Abstract
Systemic inflammation impacts outcome after traumatic brain injury (TBI), but most TBI biomarker studies have focused on brain-specific proteins. C-reactive protein (CRP) is a widely used biomarker of inflammation with potential as a prognostic biomarker after TBI. The Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study prospectively enrolled TBI patients within 24 h of injury, as well as orthopedic injury and uninjured controls; biospecimens were collected at enrollment. A subset of hospitalized participants had blood collected on day 3, day 5, and 2 weeks. High-sensitivity CRP (hsCRP) and glial fibrillary acidic protein (GFAP) were measured. Receiver operating characteristic analysis was used to evaluate the prognostic ability of hsCRP for 6-month outcome, using the Glasgow Outcome Scale-Extended (GOSE). We included 1206 TBI subjects, 122 orthopedic trauma controls (OTCs), and 209 healthy controls (HCs). Longitudinal biomarker sampling was performed in 254 hospitalized TBI subjects and 19 OTCs. hsCRP rose between days 1 and 5 for TBI and OTC subjects, and fell by 2 weeks, but remained elevated compared with HCs (p < 0.001). Longitudinally, hsCRP was significantly higher in the first 2 weeks for subjects with death/severe disability (GOSE <5) compared with those with moderate disability/good recovery (GOSE ≥5); AUC was highest at 2 weeks (AUC = 0.892). Combining hsCRP and GFAP at 2 weeks produced AUC = 0.939 for prediction of disability. Serum hsCRP measured within 2 weeks of TBI is a prognostic biomarker for disability 6 months later. hsCRP may have utility as a biomarker of target engagement for anti-inflammatory therapies.
Keywords: biomarkers, head trauma, traumatic brain injury
Introduction
The management of patients with traumatic brain injury (TBI) relies upon neurological examination and radiographical imaging for assessment of injury severity and prognosis. Although outcome after TBI can range from complete recovery to death or severe disability, clinical assessments, such as the Glasgow Coma Scale (GCS) and standard neuroimaging, explain only a small fraction of the variance in outcome and are largely non-specific for pathophysiology, limiting our ability to identify patients appropriate for clinical trials of novel therapies.1–3 Blood-based biomarkers have the potential to identify patients who may be at risk for clinical deterioration4 and confirm target engagement by novel therapies aimed at specific pathophysiological mechanisms.
C-reactive protein (CRP) is a non-specific but sensitive biomarker of systemic inflammation that is known to rise in response to numerous conditions, including infection, cancer, surgery, burns, and tissue infarction, and is routinely used in clinical assessment of these conditions.5–7 Anti-inflammatory therapies reduce CRP levels in these and other medical conditions, including cardiovascular disease,8,9 cancers,10,11 and various autoimmune diseases.12–14 CRP belongs to the pentraxin family of calcium-dependent, ligand-binding plasma proteins, which activate the classical complement pathway by binding to the phosphocholine expressed on the surface of dead or dying cells and some bacteria.15,16 As a member of the acute-phase protein class, CRP levels in serum increase up to 1000-fold in response to inflammation, often directly in proportion to injury severity.5,17
A growing body of evidence implicates CRP as a biomarker in neurological disease. Although CRP is produced primarily by hepatocytes, it can be generated by human neurons.18 It is sharply upregulated in Alzheimer's disease19 and after spontaneous intracerebral hemorrhage, proportional to hematoma volume.20 In TBI, elevated levels of serum CRP within the first 24 h post-injury is associated with more-severe injury21 and presence of intracranial lesions on neuroimaging.22,23 It is also associated with post-injury headache and fatigue up to 30 days post-injury24 and poor long-term outcomes, including premature mortality21 and persistent post-concussional, psychiatric, and neurocognitive symptoms.25 However, CRP in the acute phase is limited by a lack of specificity and is affected by concurrent polytrauma.26,27 In previous studies, CRP has been observed to rise for 3–5 days after TBI28 before gradually declining, potentially over the course of months.24 Similar trends have been reported in ischemic stroke,29–32 but no studies have explored the relationship between sustained CRP elevation and outcome after TBI. Monitoring subacute CRP elevations in these patients may provide important prognostic information for identifying patients at risk for unfavorable recovery.33
The current study is a pre-specified analysis of the prospective, multi-center Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study (ClinicalTrials.gov: NCT02119182; and see Methods). We assess the temporal evolution of high-sensitivity CRP (hsCRP) serum levels over the first 2 weeks post-injury and the utility of serum CRP as a prognostic biomarker for post-TBI outcome.
Methods
Subjects and study design
Patients presenting with TBI (GCS 3–15) to 1 of 18 participating level I U.S. trauma centers from February 26, 2014 to July 27, 2018 were identified and enrolled prospectively in the TRACK-TBI study, as previously described.34,35 Written consent was obtained from subjects or their legal authorized representatives. Eligibility criteria included presentation within 24 h of injury with TBI warranting clinical evaluation with a non-contrast head computed tomography (CT) evaluation based on practice guidelines.36 Exclusion criteria were positive pregnancy test or known pregnancy, imminent death or current life-threatening disease, incarceration, or evidence of serious psychiatric and neurological disorders that would interfere with consent or follow-up outcome assessment. The study was approved by the institutional review board of each enrolling site.
TBI subjects were stratified into three clinical groups differentiated by clinical care path: 1) emergency department and discharged (ER) stratum; 2) admission stratum (ADM): patients admitted to the hospital but not to the intensive care unit (ICU); and 3) ICU stratum (ICU): patients admitted directly from ER or another hospital to the ICU.
Subjects were eligible for inclusion as orthopedic trauma controls (OTCs) if they presented with isolated trauma to their limbs, pelvis, and/or ribs and had an Abbreviated Injury Score <4 for those body regions. OTC subjects were identified and enrolled using the same process as that for patients with TBI, except for the head CT requirement. Subjects were ineligible from enrollment as an OTC if they had loss of consciousness, disturbance of consciousness, post-traumatic amnesia/retrograde amnesia, or other clinical findings suggestive of a TBI.
Finally, healthy controls (HCs) were recruited either based on a relationship with a TRACK-TBI participant or through public outreach within TRACK-TBI institutions, and the ability to provide informed consent. HCs were ineligible for enrollment if they had a history of TBI, concussion, or any traumatic injury causing polytrauma in the 12 months preceding enrollment. In this analysis, HCs were sex- and age-matched to TBI subjects.
The TRACK-TBI Phase 1 Biomarker Cohort (n = 1706) evaluated in this study was a prespecified interim analysis that included the first half of the enrolled TBI subjects and the OTC and HC groups (Fig. 1).
FIG. 1.
TRACK-TBI phase 1 biomarker cohort CONSORT diagram. ADM = admission stratum: patients admitted to the hospital but not to the ICU. ICU = ICU stratum: patients admitted directly from ER or another hospital to the ICU. D1 = day 1. D3 = day 3. D5 = day 5. 2W = 2 weeks. Serial samples: available hsCRP samples on day 1 and 2 Weeks, and day 3 and/or day 5. GOSE = Glasgow Outcome Scale-Extended. CRP, C-reactive protein; CONSORT, Consolidated Standards of Reporting Trials; ER, emergency room; ICU, intensive care unit; TBI, traumatic brain injury; TRACK-TBI, Transforming Research and Clinical Knowledge in Traumatic Brain Injury.
Clinical data collection
Demographic, injury, and outcome variables were collected in accordance with the National Institute of Neurological Disorders and Stroke (NINDS) TBI Common Data Elements (TBI-CDE).37,38 Demographic data were obtained through a combination of medical records and patient report. Injury Severity Score (ISS) was collected for all hospitalized (ADM, ICU) subjects. Outcome assessments occurred at 2 weeks and 3, 6, and 12 months post-injury. Three-month assessments were performed by telephone; other assessments were performed in person. For this study, the primary outcome was the 6-month Glasgow Outcome Scale-Extended (GOSE) administered to assess patient-reported global disability attributable only to the TBI. Complete recovery was defined as a GOSE = 8. Incomplete recovery was defined as GOSE <8. Unfavorable outcome was defined as GOSE <5 and favorable outcome as GOSE ≥5.
Sample collection and biomarker analysis
For subjects who consented to biospecimen collection, blood samples were collected within 24 h of injury (day 1) and at 2 weeks and 6 months. For subjects admitted to the hospital (ADM, ICU), additional blood samples were collected on days 3 and 5, when possible. Patients with available samples on day 1 and 2 weeks, and additionally day 3 and/or day 5, were considered to have “serial samples” and were included in longitudinal analyses. All samples were dated and time-stamped to compare with time of injury. The TBI-CDE Biospecimens and Biomarkers Working Group consensus recommendations for plasma and serum preparation were followed.37 Plasma and serum aliquots were prepared for each subject and frozen at −80°C for future analysis. All samples were deidentified using a unique study ID, specific to site and subject, and batch-shipped in temperature-controlled overnight express freight containers to the TRACK-TBI Biospecimens Repository at the University of Pittsburgh Medical Center (Pittsburgh, PA).
Blinded sample analysis of hsCRP was carried out by a single laboratory (University College of Dublin) using the Abbott Architect c8000, MULTIGENT CRP Vario assay using the high-sensitivity method (CRP16). Anti-CRP antibody adsorbed to latex particles agglutinate when an antigen-antibody reaction occurs with CRP, resulting in a change in absorbance proportional to the quantity of CRP in the sample. Serum samples were thawed in batches at room temperature and centrifuged at 10,000 rfc for 10 min at 4°C before testing. Assays were performed in duplicate with a lower limit of quantification of 0.1 mg/L and a reportable range of 0.1–160.0 mg/L. Temporal trends of CRP were analyzed and reported. Glial fibrillary acidic protein (GFAP) concentrations were determined using prototype immunoassays on the i-STAT point-of-care platform (Abbott Laboratories, Abbott Park, IL), as previously described.34 The i-STAT GFAP test uses the sandwich enzyme-linked immunosorbent assay method with electrochemical detection of the resulting enzyme signal.
Computing tomography imaging evaluation and analysis
Initial head CT scans were deidentified and uploaded to a central imaging database at the Laboratory of NeuroImaging (LONI; University of Southern California, Los Angeles, CA) and independently evaluated by a central board-certified neuroradiologist in accordance with TBI-CDE Neuroimaging Working Group consensus recommendations.36 The study neuroradiologist was blinded to the identity and clinical information associated with each CT scan. The result of each review was uploaded to the TRACK-TBI clinical database under the respective subject's record. CT scans were read as positive (CT+) if there was any evidence of acute intracranial pathology consistent with TBI (e.g., contusion, subarachnoid hemorrhage, and subdural hematoma).
Magnetic resonance imaging methods and analysis
Magnetic resonance imaging (MRI) was obtained at 7–18 days. Image sequences included T1, T2, fluid-attenuated inversion recovery, and T2*. The MRI protocol was standardized across all sites and General Electric, Siemens, and Phillips MRI platforms (available at https://tracktbi.ucsf.edu/researchers). Baseline phantom scans were performed at all centers to quantify differences between magnets and correct geometrical variances across scanners. Structural MRI abnormalities were quantified according to CDE standards and definitions36 by a central board-certified neuroradiologist blinded to the identity and clinical history of the subject. MRI scans were read as positive (MRI+) if there was any evidence of acute intracranial pathology consistent with TBI (e.g., contusion, traumatic axonal injury, and diffuse axonal injury).
Statistical analysis
Descriptive summary statistics were used to characterize the demographics and clinical attributes of the study cohort. hsCRP levels were reported using the median and 25th/75th percentiles and were compared using a Wilcoxon's rank-sum test between TBI subjects and OTC/HC; among TBI subjects with and without intracranial lesions on CT; among TBI CT-negative subjects with and without intracranial lesions on MRI; among TBI subjects by ISS total score categories (≤9, 10–16, 17–25, and >25); and among TBI subjects by GOSE outcomes (unfavorable, <5 vs. favorable, ≥5; and complete recovery, = 8 vs. incomplete recovery, <8). Receiver operating characteristic (ROC) analysis was performed to assess the ability of hsCRP level at each time point to predict GOSE at 6 months post-injury, and area under the ROC curve (AUC) was calculated with a 95% confidence interval (CI). AUCs of >0.9 were considered excellent, 0.8–0.9 as good, 0.7–0.8 as adequate, and <0.7 as poor. All data were analyzed and plotted using statistical software R (version 3.6.1; http://www.r-project.org).
Results
The TRACK-TBI Phase 1 Biomarker Cohort included 1706 subjects (1375 TBIs, 122 OTCs, and 209 HCs; Fig. 1). Serum was available for hsCRP assay in 1206 TBI subjects. Most TBI subjects were Caucasian, male, and had mild injury (GCS 13–15). The most common cause of injury was road traffic accidents, followed by falls. Full demographic and clinical data are presented in Table 1.
Table 1.
Demographic and Clinical Characteristics of the TRACK-TBI Phase 1 Biomarker Cohort
| TBI (n = 1206) | OTC (n = 122) | p value* | HC (n = 209) | |
|---|---|---|---|---|
| Patient care pathway | ||||
| ER discharge | 343 (28.4%) | 45 (36.9%) | 0.0005 | |
| ADM, hospital admit | 437 (36.2%) | 70 (57.4%) | ||
| ICU, ICU admit | 426 (35.3%) | 7 (5.7%) | ||
| Sex | ||||
| Female | 390 (32.3%) | 43 (35.3%) | 0.5434 | 104 (50%) |
| Male | 816 (67.7%) | 79 (64.8%) | 105 (50%) | |
| Age (mean ± SD) | 40.0 ± 17.0 | 39.2 ± 15.0 | 0.9173 | 39.0 ± 17.0 |
| Years of education (mean ± SD) | 13.4 ± 2.9 | 13.8 ± 2.6 | 0.0702 | |
| Race | ||||
| White | 924 (77.3%) | 95 (81.2%) | 0.6192 | |
| Black | 196 (16.4%) | 15 (12.8%) | ||
| Other | 75 (6.3%) | 7 (6.0%) | ||
| Hispanic | ||||
| No | 934 (78.2%) | 91 (76.5%) | 0.6443 | |
| Yes | 261 (21.8%) | 28 (23.5%) | ||
| Cause of injury | ||||
| Road traffic accident | 705 (58.5%) | 43 (38.0%) | 0.0005 | |
| Incidental fall | 314 (26.1%) | 40 (35.4%) | ||
| Violence/assault | 82 (6.8%) | 1 (0.9%) | ||
| Other | 104 (8.6%) | 29 (25.7%) | ||
| GCS on ER arrival | ||||
| 3–8 | 117 (9.8%) | 0 (0%) | 0.0005 | |
| 9–12 | 43 (3.6%) | 0 (0%) | ||
| 13–15 | 1030 (86.6%) | 122 (100%) | ||
| CT | ||||
| CT– | 731 (61.3%) | |||
| CT+ | 461 (38.7%) |
Data are n (%) or mean ± SD.
p values were calculated comparing TBI and OTC using Wilcoxon's rank-sum test for continuous variables and Fisher's exact test for categorical variables.
TBI, traumatic brain injury; OTC, orthopedic trauma control; HC, healthy control; ER, emergency department and discharged (ER) stratum; ADM, admission stratum: patients admitted to the hospital, but not to the ICU; ICU, ICU stratum: patients admitted directly from the ER or another hospital to the ICU; GCS, Glasgow Coma Scale; SD, standard deviation; CT, computed tomography.
High-sensitivity C-reactive protein rises in traumatic brain injury and orthopedic trauma controls and is increased in computed tomography–positive vs. computed tomography–negative cases
Day 1 hsCRP was higher in TBI subjects compared to HC (median [interquartile range], 9.091 [2.110–30.932] vs. 1.34 [0.642–2.785] mg/L; p < 0.0001). hsCRP values rose over the first 5 days in both TBI and OTC. In those with serial samples (day 1 and 2 weeks, as well as day 3 and/or day 5), there was no significant difference in hsCRP between TBI and OTC at any time point (Fig. 2), suggesting that OTCs were well matched to TBI subjects for systemic injury severity. Among patients with mild TBI (mTBI; GCS 13–15), a slight trend toward decreased hsCRP compared with OTC was observed at all time points, which did not reach significance. Please refer to Supplementary Table S1 for hsCRP numerical values for mTBI patients.
FIG. 2.
hsCRP at days 1, 3, and 5 and 2 weeks, comparing TBI, OTC, and HC. Line plot indicates median and 25th–75th percentile. Among patients with serial hsCRP samples (day 1 and 2 weeks, and day 3 and/or day 5), Wilcoxon's rank-sum test found no significant difference in hsCRP level between TBI and OTC at any time point. Baseline hsCRP level in HC was measured at one time point and was significantly lower than TBI and OTC at all time points. HC, healthy controls; hsCRP, high-sensitivity C-reactive protein; OTC, orthopedic trauma controls; TBI, traumatic brain injury.
In TBI patients with day 1 samples, median hsCRP was higher in CT+ cases compared to CT– cases on day 1 (20.415 [5.979–54.244] vs. 4.233 [1.327–17.479] mg/L; p < 0.0001). Within CT– cases, day 1 hsCRP was higher among MRI+ cases compared with MRI– cases (3.905 [1.840–16.219] vs. 2.94 [0.800–11.349] mg/L; p = 0.0075). In those with serial samples, hsCRP remained significantly higher in CT+ versus CT– cases at all time points, increasing from days 1 to 5 in CT+ cases and plateauing between days 3 and 5 in CT– cases.
Given that future clinical trials may require enrolling subjects within time windows shorter than 24 h, we investigated hsCRP elevation by blood-draw time intervals from 0 to 6, 7 to 12, 13 to 18, and 19–25 h post-injury. hsCRP increased temporally over the first 24 h in both CT– and CT+ TBI subjects, and hsCRP was significantly higher in CT+ cases at all time points. Please refer to Supplementary Table S2 for hsCRP numerical values.
C-reactive protein rises with increasing overall Injury Severity Score
In subjects with recorded ISS and day 1 samples, day 1 hsCRP increased with ISS (Fig. 3A), with similar findings observed in mTBI subjects. In subjects with serial samples, median hsCRP increased with ISS at all time points (Fig. 3B). In mTBI subjects with serial samples, hsCRP increased with ISS at all time points, but reached significance only on days 1, 3, and 5.
FIG. 3.
Relationship between hsCRP and ISS at (A) day 1 and (B) days 1, 3, and 5 and 2 weeks. Boxplots indicate median and 25th–75th percentile (interquartile range; IQR) of hsCRP values. Upper whisker indicates the smaller value of: the maximum value or 75th percentile +1.5*IQR, and lower whisker indicates the larger value of: the minimum value or 25th percentile −1.5*IQR. ISS total score was separated into four score categories: ≤9, 10–16, 17–25, and >25. (A) Among all TBI patients with available day 1 hsCRP samples, Ddy 1 hsCRP rises with increasing ISS total score. (B) Among patients with serial hsCRP samples (day 1 and 2 weeks, and day 3 and/or day 5), hsCRP rises with increasing ISS total score at all time points. hsCRP, high-sensitivity C-reactive protein; ISS, Injury Severity Score; TBI, traumatic brain injury.
C-reactive protein is a prognostic biomarker for predicting death/severe disability (Glasgow Outcome Scale-Extended [GOSE] <5) vs. moderate disability/good recovery (GOSE ≥5)
In TBI patients with serial samples, hsCRP level at each of the four time points was significantly elevated in subjects with death/severe disability (GOSE <5) compared to those with moderate disability/good recovery (GOSE ≥5; Fig. 4A). The AUC of hsCRP for discriminating 6-month disability was highest at 2 weeks (AUC = 0.892; Table 2). The same analysis was performed in mTBI subjects and revealed similar findings with 2-week hsCRP (AUC = 0.928). When stratified based on degree of peripheral injury (peripheral ISS [excluding head/neck] ≤9, = 10–16, and ≥17), predictive value of 2-week hsCRP remained high across all stratifications (AUC = 0.897, 0.922, and 0.800, for ISS ≤9, = 10–16, and ≥17, respectively). When stratified based on GCS (3–12, 13–15), predictive value of 2-week hsCRP was found to be higher in the subset of patients with milder injury (AUC = 0.779 and 0.928 for GCS 3–12 and 13–15, respectively).
FIG. 4.
hsCRP and outcome after TBI: (A) GOSE ≥5 versus <5 (B) GOSE = 8 versus <8. Line plots indicate median and 25th–75th percentile. (A) In patients with serial hsCRP samples (day 1 and 2 weeks, and day 3 and/or day 5), hsCRP level was compared between patients with unfavorable outcome (GOSE <5, indicating death/severe disability) and favorable outcome (GOSE ≥5). Patients with favorable outcome had significantly higher hsCRP level at all time points compared to patients with unfavorable outcome. (B) In patients with serial hsCRP samples, hsCRP level was compared between patients with complete recovery (GOSE = 8) and incomplete recovery (GOSE <8). Patients with incomplete recovery had significantly higher hsCRP level at the 2-week time point compared with patients with complete recovery, but were not significantly different at any other time point. GOSE, Glasgow Outcome Scale-Extended; hsCRP, high-sensitivity C-reactive protein; TBI, traumatic brain injury.
Table 2.
Predictive Performance of Acute Measurement of hsCRP on GOSE at 6 Months after Traumatic Brain Injury
| AUC (95% CI) | |
|---|---|
| GOSE <5 vs. GOSE ≥5 | |
| Day 1 | 0.640 (0.521–0.760) |
| Day 3 | 0.800 (0.729–0.871) |
| Day 5 | 0.777 (0.691–0.862) |
| 2-week | 0.892 (0.839–0.944) |
| Multiple time points | 0.872 (0.818–0.925) |
| GOSE <8 vs. GOSE = 8 | |
| Day 1 | 0.570 (0.477–0.662) |
| Day 3 | 0.569 (0.474–0.665) |
| Day 5 | 0.607 (0.480–0.735) |
| 2-week | 0.615 (0.525–0.705) |
| Multiple time points | 0.608 (0.519–0.698) |
GOSE <5 indicates unfavorable outcome (severe disability/death). GOSE = 8 indicates complete recovery.
hsCRP, high-sensitivity C-reactive protein; GOSE, Glasgow Outcome Scale-Extended; AUC, area under the receiver operating characteristic (ROC) curve; CI, confidence interval.
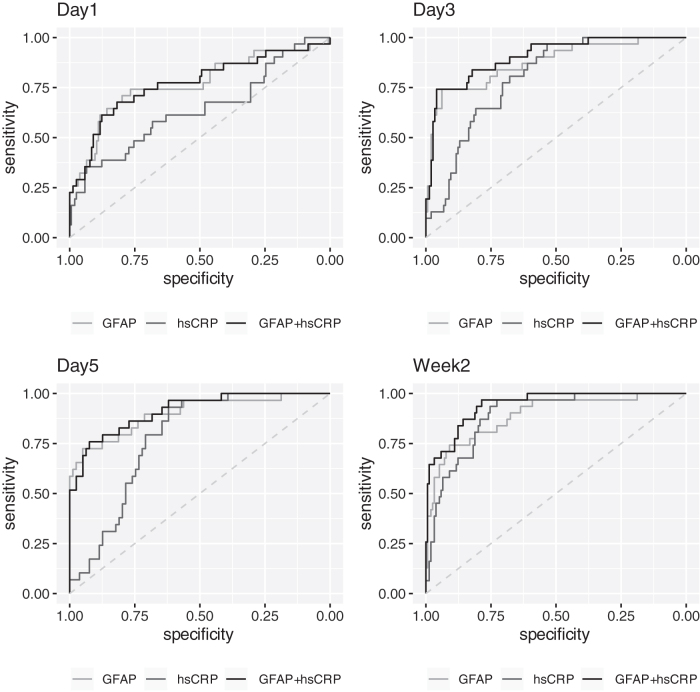
Notably, among TBI subjects, combining 2-week hsCRP (AUC = 0.892) and 2-week GFAP (AUC = 0.890) improved discrimination of 6-month GOSE <5 versus GOSE ≥5 to AUC = 0.939 (95% CI, 0.900–0.978), higher than either marker individually (Table 3; ROC curves shown in Fig. 5).
Table 3.
Combined Predictive Performance of Acute Measurement of hsCRP and GFAP on GOSE ≥5 vs. <5 at 6 Months after Traumatic Brain Injury
| AUC (95% CI) | |
|---|---|
| Day 1 | |
| logGFAP | 0.768 (0.662–0.875) |
| loghsCRP | 0.640 (0.521–0.760) |
| logGFAP + loghsCRP | 0.769 (0.663–0.876) |
| Day 3 | |
| logGFAP | 0.873 (0.798–0.949) |
| loghsCRP | 0.800 (0.729–0.871) |
| logGFAP + loghsCRP | 0.904 (0.846–0.963) |
| Day 5 | |
| logGFAP | 0.900 (0.827–0.972) |
| loghsCRP | 0.777 (0.691–0.862) |
| logGFAP + loghsCRP | 0.913 (0.853–0.973) |
| 2-week | |
| logGFAP | 0.890 (0.823–0.956) |
| loghsCRP | 0.892 (0.839–0.944) |
| logGFAP + loghsCRP | 0.942 (0.905–0.979) |
GOSE <5 indicates unfavorable outcome (severe disability/death).
hsCRP, high-sensitivity C-reactive protein; GFAP, glial fibrillary acidic protein; GOSE, Glasgow Outcome Scale-Extended; AUC, area under the receiver operating characteristic (ROC) curve; CI, confidence interval.
FIG. 5.
Receiver operating characteristic (ROC) curves for hsCRP and GFAP in predicting GOSE ≥5 versus <5 at 6 months after traumatic brain injury. ROC curves for GFAP alone, hsCRP alone, and GFAP + hsCRP at (A) day 1, (B) day 3, (C) day 5, and (D) 2 weeks post-injury. GOSE <5 indicates unfavorable outcome (severe disability/death). GFAP, glial fibrillary acidic protein; GOSE, Glasgow Outcome Scale-Extended; hsCRP, high-sensitivity C-reactive protein.
When comparing the AUC of the model using age and GCS score category only compared to the model using age, GCS score category, and hsCRP, the addition of 2-week hsCRP significantly improved predictive ability of the model (Supplementary Table S3). Imaging results were not included as CT+ versus CT– status was not significant in the age + GCS model.
C-reactive protein is a poor predictor of complete recovery (Glasgow Outcome Scale-Extended [GOSE] = 8) vs. incomplete recovery (GOSE <8)
Median hsCRP differed between subjects who experienced complete recovery (GOSE = 8) from those with incomplete recovery (GOSE <8) at only the 2-week time assessment (Fig. 4B). The AUC of hsCRP for discriminating complete recovery at 6 months increased with time after injury, but was poor at all time points (Table 2), similar to findings in the mTBI subjects with 2-week hsCRP (AUC = 0.547).
Discussion
hsCRP, a measure of systemic inflammation, is a prognostic biomarker for poor 6-month outcome after TBI when measured acutely. hsCRP is elevated in the first 2 weeks after TBI, proportional to the severity of systemic injury and more so in subjects with intracranial lesions on CT or MRI scans. Elevation of hsCRP in TBI subjects was indistinguishable from orthopedic controls at all time points after injury. In TBI subjects, hsCRP at all time points increased linearly with worsening ISS, a clinical measure of injury severity in six body regions.
Prognostically, hsCRP measured through the first 2 weeks post-injury was significantly higher in subjects with outcome of death/severe disability (GOSE <5) compared with those with favorable outcome (GOSE ≥5) at 6 months. Using ROC analysis, only 2-week hsCRP demonstrated good discriminative ability (AUC >0.8) for discriminating between favorable (GOSE ≥5) and unfavorable outcome (GOSE <5). In comparing subjects with full recovery (GOSE = 8) and those who were not fully recovered (GOSE <8), hsCRP was significantly higher in fully recovered patients only at the latest time point (2 weeks), with poor discriminative ability (AUC <0.7). These findings indicate that subacute hsCRP is a useful prognostic biomarker for detecting death/severe disability after TBI, particularly in combination with the prognostic biomarker, GFAP, and demonstrate the important association between both systemic injury and inflammation with outcome after TBI.
The mechanism of injury in TBI is characterized by an initial primary injury, during which mechanical forces lead to axonal shearing and necrosis, followed by a later secondary injury, driven by inflammation, blood–brain barrier (BBB) disruption, apoptosis, metabolic disturbances, and oxidative stress, which may have long-lasting effects.39 In patients with isolated TBI, injury to the BBB allows peripheral inflammatory factors to access the brain tissue, leading to activation of neuroinflammatory cascades.40 It is reasonable to hypothesize that concurrent polytrauma leads to greater systemic inflammation that can breach the BBB, further exacerbating inflammation both systemically and in the brain.41 Previous studies support this hypothesis. Polytrauma in TBI results in increased levels of inflammatory cytokine interleukin (IL)-6, compared with patients with isolated TBI.42,43 Animal studies in rodent models of concurrent polytrauma in TBI have also found increased levels of inflammatory markers, including IL-6, tumor necrosis factor alpha, and chemokine (C-C motif) ligand 2,44,45 in the acute phase of injury. Further, peripheral delivery of IL-1ß in a rat model of TBI led to significantly worse behavioral outcomes compared with control vehicle-treated animals,46 demonstrating a link between systemic inflammatory response and unfavorable outcome in TBI.
Several clinical studies have examined the role of concurrent polytrauma on mortality and functional outcome after TBI, as reviewed in detail by McDonald and colleagues.41 Overall findings suggest that TBI, compounded by concurrent polytrauma, results in increased mortality47–49 and worsened functional outcomes.47,48,50,51 In a large retrospective study of 39,274 TBI patients with and without major extracranial injury, van Leeuwen and colleagues found that patients with both TBI and major extracranial injury had significantly higher mortality, with a stronger effect noted in mild and moderate TBI (odds ratio of 2.14 and 1.46, respectively) than in severe TBI (odds ratio of 1.18),49 a trend that has been previously observed.52,53 Functional outcome, measured using GOS or GOSE score, was also worse in TBI patients with concurrent polytrauma,48,51,54 although a few studies have reported no difference42,55 or even improved outcome in polytrauma patients.53 This divergence may be attributable to the observation that patients with TBI and concurrent polytrauma are often significantly younger than patients with isolated TBI, which may mask the effects of concurrent polytrauma on outcome.41
A broad span of therapeutic agents have been shown to reduce systemic inflammation and hsCRP level in cardiovascular disease,56 including statins,57 cyclooxygenase inhibitors,58 angiotensin-converting enzyme inhibitors,59 and more targeted therapies, such as canakinumab9 (IL-1β monoclonal antibody). Targeting systemic inflammation in cardiovascular disease has been shown to improve outcomes, for instance reducing atherosclerotic burden57 and decreasing rate of recurrent cardiovascular events.9 Similar investigations are ongoing in the treatment of autoimmune disease13,14,60,61 and cancers,10,11,62 with growing evidence for the significant relationship between CRP level and outcomes. Future efforts may explore the application of similar therapies in TBI.
Our study has several important limitations. ISS is a crude measure of polytrauma and was only available in hospitalized TRACK-TBI participants, making it impossible to correlate ISS with hsCRP levels in less severely injured subjects who did not require in-patient care. A granular assessment of how much disability was attributable to central nervous system (CNS) versus extra-CNS injury is beyond the scope of this study. In addition, hsCRP levels are also affected by a number of other conditions, including acute and chronic inflammatory conditions, surgical interventions, and complications such as ventilator associated pneumonia, which we do not account for in this study. The role of systemic inflammation in TBI requires further exploration and is likely to have important implications for prognostication and future clinical trial design.
Conclusions
Serum hsCRP measured within 2 weeks of TBI discriminates unfavorable from favorable recovery at 6 months. Our data support the role of hsCRP as a prognostic marker with potential utility for both early identification of subjects at risk for poor outcome and as a tool for subject stratification and cohort enrichment for clinical trials of anti-inflammatory treatments.
Supplementary Material
Acknowledgments
We thank the following contributors to the development of the TRACK-TBI database and repositories by organization and in alphabetical order by last name: Peter Chiarelli, Garen Staglin (One Mind, Rutherford, CA); Vibeke Brinck, Michael Jarrett (QuesGen Systems, Burlingame, CA); and Sirimon O'Charoen (Thomson Reuters, San Francisco, CA). We also acknowledge editorial and project management support from Amy J. Markowitz.
Contributor Information
Collaborators: The TRACK-TBI Investigators, Opeolu Adeoye, Neeraj Badjatia, Kim Boase, Yelena Bodien, M. Ross Bullock, Randall Chesnut, John D. Corrigan, Karen Crawford, Ann-Christine Duhaime, Richard Ellenbogen, V. Ramana Feeser, Adam R. Ferguson, Brandon Foreman, Raquel Gardner, Etienne Gaudette, Dana Goldman, Luis Gonzalez, Shankar Gopinath, Rao Gullapalli, J. Claude Hemphill, Gillian Hotz, Joel Kramer, Natalie Kreitzer, Chris Lindsell, Joan Machamer, Christopher Madden, Alastair Martin, Thomas McAllister, Randall Merchant, Lindsay Nelson, Laura B. Ngwenya, Florence Noel, David Okonkwo, Eva Palacios, Daniel Perl, Jonathan Rosand, Angelle Sander, Gabriella Satris, David Schnyer, Seth Seabury, Arthur Toga, Alex VaOpeolu Adeoye, Neeraj Badjatia, Kim Boase, Yelena Bodien, M. Ross Bullock, Randall Chesnut, John D. Corrigan, Karen Crawford, Ann-Christine Duhaime, Richard Ellenbogen, V. Ramana Feeser, Adam R. Ferguson, Brandon Foreman, Raquel Gardner, Etienne Gaudette, Dana Goldman, Luis Gonzalez, Shankar Gopinath, Rao Gullapalli, J. Claude Hemphill, Gillian Hotz, Joel Kramer, Natalie Kreitzer, Chris Lindsell, Joan Machamer, Christopher Madden, Alastair Martin, Thomas McAllister, Randall Merchant, Lindsay Nelson, Laura B. Ngwenya, Florence Noel, David Okonkwo, Eva Palacios, Daniel Perl, Jonathan Rosand, Angelle Sander, Gabriella Satris, David Schnyer, Seth Seabury, Arthur Toga, Alex Valadka, Paul Vespa, and Ross Zafonte
The TRACK-TBI Investigators
Opeolu Adeoye, MD, University of Cincinnati; Neeraj Badjatia, MD, University of Maryland; Kim Boase, University of Washington; Yelena Bodien, PhD, Massachusetts General Hospital; M. Ross Bullock, MD, PhD, University of Miami; Randall Chesnut, MD, University of Washington; John D. Corrigan, PhD, ABPP, Ohio State University; Karen Crawford, University of Southern California; Ann-Christine Duhaime, MD, MassGeneral Hospital for Children; Richard Ellenbogen, MD, University of Washington; V. Ramana Feeser, MD, Virginia Commonwealth University; Adam R. Ferguson, PhD, University of California, San Francisco; Brandon Foreman, MD, University of Cincinnati; Raquel Gardner, University of California, San Francisco; Etienne Gaudette, PhD, University of Southern California; Dana Goldman, PhD, University of Southern California; Luis Gonzalez, TIRR Memorial Hermann; Shankar Gopinath, MD, Baylor College of Medicine; Rao Gullapalli, PhD, University of Maryland; J. Claude Hemphill, MD, University of California, San Francisco; Gillian Hotz, PhD, University of Miami; Joel Kramer, PsyD, University of California, San Francisco; Natalie Kreitzer, MD, University of Cincinnati; Chris Lindsell, PhD, Vanderbilt University; Joan Machamer, MA, University of Washington; Christopher Madden, MD, UT Southwestern; Alastair Martin, PhD, University of California, San Francisco; Thomas McAllister, MD, Indiana University; Randall Merchant, PhD, Virginia Commonwealth University; Lindsay Nelson, PhD, Medical College of Wisconsin; Laura B. Ngwenya, MD, PhD, University of Cincinnati; Florence Noel, PhD, Baylor College of Medicine; David Okonkwo, MD, PhD, University of Pittsburgh; Eva Palacios, PhD, University of California, San Francisco; Daniel Perl, MD, Uniformed Services University; Jonathan Rosand, MD, MSc, Massachusetts General Hospital; Angelle Sander, PhD, Baylor College of Medicine; Gabriella Satris, University of California, San Francisco; David Schnyer, PhD, UT Austin; Seth Seabury, PhD, University of Southern California; Arthur Toga, PhD, University of Southern California; Alex Valadka, MD, Virginia Commonwealth University; Paul Vespa, MD, University of California, Los Angeles; Esther Yuh, MD, PhD, University of California, San Francisco; Ross Zafonte, Harvard Medical School.
Funding Information
This work was supported by the following grants: NINDS 1RC2NS069409-01, 3RC2NS069409-02S1, 5RC2NS069409-02, 1U01NS086090-01, 3U01NS086090-02S1, 3U01NS086090-02S2, 3U01NS086090-03S1, 5U01NS086090-02, and 5U01NS086090-03; U.S. Department of Defense (DOD) W81XWH-13-10441, US DOD W81XWH-14-2-0176 (to G.T.M.).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Sandsmark, D.K. (2016). Clinical outcomes after traumatic brain injury. Curr. Neurol. Neurosci. Rep. 16, 52. [DOI] [PubMed] [Google Scholar]
- 2. Foreman, B.P., Caesar, R.R., Parks, J., Madden, C., Gentilello, L.M., Shafi, S., Carlile, M.C., Harper, C.R., and Diaz-Arrastia, R.R. (2007). Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. J. Trauma Acute Care Surg. 62, 946–950 [DOI] [PubMed] [Google Scholar]
- 3. Diaz-Arrastia, R., Kochanek, P.M., Bergold, P., Kenney, K., Marx, C.E., Grimes, C.J.B., Loh, L.Y., Adam, L.G.E., Oskvig, D., and Curley, K.C. (2014). Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 31, 135–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dadas, A., Washington, J., Diaz-Arrastia, R., and Janigro, D. (2018). Biomarkers in traumatic brain injury (TBI): a review. Neuropsychiatric disease and treatment 14, 2989–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabay, C., and Kushner, I. (1999). Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 [DOI] [PubMed] [Google Scholar]
- 6. Pietllä, K. O., Harmoinen, A. P., Jokiniitty, J., and Pasternack, A. I. (1996). Serum C-reactive protein concentration in acute myocardial infarction and its relationship to mortality during 24 months of follow-up in patients under thrombolytic treatment. Eur. Heart J. 17, 1345–1349 [DOI] [PubMed] [Google Scholar]
- 7. Yasojima, K., Schwab, C., McGeer, E.G., and McGeer, P.L. (2001). Generation of C-reactive protein and complement components in atherosclerotic plaques. Am. J. Pathol. 158, 1039–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albert, M.A., Danielson, E., Rifai, N., and Ridker, P.M.; Prince Investigators. (2001). Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 286, 64–70 [DOI] [PubMed] [Google Scholar]
- 9. Ridker, P.M., Everett, B.M., Thuren, T., MacFadyen, J.G., Chang, W.H., Ballantyne, C., Fonseca, F., Nicolau, J., Koenig, W., and Anker, S.D. (2017). Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 [DOI] [PubMed] [Google Scholar]
- 10. Reichle, A., Vogt, T., Coras, B., Terheyden, P., Neuber, K., Trefzer, U., Schultz, E., Berand, A., Bröcker, E.B., and Landthaler, M. (2007). Targeted combined anti-inflammatory and angiostatic therapy in advanced melanoma: a randomized phase II trial. Melanoma Res. 17, 360–364 [DOI] [PubMed] [Google Scholar]
- 11. Trikha, M., Corringham, R., Klein, B., and Rossi, J. (2003). Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin. Cancer Res. 9, 4653–4665 [PMC free article] [PubMed] [Google Scholar]
- 12. Peyrin-Biroulet, L., Reinisch, W., Colombel, J., Mantzaris, G.J., Kornbluth, A., Diamond, R., Rutgeerts, P., Tang, L.K., Cornillie, F.J., and Sandborn, W.J. (2014). Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut 63, 88–95 [DOI] [PubMed] [Google Scholar]
- 13. Strober, B., Teller, C., Yamauchi, P., Miller, J.L., Hooper, M., Yang, Y., and Dann, F. (2008). Effects of etanercept on C-reactive protein levels in psoriasis and psoriatic arthritis. Br. J. Dermatol. 159, 322–330 [DOI] [PubMed] [Google Scholar]
- 14. Soilu-Hänninen, M., Koskinen, J. O., Laaksonen, M., Hänninen, A., Lilius, E., and Waris, M. (2005). High sensitivity measurement of CRP and disease progression in multiple sclerosis. Neurology 65, 153–155 [DOI] [PubMed] [Google Scholar]
- 15. Wolbink, G.J., Brouwer, M.C., Buysmann, S., Ten Berge, I.J., and Hack, C.E. (1996). CRP-mediated activation of complement in vivo: assessment by measuring circulating complement-C-reactive protein complexes. J. Immunol. 157, 473–479 [PubMed] [Google Scholar]
- 16. Hicks, P.S., Saunero-Nava, L., Du Clos, T.W., and Mold, C. (1992). Serum amyloid P component binds to histones and activates the classical complement pathway. J. Immunol. 149, 3689–3694 [PubMed] [Google Scholar]
- 17. Thompson, D., Pepys, M.B., and Wood, S.P. (1999). The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 7, 169–177 [DOI] [PubMed] [Google Scholar]
- 18. Di Napoli, M., Parry-Jones, A.R., Smith, C.J., Hopkins, S.J., Slevin, M., Masotti, L., Campi, V., Singh, P., Papa, F., and Popa-Wagner, A. (2014). C-reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke 45, 59–65 [DOI] [PubMed] [Google Scholar]
- 19. Yasojima, K., Schwab, C., McGeer, E.G., and McGeer, P.L. (2000). Human neurons generate C-reactive protein and amyloid P: upregulation in Alzheimer's disease. Brain Res. 887, 80–89 [DOI] [PubMed] [Google Scholar]
- 20. Di Napoli, M., Godoy, D.A., Campi, V., Masotti, L., Smith, C.J., Jones, A.R.P., Hopkins, S.J., Slevin, M., Papa, F., and Mogoanta, L. (2012). C-reactive protein in intracerebral hemorrhage: time course, tissue localization, and prognosis. Neurology 79, 690–699 [DOI] [PubMed] [Google Scholar]
- 21. Sogut, O., Guloglu, C., Orak, M., Sayhan, M.B., Gokdemir, M.T., Ustundag, M., and Akkus, Z. (2010). Trauma scores and neuron-specific enolase, cytokine and C-reactive protein levels as predictors of mortality in patients with blunt head trauma. J. Int. Med. Res. 38, 1708–1720 [DOI] [PubMed] [Google Scholar]
- 22. Sharma, R., Rosenberg, A., Bennett, E.R., Laskowitz, D.T., and Acheson, S.K. (2017). A blood-based biomarker panel to risk-stratify mild traumatic brain injury. PLoS One 12, e0173798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carabias, C.S., Gomez, P.A., Panero, I., Eiriz, C., Castaño-León, A.M., Egea, J., Lagares, A., Paredes, I., Fernández, J.A., and Moreno-Gómez, L.M. (2019). YKL-40, SAA1, CRP and PCT are promising biomarkers for intracranial severity assessment of traumatic brain injury: relationship with Glasgow Coma Scale and CT volumetry. World Neurosurg. 134. doi: 10.1016/j.wneu.2019.09.143 [DOI] [PubMed] [Google Scholar]
- 24. Shetty, T., Cogsil, T., Dalal, A., Kim, E., Halvorsen, K., Cummings, K., and Nguyen, J.T. (2019). High-sensitivity C-reactive protein: retrospective study of potential blood biomarker of inflammation in acute mild traumatic brain injury. J. Head Trauma Rehabil. 34, E28–E36 [DOI] [PubMed] [Google Scholar]
- 25. Su, S., Xu, W., Li, M., Zhang, L., Wu, Y., Yu, F., and Hai, J. (2014). Elevated C-reactive protein levels may be a predictor of persistent unfavourable symptoms in patients with mild traumatic brain injury: a preliminary study. Brain Behav. Immun. 38, 111–117 [DOI] [PubMed] [Google Scholar]
- 26. Anada, R.P., Wong, K.T., Jayapalan, J.J., Hashim, O.H., and Ganesan, D. (2018). Panel of serum protein biomarkers to grade the severity of traumatic brain injury. Electrophoresis 39, 2308–2315 [DOI] [PubMed] [Google Scholar]
- 27. Lustenberger, T., Kern, M., Relja, B., Wutzler, S., Störmann, P., and Marzi, I. (2016). The effect of brain injury on the inflammatory response following severe trauma. Immunobiology 221, 427–431 [DOI] [PubMed] [Google Scholar]
- 28. Is, M., Coskun, A., Sanus, G.Z., Tanriverdi, T., Kafadar, A.M., Hanimoglu, H., Tanriover, N., Gezen, F., and Uzan, M. (2007). High-sensitivity C-reactive protein levels in cerebrospinal fluid and serum in severe head injury: relationship to tumor necrosis factor-α and interleukin-6. J. Clin. Neurosci. 14, 1163–1171 [DOI] [PubMed] [Google Scholar]
- 29. Emsley, H.C., Smith, C.J., Gavin, C.M., Georgiou, R.F., Vail, A., Barberan, E.M., Hallenbeck, J.M., Del Zoppo, G.J., Rothwell, N.J., and Tyrrell, P.J. (2003). An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J. Neuroimmunol. 139, 93–101 [DOI] [PubMed] [Google Scholar]
- 30. Winbeck, K., Poppert, H., Etgen, T., Conrad, B., and Sander, D. (2002). Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke 33, 2459–2464 [DOI] [PubMed] [Google Scholar]
- 31. Rocco, A., Ringleb, P.A., Grittner, U., Nolte, C.H., Schneider, A., and Nagel, S. (2015). Follow-up C-reactive protein level is more strongly associated with outcome in stroke patients than admission levels. Neurol. Sci. 36, 2235–2241 [DOI] [PubMed] [Google Scholar]
- 32. Di Napoli, M., Papa, F., and Bocola, V. (2001). C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke 32, 917–924 [DOI] [PubMed] [Google Scholar]
- 33. Woodcock, T., and Morganti-Kossmann, C. (2013). The role of markers of inflammation in traumatic brain injury. Front. Neurol. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yue, J.K., Yuh, E.L., Korley, F.K., Winkler, E.A., Sun, X., Puffer, R.C., Deng, H., Choy, W., Chandra, A., and Taylor, S.R. (2019). Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: a prospective multicentre study. Lancet Neurol. 18, 953–961 [DOI] [PubMed] [Google Scholar]
- 35. Nelson, L.D., Temkin, N.R., Dikmen, S., Barber, J., Giacino, J.T., Yuh, E., Levin, H.S., McCrea, M.A., Stein, M.B., and Mukherjee, P. (2019). Recovery after mild traumatic brain injury in patients presenting to US Level I trauma centers: a Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study. JAMA Neurol. 76, 1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jagoda, A.S., Bazarian, J.J., Bruns, J.J.Jr., Cantrill, S.V., Gean, A.D., Howard, P.K., Ghajar, J., Riggio, S., Wright, D.W., and Wears, R.L. (2009). Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. J. Emerg. Nurs. 35, e5–e40 [DOI] [PubMed] [Google Scholar]
- 37. Manley, G.T., Diaz-Arrastia, R., Brophy, M., Engel, D., Goodman, C., Gwinn, K., Veenstra, T.D., Ling, G., Ottens, A.K., and Tortella, F. (2010). Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch. Phys. Med. Rehabil. 91, 1667–1672 [DOI] [PubMed] [Google Scholar]
- 38. Haacke, E.M., Duhaime, A.C., Gean, A.D., Riedy, G., Wintermark, M., Mukherjee, P., Brody, D.L., DeGraba, T., Duncan, T.D., and Elovic, E. (2010). Common data elements in radiologic imaging of traumatic brain injury. J. Magn. Reson. Imaging 32, 516–543 [DOI] [PubMed] [Google Scholar]
- 39. Kumar, A., and Loane, D.J. (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 26, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 40. Lucas, S., Rothwell, N.J., and Gibson, R.M. (2006). The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 147, Suppl. 1, S232–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McDonald, S.J., Sun, M., Agoston, D.V., and Shultz, S.R. (2016). The effect of concomitant peripheral injury on traumatic brain injury pathobiology and outcome. J. Neuroinflammation 13, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar, R.G., Diamond, M.L., Boles, J.A., Berger, R.P., Tisherman, S.A., Kochanek, P.M., and Wagner, A.K. (2015). Acute CSF interleukin-6 trajectories after TBI: associations with neuroinflammation, polytrauma, and outcome. Brain Behav. Immun. 45, 253–262 [DOI] [PubMed] [Google Scholar]
- 43. Hensler, T., Sauerland, S., Bouillon, B., Raum, M., Rixen, D., Helling, H., Andermahr, J., and Neugebauer, E.A. (2002). Association between injury pattern of patients with multiple injuries and circulating levels of soluble tumor necrosis factor receptors, interleukin-6 and interleukin-10, and polymorphonuclear neutrophil elastase. J. Trauma Acute Care Surg. 52, 962–970 [DOI] [PubMed] [Google Scholar]
- 44. Weckbach, S., Perl, M., Heiland, T., Braumüller, S., Stahel, P.F., Flierl, M.A., Ignatius, A., Gebhard, F., and Huber-Lang, M. (2012). A new experimental polytrauma model in rats: molecular characterization of the early inflammatory response. Mediators Inflamm. 2012, 890816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Probst, C., Mirzayan, M.J., Mommsen, P., Zeckey, C., Tegeder, T., Geerken, L., Maegele, M., Samii, A., and Van Griensven, M. (2012). Systemic inflammatory effects of traumatic brain injury, femur fracture, and shock: an experimental murine polytrauma model. Mediators Inflamm. 2012, 136020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Utagawa, A., Truettner, J.S., Dietrich, W.D., and Bramlett, H.M. (2008). Systemic inflammation exacerbates behavioral and histopathological consequences of isolated traumatic brain injury in rats. Exp. Neurol. 211, 283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watanabe, T., Kawai, Y., Iwamura, A., Maegawa, N., Fukushima, H., and Okuchi, K. (2018). Outcomes after traumatic brain injury with concomitant severe extracranial injuries. Neurol. Med. 58, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lingsma, H., Andriessen, T.M., Haitsema, I., Horn, J., van der Naalt, J., Franschman, G., Maas, A.I., Vos, P.E., and Steyerberg, E.W. (2013). Prognosis in moderate and severe traumatic brain injury: external validation of the IMPACT models and the role of extracranial injuries. J. Trauma Acute Care Surg. 74, 639–646 [DOI] [PubMed] [Google Scholar]
- 49. van Leeuwen, N., Lingsma, H.F., Perel, P., Lecky, F., Roozenbeek, B., Lu, J., Shakur, H., Weir, J., Steyerberg, E.W., and Maas, A.I.; International Mission on Prognosis and Clinical Trial Design in TBI Study Group, Corticosteroid Randomization After Significant Head Injury Trial Collaborators, and Trauma Audit and Research Network. (2012). Prognostic value of major extracranial injury in traumatic brain injury: an individual patient data meta-analysis in 39,274 patients. Neurosurgery 70, 811–818; discussion, 818. [DOI] [PubMed] [Google Scholar]
- 50. Groswasser, Z., Cohen, M., and Blankstein, E. (1990). Polytrauma associated with traumatic brain injury: incidence, nature and impact on rehabilitation outcome. Brain Inj. 4, 161–166 [DOI] [PubMed] [Google Scholar]
- 51. Leong, B.K., Mazlan, M., Rahim, R.B.A., and Ganesan, D. (2013). Concomitant injuries and its influence on functional outcome after traumatic brain injury. Disabil. Rehabil. 35, 1546–1551 [DOI] [PubMed] [Google Scholar]
- 52. Siegel, J.H., Gens, D.R., Mamantov, T., Geisler, F.H., Goodarzi, S., and MacKenzie, E.J. (1991). Effect of associated injuries and blood volume replacement on death, rehabilitation needs, and disability in blunt traumatic brain injury. Crit. Care Med. 19, 1252–1265 [DOI] [PubMed] [Google Scholar]
- 53. Leitgeb, J., Mauritz, W., Brazinova, A., Majdan, M., and Wilbacher, I. (2013). Impact of concomitant injuries on outcomes after traumatic brain injury. Arch. Orthop. Trauma Surg. 133, 659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stulemeijer, M., Van Der Werf, S.P., Jacobs, B., Biert, J., Vugt, A. B. V., Brauer, J.M., and Vos, P.E. (2006). Impact of additional extracranial injuries on outcome after mild traumatic brain injury. J. Neurotrauma 23, 1561–1569 [DOI] [PubMed] [Google Scholar]
- 55. Sarrafzadeh, A.S., Peltonen, E.E., Kaisers, U., Küchler, I., Lanksch, W.R., and Unterberg, A.W. (2001). Secondary insults in severe head injury—do multiply injured patients do worse? Crit. Care Med. 29, 1116–1123 [DOI] [PubMed] [Google Scholar]
- 56. Prasad, K. (2006). C-reactive protein (CRP)-lowering agents. Cardiovasc. Drug Rev. 24, 33–50 [DOI] [PubMed] [Google Scholar]
- 57. Nissen, S.E., Tuzcu, E.M., Schoenhagen, P., Crowe, T., Sasiela, W.J., Tsai, J., Orazem, J., Magorien, R.D., O'Shaughnessy, C., and Ganz, P. (2005). Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 352, 29–38 [DOI] [PubMed] [Google Scholar]
- 58. Ikonomidis, I., Andreotti, F., Economou, E., Stefanadis, C., Toutouzas, P., and Nihoyannopoulos, P. (1999). Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation 100, 793–798 [DOI] [PubMed] [Google Scholar]
- 59. Koulouris, S., Symeonides, P., Triantafyllou, K., Ioannidis, G., Karabinos, I., Katostaras, T., El-Ali, M., Theodoridis, T., Vratsista, E., and Thalassinos, N. (2005). Comparison of the effects of ramipril versus telmisartan in reducing serum levels of high-sensitivity C-reactive protein and oxidized low-density lipoprotein cholesterol in patients with type 2 diabetes mellitus. Am. J. Cardiol. 95, 1386–1388 [DOI] [PubMed] [Google Scholar]
- 60. Toedter, G.P., Blank, M., Lang, Y., Chen, D., Sandborn, W.J., and De Villiers, W.J. (2009). Relationship of C-reactive protein with clinical response after therapy with ustekinumab in Crohn's disease. Am. J. Gastroenterol. 104, 2768–2773 [DOI] [PubMed] [Google Scholar]
- 61. Warren, M.S., Hughes, S.G., Singleton, W., Yamashita, M., and Genovese, M.C. (2015). Results of a proof of concept, double-blind, randomized trial of a second generation antisense oligonucleotide targeting high-sensitivity C-reactive protein (hs-CRP) in rheumatoid arthritis. Arthr. Res. Ther. 17, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lust, J.A., Lacy, M.Q., Zeldenrust, S.R., Witzig, T.E., Moon-Tasson, L.L., Dinarello, C.A., and Donovan, K.A. (2016). Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am. J. Hematol. 91, 571–574 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.