Abstract
OBJECTIVES
Using a contemporary, multicenter international single-photon emission computed tomography myocardial perfusion imaging (SPECT-MPI)registry, this study characterized the potential major adverse cardiovascular event(s) (MACE) benefit of early revascularization based on automatic quantification of ischemia.
BACKGROUND
Prior single-center data reported an association between moderate to severe ischemia SPECT-MPI and reduced cardiac death with early revascularization.
METHODS
Consecutive patients from a multicenter, international registry who underwent 99mTc SPECT-MPI between 2009 and 2014 with solid-state scanners were included. Ischemia was quantified automatically as ischemic total perfusion deficit (TPD). Early revascularization was defined as within 90 days. The primary outcome was MACE (death, myocardial infarction, and unstable angina). A propensity score was developed to adjust for nonrandomization of revascularization; then, multivariable Cox modeling adjusted for propensity score and demographics was used to predict MACE.
RESULTS
In total, 19,088 patients were included, with a mean follow-up of 4.7 ± 1.6 years, during which MACE occurred in 1,836 (9.6%) patients. There was a significant interaction between ischemic TPD modeled as a continuous variable and early revascularization (interaction p value: 0.012). In this model, there was a trend toward reduced MACE in patients with >5.4% ischemic TPD and a significant association with reduced MACE in patients with >10.2% ischemic TPD.
CONCLUSIONS
In this large, international, multicenter study reflecting contemporary cardiology practice, early revascularization of patients with >10.2% ischemia on SPECT-MPI, quantified automatically, was associated with reduced MACE.
Keywords: ischemia, MACE, revascularization
Recommendations regarding the use of revascularization in stable coronary artery disease (CAD) is a continuing challenge for clinicians and an area of debate (1–3). Revascularization is associated with symptomatic improvement and is clearly indicated in patients with symptoms refractory to optimal medical therapy (4,5). However, the evidence supporting revascularization for prognostic benefit, particularly with respect to major adverse cardiovascular event(s) (MACE), is less well established.
The decision to pursue revascularization in stable CAD depends on a patient’s symptoms, anatomic complexity, and burden of ischemia. Observational studies have suggested that early revascularization of patients with moderate to severe ischemia on single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) with a threshold of >10% ischemia may decrease short-term cardiac death and long-term all-cause mortality (1,2). However, the applicability of these studies to contemporary cardiology practice remains uncertain. The study periods pre-dated routine use of drug-eluting stents and dual-antiplatelet therapy. Additionally, the previous study relied on a subjective visual analysis of ischemia from expert readers at a single institution rather than fully automated quantitative analysis, which can be uniformly applied across laboratories (6). Finally, the results of the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial, which demonstrated no benefit of an early invasive strategy in patients with moderate or severe ischemia, have raised questions regarding the ability of noninvasive ischemia testing to predict outcome benefit with revascularization (7).
In this study, we sought to characterize the potential MACE benefit of early revascularization versus no revascularization in a contemporary cohort of patients in relation to burden of myocardial ischemia quantified automatically using an international multicenter registry of latest-generation SPECT-MPI.
METHODS
STUDY DESIGN AND PATIENT POPULATION.
We conducted an observational study of patients in REFINE SPECT (Registry of Fast Myocardial Perfusion Imaging with Next-Generation SPECT), an international multicenter cohort of patients who underwent exercise or pharmacological stress SPECT-MPI with 99mTc on the latest-generation dedicated cardiac camera systems based on solid-state cadmium-zinc-telluride detectors (8). Details of the patient inclusion and exclusion criteria are available in the Supplemental Methods section.
AUTOMATED QUANTIFICATION.
SPECT-MPI studies were acquired using either the D-SPECT camera system (Spectrum-Dynamics, Haifa, Israel) or the Discovery NM530c camera system (GE Healthcare, Milwaukee, Wisconsin) as previously described (8). Perfusion was assessed by automated quantitation of total perfusion deficit (TPD), which incorporates the extent and severity of perfusion abnormalities (6). Upright post-stress and rest images were used to assess TPD in studies obtained with a D-SPECT camera when upright rest imaging was available. Supine post-stress and rest images were used when supine, but not upright, rest imaging was performed. Supine post-stress and rest images were used for studies obtained with the Discovery NM530c. The magnitude of myocardial ischemia was quantified by ischemic TPD (stress TPD – rest TPD). When a stress-only protocol was performed, rest TPD was assumed to be 0. Additional details of image processing are available in the Supplemental Methods section.
Visual scoring for ischemia using summed difference scores (SDSs) was performed in a subset of patients (n = 11,580). In these patients, experienced clinicians scored images for ischemia using the 17-segment American Heart Association model at the time of clinical reporting using a 4-point scale (0: normal; 1: mild; 2: moderate; 3: severe; 4: absent) (9–11). SDS was calculated as summed stress score – summed rest score, and percent ischemic myocardium was calculated as (SDS/68) × 100 (1). Moderate to severe ischemia was defined as >10% ischemic myocardium (2).
OUTCOMES.
MACE was defined as death, nonfatal myocardial infarction (MI), or admission for unstable angina. MACE was verified at each site independently by an experienced cardiologist. The first event was considered as the time-to-event interval. Details of outcome definitions and ascertainment are available in the Supplemental Methods section and Supplemental Table 1.
ANALYSIS DESIGN.
Patients were categorized by revascularization status at 90 days, and a propensity analysis was used to account for selection bias. Revascularization occurring more than 90 days from SPECT MPI was not included as an outcome or treatment. A 2-step process was used with initial development of a propensity score followed by multivariable survival analysis. The MACE-free survival impact of revascularization compared with no revascularization was assessed using interaction terms. In the primary analysis, ischemia was modeled as a continuous variable. In a separate analysis, 3 ischemia levels were defined based on previous data: ≤5% (no or minimal ischemia), >5% to 10% (mild ischemia), and >10% (moderate to severe ischemia) (1,2).
PROPENSITY SCORE.
A propensity score was developed to adjust for potential biases introduced by the nonrandomization to revascularization (12). A logistic regression model was used for this approach (no revascularization vs. early revascularization) as a function of age, sex, hypertension, hyperlipidemia, diabetes, peripheral vascular disease, smoking status, presence of typical chest pain, family history of pre-mature CAD, smoking, peripheral vascular disease, type of stress (exercise or pharmacological), stress ejection fraction (EF), site, rest TPD, and ischemic TPD. Age, stress EF, and ischemic TPD were treated as continuous variables (13). In this way, the propensity score was derived as an index, ranging from 0 to 1, to summarize all measured factors to influence the decision of treatment assignment, such that high values indicate high probability of a patient being assigned to early revascularization. This score was incorporated as a covariate in the survival analysis. Supplemental Figure 1 displays the distribution of propensity scores in the early revascularization and no revascularization groups.
STATISTICAL ANALYSIS.
Event rates between categories of ischemia were compared with a simultaneous inference procedure that corrects for multiple testing after performing logistic regression (14). A multivariable Cox proportional hazards model was used to determine the benefit of early revascularization with respect to MACE-free survival. Models were adjusted for potential confounders along with the propensity score outlined earlier, using a doubly robust method described previously (15). The propensity score balance was assessed using absolute standardized mean differences, determined by estimating the variable values from regression models with early revascularization and propensity score as covariates (16). Covariate selection for model entry was based on clinical experience and identification of covariates known to be multivariable predictors and was the same as those used for the propensity score. Multiple centers were accounted for using site-specific baseline hazards. The proportional hazards assumption was verified by using Schoenfeld residuals (17). Continuous covariates were modeled either using restricted cubic splines with 3 knots defined by quantiles (10th, 50th, and 90th) or a linear effect based on a likelihood ratio test. Ischemic TPD was treated as a linear continuous and categorical covariate, with cutoffs based on previous data from published reports (1,2). The hazard ratio (HR) for a patient with x% ischemic TPD was calculated, and the logarithm of the HR was displayed as function of continuous ischemic TPD. We determined the level of ischemia at which early revascularization is associated with no difference in outcomes (adjusted HR of 1.00). Additionally, we determine the level at which there is a significant association with reduced MACE with revascularization (where the upper 95% confidence interval [CI] crosses an adjusted HR of 1.00). There was no interaction between positive findings on stress electrocardiography (ECG), early revascularization, and association with MACE (interaction p = 0.716).
The primary analysis included all MACE events; however, results were not significantly different in an analysis excluding events that occurred in the first 90 days. A sensitivity analysis was performed in the subset of patients with available expert visual segmental scoring. In this analysis, the same propensity score–adjusted analysis was performed but with percent ischemic myocardium calculated from SDS rather than ischemic TPD. The primary analysis was also repeated with all-cause mortality as the outcome. Two-way and 3-way interactions were tested using the likelihood ratio test.
All hypotheses were 2 sided, and a p value of <0.05 was considered statistically significant. Calculations were performed using R, version 3.5.3 (R Core Team, Vienna, Austria). This research was approved by the Institutional Review Boards of the participating centers and at the core laboratory at Cedars-Sinai.
RESULTS
PATIENT CHARACTERISTICS.
Of the 19,088 patients included in the study, the mean age was 63.9 ± 12.0 years, and 44.3% were women. The baseline characteristics of the patients and the differences between the revascularization and no revascularization cohorts are shown in Table 1. A total of 554 (2.9%) patients underwent early revascularization within 90 days, of whom 20.6% (n = 114) had coronary artery bypass surgery and 79.4% (n = 440) had PCI. More patients in the revascularization group underwent pharmacological stress (57.2% vs. 50.8%; p = 0.003), had abnormal stress ECG (35.9 vs. 9.6%; p < 0.001), and had a higher rate of prior PCI (21.1% vs. 11.4%; p < 0.001) compared to those who were treated medically. Men were referred for revascularization more often than women (80.3% vs. 19.7%; p < 0.001) and were more likely to have >10% ischemic TPD (5.3% vs. 1.3%; p < 0.001). Additionally, after adjusting for all variables included in the propensity score, men were more likely to be referred for revascularization (adjusted odds ratio: 1.34; 95% CI: 1.02 to 1.77; p = 0.034).
TABLE 1.
Baseline Patient Characteristics
| Variable | No Revascularization (n = 18,534) | Early Revascularization (n = 554) | p Value |
|---|---|---|---|
| Site | <0.001 | ||
| Assuta Medical Center | 7,363 (39.7) | 283 (51.1) | |
| Brigham and Women’s Hospital | 2,212 (11.9) | 49 (8.8) | |
| Cedars-Sinai Medical Center | 3,234 (17.5) | 78 (14.1) | |
| Oregon Heart and Vascular Institute | 2,536 (13.7) | 66 (11.9) | |
| Ottawa Heart Institute | 3,189 (17.2) | 78 (14.1) | |
| Demographic characteristics | |||
| Age, yrs | 63.8 ± 12.1 | 65.6 ± 10.6 | <0.001 |
| Male | 10,180 (54.9) | 445 (80.3) | <0.001 |
| BMI, kg/m2 | 28.4 ± 6.1 | 28.2 ± 4.3 | 0.264 |
| Cardiovascular risk factors | |||
| Peripheral vascular disease | 1,993 (10.8) | 72 (13.0) | 0.096 |
| Hypertension | 11,542 (62.3) | 403 (72.7) | <0.001 |
| Diabetes mellitus | 4,541 (24.5) | 199 (35.9) | <0.001 |
| Hyperlipidemia | 11,474 (61.9) | 404 (72.9) | <0.001 |
| Family history of CAD | 5,153 (27.8) | 140 (25.3) | 0.194 |
| Smoking | 3,536 (19.1) | 91 (16.4) | 0.124 |
| Prior PCI | 3,197 (17.3) | 164 (29.6) | <0.001 |
| Typical angina | 992 (5.4) | 120 (21.7) | <0.001 |
| Stress test type | 0.003 | ||
| Exercise | 9,111 (49.2) | 237 (42.8) | |
| Pharmacological | 9,423 (50.8) | 317 (57.2) | |
| Stress ECG response | <0.001 | ||
| Negative | 16,755 (90.4) | 355 (64.1) | |
| Positive | 1,779 (9.6) | 199 (35.9) | |
| Myocardial perfusion study | |||
| Ejection fraction, % | 62.6 ± 11.2 | 56.5 ± 11.3 | <0.001 |
| Ischemic TPD | 2.4 ± 2.9 | 10.2 ± 7.1 | <0.001 |
| Ischemic TPD | <0.001 | ||
| 0% to 5% | 15,776 (75.5) | 154 (27.8) | |
| >5% to 10% | 2,226 (12.0) | 146 (26.4) | |
| >10% | 532 (2.9) | 254 (45.9) | |
| MACE | 1,770 (9.6) | 66 (11.9) | 0.067 |
Values are n (%) or mean ± SD.
BMI = body mass index; CAD = coronary artery disease; MACE = major adverse cardiovascular event(s); PCI = percutaneous coronary intervention; TPD = total perfusion deficit.
Baseline characteristics according to burden of ischemia on SPECT-MPI are shown in Supplemental Table 2. Early revascularization occurred in 0.9% of patients with ischemic TPD of 0% to 5%, 6.5% of patients with ischemic TPD of 5% to 10%, and 36.6% of patients with >10% ischemic TPD.
MYOCARDIAL PERFUSION FINDINGS.
Overall, minimal ischemia (0% to 5%) was present in 15,930 (83.5%) patients, mild (>5% to 10%) in 2,372 (12.4%) patients, and moderate to severe (>10%) in 786 (4.1%) patients. Ischemic TPD was higher in patients referred for early revascularization (mean ± SD: 10.42% ± 7.1% vs. 2.4% ± 2.8%; p < 0.001). The frequency of moderate to severe ischemia was also higher (45.9% vs. 2.9%; p < 0.001). Stress left ventricular EF was lower in patients referred for early revascularization (mean: 56.5% vs. 62.6%; p < 0.001), and the proportion of patients with a left ventricular EF of <40% was higher (6.8% vs. 2.7%; p < 0.001).
MYOCARDIAL PERFUSION ANALYSIS AND OUTCOME.
Outcome events.
During a mean follow-up of 4.7 ± 1.6 years, MACE occurred in 1,836 (9.6%) patients, including death in 1,269 (6.6%) patients, nonfatal MI in 332 (1.7%) patients, and admission for unstable angina in 235 (1.2%) patients. The overall MACE event rate was 2.1% per year.
Univariable predictors of events.
The univariable Cox proportional hazards model predicting MACE is shown in Supplemental Table 3. Increasing ischemic TPD was associated with increased MACE (unadjusted HR: 2.15 per percent ischemic myocardium). Kaplan-Meier curves for survival free of MACE across groups of ischemia and revascularization status are shown in Figure 1. In patients with ≤5% ischemic TPD, annualized MACE rates were higher in patients treated with early revascularization (3.11% vs. 1.76%; p < 0.001). In patients with >10% ischemic TPD, annualized MACE rates were lower in patients treated with early revascularization (2.48% vs. 4.85%; p < 0.001). There was no difference in patients with 5% to 10% ischemic TPD (2.55% vs. 3.45%; p = 0.219).
FIGURE 1. Survival Free of MACE by Categories of Ischemic TPD and Revascularization Status.
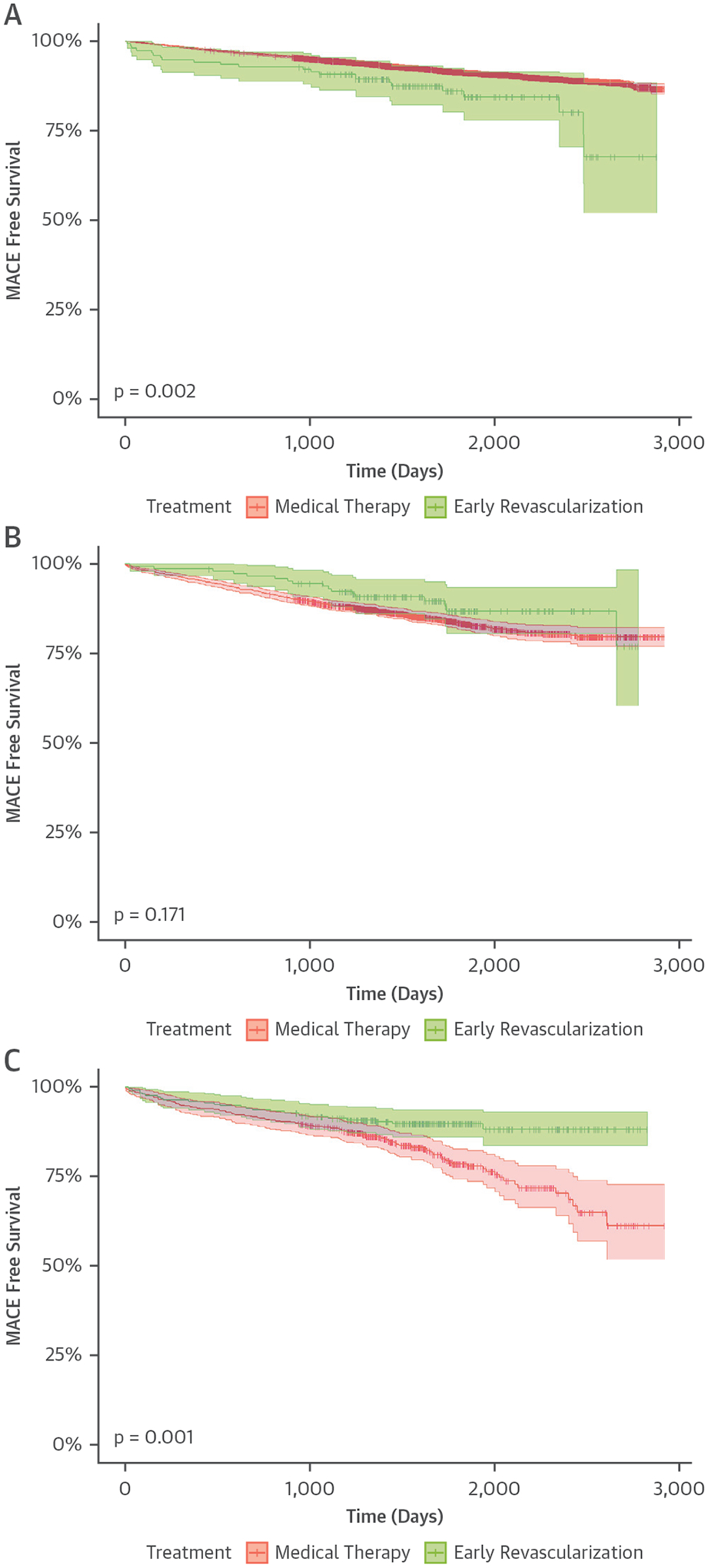
(A) Patients with 0% to 5% ischemic TPD. (B) Patients with 5% to 10% ischemic TPD.
(C) Patients with >10% ischemic TPD. MACE includes all-cause mortality, nonfatal myocardial infarction, and admission for unstable angina. MACE = major adverse cardiovascular event(s); TPD = total perfusion deficit.
Impact of early revascularization on outcomes.
Components of the propensity score are detailed in Supplemental Table 4. Absolute standardized mean differences, after adjusting for the propensity score, in patients treated with and without early revascularization are shown in Supplemental Figure 2. The multivariable Cox proportional hazard model was adjusted for propensity score, age, sex, hypertension, hyperlipidemia, diabetes, peripheral vascular disease, smoking status, presence of typical chest pain, family history of CAD, type of stress test, stress EF, site, resting TPD, and ischemic TPD. Association between early revascularization and MACE as a function of ischemia is shown in the Central Illustration. There was a significant interaction between ischemic TPD modeled as a continuous variable and early revascularization (interaction p value: 0.012). In this model, there was a trend toward reduced MACE in patients with >5.4% ischemic TPD and a significant association with reduced MACE in patients with >10.2% ischemic TPD. Results of the analysis of variables other than ischemic TPD are shown in Supplemental Figure 3.
CENTRAL ILLUSTRATION. Association Between Ischemia, Early Revascularization, and Major Adverse Cardiovascular Events.
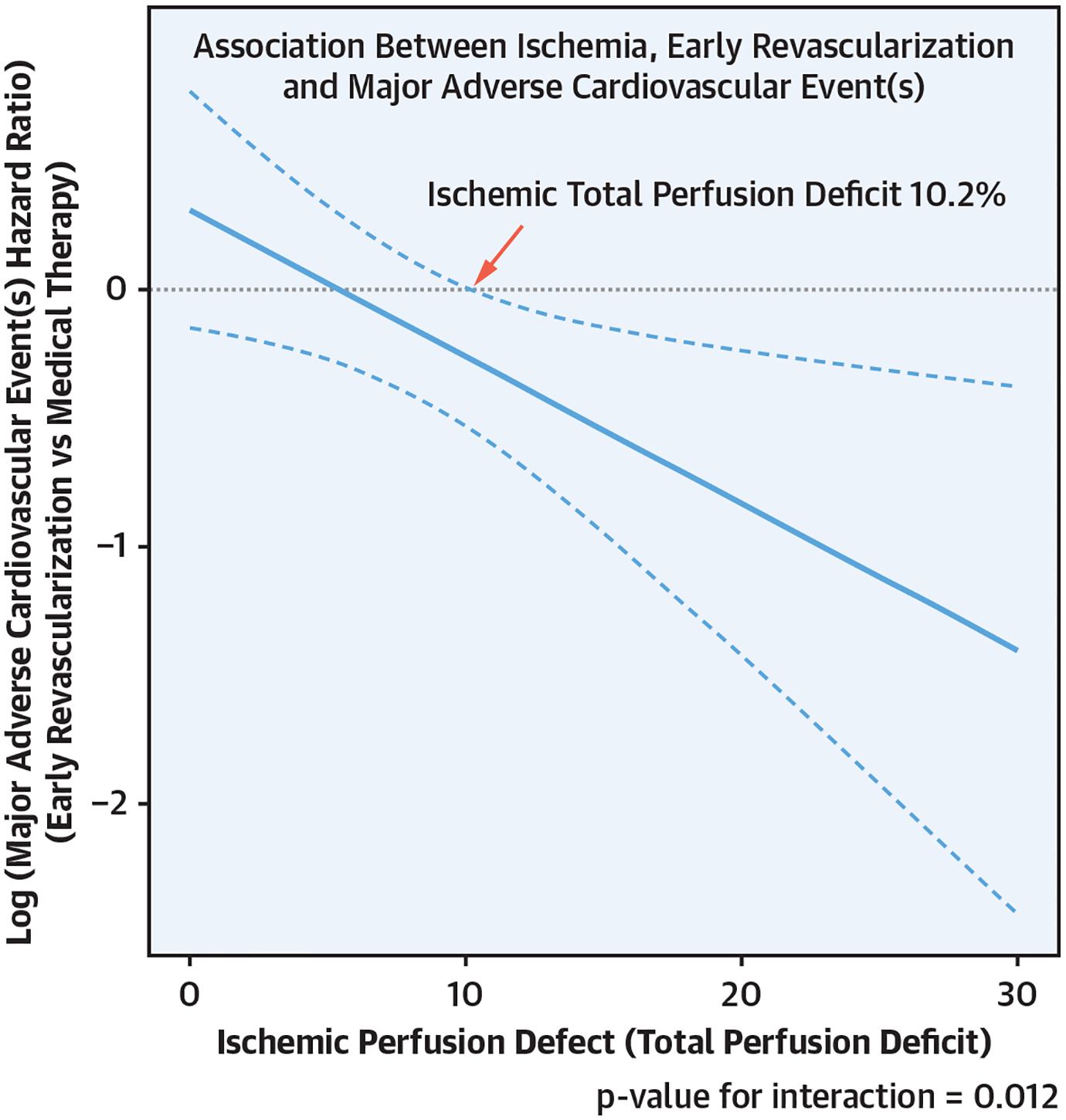
Multivariable analysis of the association between early revascularization and major adverse cardiovascular event(s) (MACE). Ischemic total perfusion deficit (TPD) is treated as a continuous variable.
Ischemia was also modeled as a categorical variable. Patients with ischemic TPD of >10% who underwent early revascularization had a MACE-free survival advantage (adjusted HR: 0.58; 95% CI: 0.37 to 0.90) compared to patients treated without early revascularization (Supplemental Figure 4). Results of the unadjusted, propensity-adjusted, and fully adjusted models are compared in Table 2.
TABLE 2.
Associations Between Early Revascularization and MACE in 3 Different Models
| Ischemia Category | Treatment Strategy | n/MACE | MACE rate per 100 patient-years | Unadjusted HR (95% CI) | Propensity-Adjusted HR (95% CI) | Fully Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| ITPD of <5% | Early revascularization | 154/20 | 3.11 | 1.98 (1.31–2.99) | 2.00 (1.32–3.02) | 1.42 (0.94–2.16) |
| No early revascularization | 15,776/1,328 | 1.76 | ||||
| ITPD of 5% to 10% | Early revascularization | 146/17 | 2.55 | 0.70 (0.43–1.14) | 0.71 (0.44–1.16) | 0.78 (0.48–1.27) |
| No early revascularization | 1,756/339 | 3.45 | ||||
| ITPD of >10% | Early revascularization | 254/28 | 2.48 | 0.48 (0.31–0.74) | 0.51 (0.32–0.80) | 0.58 (0.37–0.90) |
| No early revascularization | 380/104 | 4.85 | ||||
| Interaction between ischemia and early revascularization | p < 0.001 | p < 0.001 | p = 0.012 | |||
The fully adjusted model is adjusted for propensity score and all covariates described.
CI = confidence interval; HR = hazard ratio; ITPD = ischemic total perfusion deficit.
Women were less likely to experience MACE (adjusted HR: 0.79; p < 0.001). However, the relationship between ischemic TPD and early revascularization was not significantly different between men and women (sex interaction p value = 0.771; results in Supplemental Figure 5).
SENSITIVITY ANALYSES.
The propensity score–adjusted multivariable analysis assessing the impact of revascularization on outcomes was repeated, with ischemia assessed by using SDS in patients with SDS available. Moderate to severe ischemia (>10% myocardial ischemia) was present by both ischemic TPD and SDS in 337 (2.6%) patients. Moderate to severe ischemia was present by ischemic TPD, but not SDS, in 314 (2.4%) patients, and the opposite pattern was seen in 114 (0.9%) patients. Ischemia was modeled as a continuous variable (Supplemental Figure 6). In this model, there was a significant interaction between extent of ischemia, early revascularization, and association with MACE (p = 0.02). Ischemia was also modeled as a categorical variable, with the results given in Supplemental Figure 7.
Finally, the analysis was repeated with an outcome of all-cause mortality alone. The results of this analysis, with ischemic TPD modeled as a continuous variable, are shown in Supplemental Figure 8. In this analysis, early revascularization had a trend toward lower all-cause mortality in patients with >3.3% ischemia and a significant association with reduced all-cause mortality in patients with >8.7% ischemia.
There was no interaction between mode of stress, early revascularization, ischemic TPD, and MACE (p = 0.644).
DISCUSSION
This international multicenter observational study assessed the relationship between ischemia and revascularization benefit with respect to MACE in a large population of patients with no prior MI. Our results expand on existing observational, single-center reports by providing validation of this benefit in a contemporary, multicenter context, reflecting a range of practice settings and scanning protocols using the latest-generation SPECT cameras and automated ischemia quantitation. In patients with >5.4% ischemic TPD, there was a trend toward reduced MACE with revascularization, which became significant at >10.2% ischemic TPD. To our knowledge, t h e study is the first such analysis using the latest-generation SPECT cameras and automated quantitative ischemia assessment, the first in a multicenter setting, and the first to reflect contemporary cardiology practice.
The existing observational evidence for improved clinical outcomes with revascularization in patients with stable CAD and moderate to severe ischemia is largely based on data from a single tertiary referral center (1,2). Hachamovitch et al. (2) demonstrated that visually assessed ischemia of >10% may identify patients with a survival benefit from revascularization. However, this prior study was performed in patients studied between 1991 and 1997 (2), and substantial differences in medical therapy and revascularization have occurred since that time. Clopidogrel, prasugrel, and ticagrelor were approved by the U.S. Food and Drug Administration in 1998, 2009, and 2011, respectively, and dual antiplatelet therapy began to be used routinely only after publication of the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial in 2001 (18). The most potent statins, atorvastatin and rosuvastatin, were approved in 1996 and 2003, respectively. Despite these improvements in medical therapies since the era studied by Hachamovitch et al., in this contemporary cohort, we demonstrate that patients with >10.2% ischemic TPD may derive MACE benefit from early revascularization. The threshold in our study, however, was derived with an objective, automated method that can be repeated in any nuclear cardiology laboratory. The current report also expands on this earlier observational evidence by demonstrating similar findings in an international, multicenter cohort. Furthermore, we identified similar benefits from early revascularization when MACE and all-cause mortality were used as outcomes. Of note, a large single-center observational study demonstrated a mortality benefit with early revascularization in patients with >10% visually assessed ischemia on positron emission tomography (19).
The findings of this study contrast with those of the ISCHEMIA trial, which found no benefit regarding composite cardiovascular events from the use of an early invasive strategy based on the presence of >10% ischemia or its equivalent on stress imaging or exercise ECG testing (7). Several aspects of our study provide insight into these differences. Regarding technology and interpretation, the current study used an entirely automated quantitative analysis not used in the nuclear core laboratory in the ISCHEMIA trial. Quantitation with TPD has been shown to improve reproducibility compared to visual interpretation (20) and may potentially classify a patient’s severity of ischemia differently. REFINE-SPECT included only studies using the latest-generation solid-state SPECT camera systems at expert sites. In contrast, ISCHEMIA included all camera systems. Regarding the patient populations in these 2 studies, there are several reasons to believe that significant differences may be present. Many patients at increased risk of cardiac events were included in REFINE who would have been excluded from the ISCHEMIA trial. The ISCHEMIA trial excluded patients who had left main coronary artery disease on coronary computed tomography (present in nearly 5% of the enrolled patient group) or were considered likely to have left main disease—a high-risk group likely to show the greatest revascularization benefit when ischemia is present. Furthermore, patients with unacceptable levels of angina were excluded (7). Importantly, as with all randomized trials, physicians would be less likely to enroll patients who did not have clear clinical equipoise between invasive and conservative strategies. All these patients would be represented within our study population, given its observational nature. Whereas in the ISCHEMIA trial, most had moderate or severe ischemia, 13.9% of patients randomized with stress imaging had less than moderate ischemia on core laboratory interpretation. Although subanalysis of patients with core laboratory–determined severe ischemia by any modality showed no treatment interaction (7), analysis of patients with moderate to severe ischemia on SPECT-MPI has not been reported and may be relevant. Specific optimal medical therapy was recommended and monitored in ISCHEMIA, whereas in our registry, medical therapy was not controlled and reflects standard clinical practice. This could potentially explain the difference in results; however, medical therapy in ISCHEMIA is likely more intensive than in routine clinical practice, suggesting that the real-world benefit of revascularization may be greater than that demonstrated in the ISCHEMIA trial. Differences in event ascertainment and event type may also be contributory. Patients in ISCHEMIA were routinely assessed for procedural MI, whereas in the REFINE registry, screening for procedural MI was not routinely performed. Additionally, events in the REFINE-SPECT registry were adjudicated by a site physician, not an independent clinical event adjudication committee, which may have led to an over-estimation of events. Finally, given the limitations of retrospective analyses, unmeasured confounding factors in our dataset likely contributed to the differences in study results.
Prior randomized trial evidence suggests that basing revascularization decisions on the functional significance of CAD may improve patient outcomes. In the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) trial, fractional flow reserve–guided PCI decreased rates of death or nonfatal MI compared to angiography-guided PCI at 2 years (21) and the 5-year follow-up (22). In the FAME 2 trial, patients were randomized to receive fractional flow reserve–guided revascularization plus medical therapy or medical therapy alone (14). At 5 years of follow-up, there was a reduction in the composite outcome of death, nonfatal MI, and urgent revascularization (14). Additionally, there was a trend toward decreased MI with revascularization (8.1% vs. 12.0%) in the FAME 2 trial. This mirrors results from the ISCHEMIA trial demonstrating a decrease in non-procedural MI with early revascularization (21). These data support the concept that the functional significance of CAD should be interpreted in conjunction with anatomic significance. Fewer than 10% of the patients in our study had moderate to severe ischemia, with only 2.9% receiving early revascularization. This finding parallels data from single centers showing that the proportion of patients with ischemia has decreased considerably over time (23). Overall, there needs to be improved selection of patients for SPECT with less testing of lower-risk patients. However, SPECT-MPI remains valuable to detect and quantify ischemia in symptomatic patients for purposes of assessing whether symptoms are due to ischemia and guiding decisions regarding the need to consider an invasive strategy and the intensity of medical management.
An important novel aspect of our study is the use of automated quantification of ischemia. As noted previously, the observational data to date assessing the benefit from revascularization in patients with stable CAD with ischemia on SPECT-MPI were based on visual analysis of SPECT-MPI. Classification of the visual severity of ischemia is more prone to interobserver variability compared to stress perfusion abnormality (24). Quantitative assessment of ischemia with TPD correlates well with expert-reader interpretation (24) but with significantly higher reproducibility (25). It is not reliant on the expertise of clinical readers, which varies widely across practice settings and provides robust risk stratification (6). Our results suggest that an objective TPD approach could be used in conjunction with clinician interpretation to guide management decisions. The higher reproducibility should yield more consistent classification of patients with moderate to severe ischemia.
There was significant variation in the use of early revascularization between the sexes in our study. Men were also more likely to be referred for revascularization after adjusting for all important baseline characteristics. This finding is of critical importance, because CAD manifests differently in women compared to men (26), driving differences in treatment that may be inappropriate. This bias toward revascularization in men was also shown by Steg et al. (26) in more than 30,000 patients with stable CAD (26). In the ISCHEMIA trial, women were less likely to have severe ischemia on stress imaging and were more likely to have no obstructive CAD on computed tomography angiography (27). However, the absence of interaction between early revascularization and sex in our study provides evidence that no significant sex-based modification of the benefit from revascularization exists.
STUDY LIMITATIONS.
This was an observational study, and there are potential unmeasured confounders that have not been taken into account. The study was not randomized; however, the use of the doubly robust propensity score adjustment was used to decrease the selection bias of revascularization (15). There were residual differences between populations, which we attempted to adjust for in the outcomes analysis. Our analysis was performed in the context of a core laboratory with image quality-control processes, and the results may not be applicable in the setting of poor image quality. We included patients imaged with 2 camera systems and used the default imaging position for each camera system. Combining information from 2 imaging positions or using computed tomography–based attenuation may affect the quantification of stress, rest, and ischemic TPD and may need to be separately evaluated in further studies. Details regarding coronary anatomy were not available in most patients, and we do not have data regarding completeness of coronary revascularization. Additionally, the degree to which patients in our study were treated with optimal medical management is not known. However, given the recentness of the study data collection, the treatment is representative of current real-world clinical practice and the potential MACE benefit from revascularization within that context. All-cause mortality rather than cardiac death was used in our MACE definition. Cardiac death may be more closely related to SPECT-MPI findings and revascularization than all-cause mortality. However, data relating to cardiac death were available in this large, multicenter registry, because most of the sites did not differentiate causes of death (28). Additionally, we had limited statistical power to assess nonfatal components of the composite outcome. Physicians were not blinded to SPECT-MPI results or revascularization status when adjudicating nonfatal events. However, all events were adjudicated by sites before conception of the current study, and physicians would not be aware of the significance of early revascularization with respect to the current study. In addition, there might be patients who experienced nonfatal events that were not captured because of hospitalization at another medical center. Although we have limited information on patient symptoms and response to therapy, revascularization has been shown to decrease anginal symptoms in patients with documented ischemia (32). In the ISCHEMIA trial, patients randomized to early invasive therapy had greater improvement in anginal symptoms as measured by the Seattle Angina Questionnaire (33).
CONCLUSIONS
In this large, international, multicenter study reflecting contemporary cardiology practice, early coronary revascularization of patients with >10.2% ischemia on SPECT-MPI, quantified by automatic TPD, was associated with MACE-free survival benefit.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE 1:
Our results expand on prior single-center data by demonstrating an association between early revascularization and reduced cardiovascular events in patients with moderate to severe ischemia.
COMPETENCY IN MEDICAL KNOWLEDGE 2:
Automatic quantification of ischemia may identify patients who benefit from revascularization.
TRANSLATIONAL OUTLOOK:
Given the existing randomized trial evidence, it may be reasonable to determine if significant differences in patient selection for revascularization exist between quantitative interpretation and expert visual interpretation.
Acknowledgments
This research was supported in part by grant R01HL089765 from the National Heart, Lung, and Blood Institute/National Institutes of Health (principal investigator: Dr. Slomka). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work was also supported in part by the Miriam and Sheldon Adelson Medical Research Foundation. Dr. R.J.H. Miller received funding support from the Arthur J E Child Fellowship grant. Dr. Einstein has served as a consultant for GE Healthcare, and his institution has received research support from Toshiba America Medical Systems and Roche Medical Systems. Dr. Ruddy has received research grant support from GE Healthcare and Advanced Accelerator Applications. Dr. E. Miller has served as a consultant for GE Healthcare and Bracco Inc., and he and his institution have received grant support from Bracco Inc. Dr. Dorbala has served as a consultant for GE Healthcare and Bracco Diagnostics, and her institution has received grant support from Astellas. Dr. Di Carli has received research grant support from Spectrum Dynamics and consulting honoraria from Sanofi and GE Healthcare. Dr. Berman participates in software royalties for QPS software at Cedars-Sinai Medical Center and has served as a consultant for GE Healthcare, and his institution has received grant support from HeartFlow. Drs. Slomka participates in software royalties for QPS software at Cedars-Sinai Medical Center and has received research grant support from Siemens Medical Systems. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CI
confidence interval
- ECG
electrocardiography
- EF
ejection fraction
- HR
hazard ratio
- MACE
major adverse cardiovascular event(s)
- MI
myocardial infarction
- MPI
myocardial perfusion imaging
- PCI
percutaneous coronary intervention
- SDS
summed difference score
- SPECT
single-photon emission computed tomography
- TPD
total perfusion deficit
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Cardiovascular Imaging author instructions page.
APPENDIX For an expanded Methods section as well as supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900–7. [DOI] [PubMed] [Google Scholar]
- 2.Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012–24. [DOI] [PubMed] [Google Scholar]
- 3.Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 2005;111:2906–12. [DOI] [PubMed] [Google Scholar]
- 4.Kastrati A, Banning AP, Koller A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2018;40:87–165. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease. J Am Coll Cardiol 2017;69:2212–41. [DOI] [PubMed] [Google Scholar]
- 6.Otaki Y, Betancur J, Sharir T, et al. 5-year prognostic value of quantitative vs visual myocardial perfusion imaging in subtle perfusion defects: results from the REFINE SPECT Registry. J Am Coll Cardiol Img 2019;13:774–85. [Google Scholar]
- 7.Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382: 1395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slomka PJ, Betancur J, Liang JX, et al. Rationale and design of the REgistry of Fast Myocardial Perfusion Imaging with NExt generation SPECT (REFINE SPECT). J Nucl Cardiol 2020;27:1010–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman DS, Kiat H, Friedman JD, et al. Separate acquisition rest thallium-201/stress technetium-99m sestamibi dual-isotope myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol 1993;22:1455–64. [DOI] [PubMed] [Google Scholar]
- 10.Berman DS, Abidov A, Kang X, et al. Prognostic validation of a 17-segment score derived from a 20-segment score for myocardial perfusion SPECT interpretation. J Nucl Cardiol 2004;11:414–23. [DOI] [PubMed] [Google Scholar]
- 11.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 12.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 13.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometric J 2008;50:346–63. [DOI] [PubMed] [Google Scholar]
- 15.Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol 2017;69:345–57. [DOI] [PubMed] [Google Scholar]
- 16.Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf 2008;17:1202–17. [DOI] [PubMed] [Google Scholar]
- 17.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–41. [Google Scholar]
- 18.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 19.Patel KK, Spertus JA, Chan PS, et al. Extent of myocardial ischemia on positron emission tomography and survival benefit with early revascularization. J Am Coll Cardiol 2019;74:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slomka PJ, Dey D, Sitek A, Motwani M, Berman DS, Germano G. Cardiac imaging: working towards fully-automated machine analysis & interpretation. Exp Rev Med Dev 2017;14: 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME. J Am Coll Cardiol 2010; 56:177–84. [DOI] [PubMed] [Google Scholar]
- 22.Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP–ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations. Circulation 1999; 99:2829–48. [DOI] [PubMed] [Google Scholar]
- 23.Rozanski A, Gransar H, Hayes SW, et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol 2013;61: 1054–65. [DOI] [PubMed] [Google Scholar]
- 24.Arsanjani R, Xu Y, Hayes SW, et al. Comparison of fully automated computer analysis and visual scoring for detection of coronary artery disease from myocardial perfusion SPECT in a large population. J Nucl Med 2013;54:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berman DS, Kang X, Gransar H, et al. Quantitative assessment of myocardial perfusion abnormality on SPECT myocardial perfusion imaging is more reproducible than expert visual analysis. J Nucl Cardiol 2009;16:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steg PG, Greenlaw N, Tardif JC, et al. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J 2012;33:2831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds HR, Shaw LJ, Min JK, et al. Association of sex with severity of coronary artery disease, ischemia, and symptom burden in patients with moderate or severe ischemia: secondary. JAMA Cardiol 2020. March 30 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation 2013;127:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


