risk for vincristine-associated toxicities [18]. However, details regarding the precise contribution of metabolizing enzymes to the observed drug-drug interactions are lacking, and recent preliminary data indicate that there might be no relationship between vincristine pharmacokinetics and azole treatment [19].
In order to directly address the mechanistic basis of the reported interactions, we have recently initiated a project to identify sources of inter-individual pharmacokinetic variability associated with vincristine-induced peripheral neuropathy using a murine model that lacks all functional CYP3A genes [20]. In support of this project, we report here the development and validation of a rapid and sensitive analytical method for vincristine in mouse plasma with the use of liquid chromatography-tandem mass spectrometry (LC-MS/MS), and demonstrate its utility in a pharmacokinetic study of vincristine in wild-type mice and CYP3A-deficient mice.
2. Materials and methods
2.1. Chemical and reagents
Vincristine sulfate was purchased from MedChemExpress (99.7% purity, Monmouth Junction, NJ, USA). An internal standard of vincristine, [2H3]-vincristine sulfate (vincristine-d3), was purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). LC-MS-grade formic acid (FA), methanol and acetonitrile were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Blank plasma was obtained from wild-type mice on an FVB strain (Taconic Biosciences, Cambridge City, IN, USA). Whole blood was obtained from untreated wild-type mice, collected in 1.3-mL microtubes containing heparin, which were centrifuged at 1,500 × g for 5 min to collect the plasma supernatant.
2.2. Instrument and chromatographic conditions
A Vanquish UHPLC coupled with a Quantiva triple quadrupole mass spectrometer from Thermo Fisher Scientific was used for analysis. An Accucore aQ column (50 mm × 2.1 mm, dp =2.6 μm, Thermo Fisher Scientific) protected by a C18 AQUASIL guard cartridge (2.1 mm × 10 mm, dp =3 μm, Thermo Fisher Scientific) was employed for separation of analytes. The column and autosampler were maintained at 50°C and 4°C, respectively. The mobile phase was composed of solvent A (0.1% FA in H2O) and solvent B (0.1% FA in acetonitrile), and gradient elution was used at a flow rate of 0.4 mL/min for a total run time of 2.2 min. The gradient conditions were as follows: 0–0.5 min, 10% B; 0.5–0.51 min, 10 to 50%, 0.51–1.8 min, 50% to 95% B;1.8–1.81 min, 95% to 10% B; 1.81–2.2 min, 10% B. Aliquots of 2 μL of the extracted plasma samples were injected.
The following parameters were set for the mass spectrometer: 41.4 Arb, 3.6 Arb, 10 Arb, 375°C and 450°C for sheath gas, aux gas, sweep gas, ion transfer tube, and vaporizer temperature, respectively. The ion source was operated using heated ESI with the ion spray voltage set at 3536.36 V in positive ion mode. The collision gas argon was used at a pressure of 1.5 mTorr. Scheduled multiple reaction monitoring (MRM) was employed for analysis of vincristine and the internal standard. The optimized selective reaction monitoring (SRM) transitions and their respective collision energies are listed in Table 1. LCquan (version 3.0, Thermo Fisher Scientific) was used for data acquisition and processing.
Table 1.
List of LC retention time and MRM transitions for analytes included in the assay
| Analyte | Precursor (m/z) | Product (m/z) | Collision Energy (V) | RF Lens (V) |
|---|---|---|---|---|
| vincristine | 825.438 | 765.354 | 35.74 | 79.6 |
| vincristine-d3 | 828.488 | 768.275 | 36.08 | 14.3 |
2.3. Drug solutions, calibration, and quality control samples
Methanolic stock solutions of 5 mg/mL were prepared for vincristine and the internal standard, and the working solution of vincristine was serially diluted (10–1,000 ng/mL) with methanol. Calibration curves of vincristine were prepared from the working stock solution at concentrations of 2.5, 5, 12.5, 25, 50, 125, and 250 ng/mL. The lower limit of quantitation (LLOQ), low quality-control (LQC), medium quality-control (MQC), and high quality-control (HQC) were prepared at vincristine concentrations of 2.5, 7.5, 125, 212.5 ng/mL, respectively. An internal standard solution (100 ng/mL) was prepared by diluting a stock solution with methanol. All the solutions were stored at −20°C and brought to room temperature before use.
2.4. Method validation
2.4.1. Linearity, accuracy and precision
Calibration curves were analyzed on four consecutive days by calculating the vincristine to internal standard peak area ratios and applying a weighted (1/x2) least-squares linear regression analysis. Accuracy was expressed as the relative error of the measured mean deviation from the nominal concentrations and analysis of variance was used to assess intra-day and inter-day precision, as follows:
| (1) |
| (2) |
In these equations, MSintra and MSinter are the mean squares within and between groups, respectively, n is the number of determinations per group, and Meanall is the mean for all determinations at each concentration [21]. Statistical analysis was performed using SPSS version 25 (SPSS, Inc., Chicago IL, USA).
Precision and accuracy for the interpolated concentrations of the calibration points were considered acceptable if deviations were within ±15% of their nominal values, except for the LLOQ (within ±20%). The intra-day precision, inter-day precision, and accuracy of LLOQ and other quality control samples were measured in quintuplicate at each concentration on four consecutive days.
2.4.2. Specificity and selectivity
Specificity was tested by analyzing six different lots of plasma from untreated mice to ensure that no endogenous substances in plasma interfere with vincristine or the internal standard. Selectivity was evaluated by comparing chromatograms obtained from blank, vincristine-free plasma and plasma spiked with vincristine.
2.4.3. Dilution, matrix effect and recovery
A dilution test was performed to evaluate accuracy at a vincristine concentration of 2,125 ng/mL followed by a 1:10 (v/v) dilution in the blank plasma. Accuracy were considered acceptable with results were within ±15% of the nominal value. A matrix effect test was performed to evaluate the suppression or enhancement of the ionization of vincristine by the presence of matrix components in biological samples. In the present study, the matrix effect experiment was carried out using the ratio between spiked mobile phase solutions and un-extracted samples. The extraction recoveries were evaluated by dividing the extracted sample mean response by the un-extracted (spiked blank plasma extract) sample mean of the corresponding concentration. Three replicates each of quality control samples were prepared according to the established extraction procedure.
2.4.4. Stability
Stability was assessed at LQC and HQC levels, and included bench-top, autosampler, re-injection, and freeze-thaw stabilities. The bench-top stability was assessed by keeping QC samples at ambient temperature for 6 hours. The auto-sampler stability was determined by analyzing the quality control samples stored at the auto-sampler at 4°C for 24 hours. Re-injection stability was evaluated by reanalyzing the processed quality control samples stored at 4°C after 24 hours. For freeze-thaw stability, quality control samples were stored at −80°C for 12 hours, thawed at room temperature, and then frozen again at −80°C. The cycle was repeated three times. The stock solution stabilities of Vincristine and IS in MeOH at refrigerated conditions (−20°C) were demonstrated by comparing the area response of the analyte with the response of the sample prepared from fresh stock solution.
2.5. In vivo pharmacokinetics studies
2.5.1. Animal studies
The developed method was applied to pharmacokinetic studies of vincristine in male FVB wild-type mice and sex- and age-matched CYP3A-deficient [CYP3A(−/−)] mice that lack all 8 murine CYP3A genes (Taconic Biosciences, Cambridge City, IN, USA) on the same background strain. Mice were maintained under pathogen-free conditions in the Ohio State University Laboratory Animal Resources, and all in vivo experiments were approved by University Animal Care and Use Committee. Mice were housed in a temperature- and the light-controlled environment with free access to water and a standard diet. For in vivo studies, vincristine sulfate was dissolved in normal saline (0.2 mg/mL) and administered as a single 1 mg/kg dose by intraperitoneal injection. In select experiments, a single oral dose of ketoconazole (50 mg/kg) or its vehicle polyethylene glycol 400 (PEG400) was given 30 min before vincristine administration.
Pharmacokinetic studies were performed as previously described [22]. Briefly, whole blood samples of about 30 μL were collected from each mouse at 5 min, 15 min, 30 min, 1 h and 4 h after the administration of vincristine. Samples obtained at 5, 15, and 30 min were collected from a submandibular vein using a sterile 5-mm Goldenrod animal lancet in heparinized capillary tubes. For samples obtained at 1 h, mice were anesthetized using 2% isoflurane and whole blood was collected from the retro-orbital venous plexus using capillary tubes. The final sample at 4 h was collected by cardiac puncture using a syringe and needle. Whole blood samples were centrifuged at 13,000 rpm for 5 min. The plasma supernatant was then collected, immediately placed on dry ice, and stored at −80°C until analysis.
2.5.2. Sample preparation
A single protein precipitation step was used to extract vincristine from plasma samples. Prior to analysis, frozen samples were thawed at room temperature, 5-μL aliquots of plasma were transferred into a 0.5-mL Eppendorf tube, followed by the addition of 40 μL of internal standard working solution and 55 μL of neat methanol. The samples were vortex-mixed for 30 s and centrifuged at 13,000 rpm for 10 min at 4°C. Next, 75-μL aliquots of the supernatant were added to autosampler vials (Agilent Technologies, Palo Alto, CA), and a 2-μL volume was injected into the LC-MS/MS system.
2.5.3. Pharmacokinetic data analysis
Pharmacokinetic parameters were calculated by non-compartmental analysis using Phoenix WinNonlin version 8.0 (Certara, USA). Peak plasma concentration (Cmax) was determined by visual inspection of the data from the concentration-time curves. The linear trapezoidal rule was used to obtain the area under the plasma concentration-time curve (AUC). An unpaired, 2-sided Student’s t-test with Welch’s correction was performed to compare the pharmacokinetic parameters between the two mouse genotypes (wild-type and CYP3A deficiency). A one-way analysis of variance with Tukey’s multiple comparison test was used to compare the Cmax and AUC0–4h between each of four treatment groups (vehicle or ketoconazole in wild-type mice or CYP3A-deficient mice).
3. Results and discussion
3.1. Chromatographic and mass spectrometric conditions
The analytes of interest were ionized with an ESI source operating in the positive ion mode. Detection was performed in the MRM mode at m/z 825.438→765.354 for vincristine and m/z 828.488→768.275 for the isotope-labeled internal standard. Chromatographic conditions were optimized in several trials to achieve high resolution and symmetrical peak shapes, and the retention times were 1.3 min for both vincristine and the internal standard, with a total run time of 2.2 min (Fig. 1).
Figure. 1. Chromatograms of vincristine and vincristine-d3 in mouse plasma:
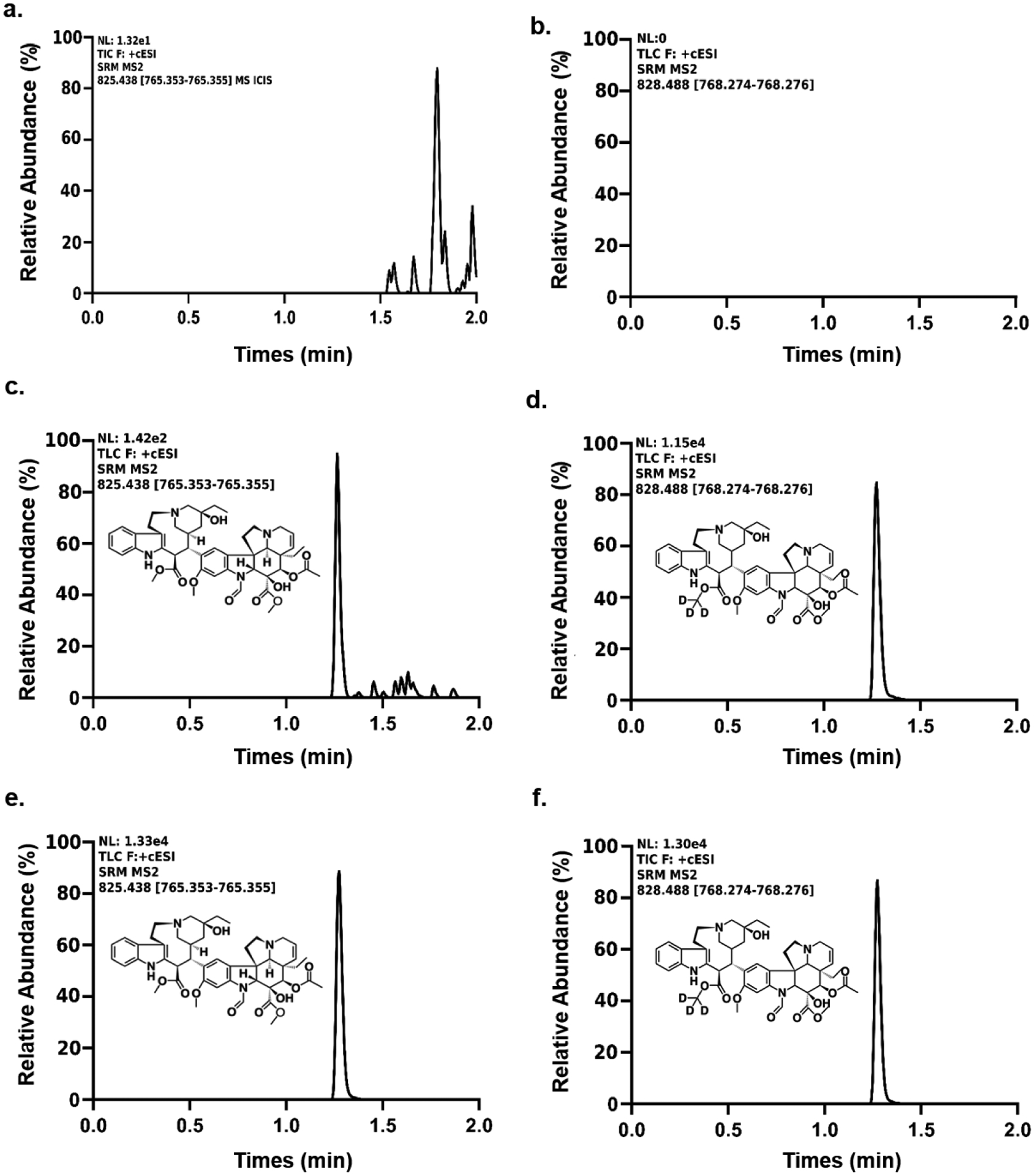
Monitoring (a) vincristine and (b) vincristine-d3 in the blank plasma, (c) 2.5 ng/mL vincristine spiked into the blank plasma, (d) 40 ng/mL vincristine-d3 spiked into the blank plasma, (e) vincristine in treated sample processed according to the sample preparation after oral administration, (f) 40 ng/mL vincristine-d3 added into the treated sample processed according to the sample preparation.
3.2. Method validation
3.2.1. Specificity, selectivity, matrix effect, recovery and dilution
Typical MRM chromatograms of untreated plasma spiked at the LLOQ of vincristine and the internal standard, as well as samples from treated mice are shown in Fig. 1. No endogenous interferences were found at the retention time of vincristine during analysis, supporting the specificity of the method. The method was also found to distinguish and quantify the analyte in the presence of endogenous and/or exogenous interferences (Fig. 1), demonstrating sufficient selectivity was achieved. Quality control samples (n=3) at LQC, MQC and HQC levels were assayed for matrix effect and extraction recovery. The results were found to be well within the acceptable limit of ±15%, without adverse matrix effects or compromised recovery (Table 2). Additionally, the intra-day precision, inter-day precision, and accuracy observed for the 10-fold diluted quality control samples were 2.12%, 5.20%, and 101% (Table 3), respectively. This established that samples containing concentrations higher than the upper limit of the calibration curve can be diluted with blank plasma and re-analyzed to fall within the validated concentration range of the calibration curve.
Table 2.
Recovery and matrix effect of vincristine in mouse plasma
| Matrix Effect | Recovery | ||||
|---|---|---|---|---|---|
| Conc. (ng/mL) | N | Mean Matrix Effect (%) | RSD (%) | Mean Recovery (%) | RSD (%) |
| 7.5 | 3 | 109 | 7.16 | 88.4 | 16.5 |
| 125 | 3 | 110 | 9.13 | 107 | 4.91 |
| 212.5 | 3 | 108 | 7.59 | 89.3 | 13.6 |
Abbreviation: RSD, relative standard deviation.
Table 3.
Assay performance data for the quantitation of vincristine in mouse plasma
| N | Conc. (ng/mL) | RSD (%) | DEV (%) | Intra-assay (%) | Inter-Assay (%) | Accuracy (%) | |
|---|---|---|---|---|---|---|---|
| LLOQ | 20 | 2.5 | 3.44 | 6.57 | 3.14 | 1.87 | 107 |
| LQC | 20 | 7.5 | 4.41 | −8.30 | 3.89 | 2.59 | 91.7 |
| MQC | 20 | 125 | 4.06 | 4.54 | 3.33 | 2.96 | 104 |
| HQC | 20 | 212.5 | 4.85 | −0.16 | 4.14 | 3.06 | 99.8 |
| 8500 (dilution 10x) | 20 | 212.5 | 4.62 | 1.39 | 2.12 | 5.20 | 101 |
Abbreviations: RSD, relative standard deviation; DEV, deviation from the nominal value; LLOQ, The lower limit of quantification; LQC, low quality-control; MQC, medium quality-control, and HQC, high quality-control
3.2.2. Linearity, accuracy and precision
The calibration curves were linear in the concertation range of 2.5–250 ng/mL, with a mean (± SD) correlation coefficient for regression equations generated on four consecutive days of 0.99 ± 0.001. The percent deviation from nominal values determined for mean back-calculated concentrations (Table 4), for each standard, ranged from −10.1 to 12.4%, indicating that a weighted quadratic regression equation provides acceptable fits of the data. The accuracy and precision results for vincristine in quality control samples are summarized in Table 3. All intra-day precision, inter-day precision, and accuracy results were within the standard as specified in the FDA and EMA guidance for bioanalytical method validation.
Table 4.
Back calculated concentrations from calibrators run in duplicate on four consecutive days
| Nominal Conc. (ng/mL) | N | Mean (ng/mL) | RSD (%) | DEV (%) |
|---|---|---|---|---|
| 2.5 | 8 | 2.42 | 5.16 | −3.07 |
| 5 | 8 | 5.41 | 3.27 | 8.21 |
| 12.5 | 8 | 11.9 | 2.51 | −5.14 |
| 25 | 8 | 25.0 | 5.92 | −0.22 |
| 50 | 8 | 56.2 | 3.18 | 12.4 |
| 125 | 8 | 124 | 3.35 | −0.69 |
| 250 | 8 | 225 | 2.67 | −10.1 |
Abbreviations: RSD, relative standard deviation; DEV, deviation from the nominal value; N, total number of observations during validation.
3.2.3. Stability
The stock solutions of vincristine and IS in MeOH were confirmed to be stable for 40 days at −20 °C (data not shown). The experimental conditions during the actual sample analysis of our ongoing pharmacokinetic project were simulated, including bench-top, autosampler, re-injection, and freeze-thaw stabilities. The results of these studies are enumerated in Table 5. For bench-top stability, the results indicate that sample preparation times of even 6 h do not result in significant degradation of vincristine thus allowing large numbers of samples to be processed for a single analytical run. Results from autosampler and re-injection stability studies indicate that vincristine in reconstituted samples is not degraded when kept at 4°C in a temperature-controlled autosampler for up to 24 hours, and that samples can be re-injected within a 24-h window should that be needed without loss of assay performance. Freeze-thaw stability results suggest that 3 consecutive freeze-thaw cycles will not affect the integrity of vincristine in mouse plasma samples. And it is previously reported that vincristine concentration in QC samples after 6 months of storage at −40 °C did not show significant degradation [23]
Table 5.
Stability in mouse plasma
| Con. (ng/mL) | N | Sample Conditions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bench-Top Stabilitya | Auto-Sampler Stabilityb | Re-injection Stabilityc | Freeze-Thaw Stabilityd | ||||||
| Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | ||
| 7.5 | 3 | 95.8 | 5.54 | 89.8 | 5.52 | 96.6 | 4.92 | 92.3 | 4.79 |
| 212.5 | 3 | 95.6 | 5.65 | 95.8 | 9.39 | 90.1 | 5.67 | 101 | 5.07 |
Stored at ambient temperature (25 °C) for 6 hours
Stored the fresh samples at auto-sampler (4°C) for 24 hours
Stored the analyzed samples at auto-sampler (4°C) and reanalyzed samples after 24 hours
After three freeze-thaw cycles
3.3. Pharmacokinetic studies
Previous studies have demonstrated that the systemic exposure to vincristine is subject to extensive inter-individual variability in both adult and pediatric patients [7,8], and it has been speculated that this variability is causally connected with genetic and/or environmental factors [24] that influence vincristine-mediated metabolism by CYP3A isoforms [12]. To directly test this hypothesis, we applied the developed analytical method to a comparative pharmacokinetic study of vincristine in wild-type and CYP3A(−/−) mice, based on the expectation that complete deficiency of all CYP3A isoforms would result in diminished vincristine metabolism, which in turn would decrease clearance. The observed pharmacokinetic profiles in our study from wild-type mice was consistent with previously reported result [25]. Compared to wild-type mice, the systemic exposure to vincristine was increased by about 20% (P=0.065) in CYP3A-deficient mice (Fig. 2a), suggesting that the clearance of vincristine in mice is largely independent of CYP3A function. Since CYP2C enzymes are up-regulated in the liver of CYP3A(−/−) mice [26] and provide a compensatory mechanism of xenobiotic metabolism of some [26] but not all xenobiotics [27], we also evaluated the murine pharmacokinetics of vincristine in the presence of the dual CYP2C/CYP3A inhibitor, ketoconazole [28]. This study demonstrated that the lack of a profound phenotypic change in the handling of vincristine in CYP3A(−/−) mice is unrelated to a known escape mechanism in these animals (Fig. 2b). Although these collective findings are unexpected and suggest that the contribution of CYP3A-mediated metabolism to vincristine elimination may be much smaller than held previously, it is conceivable that, compared to mice, the clearance of vincristine in humans is rate-limited predominantly by transport except for individuals with high hepatic expression of CYP3A5. This possibility is currently under further investigation in a transgenic mouse model with hepatic expression of human CYP3A5 [29].
Figure. 2. Pharmacokinetic profile of vincristine:
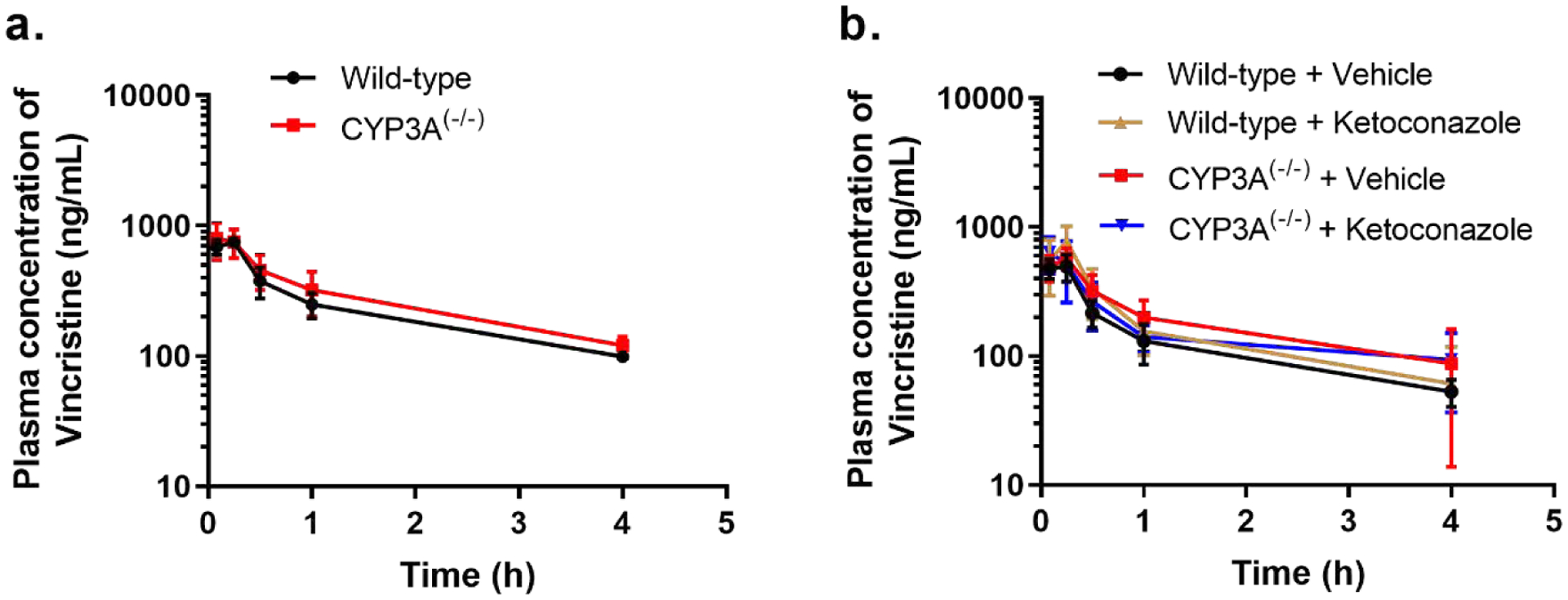
mean plasma concentration-time profiles of vincristine (1 mg/kg; i.p.) in wild-type and CYP3A(−/−) mice (a), and 30 min after vehicle (PEG400; p.o.) or ketoconazole (50 mg/kg; p.o.) administration (b), mean ± 95% CI, n = 5–9.
3.4. Comparison with reported methods
Available methods for the measurement of vincristine are based on the use of HPLC-UV[30,31] and LC-MS/MS [23,32–35]. These methods require longer run times with a high plasma volume [32,34] and extended procedure steps [23,33,35]. Compared to previously reported analytical methods for vincristine, our assay does not require solid-phase extraction, and only needs 5 μL plasma per sample with an optimized shorter run time of 2.2 min. Together, these advantages suggest that our method is suitable for serial sampling and constructing the complete concentration time profiles in individual mouse.
4. Conclusion
In the current study, we developed and validated a sensitive and reproducible UHPLC-MS/MS method to quantitatively determine concentrations of vincristine in mouse plasma. The described method was linear over a wide range of translationally-relevant concentrations, provides high extraction efficiency using a single protein-precipitation step, does not require additional drying or reconstitution steps, and is suitable for the use of micro-volumes of mouse plasma. The method was successfully applied to a murine pharmacokinetic study that provided previously unavailable data into the role of CYP3A-mediated metabolism as a pathway of vincristine elimination. Our ongoing studies will employ the method to gain mechanistic insights into the role of vincristine metabolism in drug-drug interactions and to identify additional sources of inter-individual pharmacokinetic variability associated with vincristine-induced peripheral neuropathy.
Supplementary Material
Highlights.
We have developed a simple UHPLC-MS/MS method for determining vincristine in mouse plasma.
Convenient for animal studies, our method only requires 5 μL of plasma per sample.
Our method is highly sensitive and effective with a sample run time of only 2.2 min.
The method was validated and applied to vincristine murine pharmacokinetic study in CYP3A KO mice.
Pharmacokinetic studies provided novel data of CYP3A metabolism in vincristine elimination.
Acknowledgements
This work was supported by funds from The Ohio State University Comprehensive Cancer Center Pelotonia foundation (AS and SH), by the Pelotonia Fellowship Program (YL), and in part by NIH grants R01CA215802 (AS) and R01CA238946 (SH). Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Den Boer ML, Harms DO, Pieters R, Kazemier KM, Gobel U, Körholz D, Graubner U, Haas RJ, Jorch N, Spaar HJ, Kaspers GJL, Kamps WA, Van der Does-Van den Berg A, Van Wering ER, Veerman AJP, Janka-Schaub GE, Patient stratification based on prednisolone-vincristine-asparaginase resistance profiles in children with acute lymphoblastic leukemia., J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 21 (2003) 3262–3268. 10.1200/JCO.2003.11.031. [DOI] [PubMed] [Google Scholar]
- [2].Diouf B, Crews KR, Lew G, Pei D, Cheng C, Bao J, Zheng JJ, Yang W, Fan Y, Wheeler HE, Wing C, Delaney SM, Komatsu M, Paugh SW, McCorkle JR, Lu X, Winick NJ, Carroll WL, Loh ML, Hunger SP, Devidas M, Pui C-H, Dolan ME, V Relling M, Evans WE, Association of an Inherited Genetic Variant With Vincristine-Related Peripheral Neuropathy in Children With Acute Lymphoblastic Leukemia, JAMA. 313 (2015) 815–823. 10.1001/jama.2015.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Verstappen CCP, Koeppen S, Heimans JJ, Huijgens PC, Scheulen ME, Strumberg D, Kiburg B, Postma TJ, Dose-related vincristine-induced peripheral neuropathy with unexpected off-therapy worsening, Neurology. 64 (2005) 1076 LP–1077. 10.1212/01.WNL.0000154642.45474.28. [DOI] [PubMed] [Google Scholar]
- [4].Mora E, Smith EML, Donohoe C, Hertz DL, Vincristine-induced peripheral neuropathy in pediatric cancer patients., Am. J. Cancer Res 6 (2016) 2416–2430. [PMC free article] [PubMed] [Google Scholar]
- [5].van de Velde ME, Kaspers GL, Abbink FCH, Wilhelm AJ, Ket JCF, van den Berg MH, Vincristine-induced peripheral neuropathy in children with cancer: A systematic review., Crit. Rev. Oncol. Hematol 114 (2017) 114–130. 10.1016/j.critrevonc.2017.04.004. [DOI] [PubMed] [Google Scholar]
- [6].Groninger E, Meeuwsen-de Boar T, Koopmans P, Uges D, Sluiter W, Veerman A, Kamps W, de Graaf ffS., Pharmacokinetics of vincristine monotherapy in childhood acute lymphoblastic leukemia., Pediatr. Res 52 (2002) 113–118. 10.1203/00006450-200207000-00021. [DOI] [PubMed] [Google Scholar]
- [7].Van den Berg HW, Desai ZR, Wilson R, Kennedy G, Bridges JM, Shanks RG, The pharmacokinetics of vincristine in man: reduced drug clearance associated with raised serum alkaline phosphatase and dose-limited elimination., Cancer Chemother. Pharmacol 8 (1982) 215–219. 10.1007/BF00255487. [DOI] [PubMed] [Google Scholar]
- [8].Frost BM, Lönnerholm G, Koopmans P, Abrahamsson J, Behrendtz M, Castor A, Forestier E, Uges DRA, de Graaf SSN, Vincristine in childhood leukaemia: no pharmacokinetic rationale for dose reduction in adolescents., Acta Paediatr. 92 (2003) 551–557. [PubMed] [Google Scholar]
- [9].Zhou XJ, Zhou-Pan XR, Gauthier T, Placidi M, Maurel P, Rahmani R, Human liver microsomal cytochrome P450 3A isozymes mediated vindesine biotransformation. Metabolic drug interactions., Biochem. Pharmacol 45 (1993) 853–861. 10.1016/0006-2952(93)90169-w. [DOI] [PubMed] [Google Scholar]
- [10].Zhou-Pan XR, Sérée E, Zhou XJ, Placidi M, Maurel P, Barra Y, Rahmani R, Involvement of human liver cytochrome P450 3A in vinblastine metabolism: drug interactions., Cancer Res. 53 (1993) 5121–5126. [PubMed] [Google Scholar]
- [11].Dennison JB, Jones DR, Renbarger JL, Hall SD, Effect of CYP3A5 expression on vincristine metabolism with human liver microsomes., J. Pharmacol. Exp. Ther 321 (2007) 553–563. 10.1124/jpet.106.118471. [DOI] [PubMed] [Google Scholar]
- [12].Dennison JB, Kulanthaivel P, Barbuch RJ, Renbarger JL, Ehlhardt WJ, Hall SD, Selective metabolism of vincristine in vitro by CYP3A5., Drug Metab. Dispos 34 (2006) 1317–1327. 10.1124/dmd.106.009902. [DOI] [PubMed] [Google Scholar]
- [13].Dennison JB, Mohutsky MA, Barbuch RJ, Wrighton SA, Hall SD, Apparent high CYP3A5 expression is required for significant metabolism of vincristine by human cryopreserved hepatocytes., J. Pharmacol. Exp. Ther 327 (2008) 248–257. 10.1124/jpet.108.139998. [DOI] [PubMed] [Google Scholar]
- [14].Bosilkovska M, Ing Lorenzini K, Uppugunduri CRS, Desmeules J, Daali Y, Escher M, Severe Vincristine-induced Neuropathic Pain in a CYP3A5 Nonexpressor With Reduced CYP3A4/5 Activity: Case Study., Clin. Ther 38 (2016) 216–220. 10.1016/j.clinthera.2015.10.017. [DOI] [PubMed] [Google Scholar]
- [15].Egbelakin A, Ferguson MJ, MacGill EA, Lehmann AS, Topletz AR, Quinney SK, Li L, McCammack KC, Hall SD, Renbarger JL, Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia., Pediatr. Blood Cancer 56 (2011) 361–367. 10.1002/pbc.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nikanjam M, Sun A, Albers M, Mangalindin K, Song E, Vempaty H, Sam D, V Capparelli E, Vincristine-associated Neuropathy With Antifungal Usage: A Kaiser Northern California Experience., J. Pediatr. Hematol. Oncol 40 (2018) e273–e277. 10.1097/MPH.0000000000001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moriyama B, Henning SA, Leung J, Falade-Nwulia O, Jarosinski P, Penzak SR, Walsh TJ, Adverse interactions between antifungal azoles and vincristine: review and analysis of cases., Mycoses. 55 (2012) 290–297. 10.1111/j.1439-0507.2011.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Smitherman AB, Faircloth CB, Deal A, Troy M, Gold SH, Vincristine toxicity with co-administration of fluconazole during induction therapy for pediatric acute lymphoblastic leukemia., Pediatr. Blood Cancer 64 (2017). 10.1002/pbc.26525. [DOI] [PubMed] [Google Scholar]
- [19].van de Velde ME, Panetta JC, Wilhelm AJ, van den Berg MH, van der Sluis IM, van den Bos C, Abbink FCH, van den Heuvel-Eibrink MM, Segers H, Chantrain C, van der Werff Ten Bosch J, Willems L, Evans WE, Kaspers GL, Population Pharmacokinetics of Vincristine Related to Infusion Duration and Peripheral Neuropathy in Pediatric Oncology Patients., Cancers (Basel). 12 (2020). 10.3390/cancers12071789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Herwaarden AE, Wagenaar E, van der Kruijssen CMM, van Waterschoot RAB, Smit JW, Song J-Y, van der Valk MA, van Tellingen O, van der Hoorn JWA, Rosing H, Beijnen JH, Schinkel AH, Knockout of cytochrome P450 3A yields new mouse models for understanding xenobiotic metabolism, J. Clin. Invest 117 (2007) 3583–3592. 10.1172/JCI33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tohnya TM, Hwang K, Lepper ER, Fine HA, Dahut WL, Venitz J, Sparreboom A, Figg WD, Determination of CC-5013, an analogue of thalidomide, in human plasma by liquid chromatography–mass spectrometry, J. Chromatogr. B 811 (2004) 135–141. 10.1016/J.JCHROMB.2004.08.022. [DOI] [PubMed] [Google Scholar]
- [22].Leblanc AF, Huang KM, Uddin ME, Anderson JT, Chen M, Hu S, Murine Pharmacokinetic Studies, Bio-Protocol. 8 (2018). 10.21769/BioProtoc.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guilhaumou R, Solas C, Rome A, Giocanti M, Andre N, Lacarelle B, Validation of an electrospray ionization LC/MS/MS method for quantitative analysis of vincristine in human plasma samples., J. Chromatogr. B, Anal. Technol. Biomed. Life Sci 878 (2010) 423–427. 10.1016/j.jchromb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- [24].Skiles JL, Chiang C, Li CH, Martin S, Smith EL, Olbara G, Jones DR, Vik TA, Mostert S, Abbink ffF., Kaspers GJ, Li L, Njuguna F, Sajdyk TJ, Renbarger JL, CYP3A5 genotype and its impact on vincristine pharmacokinetics and development of neuropathy in Kenyan children with cancer., Pediatr. Blood Cancer 65 (2018). 10.1002/pbc.26854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].El Dareer SM, White VM, Chen FP, Mellet LB, Hill DL, Distribution and metabolism of vincristine in mice, rats, dogs, and monkeys., Cancer Treat. Rep 61 (1977) 1269–1277. [PubMed] [Google Scholar]
- [26].van Waterschoot RAB, van Herwaarden AE, Lagas JS, Sparidans RW, Wagenaar E, van der Kruijssen CMM, Goldstein JA, Zeldin DC, Beijnen JH, Schinkel AH, Midazolam metabolism in cytochrome P450 3A knockout mice can be attributed to up-regulated CYP2C enzymes., Mol. Pharmacol 73 (2008) 1029–1036. 10.1124/mol.107.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].van Waterschoot RAB, Lagas JS, Wagenaar E, van der Kruijssen CMM, van Herwaarden AE, Song J-Y, Rooswinkel RW, van Tellingen O, Rosing H, Beijnen JH, Schinkel AH, Absence of both cytochrome P450 3A and P-glycoprotein dramatically increases docetaxel oral bioavailability and risk of intestinal toxicity., Cancer Res. 69 (2009) 8996–9002. 10.1158/0008-5472.CAN-09-2915. [DOI] [PubMed] [Google Scholar]
- [28].Ong CE, Coulter S, Birkett DJ, Bhasker CR, Miners JO, The xenobiotic inhibitor profile of cytochrome P4502C8., Br. J. Clin. Pharmacol 50 (2000) 573–580. 10.1046/j.1365-2125.2000.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Abe S, Kobayashi K, Oji A, Sakuma T, Kazuki K, Takehara S, Nakamura K, Okada A, Tsukazaki Y, Senda N, Honma K, Yamamoto T, Ikawa M, Chiba K, Oshimura M, Kazuki Y, Modification of single-nucleotide polymorphism in a fully humanized CYP3A mouse by genome editing technology., Sci. Rep 7 (2017) 15189. 10.1038/s41598-017-15033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Embree L, Gelmon KA, Tolcher AW, Hudon NJ, Heggie JR, Dedhar C, Webb MS, Bally MB, Mayer LD, Validation of a high-performance liquid chromatographic assay method for quantification of total vincristine sulfate in human plasma following administration of vincristine sulfate liposome injection, J. Pharm. Biomed. Anal 16 (1997) 675–687. 10.1016/S0731-7085(97)00087-3. [DOI] [PubMed] [Google Scholar]
- [31].Koopmans P, Gidding CE, de Graaf SS, Uges DR, An automated method for the bioanalysis of vincristine suitable for therapeutic drug monitoring and pharmacokinetic studies in young children., Ther. Drug Monit 23 (2001) 406–409. 10.1097/00007691-200108000-00014. [DOI] [PubMed] [Google Scholar]
- [32].Corona G, Casetta B, Sandron S, Vaccher E, Toffoli G, Rapid and sensitive analysis of vincristine in human plasma using on-line extraction combined with liquid chromatography/tandem mass spectrometry., Rapid Commun. Mass Spectrom 22 (2008) 519–525. 10.1002/rcm.3390. [DOI] [PubMed] [Google Scholar]
- [33].Guo P, Wang X, Zhou F, Gallo JM, Determination of vincristine in mouse plasma and brain tissues by liquid chromatography-electrospray mass spectrometry., J. Chromatogr. B, Anal. Technol. Biomed. Life Sci 809 (2004) 273–278. 10.1016/j.jchromb.2004.06.025. [DOI] [PubMed] [Google Scholar]
- [34].Dennison JB, Renbarger JL, Walterhouse DO, Jones DR, Hall SD, Quantification of vincristine and its major metabolite in human plasma by high-performance liquid chromatography/tandem mass spectrometry., Ther. Drug Monit 30 (2008) 357–364. 10.1097/FTD.0b013e31816b92c9. [DOI] [PubMed] [Google Scholar]
- [35].Hantrakul S, Klangkaew N, Kunakornsawat S, Tansatit T, Poapolathep A, Kumagai S, Poapolathep S, Clinical pharmacokinetics and effects of vincristine sulfate in dogs with transmissible venereal tumor (TVT)., J. Vet. Med. Sci 76 (2014) 1549–1553. 10.1292/jvms.14-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


