Abstract
Traditional methods for diabetes management require constant and tedious glucose monitoring and insulin injections, impacting quality of life. The global diabetic population is expected to increase to 439 million, with approximately USD 490 billion in healthcare expenditures by 2030, imposing a significant burden on healthcare systems worldwide. Recent advances in nanotechnology have emerged as promising alternative strategies for the management of diabetes. For example, implantable nano-sensors are being developed for continuous glucose monitoring, new nanoparticle-based imaging approaches that quantify subtle changes in β cell mass can facilitate early diagnosis, and nanotechnology-based insulin delivery methods are being explored as novel therapies. Here, we provide a holistic summary of this rapidly advancing field compiling all aspects pertaining to the management of diabetes.
Keywords: Nanotechnology, Diabetes, Magnetic Nanoparticles, Carbon Nanotubes, Quantum Dots, Nanogels, Scaffolds, Cell Therapies
Diabetes mellitus is a group of metabolic disorders that is characterized by elevated blood glucose levels driven by insulin deficiency or resistance [1]. The number of individuals affected by this disorder is steadily increasing with an estimated 7.7% of the world-wide adult population (439 million people) suffering from the disorder by 2030, bringing with it an associated increase in the world-wide financial burden from USD 376 billion in 2010 to USD 490 billion by 2030 [2].
The two types of diabetes were first classified by Percival Hemsworth in 1936 [3]. Type 1 diabetes accounts for ~10% of the diabetic patient population, and is associated with a deficiency in insulin [4] caused by autoimmune destruction of islet β cells in the pancreas [5]. Type 2 diabetes, on the other hand, is associated with insulin resistance, the dampening of the body’s response to insulin, which consequently leads to hyperglycemia. This form of diabetes has been closely connected to lifestyle habits and can develop over time in an individual [6].
According to the American Diabetes Association, proper glucose control (i.e., <150 mg/dL fasting glucose or 7% A1C) is important for preventing downstream complications in diabetic patients [7]. Prolonged hyperglycemia, for example, has been linked to a multitude of microvascular (e.g., eye, nerve and kidney disease) and macrovascular complications (cardiovascular disease) [7]. Thus, conventional therapies for both types of diabetes typically consist of frequent glucose monitoring and insulin administration (e.g., through subcutaneous injections or insulin pumps) throughout the day. However, sporadic glucose monitoring and poor patient adherence, driven by a multitude of factors including pain and the tediousness of the procedure [8], often lead to unpredictable insulin dosages, which could result in uncontrolled hyperglycemia, hypoglycemia, seizures, unconsciousness or death [9]. Additionally, in youth with diabetes, developmental, psychosocial, and caregiver challenges add further to complexities in diabetes care [10]. Although continuous glucose monitors and insulin pumps have been developed to address these issues, and recent “closed loop systems” that integrate the two devices (wherein pumps intelligently respond by delivering appropriate insulin amounts in response to glucose data) have been introduced, glucose management within an acceptable range has been shown to occur at best 70% of the time using these systems [11, 12]. As such, there is still a need for improved diabetes management tools. Recent advances in nanotechnology have shown promise for the management of a wide variety of medical conditions [13–17]. Here, we provide a holistic overview on this rapidly growing field, compiling all aspects pertaining to the management of diabetes, including diagnosis, monitoring and treatment (Figure 1).
Figure 1. Nanotechnology applications in diabetes management.
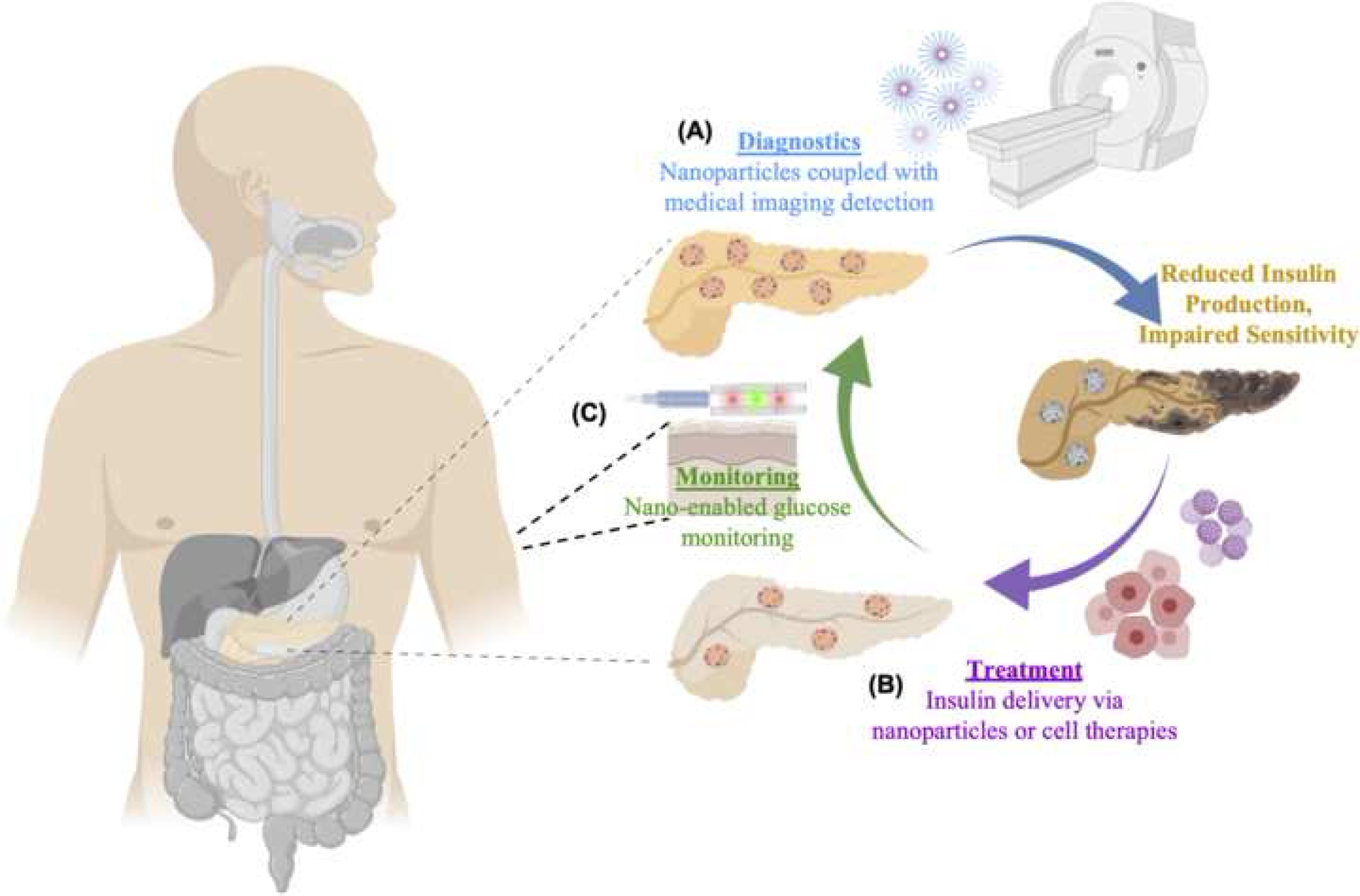
Schematic diagram depicting the use of nanotechnology in all aspects of diabetes management, including (A) nanoparticles in conjunction with medical imaging techniques for potential applications in diagnosis, (B) nanoparticle- or cell therapy-enabled insulin delivery for treatment, and (C) carbon nanotubes and quantum dots for glucose monitoring.
Nanotechnology Applications in Diagnostics
Accurate and timely diagnosis of diabetes is vital for optimal patient outcomes. For example, early and reliable diagnostic testing might identify individuals in whom early intervention with lifestyle management or pharmacological approaches might prevent dysglycemia or even the onset of disease. Previous studies demonstrated that early glycemia management prevented or delayed a number of disease-related complications [18, 19], suggesting that earlier diagnosis and subsequent management should be of priority. However, traditional testing methods often fall short in this regard [20]. Conventional diagnostic techniques for diabetes include analyzing fasting glucose levels, A1c levels, or oral glucose tolerance tests. In the setting of clinical trials, the measurement of autoantibodies is often used as a diagnostic test to identify individuals at high risk for the development of diabetes, and in the clinical setting autoantibodies are sometimes used to distinguish those with type 1 diabetes when the nature of diabetes is unclear [21]. These methods are deemed painful by some patients, and rely on glucose measurements or antibody titers, which can vary based on many factors, including age, time of testing, and other physiological conditions [1]. Moreover, the manifestation of disease symptoms such as hyperglycemia, often does not become clinically evident until years after disease onset [22], which precludes early intervention. To address the weaknesses of traditional diagnostic tools, different types of nanotechnologies have been developed to potentially enable earlier and non-invasive detection of diabetes.
Characterization of Immune Cell Activity and β Cell Mass
Whereas alterations in β cell function (i.e., loss of insulin secretion) are evident in both type 1 and type 2 diabetes, assessment of β cell mass has the potential to identify those individuals in whom potential β cell restoration therapies may be useful. Alterations in β cell mass are not only a hallmark of autoimmune type 1 diabetes, but could also be precipitated by sustained insulin resistance under type 2 diabetes [4, 23]. New technologies capable of measuring changes in β cell mass and/or associated immune cell activity could potentially lead to improved detection and earlier prognosis [23], prior to the manifestation of disease-related symptoms. Recent advances in nanoparticle-based imaging technologies have provided a novel route for quantifying β cell mass in diabetic patients [24–27]. Magnetic nanoparticles (MNPs), for example, possess unique physical properties that make them excellent contrast agents for magnetic resonance imaging (MRI) [28, 29]. Different approaches have thus been developed to enhance their contrast and biocompatibility (BOX 1) [30, 31]. To date, MNPs have already been implemented in a wide range of medical conditions, including cancer [32] and cardiovascular diseases [33], potentially making them an attractive diagnostics tool for diabetes. Different types of MNPs have now been fashioned as contrast agents for β cell monitoring under MRI [34]. Once these nanoparticles are internalized by the host’s cells, the MRI can accurately and efficiently detect the particles to facilitate non-invasive visualization of the β cells or pancreas [35]. Superparamagnetic iron oxide nanoparticles (SPIONs), which are highly biocompatible [36], have been conjugated with ferumoxran-10 or exendin-4 for immune or β cell targeting, respectively [24–26]. Fermoxran-10-conjugated SPIONs have been developed and tested in preclinical and clinical studies to monitor immune cell infiltration into the pancreas as an early diagnostic tool for type 1 diabetes. Such particles were infused into healthy volunteers and early-stage diabetic patients, and MRI was used to monitor immune cell accumulation/activity in the pancreas [24]. The particles were readily engulfed by macrophages, which enabled visualization of islet inflammation as well as alterations in the microvasculature, clearly distinguishing patients with recent-onset diabetes from healthy individuals (Figure 2 A–B) [24]. In more recent studies, exendin-4-conjugated SPIONs, which target the Glucagon-like peptide 1 receptor (GLP-1R) in β cells, were used to accurately monitor changes in β cell mass under diabetes in preclinical studies. However, while promising, exendin-4-conjugated SPIONS could also be captured by the liver and spleen, decreasing the overall output signal from the pancreatic islets. Therefore, future modifications focused on reducing off-target organ accumulation will be key to improving the performance of this platform nanotechnology [25, 26]. Nevertheless, the ability to target MNPs to the pancreas opens new avenues for non-invasive imaging methods that can trace the progression of the disease as well as treatment modalities.
BOX 1: Advancements made in Magnetic Nanoparticle Contrast and Biocompatibility.
Magnetic Nanoparticles (MNPs) are often used as contrast agents in magnetic resonance imaging (MRI). These agents are typically developed from a paramagnetic metal core and coated with specific materials to improve biocompatibility [30, 31].
a) Improving Contrast in Magnetic Nanoparticles
Contrast agents and other magnetic biological molecules are aligned and excited from the strong magnetic field and radiofrequency pulse during a typical MRI. The time taken for the affected particles to revert to their lower energy levels or ground state is called the “relaxation time” and is directly associated with contrast in MRI [30, 31]. Relaxation properties are different for every metal. Metals such as gadolinium and iron have been widely used for contrast agents due to their relatively short relaxation times compared to other metals. MNP coatings, often used for biocompatibility enhancements, have also been used to improve relaxation times. β-cyclodextrin-and hyaluronic acid-based coatings have demonstrated improved relaxation times and contrast [30].
b) Improving Biocompatibility in Magnetic Nanoparticles
MNPs have been developed from biocompatible metals or coated with hydrophilic materials to improve biocompatibility [30, 31]. Magnetic metals such as iron oxides and manganese provide low toxicity levels. Additionally, thin polymer or carbohydrate coatings are often used to prevent aggregate formation, alterations in original structure, biological degradation of the metal and negative biological reactions. Studies investigating MNP coatings have provided valuable insight into material/molecular properties that can provide improved biological interactions [30]. Polyethylene glycol, dextran, silica and gold coatings have been found to improve MNP biocompatibility [31]. Additionally, negatively charged materials were found to be less genotoxic than positively charged materials in in vitro studies [30].
Figure 2. Potential applications in diagnostics.
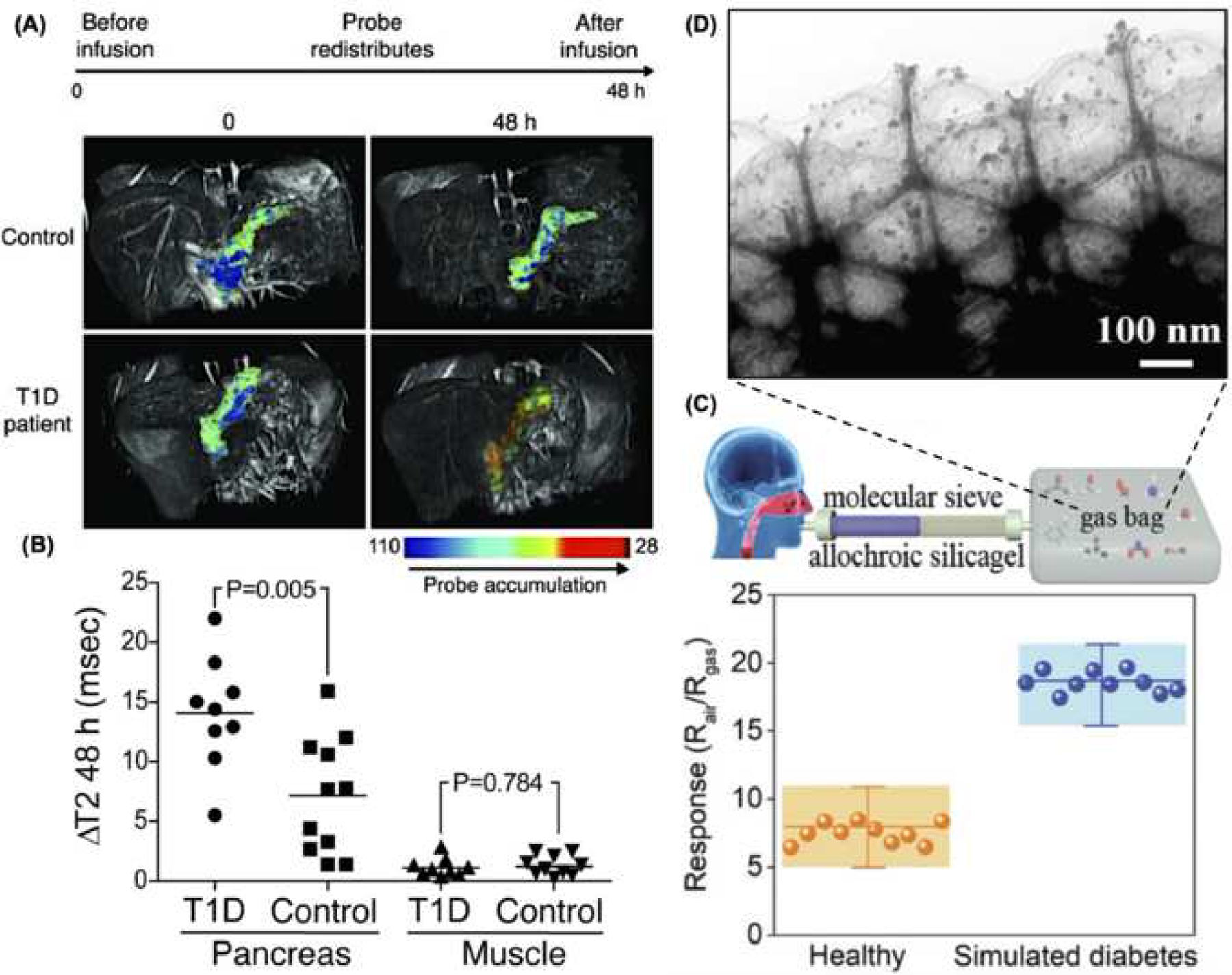
(A) MRI images of pancreatic β cells of healthy individuals and type 1 diabetic patients before and 48 hours after ferumoxtran-10 nanoparticle infusion demonstrate an increase in nanoparticle accumulation (colored in red) in diabetic patients (adapted from Figure 2, reference 24). (B) A significant difference in nanoparticle accumulation was detected between groups (adapted from Figure 3, reference 24). (C) Representation of the acetone gas sensor device and associated response detected from healthy and simulated diabetic patients (adapted from Figure 3, reference 38). (D) Transmission electron micrograph of the sensing component of an acetone gas detector with quantum dots (adapted from Figure 1, reference 38).
Acetone Gas Sensor
Recent studies have looked at the use of quantum dots (QDs) for the development of alternative diagnostic approaches for diabetes. QDs are highly sensitive and cost-effective fluorescent semiconductor nanoparticles that range in size from 2 to 10 nm [37–39]. While their biocompatibility still needs to be studied further [40], they have the ability to emit fluorescence in the near-infrared range (NIR), making them ideal tools for bio-imaging applications due to reduced photon scattering, decreased tissue absorption, and reduced background autofluorescence, which leads to enhanced contrast and tissue penetration [41, 42]. Overall, QDs are of great interest in nanotechnology-based imaging/detection applications due to their wide range of optoelectronic properties (e.g., narrow-band emissions), which differ significantly from the corresponding bulk material, and can be finely tuned based on size and shape [43]. Moreover, additional surface modifications (e.g., zinc sulfide coatings) can enable enhanced properties, including biocompatibility and optical performance [44], for a wide range of medical applications. Beyond diabetes, QDs are actively being investigated for other indications such as cancer theranostics [45], tissue engineering [46], and pathogen detection [47]. Liu et al developed a QD-driven breath sensor that could diagnose diabetes based on the acetone levels (Figure 2 C), which could increase from ~300–900ppb for a healthy individual, to ~1.8 ppm for a diabetic patient [38]. Briefly, the QD metal oxide surface supports the adsorption of acetone molecules, which lowers the voltage barrier by moving electrons up the conduction band, resulting in an increased flow of electrons. Such changes lead to alterations in the electrical resistance/conductivity of the sensor, which facilitates accurate quantification of the acetone levels present in a single breath under simulated diabetic vs. healthy conditions (Figure 2 D). However, while the sensor exhibited high selectively to acetone compared to other potentially interfering analyte gases (e.g, ethanol, methanol, etc.), changes in relative humidity could have a significant and inversely proportional dampening effect on the sensor’s response [38]. Thus, future studies aimed at better controlling fluctuations in relative humidity will be central to enabling clinical implementation of this nanotechnology.
Nanotechnology Applications in Monitoring
The most common approach to monitoring under diabetes involves the use of standard glucose meters, which require patients to manually “prick” themselves constantly throughout the day in order to track changes in glucose levels. Although time-tested, this method has several limitations, including poor patient compliance and unreliable glucose measurements due to a variety of factors, including mealtimes, age, etc. [1]. Additionally, standard glucose monitoring cannot be conducted during certain everyday activities, such as sleeping or driving. Thus, the intermittent nature of conventional monitoring methods imply that a patient can miss potentially dangerous glycemic fluctuations between tests, putting patients at risk for severe complications [7, 9]. Over the last three decades various attempts have been made towards developing a hassle-free method for glucose monitoring. With the eventual development of implantable biosensors, this idea became feasible and resulted in continuous glucose monitoring (CGM) systems, which can provide constant glucose monitoring for up to 10 days [48]. The first generation of these devices included amperometric sensors that are implanted subcutaneously. These sensors emit a detectable electric current as a function of glucose concentration [49]. While a step forward in CGM, these devices have several drawbacks including (1) lack of stability due to signal lag and sensor drift, (2) the need for weekly subcutaneous implantation and calibration procedures, and (3) high sensitivity to changes in various physiological parameters such as pH and temperature [49, 50]. Nanotechnology-based biosensors possess capabilities that can potentially circumvent these limitations.
Glucose Monitoring
Fluorescence-based nanosensors emit a particular fluorescence signal upon binding glucose. These sensors typically are associated with glucose-binding molecules linked to QDs [51], carbon nanotubes (CNTs) [52], or nano-optodes [40, 53], which convert the fluorescence energy associated with the binding event into a shift in spectra or voltammetric output. Several glucose-binding molecules from natural (e.g., lectins) and synthetic (e.g., phenylboronic acid) compounds have been used in these sensors [51, 54]. Two of the most common proteins used for glucose sensing are concanavalin A (ConA), a lectin which has a high specificity for glucose, and glucose/galactose-binding protein, which alters its conformation upon binding glucose [51, 55]. A major advantage of these devices compared to current systems is that they are not dependent on battery life, thus possessing the ability to potentially function almost continuously for prolonged periods of time [55]. Liao et al developed a fiber-optic glucose sensor based on ConA-functionalized QDs [51], with hair-like size and flexibility, which enables discrete implantation in the skin for continuous monitoring of glucose in the interstitial fluid. Briefly, glucose diffuses freely into the sensor and binds to ConA, which causes a shift in fluorescence that can be correlated with glucose concentration (Figure 3 A). This sensor was successfully used to rapidly and accurately detect changes in glucose concentration (0 – 500mg/dL) in vitro in physiological solutions for up to 7 weeks (Figure 3 B). More recently, amino-functionalized silicon QDs have also been demonstrated as effective glucose detectors [56]. Exercising similar principles as the CoA-functionalized QDs, the amino-functionalized QDs showed clear changes in intensity as a function of glucose concentration in diluted human blood, as well as high specificity for glucose over other relevant/similar molecules such as sucrose, Na+, etc. [56]. However, while this system shows high sensitivity, limitations in the detection rage of the sensor and the need for diluted blood samples could pose a challenge to clinical deployment. As such, additional technological advancements, possibly driven by micro/nanofluidic platforms, will be key to enabling automated sample pre-processing (e.g., blood dilution) for proper monitoring of glucose fluctuations.
Figure 3. Potential applications in monitoring.
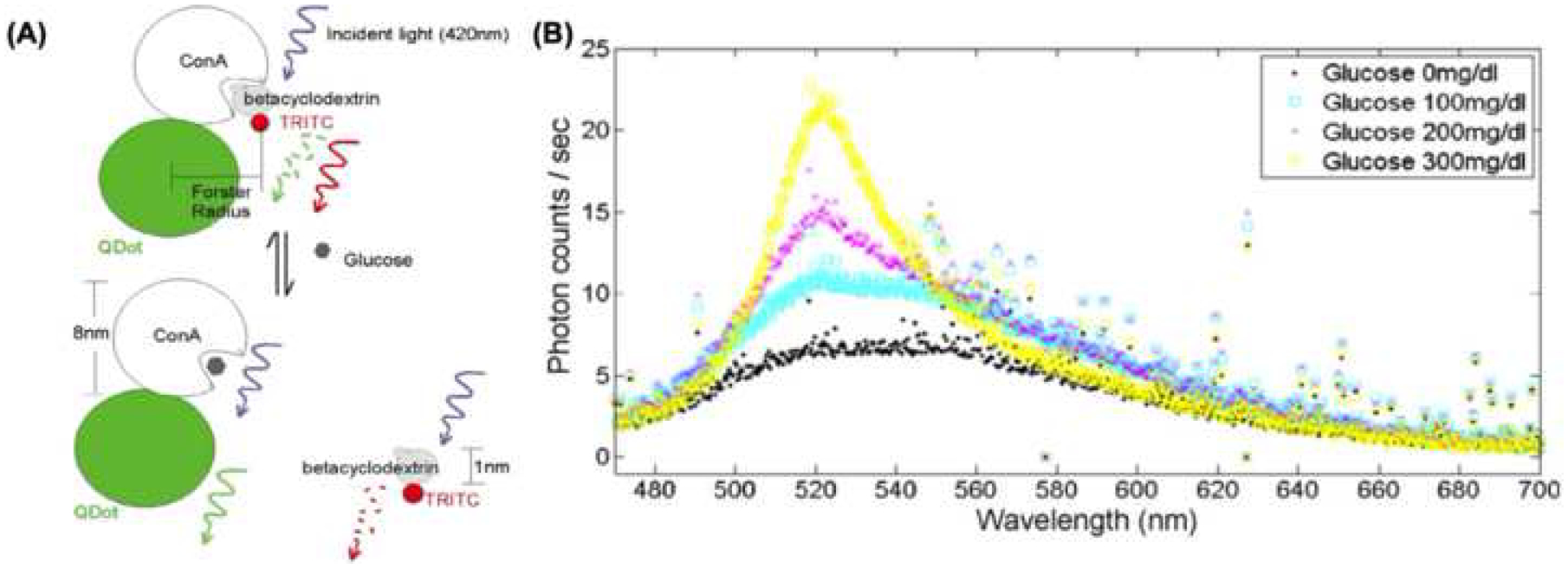
(A) Schematic diagram depicting the concept of QD coupling with ConA, and associated changes in fluorescence wavelength depending on the competitive binding between glucose and betacyclodextrin. The concentration of glucose can be extracted from the ratio between signals from the tetramethylrhodamine isothiocyanate (TRITC) fluorophore and QDs (adapted from Figure 2, reference 51). (B) Results from the optical sensor showing the readout for different concentrations of glucose (adapted from Figure 4, reference 51).
Insulin Monitoring
Current insulin detection methods for the most part require cumbersome and lengthy ex vivo analyses based on ELISA or fluorescence spectroscopy [57, 58]. Simplifying insulin detection and monitoring, however, may enable improved tracking of disease progression and/or therapeutic delivery in both type 1 and type 2 diabetes [1, 23, 59]. Previous studies have reported on the development of improved technologies for continuous insulin monitoring based on multi-walled carbon nanotubes (MWCNT), which are basically flat sheets of carbon atoms stacked and rolled into nanoscale tubes, and can be tailored to show enhanced sensitivity to different biomolecules [60, 61]. Insulin concentrations as low as 1 μM can then be monitored based on electrochemical detection driven by insulin oxidation. More recent studies have also looked at the use of graphene-polypyrrole nanocomposites for electrochemical insulin detection [62]. Improved insulin monitoring technologies will not only be key to facilitating indirect tracking of disease progression (i.e., β cell mass/activity) or therapy delivery, but may also find applications in the screening of islet preparations prior to transplantation for therapeutic purposes.
Nanotechnology Applications in Therapy
Traditional treatments for diabetes consist of administering synthetic insulin or insulin-based therapies [59]. While many forms of insulin (e.g, injectable, oral, inhalable, etc.) [63] and insulin analogs (e.g., insulin degludec U-100 and U200, insulin aspart, etc.) [64] are currently available to treat type 1 diabetes, total recovery from this condition has not yet been reported. Most current treatments carry the risk of adverse side-effects (e.g., hypoglycemia) [65, 66], and are often incapable of maintaining euglycemia for prolonged periods of time [59]. The first description of “smart” glucose-responsive insulin-based therapies, which only activate insulin release when glucose levels are high, was published in 1979 [67]. Since then, nanotechnology has played a significant role in increasing the efficiency, ease of use, and safety of insulin replacement therapies. Many long-lasting nanotechnology-enabled therapeutic methods have now been developed to provide tighter glycemic control for patients and minimize the need for continuous and tedious manual injections [67]. Here we will discuss a few of these novel insulin delivery methods, including cellular therapies, for the treatment of diabetes.
Insulin Patch
Non-invasive delivery of insulin has the potential to address non-compliance issues and avoid complications associated with poor glycemic control. The transdermal [68] and oral routes [69] have been recognized as “patient-friendly” due to their non-invasive, relatively painless, and simple nature. However, limited transport across epithelial barriers, poor bioavailability, and the harsh environment of the gastrointestinal tract have limited the success of previous attempts at insulin delivery via these routes [69]. Yu et al. developed a microneedle-based patch with glucose-responsive insulin-laden nanoscale vesicles for minimally invasive delivery of insulin [70]. The patch can be applied on the skin to controllably release insulin in response to changes in glucose levels, providing tighter glycemic control (Figure 4 A) [71]. The glucose-responsive vesicles that contain insulin and glucose oxidase are made from hyaluronic acid conjugated with a hypoxia-sensitive component, 2-nitroinidazole. Under hyperglycemia, the oxygen is quickly consumed due to glucose oxidation, which is catalyzed by the glucose oxidase within the vesicles. This then results in localized hypoxia, triggering the breakdown of the vesicles and subsequent release of insulin via the reduction of 2-nitroindazole [70]. However, while promising, future modifications may still be needed to prevent excessive biofouling under long-term implementation, possibly through nanocoating or nanofabrication technologies [72, 73].
Figure 4. Potential applications in therapy.
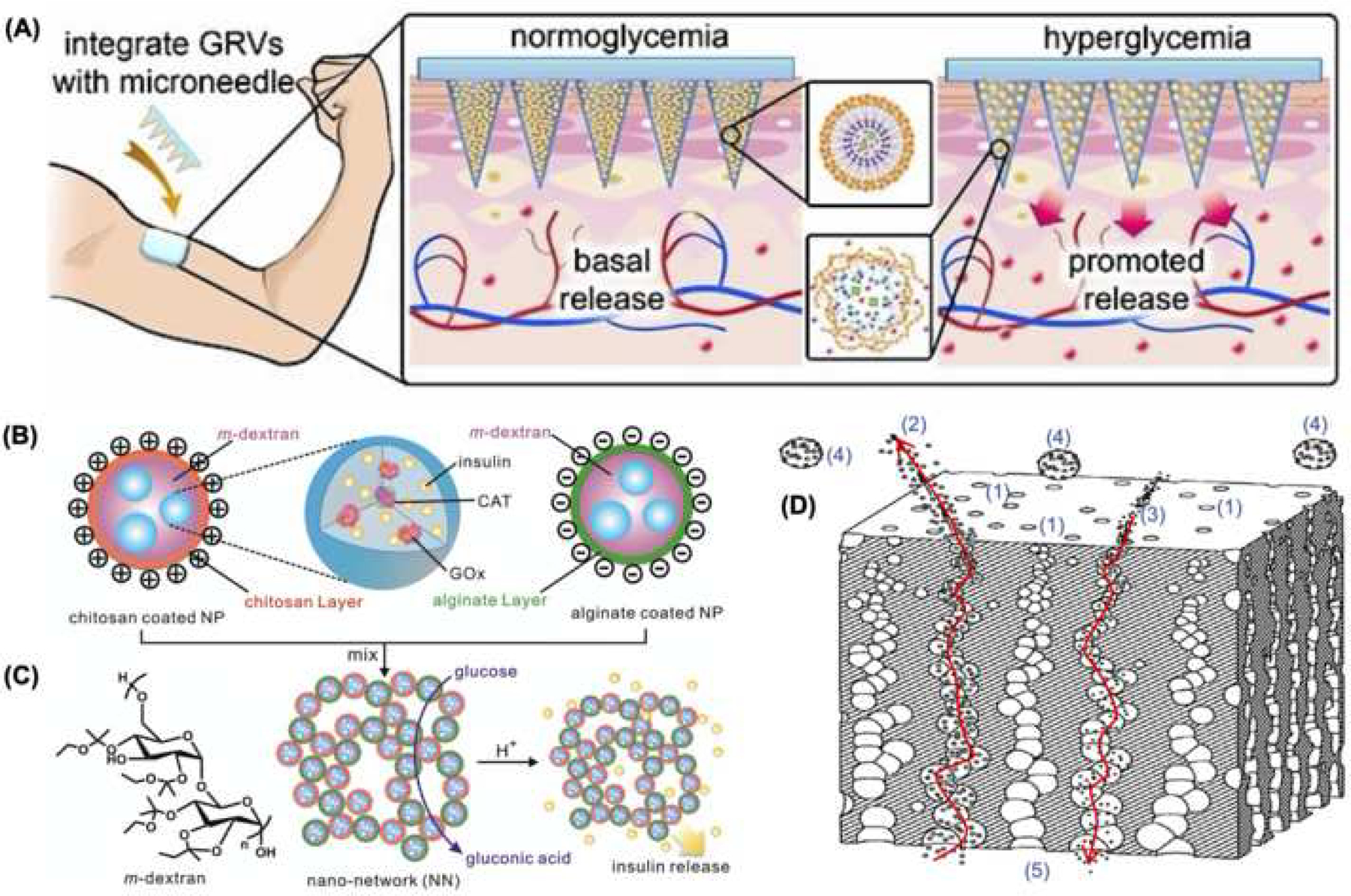
(A) Schematic diagram of the microneedle insulin patch with glucose responsive vesicles (adapted from Figure 1, reference 70). (B) Schematic diagram of dextran nanoparticles coated with chitosan or alginate, and loaded with insulin, GOx (glucose oxidase), CAT (catalase) and m-dextran chemical structure. (C) Gel formation based on electrostatic interactions capable of responding to the local environment with controlled insulin release (adapted from Figure 1, reference 74). (D) Cross section of encapsulation coating with (1) tapered nanopores permitting (2) insulin and (3) nutrient translocation across the membrane, while also blocking (4) immune cells from reaching the (5) insulin-producing cell compartment (adapted from Figure 2, reference 88).
Insulin Nano-Gel
Glucose-responsive insulin-loaded hydrogels have emerged as an alternative strategy for insulin delivery. Previous gel-based systems showed slow response times, lacked mechanical strength, and had problems with the insulin leaking out uncontrollably [74]. Gu et al. introduced a highly effective insulin delivery system based on an injectable gel made from pH-sensitive dextran nanoparticles loaded with glucose oxidase, which converts glucose into gluconic acid [75]. When glucose levels are high, enhanced glucose diffusion into the gel results in large quantities of gluconic acid. The resulting acidic microenvironment then causes the dextran spheres to gradually degrade and release insulin (Figure 4 B–C). Such systems have been used successfully to maintain glycemic control in diabetic mice for up to 10 days after a single injection [74]. More recent studies have reported on analogous systems but with different and more diverse chemistries (e.g., polyethene glycol-based nanogels) [76], which induced adequate glucose responsiveness in diabetic mice for approximately two hours during a glucose tolerance test.
Cellular Therapies
Although allogeneic islet transplantation has been shown to lead to improved glycemic control in diabetic patients, donor tissue scarcity and the need for systemic immunosuppression regimens have hindered widespread implementation [77, 78]. To address these issues, some groups have worked on developing improved strategies to differentiate induced pluripotent stem cells (iPSCs), which can be autologous in nature, into insulin-producing cells (IPCs) [79, 80]. Nanofiber-based polymeric (e.g., PLLA/PVA, PES) scaffolds, for example, have been successfully used to improve the functionality (e.g., glucose responsiveness) and differentiation efficiencies of IPCs [81, 82]. On the other hand, to minimize the need for systemic immunosuppression associated with donor-derived cellular therapies, a number of groups have looked at different encapsulation approaches to protect cellular grafts (e.g., allogeneic islets) from immune cell rejection [83]. Alginate is commonly used in encapsulation strategies, however, recent studies have shown that alginate alone can evoke adverse immune responses by itself [83]. Nevertheless, Vegas et al. showed that nanoscale surface modifications with triazole deposits could curtail such responses, permitting implantation of alginate microspheres in both rodents and nonhuman primates with significantly reduced foreign body response (e.g., fibrotic tissue deposition, associated macrophages, etc.) for up to 6-months [84]. Additional studies have focused on the development of thin (i.e., nanometer scale) encapsulation membranes with nanoscale pores for immunoisolation purposes [85–87]. The nanoscale thickness of the membrane in combination with the small pore size is key to enabling islet graft shielding from the immune system, while at the same time facilitating proper oxygen, glucose and insulin transport across the membrane, which is an improvement over previous alginate-based designs [86, 87]. Wang et al, for example, reported successful islet transplantation without the use of immunosuppressive drugs in a nonhuman primate model for 90 days, using nanoscale coatings with a tapered nanopore conduit system that significantly reduced immune cell as well as cytokine interactions with the implanted islets, without impacting mass transport mechanisms key to graft survivability and functionality (Figure 4 D) [88].
Current Outstanding Questions in Nanotechnology for Diabetes Management
Although nanoscale technologies have clearly enabled multiple technological advancements in diabetes management, potential hurdles for widespread implementation vs. the continued use of traditional methods include cost-benefit considerations, as well as the lack of clarity in some cases regarding regulatory guidelines and/or success metrics/standards (see outstanding questions box) [89]. Additional pre-clinical and larger scale clinical studies are still needed to fully evaluate the extent to which different types of nanotechnologies can lead to improved diabetic patient outcomes. For example, while magnetic nanoparticles facilitate minimally invasive intrapancreatic imaging, more evidence is still needed to determine whether subtle changes in β cell mas and/or immune cell activity can be reliably used to diagnose diabetes earlier. Similarly, although blood glucose measurements have been the gold standard in terms of diagnostics and monitoring, further research is warranted to evaluate not only the advantages, cost-effectiveness and clinical relevance of using nanotechnology in terms of increased glucose sensitivity, precision, accuracy and durability, but also as a tool to evaluate other metabolic outputs, besides glucose, that could be linked to disease onset/progression. On the other hand, in terms of treatment, there is a lot of preclinical evidence suggesting that nanotechnology can lead to improved systems for glucose sensing coupled with insulin delivery. However, more studies are required to evaluate the long-term effectiveness of such systems to safely control glycemic excursions in diabetic patients. In addition, gene and cell therapies may enable novel solutions for diabetes by modulating immune cell responses and/or facilitating the development of insulin-producing β-like cells through controlled cellular differentiation or reprogramming [90]. And while a wide variety of nanoscale technologies have been developed for controlled gene delivery and cellular differentiation in vitro and in vivo [91–93], there is still limited information regarding the use of these approaches within the context of diabetes therapies. As the knowledge and breadth of the nanotechnology field increases, the potential applications for diabetes management become increasingly more realistic and promising.
Outstanding Questions.
Will a lack of precise regulatory guidelines on nanotechnology negatively impact clinical translation and widespread implementation for some of the most promising nanotechnologies for diabetes management? [83]
Should we be putting more effort into establishing clear and widely accepted metrics/standards for risk/cost-benefit considerations to determine which nanotechnologies continue to move along the translational pipeline? [83]
Will some of these nanotechnologies end up being cost-prohibitive for certain pockets of the diabetic/pre-diabetic patient population, and/or for nations with limited healthcare budgets?
What should be the role of governments, industry, academia and non-profits in terms of making sure these nanotechnologies are more affordable?
Should the field be equally vested in developing nanotechnologies that are solely aimed at helping us understand better the fundamentals of diabetes? Or should the field focus instead on developing translational nanotechnologies with more commercial potential?
Highlights.
Following success in other medical areas, nanotechnology is currently generating immense promise for alternative strategies for diabetes management.
Magnetic nanoparticle-based imaging of β cell mass and immune cell activity, as well as quantum dot-enabled gas sensors, offer potential new avenues for early detection.
Multiwalled-carbon nanotubes, graphene nanocomposites and quantum dots provide discrete sensors with improved sensitivity and efficiency for glucose and insulin monitoring.
Cell therapies supported by nanofiber-based scaffolds or immunoisolation membranes, and glucose-responsive nanogels and nanovesicles offer novel approaches to controlled insulin release.
Acknowledgements
Some of the illustrations were created with BioRender.com. The authors would also like to thank Ian Risser and Aidan Maxwell for their assistance with manuscript preparation.
References
- 1.Association A.D., 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care, 2019. 42(Supplement 1): p. S13–S28. [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, and Zimmet PZ, Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice, 2010. 87(1): p. 4–14. [DOI] [PubMed] [Google Scholar]
- 3.Karamanou M, et al. , Milestones in the history of diabetes mellitus: The main contributors. World journal of diabetes, 2016. 7(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabelea D, The accelerating epidemic of childhood diabetes. The Lancet, 2009. 373(9680): p. 1999–2000. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman S and DiLorenzo TP, A comprehensive guide to antibody and T‐ cell responses in type 1 diabetes. Tissue antigens, 2003. 62(5): p. 359–377. [DOI] [PubMed] [Google Scholar]
- 6.Donath MY and Shoelson SE, Type 2 diabetes as an inflammatory disease. Nature Reviews Immunology, 2011. 11(2): p. 98. [DOI] [PubMed] [Google Scholar]
- 7.Association A.D., 6. Glycemic targets: standards of medical care in diabetes—2019. Diabetes Care, 2019. 42(Supplement 1): p. S61–S70. [DOI] [PubMed] [Google Scholar]
- 8.Association A.D., Standards of medical care in diabetes—2014. Diabetes care, 2014. 37(Supplement 1): p. S14–S80. [DOI] [PubMed] [Google Scholar]
- 9.Schulman R, et al. , Association of glycemic control parameters with clinical outcomes in chronic critical illness. Endocrine Practice, 2014. 20(9): p. 884–893. [DOI] [PubMed] [Google Scholar]
- 10.Streisand R and Monaghan M, Young children with type 1 diabetes: challenges, research, and future directions. Current diabetes reports, 2014. 14(9): p. 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickup JC, Insulin-pump therapy for type 1 diabetes mellitus. New England Journal of Medicine, 2012. 366(17): p. 1616–1624. [DOI] [PubMed] [Google Scholar]
- 12.Brown SA, et al. , Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. New England Journal of Medicine, 2019. 381(18): p. 1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis ME, et al. , Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature, 2010. 464(7291): p. 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peer D, et al. , Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology, 2007. 2(12): p. 751. [DOI] [PubMed] [Google Scholar]
- 15.Kim BY, Rutka JT, and Chan WC, Nanomedicine. New England Journal of Medicine, 2010. 363(25): p. 2434–2443. [DOI] [PubMed] [Google Scholar]
- 16.LaVan DA, Lynn DM, and Langer R, Moving smaller in drug discovery and delivery. Nature Reviews Drug Discovery, 2002. 1(1): p. 77. [DOI] [PubMed] [Google Scholar]
- 17.Whitesides GM, The’right’size in nanobiotechnology. Nature biotechnology, 2003. 21(10): p. 1161. [DOI] [PubMed] [Google Scholar]
- 18.Control D. and Group C.T.R., Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Ophthalmology, 1995. 102(4): p. 647–661. [DOI] [PubMed] [Google Scholar]
- 19.Evans M, The UK prospective diabetes study. The Lancet, 1998. 352(9144): p. 1932–1933. [DOI] [PubMed] [Google Scholar]
- 20.Dallo FJ and Weller SC, Effectiveness of diabetes mellitus screening recommendations. Proceedings of the National Academy of Sciences, 2003. 100(18): p. 10574–10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regnell SE and Lernmark Å, Early prediction of autoimmune (type 1) diabetes. Diabetologia, 2017. 60(8): p. 1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bleich D, et al. , Analysis of metabolic progression to type I diabetes in ICA+ relatives of patients with type I diabetes. Diabetes Care, 1990. 13(2): p. 111–118. [DOI] [PubMed] [Google Scholar]
- 23.Weir GC and Bonner-Weir S, Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes, 2004. 53(suppl 3): p. S16–S21. [DOI] [PubMed] [Google Scholar]
- 24.Gaglia JL, et al. , Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. The Journal of clinical investigation, 2011. 121(1): p. 442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, et al. , The role of exendin-4-conjugated superparamagnetic iron oxide nanoparticles in beta-cell-targeted MRI. Biomaterials, 2013. 34(23): p. 5843–5852. [DOI] [PubMed] [Google Scholar]
- 26.Wang P, et al. , GLP-1R–targeting magnetic nanoparticles for pancreatic islet imaging. Diabetes, 2014. 63(5): p. 1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh P, et al. , Gold nanoparticles in delivery applications. Advanced drug delivery reviews, 2008. 60(11): p. 1307–1315. [DOI] [PubMed] [Google Scholar]
- 28.Alavijeh AA, et al. , The Potential of Magnetic Nanoparticles for Diagnosis and Treatment of Cancer Based on Body Magnetic Field and Organ-on-the-Chip. Advanced pharmaceutical bulletin, 2019. 9(3): p. 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corot C, et al. , Recent advances in iron oxide nanocrystal technology for medical imaging. Advanced drug delivery reviews, 2006. 58(14): p. 1471–1504. [DOI] [PubMed] [Google Scholar]
- 30.Felton C, et al. , Magnetic nanoparticles as contrast agents in biomedical imaging: recent advances in iron-and manganese-based magnetic nanoparticles. Drug metabolism reviews, 2014. 46(2): p. 142–154. [DOI] [PubMed] [Google Scholar]
- 31.McNamara K and Tofail SA, Nanosystems: the use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Physical Chemistry Chemical Physics, 2015. 17(42): p. 27981–27995. [DOI] [PubMed] [Google Scholar]
- 32.Hrkach J, et al. , Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Science translational medicine, 2012. 4(128): p. 128ra39–128ra39. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy JR, Nanomedicine and cardiovascular disease. Current cardiovascular imaging reports, 2010. 3(1): p. 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z and Kandeel F, Radionuclide probes for molecular imaging of pancreatic beta-cells. Advanced drug delivery reviews, 2010. 62(11): p. 1125–1138. [DOI] [PubMed] [Google Scholar]
- 35.Andralojc K, et al. , Obstacles on the way to the clinical visualisation of beta cells: looking for the Aeneas of molecular imaging to navigate between Scylla and Charybdis. Diabetologia, 2012. 55(5): p. 1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veiseh O, Gunn JW, and Zhang M, Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Advanced drug delivery reviews, 2010. 62(3): p. 284–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matea CT, et al. , Quantum dots in imaging, drug delivery and sensor applications. International journal of nanomedicine, 2017. 12: p. 5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W, et al. , Graphene quantum dot-functionalized three-dimensional ordered mesoporous ZnO for acetone detection toward diagnosis of diabetes. Nanoscale, 2019. [DOI] [PubMed] [Google Scholar]
- 39.Hu M, et al. , H2O2-sensitive quantum dots for the label-free detection of glucose. Talanta, 2010. 82(3): p. 997–1002. [DOI] [PubMed] [Google Scholar]
- 40.Billingsley K, et al. , Fluorescent nano-optodes for glucose detection. Analytical chemistry, 2010. 82(9): p. 3707–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinnathambi S and Shirahata N, Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Science and technology of advanced materials, 2019. 20(1): p. 337–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J-B, et al. , Recent progress in small-molecule near-IR probes for bioimaging. Trends in Chemistry, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi-Ge X, et al. , Recent research advances of antibody-conjugated quantum dots. Chinese Journal of Analytical Chemistry, 2013. 41(6): p. 949–954. [Google Scholar]
- 44.Wang Y, et al. , Functionalized quantum dots for biosensing and bioimaging and concerns on toxicity. ACS applied materials & interfaces, 2013. 5(8): p. 2786–2799. [DOI] [PubMed] [Google Scholar]
- 45.Zayed DG, et al. , Hybrid quantum dot-based theranostic nanomedicines for tumor-targeted drug delivery and cancer imaging. 2019, Future Medicine. [DOI] [PubMed] [Google Scholar]
- 46.Wei K, et al. , Engineered-Macrophage Induced Endothelialization and Neutralization via Graphene Quantum Dot-Mediated MicroRNA Delivery to Construct Small-Diameter Tissue-Engineered Vascular Grafts. Journal of biomedical nanotechnology, 2019. 15(7): p. 1492–1505. [DOI] [PubMed] [Google Scholar]
- 47.Yuan H, et al. , Conjugated Polymer-Quantum Dot Hybrid Materials for Pathogen Discrimination and Disinfection. ACS Applied Materials & Interfaces, 2019. [DOI] [PubMed] [Google Scholar]
- 48.Edelman SV, et al. , Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care, 2018. 41(11): p. 2265–2274. [DOI] [PubMed] [Google Scholar]
- 49.Hovorka R, et al. , Assessing performance of closed-loop insulin delivery systems by continuous glucose monitoring: drawbacks and way forward. Diabetes technology & therapeutics, 2013. 15(1): p. 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmid C, et al. , System accuracy of blood glucose monitoring systems: impact of use by patients and ambient conditions. Diabetes technology & therapeutics, 2013. 15(10): p. 889–896. [DOI] [PubMed] [Google Scholar]
- 51.Liao K-C, et al. , Percutaneous fiber-optic sensor for chronic glucose monitoring in vivo. Biosensors and Bioelectronics, 2008. 23(10): p. 1458–1465. [DOI] [PubMed] [Google Scholar]
- 52.Barone PW and Strano MS, Single walled carbon nanotubes as reporters for the optical detection of glucose. 2009, SAGE Publications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balaconis MK, et al. , The design and development of fluorescent nano-optodes for in vivo glucose monitoring. Journal of diabetes science and technology, 2011. 5(1): p. 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schultz JS, Mansouri S, and Goldstein IJ, Affinity sensor: a new technique for developing implantable sensors for glucose and other metabolites. Diabetes Care, 1982. 5(3): p. 245–253. [DOI] [PubMed] [Google Scholar]
- 55.Klonoff DC, Overview of fluorescence glucose sensing: a technology with a bright future. 2012, SAGE Publications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du L, et al. , Enzyme free glucose sensing by amino-functionalized silicon quantum dot. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2019. 216: p. 303–309. [DOI] [PubMed] [Google Scholar]
- 57.Pørksen N, et al. , In humans at least 75% of insulin secretion arises from punctuated insulin secretory bursts. American Journal of Physiology-Endocrinology And Metabolism, 1997. 273(5): p. E908–E914. [DOI] [PubMed] [Google Scholar]
- 58.Qian W-J, et al. , Simultaneous monitoring of Zn2+ secretion and intracellular Ca2+ from islets and islet cells by fluorescence microscopy. Biotechniques, 2004. 37(6): p. 922–933. [DOI] [PubMed] [Google Scholar]
- 59.Association AD, 7. Diabetes technology: standards of medical care in diabetes—2019. Diabetes Care, 2019. 42(Supplement 1): p. S71–S80. [DOI] [PubMed] [Google Scholar]
- 60.Snider RM, et al. , A multiwalled carbon nanotube/dihydropyran composite film electrode for insulin detection in a microphysiometer chamber. Analytica chimica acta, 2008. 609(1): p. 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meetoo D and Lappin M, Nanotechnology and the future of diabetes management. Journal of Diabetes Nursing, 2009. 13(8): p. 288–297. [Google Scholar]
- 62.Ebrahimiasl S, Fathi E, and Ahmad M, Electrochemical Detection of Insulin in Blood serum using Ppy/GF Nanocomposite Modified Pencil Graphite Electrode. Nanomedicine Research Journal, 2019. 3(4): p. 219–228. [Google Scholar]
- 63.Amulya C, Gupta ME, and Babu IS, A REVIEW ON ALTERNATIVE ROUTES FOR INSULIN ADMINISTRATION. 2019. [Google Scholar]
- 64.Rodbard H and Rodbard D, Biosynthetic Human Insulin and Insulin Analogs. American Journal of Therapeutics, 2020. 27(1). [DOI] [PubMed] [Google Scholar]
- 65.McCrimmon R and Frier BM, Hypoglycaemia, the most feared complication of insulin therapy. Diabete & metabolisme, 1994. 20(6): p. 503–512. [PubMed] [Google Scholar]
- 66.Johnson‐ Rabbett B and Seaquist ER, Hypoglycemia in diabetes: The dark side of diabetes treatment. A patient‐ centered review. Journal of diabetes, 2019. 11(9): p. 711–718. [DOI] [PubMed] [Google Scholar]
- 67.Brownlee M and Cerami A, A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science, 1979. 206(4423): p. 1190–1191. [DOI] [PubMed] [Google Scholar]
- 68.Pegoraro C, MacNeil S, and Battaglia G, Transdermal drug delivery: from micro to nano. Nanoscale, 2012. 4(6): p. 1881–1894. [DOI] [PubMed] [Google Scholar]
- 69.Ensign LM, Cone R, and Hanes J, Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Advanced drug delivery reviews, 2012. 64(6): p. 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu J, et al. , Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proceedings of the National Academy of Sciences, 2015. 112(27): p. 8260–8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schoellhammer CM, Blankschtein D, and Langer R, Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert opinion on drug delivery, 2014. 11(3): p. 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hang T, et al. , Hierarchical graphene/nanorods-based H2O2 electrochemical sensor with self-cleaning and anti-biofouling properties. Sensors and Actuators B: Chemical, 2019. 289: p. 15–23. [Google Scholar]
- 73.Archana S and Sundaramoorthy B, Review on biofouling prevention using nanotechnology. 2019. [Google Scholar]
- 74.Gu Z, et al. , Injectable nano-network for glucose-mediated insulin delivery. ACS nano, 2013. 7(5): p. 4194–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilchrist JF, et al. , Phase Behavior and 3D Structure of Strongly Attractive Microsphere- Nanoparticle Mixtures. Langmuir, 2005. 21(24): p. 11040–11047. [DOI] [PubMed] [Google Scholar]
- 76.Li C, et al. , Glucose and H 2 O 2 dual-sensitive nanogels for enhanced glucose-responsive insulin delivery. Nanoscale, 2019. 11(18): p. 9163–9175. [DOI] [PubMed] [Google Scholar]
- 77.Habener JF, A perspective on pancreatic stem/progenitor cells. Pediatric diabetes, 2004. 5: p. 29–37. [DOI] [PubMed] [Google Scholar]
- 78.Shapiro AMJ, et al. , Strategic opportunities in clinical islet transplantation. Transplantation, 2005. 79(10): p. 1304–1307. [DOI] [PubMed] [Google Scholar]
- 79.Enderami SE, et al. , Generation of insulin‐ producing cells from human‐ induced pluripotent stem cells using a stepwise differentiation protocol optimized with platelet‐rich plasma. Journal of cellular physiology, 2017. 232(10): p. 2878–2886. [DOI] [PubMed] [Google Scholar]
- 80.Abazari MF, et al. , PCL/PVA nanofibrous scaffold improve insulin-producing cells generation from human induced pluripotent stem cells. Gene, 2018. 671: p. 50–57. [DOI] [PubMed] [Google Scholar]
- 81.Mansour RN, et al. , Collagen coated electrospun polyethersulfon nanofibers improved insulin producing cells differentiation potential of human induced pluripotent stem cells. Artificial cells, nanomedicine, and biotechnology, 2018. 46(sup3): p. S734–S739. [DOI] [PubMed] [Google Scholar]
- 82.Enderami SE, et al. , Generation of insulin-producing cells from human induced pluripotent stem cells on PLLA/PVA nanofiber scaffold. Artificial cells, nanomedicine, and biotechnology, 2018. 46(sup1): p. 1062–1069. [DOI] [PubMed] [Google Scholar]
- 83.de Vos P, et al. , Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials, 2006. 27(32): p. 5603–5617. [DOI] [PubMed] [Google Scholar]
- 84.Vegas AJ, et al. , Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nature biotechnology, 2016. 34(3): p. 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lou S, et al. , Pancreatic islet surface bioengineering with a heparin-incorporated starPEG nanofilm. Materials Science and Engineering: C, 2017. 78: p. 24–31. [DOI] [PubMed] [Google Scholar]
- 86.Song S, et al. , Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport. Scientific reports, 2016. 6: p. 23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang J, et al. , Pancreatic islet surface engineering with a starPEG-chondroitin sulfate nanocoating. Biomaterials science, 2019. 7(6): p. 2308–2316. [DOI] [PubMed] [Google Scholar]
- 88.Wang T, Successful diabetes management without immunosuppressivedrugs in NHP model has been demonstrated. Encapsulation system with taperednanopore conduits achieved normal glycaemia with regulated insulin release. Artificial cells, nanomedicine, and biotechnology, 2018. 46(sup3): p. S1162–S1168. [DOI] [PubMed] [Google Scholar]
- 89.Havel H, et al. , Nanomedicines: from bench to bedside and beyond. The AAPS journal, 2016. 18(6): p. 1373–1378. [DOI] [PubMed] [Google Scholar]
- 90.Xiao X, et al. , Endogenous reprogramming of alpha cells into beta cells, induced by viral gene therapy, reverses autoimmune diabetes. Cell Stem Cell, 2018. 22(1): p. 78–90. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whitehead KA, Langer R, and Anderson DG, Knocking down barriers: advances in siRNA delivery. Nature reviews Drug discovery, 2009. 8(2): p. 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gallego-Perez D, et al. , Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nature nanotechnology, 2017. 12(10): p. 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gallego-Perez D, et al. , Deterministic transfection drives efficient nonviral reprogramming and uncovers reprogramming barriers. Nanomedicine: Nanotechnology, Biology and Medicine, 2016. 12(2): p. 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]


