Abstract
SETTING:
Hypertension, diabetes mellitus and asthma are on the rise in developing countries, including Rwanda; there is thus a need to ensure uninterrupted drug availability.
OBJECTIVES:
To assess 1) the frequency and duration of drug stock-outs; 2) lead time duration 3) monthly stock levels; and 4) drug quantities requested vs. quantity delivered for captopril, metformin and inhaled salbutamol between January and December 2018 Kirehe District, Rwanda.
DESIGN:
This was a cross-sectional study using secondary programme data.
RESULTS:
The median annual stock-outs for captopril, metformin and inhaled salbutamol were respectively 4 (IQR 3–4), 3 (IQR 2–3) and 4 (IQR 4–5) at rural health facilities (RHCs); no stock-outs occurred at the district hospital. For all three drugs, the median lead time was 7.5 days (IQR 5.5–11.5) at the hospital vs. 5 days (IQR 3–6) in RHCs. Stock status for captopril was below the 4-week minimum stock level for 2/12 months at the hospital vs. 7/12 months at the RHCs, while metformin and inhaled salbutamol were below the 4-week minimum stock levels for respectively 1/12 and 4/12 months at both hospital and RHCs. Total drug quantities delivered were less than the combined total quantities requested in respectively 8/12, 5/12 and 8/12 months for captopril, metformin and inhaled salbutamol.
CONCLUSION:
There is a need to regularly and effectively monitor drug stock levels and ensure timely and sufficient stock replenishment to avert stock-outs.
Keywords: Rwanda, SORT IT, operational research, supply chain, non-communicable diseases
Abstract
CONTEXTE :
L’hypertension, le diabète sucré et l’asthme augmentent progressivement dans les pays en développement, y compris le Rwanda ; il est donc nécessaire d’assurer une disponibilité ininterrompue des médicaments.
OBJECTIFS :
Évaluer 1) la fréquence et la durée des ruptures de stock de médicaments ; 2) durée du délai d’approvisionnement ; 3) niveaux de stock mensuels ; et 4) quantités de médicaments demandées par rapport aux quantités livrées pour le captopril, la metformine et le salbutamol inhalé ; entre janvier et décembre 2018 dans le district rural de Kirehe, au Rwanda.
METHODE :
Il s’agissait d’une étude transversale utilisant des données secondaires de programme.
RÉSULTATS :
Les ruptures de stock annuelles médianes pour le captopril, la metformine et le salbutamol inhalé étaient respectivement de 4 (intervalle interquartile [IQR] 3–4), 3 (IQR 2–3) et 4 (IQR 4–5) dans les Centre de Santé ruraux et aucune rupture de stock n’est survenue à l’hôpital de district. Pour les trois médicaments, le délai d’approvisionnement médian était de 7,5 jours (IQR 5,5–11,5) à l’hôpital contre 5 jours (IQR 3–6) dans les services sanitaires ruraux. L’état des stocks de captopril était inférieur au niveau de stock minimum de 4 semaines pendant 2/12 mois à l’hôpital contre 7/12 mois aux services sanitaires ruraux, tandis que pour la metformine et le salbutamol inhalé ils étaient inférieurs aux niveaux de stock minimum de 4 semaines pour 1/12 et 4/12 mois à l’hôpital et dans les Centre de Santé ruraux, respectivement. Les quantités totales de médicaments livrées étaient inférieures aux quantités totales combinées et demandées en 8/12, 5/12 et 8/12 mois pour le captopril, la metformine et le salbutamol inhalé, respectivement.
CONCLUSION :
Il est indispensable de surveiller régulièrement et convenablement les niveaux des stocks de médicaments et d’assurer un réapprovisionnement en temps propice et en quantité appropriée pour éviter les ruptures de stock.
The global burden of non-communicable diseases (NCDs) remains unacceptably high, especially among people living in low- and middle-income countries (LMICs). In 2016, approximately 41 million people died due to NCDs, representing 71% of all causes of deaths globally,1 78% of which and 85% of premature adult deaths (i.e., deaths among people aged 30–69 years) occurred in LMICs. Four main chronic NCDs are responsible for 80% of all premature deaths, and these include cardiovascular diseases, cancers, chronic respiratory diseases (including asthma) and diabetes mellitus (DM).2,3 In LMICs, high mortality is often due to inaccessibility of health care services and inadequate access to essential drugs for their appropriate treatment.4 Unfortunately, in many LMICs where these diseases have been neglected in the past, their prevalence is increasing due to progressive changes in lifestyles of populations, including tobacco use, physical inactivity, harmful use of alcohol and unhealthy diets.5–7
While we are experiencing evolving global health challenges and increased NCD cases, shortages of essential drugs are reportedly frequent, especially in rural health settings. Challenges that appear to hinder a continuous supply of NCD drugs in LMICs include inequitable distribution of drugs, limited resources and poor stock management, coupled with the ever-increasing numbers of new NCD cases.8–10 A recent qualitative study conducted in Kenya showed that patients often buy NCD medicines from private pharmacies, where the price is higher due to frequent unavailability of these products in public health facilities where they are cheaper or provided free of charge.4
Much of the global focus and financing mechanisms have been channelled towards mitigating the high burden of communicable diseases such as HIV/AIDS, TB and malaria which affect LMICs particularly sub-Saharan Africa. This has resulted in improved coverage of healthcare provision, improved outcomes and health impact.11 However, this has diverted attention away from NCDs, which are on the rise in sub-Saharan Africa. In Rwanda, NCDs are estimated to account for 44% of all deaths, and 18% of adults aged 30–70 years are at risk of premature death from NCDs. Risk factors for NCDs such as raised blood pressure and blood glucose are estimated to affect respectively 20% and 3% of adults aged ⩾18 years.12 In 2013, NCDs accounted for approximately 52% of all hospital outpatient consultations and 22% of hospitalisations in Rwanda. Much effort is now being expended to mitigating the burden of NCDs in the country.13
In 2017, Rwanda was among half of the worldwide countries reported to have all 10 NCD essential medicines as “generally available” in public primary care facilities.12 However, this may not always translate into uninterrupted availability of drugs in required quantities at the health facility level due to various constraints, including supply chain-related challenges. According to a previous study conducted in northern Rwanda on the availability of drugs for the management of communicable diseases such as diarrheal diseases and malaria, the average stock-out duration ranged between 7 and 30 days for oral rehydration salt and quinine 300 mg, which are common essential drugs.14 To our knowledge, no study has been conducted to assess the availability of NCD drugs in Rwanda, particularly captopril, metformin and inhaled salbutamol, which are among the essential drugs for the management of hypertension, DM and asthma, respectively.
The aim of the present study was to investigate the availability of essential drugs used in the treatment of selected NCDs such as captopril, metformin and inhaled salbutamol. Specific objectives were to compare between the district hospital and selected rural health centres (RHCs) of Kirehe District, Rwanda, from January to December 2018 and stratified by NCD drug: 1) the annual frequency and duration of drug stock-outs; 2) the average lead time duration (time between the placement of an order and delivery of new stock by the supplier); 3) the monthly trends in drug stock levels in relation to maximum-, minimum- and emergency- stock levels; and 4) the number of drug requests from health facilities vs. the total number of drug deliveries from the district pharmacy.
STUDY POPULATION, DESIGN AND METHODS
Study design
This was a cross-sectional study using routinely collected programme data.
Settings
Rwanda (surface area: 26,338 km2; 2017 population: 12.2 million) is a small landlocked country located in East Africa. At the end of 2016, there were 30 district pharmacies, 8 referral hospitals, 4 provincial hospitals, 36 district hospitals and 499 health centres.15 Kirehe District, a rural setting located in the Eastern Province, has a population of approximately 340,400 inhabitants and the district has 16 rural health centres (RHCs), 1 district hospital and 1 district pharmacy.
Pharmaceutical supply chain management in Rwanda
In Rwanda, captopril, metformin and inhaled salbutamol are part of the essential NCD drugs that are commonly dispensed for managing patients with hypertension, DM and asthma, respectively, in line with national treatment guidelines.16,17
According to the Rwanda pharmaceutical supply chain structure, all public health facilities located in the district procure all drugs from the district pharmacy through monthly orders.18 They also report on drug consumption on a monthly basis using the electronic Logistics Management Information System (eLMIS) which connects the Ministry of Health, the national medical store, district pharmacies and health facilities. A health facility drug request order is based on historical data, especially consumption and available stock. Drug stocks should not normally fall below the minimum- and emergency- stock level thresholds estimated according to previous consumption data.19
The district pharmacy procures drugs from the national central medical stores which are public and may alternatively procure from faith-based organisations; if not available, medical products can be procured from private pharmaceutical wholesalers. While the Ministry of Health has an overall supervisory role, the district pharmacy has the mandate to distribute drugs to health facilities, supervise and ensure their rational use within the district catchment area.
Study population
This study focused on aggregate monthly data for captopril, metformin and inhaled salbutamol at the district pharmacy, district hospital and five randomly selected RHCs in Kirehe District from 1 January to 31 December 2018.
Data variables and sources of data
Data variables collected by each facility and for each drug included the following: month, monthly drug stock status, monthly drug consumption, frequency of stock-outs, stock-out duration, date of drug orders, delivery date, lead time, average monthly consumption, maximum, minimum and emergency stock levels. Data were extracted into an Excel database (MicroSoft, Redmond, WA, USA) from the eLMIS database and from stock cards during field data collection visits.
Data analysis and statistics
Data were imported into Stata v15 (Stata Corporation, College Station, TX, USA) for data cleaning and analysis. Descriptive statistics comprised numbers and proportions for categorical variables and medians and their respective interquartile ranges (IQRs) for skewed continuous variables. Graphs were generated using Excel and Stata. Median monthly drug consumption patterns and median lead time duration were compared using Wilcoxon’s rank-sum test for each drug. Levels of significance were set at 5%.
Ethics and consent
Ethical approval was obtained from the Rwanda National Ethics Committee, Kigali, Rwanda (Approval Number 683/RNEC/2019) and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Diseases, Paris, France (EAG Number 31/19). The study used secondary programme data and consent was not required.
RESULTS
Table 1 shows that in comparison to RHCs, the median monthly consumption for the three NCD drugs was significantly higher at the district hospital (P < 0.01). Figure 1 shows that there were no stock-outs recorded at the district hospital. In contrast, the median annual stock-outs for captopril, metformin and inhaled salbutamol at RHCs were respectively 4 (IQR 3–4), 3 (IQR 2–3) and 4 (IQR 4–5). At the district pharmacy, there were respectively 13, 3 and 4 drug stock-outs observed throughout the year for captopril, metformin and inhaled salbutamol.
TABLE 1.
Median monthly consumption of NCD drugs stratified by type of health facility in Kirehe District, Rwanda, January–December 2018
| Medical product | Monthly consumption | P value | |
|---|---|---|---|
| District hospital Median [IQR] | Rural health centres Median [IQR] | ||
| Captopril tablet 250 mg | 27,762.5 [20,000–38,500] | 1950 [1309–3233] | <0.01 |
| Metformin 500 mg | 9750 [6500–13,750] | 1000 [500–1500] | <0.01 |
| Inhaled salbutamol 250 mcg | 225 [158–372] | 21 [11.5–40.5] | <0.01 |
NCD = non-communicable diseases; IQR = interquartile range.
FIGURE 1.
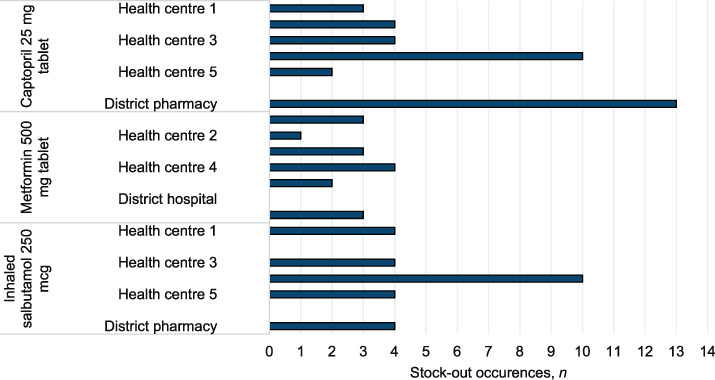
Annual frequency of non-communicable disease drug stock-outs stratified health facility in Kirehe District, Rwanda, January–December 2018.
Table 2 shows that the overall median lead time for all three NCD drugs was significantly longer at the hospital than in RHCs (7.5 days, IQR 5.5–11.5 vs. 5 days, IQR 3–6; P < 0.001). The median drug stock-out duration for all three NCD drugs at RHCs was 7 days (IQR 4–11) but it was longer, up to 10 days (IQR 6–18), for captopril.
TABLE 2.
The lead time and stock-out duration for district hospital and health facilities respectively, stratified by NCD drug in Kirehe District, Rwanda, January–December 2018
| Medical products | Days, n | P value | |||||
|---|---|---|---|---|---|---|---|
| Total | Rural health centres | District hospital | |||||
| n* | Median [IQR] | n | Median [*IQR] | n | Median [IQR] | ||
| Lead time | |||||||
| Captopril tablet 250 mg | 58 | 5 [4–6] | 50 | 5 [4–6] | 8 | 6 [5.5–9.5] | 0.05 |
| Metformin 500 mg | 34 | 5 [4–7] | 30 | 5 [4–6] | 4 | 10 [6–13] | 0.09 |
| Inhaled salbutamol 250 mcg | 47 | 5 [3–7] | 43 | 5 [3–7] | 4 | 9.5 [6–11] | 0.06 |
| Total | 139 | 5 [4–7] | 123 | 5 [3–6] | 16 | 7.5 [5.5–11.5] | <0.01 |
| Duration of stock-outs | |||||||
| Captopril tablet 250 mg | 19 | 10 [6–18] | 19 | 10 [6–18] | — | — | — |
| Metformin 500 mg | 13 | 6 [4–7] | 13 | 6 [4–7] | — | — | — |
| Inhaled salbutamol 250 mcg | 21 | 6 [5–11] | 21 | 6 [5–11] | — | — | — |
| Total | 53 | 7 [4–11] | 53 | 7 [4–11] | — | — | — |
* Number of periods with complete data across the different health facilities by type of medical product for the months from January to December 2018 out of 72 periods for each of captopril, metformin and inhaled salbutamol and 216 periods for all three medical products combined.
IQR = interquartile range.
Stock status for captopril was below the 4-week minimum stock level in 2/12 (16%) months at the hospital vs. 7/12 (58%) months at RHCs. However, metformin and inhaled salbutamol were only below the 4-week minimum stock levels at both the hospital and RHCs in 1/12 (8%) months and 4/12 (33%) months, respectively (Figure 2).
FIGURE 2.
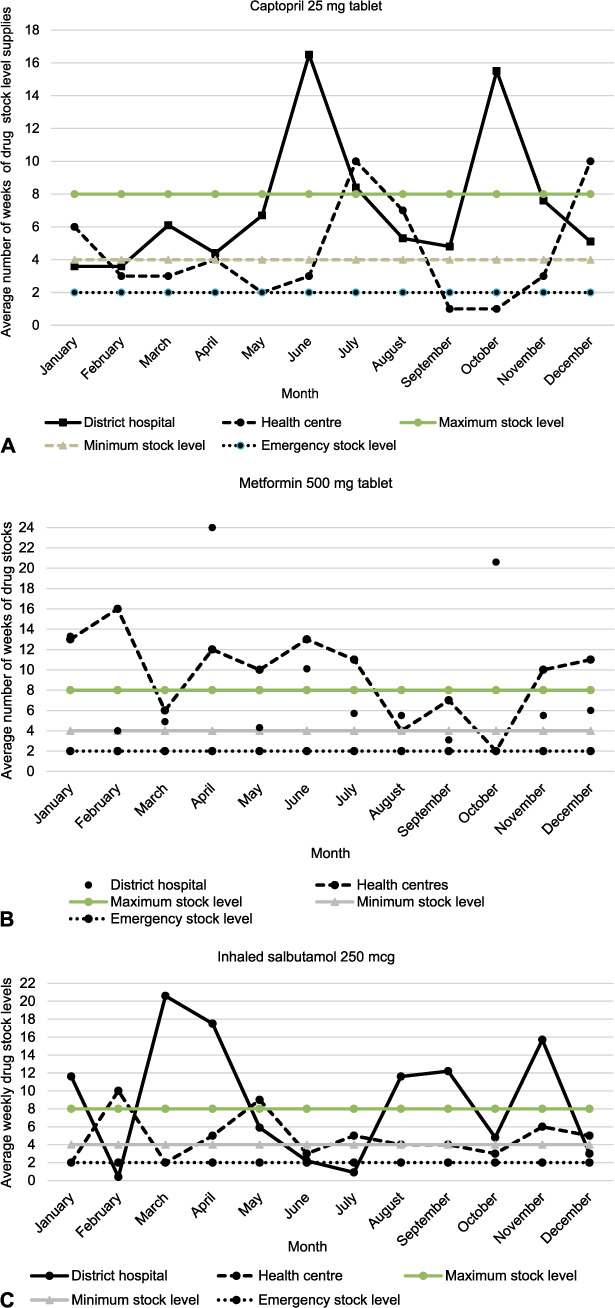
Average weeks of drug stock levels of three NCD drugs in comparison to maximum-, minimum- and emergency- stock level thresholds, stratified by type of health facility in Kirehe District, Rwanda, January–December 2018. NCD = non-communicable diseases.
Total quantities of drugs delivered from the district pharmacy to health facilities were less than the combined total quantities requested for 8/12 (67%), 5/12 (42%), 8/12 (67%) months for respectively captopril, metformin and inhaled salbutamol (Figure 3).
FIGURE 3.
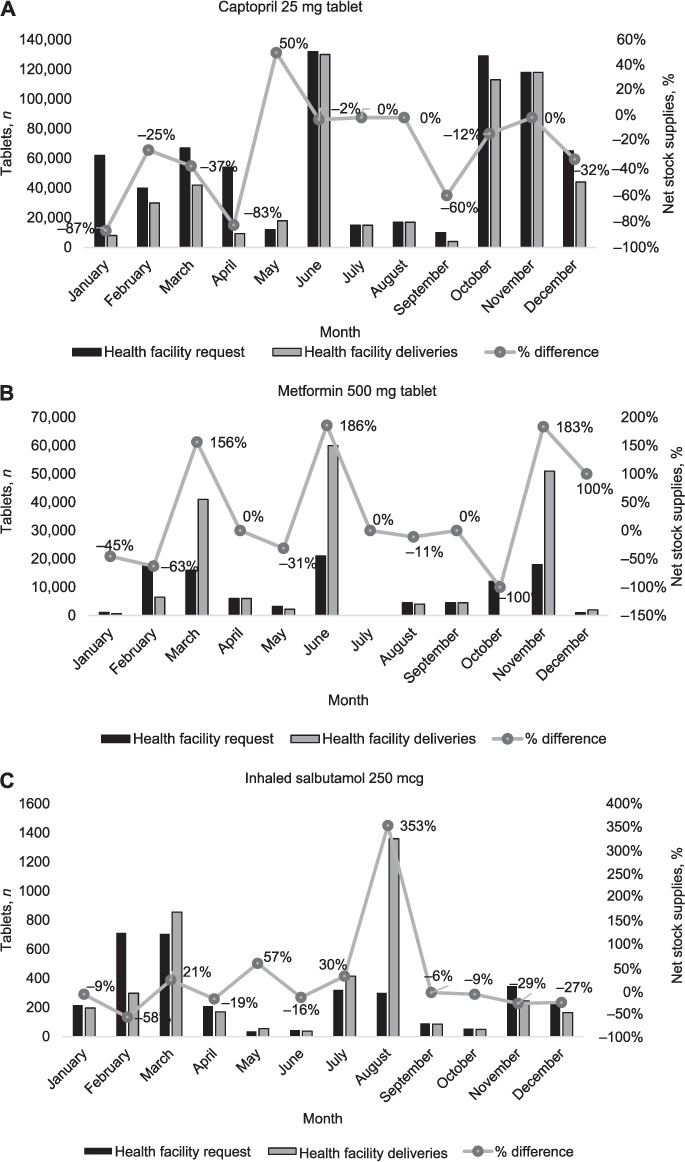
Monthly trends for quantity of drugs requested by health facilities vs. total quantities of drugs delivered from the district pharmacy and the percentage net stock supplies stratified by type of NCD drug in Kirehe District, Rwanda, January–December 2018. The proportion of net stock supplies is the percentage difference between the total quantities of drugs delivered minus the total quantity requested divided by the total quantity requested on a monthly basis. NCD = non-communicable diseases.
DISCUSSION
We assessed availability of drugs for hypertension, DM and asthma in rural health settings of Rwanda, and there were some notable findings. Compared to RHCs, NCD drug consumption was higher at the hospital, with no drug stock-outs observed. At RHCs, stock-outs were frequent, with an average duration of 1 week and particularly longer for captopril. Drug stock-outs were also observed at the district pharmacy warehouse and were also more frequent for captopril than for other drugs. The lead time was on average therefore nearly ⩾1 week at the hospital compared to RHCs. Unlike metformin and inhaled salbutamol, which were mostly well-stocked at all RHCs, captopril was frequently below the minimum recommended levels at health centres, while above or within the recommended stock levels at the district hospital. Overall, monthly drug stock deliveries were lower than health facility requests, with isolated months where drugs were oversupplied, which was subsequently followed by lower health facility requests, particularly for metformin.
The major strength of our study was the use of routine programme data; our findings can thus be extrapolated to other similar rural health settings within the public health system in Rwanda. However, our study had some limitations, as we did not assess data on the number of patients receiving treatment for the three NCD conditions as a means of verifying drug consumption accuracy; we also did not assess drug stock status at the central medical stores in relation to drug stock availability at the district pharmacy.
There are a number of possible reasons for our study findings. First, the higher consumption of all NCD drugs at the hospital is a result of more patients seeking treatment and care for these conditions at the hospital level, where doctors and relevant medical diagnostic tools are available. In Rwanda, as in many other settings, NCD care has traditionally been doctor-led, but in recent years there has been task-shifting towards nurse-led NCD patient care.20 The stock-outs observed at the health centre-level, particularly for captopril, is an indication that hypertension is the most common NCD condition treated; this is also reflected by the higher drug consumption of captopril. This is not surprising given that in Rwanda, an estimated one in five adults are reported to have elevated blood pressure, while only three per 100 persons have raised blood glucose levels, which are risk factors for hypertension and DM, respectively.12
The stock-outs at RHCs may also be associated with submissions of incorrect order requests and poor recording of management tools due to health workers not adhering to pharmacy standard operating procedures (SOPs) for accurate drug quantification.21 In addition, while there are designated nurses responsible for pharmacy stock management in RHCs, they are often overwhelmed with the provision of integrated health services.21,22
Unlike antiretroviral or anti-TB drugs, which are offered free of charge, NCD drugs (among other essential medicines and their associated health services) are paid for by the patients, mostly using the Community Based Health Insurance (CBHI) scheme, which covers the majority of the Rwanda’s population.23 While the CBHI is a good intervention, occasional payment delays by the CBHI have led to increasing drugs debts among health facilities, and this can further limit their ability to place adequate stock orders and comply with re-supply frequency among both district pharmacy and health facilities.
The stock-out durations observed in RHCs may be explained by late notices of low drug stocks or submission of emergency stock order requests to the district pharmacy. This may be coupled with transport unavailability at the health facility for timely collection of ad-hoc emergency supplies from the district pharmacy if stocks are available. Alternatively, RHCs may borrow stocks from neighbouring RHCs with adequate stocks until they receive scheduled deliveries from the district pharmacy. The longer drug delivery lead time at the district hospital compared to RHCs, despite its proximity to the district pharmacy, is likely due to the greater priority being accorded to RHCs who encounter more frequent stock-outs.
Drug stock-out occurrences are not only unique to Rwanda’s health setting. Our findings are similar to those reported in a study conducted in Swaziland in which stock-outs at centralised medical stores ranged from 3 to 6 months.24 Drug stock-outs have also been reported in other sub-Saharan African countries for free-of-charge HIV commodities, where approximately half of all assessed health facilities encountered a stock-out occurrence with a median duration of >1 month, which was associated with challenges in managing supply chain.25
There are important implications arising from this study. First, the occurrence of drug stock-outs is detrimental to patient treatment adherence and further retention in care which often leads to poor related health outcomes. In a study from South Africa, health workers reported patients becoming lost from care when advised to return to the health facility at a later date for drug re-supplies.26 In Rwanda, the presence of a digital tool (eLMIS), gives the district pharmacy as well as the Ministry of Health the ability to more regularly monitor real-time data on health facility NCD drug consumption and stock levels. This should guide performance-informed support and supervision visits to assess the accuracy of drug quantity requests submitted to the district pharmacy and to assess bottlenecks influencing drug stock-out occurrences.
Second, while there is guidance from the district pharmacy on ensuring that drug stock levels do not fall below the minimum-or emergency- stock level thresholds, this practice is not being adhered to. This often leads to reactionary submission of emergency order requests that are subsequently followed by stock-outs of long durations. Therefore, there is need for effective implementation and compliance with SOPs on health commodity management coupled with availability of skilled staffs and a well-functioning digital supply chain.
Third, the insufficient drug deliveries in relation to submitted stock order requests which often culminates in drug stock-outs suggest the need to investigate the challenges related to delayed CBHI payments to health facilities. These delays often lead to limited funds to procure adequate quantities of NCD drugs that match health facility needs.
In conclusion, we observed frequent drug stock-outs of long duration at RHCs, particularly for captopril. This is coupled with delayed and insufficient drug deliveries compared to health facility needs. Regular tracking of NCD drug stock levels need to be reinforced to avert stock-outs and ensure an optimised availability of NCDs drugs, especially captopril.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union; Paris, France) and Medécins Sans Frontières (MSF/Doctors Without Border; Paris, France). The specific SORT IT programme which resulted in this publication was jointly organised, implemented and mentored by the Centre for Operational Research, The Union; MSF-Luxembourg (MSF LuxOR; Luxembourg); MSF-Belgium (MSF-OCB; Brussels, Belgium); the University of Bergen, Bergen, Norway and the London School of Hygiene & Tropical Medicine, London, UK. Funding was provided by the UK Department for International Development (DFID) and La Fondation Veuve Emile Metz-Tesch, Luxembourg.
Footnotes
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of interest: none declared.
References
- 1.Bennett J E, et al. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet. 2018;392(10152):1072–1088. doi: 10.1016/S0140-6736(18)31992-5. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutayeb A. New York, NY, USA: Springer-Verlag; 2010. The burden of communicable and non-communicable diseases in developing countries. Handbook of disease burdens and quality of life measures; pp. 531–546. [Google Scholar]
- 4.Onyango M A, et al. Perceptions of Kenyan adults on access to medicines for non-communicable diseases: a qualitative study. PloS One. 2018;13(8):e0201917. doi: 10.1371/journal.pone.0201917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaman M M, Choudhury S R, Rahman S, Ahmed J. Prevalence of rheumatic fever and rheumatic heart disease in Bangladeshi children. Indian Heart J. 2015;67(1):45–49. doi: 10.1016/j.ihj.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappuccio FP, Miller MA. Cardiovascular disease and hypertension in sub-Saharan Africa: burden, risk and interventions. Intern Emerg Med. 2016;11(3):299–305. doi: 10.1007/s11739-016-1423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouda H N, et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375–e1387. doi: 10.1016/S2214-109X(19)30374-2. [DOI] [PubMed] [Google Scholar]
- 8.Doctors Without Borders (MSF), the Rural Health Advocacy Project (RHAP), Treatment Action Campaign (TAC) and SECTION27 The chronic crisis: essential drug stock-outs risk unnecessary death and drug resistance in South Africa. https://www.msf.org.za/system/tdf/publications/ec_drug_stockouts_5_months_on_june_2013_1.pdf?file=1&type=node&id=6699&force=/ Accessed November 2019.
- 9.Mattke S, et al. Improving access to medicines for non-communicable diseases in the developing world. https://www.rand.org/pubs/occasional_papers/OP349.html/ Accessed November 2019. [PMC free article] [PubMed]
- 10.Hodes R, et al. How front-line healthcare workers respond to stock-outs of essential medicines in the Eastern Cape Province of South Africa. S Afr Med J. 2017;107(9):738–740. doi: 10.7196/SAMJ.2017.v107i9.12476. [DOI] [PubMed] [Google Scholar]
- 11.De Jongh T E et al. Health impact of external funding for HIV, tuberculosis and malaria: systematic review. Health Policy Plan. 2014;29(5):650–662. doi: 10.1093/heapol/czt051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Non-communicable diseases country profiles 2018. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 13.Rwanda Ministry of Health Rwanda noncommunicable disease policy. Kigali, Rwanda: Rwanda Ministry of Health; 2015. [Google Scholar]
- 14.Nditunze L, et al. Assessment of essential medicines stock-outs at health centers in Burera District in Northern Rwanda. Rwanda J. 2015;2(1):85–88. [Google Scholar]
- 15.Ministry of Health Annual health statistics booklet, key statistics in health sector for year, 2016. Kigali, Rwanda: MoH; 2016. http://www.moh.gov.rw/fileadmin/user_upload/HMIS/2016_Annual_Statistical_booklets_V9_08_03_2018.pdf/ Accessed November 2019. [Google Scholar]
- 16.Ministry of Health, Rwanda National List of Essential Medicines for Adults, Rwanda. 6th ed. Kigali, Rwanda: Rwanda MoH; 2015. https://www.medbox.org/preview/59eeec8b-0000-430b-80fd-3f871fcc7b87/doc.pdf Accessed November 2019. [Google Scholar]
- 17.Ministry of Health, Rwanda Internal medicine clinical treatment guidelines. Kigali, Rwanda: Rwanda MoH; 2012. https://www.medbox.org/preview/5332d567-076c-4818-90ff-3f351fcc7b89/doc.pdf Accessed November 2019. [Google Scholar]
- 18.Ministry of Health,- Rwanda National Pharmacy Policy. Kigali, Rwanda: Rwanda MoH; 2016. https://www.medbox.org/preview/5b30ce8d-9d7c-42e1-b42a-102a1fcc7b87/doc.pdf Accessed November 2019. [Google Scholar]
- 19.Ministry of Health Rwanda NSCA and pharmaceutical supply chain strategic plan technical report. Kigali, Rwanda: MoH; 2013. Report. https://docplayer.net/19741371-Rwanda-nsca-and-pharmaceutical-supply-chain-strategic-plan-technical-report.html/ Accessed November 2019. [Google Scholar]
- 20.Tapela N M, et al. Treatment of non-communicable disease in rural resource-constrained settings: a comprehensive, integrated, nurse-led care model at public facilities in Rwanda. Lancet Glob Health. 2015;3:S36. [Google Scholar]
- 21.Republic of Rwanda Ministry of Health Standard operating procedures for health commodities management in health facilities. Kigali, Rwanda: MoH; 2014. Accessed November 2019. [Google Scholar]
- 22.Wagenaar BH, et al. Stock-outs of essential health products in Mozambique -longitudinal analyses from 2011 to 2013. Trop Med Int Health. 2014;19(7):791–801. doi: 10.1111/tmi.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United States Agency for International Development Health insurance profile: Rwanda. Washington DC, USA: USAID; 2015. http://www.africanstrategies4health.org/uploads/1/3/5/3/13538666/country_profile_-_rwanda_-_us_letter.pdf/ Accessed October 2019. [Google Scholar]
- 24.Shabangu K, Suleman F. Medicines availability at a Swaziland hospital and impact on patients. Afr J Prim Health Care Fam Med. 2015;7(1):e1–e6. doi: 10.4102/phcfm.v7i1.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gils T, et al. Stockouts of HIV commodities in public health facilities in Kinshasa: Barriers to end HIV. PLoS One. 2018;13(1):e0191294. doi: 10.1371/journal.pone.0191294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magadzire B P, Ward K, Leng H M J, Sanders D. Inefficient procurement processes undermine access to medicines in the Western Cape Province of South Africa. S Afr Med J. 2017;107(7):581–584. doi: 10.7196/SAMJ.2017.v107i7.11356. [DOI] [PubMed] [Google Scholar]


