Abstract
Background
Studies have found different waning rates of neutralising antibodies compared with binding antibodies against SARS-CoV-2. The impact of neutralising antibody waning rate at the individual patient level on the longevity of immunity remains unknown. We aimed to investigate the peak levels and dynamics of neutralising antibody waning and IgG avidity maturation over time, and correlate this with clinical parameters, cytokines, and T-cell responses.
Methods
We did a longitudinal study of patients who had recovered from COVID-19 up to day 180 post-symptom onset by monitoring changes in neutralising antibody levels using a previously validated surrogate virus neutralisation test. Changes in antibody avidities and other immune markers at different convalescent stages were determined and correlated with clinical features. Using a machine learning algorithm, temporal change in neutralising antibody levels was classified into five groups and used to predict the longevity of neutralising antibody-mediated immunity.
Findings
We approached 517 patients for participation in the study, of whom 288 consented for outpatient follow-up and collection of serial blood samples. 164 patients were followed up and had adequate blood samples collected for analysis, with a total of 546 serum samples collected, including 128 blood samples taken up to 180 days post-symptom onset. We identified five distinctive patterns of neutralising antibody dynamics as follows: negative, individuals who did not, at our intervals of sampling, develop neutralising antibodies at the 30% inhibition level (19 [12%] of 164 patients); rapid waning, individuals who had varying levels of neutralising antibodies from around 20 days after symptom onset, but seroreverted in less than 180 days (44 [27%] of 164 patients); slow waning, individuals who remained neutralising antibody-positive at 180 days post-symptom onset (46 [28%] of 164 patients); persistent, although with varying peak neutralising antibody levels, these individuals had minimal neutralising antibody decay (52 [32%] of 164 patients); and delayed response, a small group that showed an unexpected increase of neutralising antibodies during late convalescence (at 90 or 180 days after symptom onset; three [2%] of 164 patients). Persistence of neutralising antibodies was associated with disease severity and sustained level of pro-inflammatory cytokines, chemokines, and growth factors. By contrast, T-cell responses were similar among the different neutralising antibody dynamics groups. On the basis of the different decay dynamics, we established a prediction algorithm that revealed a wide range of neutralising antibody longevity, varying from around 40 days to many decades.
Interpretation
Neutralising antibody response dynamics in patients who have recovered from COVID-19 vary greatly, and prediction of immune longevity can only be accurately determined at the individual level. Our findings emphasise the importance of public health and social measures in the ongoing pandemic outbreak response, and might have implications for longevity of immunity after vaccination.
Funding
National Medical Research Council, Biomedical Research Council, and A*STAR, Singapore.
Introduction
The COVID-19 pandemic,1 caused by SARS-CoV-2,2 has lasted more than a year with no sign of ending. The pandemic has resulted in more than 114 million cases and close to 2·5 million deaths as of March 3, 2021.3 Several key unanswered scientific questions remain concerning the pandemic. One of these questions is the nature and longevity of protective immunity, which is highly important in the context of risk assessment for reinfection and vaccine development.4, 5
In any viral infection, it is expected that both antibody and T-cell responses will play roles in protective immunity and there are published studies to suggest that this might also be true for SARS-CoV-2 infection.4, 6, 7 In patients who have recovered from COVID-19, some individuals have very low levels or absence of neutralising antibodies, indicating that T-cell immunity could be the dominant mechanism, at least in some individuals.8, 9 However, high levels of neutralising antibodies appear to be correlated with protection against reinfection.7
Research in context.
Evidence before this study
We searched PubMed on Sept 16, 2020, with no restrictions, using the terms (“SARS-CoV-2” OR “COVID-19”) AND (“neutralizing antibody” OR “neutralising antibody”) AND “longevity”. Our search retrieved no published papers. We searched medRixv and bioRixv and found 22 and 18 preprints, respectively. Most studies were not directly related to longitudinal cohort investigation. A few studies followed antibody responses using ELISA or virus neutralisation tests using live virus in Biosafety Level (BSL) 3 laboratories or pseudovirus in BSL2 laboratories, and few had a duration of 6 months after symptom onset. A common limitation was cohort size (mostly less than 100 participants) and irregular sampling frequency. None of the studies investigated the avidity of SARS-CoV-2-specific antibodies in the context of peak antibody responses and neutralising antibody longevity.
Added value of this study
In this 180-day longitudinal survey, we examined the dynamics of antibody changes, focusing on the level of neutralising antibodies, as they are better correlated with protective immunity than are total binding antibodies. We further investigated the change in avidity as an additional biomarker for the quality of antibody responses in different individuals, and found that rapid avidity maturation played an important part in determining not only the level of neutralising antibodies, but also the waning rate of neutralising antibodies. Antibody level and avidity were further correlated with other immune markers, including cytokines and T-cell immunity. Using machine learning algorithms, we established a prediction model that indicated that the longevity of neutralising antibody immunity for patients with COVID-19 could vary from weeks to decades.
Implications of all the available evidence
Although we are not at a stage to conclusively correlate the level of antibody responses with protective immunity, we are in a much better position to assess the dynamics of antibody responses with data from a cohort who have been in convalescence for more than 6 months. Our findings show that the level and quality of neutralising antibodies can vary greatly from patient to patient, and that neutralising antibodies can last for a long period in certain patient populations, so it is important to monitor this at an individual level. This work might have implications for longevity of immunity after vaccination.
Previous studies of other coronaviruses offer little guidance for SARS-CoV-2 serology. Antibody responses to the four seasonal human coronaviruses are generally short-lived and recovered individuals are prone to reinfection.5, 10 Current knowledge for SARS-CoV and Middle East respiratory syndrome coronavirus is less conclusive, with some studies suggesting rapidly waning antibodies and others indicating long-lasting antibody-mediated immunity.11, 12, 13 For SARS-CoV-2, several serological studies have assessed the dynamics and duration of antibody responses. These findings are not uniform, with some claiming rapid waning and others showing antibody persistence, partly due to the fact that different groups have measured different antibodies and most studies were done at an early stage of convalescence.14, 15, 16, 17, 18
Longitudinal serological studies of patients who have recovered from COVID-19 are vital to providing key information that is lacking in the context of acquisition of protective immunity and longevity of neutralising antibodies for SARS-CoV-2. We aimed to investigate the peak levels and dynamics of neutralising antibody waning and IgG avidity maturation over time, and correlate this with clinical parameters, cytokines, and T-cell responses. Here, we present our findings from a 180-day cohort study in Singapore.
Methods
Patient selection and data collection
The inclusion criterion for this prospective cohort study was confirmed COVID-19 infection, defined as positive SARS-CoV-2 PCR from any respiratory sample. There were no exclusion criteria. Electronic medical records of enrolled patients were reviewed and data entered onto a standardised collection form adapted from the International Severe Acute Respiratory and Emerging Infection Consortium case record form for emerging severe acute respiratory infections. Disease severity was defined as follows: mild (no pneumonia on chest radiography), moderate (pneumonia on chest radiography but not requiring supplemental oxygen), and severe (requiring supplemental oxygen, intensive care unit admission, or mechanical ventilation). Serial blood samples were collected weekly during hospitalisation and after hospital discharge between days 30–60, on day 90, and on day 180. A more detailed description of the patient cohort is given in the appendix (p 1). The patient cohort was recruited from Jan 30, 2020, to Aug 14, 2020, and had confirmed SARS-CoV-2 infection as detected by nasal swab PCR test.
Written informed consent was obtained from patients as part of a larger multicentre observational cohort study characterising emerging infectious diseases (PROTECT study;19 National Healthcare Group Domain Specific Review Board [DSRB] reference number 2012/00917; NUS-IRB reference code H-20-006). SARS-CoV recall participants were recruited under the ethics approval numbers DSRB E 2020/00091 and healthy individuals under NUS-IRB reference codes H-18-029 and 04-140.
Neutralisation antibody level and IgG avidity assays
We used the surrogate virus neutralisation test for both SARS-CoV-2 and SARS-CoV. The development and validation of the surrogate virus neutralisation test assay from our group was previously reported20 and detailed protocols are provided in the appendix (p 2). Briefly, a biochemical measurement of the amounts of neutralising antibody present in the test sera was done by inhibition ELISA, whereby the test sera were first pre-incubated with SARS-CoV-2 or SARS-CoV receptor binding domain-horseradish peroxidase, then added to angiotensin-converting enzyme 2-coated plates. Total IgG avidity was determined using ELISA in the presence and absence of urea (see appendix p 2 for details).
Multiplex microbead-based immunoassays
Plasma samples were treated with 1% Triton X-100 solvent–detergent mix for virus inactivation.21 Immune mediator levels in plasma of patients with COVID-19 at 30 days post-symptom onset and 180 days post-symptom onset were measured with the Luminex assay using the Cytokine/Chemokine/Growth Factor 45-plex Human ProcartaPlex Panel 1 (ThermoFisher Scientific; Waltham, MA, USA; see appendix pp 2–3 for detailed method).
SARS-CoV-2-specific T-cell analysis
SARS-CoV-2-specific T cells were tested as described previously.6 Briefly, peripheral blood mononuclear cells were isolated and directly tested by IFN-γ-ELISpot assay for reactivity to six SARS-CoV-2 peptide pools of 15-mers (appendix pp 15–19) covering nucleoprotein (NP-1, NP-2), membrane (M), open reading frame (ORF)3a, ORF7, and ORF8 combined, and one pool of 55 peptides covering the most immunogenic regions of spike (S).
Data processing, bioinformatics, and statistical analysis
Data processing and analysis were done in R (version 4.0.2) with the tidyverse package (version 1.3.0). Continuous variables were compared using Kruskal-Wallis test or Wilcoxon signed-rank test as indicated, and categorical variables were compared using Fisher's exact test or Wilcoxon signed-rank test as appropriate. All tests were two sided, and p<0·05 was considered statistically significant. Scatter plots and heatmaps were generated using GraphPad Prism version 8 or ggplot2 package in R (version 3.3.2). Generalised linear models in different settings (Gaussian, Poisson, Gamma, and Inverted Gaussian) was applied and Akaike information criterion for the different groups were compared. Levels of immune mediators were scaled between 0 and 1 for visualisation in the heatmap. Prediction of neutralising antibody longevity was calculated as the time that neutralising antibody surrogate virus neutralisation test inhibition percentage reached 30% by extracting the intercept for the generalised linear modelling (R package stats version 4.0.2) for days post-symptom onset and the difference between surrogate virus neutralisation test inhibition percentage and 30%. Samples were grouped by binning the neutralising antibody longevity and a decision tree made by rpart in R (version 4.1-15). Logistic regression with Firth's bias reduction method (logistf package in R version 1.24) was used to examine the association between clinical features and antibody persistence. The following covariates were chosen for inclusion in the multivariable model as they were significantly different when comparing the persistent antibody group versus the other three groups: age group (<45 years, 45–64 years, or ≥65 years), sex (male vs female), Charlson's comorbidity index group (0 vs ≥1), hypertension (present vs absent), and infection severity (mild, moderate, or severe). A multivariate ordinal logistic regression was used to examine the association between the same covariates and serological group (outcome ordered from 1=negative, 2=rapid waning, 3=slow waning, and 4=persistent). Proportional odds assumption was examined using Brant test.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
We approached 517 patients for participation in the study, of whom 288 consented for outpatient follow-up and collection of serial blood samples. 164 patients were followed up and had adequate blood samples collected for analysis, with a total of 546 serum samples collected (appendix pp 7–12) during treatment in hospital and post-discharge, up to 180 days post-symptom onset. The breakdown of the number of samples at each timepoint was as follows: 64 samples at 14 days post-symptom onset, 39 samples at 21 days post-symptom onset, 127 samples at 28 days post-symptom onset, 30 samples at 60 days post-symptom onset, 158 samples at 90 days post-symptom onset, and 128 samples at 180 days post-symptom onset. 42 (26%) of 164 patients were women, and the median age was 44 years (IQR 34·5–56; range 21–74). 72 (44%) of 164 patients had at least one comorbidity, 47 (29%) had hypertension, and 27 (16%) had diabetes. 34 (21%) of 164 patients were asymptomatic at presentation. No patients had a documented history of previous SARS infection.
Based on the slope of the regression line and whether the samples crossed the significance threshold of 30% inhibition (figure 1A ), we identified five distinctive patterns of neutralising antibody dynamics as follows: negative, individuals who did not, at our intervals of sampling, develop neutralising antibodies at the 30% inhibition level (19 [12%] of 164 patients); rapid waning, individuals who had varying levels of neutralising antibodies early on (around 20 days post-symptom onset), but seroreverted in less than 180 days (44 [27%] of 164 patients); slow waning, individuals who remained neutralising antibody-positive at 180 days post-symptom onset (46 [28%] of 164 patients); persistent, although with varying peak neutralising antibody levels, these individuals had minimal neutralising antibody decay (52 [32%] of 164 patients); and delayed response, a small group that showed an unexpected increase of neutralising antibodies during late convalescence (≥90 days post-symptom onset; three [2%] of 164 patients). These classifications of samples could be determined using a decision tree to evaluate the neutralising antibody levels at 28 days post-symptom onset, 90 days post-symptom onset, and 180 days post-symptom onset (appendix p 4).
Figure 1.
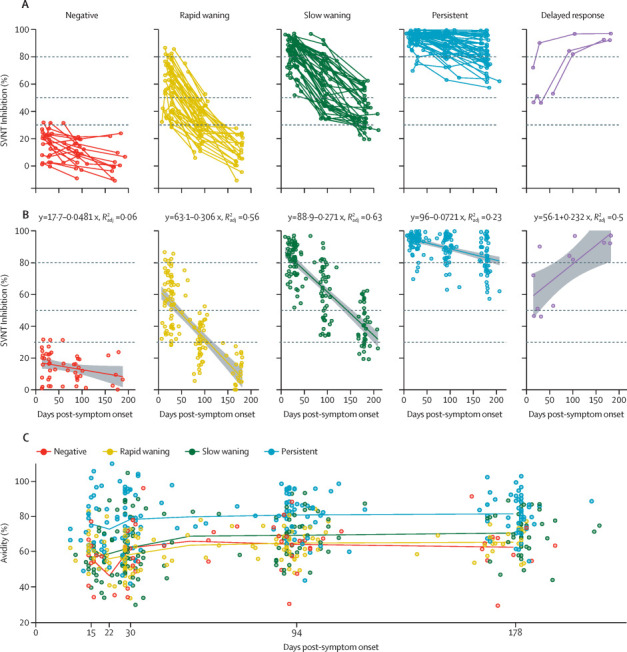
Longitudinal dynamics of neutralising antibodies
(A) Neutralising antibody level, measured by percentage inhibition of sVNT readings. (B) Linear regression model of each grouping for neutralising antibody level. Dashed lines represents 30%, 50%, and 80% of sVNT percentage inhibition. (C) Group mean of IgG avidity percentage is connected at days 14, 21, 30, 90 and 180. Since each patient blood sample was taken at a different timepoint in practice, we marked the mean days post-symptom onset of the samples within the same group but the definition of the time groups remains 14, 21, 30, 90, and 180 days post-symptom onset. Each point represents a single patient. sVNT=surrogate virus neutralisation test.
To better characterise the distinguishing features of each group, we applied a linear regression analysis to illustrate the slopes of change for each group (figure 1B). For groups 2, 3, and 4, although they all showed a general trend of waning, the speed of waning (ie, the slope of change) was very different, which resulted in very different neutralising antibody levels at 180 days post-symptom onset, with the rapid waning group showing almost all samples at less than 20% inhibition, the slow waning group at 40% inhibition or above, and the persistent group at 80% inhibition or above.
The fifth group, the delayed response group, showed an unusual increase of neutralising antibodies during the convalescent period. The mechanism and significance of this finding is unclear. Two [67%] of three patients in this group had pneumonia in hospital, but none required oxygen and one was treated with remdesivir. Since hospital discharge, two (67%) patients in the delayed response group did not report any febrile illness or acute respiratory infection, and one (33%) patient reported three episodes of asthma exacerbation. None of the patients in the delayed response group reported exposure to known patients with COVID-19 or migrant workers, who comprised most patients with COVID-19 in Singapore. As the sample number is very small (three patients), these individuals were excluded from further analysis in the current study and will be followed up in future studies if we encounter more samples in this category.
IgG maturation (ie, increase in avidity) may play a part in our observations. All samples were subjected to avidity testing and the data revealed three important findings (figure 1C). First, levels of receptor binding domain (RBD)-binding IgG antibody avidity correlated with the levels and waning rates of neutralising antibody across all patient groups. Second, for the negative, rapid waning, and slow waning groups, there was a corresponding biphasic kinetics for avidity change, with more rapid rise in the first phase (from days 15–30 post-symptom onset) than the second phase (from days 31–180 post-symptom onset). Third, for the persistent group, avidity reached a high level very early (15–30 days post-symptom onset) and showed a less obvious biphasic change.
To investigate if cytokine levels correlated with antibody waning patterns in patients with COVID-19, we profiled concentrations of cytokines and chemokines in the plasma at 30 days post-symptom onset and 180 days post-symptom onset (appendix p 5). At the late convalescent timepoint of 180 days post-symptom onset, higher levels of pro-inflammatory cytokines (IFN-γ, IL-12p70, and IL-17A), pro-inflammatory chemokine (IP-10), and growth factors (human growth factor) were observed in the persistent group compared with all other groups. This result contrasted with patients in the negative group, with lower concentrations of pro-inflammatory IFN-γ, IL-12p70, and IL-17A at 180 days post-symptom onset compared with all other groups. There was no difference in IL-6 levels across the different groups (data not shown).
For a subset of 23 samples randomly selected from each group at day 180, we tested T cells that were reactive to peptides of S, M, NP, ORF3a, and ORF7/8 proteins to investigate if there was a correlation between T-cell immunity and different antibody kinetics. We made two observations (appendix p 6). First, all patients in each group maintained substantial specific T-cells at 180 days post-symptom onset and the T-cell response was multi-specific, with most donors having T-cells reactive to NP, M, and S. Second, there was no clear difference in T-cell immunity between the groups, consistent with previous findings.6, 22
We found significant differences in terms of age, presence of comorbidities, baseline symptoms, investigations, and clinical outcomes when comparing all four groups against each other and in the persistent antibody group compared with the other three groups with waning or absent antibodies (table 1 ). We observed a distinct stepwise progression from the negative group to the persistent group, whereby patients with persistent antibodies were older and had more comorbidities, including hypertension and diabetes mellitus.
Table 1.
Demographic and clinical characteristics of patients, grouped by antibody dynamics
| Persistent (n=52)* | Slow waning (n=46) | Rapid waning (n=44) | Negative (n=19) | p value† | p value‡ | ||
|---|---|---|---|---|---|---|---|
| Patient characteristics | |||||||
| Age, years | 52 (43–60·5) | 44 (33–55) | 25·5 (27–48·5) | 42 (37–52) | 0·0001 | 0·0001 | |
| Sex | 0·76 | 0·70 | |||||
| Female | 14 (27%) | 13 (28%) | 10 (23%) | 3 (16%) | .. | .. | |
| Male | 38 (73%) | 33 (72%) | 34 (77%) | 16 (84%) | .. | .. | |
| Ethnicity | .. | .. | .. | .. | 0·020 | 0·065 | |
| Chinese | 31 (60%) | 27 (59%) | 19 (43%) | 4 (21%) | .. | .. | |
| Malay | 7 (13%) | 2 (4%) | 5 (11%) | 0 | .. | .. | |
| South Asian (Indian or Bangladeshi) | 9 (17%) | 11 (24%) | 14 (32%) | 11 (58%) | .. | .. | |
| Other | 5 (10%) | 6 (13%) | 6 (14%) | 4 (21%) | .. | .. | |
| Charlson comorbidity index | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0·032 | 0·012 | |
| Any comorbidity | 29 (56%) | 18 (39%) | 15 (34%) | 7 (37%) | 0·15 | 0·027 | |
| Diabetes | 17 (33%) | 6 (13%) | 3 (7%) | 1 (5%) | 0·0030 | 0·0005 | |
| Hypertension | 22 (42%) | 12 (26%) | 8 (18%) | 3 (16%) | 0·037 | 0·0081 | |
| Baseline symptoms | |||||||
| Duration of symptoms, days (n=158) | |||||||
| n | 50 | 46 | 44 | 18 | .. | .. | |
| Median (IQR) | 4 (2–7) | 2 (1–5) | 2 (0–6) | 0 (0–3) | 0·0008 | 0·0006 | |
| Fever (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| n (%) | 39 (78%) | 34 (74%) | 18 (41%) | 5 (26%) | <0·0001 | 0·0028 | |
| Cough (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| n (%) | 34 (68%) | 25 (54%) | 22 (50%) | 5 (26%) | 0·017 | 0·025 | |
| Dyspnoea (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| n (%) | 11 (22%) | 3 (7%) | 1 (2%) | 1 (5%) | 0·011 | 0·0014 | |
| Sore throat (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| n (%) | 18 (36%) | 15 (33%) | 19 (43%) | 3 (16%) | 0·21 | 0·86 | |
| Rhinorrhoea (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| n (%) | 13 (26%) | 9 (20%) | 13 (30%) | 3 (16%) | 0·60 | 0·69 | |
| Asymptomatic (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| n (%) | 3 (6%) | 7 (15%) | 13 (30%) | 11 (58%) | <0·0001 | 0·0008 | |
| Baseline investigations | |||||||
| White blood count, ×109/L (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| Median (IQR) | 5·40 (4·10–6·70) | 5·15 (4·50–6·90) | 6·35 (4·65–8·45) | 7·20 (4·60–9·90) | 0·079 | 0·19 | |
| Neutrophil count, ×109/L (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| Median (IQR) | 3·84 (2·62–5·40) | 3·63 (2·40–4·73) | 3·78 (2·51–5·12) | 4·14 (3·01–5·68) | 0·77 | 0·95 | |
| Lymphocyte count, ×109/L (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| Median (IQR) | 1·08 (0·79–1·44) | 1·13 (0·85–1·58) | 1·79 (1·22–2·56) | 1·73 (1·58–2·34) | 0·0001 | 0·0004 | |
| C-reactive protein, mg/L (n=153) | |||||||
| n | 49 | 44 | 42 | 18 | .. | .. | |
| Median (IQR) | 50·3 (13·0–76·6) | 9·85 (1·8–50·7) | 3·3 (1·4–7·6) | 1·85 (1·2–4·1) | 0·0001 | 0·0001 | |
| Lactate dehydrogenase, U/L (n=148) | |||||||
| n | 47 | 41 | 41 | 19 | .. | .. | |
| Median (IQR) | 589 (409–721) | 423 (364–538) | 362 (314–421) | 374 (319–439) | 0·0001 | 0·0001 | |
| Creatinine (μmol/L) (n=159) | |||||||
| n | 50 | 46 | 44 | 19 | .. | .. | |
| Median (IQR) | 79·5 (61–87) | 73 (62–83) | 72 (62–87) | 71 (64–81) | 0·70 | 0·26 | |
| Outcomes | |||||||
| Pneumonia | 47 (90%) | 28 (61%) | 12 (27%) | 1 (5%) | <0·0001 | <0·0001 | |
| Supplemental oxygen requirement | 33 (63%) | 9 (20%) | 2 (5%) | 0 | <0·0001 | <0·0001 | |
| Intensive care unit admission | 21 (40%) | 5 (11%) | 1 (2%) | 0 | <0·0001 | <0·0001 | |
| Mechanical ventilation | 8 (15%) | 0 | 0 | 0 | 0·0008 | <0·0001 | |
Data are median (IQR) or n (%). Categorical variables represented as number (percentage) and compared using Fisher's exact test. Continuous variables represented as median (interquartile range) and compared using Kruskal-Wallis test.
Two of 52 patients had missing data for baseline symptoms and baseline investigations; the rest of the data (demographics, medical history, and clinical outcomes) were complete.
Comparing all four groups independently.
Persistent antibody group versus all other three groups (slow waning, rapid waning, and negative).
The demographic differences we observed were probably related to increased disease severity, as patients in the persistent group had poorer clinical outcomes, including pneumonia, supplemental oxygen requirement, intensive care unit admission, and mechanical ventilation. Baseline symptoms and investigations reflected this increased disease severity, with a greater proportion of patients with fever, cough, dyspnoea, reduced lymphocyte count, increased C-reactive protein, and increased lactate dehydrogenase in the persistent group. We observed a greater proportion of asymptomatic individuals in the negative group (11 [58%] of 19 patients) compared with the persistent group (three [6%] of 52 patients).
Viral load data, in the form of quantitative PCR results, were available for 70 patients and, in this subgroup, baseline earliest nasopharyngeal PCR cycle threshold values on admission were not associated with antibody response (data not shown), although there are limitations to this data as sample types varied (eg, nasopharyngeal swab, oropharyngeal swab, or sputum).
In the multivariable model that incorporated age, sex, and presence of comorbidities, only disease severity was independently associated with persistent antibody levels, with an adjusted odds ratio of 5·20 (95% CI 1·83–16·7) for moderate disease severity and 30·3 (10·0–107·9) for severe disease severity, both compared with patients with mild disease (table 2 ).
Table 2.
Logistic regression analysis of predictors of persistent antibody trend (n=161)
|
Univariable model (persistence)* |
Multivariable model (persistence)* |
Ordinal multivariable logistic regression model† |
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | ||
| Age group, years | |||||||
| <45 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| 45–65 | 3·19 (1·55–6·76) | 0·0015 | 1·81 (0·72–4·58) | 0·21 | 1·38 (0·68–2·80) | 0·37 | |
| >65 | 16·8 (4·23–94·9) | <0·0001 | 5·37 (0·90–41·7) | 0·065 | 3·18 (0·61–25·2) | 0·20 | |
| Female sex | 1·19 (0·55–2·48) | 0·65 | 1·84 (0·68–5·1) | 0·23 | 2·28 (1·08–4·92) | 0·032 | |
| Charlson comobidity index score ≥1 | 2·51 (1·23–5·15) | 0·012 | 0·92 (0·27–2·91) | 0·89 | 1·26 (0·53–3·03) | 0·60 | |
| Hypertension | 2·72 (1·34–5·56) | 0·0059 | 0·90 (0·26–2·98) | 0·86 | 0·73 (0·29–1·82) | 0·50 | |
| Severity | |||||||
| Mild (no pneumonia) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Moderate (pneumonia, no supplemental O2) | 5·73 (2·06–18·1) | 0·0007 | 5·20 (1·83–16·7) | 0·0017 | 6·45 (2·98–14·48) | <0·0001 | |
| Severe (supplemental O2 or intensive care unit admission) | 39·7 (14·0–132·8) | <0·0001 | 30·3 (10·0–107·9) | <0·0001 | 51·1 (18·5–154·4) | <0·0001 | |
OR=odds ratio.
Firth logistic regression.
Ordinal logistic regression. The proportional odds assumption was examined with Brant test: parallel regression assumptions hold for individual variables and the overall model (p=0·99).
The longevity of neutralising antibodies (in days) was calculated by the slope and intercept of linear modelling of the different groups and individuals. Generalised linear models in different settings were applied and areas under the curve for the different groups were compared. Gaussian distribution had the lowest Akaike information criterion in all groups except for negative samples, but that group was not used for longevity prediction (data not shown). The median neutralising antibody positive days for the rapid waning, slow waning, and persistent groups were 96 days, 201 days, and 580 days, respectively (figure 2A ; appendix pp 7–12). The individual neutralising antibody-positive days within each group had substantial variations. The persistent group had the greatest variation of longevity, as the minimum duration of neutralising antibody longevity was predicted to be 326 days and the maximum was more than 14 881 days. 56 patients available for 270 days post-symptom onset sampling were used to test the correlation of modelling and actual data. The predicted and actual 270 days data had a Pearson correlation of 0·84, showing robustness and validating our neutralising antibody longevity prediction algorithm (figure 2B).
Figure 2.
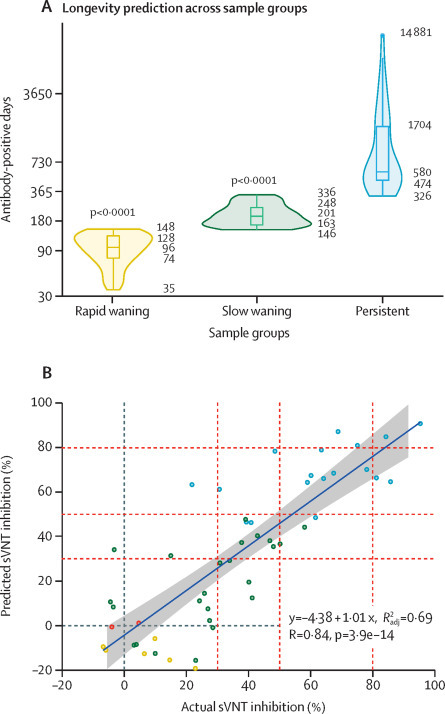
Prediction of neutralising antibody longevity using linear regression modelling for different groups
(A) Violin plots and box plots showing neutralising antibody positive days. p-value was calculated by Wilcoxon signed-rank test with the persistent group as the reference. For each group, the 0th, 25th, 50th, 75th, and 100th percentile are marked. (B) Correlation of predicted sVNT inhibition percentage compared with actual sVNT inhibition percentage for a subset of the returning cohort at 270 days post-symptom onset. sVNT=surrogate virus neutralisation test.
We observed some patients in the persistent and delayed response groups had increasing antibody levels many months after they recovered from acute disease. This increase affected our prediction modelling for these patients, as the modelling was developed for a downward trendline, resulting in the infinite prediction of antibody longevity (more than half a million days).
We extended the cohort study to a total of 20 SARS survivors recruited 17 years after their disease recovery (appendix p 13). 90% of the recalled individuals showed surrogate virus neutralisation test percentage inhibition at 30% or above. This pattern of neutralising antibody waning dynamics is similar to the one predicted for the persistent group of the patients who recovered from SARS-CoV-2.
Discussion
Previous published studies indicated that S-specific antibodies waned more slowly than did N-specific antibodies,16, 20 and that the level of neutralising antibodies could be an important indicator of protection.5, 7 In this 180-day longitudinal study of SARS-CoV-2 antibody dynamics, we focused specifically on functional neutralising antibodies using the operator-friendly surrogate virus neutralisation test assay platform, which has an excellent concordance with the live virus neutralisation test and has been successfully applied in multiple studies from different countries.6, 7, 20, 23 The surrogate virus neutralisation test offers advantages at the operational level for large numbers of samples and when repeated testing is required, as was the case for this study.
Our study covered a 180-day period after infection, with a subset of samples extended to 270 days. The extended timeframe and multiple samplings for many of the individuals in the study allowed us to have a more reliable and in-depth dissection of the multifaceted nature of neutralising antibody dynamics. In contrast to previously published studies,14, 15, 17, 18 which generally focused on antibody decay among different cohorts and in different geographical regions, we showed the diversity of neutralising antibody dynamics in five distinctive patterns. These patterns differed in the peak level of neutralising antibodies, the speed of decay, and the IgG avidity maturation process and immune modulator profiles.
Neutralising antibody longevity was associated with sustained levels of inflammatory cytokines up to at least 180 days post-symptom onset in patients who had recovered from COVID-19. Patients in the persistent group maintained high systemic concentrations of pro-inflammatory cytokines, even at 6 months post-symptom onset. This pro-inflammatory cytokine milieu has been shown to correlate strongly with antibody levels in COVID-19.24 Pro-inflammatory IFN-γ,25 IL-12,26 and IL-1727 have been indicated to play a part in B-cell development. In this context, it is interesting to note that there was no significant difference in IL-6 levels across the different groups, although IL-6 has been shown to be important for IgG production.28 By contrast, T-cell responses seem to have no clear correlation with the different patterns of neutralising antibody dynamics. Patients from all groups, including the negative group, showed sustained T-cell immunity 6 months after initial infection. These data indicate that the pro-inflammatory environment during late convalescence (≥90 days after symptom onset) could be important in maintaining long-term COVID-19 specific neutralising antibody levels.
With regard to clinical parameters, the persistent group had the strongest correlation with disease severity, consistent with previous findings of a correlation between neutralising antibody level and disease severity at the neutralising antibody peak. Greater disease severity was independently associated with persistent neutralising antibody level, and patients with milder disease appeared to have more rapid neutralising antibody waning. This finding could have substantial implications in terms of population-level or herd immunity, especially if ongoing viral mutation attenuates SARS-CoV-2 virulence, with a consequent reduction in the proportion of patients with severe disease. Asymptomatic individuals appear to have lower levels of seroconversion or antibody persistence,29 although this needs further investigation in large cohort studies.
By modelling the rate of neutralising antibody waning in different groups, we were able to establish prediction models to estimate the longevity of responses in individuals in the three groups showing different neutralising antibody waning rates. The rate of waning suggests reinfection during second and later waves of infection is likely to occur, limiting the viability of a herd immunity strategy before an effective vaccine.30 Assuming similar rates of waning after vaccination, annual administration is likely to be necessary to prevent large outbreaks as population immunity declines. However, contrary to some previously published studies suggesting a short lifespan of SARS-CoV-2 RBD-specific antibodies, we showed that neutralising antibodies might persist for many years in some patients who have recovered from COVID-19. Although such predictions can only be confirmed over the next 5–10 years, we believe our predictions are not unrealistic considering that patients infected with SARS-CoV showed long lasting neutralising antibodies 17 years after initial infection, also observed in our previous studies.13, 31 As most patients with SARS developed severe disease, it is unsurprising that their neutralising antibody waning pattern was more aligned to the persistent group of patients who recovered from SARS-CoV-2.
Our study has several limitations. This was an observational cohort study, and although individuals enrolled in the study were representative of the community pandemic in Singapore, there might be host or environmental conditions in other populations that affect immune responses that we are unable to account for. The largest samples available in this cohort were for individuals of Chinese ethnicity, and given that there are differences in outcomes and disease progression by different ethnic groups observed in some countries, the results of this study might only be generalisable to Chinese people. The median age of the cohort was 44 years; thus the results might not be generalisable to older adults or children, who might have different immune profiles. We enrolled individuals with various disease severities, but asymptomatic infections were few in number, limiting our ability to study this important group. Some individuals were lost to follow-up and the timing of sample collection also varied. Although such issues can be adjusted by using statistical models to correct survivor bias, without knowing who was lost to follow-up permanently (as a participant could miss one timepoint but come back for the next), this is not feasible.
In conclusion, our study showed that neutralising antibody dynamics vary greatly among individual patients with COVID-19, in peak antibody level and rate of waning and longevity of neutralising antibodies. We found an association between persistent neutralising antibodies and severe COVID-19 clinical symptoms and higher levels of pro-inflammatory cytokines and chemokines. In a subset of tested patients, SARS-CoV-2 specific T cells were detected regardless of waning patterns of neutralising antibodies. Clinical and epidemiological studies of reinfection among patients who recovered from COVID-19 with and without persistent neutralising antibodies are needed to answer important clinical questions regarding long-term protective immunity and the level of neutralising antibodies that correspond to protection. In this context, it is important to conduct similar large cohort and longitudinal studies among people who have been vaccinated to examine immunity dynamics and longevity.
This online publication has been corrected. The corrected version first appeared at thelancet.com/microbe on April 9, 2021
Data sharing
The data supporting the findings of this study are available in the manuscript or appendix. Further requests might require ethical approval and should contact the corresponding authors.
Acknowledgments
Acknowledgments
We thank all clinical and nursing staff who provided care for the patients; staff at the Communicable Diseases Division, Ministry of Health (Singapore) who contributed to outbreak response and contact tracing; staff at the National Public Health and Epidemiology Unit, National Centre for Infectious Diseases (Singapore) who assisted with data analysis; and staff in Singapore Infectious Disease Clinical Research Network and Infectious Disease Research and Training Office, National Centre for Infectious Diseases for coordinating patient recruitment. We also thank Danielle Anderson and her team at Duke-NUS Medical School (Singapore), for their technical assistance in serum inactivation procedures with Triton X-100, and Olaf Rötzschke, Bernett Lee, Wilson How, and Norman Leo Fernandez from the Singapore Immunology Network Multiplex Analysis of Proteins platform for their assistance in running multiplex microbead-based immunoassay. No compensation was received for their role in the study. This study was funded by grants from the Singapore National Medical Research Council (COVID19RF-001 and COVID19RF-060) and COVID-19 fund (project number H20/04/g1/006) provided to Singapore Immunology Network by the Biomedical Research Council. The SIgN flow cytometry and the Multiplex Analysis of Protein platforms were supported by a grant from the National Research Foundation (NRF), and Immunomonitoring Service Platform (#NRF2017_SISFP09) is funded by the NRF Singapore.
Contributors
L-FW, DCL, Y-SL, LR, LFPN, and AB conceived and designed the study. SWXO, BEY, S-WF, SYT, SP, MI-CC, JGHL, and DCL collected clinical samples and data. WNC, FZ, SWXO, BEY, S-WF, NLB, CWT, CT, JZ, Y-HC, CYLT, and KK did the experiments and analysed the data. WNC, FZ, and L-FW wrote the first draft of the manuscript. All authors contributed to data interpretation, critically reviewed the manuscript, and approved the final manuscript for submission. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. WNC, FZ, SWXO, S-WF, and L-FW have accessed and verified the data.
Declaration of interests
L-FW, CWT, and WNC are co-inventors on a patent application for the surrogate virus neutralisation test technology and a commercial kit, cPass, is being marketed by GenScript Biotech. BEY reports personal fees from Roche and Sanofi, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO COVID-19 situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 4.Hellerstein M. What are the roles of antibodies versus a durable, high quality T-cell response in protective immunity against SARS-CoV-2? Vaccine X. 2020;6 doi: 10.1016/j.jvacx.2020.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang AT, Garcia-Carreras B, Hitchings MDT. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11 doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Bert N, Tan AT, Kunasegaran K. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 7.Addetia A, Crawford KHD, Dingens A. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with high attack rate. J Clin Microbiol. 2020;58:e02107–e02120. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rydyznski Moderbacher C, Ramirez SI, Dan JM. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Ellingson MK, Wong P. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edridge AWD, Kaczorowska J, Hoste ACR. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 11.Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol. 2020;101:791–797. doi: 10.1099/jgv.0.001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357:1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DE, Tan CW, Chia WN. Lack of cross-neutralization by SARS patient sera towards SARS-CoV-2. Emerg Microbes Infect. 2020;9:900–902. doi: 10.1080/22221751.2020.1761267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibarrondo FJ, Fulcher JA, Goodman-Meza D. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudbjartsson DF, Norddahl GL, Melsted P. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripperger TJ, Uhrlaub JL, Watanabe M. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925–933. doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perreault J, Tremblay T, Fournier MJ. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood. 2020;136:2588–2591. doi: 10.1182/blood.2020008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer AS, Jones FK, Nodoushani A. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young BE, Ong SWX, Ng LFP. Viral dynamics and immune correlates of COVID-19 disease severity. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1280. published online Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan CW, Chia WN, Qin X. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 21.Darnell ME, Taylor DR. Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products. Transfusion. 2006;46:1770–1777. doi: 10.1111/j.1537-2995.2006.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds CJ, Swadling L, Gibbons JM. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abf3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera R, Ko R, Tsang OTY. Evaluation of a SARS-CoV-2 surrogate virus neutralization test for detection of antibody in human, canine, cat and hamster sera. J Clin Microbiol. 2020;59:e02504–e02520. doi: 10.1128/JCM.02504-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu F, Liu M, Wang A. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med. 2020;180:1356–1362. doi: 10.1001/jamainternmed.2020.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abed NS, Chace JH, Fleming AL, Cowdery JS. Interferon-gamma regulation of B lymphocyte differentiation: activation of B cells is a prerequisite for IFN-gamma-mediated inhibition of B cell differentiation. Cell Immunol. 1994;153:356–366. doi: 10.1006/cimm.1994.1034. [DOI] [PubMed] [Google Scholar]
- 26.Metzger DW. Interleukin-12 as an adjuvant for induction of protective antibody responses. Cytokine. 2010;52:102–107. doi: 10.1016/j.cyto.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibui A, Shimura E, Nambu A. Th17 cell-derived IL-17 is dispensable for B cell antibody production. Cytokine. 2012;59:108–114. doi: 10.1016/j.cyto.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda K, Mehta H, Drevets DA, Coggeshall KM. IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood. 2010;115:4699–4706. doi: 10.1182/blood-2009-07-230631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long QX, Tang XJ, Shi QL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 30.Alwan NA, Burgess RA, Ashworth S. Scientific consensus on the COVID-19 pandemic: we need to act now. Lancet. 2020;396:e71–e72. doi: 10.1016/S0140-6736(20)32153-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chia WN, Tan CW, Foo R. Serological differentiation between COVID-19 and SARS infections. Emerg Microbes Infect. 2020;9:1497–1505. doi: 10.1080/22221751.2020.1780951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available in the manuscript or appendix. Further requests might require ethical approval and should contact the corresponding authors.


