Abstract
Veno-arterial extracorporeal membrane oxygenation (V-A ECMO) is established therapy for short-term circulatory support for children with life-treating cardiorespiratory dysfunction. In children with congenital heart disease (CHD), ECMO is commonly used to support patients with post-cardiotomy shock or complications including intractable arrhythmias, cardiac arrest, and acute respiratory failure. Cannulation configurations include central, when the right atrium and aorta are utilized in patients with recent sternotomy, or peripheral, when cannulation of the neck or femoral vessels are used in nonoperative patients. ECMO can be used to support any form of cardiac disease including univentricular palliated circulation. Although V-A ECMO is commonly used to support children with CHD, veno-venous ECMO (V-V ECMO) has been used in selected patients with hypoxemia or ventilatory failure in the presence of good cardiac function. ECMO use and outcomes in the CHD population are mainly informed by single-center studies and reports from collated registry data. Significant knowledge gaps remain, including optimal patient selection, timing of ECMO deployment, duration of support, anticoagulation, complications, and the impact of these factors on short- and long-term outcomes. This report, therefore, aims to present a comprehensive overview of the available literature informing patient selection, ECMO management, and in-hospital and early post-discharge outcomes in pediatric patients treated with ECMO for post-cardiotomy cardiorespiratory failure.
Keywords: Extracorporeal membrane oxygenation, Cardiac surgical procedures, Child, Congenital
Introduction
Mechanical circulatory support (MCS) is well-established therapy for children with severe refractory pulmonary or cardiac failure.1 In the 1970s, the first use of extracorporeal circulatory support in infants with congenital heart disease (CHD) was reported, followed by a longer extracorporeal membrane oxygenation (ECMO) run after surgical correction of Tetralogy of Fallot (TOF)2,3. Despite the availability of other modes of support, including ventricular assist devices, ECMO remains the most commonly used form of MCS in the pediatric population4. In January 2019 the Extracorporeal Life Support Organization (ELSO) Registry reported 19,629 cardiac ECMO cases in neonates and children from 350 international centers between 1990 and 2019 (https://www.elso.org/Registry/Statistics/International Summary.aspx). According to the ELSO registry, hypoplastic left heart syndrome (HLHS) was the most common CHD diagnosis for neonates supported with ECMO, and cyanotic CHD with decreased pulmonary flow (e.g. TOF, double outlet right ventricle, and Ebstein’s anomaly of the tricuspid valve) were the most common CHD diagnoses associated with cardiac ECMO in children.
Veno-Arterial ECMO (V-A ECMO) is utilized in children with cardiac failure after CHD surgery, in order to augment cardiac output and facilitate respiratory gas exchange. Indications for V-A ECMO in this population include failure to wean from cardiopulmonary bypass (CPB), thrombosis of systemic-to-pulmonary artery shunts in patients with palliated single ventricle circulation, intractable arrhythmias, postoperative low cardiac output syndrome, and cardiac arrest.1 Post-cardiotomy ECMO (PC-ECMO) may additionally bridge patients to myocardial recovery, or provide temporary MCS support as a bridge to cardiac transplantation or durable MCS. 8,9, 10 V-A ECMO has also been described as bridge to CHD surgery in the setting of profound cyanosis, cardiogenic shock or pre-operative cardiopulmonary arrest (CPA).5,6,7
The use of ECMO to support children following CHD surgery has increased steadily during the past 3 decades.11-15 This increased use reflects growing experience with repair or palliation of complex forms of CHD, readily available ECMO equipment, point of care ECMO deployment, and the accumulated experience of ECMO management. Furthermore, advances in ECMO pump and oxygenator design, reduction of blood-prosthetic surface interaction with coated ECMO circuit tubing, and improved anticoagulation protocols have resulted in increased ECMO use.16 Despite increasing experience and improved ECMO technology, mortality in pediatric patients requiring ECMO support following CHD surgery is high and has remained unchanged over the last several decades.12
We aimed to summarize the current literature regarding PC-ECMO in pediatric patients with CHD. We provide a detailed and comprehensive summary of patient characteristics, ECMO management and complications, and short- and long-term outcomes of these patients. Future perspectives including novel indications, targets for clinical education, ethical considerations and optimal resource use will be highlighted.
Characteristics of PC-ECMO
Trends in ECMO use
Utilization of PC-ECMO is variable among institutions performing surgery for CHD. Differences in ECMO utilization may reflect variation in technical performance, chosen operative interventions as well as ECMO availability, local indications for use, and the cost of ECMO. Prior to 1990, several authors reported that 1.5 -13% of children who underwent cardiac surgery for CHD were supported with ECMO (Table 1).13, 17-19 Using data from the Pediatric Health Information System, which contains administrative data from 42 children’s hospitals in the United States, Bratton and colleagues reported that from 2003-2014, 0.5% to 6% of children who underwent cardiac surgery for CHD were supported with ECMO.20 A recent analysis using the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database identified 2287 children (2.4%) supported postoperatively with MCS from the 96,596 operations performed for CHD from 2000-2010.21 Most were supported with ECMO (>95%). The report showed ECMO was most commonly used in children undergoing the Norwood single ventricle palliation operation for HLHS (17%) or complex biventricular repairs (14%). The findings illustrated the wide variability in ECMO utilization across the CHD centers reporting to the STS database.
TABLE 1.
BASELINE CHARACTERISTICS
| Authorreference – Year of Publication |
Number of Patients (male/female) |
ECMO use post- cardioto my, % |
Weight, in kg |
Age (range) | Anomalies, %: with normal segmental connections (1); of the atrioventricular valves (2); of the arterial valves and outflow tracts (3); with abnormal segmental connections (4); of the great vessels (5); of the coronary arteries (6); HTx (7); combined surgery (8); other (9). |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||||
| Klein17 – 1990 | 36* (NA) | 12.5%** | 7.15± 2.41 | 13.6 months (1 day-7 years) | 47.2 | 2.8 | 11.1 | 2.8 | 2.8 | 5.6 | NA | 25.0 | 2.8 |
| Ferrazzi 75 - 1991 | 6 (3/3) | NA | 18.1±11.25 (5.7-35) | 4.6±3.95 years (9 months-12 years) | 17% | 0 | 67 | 0 | 0 | 0 | 17 | 0 | 0 |
| del Nido76 - 1992 | 11 (NA) | NA | 5.9 (median) | 15 ± 7 months (0.75-72) | 54.5 | NA | 18.2 | 9.1 | NA | 9.1 | NA | 9.1 | |
| Raithel77 - 1992 | 65 (32/33) | 8.3% | 9.65 (median) | 28.6 months | 13.8 | 3.1 | 21.5 | 26.2 | 7.7 | 4.6 | 7.7 | 6.2 | 9.2 |
| Ziomek19 - 1992 | 24 (12/12) | NA | 5.7 (2.9 – 12) | 12.5 months (0 day-6 years) | 4.2 | 8.3 | 8.3 | NA | 8.3 | 4.2 | NA | 50.0 | 16.7 |
| Dalton13 - 1993 | 25 PC, 4 no PC (13/16) | 1.5% | Overall 8 ± 7 (2.5-35) | 17 ± 23 months (2 weeks -7 years) | 11*** | 1 | 10 | 5 | 2 | 1 | 2 | NA | 2 |
| Black78 - 1995 | PC 25, 6 no PC (11/14) | NA | 6.13 ± 1.75† | 6.95 ± 2.22 months† | NS | ||||||||
| Walters29 - 1995 | 73 (44/29) | 1.6% | 5.6 (median) | 7.2 months (median) | 19.7 | NA | 13.6 | 7.6 | 7.6 | NA | 51.5 | NA | |
| Kulik50 - 1996 | 64 (NA) | NA | 7.28±5.0 | 14 ± 20.2 months | NA | 2.4 | 24.4 | 14.6 | 7.3 | 4.9 | 2.4 | 7.3 | 36.6 |
| Langley67 - 1998 | 9 (7/2) | 1.2% | 6.6 (3.0–16.0) | 7.2 months (2 weeks–3 years) | 44.4 | 0 | 11.1 | 22.2 | 22.2 | 0 | 0 | 0 | 11.1 |
| Jaggers79 - 2000 | 35 (NA) | 3.4% | 3.9 (median, 4.7) | 89 days (median, 19 days) | 7*** | 3 | 9 | 7 | 9 | 1 | NA | 11 | |
| Aharon80 - 2001 | 50 (24/26) | 4% | 6.2 (1.6-47.9) | 40 days (median) | NA | 8 | 20 | 20 | 4 | 2 | NA | 46 | |
| Pizarro68 – 2001 | 12 (NA) | NA | 2.6 (1.4-3.8) | 3.9 days (1-14) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| Kolovos18 – 2003 | 74 (45/29) | 2.2% | NA | 17 days (median) (7-272) | NA | 8.1 | 4.1 | 8.1 | NA | 2.7 | 35.1 | 27.0 | NA |
| Chaturvedi33 - 2004 | 81 (NA) | 1–2.5%‡ | 4.5 (median) (3.3–8.7) | 2.4 months (median) (0.6–13) | 4.9 | 11.1 | 25.9 | 34.6 | 9.9 | 4.9 | 4.9 | NA | 3.7 |
| Chow51 - 2004 | 90 (42/48) | NA | 0.77 years (median) 1 day – 17 years, 4 months | Univentricular repairs 7 (7.7%) Biventricular repairs 58 (64.4%) | 6.6 | ||||||||
| Morris26 – 2004 | 137 (71/66) | 2.6% | 5.1 (1.9–110) | 4.7 months (1 day–42 yrs) | |||||||||
| Huang34 - 2005 | 68 (41/27) | 3.2% | 3.4 (median) (2.3–31)† | 1 month (median) (1 day - 13.1 years)† | SVP: 46 BVP: 22 |
||||||||
| Ghez81 - 2005 | 15§ (NA) | 3.2% | NA | 4.97 ±7 years | 6.7 | NA | 13.3 | 20.0 | NA | 6.7 | 26.7 | 26.7 | |
| Mahle71 - 2005 | 32 | NA | NA | 2.0 months (4 days – 5.1 years) | 0 | 6.3 | 6.3 | 9.4 | 12.5 | 6.3 | 3.1 | 0 | 46.9 |
| Baslaim25 - 2006 | 26 (NA) | 4% | 3.7 (median) (2.2-26) | 4.3 months (median) (2 weeks–144 months) | NA | 34.6 | 11.5 | NA | 30.8 | 23.1 | |||
| Thourani28 - 2006 | 27 (27/8) | 2.8% | 5.4±3.1 (1.8-11.3) | 139.3-183.6 days (1-640 days) | 18.5 | 0 | 0 | 37.0 | |||||
| Allan41-2007 | 44 (NA) | 15% | 3.1±0.1 | 8.0±2.3 days | SVP:100% | ||||||||
| Alsoufi42 – 2009 | 180 (91/89) | NA | 4.3 (median) (1.7-75) | 109 days (median) (1day -16.9 years) | SVP:34% BVP: 66% |
||||||||
| Delmo Walter82 - 2010 | 27 PC (17/10) | NA | 7.1 (median) (2.7–80) | 0.74 years (median) (1–17.8) | 14.8 | ||||||||
| Polimenakos27 - 2011 | 21 (NA) | NA | 3.57 ± 1.7 | 7.5 ± 2.7 days | HLHS 66,6% SVP: 33.3% |
||||||||
| Bhat35 – 2013 | 64 (34/30) | NA | 2.7 (IQR,2.3-2.9) | 7 days (IQR, 4-9) | 1.6 | 3.1 | 12.5 | 17.2 | 10.9 | NA | 1.6 | 53.1 | |
| Sasson83 – 2013 | 62 (41/21) | 3.2% | 4.3 (median) (1.9-51) | 3 months (median) (0-216) | NS | ||||||||
| Agarwal16 - 2014 | 119 (67/52) | 3.4% | 3.3 (IQR 2.7-4.1) | 12 days (IQR,6-79) | NS | ||||||||
| Alsoufi43 - 2014 | 100 (63/27) | NA | 4.1 (median) (1.8-60) | 73 days (median) (4 days-16.2 years) | SVP: 31% | ||||||||
| Sasaki37 – 2014 | 36 (24/12) | 1.4% | 3.1 (2.1–10.8) | 64 days (0 days–4.1 years) | NA | 2.8 | 2.8 | 5.6 | 2.7 | NA | 86.1 | NA | |
| Jolley 48 - 2014 | 103 (54/49) | 4.7% | 5.6 kg (IQR, 5.0-6.4) | 158 days (IQR, 124-214) | SVP: 100% | ||||||||
| Miana84 - 2015 | 56 (33/23) | 0.5% | 5.6 (median) (IQR 3.2-16.1) | 240 days (IQR 67.5-2045 days) | 12.5 | 8.9 | 16.0 | 16.1 | 12.5 | 3.6 | NA | 3.6 | 19.7+7.1 NS |
| Gupta40 – 2015 | 998 (562/436) | NA | 3 (2.5-3.4) | 14 days (median) (IQR, 5-148 days) | NS | ||||||||
| Lou85 – 2015 | 96 (49/47) | NA | 3.7 (median) (1.7–115) | 0.06 years (median) (0.002–17) | NS | ||||||||
| Sznycer-Taub23 - 2016 | 93 (54/39) | NA | 3.3 (median) (IQR,2.9-3.8) | 7 days (median) (IQR 5–20) | NS | ||||||||
| Aydin11 – 2016 | 89 (47/42) | 4 (median) (IQR, 3.2-6.8) | 66 days (IQR, 8-221) | SVP: 100% | |||||||||
| Howard46 -2016 | 84 (47/37) | 8.8% | 3.0 (2.5-3.3) | 5.5 days (3.5-12days) | SVP:48.8% BVP:51.2% |
||||||||
| ElMahrouk86 - 2017 | 113 (67/46) | 3.34% | 3.5 (median) (2.2–42.5) | 3 months (median) (4 days–15 years) | SVP:45.1% BVP:54.9% |
||||||||
| Mistry87 - 2018 | 68 (44/24) | NA | 11 (3.95-37.75) | 1.45 years (0.18-8.70) | 0 | 0 | 8.8 | 0 | 4.4 | 0 | 7.4 | 0 | 79.4 |
Additionally, 3 patients were supported with ECMO before surgery
including 39 patients
multiple combinations per patient
data reported for survivors only
all patients in Cardiac Intensive Care Unit
15 patients with 19 ECMO devices implanted (16 veno-arterial, 2 veno-venous, 1 x Biventricular Support)
Extracorporeal cardiopulmonary resuscitation (ECPR) in 41 postcardiotomy cases
BVP, biventricular pathology; ECMO, extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; HTx, heart transplantation; IQR, interquartile range; NA, not available; NS, not specified; PC, post-cardiotomy; SVP, single ventricular pathology. Values for weight and age are mean+SD unless specified otherwise
Patient characteristics
ECMO has been successfully deployed to support children of all ages, from newborn to adult-sized patients with CHD requiring cardiac surgery.16, 18, 22-24 Similarly, PC-ECMO support has been utilized in children of all sizes, although the small vessels in premature and low birthweight infants can make the placement of appropriately sized ECMO cannula challenging. PC-ECMO has been used to rescue children after surgery for all forms of CHD, although it is more frequently used to support children undergoing more complex procedures (Table 1).23, 25-27
Indications for ECMO
The indications for and rates of ECMO implantation in pediatric patients vary among different studies; however, common indications include failure to wean from CPB, cardiac arrest, low cardiac output syndrome or respiratory failure (Table 2). Klein and colleagues reported pathophysiology resulting in ECMO support included biventricular failure (36%), right ventricular failure (14%), left ventricular failure (33%), and pulmonary hypertensive crisis (17%).17 Some populations are particularly high risk, for example, in a study of single ventricle patients palliated with systemic-to-pulmonary artery shunts, nearly half of 27 patients required ECMO implantation. 28 And other studies appear to represent different population, for example, in two studies of PC-ECMO in children, low cardiac output as an indication for ECMO was present in 17% of 93 patients, and 92% of 73 patients.29, 23 Cardiac or cardiopulmonary arrest occurred in 6%, or 28% of the respective populations. Pulmonary arterial hypertension, arrythmia and failure to wean from CPB are also represented in different proportions (Table 2).
TABLE 2.
ECMO IMPLANT INDICATION, IMPLANT LOCATION, AND IMPLANT ACCESS.
| Study | Indications for ECMO implant, n (%) |
ECMO implant location, n (%) | ECMO implant access, n (%) | ECMO venting, n (%) |
|---|---|---|---|---|
| Klein17 - 1990 | BVF 13 (36%) RVF 5 (14%) LVF 12 (33%) PVRC 6 (17%) |
OR 9 (25%) ICU 27 (75%) |
Neck 30 (83%) Chest 6 (17%) |
NA |
| Ferrazzi75 - 1991 | LVF 1 (16.6%) RVF 4 (66.6%) BVF 1 (16.6) |
OR 2 (33%) ICU 4 (66%) |
Chest 6 (100%) | NA |
| del Nido76 - 1992 | Cardiac arrest 9 (82%) Not specified 2 (18%) |
NA | Neck 1 (9%) Chest 10 (91%) |
3** (27%) |
| Raithel77 - 1992 | Failure to wean from CPB 20 (31%) Cardiac failure 45 (69%) |
OR 22 (34%) ICU 43 (66%) |
Chest 59 (91%) Femoral 6 (9%) |
LA vent 11 (17%) LV vent 4 (6%) |
| Ziomek19 - 1992 | Ventricular failure 17 (71%) Pulmonary hypertension 6 (25%) Hypoxemia 1 (4%) |
OR 17 (71%) ICU 7 (29%) |
Neck 9 (38%) Chest 15 (62%) |
Pulmonary artery vent 1 (4%) |
| Dalton13 - 1993 | BVF + arrest 4 (15%) BVF 8 (30%) RVF 3 (11%) LVF 2 (7%) Cardiac arrest 9 (33%) Arrhythmia 1 (4%) |
NA | Neck 5 (19%) Chest 22 (81%) |
LA vent 2 *** (7%) |
| Black78 - 1995 | Myocardial failure 21 (84%) Respiratory failure 3 (12%) Cardiac arrest 1 (4%) |
NA | Chest 28 (90%) Femoral 3 (10%)† |
NA |
| Walters29 - 1995 | Arrhythmia 1 (1.5%) Cardiac arrest 4 (6.1%) Low cardiac output 61 (92.4%) No spontaneous electrical activity 1 (1.5%) Pulmonary artery hypertension 14 (21.2%) |
NA | Neck 48 (73%) Chest 18 (27%) |
NA |
| Kulik50 - 1996 | Ventricular dysfunction 26 (41%) Pulmonary failure 13 (20%) Pulmonary hypertension 7 (11%) Combination 10 (16%) Cause of hemodynamic instability unknown 8 (13%) |
NA | Neck 37 (58%) Chest 20 (31%) Femoral 2 (3%) Multiple 5 (8%) |
LA vent (19%) |
| Langley67 - 1998 | LVF (33%) RVF (11%) BVF (44%) Cardiac arrest (11%) |
OR 7 (78%) ICU 2 (22%) |
Chest 9 (100%) | 0% |
| Jaggers79 - 2000 | Low cardiac output 17 (49%) Failure to wean from CPB 10 (29%) Cardiac arrest 5 (14%) Arrhythmia 3 (9%) Pulmonary Hypertension 2 (6%) Hypoxia 1 (3%) Biventricular dysfunction 1 (3%) |
OR 15 (43%) ICU 20 (57%) |
NA | LA vent 1 (3%) |
| Aharon80 - 2001 | Failure to wean from CPB 22 (44%) Low cardiac output 11 (22%) Pulmonary hypertension 7 (14%) Cardiac arrest 10 (20%) |
OR 23 (46%) ICU 27 (54%) |
Neck 1 (2%) Chest 49 (98%) |
NA |
| Pizarro68 - 2001 | Low cardiac output 6 (50%) Cardiac arrest 2 (16.6%) Respiratory failure 2 (16.6%) Unbalanced pulmonary/systemic circulation 1 (8.3%) Supraventricular tachycardia 1 (8.3%) |
OR 9 (75%) ICU 3 (25%) |
Chest 12 (100%) | NA |
| Kolovos18 - 2003 | Ventricular failure 51 (69%) Respiratory failure 9 (12%) Pulmonary hypertension 4 (5%) Multiple indications (not specified) 6 (8%) Shunt occlusion 4 (5%) |
NA | Neck 26 (35%) Chest 47 (64%) Groin 1 (1%) |
LA vent 12 (16%) Atrial septostomy 1 (1%) |
| Chaturvedi33 - 2004 | Failure to wean from CPB Low cardiac output No data available |
OR 47 (58%) ICU 34 (42%) |
Neck 5 (6%) Chest 76 (94%) |
NA |
| Chow51 - 2004 | Myocarditis 10 (11%) Cardiomyopathy 9 (10%) Congenital Heart Disease 71 (79%) |
NA | NA | NA |
| Morris26 - 2004 | Cardiac arrest Failure to wean from CPB Low cardiac output |
|||
| Huang34 - 2005 | Failure to wean from CPB 46 (67%) Low cardiac output 11 (16%) Cardiac arrest 11 (16%) |
OR 46 (67%) ICU 22 (33%) |
Chest 66 (97%) Femoral 2 (3%) |
LA vent 12 (18%) |
| Ghez81 - 2005 | Hemodynamic failure 12 (63%) Respiratory failure 2 (11%) Mixed failure 5 (8%) |
OR 4 (27%) ICU 11 (73%) |
Neck 4 (21%) Chest 11 (58%) Femoral 4‡ (21%) |
NA |
| Mahle71 - 2005 | Cardiopulmonary arrest 18 (56%) Failure to wean from CPB 11 (34%) Postoperative LCOS 2 (6%) Pulmonary hypertension 1 (3%) |
NA | NA | NA |
| Baslaim25 - 2006 | Ventricular failure 17 (65%) Respiratory failure 6 (23%) Pulmonary hypertension 1 (4%) Allergic reaction to blood products 1 (4%) Postoperative distal pulmonary artery stenting 1 (4%) |
OR (not specified) ICU (not specified) |
Chest 26 (100%) | NA |
| Thourani28 - 2006 | Cardiomyopathy-myocarditis 8 (30%) Systemic-to-pulmonary artery shunt dependent single ventricle 12 (44%) Postcardiotomy for biventricular repair 6 (22%) Arrhythmias 1 (4%) |
NA | NA | NA |
| Allan41 - 2008 | Myocardial failure 22 (50%) Cardiac arrest 4 (9.1%) Tamponade 2 (4.5%) Pulmonary hypertension 1 (2.3%) Respiratory failure 3 (6.8%) Shunt thrombosis/stenosis 12 (27.2%) |
NA | NS | NA |
| Alsoufi42 - 2009 | Failure to wean from CPB 83 (46%) Low cardiac output 97 (54%) Cardiac arrest 48 (27%) |
OR 83 (46%) ICU 92 (51%) Catherization laboratory 5 (3%) |
Neck 12 (6%) Chest 168 (94%) |
NA |
| Delmo Walter82 - 2010 | Cardiac Arrest 27 (100%) | NA | NA | LV vent 5 (19%) |
| Polimenakos27 - 2011 | Cardiac arrest 17 (81%) Respiratory failure followed by cardiac arrest 4 (19%) |
NA | NA | NA |
| Bhat35 - 2013 | Failure to wean from CPB 39 (61%) Low cardiac output 9 (14%) Cardiac arrest 16 (25%) |
OR 39 (61%) ICU 25 (39%) |
Chest 59 (92%) | NA |
| Sasson83 - 2013 | Failure to wean from CPB 53 (83%) Cardiac arrest 9 (17%) |
OR 53 (83%) ICU 9 (17%) |
Neck 2 (3%) Chest 60 (97%) |
NA |
| Agarwal16 - 2014 | Failure to wean from CPB 50 (42%) Low cardiac output 25 (21%) Cardiac arrest 34 (29%) Other (including arrhythmia, respiratory failure and pulmonary hypertension) 10 (8%) |
OR 58 (49%) ICU 61 (51%) |
NA | NA |
| Alsoufi43 - 2014 | Failure to wean from CPB 34 (34%) Low cardiac output 29 (29%) Cardiac arrest 37 (37%) |
OR 34 (34%) ICU 66 (66%) |
NA | NA |
| Sasaki37 - 2014 | Failure to wean from CPB 14 (39%) Low cardiac output 15 (42%) Other (not specified) 7 (19%) |
OR 18 (50%) ICU 18 (50%) |
Chest 36 (100%) | NA |
| Jolley 48 - 2014 | cardiac 90 (87%) ECPR 9, (9%) pulmonary 4 (4%). |
NA | Chest 89 (86%) Neck 14 (103%) |
NA |
| Miana84 - 2015 | Failure to wean from CPB 56 (100%) | OR 56 (100%) | Chest 56 (100%) | Interatrial vent LA vent (not specified) |
| Gupta22 - 2015 | NA | NA | NA | NA |
| Lou85 - 2015 | Failure to wean from CPB 24 (25%) Low cardiac output 26 (27%) Cardiac arrest 46 (48%) |
NA | Chest 90 (94%) Peripheral (not specified) 3 (3%) Both 3 (3%) |
NA |
| Sznycer-Taub23 - 2016 | Low cardiac output 16 (17%) Failure to wean from CPB 44 (47%) Pulmonary hypertension 2 (2%) Combined cardiac and respiratory failure 26 (28%) Respiratory failure 2 (2%) Shunt occlusion 3 (3%) |
OR 42 (45%) ICU 51 (55%) |
Neck 13 (14%) Chest 80 (86%) |
NA |
| Aydin11 – 2016 | Respiratory failure 59 (63%) Cardiac failure 30 (32%) |
NA | Neck 57 (64%) Chest 24 (27%) Femoral 10 (11%) |
NA |
| Howard46-2016 | Cardiac arrest 39 (46%) Failure to wean from CPB 21 (25%) LCOS 18 (21%) Hypoxemia 6 (7%) |
NA | Chest 82 (98%) | NA |
| ElMahrouk86 - 2017 | Ventricular dysfunction 59 (52%) Pulmonary Failure 34 (30%) Cardiac arrest 10 (9%) Pulmonary hypertension 7 (6%) Other 3 (3%)* |
OR 88 (78%) ICU 25 (22%) |
NA | NA |
| Mistry87 - 2018 | Cardiomyopathy 28 (41%) Cardiorespiratory failure 28 (41%) Congenital heart disease 9 (13%) Posttransplant rejection 5 (7.4%) |
NA | NA | 16 (23.5%) |
Arrhythmia, allergic reaction to blood products, and Fontan circuit thrombus.
Vent type not specified.
Data on venting was only given for 2 patients.
No data on 25 PC patients
15 patients, 19 ECLS runs
BVF; biventricular failure, CPB, cardiopulmonary bypass; ECLS, extracorporeal life support; ECPR, extracorporeal cardiopulmonary resuscitation; ICU, intensive care unit; LA, left atrial; LCOS, low cardiac output syndrome; LV, left ventricular; LVF; left ventricular failure; NA, not available; OR, operating room; PC; post-cardiotomy; PVRC; pulmonary vasoreactive crisis; RVF; right ventricular failure.
Cannulation for ECMO
ECMO cannulation strategy is determined by underlying the anatomy and physiology of CHD. An analysis of all pediatric patients (0-18 years old) reported to the ELSO Registry demonstrated carotid cannulation in 64% of patients, aortic cannulation in 32%, and femoral cannulation in only 4%.30 In many centers, central cannulation of the right atrium for venous drainage and aorta for arterial return is commonly used in the presence of a recent sternotomy (Figures 1-3). In many circumstances, peripheral vessel cannulation may be the preferred approach to reduce the risk of major bleeding and infections1. Vascular access for peripheral V-A ECMO cannulation can be achieved through the neck vessels (internal jugular vein and carotid artery) or through the femoral vessels (femoral vein and artery) in children weighing >15 kg. Children with single ventricle circulation palliated with cavopulmonary connections (bidirectional Glenn and Fontan circulations) frequently need multisite cannulation for venous drainage. In rare instance when patients have adequate cardiac function and only require lung support, veno-venous ECMO (V-V ECMO) can be used, and the cannulas for drainage and return are both placed in the venous circulation.
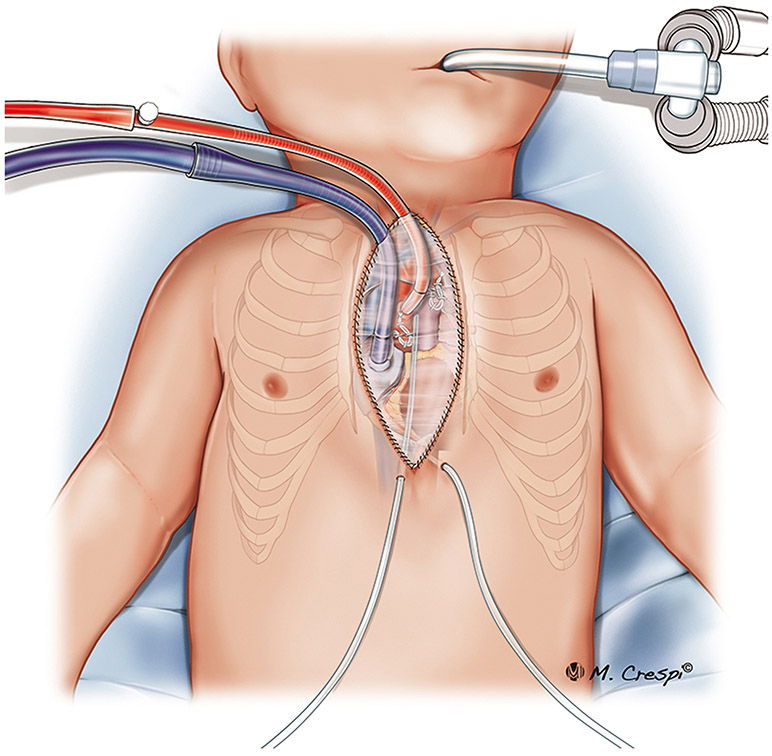
Figure 1.
Post-cardiotomy ECMO approaches for cannulation: central cannulation (right atrium and ascending aorta cannulation) with subxyphoid exit port for cannulas, and the sternum closed.
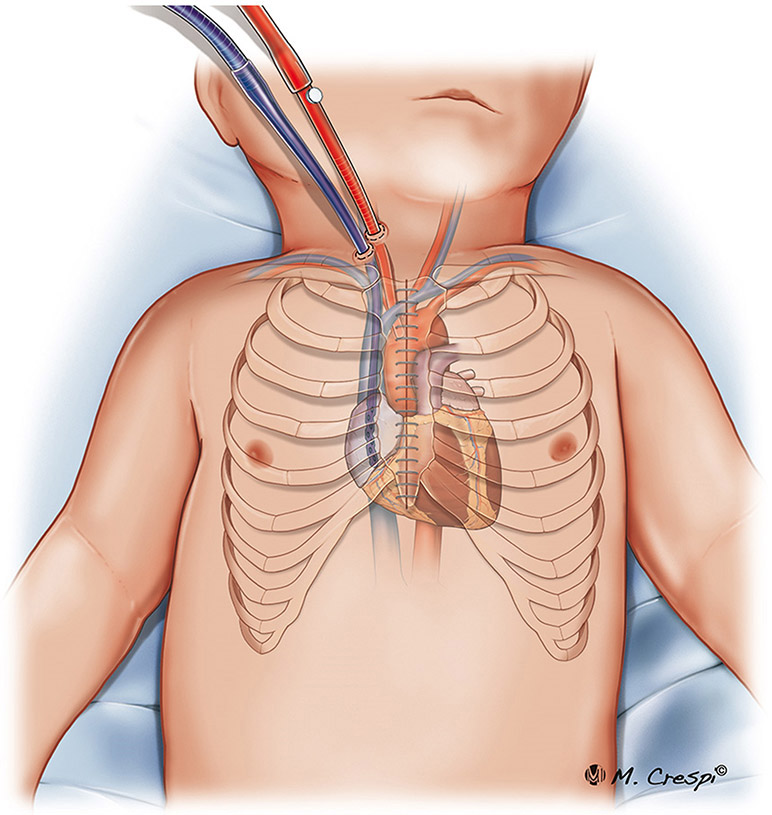
Figure 3.
Post-cardiotomy ECMO approaches for cannulation: central cannulation with the sternum opened.
Children with complex congenital heart defects are at risk for occlusion of peripheral vessels used for ECMO cannulation, because the vessels may have been accessed previously for cardiac catheterization. Thus, knowledge of vessel patency is important in children with complex CHD and previous history of multiple cardiac catheterization procedures. Chan and colleagues, in a report of 492 children with CHD supported with ECMO following cardiac arrest, showed that the use of the right carotid artery for V-A ECMO was associated with improved survival to hospital discharge as compared with transthoracic cannulation. The authors speculate that the reason for improved survival may be related to fewer cardiopulmonary resuscitation (CPR) interruptions during neck cannulation.31 Finally, the American Heart Association recently published a statement on cardiopulmonary resuscitation in children with CHD, which includes a table of suggested cannulation sites for ECMO based on the underlying circulation.32
Post-cardiotomy ECMO Outcomes
Survival and duration of ECMO support
After PC-ECMO, survival-to-hospital-discharge ranges from 40 to 60% in most studies of pediatric patients (Table 3). Many factors influence the duration of PC-ECMO support in children, including the underlying cardiac lesion, presence of residual lesion, the family’s wishes, cardiac surgical recovery time, and the applicability of bridging to transplant if recovery does not occur. Thus, the number of hours of PC-ECMO support varies greatly among different studies, ranging from 17 to more than 200 hours (Table 3).17,19 Survival to ECMO decannulation and hospital discharge also varies in the reported literature, with between 49 to 58% making it home alive.17,19,33 These mainly single center studies report higher survival than typically found in ELSO registry reports of the pediatric cardiac population.12 Longer-term survival, for example at 1 year post PC-ECMO has been reported as high as 41% (Table 3). 33
TABLE 3.
DURATION OF ECMO SUPPORT, WEANING RATE, IN-HOSPITAL SURVIVAL, 1-YEAR SURVIVAL AND PREDICTORS OF IN-HOSPITAL MORTALITY.
| Study | Duration of ECMO, in hours or days |
Weaning n (%) |
In-Hospital Survival n (%) |
1-Year Survival n (%) |
Predictors of In-Hospital Mortality |
|---|---|---|---|---|---|
| Klein17 - 1990 | Total: 105 ± 46 hours Survivors: 110 ± 27 hours (mean ± SD) |
22 (61%) | 21 (58%) | NA | NA |
| Ferrazzi75 - 1991 | 126±47 hours (range: 67-173 hours) | 3 (50%) | 2 (33%) | NA | NA |
| del Nido76 - 1992 | 112 ± 18 hours | 7 (64%) 1 HTx included |
7 (64%) | 6 (55%) | NA |
| Raithel77 - 1992 | Survivors: 87.9 hours, 45-197.5 hours (mean + range) | 44 (68%) | 23 (35%) | NA | Duration of ECMO support, renal failure/dialysis, sepsis |
| Ziomek19 - 1992 | 96 hours, 17-198 hours (mean + range) | 16 (75%) | 13 (54%) | NA | Sepsis |
| Dalton13 - 1993 | 113 ± 62 hours (mean ± SD) | 14 (67%) | 9 (43%) | 7 (33%) | Longer CPB time and shorter time on ECMO |
| Black78 - 1995 | 5.7 days (mean) | 10 (40%) | 10 (40%) | 10 (40%) | NA |
| Walters29 - 1995 | 115 ± 6 hours (mean ± SD) | 44 (67%) | 38 (58%) | NA | Longer CPB time, patients who couldn’t be weaned from CPB, Elevated BUN 48 h after ECMO cannulation, Elevated creatinine 48 h after ECMO cannulation, Need of RBC’s on ECMO, Need of plasma on ECMO Creatinine 48 h after ECMO decannulation and average right atrial pressure 8 h after ECMO decannulation |
| Kulik50 - 1996 | NA | 31 (48%) | 21 (33%) | NA | No significant variables |
| Langley67 - 1998 | 121 hours (15–648) | 4 (44.4%) | 3 (33%) | 2 (22%) | Duration of ECMO |
| Jaggers79 - 2000 | 5.6 days (median 5 days, range 0.7-16 days) | NA | 21 (61%) | 17 (49%) | Longer time on ECMO, development of renal failure, shunt left open on ECMO |
| Aharon80 - 2001 | Survivors: 89 hours (mean) (range 20-192 hours) | 30 (60%) | 25 (50%) | 23 (45%) | Renal failure requiring hemodialysis, ECMO duration >72 hours, prolonged CPR times (>45 minutes) |
| Pizarro 68 - 2001 | 67 hours (median 48 hours) range (24-192 hours) | 12 (100%) | 6 (50%) | 6 (50%) | ECMO initiated outside OR |
| Kolovos18 - 2003 | 127 hours (median) (IQR 73-209 hours) | 50 (68%) | 37 (50%) | NA | CPR during ECMO cannulation, Renal failure/dialysis, single ventricle physiology, lactate within 48 hours of ECMO initiation |
| Chaturvedi33 - 2004 | 144 hours (median) (IQR 70-226 hours) | 47 (58%) | 40 (49%) | 33 (41%) | Circuit problems, renal failure/dialysis, residual cardiac lesions, ECMO duration, blood product transfusion and cross clamp time |
| Chow 51 - 2004 | 90 hours (median) 6-394 hours | NA | 41 (46%) | 21 (38%)** | previous CPR |
| Morris26 - 2004 | Survivors: 94.5 (median) (range 7 hours -15 days) | NA | 36 (40%) | NA | Age <1 month, male sex |
| Huang34 - 2005 | Survivors: 75.3 hours (median) (range 23-234 hours) | 45 (66.2%) | 22 (32.4%) | 22 (32.4%) (2 year survival) | Univentricular physiology, acute renal failure, duration of ECMO, lowest lactate |
| Ghez81 - 2005 | 145 ±72 hours (mean ± SD) | 13 (87%) | 12 (80%) | NA | NA |
| Mahle71 - 2005 | 5.1±4.1 days | NA | 16 (50%) | 15 (47%) | NA |
| Baslaim25 - 2006 | Survivors: 74.5 hours (mean) (range 12-189 hours) | NA | 12 (46%) | NA | Stroke, DIC, renal failure |
| Thourani28 - 2006 | 151.5±201.4 hours (18-986 hours) | 20 (74.1%) | 16 (59.3%) | 13 (48.1%) | NA |
| Allan41 -2007 | Survivors 56±15 hours Non survivors 160±23 |
30 (68.2%) | 21 (48%) | 12 (27.2%) | NA |
| Alsoufi42 - 2009 | Survivors: 3 days (median) (range 2.8-4.2 days) | 109 (61%) | 68 (38%) | NA | Duration of ECMO, repeat ECMO, bleeding complications, neurological complications, renal dysfunction |
| Delmo Walter82 - 2010 | 4.97 ± 0.68 days (mean ± SD) | 18 (66.7%) | 9 (33.3%) | NA | Duration of CPR, High doses of inotropes |
| Polimenakos27 - 2011 | 7 days (median) (IQR 4-21 days) | 15 (72%) | 13 (62%) | 10 (47%) | High serum peak lactate (first 24 hours), longer ECMO duration |
| Bhat35 - 2013 | Total: 164 hours (median) (IQR 95-231) Survivors: 134 hours (IQR 95-160 hours) |
33 (52%) | 18 (28.1%) | NA | Renal replacement therapy on ECMO, ECMO duration >231 hours |
| Sasson83 - 2013 | Total: 4 days (median) (range 1-13 days) Survivors: 3 days (range 1-13 days) |
29 (46.8%) | 24 (38.7%) | NA | Total anomalous pulmonary venous return |
| Agarwal16 - 2014 | 4 days (median) (IQR 2-7 days) | 75 (63%) | 49 (41%) | NA | NA |
| Alsoufi43 - 2014 | Total: 4 days (median) (IQR 3-6 days) Survivors: 3 days (IQR 2-5 days) |
62 (62%) | 37 (37%) | NA | Renal failure requiring dialysis, maximum creatinine, bleeding requiring re-exploration, ECMO duration, hours to lactate normalization, Immediate post-ECMO lactate, Peak post-ECMO lactate, maximum bilirubin, Sepsis |
| Sasaki37 - 2014 | 4.9 ± 4.2 days (mean ± SD) | 21 (58%) | 17 (47%) | NA | Univentricular anatomy, younger age, longer ECMO duration, higher lactate, pulmonary haemorrhage |
| Jolley 48 - 2014 | survivors 88 hours (48-132) non survivors 136 hours (73-267) |
68 (66%) | 42 (41%) | NA | inotrope requirement, longer duration of ECMO support, combined cardiopulmonary indication for ECMO, and renal failure |
| Miana84 - 2015 | 182.2 ± 117 hours (mean ± SD) | 26 (46.4%) | 11 (19.6%) | NA | NA |
| Gupta22 - 2015 | 4 days (median) (IQR 1-7 days) | NA | 518 (51.9%) | NA | Longer ECMO duration |
| Lou85 - 2015 | Group 1 (ECMO without therapeutic hypothermia): 83 hours (median) (range 26-332 hours) Group 2 (ECMO with therapeutic hypothermia): 106 hours (median) (range 24-367 hours) |
77 (80.2%) | 55 (57.3%) | NA | NA |
| Sznycer-Taub23 - 2016 | Total: 5 days (median) (IQR 3-7 days) 30 day survivors: 4 days (IQR 3-6 days) |
NA | 46 (49%) | NA | Longer ECMO duration, High mean PaO2 (>193 mmHg) (first 48 hours) |
| Aydin11 – 2016 | survuvors: 88.5 hours(57-116) non survivors: 183 hours (71-288) |
NA | 46 (48%) | NA | The duration of intubation, partial pressure carbon dioxide, mean airway pressure, and renal injury |
| Howard46-2016 | 7.5 (3-11) days | NA | 42 (50%) | 34 (40%) | Prematurity, pH<7.17 pre ECMO, inotropic support, ECMO duration >168hours |
| ElMahrouk86 - 2017 | 4 days (median) (range 1-14 days) | 53 (47%) | 42 (37%) | NA | Longer ECMO duration, renal failure, stroke |
| Mistry87 - 2018 | 126.97 hours (78.89-216.64) | NA | 48 (70.6%) | 48 (69.2%)* | Low body weight, serum lactate and creatinine, prior cardiac surgery, inotropes use. |
3 patients lost to follow-up
mean follow-up 4.5 years
BUN, blood urea nitrogen; CPB, cardiopulmonary bypass; CPR, cardiopulmonary resuscitation; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; h, hours; HTx, heart transplantation; IQR, interquartile range; OR, operating room; NA, not available; PaO2, partial pressure of oxygen; RBC, red blood cells; SD, standard deviation.
Survival to hospital discharge may also vary by age and weight, with higher risk of death in neonates.16, 18, 22-24 Infants weighing <3 kg have been reported to have high risk of death after ECMO support.35-37 In a study of 4,471pediatric patients supported with ECMO for cardiac indications reported to the ELSO registry, there were no survivors among 9 patients weighing < 1.5 kg, and survival was 25% among those who weighed 1.5 – 2 kg.36 In premature infants, immaturity of the choroid plexus may result in the higher incidence of intracranial hemorrhage. Birthweight < 3 kg has been associated with increased risk of neurological complications in PC-ECMO for CHD. 38 Indeed, both prematurity and lower birthweight were associated with increased mortality and a higher incidence of neurological complications in a study of 641 neonates supported with ECMO following cardiac arrest by McMullan and colleagues.39 Bhat and colleagues also examined PC-ECMO used in infants weighing 3 kg or less; 52% of the patients were decannulated from ECMO and 28.1% survived until discharge.35 This study reported one of the longest durations of ECMO support with median 164 hours (interquartile range 95-231). (Table 3). Thus, the ability to provide adequate ECMO support is affected by size, and neurological complications may limit survival in premature neonates (< 34 weeks gestation or birthweight < 2 kg).
Survival to hospital discharge also varies widely based on the complexity of the underlying cardiac surgical procedure.25, 27, 33, 40 Allan and colleagues compared the indications for initiation of ECMO in infants with shunted single-ventricle physiology to the survival; 81% of patients cannulated for hypoxemia, but only 29% of those cannulated for hypotension survived to hospital discharge.41 Patients cannulated for shunt obstruction had the highest survival (83%). In an STS Congenital Heart Surgery Database study of 2287 children supported postoperatively with MCS by Mascio and colleagues21, in-hospital mortality was highest among in patients supported after repair of truncus arteriosus, the Norwood single ventricle palliation operation for HLHS, or the Ross-Konno operation for repair of left ventricular outflow tract obstruction. In these circumstances, poor outcomes may be due to the accidental damage of the coronaries during surgery, serious aortic regurgitation after an incomplete repair, or an inherently poor systemic ventricle due to congenital aortic stenosis. Patients undergoing repair of an anomalous coronary artery from the left pulmonary artery had the best survival.21
Predictors of mortality
Many studies have identified similar predictors of in-hospital mortality, summarized by Walters and colleagues to include longer CPB time, the inability to separate from CPB, elevated blood nitrogen urea 48 hours after ECMO cannulation, elevated creatinine 48 hours after ECMO cannulation, the need for red blood cells or plasma, and elevated right atrial pressure 8 hours after ECMO decannulation (Table 3).29 Kolovos and colleagues18 found that CPR during ECMO cannulation, renal failure/dialysis, single ventricle palliated circulation, and a lactate trend within 48 hours of ECMO initiation to be associated with in-hospital mortality. Alsoufi and colleagues42, 43 found that duration of ECMO, repeat ECMO, neurological complications, renal dysfunction, and mechanical complications were associated with in-hospital mortality. In Bhat’s study of PC-ECMO neonates, renal replacement therapy on ECMO and duration of ECMO support > 231 hours were predictors of poor prognosis.35 Renal failure is commonly associated with poorer prognosis in pediatric patients receiving PC-ECMO, which is in line with studies conducted in adults.44 Of note, continuous renal replacement therapy (CRRT) before ECMO is associated with worse outcomes than either no CRRT or CRRT started after ECMO. The former is indicative of underlying renal disease, while the latter may reflect an attempt to prevent fluid overload.45 Additionally, worse outcomes may be expected in patients with palliated single ventricle circulation with Glenn or Fontan operations, due to the ineffectiveness of conventional CPR and the high risk of brain injury unless the bidirectional cavopulmonary connection is cannulated along with the inferior vena cava (ie. bicaval or central cannulation may be required).36,46
Extracorporeal membrane oxygenation for extracorporeal cardiopulmonary resuscitation
Extracorporeal cardiopulmonary resuscitation (ECPR), or ECMO to support CPA, is an important and increasing use of ECMO in children who undergo cardiac surgery. Nonetheless, early institution of ECMO support prior to CPA in children with deteriorating hemodynamics in the postoperative period is preferable. In a study of 81 children supported with ECMO following cardiac surgery for CHD, Chaturvedi and colleagues reported that patients who underwent ECMO deployment in the operating room had improved survival to hospital discharge as compared with patients with ECMO was deployed in the intensive care unit (64% vs. 29%) suggesting that early institution of ECMO prevented exposure to a prolonged period of low cardiac output.33
The ELSO International Summary, January 2019 shows similar survival to discharge for neonates supported with ECMO for ECPR as compared with ECMO support for other cardiac indications (41% vs. 42%), but lower survival to discharge for pediatric ECPR (42%) as compared to ECMO for other cardiac indications (52%) once the neonatal period is complete. Other studies have reported similar survival for ECPR and non-ECPR ECMO.47-49 Notably, the risk of neurological injury may be higher in children supported with ECPR.48, 49 Survival-to-discharge for both ECPR and non-ECPR ECMO patients is heavily influenced by the ability to reverse the postoperative cardiorespiratory failure that necessitated ECMO support.
Complications of PC-ECMO in pediatric patients
Important complications of PC-ECMO in children and neonates include bleeding, mechanical complications, liver failure, sepsis, central nervous system events, and renal failure which are reported at different rates in various studies (Table 4).17, 50, 33 Mechanical complications of ECMO are also common. 33
TABLE 4.
ECMO COMPLICATIONS.
| Study | Bleeding, n (%) |
ECMO system failure, n (%) |
Liver Failure, n (%) |
Sepsis/ infection/ bacteremia, n (%) |
CNS events, n (%) |
Kidney failure, n (%) |
|---|---|---|---|---|---|---|
| Klein17 - 1990 | 17 (47%) | NA | NA | NA | NA | NA |
| Ferrazzi75 - 1991 | 6 (100%) | NA | NA | 3 (50%) | 1 (17%) | NA |
| del Nido76 - 1992 | 1 (9%) | NA | NA | 1 (9%) | 3 (27%) | NA |
| Raithel77 - 1992 | 44 (68%) | 7 (11%) | NA | 20 (31%) | 18 (28%) | 7 (11%) |
| Ziomek19 - 1992 | NA | 3 (13%) | NA | 8 (33%) | 1 (4%) | 4 (17%) |
| Dalton13 - 1993 | 6 (22%) | NA | NA | 3 (11%) | 6 (22%) | NA |
| Walters29 - 1995 | 5 (13%) | NA | 2 (5%) | 4 (10%) | 9 (24%) | 3 (8%) |
| Kulik50 - 1996 | 28 (44%) | NA | 18 (28%) | 12 (19%) | 29 (45%) | 28 (44%) |
| Langley67 - 1998 | 4 (44%) | NA | NA | 5 (56%) | 1 (11%) | 7 (78%) |
| Jaggers79 - 2000 | 15 (35%) | NA | NA | 9 (26%) | 18 (51%) | 9 (26%) |
| Aharon80 - 2001 | NA | NA | NA | 7 (14%) | NA | 4 (8%) |
| Pizarro68 - 2001 | 2 (17%) | 1 (8%) | NA | 5 (42%) | 3 (25%) | 5 (42%) |
| Kolovos18 - 2003 | NA | NA | NA | 11 (15%) | 16 (22%) | 26 (35%) |
| Chaturvedi33 - 2004 | 51 (63%) | 22 (27%) | NA | 22 (27%) | NA | 22 (27%) |
| Chow51 - 2004 | NA | NA | NA | NA | 20 (22%) | NA |
| Morris26 - 2004 | 31 (35%) | NA | NA | NA | 10 (19%)* | NA |
| Huang34 - 2005 | 34 (50%) | NA | NA | NA | NA | 47 (69%) |
| Ghez81 - 2005 | NA | NA | NA | 2 (13%) | 0 (0%) | 6 (40%) |
| Mahle71 - 2005 | NA | 1 (3.1%) | NA | 1 (3.1%) | 7 (21.9%) | NA |
| Baslaim25 - 2006 | 17 (65%) | NA | NA | 4 (15%) | 5 (19%) | 8 (31%) |
| Allan41 - 2008 | NA | NA | NA | NA | 10 (22.7%) | NA |
| Alsoufi42 - 2009 | 100 (56%) | 68 (38%) | NA | NA | 32 (18%) | 18 (10%) |
| Polimenakos27 - 2011 | NA | NA | NA | 4 (19%) | 9 (43%) | 7 (33%) |
| Bhat35 - 2013 | NA | NA | NA | NA | NA | 36 (56%) |
| Agarwal16 - 2014 | NA | NA | NA | NA | NA | 41 (34%) |
| Alsoufi43 2014 | 49 (49%) | 9 (9%) | NA | 21 (21%) | 17 (17%) | 55 (56%) |
| Sasaki37 - 2014 | 17 (47%) | 7 (19%) | NA | 10 (28%) | 10 (28%) | 19 (53%) |
| Jolley 48 - 2014 | 48 (46%) | 46 (44%) | 7 (6%) | 12 (11%) | 24 (23%) | 34 (33%) |
| Miana84 - 2015 | 19 (34%) | NA | NA | NA | 6 (11%) | 5 (9%) |
| Lou85 - 2015 | 29 (30%) | 11 (11%) | NA | 40 (42%) | 11 (11%) | 51 (53%) |
| Sznycer-Taub23 - 2016 | NA | NA | NA | NA | 39 (42%) | 35 (38%) |
| Aydin11 – 2016 | 27 (30%) | 41 (46%) | NA | 9 (10%) | 15 (16%) | 37 (41%) |
| Howard46 – 2016 | 32 (43%) | 41 (49%) | 16 (19%) | NA | 21 (25%) | 16 (19%) |
| ElMahrouk86 - 2017 | 78 (69%) | NA | NA | 35 (31%) | 18 (16%) | 41 (36%) |
Neurological sequalae occur frequently in children supported with ECMO. In a study of 90 patients by Chow and colleagues,51 only 15 children survived without neurological sequelae. There were short-term neurological events (22%) and long-term neurological sequelae (12%), accounting for 39% of survivors. In a study of 1,898 neonates with CHD reported to the ELSO Registry, 14% suffered a neurological injury.38 Risk factors for neurological injury included birth weight <3kg, pH <7.15 pre-ECMO, and the need for CPR prior to ECMO. Importantly, the patients who suffered neurological injury had higher in-hospital mortality (73%) as compared with those without neurological complications (53%). Khan and colleagues52 reported that 17.5% of neonates supported on ECMO had intraventricular hemorrhage detected by cranial ultrasound (CUS). The investigators performed routine daily CUS on all neonatal patients and found that almost all intracranial hemorrhages occurred in the first 5 days after surgery (including pre-ECMO), and any hemorrhage after that time was associated with clinical symptoms. In infants with an open fontanelle, CUS is a safe bedside screening tool, which can be performed regularly when increased vigilance for neurological complications after ECMO cannulation is warranted.
Although femoral cannulation is less common than carotid cannulation in pediatric patients, when the femoral approach is used, limb ischemia may be a serious complication.53 Methods to prevent limb ischemia in this setting include the use of contralateral femoral vessels for arterial and venous cannulation, the use of the smallest arterial cannula for the desired flow rate, incorporation of a chimney graft, and the use of a distal reperfusion cannula in an antegrade manner in the femoral artery or in a retrograde manner in the dorsalis pedis artery. In a single-center study of 29 children with femoral cannulation for V-A ECMO, Schad and colleagues54 found that 29% of those without routine distal perfusion catheter placement suffered ischemic complications, compared with only 12% when distal perfusion catheters were routinely placed. In addition, non-invasive limb perfusion monitoring with near infrared spectroscopy has translated to better outcomes.55
Post-Cardiotomy V-V ECMO for Respiratory Dysfunction in Pediatric Patients
Despite advances in CPB techniques and in preventive measures aimed at decreasing respiratory complications after cardiac surgery, postoperative acute respiratory distress syndrome (ARDS) occurs in 1–20% of patients, depending on inclusion criteria.56-59 The use of V-A ECMO for refractory cardiovascular dysfunction after pediatric cardiac surgery has been described, but there is paucity of data on the use of postcardiotomy V-V ECMO. Respiratory distress and hypoxia are reported as indications for ECMO support in 2-30% of pediatric patients.11, 13-15 V-V ECMO is an uncommon mode of support for patients with underlying cardiac disease, however in selected patients, it may be the mode of choice to facilitate oxygenation and decrease pulmonary vascular resistance.13
Controversial Issues and Future Perspectives
Cardiac catherization during PC-ECMO
Diagnostic or therapeutic cardiac catheterization can be safely performed on patients receiving ECMO support.60 Early detection and correction of residual cardiac lesions is associated with improved survival.16,61 Catheter-based diagnostic procedures should be considered when non-invasive diagnostic studies fail to identify a reason for failure to wean from ECMO and also, to evaluate decompression of the left side of the heart.62 Callahan and colleagues reported the results of cardiac catheterization on 36 pediatric patients supported by ECMO. 61 They found that the catheterization investigation excluded a residual lesion in 18% of the patients, confirmed a residual lesion in 15%, and identified unexpected residual cardiac lesions in 52%. Interventions to manage the residual lesion were performed in 50% of cases, including stenting, device closure, or thrombolysis. After the cardiac catheterization procedure, 86% of patients were weaned from ECMO and 72% survived to discharge. Of note, catheter-based diagnostic procedures performed during the first or second day of ECMO support (day 0 or 1) significantly reduced the duration of ECMO without impacting survival. Recently, Another single center report of cardiac catheterization on 51 children on ECMO support demonstrated a low rate of serious complications (5.6%), and subsequent decannulation/weaning and survival rates were 71% and 54%, respectively. 63 These studies demonstrate the benefit of cardiac catheterization in evaluating PC-ECMO-supported patient, despite the complexity of interpreting hemodynamic measurements with ECMO cannulae in situ, with or without cessation of ECMO flow for the procedure. Transport of the ECMO patient for cardiac catheterization was reported as uncomplicated, and overall, the complication rate was low.61, 63
Decompression of the left side of the heart
Assessment and management of left heart decompression is a common indication for cardiac catheterization.61, 63 Left heart hypertension of patients managed on ECMO can be addressed by atrial septostomy, use of an axial trans-aortic valve pump (Impella), direct left ventricular venting via an open approach, or with left atrial cannulation, either directly or via catheter crossing the atrial septum. Eastaugh and colleagues reported percutaneous left heart decompression in 44 of 419 patients managed on V-A ECMO, via atrial septostomy, stenting of the atrial septum or left atrial venting across the atrial septum.64 All techniques were equally successful at reducing left atrial pressure and decreasing pulmonary edema. Another single center study reported left heart decompression in 49 children managed on central V-A ECMO with left atrial venting, atrial septostomy, and left ventricle cannulation.65 Elective left heart decompression was associated with reduced duration of ECMO support, but was not associated with improved survival. Recently, an Impella device was used to decompress the left ventricle of 4 children on V-A ECMO.66 The device reduced left atrial pressure and increased tissue perfusion as observed by near infrared spectroscopy. Institutional preferences for assessment, timing, and mechanism of left heart decompression vary.
Single ventricle physiology
Despite advances in care, mortality after ECMO support in patients with single ventricle palliated circulation remains greater than 50%.18,67,28 The higher mortality in this subset of patients with cardiac palliation has been hypothesized as an imbalance between systemic and pulmonary blood flow and associated suboptimal coronary perfusion and increased probability of ventricular distension.28, 68 Indications for ECMO in pediatric patients with single ventricle palliated circulation are comparable to those in patients with biventricular circulation, but additional complications are associated with systemic-to-pulmonary artery shunts.62 When the pulmonary circulation is supplied by a systemic-to-pulmonary artery shunt and ECMO support is instituted, the shunt is most often left open. Adequate support of this circulation may require an augmented circuit blood flow of 150-200 ml/Kg/min to allow for diastolic run-off to the pulmonary circulation. In patients with single ventricle palliated circulation, cannulation strategy is an important issue.32, 41, 48 In-hospital mortality rates with the use of ECMO after the Norwood single ventricle palliation procedure exceed 50%.69, 70 ECMO support of patients with Glenn and Fontan circulation is associated with additional complexity due to the surgical anatomy and resulting physiology. Multiple drainage cannulae may be required to optimize support.32 In all stages of single-ventricle palliation, long-duration ECMO, inotropic support, and renal failure are associated with higher mortality.48,49 The adequacy of the cannulation strategy should be questioned if the ECMO-supported patient with a single ventricle palliated circulation has evidence of ongoing poor end-organ oxygen delivery.
ECMO costs
Against the background of high mortality prior to hospital discharge, the cost of ECMO support requires some consideration. Mahle and colleagues focused on hospital costs in 32 pediatric patients with CHD who received salvage ECMO (18 for cardiopulmonary arrest and 14 PC shock).71 Survival to hospital discharge was 50% and 1-year survival was 47%. The quality-of-life of the survivors was determined with the Health Utilities Index Mark II, and median cost for hospital stay after institution of ECMO was $156,324 per patient. The calculated cost-utility for salvage ECMO in this population was $24,386 per quality-adjusted life-year saved, which would be considered within the range of accepted cost-efficacy (<$50,000 per quality-adjusted life-year saved). The authors note, however, that although the hospital costs for salvage cardiac ECMO are similar to those for neonatal ECMO for noncardiac indications, the calculated cost-utility is slightly less favorable. Salvage cardiac ECMO may be somewhat less cost-effective than noncardiac ECMO since the survival to hospital discharge is lower for cardiac patients. In addition, life expectancy for children with complex CHD, such as those with single ventricle palliated circulation, is lower than for children with respiratory distress in the neonatal period. Other investigators also noted a greater cost was associated with smaller hospitals and hospital location.72
Bridging to heart transplantation
ECMO support for children with circulatory failure awaiting heart transplantation has been analyzed using combined data from the ELSO Registry and the United States Organ Procurement Transplant Network (OPTN).10 The authors demonstrated that ECMO was associated with high mortality while on the waiting list, and one-third of the patients who received a heart transplant died before hospital discharge. Overall, survival to hospital discharge of this population was 47%. In this context, other forms of temporary MCS are often considered for children awaiting heart transplantation.73 While bridging to transplantation represents a different clinical challenge than PC-ECMO, these studies may lead to innovative techniques for post-cardiotomy circulatory support that will advance the field.
Limitations of the Review
Pediatric PC-ECMO, particularly in the setting of CHD, is a technically challenging but potentially life-saving mode of support. There is limited evidence to inform clinical practice. We acknowledge several limitations to the current systematic review that are inherent to retrospective, observational studies. Indeed, most of the studies assembled here are single-center case series reports, precluding statistical analyses and lacking the power to detect some clinically significant differences in outcome. Secondly, the analyses were seldom adjusted for underlying confounders, such as duration of ECMO, cannulation strategy (peripheral vs central), cannula types and ECMO weaning protocols. Finally, although studies with large data sets coming from clinical registries (STS, ELSO, OPTN and PHIS) are discussed in the text, they were not included in the tables.20, 21, 36, 38, 74
Conclusion
V-A ECMO is the optimal support technique in children with CHD and post-cardiotomy shock. ECMO facilitates augmented cardiac output and respiratory gas exchange to improve the metabolic status of both preoperative and post-cardiotomy patients. Although PC-ECMO can improve survival of this vulnerable population, mortality and morbidity remain high. Complications related to bleeding, thrombosis, and infections increase mortality and are major areas for improvement. Neurological injury and neurodevelopmental impairment are common in pediatric patients post PC-ECMO and reflect the severity of critical illness, complexity of cardiac surgery and complications of ECMO support. Bridging to cardiac transplantation can be successful if PC-ECMO does not lead to cardiac recovery, but the availability of organs and waiting list duration are ultimately factors in survival. The paucity of standardized care processes, informed patient selection, optimal timing of ECMO deployment, anti-coagulation strategies or weaning proto- cols, and the lack of long-term follow-up of survivors after PC-ECMO demonstrated on systematic review informs the urgent need for further studies to inform best management of this high-risk population.
Supplementary Material
Figure 2.
Post-cardiotomy ECMO approaches for cannulation: central cannulation (right atrium and ascending aorta cannulation) with a jugular exit port for cannulas, and the sternum closed.
Acknowledgments
Source of Funding. No funds were received.
Footnotes
Disclosure.
Dr. Lorusso is consultant and conducts clinical trial for LivaNova (London, UK)
Dr. Brodie is currently the co-chair of the Trial Steering Committee for the VENT-AVOID trial sponsored by ALung Technologies, he was previously on the medical advisory board of ALung Technologies and Kadence (Johnson & Johnson). All compensation for these activities is paid to Columbia University.
The other Authors report no conflict of interest
References
- 1.Cooper DS, Jacobs JP, Moore L, Stock A, Gaynor JW, Chancy T, Parpard M, Griffin DA, Owens T, Checchia PA, Thiagarajan RR, Spray TL and Ravishankar C. Cardiac extracorporeal life support: state of the art in 2007. Cardiology in the young. 2007;17 Suppl 2:104–15. [DOI] [PubMed] [Google Scholar]
- 2.Baffes TG, Fridman JL, Bicoff JP and Whitehill JL. Extracorporeal circulation for support of palliative cardiac surgery in infants. The Annals of thoracic surgery. 1970;10:354–63. [DOI] [PubMed] [Google Scholar]
- 3.Soeter JR, Mamiya RT, Sprague AY and McNamara JJ. Prolonged extracorporeal oxygenation for cardiorespiratory failure after tetralogy correction. The Journal of thoracic and cardiovascular surgery. 1973;66:214–8. [PubMed] [Google Scholar]
- 4.Duncan BW. Pediatric mechanical circulatory support. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2005;51:ix–xiv. [DOI] [PubMed] [Google Scholar]
- 5.Chang AC, Wernovsky G, Kulik TJ, Jonas RA and Wessel DL. Management of the neonate with transposition of the great arteries and persistent pulmonary hypertension. The American journal of cardiology. 1991;68:1253–5. [DOI] [PubMed] [Google Scholar]
- 6.Di Russo GB, Clark BJ, Bridges ND, Godinez RI, Paridon SM, Spray TL and Gaynor JW. Prolonged extracorporeal membrane oxygenation as a bridge to cardiac transplantation. The Annals of thoracic surgery. 2000;69:925–7. [DOI] [PubMed] [Google Scholar]
- 7.Bautista-Hernandez V, Thiagarajan RR, Fynn-Thompson F, Rajagopal SK, Nento DE, Yarlagadda V, Teele SA, Allan CK, Emani SM, Laussen PC, Pigula FA and Bacha EA. Preoperative extracorporeal membrane oxygenation as a bridge to cardiac surgery in children with congenital heart disease. The Annals of thoracic surgery. 2009;88:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossano JW, Cherikh WS, Chambers DC, Goldfarb S, Khush K, Kucheryavaya AY, Levvey BJ, Lund LH, Meiser B, Yusen RD and Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: Twentieth Pediatric Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2017;36:1060–1069. [DOI] [PubMed] [Google Scholar]
- 9.Lorts A, Eghtesady P, Mehegan M, Adachi I, Villa C, Davies R, Gossett JG, Kanter K, Alejos J, Koehl D, Cantor RS and Morales DLS. Outcomes of children supported with devices labeled as "temporary" or short term: A report from the Pediatric Interagency Registry for Mechanical Circulatory Support. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2018;37:54–60. [DOI] [PubMed] [Google Scholar]
- 10.Almond CS, Singh TP, Gauvreau K, Piercey GE, Fynn-Thompson F, Rycus PT, Bartlett RH and Thiagarajan RR. Extracorporeal membrane oxygenation for bridge to heart transplantation among children in the United States: analysis of data from the Organ Procurement and Transplant Network and Extracorporeal Life Support Organization Registry. Circulation. 2011;123:2975–84. [DOI] [PubMed] [Google Scholar]
- 11.Aydin SI, Duffy M, Rodriguez D, Rycus PT, Friedman P, Thiagarajan RR and Weinstein S. Venovenous extracorporeal membrane oxygenation for patients with single-ventricle anatomy: A registry report. The Journal of thoracic and cardiovascular surgery. 2016;151:1730–6. [DOI] [PubMed] [Google Scholar]
- 12.Barbaro RP, Paden ML, Guner YS, Raman L, Ryerson LM, Alexander P, Nasr VG, Bembea MM, Rycus PT, Thiagarajan RR and centers Em. Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2017;63:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton HJ, Siewers RD, Fuhrman BP, Del Nido P, Thompson AE, Shaver MG and Dowhy M. Extracorporeal membrane oxygenation for cardiac rescue in children with severe myocardial dysfunction. Critical care medicine. 1993;21:1020–8. [DOI] [PubMed] [Google Scholar]
- 14.Dhillon R, Pearson GA, Firmin RK, Chan KC and Leanage R. Extracorporeal membrane oxygenation and the treatment of critical pulmonary hypertension in congenital heart disease. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1995;9:553–6. [DOI] [PubMed] [Google Scholar]
- 15.Kim K, Mazor RL, Rycus PT and Brogan TV. Use of venovenous extracorporeal life support in pediatric patients for cardiac indications: a review of the Extracorporeal Life Support Organization registry. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012;13:285–9. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal HS, Hardison DC, Saville BR, Donahue BS, Lamb FS, Bichell DP and Harris ZL. Residual lesions in postoperative pediatric cardiac surgery patients receiving extracorporeal membrane oxygenation support. The Journal of thoracic and cardiovascular surgery. 2014;147:434–41. [DOI] [PubMed] [Google Scholar]
- 17.Klein MD, Shaheen KW, Whittlesey GC, Pinsky WW and Arciniegas E. Extracorporeal membrane oxygenation for the circulatory support of children after repair of congenital heart disease. The Journal of thoracic and cardiovascular surgery. 1990;100:498–505. [PubMed] [Google Scholar]
- 18.Kolovos NS, Bratton SL, Moler FW, Bove EL, Ohye RG, Bartlett RH and Kulik TJ. Outcome of pediatric patients treated with extracorporeal life support after cardiac surgery. The Annals of thoracic surgery. 2003;76:1435–41; discussion 1441-2. [DOI] [PubMed] [Google Scholar]
- 19.Ziomek S, Harrell JE Jr., Fasules JW, Faulkner SC, Chipman CW, Moss M, Frazier E and Van Devanter SH. Extracorporeal membrane oxygenation for cardiac failure after congenital heart operation. The Annals of thoracic surgery. 1992;54:861–7; discussion 867-8. [DOI] [PubMed] [Google Scholar]
- 20.Bratton SL, Chan T, Barrett CS, Wilkes J, Ibsen LM and Thiagarajan RR. Metrics to Assess Extracorporeal Membrane Oxygenation Utilization in Pediatric Cardiac Surgery Programs. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2017;18:779–786. [DOI] [PubMed] [Google Scholar]
- 21.Mascio CE, Austin EH, Jacobs JP, Jacobs ML, Wallace AS, He X and Pasquali SK. Perioperative mechanical circulatory support in children: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. The Journal of thoracic and cardiovascular surgery. 2014;147:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta P, DasGupta R, Best D, Chu CB, Elsalloukh H, Gossett JM, Imamura M and Butt W. Delayed extracorporeal membrane oxygenation in children after cardiac surgery: two-institution experience. Cardiology in the young. 2015;25:248–54. [DOI] [PubMed] [Google Scholar]
- 23.Sznycer-Taub NR, Lowery R, Yu S, Owens ST, Hirsch-Romano JC and Owens GE. Hyperoxia Is Associated With Poor Outcomes in Pediatric Cardiac Patients Supported on Venoarterial Extracorporeal Membrane Oxygenation. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2016;17:350–8. [DOI] [PubMed] [Google Scholar]
- 24.Polimenakos AC, Rizzo V, El-Zein CF and Ilbawi MN. Post-cardiotomy Rescue Extracorporeal Cardiopulmonary Resuscitation in Neonates with Single Ventricle After Intractable Cardiac Arrest: Attrition After Hospital Discharge and Predictors of Outcome. Pediatric cardiology. 2017;38:314–323. [DOI] [PubMed] [Google Scholar]
- 25.Baslaim G, Bashore J, Al-Malki F and Jamjoom A. Can the outcome of pediatric extracorporeal membrane oxygenation after cardiac surgery be predicted? Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2006;12:21–7. [PubMed] [Google Scholar]
- 26.Morris MC, Ittenbach RF, Godinez RI, Portnoy JD, Tabbutt S, Hanna BD, Hoffman TM, Gaynor JW, Connelly JT, Helfaer MA, Spray TL and Wernovsky G. Risk factors for mortality in 137 pediatric cardiac intensive care unit patients managed with extracorporeal membrane oxygenation. Critical care medicine. 2004;32:1061–9. [DOI] [PubMed] [Google Scholar]
- 27.Polimenakos AC, Wojtyla P, Smith PJ, Rizzo V, Nater M, El Zein CF and Ilbawi MN. Post-cardiotomy extracorporeal cardiopulmonary resuscitation in neonates with complex single ventricle: analysis of outcomes. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011;40:1396–405; discussion 1405. [DOI] [PubMed] [Google Scholar]
- 28.Thourani VH, Kirshbom PM, Kanter KR, Simsic J, Kogon BE, Wagoner S, Dykes F, Fortenberry J and Forbess JM. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) in pediatric cardiac support. The Annals of thoracic surgery. 2006;82:138–44; discussion 144-5. [DOI] [PubMed] [Google Scholar]
- 29.Walters HL 3rd, Hakimi M, Rice MD, Lyons JM, Whittlesey GC and Klein MD. Pediatric cardiac surgical ECMO: multivariate analysis of risk factors for hospital death. The Annals of thoracic surgery. 1995;60:329–36; discussion 336-7. [DOI] [PubMed] [Google Scholar]
- 30.Johnson K, Jarboe MD, Mychaliska GB, Barbaro RP, Rycus P, Hirschl RB, Gadepalli SK and Group ELE-ENOW. Is there a best approach for extracorporeal life support cannulation: a review of the extracorporeal life support organization. J Pediatr Surg. 2018;53:1301–1304. [DOI] [PubMed] [Google Scholar]
- 31.Chan T, Thiagarajan RR, Frank D and Bratton SL. Survival after extracorporeal cardiopulmonary resuscitation in infants and children with heart disease. The Journal of thoracic and cardiovascular surgery. 2008;136:984–92. [DOI] [PubMed] [Google Scholar]
- 32.Marino BS, Tabbutt S, MacLaren G, Hazinski MF, Adatia I, Atkins DL, Checchia PA, DeCaen A, Fink EL, Hoffman GM, Jefferies JL, Kleinman M, Krawczeski CD, Licht DJ, Macrae D, Ravishankar C, Samson RA, Thiagarajan RR, Toms R, Tweddell J, Laussen PC, American Heart Association Congenital Cardiac Defects Committee of the Council on Cardiovascular Disease in the Y, Council on Clinical C, Council on C, Stroke N, Council on Cardiovascular S, Anesthesia and Emergency Cardiovascular Care C. Cardiopulmonary Resuscitation in Infants and Children With Cardiac Disease: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e691–e782. [DOI] [PubMed] [Google Scholar]
- 33.Chaturvedi RR, Macrae D, Brown KL, Schindler M, Smith EC, Davis KB, Cohen G, Tsang V, Elliott M, de Leval M, Gallivan S and Goldman AP. Cardiac ECMO for biventricular hearts after paediatric open heart surgery. Heart (British Cardiac Society). 2004;90:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang SC, Wu ET, Chen YS, Chang CI, Chiu IS, Chi NH, Wu MH, Wang SS, Lin FY and Ko WJ. Experience with extracorporeal life support in pediatric patients after cardiac surgery. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2005;51:517–21. [DOI] [PubMed] [Google Scholar]
- 35.Bhat P, Hirsch JC, Gelehrter S, Cooley E, Donohue J, King K and Gajarski RJ. Outcomes of infants weighing three kilograms or less requiring extracorporeal membrane oxygenation after cardiac surgery. The Annals of thoracic surgery. 2013;95:656–61. [DOI] [PubMed] [Google Scholar]
- 36.Ford MA, Gauvreau K, McMullan DM, Almodovar MC, Cooper DS, Rycus PT and Thiagarajan R. Factors Associated With Mortality in Neonates Requiring Extracorporeal Membrane Oxygenation for Cardiac Indications: Analysis of the Extracorporeal Life Support Organization Registry Data. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2016;17:860–70. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki T, Asou T, Takeda Y, Onakatomi Y, Tominaga T and Yamamoto Y. Extracorporeal life support after cardiac surgery in children: outcomes from a single institution. Artificial organs. 2014;38:34–40. [DOI] [PubMed] [Google Scholar]
- 38.Polito A, Barrett CS, Rycus PT, Favia I, Cogo PE and Thiagarajan RR. Neurologic injury in neonates with congenital heart disease during extracorporeal membrane oxygenation: an analysis of extracorporeal life support organization registry data. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2015;61:43–8. [DOI] [PubMed] [Google Scholar]
- 39.McMullan DM, Thiagarajan RR, Smith KM, Rycus PT and Brogan TV. Extracorporeal cardiopulmonary resuscitation outcomes in term and premature neonates*. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2014;15:e9–e16. [DOI] [PubMed] [Google Scholar]
- 40.Gupta P, Robertson MJ, Beam B, Gossett JM, Schmitz ML, Carroll CL, Edwards JD, Fortenberry JD and Butt W. Relationship of ECMO duration with outcomes after pediatric cardiac surgery: a multi-institutional analysis. Minerva anestesiologica. 2015;81:619–27. [PubMed] [Google Scholar]
- 41.Allan CK, Thiagarajan RR, del Nido PJ, Roth SJ, Almodovar MC and Laussen PC. Indication for initiation of mechanical circulatory support impacts survival of infants with shunted single-ventricle circulation supported with extracorporeal membrane oxygenation. The Journal of thoracic and cardiovascular surgery. 2007;133:660–7. [DOI] [PubMed] [Google Scholar]
- 42.Alsoufi B, Al-Radi OO, Gruenwald C, Lean L, Williams WG, McCrindle BW, Caldarone CA and Van Arsdell GS. Extra-corporeal life support following cardiac surgery in children: analysis of risk factors and survival in a single institution. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2009;35:1004–11; discussion 1011. [DOI] [PubMed] [Google Scholar]
- 43.Alsoufi B, Awan A, Manlhiot C, Al-Halees Z, Al-Ahmadi M, McCrindle BW and Alwadai A. Does single ventricle physiology affect survival of children requiring extracorporeal membrane oxygenation support following cardiac surgery? World journal for pediatric & congenital heart surgery. 2014;5:7–15. [DOI] [PubMed] [Google Scholar]
- 44.Yan X, Jia S, Meng X, Dong P, Jia M, Wan J and Hou X. Acute kidney injury in adult postcardiotomy patients with extracorporeal membrane oxygenation: evaluation of the RIFLE classification and the Acute Kidney Injury Network criteria. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2010;37:334–8. [DOI] [PubMed] [Google Scholar]
- 45.Haneya A, Diez C, Philipp A, Bein T, Mueller T, Schmid C and Lubnow M. Impact of Acute Kidney Injury on Outcome in Patients With Severe Acute Respiratory Failure Receiving Extracorporeal Membrane Oxygenation. Critical care medicine. 2015;43:1898–906. [DOI] [PubMed] [Google Scholar]
- 46.Howard TS, Kalish BT, Wigmore D, Nathan M, Kulik TJ, Kaza AK, Williams K and Thiagarajan RR. Association of Extracorporeal Membrane Oxygenation Support Adequacy and Residual Lesions With Outcomes in Neonates Supported After Cardiac Surgery. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2016;17:1045–1054. [DOI] [PubMed] [Google Scholar]
- 47.Chrysostomou C, Morell VO, Kuch BA, O'Malley E, Munoz R and Wearden PD. Short- and intermediate-term survival after extracorporeal membrane oxygenation in children with cardiac disease. The Journal of thoracic and cardiovascular surgery. 2013;146:317–25. [DOI] [PubMed] [Google Scholar]
- 48.Jolley M, Thiagarajan RR, Barrett CS, Salvin JW, Cooper DS, Rycus PT and Teele SA. Extracorporeal membrane oxygenation in patients undergoing superior cavopulmonary anastomosis. The Journal of thoracic and cardiovascular surgery. 2014;148:1512–8. [DOI] [PubMed] [Google Scholar]
- 49.Rood KL, Teele SA, Barrett CS, Salvin JW, Rycus PT, Fynn-Thompson F, Laussen PC and Thiagarajan RR. Extracorporeal membrane oxygenation support after the Fontan operation. The Journal of thoracic and cardiovascular surgery. 2011;142:504–10. [DOI] [PubMed] [Google Scholar]
- 50.Kulik TJ, Moler FW, Palmisano JM, Custer JR, Mosca RS, Bove EL and Bartlett RH. Outcome-associated factors in pediatric patients treated with extracorporeal membrane oxygenator after cardiac surgery. Circulation. 1996;94:Ii63–8. [PubMed] [Google Scholar]
- 51.Chow G, Koirala B, Armstrong D, McCrindle B, Bohn D, Edgell D, Coles J and de Veber G. Predictors of mortality and neurological morbidity in children undergoing extracorporeal life support for cardiac disease. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2004;26:38–43. [DOI] [PubMed] [Google Scholar]
- 52.Khan AM, Shabarek FM, Zwischenberger JB, Warner BW, Cheu HW, Jaksic T, Goretsky MJ, Meyer TA, Doski J and Lally KP. Utility of daily head ultrasonography for infants on extracorporeal membrane oxygenation. J Pediatr Surg. 1998;33:1229–32. [DOI] [PubMed] [Google Scholar]
- 53.Fraser CD 3rd, Kovler ML, Guzman W Jr., Rhee DS, Lum YW, Alaish SM and Garcia AV. Pediatric Femoral Arterial Cannulations in Extracorporeal Membrane Oxygenation: A Review and Strategies for Optimization. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2018. [DOI] [PubMed] [Google Scholar]
- 54.Schad CA, Fallon BP, Monteagudo J, Okochi S, Cheung EW, Morrissey NJ, Kadenhe-Chiweshe AV, Aspelund G, Stylianos S and Middlesworth W. Routine Use of Distal Arterial Perfusion in Pediatric Femoral Venoarterial Extracorporeal Membrane Oxygenation. Artificial organs. 2017;41:11–16. [DOI] [PubMed] [Google Scholar]
- 55.Kim DJ, Cho YJ, Park SH, Lim C, Park KH, Jheon S and Kim JS. Near-Infrared Spectroscopy Monitoring for Early Detection of Limb Ischemia in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2017;63:613–617. [DOI] [PubMed] [Google Scholar]
- 56.Stephens RS, Shah AS and Whitman GJ. Lung injury and acute respiratory distress syndrome after cardiac surgery. The Annals of thoracic surgery. 2013;95:1122–9. [DOI] [PubMed] [Google Scholar]
- 57.Rahmanian PB, Adams DH, Castillo JG, Carpentier A and Filsoufi F. Predicting hospital mortality and analysis of long-term survival after major noncardiac complications in cardiac surgery patients. The Annals of thoracic surgery. 2010;90:1221–9. [DOI] [PubMed] [Google Scholar]
- 58.Rong LQ, Di Franco A and Gaudino M. Acute respiratory distress syndrome after cardiac surgery. J Thorac Dis. 2016;8:E1177–E1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK and Elbourne D. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet (London, England). 2009;374:1351–63. [DOI] [PubMed] [Google Scholar]
- 60.Booth KL, Roth SJ, Perry SB, del Nido PJ, Wessel DL and Laussen PC. Cardiac catheterization of patients supported by extracorporeal membrane oxygenation. Journal of the American College of Cardiology. 2002;40:1681–6. [DOI] [PubMed] [Google Scholar]
- 61.Callahan R, Trucco SM, Wearden PD, Beerman LB, Arora G and Kreutzer J. Outcomes of pediatric patients undergoing cardiac catheterization while on extracorporeal membrane oxygenation. Pediatric cardiology. 2015;36:625–32. [DOI] [PubMed] [Google Scholar]
- 62.Di Nardo M, MacLaren G, Marano M, Cecchetti C, Bernaschi P and Amodeo A. ECLS in Pediatric Cardiac Patients. Frontiers in pediatrics. 2016;4:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boscamp NS, Turner ME, Crystal M, Anderson B, Vincent JA and Torres AJ. Cardiac Catheterization in Pediatric Patients Supported by Extracorporeal Membrane Oxygenation: A 15-Year Experience. Pediatric cardiology. 2017;38:332–337. [DOI] [PubMed] [Google Scholar]
- 64.Eastaugh LJ, Thiagarajan RR, Darst JR, McElhinney DB, Lock JE and Marshall AC. Percutaneous left atrial decompression in patients supported with extracorporeal membrane oxygenation for cardiac disease. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2015;16:59–65. [DOI] [PubMed] [Google Scholar]
- 65.Hacking DF, Best D, d'Udekem Y, Brizard CP, Konstantinov IE, Millar J and Butt W. Elective decompression of the left ventricle in pediatric patients may reduce the duration of venoarterial extracorporeal membrane oxygenation. Artificial organs. 2015;39:319–26. [DOI] [PubMed] [Google Scholar]
- 66.Parekh D, Jeewa A, Tume SC, Dreyer WJ, Pignatelli R, Horne D, Justino H and Qureshi AM. Percutaneous Mechanical Circulatory Support Using Impella Devices for Decompensated Cardiogenic Shock: A Pediatric Heart Center Experience. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2018;64:98–104. [DOI] [PubMed] [Google Scholar]
- 67.Langley SM, Sheppard SV, Tsang VT, Monro JL and Lamb RK. When is extracorporeal life support worthwhile following repair of congenital heart disease in children? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1998;13:520–5. [DOI] [PubMed] [Google Scholar]
- 68.Pizarro C, Davis DA, Healy RM, Kerins PJ and Norwood WI. Is there a role for extracorporeal life support after stage I Norwood? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2001;19:294–301. [DOI] [PubMed] [Google Scholar]
- 69.Friedland-Little JM, Aiyagari R, Yu S, Donohue JE and Hirsch-Romano JC. Survival through staged palliation: fate of infants supported by extracorporeal membrane oxygenation after the Norwood operation. The Annals of thoracic surgery. 2014;97:659–65. [DOI] [PubMed] [Google Scholar]
- 70.Tabbutt S, Ghanayem N, Ravishankar C, Sleeper LA, Cooper DS, Frank DU, Lu M, Pizarro C, Frommelt P, Goldberg CS, Graham EM, Krawczeski CD, Lai WW, Lewis A, Kirsh JA, Mahony L, Ohye RG, Simsic J, Lodge AJ, Spurrier E, Stylianou M, Laussen P and Pediatric Heart Network I. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. The Journal of thoracic and cardiovascular surgery. 2012;144:882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahle WT, Forbess JM, Kirshbom PM, Cuadrado AR, Simsic JM and Kanter KR. Cost-utility analysis of salvage cardiac extracorporeal membrane oxygenation in children. The Journal of thoracic and cardiovascular surgery. 2005;129:1084–90. [DOI] [PubMed] [Google Scholar]
- 72.Faraoni D, Nasr VG, DiNardo JA and Thiagarajan RR. Hospital Costs for Neonates and Children Supported with Extracorporeal Membrane Oxygenation. The Journal of pediatrics. 2016;169:69–75 e1. [DOI] [PubMed] [Google Scholar]
- 73.Yarlagadda VV, Maeda K, Zhang Y, Chen S, Dykes JC, Gowen MA, Shuttleworth P, Murray JM, Shin AY, Reinhartz O, Rosenthal DN, McElhinney DB and Almond CS. Temporary Circulatory Support in U.S. Children Awaiting Heart Transplantation. Journal of the American College of Cardiology. 2017;70:2250–2260. [DOI] [PubMed] [Google Scholar]
- 74.Barrett CS, Chan TT, Wilkes J, Bratton SL and Thiagarajan RR. Association of Pediatric Cardiac Surgical Volume and Mortality After Cardiac ECMO. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2017;63:802–809. [DOI] [PubMed] [Google Scholar]
- 75.Ferrazzi P, Glauber M, Di Domenico A, Fiocchi R, Mamprin F, Gamba A, Crupi G, Cossolini M and Parenzan L. Assisted circulation for myocardial recovery after repair of congenital heart disease. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1991;5:419–23; discussion 424. [DOI] [PubMed] [Google Scholar]
- 76.del Nido PJ, Dalton HJ, Thompson AE and Siewers RD. Extracorporeal membrane oxygenator rescue in children during cardiac arrest after cardiac surgery. Circulation. 1992;86:Ii300–4. [PubMed] [Google Scholar]
- 77.Raithel SC, Pennington DG, Boegner E, Fiore A and Weber TR. Extracorporeal membrane oxygenation in children after cardiac surgery. Circulation. 1992;86:Ii305–10. [PubMed] [Google Scholar]
- 78.Black MD, Coles JG, Williams WG, Rebeyka IM, Trusler GA, Bohn D, Gruenwald C and Freedom RM. Determinants of success in pediatric cardiac patients undergoing extracorporeal membrane oxygenation. The Annals of thoracic surgery. 1995;60:133–8. [PubMed] [Google Scholar]
- 79.Jaggers JJ, Forbess JM, Shah AS, Meliones JN, Kirshbom PM, Miller CE and Ungerleider RM. Extracorporeal membrane oxygenation for infant postcardiotomy support: significance of shunt management. The Annals of thoracic surgery. 2000;69:1476–83. [DOI] [PubMed] [Google Scholar]
- 80.Aharon AS, Drinkwater DC Jr., Churchwell KB, Quisling SV, Reddy VS, Taylor M, Hix S, Christian KG, Pietsch JB, Deshpande JK, Kambam J, Graham TP and Chang PA. Extracorporeal membrane oxygenation in children after repair of congenital cardiac lesions. The Annals of thoracic surgery. 2001;72:2095–101; discussion 2101-2. [DOI] [PubMed] [Google Scholar]
- 81.Ghez O, Feier H, Ughetto F, Fraisse A, Kreitmann B and Metras D. Postoperative extracorporeal life support in pediatric cardiac surgery: recent results. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2005;51:513–6. [DOI] [PubMed] [Google Scholar]
- 82.Delmo Walter EM, Alexi-Meskishvili V, Huebler M, Loforte A, Stiller B, Weng Y, Berger F and Hetzer R. Extracorporeal membrane oxygenation for intraoperative cardiac support in children with congenital heart disease. Interactive cardiovascular and thoracic surgery. 2010;10:753–8. [DOI] [PubMed] [Google Scholar]
- 83.Sasson L, Cohen I, Tamir A, Sternfeld AR, Berlowitz Y, Lenczner O and Houri S. Extracorporeal membrane oxygenation in pediatric patients: our experience in the last ten years. The Israel Medical Association journal : IMAJ. 2013;15:13–6. [PubMed] [Google Scholar]
- 84.Miana LA, Caneo LF, Tanamati C, Penha JG, Guimaraes VA, Miura N, Galas FR and Jatene MB. Post-cardiotomy ECMO in pediatric and congenital heart surgery: impact of team training and equipment in the results. Revista brasileira de cirurgia cardiovascular : orgao oficial da Sociedade Brasileira de Cirurgia Cardiovascular. 2015;30:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lou S, MacLaren G, Clark J, Paul E, Best D, Delzoppo C, d'Udekem Y and Butt W. Safety of therapeutic hypothermia in children on veno-arterial extracorporeal membrane oxygenation after cardiac surgery. Cardiology in the young. 2015;25:1367–73. [DOI] [PubMed] [Google Scholar]
- 86.ElMahrouk AF, Ismail MF, Hamouda T, Shaikh R, Mahmoud A, Shihata MS, Alradi O and Jamjoom A. Extracorporeal Membrane Oxygenation in Postcardiotomy Pediatric Patients-15 Years of Experience Outside Europe and North America. The Thoracic and cardiovascular surgeon. 2017. [DOI] [PubMed] [Google Scholar]
- 87.Mistry MS, Trucco SM, Maul T, Sharma MS, Wang L and West S. Predictors of Poor Outcomes in Pediatric Venoarterial Extracorporeal Membrane Oxygenation. World journal for pediatric & congenital heart surgery. 2018:2150135118762391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.