Abstract
Knee meniscus injury is frequent, resulting in over 1 million surgeries annually in the United States and Europe. Because of the near-avascularity of this fibrocartilaginous tissue and its intrinsic lack of healing, tissue engineering has been proposed as a solution for meniscus repair and replacement. This study describes an approach employing bioactive stimuli to enhance both extracellular matrix content and organization of neomenisci toward augmenting their mechanical properties. Self-assembled fibrocartilages were treated with TGF-β1, chondroitinase ABC, and lysyl oxidase-like 2 (collectively termed TCL) in addition to lysophosphatidic acid (LPA). TCL+LPA treatment synergistically improved circumferential tensile stiffness and strength, significantly enhanced collagen and pyridinoline crosslink content per dry weight, and achieved tensile anisotropy (circumferential/radial) values of neomenisci close to 4. This study utilizes a combination of bioactive stimuli for use in tissue engineering studies, providing a promising path toward deploying these neomenisci as functional repair and replacement tissues.
Keywords: Knee meniscus, tissue engineering, fibrocartilage, extracellular matrix, anisotropy, biomechanics
Graphical Abstract
1. Introduction
Fibrocartilage is found in the knee meniscus, temporomandibular joint disc, pubic symphysis, annulus fibrosus of intervertebral discs, tendons, and ligaments. Damage to these tissues is common and can result in persistent pain while impeding daily activity. The knee meniscus is a wedge-shaped, semi-circular fibrocartilaginous tissue that is situated between the distal femur and tibial plateau; the meniscus protects articular cartilage via load distribution. Injury to the meniscus is responsible for approximately 1 million surgeries annually in the U.S. and Europe, making it the most common orthopedic surgical procedure [1].
Meniscal function arises from collagen content, crosslinks, and organization. Under compressive load, menisci function by using their wedge shape to develop tension, which is resisted by circumferentially aligned collagen. The surface of the meniscus tissue exhibits random collagen alignment, while its middle depths show circumferentially aligned collagen fibers supported by tie fibers in the radial direction [2,3]. Pyridinoline, a trivalent crosslink of collagen fibrils, contributes to mechanical properties of the knee meniscus [4] and has been shown to strongly correlate with tensile properties of various collagenous tissues [5,6]. Collagen content, crosslinking, and organization are, thus, critical to the tensile properties of menisci and their function.
The knee meniscus exhibits a gradient of healing capacity which decreases from its outer, fibrous portion toward the inner portion, which is more akin to hyaline articular cartilage and lacks vascularization [7]. Injuries may lead to surgery such as meniscectomy, i.e., tissue resection, especially if the injury is in the inner portion of the tissue, which can temporarily alleviate pain symptoms but is virtually guaranteed to result in osteoarthritis of the knee joint [8]. This lack of intrinsic healing properties makes the knee meniscus a prime candidate for repair or replacement via tissue engineering methods.
Both scaffold and scaffold-free strategies for tissue engineering the knee meniscus have been investigated. A scaffold-free method known as the self-assembling process has emerged for forming neotissue rings [9–14] while resolving issues that may result from scaffold use such as stress shielding and scaffold degradation byproducts [15]. The self-assembling process depends on both integrin-matrix binding and cell-to-cell communication via cadherin binding [16], resulting in a sequence of extracellular matrix (ECM) accumulation that is reminiscent to the sequence seen for native cartilage [17]. The neotissue rings are then cut into two meniscus-shaped constructs (termed “neomenisci”) that resemble the morphology of the native leporine knee meniscus. Mechanical properties are directly related to ECM content and organization and, as such, our group has utilized bioactive agents during culture to enhance these biochemical features and create self-assembled fibrocartilage with compressive properties that reach levels seen in native tissue [18]. However, tensile mechanical properties of neomenisci are still lacking, especially in the circumferential direction. Tensile properties still require improvement before these engineered tissues can be deployed as functional repair or replacement alternatives; as such, this study aims to improve tensile properties of engineered neomenisci through the addition of bioactive stimuli during in vitro culture.
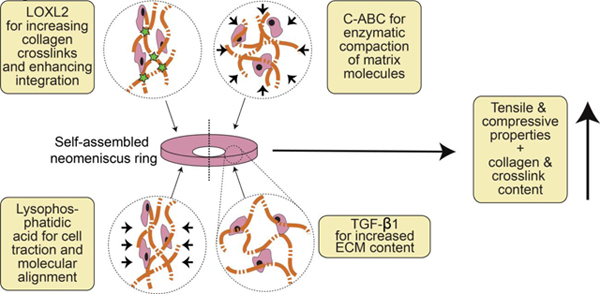
Bioactive stimuli have frequently been used to augment ECM content, leading to enhanced mechanical properties. Growth factors such as TGF-β1 [14,18–20], TGF-β3 [21–23], CTGF [23], PDGF [24–26], bFGF [24,27,28], IGF-1 [24,25,27–30], and EGF [25,26] have shown efficacy toward enhancing tissue engineered fibrocartilage formation. In addition, our group has previously used anabolic growth factor TGF-β1 to enhance ECM production, glycosaminoglycan (GAG)-cleaving enzyme chondroitinase ABC (C-ABC) to temporarily remove GAG, and collagen crosslinking enzyme lysyl oxidase-like 2 (LOXL2) to increase collagen crosslinking. These bioactive agents have been used alone or in combination to enhance mechanical properties of cartilaginous and fibrocartilaginous tissues [13,18,19,31,32]. A treatment based on this cocktail of bioactive agents (collectively termed TCL) results in matrix augmentation contributing to more robust engineered tissues.
Less frequent than the use of bioactive stimuli are strategies to induce or to accentuate ECM organization of engineered neomenisci, resulting in anisotropy. An example is lysophosphatidic acid (LPA), a phospholipid mediator that induces contractions in fibroblasts [33], which has been used to induce contraction of collagen matrices seeded with fibroblasts or myofibroblasts [34–36]. In addition to inducing contraction in fibroblasts, LPA has also been shown to be an anti-apoptotic and pro-survival factor in both fibroblasts [37] and chondrocytes [38]. Because we have observed that meniscal fibrochondrocytes seeded in a ring-shaped mold create circumferential hoop stress [9], we have used LPA in the past to enhance this hoop stress and, thus, circumferential ECM organization, by accentuating cellular traction forces that give rise to hoop stresses [10]. Thus, while self-assembled neomenisci already exhibit anisotropic tensile properties, indicating a certain degree of collagen alignment, cytoskeletal contraction induced by LPA enhances this anisotropy by increasing collagen organization.
Because TCL and LPA do not have overlapping functions, their use in combination may act synergistically toward bolstering tissue properties. Matrix augmentation has been observed in self-assembled cartilaginous tissues when applying TCL treatment, while LPA has induced directional remodeling in self-assembled neomenisci to enhance organization and anisotropy. The objective of this study was to increase the tensile properties of self-assembled neomeniscus constructs in an anisotropic fashion by combining TCL with LPA to increase and to remodel ECM content. Neomenisci were cultured for 5 weeks and treated with TCL and LPA alone or in combination. Unstimulated neomenisci served as controls. It was hypothesized that 1) treatment of constructs with TCL and LPA would lead to a synergistic increase in tensile properties, 2) an increase in tensile properties would be accompanied by an increase in the amount of pyridinoline collagen crosslinks, and 3) TCL+LPA treatment would produce neomenisci with anisotropic tensile mechanical properties.
2. Materials and methods
2.1. Chondrocyte and meniscus cell isolation
Primary bovine articular chondrocytes and meniscus cells were isolated from juvenile bovine knee joints (Research 87). Articular cartilage resected from the distal femur was minced and digested in 0.2% collagenase type II (Worthington) for 18 hours at 37°C. Meniscus tissue was isolated from the knee joint and digested as previously described [10]. Briefly, the outer rim was removed, and remaining meniscus tissue was minced and digested in 0.25% pronase (Sigma-Aldrich) for 2 hours followed by 0.2% collagenase type II for 18 hours. After isolation, cells were frozen at −80°C for 24 hours in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum and 10% dimethyl sulfoxide medium at 30 million cells per milliliter and stored in liquid nitrogen until seeding.
2.2. Self-assembly and culture of constructs
Non-adherent agarose wells in the shape of the native leporine meniscus were prepared as previously described [10–12]. Wells were saturated for five days prior to seeding with serum-free chondrogenic medium and throughout culture. Chondrogenic medium consists of: DMEM with GlutaMAX (Gibco, Grand Island, NY, USA); 1% nonessential amino acids (Gibco); 1% insulin, human transferrin, and selenous acid (ITS+; BD Biosciences, San Jose, CA, USA); 1% penicillin-streptomycin-fungizone (Lonza BioWhittaker, Walkersville, MD, USA); 3.5 nM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA); 50 μg/mL ascorbate-2-phosphate (Sigma-Aldrich); 100 μg/mL sodium pyruvate (Sigma-Aldrich) and 40 μg/mL L-proline (Sigma-Aldrich). A 1:1 co-culture of primary articular chondrocytes and meniscal cells was seeded at a density of 20 million total cells per 180 μL as previously described [39]. An additional 2 mL of media was added 4 hours after construct seeding, and all medium was changed daily. Constructs were removed from the agarose wells after 7 days and kept in culture, during which medium was changed every other day (5 mL).
2.3. Treatments
Neomenisci treatment groups were as follows: 1) unstimulated controls, 2) LPA only, 3) TCL only, 4) TCL+LPA. TCL groups were treated with the combination of 1) TGF-β1 continuously throughout culture at 10 ng/mL, 2) a one-time C-ABC treatment, added to medium with a 0.05 M sodium acetate activator, at day 7 of culture at 2 U/mL for a duration of 4 hours, and 3) LOXL2 applied continuously from days 7–21 (weeks 2–3) at 0.15 ng/mL as previously described [19,32,40]. Neomenisci that were treated with LPA (Enzo Life Sciences) were stimulated during days 21–28 (week 4) at a final concentration of 10 μM as previously described [10]. All constructs were cultured for 5 weeks at 37°C and 5% CO2.
2.4. Tissue gross morphology, histology, and macroscopic characterization
At day 35, neomenisci were removed from culture, photographed, and measured using ImageJ (NIH). Wet weights were measured before resecting pieces for mechanical testing, biochemical analysis, and histology. For histology, construct samples were fixed in 10% neutral buffered formalin then embedded in paraffin and sectioned at 5 μm. Safranin O/Fast Green, Picrosirius Red, and hematoxylin and eosin (H&E) stains were conducted to visualize GAG, collagen, and cell distributions, respectively. Collagen fiber alignment of samples was examined using second-harmonic generation (SHG) imaging microscopy (Zeiss, Jena, Germany). The acquisition of SHG images was conducted using an excitation wavelength of 800 nm and a 20x objective.
2.5. Tensile and compressive testing
Tensile properties were assessed using uniaxial, strain-to-failure testing. Using custom-made jigs, samples were cut to a depth of 1.0 mm and gauge length of 0.95 mm and 0.40 mm for circumferential and radial tensile samples, respectively. Samples were then photographed, cut into dog-bone shapes, measured with ImageJ, and adhered to paper strips with cyanoacrylate glue at their ends to ensure that a glue bridge would not form across the gauge length. Samples were then clamped within a uniaxial testing machine (Instron model 5565) and subjected to a 1% s−1 strain rate until failure. Young’s modulus (EY) was calculated from the linear portion of the stress-strain curve, and ultimate tensile strength (UTS) was calculated from the maximum stress (Supplementary Figure 1).
Compressive properties were assessed using unconfined stress-relaxation testing, with a non-porous, stainless steel platen larger in diameter than the tissue sample. Punches measuring 2–3 mm in diameter were resected from neomenisci. Sample thickness was determined via a custom program on the testing machine (Instron model 5565). Samples were subjected to stress relaxation via a 20% step-strain while submerged in a PBS bath. Viscoelastic properties such as instantaneous modulus (EI), relaxation modulus (ER), and coefficient of viscosity (μ) were calculated by fitting data curves to a standard Kelvin solid model [41].
2.6. Analysis of tissue biochemical content
Biochemistry samples were weighed wet, then frozen and lyophilized to acquire dry weights. DNA content was calculated with the use of PicoGreen dsDNA reagent (Invitrogen), assuming a conversion factor of 7.7 pg DNA/cell. Collagen content was measured with the use of a Sircol standard (Biocolor) and a Chloramine-T colorimetric hydroxyproline assay [42]. GAG content was quantified using the Blyscan assay kit (Invitrogen). All quantification measurements for DNA, GAG, and collagen content were performed with a GENios spectrophotometer/spectrofluorometer (TECAN).
Quantification of pyridinoline crosslink content was performed via a liquid chromatography mass spectrometry (LC-MS) assay [43]. Lyophilized samples were hydrolyzed in 6N HCl at 105°C for 18 hours, then acid was evaporated by SpeedVac (Labconco). Dried hydrolysates were resuspended in 25% (v/v) acetonitrile and 0.1% (v/v) formic acid in water, centrifuged at 15,000g for 5 min, and the supernatant was transferred to a LCMS autosampler vial. Liquid chromatography was carried out on a Cogent Diagmond Hydride HPLC Column (2.1 mm × 150 mm, particle size 2.2 μm, pore size 120 Å, MicroSolv). The elution gradient used 0.1% (v/v) formic acid in water as solvent A, and 100% acetonitrile as solvent B. The 5-minute elution gradient ran at 300 μL/min as follows: 0 min 25% B, 2 min 25% B, 2.2 min 5% B, 3 min 25% B. Mass spectrometry was performed on a Quadrupole Mass Detector (ACQUITY QDa, Waters) in ESI+ MS scan mode. The quadrupole range was set to 150 – 450 m/z with cone voltage 12.5 V. MassLynx software version 4.1 with TargetLynx was used to quantify pyridinoline in 10 μL injections of neomeniscus hydrolysate by integrating the extracted ion chromatogram of double-charged pyridinoline (m/z=215.1) into a standard curve of serially diluted pyridinoline standard (BOC Sciences). The standard curve contained 6 standards and was linear with R2 > 0.999 on a range of 1,000 to 4.115 pg/μL pyridinoline.
2.7. Statistical analysis
For each biomechanical and biochemical test, n=6–7 samples were used. Results were analyzed with single-factor analysis of variance (ANOVA) followed by a Tukey’s HSD post hoc test when merited (p<0.05). TCL and LPA effect on anisotropy was analyzed with two-factor ANOVA followed by a Tukey’s HSD post hoc test when merited (p<0.05). All data are presented as means ± standard deviations. For all figures, statistical significance is indicated by groups not sharing the same letters.
3. Results
3.1. Gross morphology, histology, and immunohistochemistry
Neomenisci resembled native meniscus fibrocartilage [44,45] in terms of gross appearance (Figure 1) [45–47]. The characteristic wedge-shaped profile was maintained after 5 weeks of culture (Supplementary Figure 2). Morphologically, TCL and TCL+LPA treated groups were significantly smaller (p<0.0005) than controls and constructs treated with LPA alone in terms of outer major and minor diameters, inner minor diameter, and height (Table 1). TCL+LPA was significantly smaller in terms of inner major diameter when compared to all other groups (p=0.03). Both outer (p=0.04) and inner (p=0.007) aspect ratios (defined as major diameter/minor diameter) were also significantly higher in TCL+LPA treated neomenisci when compared to controls. Wet weights were significantly different among groups, with TCL and TCL+LPA groups both weighing significantly less (p<0.0001) than controls and LPA treated groups (Table 1). Hydration percentages were also significantly lower (p<0.0001) in TCL and TCL+LPA treated groups when compared to control and LPA-only groups (Table 1). Dry weights for TCL and TCL+LPA groups, in turn, were significantly lower than control and LPA-treated constructs.
Figure 1: Gross morphology and histological staining of self-assembled neomenisci.
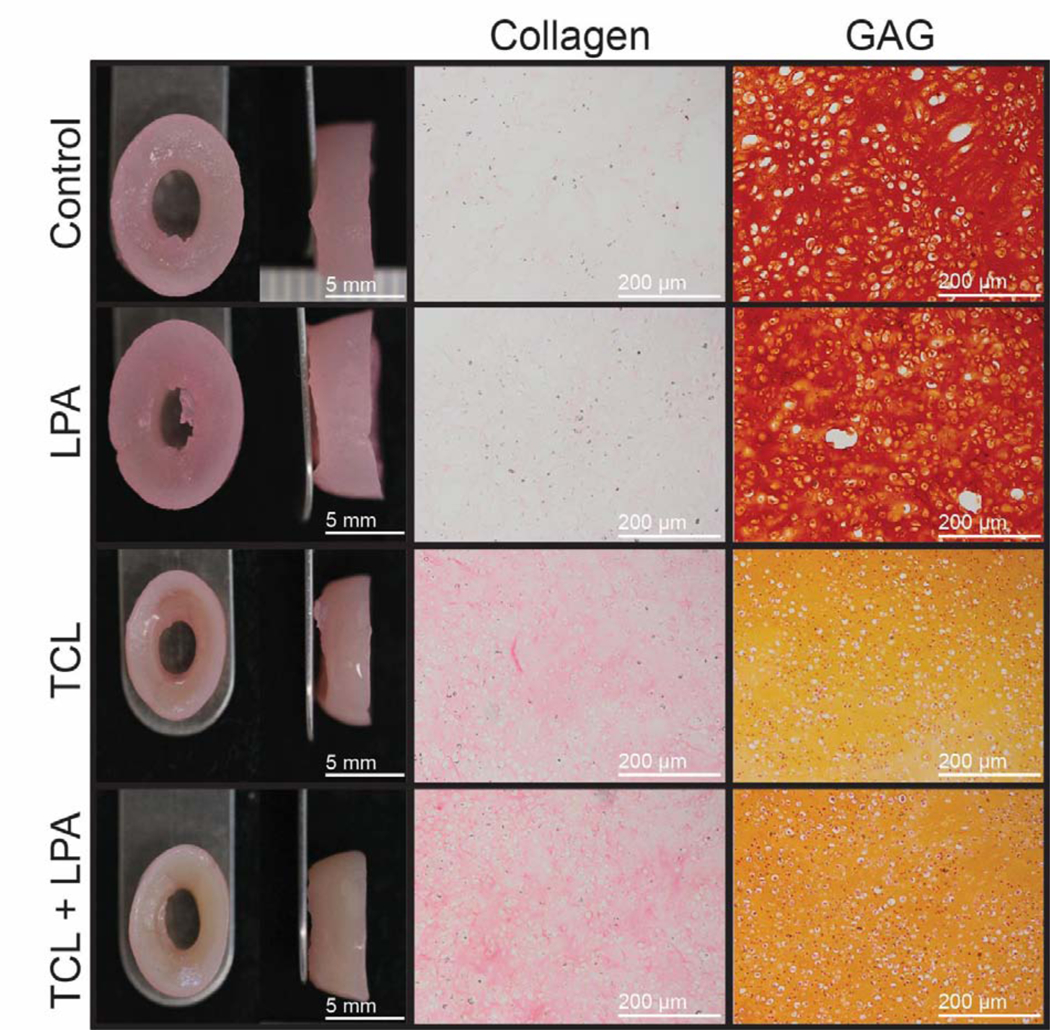
Tissue engineered constructs retained the characteristic wedge-shape of the native knee meniscus during and after culture. Slight contraction occurred in TCL+LPA groups when compared to controls. Collagen staining of TCL+LPA treated constructs appeared more intense than that of control constructs, while GAG staining in the TCL and TCL+LPA groups was less intense than controls.
Table 1: Morphological properties of neomenisci.
Values marked with different letters within each category are significantly different (p<0.05), n=7 per group.
| Group | Wet Weight (mg) | Dry Weight (mg) | Hydration (%) | Cells/construct (millions) | Outer Major Diameter (mm) | Outer Minor Diameter (mm) | Inner Major Diameter (mm) | Inner Minor Diameter (mm) | Outer Aspect Ratio | Inner Aspect Ratio | Height (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 207 ± 8C | 25 ± 1B | 88 ± 0B | 13 ± 4 | 11 ± 0B | 8 ± 0B | 4 ± 0B | 3 ± 0B | 1 ± 0B | 1 ± 0B | 4 ± 0B |
| LPA | 224 ± 10B | 25 ± 2B | 89 ± 1B | 15 ± 4 | 11 ± 0B | 8 ± 0B | 4 ± 0B | 2 ± 0B | 1 ± 0B | 2 ± 0AB | 4 ± 0B |
| TCL | 89 ± 5A | 15 ± 1A | 83 ± 1A | 15 ± 1 | 9 ± 1A | 6 ± 0A | 3 ± 0B | 2 ± 0A | 1 ± 0AB | 2 ± 0AB | 3 ± 0A |
| TCL+LPA | 91 ± 5A | 15 ± 1A | 83 ± 1A | 16 ± 1 | 9 ± 0A | 6 ± 1A | 3 ± 0A | 2 ± 0A | 2 ± 0A | 2 ± 0A | 3 ± 0A |
3.2. Tissue biomechanics
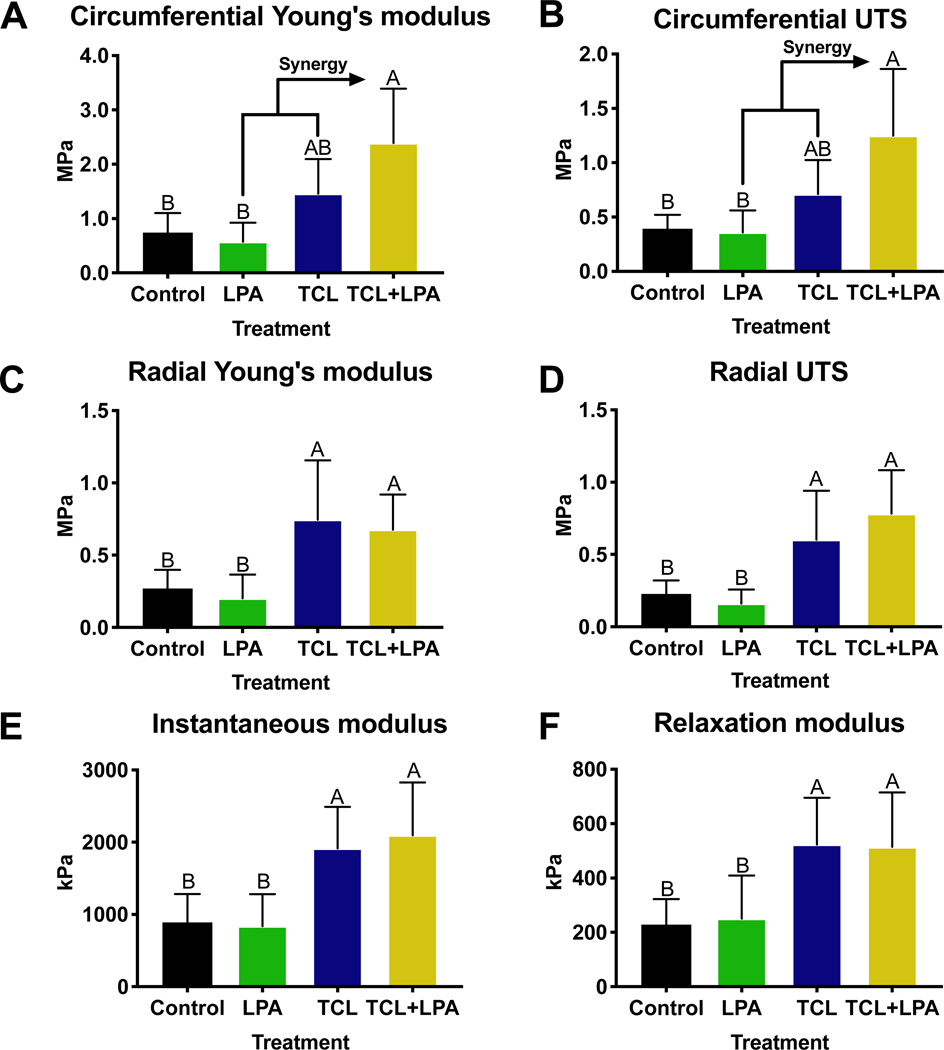
Biomechanical data revealed a significant difference among control and treated constructs, especially those that were treated with TCL+LPA (Figure 2). Application of TCL+LPA resulted in increased tensile properties over both unstimulated control and LPA treatment groups. Circumferential Young’s modulus and ultimate tensile strength (UTS) were highest (p<0.0001) in TCL+LPA treated groups (Figure 2A, 2B). TCL+LPA treated constructs increased circumferential Young’s modulus and UTS to 221% and 218% higher than control values, respectively. In addition, TCL+LPA treated constructs displayed increases to the radial Young’s modulus (p=0.03) and UTS (p=0.001) of 152% and 239% over controls, respectively (Figure 2C, 2D).
Figure 2: Mechanical properties of neomenisci.
TCL+LPA synergistically increased circumferential tensile properties over controls. TCL+LPA and TCL treatments both increased radial tensile properties and compressive properties over controls and LPA only groups. All data are presented as means ± standard deviations. For all figures, statistical significance is indicated by bars not sharing the same letters.
In addition to increases in tensile properties, compressive properties also were significantly higher in constructs treated with TCL+LPA when compared to non-treated controls. When quantifying compressive properties, TCL+LPA stimulated constructs yielded increases over controls in the instantaneous modulus (p=0.003) and relaxation modulus (p=0.02), by 135% and 125%, respectively (Figure 2E, 2F). TCL treatment also significantly increased (p=0.01) compressive properties over controls. No significant differences in coefficient of viscosity were seen among treatment groups (Supplementary Figure 3).
3.3. Tissue biochemistry
Biochemical treatment led to significant increases in collagen content and pyridinoline crosslink content (Figure 3A, 3C). TCL+LPA treatment enhanced (p<0.0001) collagen and pyridinoline content per wet weight by 125% and 185% over control values, respectively (Supplementary Figure 4A, 4B). There were no significant differences in GAG content per wet weight among treatment groups (Supplementary Figure 4C). Collagen and pyridinoline content normalized by dry weight also showed increases (p<0.003) over controls when treating with TCL+LPA, by 61% and 81% of control values, respectively. However, there were no significant differences among groups in terms of pyridinoline normalized to collagen content (Figure 3D). GAG content normalized to dry weight was significantly lower (p<0.0001) for TCL+LPA treated constructs (by 39% of controls). TCL treatment also significantly decreased (p<0.0001) GAG content per dry weight when compared to control groups (Figure 3B). There were no significant differences in DNA content among groups (Table 1).
Figure 3: Biochemical properties of self-assembled neomenisci.
TCL+LPA treatment enhanced collagen content over all other groups. GAG content was lowered closer to native tissue values in TCL+LPA and TCL groups. Pyridinoline crosslink content in TCL+LPA treated constructs increased over controls, while there were no differences among groups in pyridinoline crosslink content normalized to collagen content.
3.4. Tissue organization
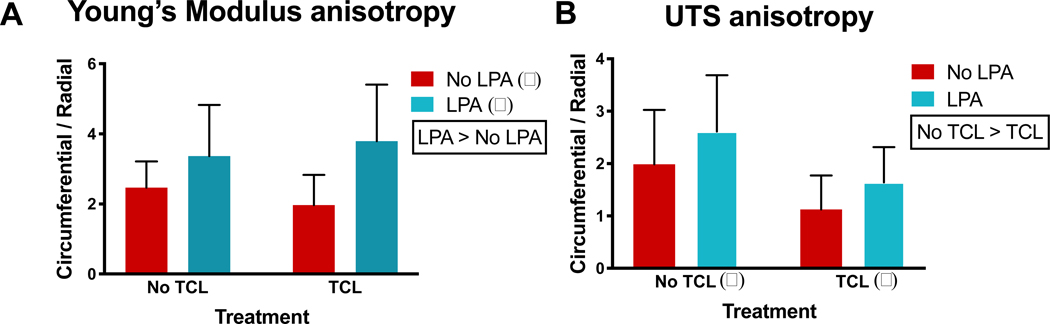
Two-way ANOVA tests were conducted to examine the effect of TCL and LPA treatments on anisotropy of tensile properties, namely, tensile Young’s modulus and UTS (Figure 4A, 4B). These tests showed that LPA treatment significantly increased (p=0.02) anisotropy, as indicated by differences of the Young’s modulus values in the circumferential versus the radial directions, while TCL significantly decreased (p=0.02) UTS anisotropy. Anisotropy ratios of tensile properties (circumferential/radial values) reached as high as 4-fold with TCL+LPA treatment. In addition, SHG signals in LPA (Supplementary Figure 5B) and TCL+LPA (Supplementary Figure 5D) treated groups appeared more intense than those of control and TCL treatment groups.
Figure 4: TCL+LPA treatment enhances anisotropy.
Two-way analysis of variance shows LPA treatment significantly contributed to increases in Young’s modulus anisotropy, while TCL significantly contributed to decreases in UTS anisotropy. Tukey’s post hoc showed that none of the groups differed from one another (i.e., there were no statistical differences among the bars). Control groups are represented by the “No TCL” and “No LPA” bar. The alpha and beta represent statistical significance between treatment levels; treatments without these symbols indicate no significant differences between levels.
4. Discussion
Building upon prior work that showed increases in mechanical properties of self-assembled fibrocartilage treated with TCL [18] and directionality imparted by LPA in neomenisci [10], this study sought to promote matrix augmentation and directional remodeling in order to enhance tensile properties. Experimental results supported the three hypotheses underlying this study, namely: 1) TCL+LPA treatment would enhance the tensile properties of self-assembling neomenisci, 2) these increases in tensile properties would be accompanied by an increase in the amount of pyridinoline collagen crosslinks, and 3) TCL+LPA treatment would produce neomenisci with anisotropic tensile mechanical properties. To the best of our knowledge, this is the first study to use a treatment that both augments matrix content of engineered neomenisci and utilizes a soluble factor to enhance matrix organization and anisotropy. This study demonstrates the combination of matrix augmentation and directional remodeling as a beneficial strategy in tissue engineering of the knee meniscus.
The knee meniscus functions by developing tension when under compressive load, highlighting the importance of attaining tensile properties akin to native tissue in engineered neomenisci. It was found that TCL+LPA treatment resulted in synergistic enhancements to tensile properties of engineered neomenisci, increasing circumferential stiffness and strength by more than 2-fold. In addition, radial stiffness and strength were also increased by more than 1.5- and 2-fold, respectively. Values for tensile strength and stiffness in the circumferential direction were approximately 4-fold higher when compared to values from neomenisci in a study by our group that were treated with only LPA [10]. Also, tissue circumferential tensile properties were on par with those of self-assembled neomenisci that used both biochemical and mechanical stimulation to enhance matrix content organization [13]. However, circumferential tensile modulus values in neomenisci treated with TCL+LPA still fall short of native tissue values (100–300 MPa) [48], denoting the importance of research that aims to enhance this mechanical property that is crucial to meniscus function. In the radial direction, tensile strength and stiffness were approximately one-tenth of values derived from native tissue (10–30 MPa) [48,49], respectively. Increasing tensile properties of engineered neomenisci is a critical step toward deploying them as functional tissues in vivo; ultimately, TCL+LPA treatment was effective in elevating tissue tensile properties, which results from its positive effects on matrix content and organization.
In addition to TCL+LPA treatment having beneficial increases to tensile properties, an accompanying increase in collagen content was also seen over all other groups (Figure 3). The significant difference in collagen content between TCL+LPA and TCL groups likely stems from the addition of LPA, which has been shown to increase collagen deposition in engineered tissue [50]. Overall, collagen per dry weight in TCL+LPA groups reached values approximately 2-fold greater than those reported for self-assembled neomenisci treated with only LPA [10]. After being able to increase collagen content, it would be important to increase the degree of crosslinking and fiber organization to improve tensile properties. The TCL+LPA treatment used by this study provides a firm foundation for crosslinks by increasing collagen content closer to that of the native meniscus, in agreement with increases in tensile properties. The native meniscus contains mostly collagen types I and II, with some minor collagens such as types III, IV, VI, and XVIII [44]. While the hydroxyproline assay only measures overall collagen, without discriminating individual collagen types, self-assembled neomenisci have been shown to contain both collagens I and II via IHC [9]. Other self-assembled fibrocartilages have also been shown to contain different ratios of collagen types I and II [51], and future work on self-assembled neomenisci may include methods to better determine the similarities and differences between different collagen types of native menisci and self-assembled neomenisci.
A significant increase to pyridinoline crosslink content over controls was seen in TCL+LPA treated constructs, which follows previous results seen when applying LOXL2 in self-assembled fibrocartilage [18]. This significant increase in crosslinking was observed in conjunction with significant increases in collagen content, indicating that collagen maturation and crosslinking are occurring at the same rate. The TCL+LPA group contained 1.42 ng/μg pyridinoline per dry weight on average, which is on par with historical values for pyridinoline content of native fibrocartilages. For example, calculation using the pyridinoline per hydroxyproline and hydroxyproline per dry weight measurements of prior work [52] shows that human menisci have 1.16 ng/μg pyridinoline per dry weight. Similarly, human intervertebral discs have been reported to contain 1.10 ng/μg pyridinoline per dry weight [53]. It is worth noting that these prior studies used an HPLC fluorescence detection assay for pyridinoline quantification as opposed to the LC-MS technique used in this paper. Because LC-MS methods have repeatedly been shown to be more precise and accurate than HPLC fluorescence detection methods [54–56], future characterization studies on native fibrocartilage crosslinks using LC-MS would yield more comparable data to those collected by our study. Overall, TCL+LPA treated neomenisci exhibited crosslink content that appears to meet or exceed values reported in the literature, though characterization of native tissue via LC-MS methods is warranted.
The anisotropic organization of ECM within the meniscus, with circumferential tensile stiffness being approximately 10-fold that of the radial direction [49], is crucial to the tissue’s function. In this study, we showed that LPA was significant toward enhancing anisotropy, while TCL’s robust matrix augmentation effect brought anisotropy ratios (circumferential/radial) closer to 1 (Figure 4). In addition, the SHG signal of TCL+LPA treated constructs appeared more intense than controls, indicating a higher degree of circumferentially aligned collagen fibers (Supplementary Figure 5). While this study utilized soluble bioactive agents to induce or enhance anisotropy, other methods to induce or enhance anisotropy within engineered menisci include mechanical stimuli via a bioreactor [13], scaffolds [57,58], or their use in combination [59]. While these methods have proven to be effective toward inducing anisotropy, LPA can be seen as a chemical analogue to these techniques that simplifies the tissue engineering process. For example, bioreactor manufacturing can become expensive and time-consuming; in addition, the use of scaffolds can result in harmful effects such as degradation byproducts or stress shielding of cells, further highlighting the benefits of using chemical analogues to achieve proper matrix organization in engineered tissues. Despite financial and temporal considerations, bioreactors that apply mechanical stimulation may further enhance matrix organization, and have previously led to increased tensile properties of self-assembled cartilage [32]. Without proper collagen fiber alignment, augmenting ECM content would likely not improve mechanical properties substantially, rendering the tissue unable to behave as it would in its native environment. Tissue engineering of the meniscus should focus on enhancing both mechanical and anisotropic properties to levels of native tissue. Thus, future work may examine the use of TCL+LPA treatment, in conjunction with additional bioactive factors or mechanical stimuli to increase ECM alignment and, consequently, tensile properties.
Interestingly, while the combination of TCL+LPA induced improvements of neomenisci tensile properties, the LPA only group did not differ mechanically or morphologically from the control group. This contradicts previous results shown when using LPA in the tissue engineering of self-assembled neomenisci [10]. No increases in tensile properties over controls in the LPA treatment group may have resulted due to the higher amounts of GAG in non-TCL treated tissues, which may have induced more swelling pressure and caused resistance to contraction by the collagen network. The use of C-ABC, which temporarily depletes GAG within the ECM and allows for more dense organization of collagen fibers [19], could have relieved this swelling pressure and allowed cell traction forces to align collagen fibers. In addition, this depletion of GAG due to C-ABC treatment likely contributed to lower wet weights and hydration percentages in TCL-treated groups, as negatively charged GAG attract water into the ECM [14]. Lower dry weights in groups treated with TCL may likely also stem from decreased GAG content as a result of C-ABC treatment. Also, despite morphological differences, a significant difference in circumferential tensile properties was not seen between TCL+LPA and TCL groups (p= 0.06), although neomenisci treated with TCL+LPA trended higher. This trend may result from a significantly higher amount of collagen in the TCL+LPA group over TCL treated neomenisci, in addition to morphological differences that imply a degree of difference in organization.
Due to the prevalence and economic impact of meniscal injuries, creating engineered meniscal tissue that recapitulates native tissue properties is crucial for its successful translation from the benchtop to the clinic. Enhancements to mechanical, biochemical, and anisotropic tensile properties of neomenisci, induced by TCL+LPA treatment, narrow the gap between native and engineered tissue properties and provide a promising path toward translation. While this study utilized bioactive agents to induce anisotropic tensile properties in neomenisci, other groups have utilized techniques such as 3D-printed scaffolds to replicate this property with the consideration of translation as the end goal [59]. In addition, as the native knee meniscus is zonally inhomogeneous, anisotropic engineered neomenisci with zonal variations have been created using the self-assembling process [60]. Since an entire neomeniscus might not be required for a repair procedure, a portion may be cut out to interface with native tissue to achieve a healing response. Investigation for this sort of engineered-native tissue interaction would require visualization of cell migration, likely through fluorescent tagging of chondrocytes and meniscal cells used to engineer self-assembled neomenisci. A previous study in which our group implanted tissue engineered constructs into fibrocartilage of the temporomandibular joint (TMJ) disc showed host cells infiltrating the implant [61]; due to the fibrocartilaginous nature and similarities between the knee meniscus and TMJ discs, we expect that a similar in vivo response may be seen when implanting neomenisci. Further in vivo work showing the safety and efficacy of engineered neomenisci toward repairing or replacing injured meniscal tissue, especially in large animal models, is required to navigate the Food and Drug Administration paradigm [62] and translate these tissues to the clinic.
5. Conclusions
This work demonstrates that treatment with TCL+LPA can augment and align the mechanical properties of engineered fibrocartilaginous tissues. Higher collagen and pyridinoline crosslink content accompanied a synergistic increase in tensile properties, and anisotropic mechanical properties were observed in treated tissues. This work highlights the use of a combination of bioactive stimuli to achieve synergistic improvements in properties of engineered knee meniscus tissue. Future studies combining TCL+LPA with mechanical stimuli or additional bioactive agents to further enhance tensile properties of neomenisci are warranted to ensure functionality of these tissues in vivo.
Supplementary Material
Statement of Significance.
This study utilizes a scaffold-free approach, which strays from the tissue engineering paradigm of using scaffolds with cells and bioactive factors to engineer neotissue. While self-assembled neomenisci have attained compressive properties akin to native tissue, tensile properties still require improvement before being able to deploy engineered neomenisci as functional tissue repair or replacement options. In order to augment tensile properties, this study utilized bioactive factors known to augment matrix content in combination with a soluble factor that enhances matrix organization and anisotropy via cell traction forces. Using a bioactive factor to enhance matrix organization mitigates the need for bioreactors used to apply mechanical stimuli or scaffolds to induce proper fiber alignment.
Acknowledgement
The authors would like to acknowledge support from the following funding sources: National Institutes of Health R01AR071457, HHMI Gilliam Fellowship (for EGL), National Science Foundation GRFP (for EGL), and University of California, Irvine Eugene Cota Robles Fellowship (for EGL)
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erik A. Gonzalez-Leon, Department of Biomedical Engineering, University of California, Irvine, Irvine, CA 92697
Benjamin J. Bielajew, Department of Biomedical Engineering, University of California, Irvine, Irvine, CA 92697
Jerry C. Hu, Department of Biomedical Engineering, University of California, Irvine, Irvine, CA 92697
Kyriacos A. Athanasiou, Department of Biomedical Engineering, University of California, Irvine, Irvine, CA 92697.
References
- [1].Salata MJ, Gibbs AE, Sekiya JK, A systematic review of clinical outcomes in patients undergoing meniscectomy, Am. J. Sports Med. 38 (2010) 1907–1916. 10.1177/0363546510370196. [DOI] [PubMed] [Google Scholar]
- [2].Petersen W, Tillmann B, Collagenous fibril texture of the human knee joint menisci, Anat. Embryol. (Berl). 197 (1998) 317–324. 10.1007/s004290050141. [DOI] [PubMed] [Google Scholar]
- [3].Andrews SHJ, Rattner JB, Abusara Z, Adesida A, Shrive NG, Ronsky JL, Tie-fibre structure and organization in the knee menisci, J. Anat. 224 (2014) 531–537. 10.1111/joa.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Eleswarapu SV, Responte DJ, Athanasiou KA, Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint, PLoS One. 6 (2011) e26178. 10.1371/journal.pone.0026178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Williamson AK, Chen AC, Masuda K, Thonar EJMA, Sah RL, Tensile mechanical properties of bovine articular cartilage: Variations with growth and relationships to collagen network components, J. Orthop. Res. 21 (2003) 872–880. 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- [6].Chan BP, Fu SC, Qin L, Rolf C, Chan KM, Pyridinoline in relation to ultimate stress of the patellar tendon during healing: An animal study, J. Orthop. Res. 16 (1998) 597–603. 10.1002/jor.1100160512. [DOI] [PubMed] [Google Scholar]
- [7].Arnoczky SP, Warren RF, Microvasculature of the human meniscus, Am. J. Sports Med. 10 (1982) 90–95. 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- [8].Rangger C, Kathrein A, Klestil T, Glötzer W, Partial meniscectomy and osteoarthritis. Implications for treatment of athletes., Sports Med. 23 (1997) 61–68. 10.2165/00007256-199723010-00006. [DOI] [PubMed] [Google Scholar]
- [9].Aufderheide AC, Athanasiou KA, Assessment of a Bovine Co-culture, Scaffold-Free Method for Growing Meniscus-Shaped Constructs, Tissue Eng. 13 (2007) 2195–2205. 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- [10].Hadidi P, Athanasiou KA, Enhancing the mechanical properties of engineered tissue through matrix remodeling via the signaling phospholipid lysophosphatidic acid, Biochem. Biophys. Res. Commun. 433 (2013) 133–138. 10.1016/j.bbrc.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hadidi P, Yeh TC, Hu JC, Athanasiou KA, Critical seeding density improves the properties and translatability of self-assembling anatomically shaped knee menisci, Acta Biomater. 11 (2015) 173–182. 10.1016/j.actbio.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hadidi P, Paschos NK, Huang BJ, Aryaei A, Hu JC, Athanasiou KA, Tendon and ligament as novel cell sources for engineering the knee meniscus, Osteoarthr. Cartil. 24 (2016) 2126–2134. 10.1016/j.joca.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huey DJ, Athanasiou KA, Tension-compression loading with chemical stimulation results in additive increases to functional properties of anatomic meniscal constructs, PLoS One. 6 (2011) 1–9. 10.1371/journal.pone.0027857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huey DJ, Athanasiou KA, Maturational growth of self-assembled, functional menisci as a result of TGF-β1 and enzymatic chondroitinase-ABC stimulation, Biomaterials. 32 (2011) 2052–2058. 10.1016/j.biomaterials.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu JC, Athanasiou KA, A Self-Assembling Process in Articular Cartilage Tissue Engineering, Tissue Eng. 12 (2006) 969–979. 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- [16].Lee JK, Hu JCY, Yamada S, Athanasiou KA, Initiation of Chondrocyte Self-Assembly Requires an Intact Cytoskeletal Network, Tissue Eng. Part A. 22 (2016) 318–325. 10.1089/ten.tea.2015.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA, Matrix development in self-assembly of articular cartilage, PLoS One. 3 (2008). 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Makris EA, MacBarb RF, Paschos NK, Hu JC, Athanasiou KA, Combined use of chondroitinase-ABC, TGF-β1, and collagen crosslinking agent lysyl oxidase to engineer functional neotissues for fibrocartilage repair, Biomaterials. 35 (2014) 6787–6796. 10.1016/j.biomaterials.2014.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].MacBarb RF, Makris EA, Hu JC, Athanasiou KA, A chondroitinase-ABC and TGF-β1 treatment regimen for enhancing the mechanical properties of tissue-engineered fibrocartilage, Acta Biomater. 9 (2013) 4626–4634. 10.1016/j.actbio.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gunja NJ, Uthamanthil RK, Athanasiou KA, Effects of TGF-β1 and hydrostatic pressure on meniscus cell-seeded scaffolds, Biomaterials. 30 (2009) 565–573. 10.1016/j.biomaterials.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Allen KD, Athanasiou KA, Scaffold and growth factor selection in temporomandibular joint disc engineering, J. Dent. Res. 87 (2008) 180–185. 10.1177/154405910808700205. [DOI] [PubMed] [Google Scholar]
- [22].Wang C-H, Wang S, Zhang B, Zhang X-Y, Tong X-J, Peng H-M, Han X-Z, Liu C, Layering Poly (lactic-co-glycolic acid)-based electrospun membranes and co-culture cell sheets for engineering temporomandibular joint disc, J. Biol. Regul. Homeost. Agents. 32 (2018) 55–61. http://www.ncbi.nlm.nih.gov/pubmed/29504365 (accessed April 23, 2018). [PubMed] [Google Scholar]
- [23].Legemate K, Tarafder S, Jun Y, Lee CH, Engineering Human TMJ Discs with Protein-Releasing 3D-Printed Scaffolds, J. Dent. Res. 95 (2016) 800–807. 10.1177/0022034516642404. [DOI] [PubMed] [Google Scholar]
- [24].Detamore MS, Athanasiou KA, Effects of growth factors on temporomandibular joint disc cells, Arch. Oral Biol. 49 (2004) 577–583. 10.1016/j.archoralbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- [25].Johns DE, Athanasiou KA, Growth factor effects on costal chondrocytes for tissue engineering fibrocartilage, Cell Tissue Res. 333 (2008) 439–447. 10.1007/s00441-008-0652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kasemkijwattana C, Menetrey J, Goto H, Niyibizi C, Fu FH, Huard J, The use of growth factors, gene therapy and tissue engineering to improve meniscal healing, Mater. Sci. Eng. C. 13 (2000) 19–28. 10.1016/S0928-4931(00)00172-7. [DOI] [Google Scholar]
- [27].Detamore MS, Athanasiou KA, Evaluation of three growth factors for TMJ disc tissue engineering, Ann. Biomed. Eng. 33 (2005) 383–390. 10.1007/s10439-005-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Almarza AJ, Athanasiou KA, Evaluation of three growth factors in combinations of two for temporomandibular joint disc tissue engineering, Arch. Oral Biol. 51 (2006) 215–221. 10.1016/j.archoralbio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- [29].Kalpakci KN, Kim EJ, Athanasiou KA, Assessment of growth factor treatment on fibrochondrocyte and chondrocyte co-cultures for TMJ fibrocartilage engineering, Acta Biomater. 7 (2011) 1710–1718. 10.1016/j.actbio.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bhargava MM, Attia ET, Murrell GAC, Dolan MM, Warren RF, Hannafin JA, The Effect of Cytokines on the Proliferation and Migration of Bovine Meniscal Cells, Am. J. Sports Med. 27 (1999) 636–643. 10.1177/03635465990270051601. [DOI] [PubMed] [Google Scholar]
- [31].Makris EA, MacBarb RF, Responte DJ, Hu JC, Athanasiou KA, A copper sulfate and hydroxylysine treatment regimen for enhancing collagen cross-linking and biomechanical properties in engineered neocartilage., FASEB J. 27 (2013) 2421–30. 10.1096/fj.12-224030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee JK, Huwe LW, Paschos N, Aryaei A, Gegg CA, Hu JC, Athanasiou KA, Tension stimulation drives tissue formation in scaffold-free systems, Nat. Mater. 16 (2017) 864–873. 10.1038/nmat4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grinnell F, Fibroblast biology in three-dimensional collagen matrices, Trends Cell Biol. 13 (2003) 264–269. 10.1016/S0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- [34].Abe M, Ho CH, Kamm KE, Grinnell F, Different Molecular Motors Mediate Platelet-derived Growth Factor and Lysophosphatidic Acid-stimulated Floating Collagen Matrix Contraction, J. Biol. Chem. 278 (2003) 47707–47712. 10.1074/jbc.M306228200. [DOI] [PubMed] [Google Scholar]
- [35].Abe M, Sogabe Y, Syuto T, Yokoyama Y, Ishikawa O, Evidence that PI3K, Rac, Rho, and Rho kinase are involved in basic fibroblast growth factor-stimulated fibroblast-collagen matrix contraction, J. Cell. Biochem. 102 (2007) 1290–1299. 10.1002/jcb.21359. [DOI] [PubMed] [Google Scholar]
- [36].Parizi M, Howard EW, Tomasek JJ, Regulation of LPA-promoted myofibroblast contraction: Role of Rho, myosin light chain kinase, and myosin light chain phosphatase, Exp. Cell Res. 254 (2000) 210–220. 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- [37].Tigyi G, Dyer DL, Miledi R, Lysophosphatidic acid possesses dual action in cell proliferation., Proc. Natl. Acad. Sci. 91 (1994) 1908–1912. 10.1073/pnas.91.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hurst-Kennedy J, Boyan BD, Schwartz Z, Lysophosphatidic acid signaling promotes proliferation, differentiation, and cell survival in rat growth plate chondrocytes, Biochim. Biophys. Acta - Mol. Cell Res. 1793 (2009) 836–846. 10.1016/j.bbamcr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- [39].Aufderheide AC, Athanasiou KA, Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs, Tissue Eng. 13 (2007) 2195–2205. 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- [40].Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA, Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking, Proc. Natl. Acad. Sci. 111 (2014) E4832–E4841. 10.1073/pnas.1414271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Allen KD, Athanasiou KA, Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression, J. Biomech. 39 (2006) 312–322. 10.1016/j.jbiomech.2004.11.012. [DOI] [PubMed] [Google Scholar]
- [42].Cissell DD, Link JM, Hu JC, Athanasiou KA, A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich’s Solution Accurately Measures Tissue Collagen Content, Tissue Eng. Part C Methods. 23 (2017) 243–250. 10.1089/ten.tec.2017.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Naffa R, Watanabe S, Zhang W, Maidment C, Singh P, Chamber P, Matyska MT, Pesek JJ, Rapid analysis of pyridinoline and deoxypyridinoline in biological samples by liquid chromatography with mass spectrometry and a silica hydride column, J. Sep. Sci. 42 (2019) 1482–1488. 10.1002/jssc.201801292. [DOI] [PubMed] [Google Scholar]
- [44].Makris EA, Hadidi P, Athanasiou KA, The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration, Biomaterials. 32 (2011) 7411–7431. 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, Lotz MK, D’Lima DD, Macroscopic And Histopathologic Analysis Of Human Knee Menisci In Aging And Osteoarthritis, Osteoarthr. Cartil. 19 (2011) 1132–1141. 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ribitsch I, Peham C, Ade N, Dürr J, Handschuh S, Schramel JP, Vogl C, Walles H, Egerbacher M, Jenner F, Structure-Function relationships of equine menisci, PLoS One. 13 (2018) 1–17. 10.1371/journal.pone.0194052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nakagawa K, Otsuki S, Murakami T, Okamoto Y, Okuno N, Wakama H, Sezaki S, Ikeda K, Okayoshi T, Neo M, Histological Analysis of the Wrapping Treatment for Meniscal Horizontal Tears in Rabbits, Cartilage. (2019). 10.1177/1947603519870838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fithian DC, Kelly MA, Mow VC, Material properties and structure-function relationships in the menisci., Clin. Orthop. Relat. Res. (1990) 19–31. http://www.ncbi.nlm.nih.gov/pubmed/2406069 (accessed November 15, 2018). [PubMed] [Google Scholar]
- [49].Tissakht M, Ahmed AM, Tensile stress-strain characteristics of the human meniscal material, J. Biomech. 28 (1995) 411–422. 10.1016/0021-9290(94)00081-E. [DOI] [PubMed] [Google Scholar]
- [50].Chabaud S, Marcoux TL, Deschênes-Rompré MP, Rousseau A, Morissette A, Bouhout S, Bernard G, Bolduc S, Lysophosphatidic acid enhances collagen deposition and matrix thickening in engineered tissue, J. Tissue Eng. Regen. Med. 9 (2015) E65–E75. 10.1002/term.1711. [DOI] [PubMed] [Google Scholar]
- [51].Murphy MK, Masters TE, Hu JC, Athanasiou KA, Engineering a fibrocartilage spectrum through modulation of aggregate redifferentiation, Cell Transplant. 24 (2015) 235–245. 10.3727/096368913X676204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Takahashi M, Suzuki M, Kushida K, Hoshino H, Inoue T, The effect of aging and osteoarthritis on the mature and senescent cross-links of collagen in human meniscus, Arthrosc. - J. Arthrosc. Relat. Surg. 14 (1998) 366–372. 10.1016/S0749-8063(98)70003-9. [DOI] [PubMed] [Google Scholar]
- [53].Tan CI, Neil Kent G, Randall AG, Edmondston SJ, Singer KP, Age-Related Changes in Collagen, Pyridinoline, and Deoxypyridinoline in Normal Human Thoracic Intervertebral Discs, Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 58 (2003) B387–B393. 10.1093/gerona/58.5.b387. [DOI] [PubMed] [Google Scholar]
- [54].da C.C. Bandeira RD, Uekane TM, da Cunha CP, Rodrigues JM, de la Cruz MHC, de Oliveira Godoy RL, de L. Fioravante A, Comparison of high performance liquid chromatography with fluorescence detector and with tandem mass spectrometry methods for detection and quantification of ochratoxin A in green and roasted coffee beans, Brazilian Arch. Biol. Technol. 56 (2013) 911–920. 10.1590/S1516-89132013000600004. [DOI] [Google Scholar]
- [55].Wang H, Walaszczyk EJ, Li K, Chung-Davidson YW, Li W, High-performance liquid chromatography with fluorescence detection and ultra-performance liquid chromatography with electrospray tandem mass spectrometry method for the determination of indoleamine neurotransmitters and their metabolites in sea lamprey pl, Anal. Chim. Acta. 721 (2012) 147–153. 10.1016/j.aca.2012.01.025. [DOI] [PubMed] [Google Scholar]
- [56].Milićević D, Jurić V, Stefanović S, Baltić T, Janković S, Evaluation and validation of two chromatographic methods (HPLC-Fluorescence and LC-MS/MS) for the determination and confirmation of ochratoxin A in pig tissues, Arch. Environ. Contam. Toxicol. 58 (2010) 1074–1081. 10.1007/s00244-009-9436-2. [DOI] [PubMed] [Google Scholar]
- [57].Zhang ZZ, Jiang D, Ding JX, Wang SJ, Zhang L, Zhang JY, Qi YS, Chen XS, Yu JK, Role of scaffold mean pore size in meniscus regeneration, Acta Biomater. 43 (2016) 314–326. 10.1016/j.actbio.2016.07.050. [DOI] [PubMed] [Google Scholar]
- [58].Baek J, Sovani S, Glembotski NE, Du J, Jin S, Grogan SP, D’Lima DD, Repair of Avascular Meniscus Tears with Electrospun Collagen Scaffolds Seeded with Human Cells, Tissue Eng. Part A. 22 (2016) 436–448. 10.1089/ten.tea.2015.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang ZZ, Chen YR, Wang SJ, Zhao F, Wang XG, Yang F, Shi JJ, Ge ZG, Ding WY, Yang YC, Zou TQ, Zhang JY, Yu JK, Jiang D, Orchestrated biomechanical, structural, and biochemical stimuli for engineering anisotropic meniscus, Sci. Transl. Med. 11 (2019). 10.1126/scitranslmed.aao0750. [DOI] [PubMed] [Google Scholar]
- [60].Higashioka MM, Chen JA, Hu JC, Athanasiou KA, Building an Anisotropic Meniscus with Zonal Variations, Tissue Eng. Part A. 20 (2014) 294–302. 10.1089/ten.tea.2013.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vapniarsky N, Huwe LW, Arzi B, Houghton MK, Wong ME, Wilson JW, Hatcher DC, Hu JC, Athanasiou KA, Tissue engineering toward temporomandibular joint disc regeneration, Sci. Transl. Med. 10 (2018) 1–10. 10.1126/scitranslmed.aaq1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Donahue RP, Gonzalez-Leon EA, Hu JC, Athanasiou KA, Considerations for translation of tissue engineered fibrocartilage from bench to bedside, J. Biomech. Eng. 141 (2019). 10.1115/1.4042201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.