Abstract
The systematic classification of vascular disease as proposed and refined by the International Society for the Study of Vascular Anomalies (ISSVA) divides vascular pathology first into tumors and malformations. Malformations are described as simple and complex, where simple malformations contain a single vascular system and complex malformations comprised of multiple vascular systems. Arteriovenous malformations are considered in terms of inflow characteristics which are primarily responsible for the key management challenges. Management utilizing endovascular embolization and/or surgical resection is often employed; however, recurrence can occur, particularly in diffuse cases. There may be an increasing role for systemic antiangiogenic therapy in such cases. Lymphaticovenous malformations are divided into the principle components on the lymphatic and venous sides for clarity of discussion. Lymphatic malformations are described morphologically as macrocystic and microcystic, and physiologically in terms of the processes responsible for growth. In both cases, surgical options are challenging and local therapeutics intended to close large luminal spaces in the case of macrocystic and to slow biological signaling for growth in microcystic. Venous malformations are described physiologically in terms of flow and distensibility, as volume plays a critical role in the limited space of the orbital cavity. Combined embolic-surgical approaches can be effective for management. More complicated, combined lesions can be managed by dividing the lesion into principal components and treating each appropriately.
Keywords: orbital neoplasms, arteriovenous malformations, venous malformations, lymphaticovenous malformation, lymphatic malformation, sclerotherapy, interventional neuroradiology, intravascular embolization
Introduction
The current International Society for The Study of Vascular Anomalies (ISSVA) classification from 2018 1 has its root in the Mulliken and Glowacki classification proposed in 1982. 2 This landmark study defined the initial binary reclassification of vascular lesions into hemangioma and malformations by endothelial cell characteristics, fundamentally separating these diseases based on their biologic nature.
Over time, both sides of the schema have evolved. 3 4 The hemangioma, or tumor, side has broadened to include a wide range of benign and malignant vascular neoplastic lesions. The malformation group has been further subcharacterized by the tissues of origin (arterial, venous, and lymphatic) and, in a sense, flow characteristics. The modern classification system maintains these central tenets, while increasingly emphasizing the spectrum of vascular disease.
The current ISSVA classification ( Table 1 ) discusses the proliferative tumor arm in terms of cellular and biologic behavior, on a spectrum ranging from benign to locally aggressive to malignant. Malformations are understood as a range of diseases that are either simple (single vascular system) or complex (multiple vascular systems), describing simple components that can be combined in almost any manner into more complex malformations.
Table 1. International Society for The Study of Vascular Anomalies classification 2018 International Society for the Study of Vascular Anomalies.
| Tumors | Malformations | |
|---|---|---|
| Benign | Simple | Complex |
| Infantile hemangioma | Capillary malformation | Capillary lymphatic malformation |
| Hemangioma of infancy | ||
| Congenital hemangioma | Lymphatic malformation | Capillary arteriovenous malformation |
| Tufted angioma a b | ||
| Spindle-cell hemangioma | Venous malformation | Lymphaticovenous malformation |
| Epithelioid hemangioma | ||
| Pyogenic granuloma (or lobular capillary hemangioma) | Arteriovenous malformation | Capillary lymphaticovenous malformation |
| Locally aggressive | ||
| Kaposiform hemangioendothelioma a b | Arteriovenous fistula | Capillary lymphatic-arteriovenous malformation |
| Retiform hemangioendothelioma | ||
| Papillary intralymphatic angioendothelioma | Capillary venous-arteriovenous malformation | |
| Dabska's tumor | ||
| Composite hemangioendothelioma | Capillary lymphatic-venous-arteriovenous malformation | |
| Kaposi's sarcoma | ||
| Others | ||
| Malignant | ||
| Angiosarcoma | ||
| Epitheloid hemangioendothelioma | ||
Some lesions may be associated with thrombocytopenia and/or consumptive coagulopathy.
Many experts believe that tufted angioma and Kaposiform hemangioendothelioma are part of a spectrum rather than distinct entities.
The present discussion will focus primarily on the malformation side of vascular disease and will generally follow ISSVA classification in terms of understanding the physiology, morphology, and ultimately, therapy of periorbital vascular lesions. Conceptually, complex malformations can be broken down into subunits and each managed (or not) according to specific symptoms. Thus, discussion of each individual component can provide the basis for combined or multifactorial management for more complex malformations.
Arteriovenous Malformations
Pathophysiology
Arteriovenous malformations (AVM) are vascular hamartomas characterized as a tangle of blood vessels connecting arteries and veins without passing through a capillary bed. They are by definition high-flow lesions. Although they are thought to be congenital, 5 they are not generally inherited in a Mendelian sense and tend to appear sporadically. These lesions are extremely rare in the orbit, with even larger case series describing fewer than 10 cases. 6 Nevertheless, more than 50% of head and neck AVMs involve branches of the internal carotid artery, particularly the ophthalmic branch, 7 which suggests that the orbit may be an important nidus for their development. AVMs in the region are often described anatomically as intraorbital, transorbital, or periorbital.
The mechanisms underlying AVM origin and expansion remain to be fully elucidated. However, certain genetic associations have been described, including in genes coding for signaling proteins in the TGF-β, RAS/MAPK, and PI3K/AKT pathways. 8 The importance of TGF-β in vasculogenesis and angiogenesis is well established, 9 and evidence suggests that vascular dysgeneses syndromes, such as hereditary hemorrhagic telangiectasia (HHT), are related to mutations in this signaling pathway. 10 There is also ample evidence for the importance of NOTCH signaling in vascular development, and altered expression of NOTCH-4 is important for vascular remodeling, particularly in the clinically high-risk group for hemorrhages. 11 12 Matrix metalloproteinase-9 (MMP9) has additionally been implicated in angiogenesis, and increased expression has been demonstrated in actively expanding AVM. 13 The processes of vascular remodeling, hormonal stimulation, biomechanical activation, extracellular matrix dysregulation, and pericyte dysfunction may all play roles in the long-term development of AVM. 8
Clinical Presentation
Orbital AVMs may be asymptomatic during infancy. They tend to gradually enlarge over time, often leading to symptoms in early adulthood. Typically, they present with proptosis, a visible mass lesion and/or color changes in the region. Palpable pulsation and bruit are common. Other associated signs including episcleral and conjunctival congestion, secondary glaucoma, restriction of extraocular movements, diplopia, and eyelid swelling are all variable in incidence. Some patients report pain, either at rest or following Valsalva's maneuver due to engorgement of the pathological vessels. Hemorrhage is rare and usually associated with trauma.
Diagnosis is based on selective angiography depicting an anastomotic network of vessels linking two adjacent angiosomes. 7 Early venous drainage in the arterial phase is characteristic. The images are characterized by a rapidly filling proximal arterial system leading to the malformation and the dilated distal venous outflow beyond. Doppler studies may demonstrate high-flow regions and magnetic resonance imaging (MRI) typically demonstrates flow voids ( Fig. 1A–D ).
Fig. 1.

( A ) Clinical photograph of a 48-year-old man with arteriovenous malformation (AVM) of the left orbit, involving medial preseptal soft tissues. ( B ) T1-weighted magnetic resonance imaging (MRI) with fat saturation illustrates flow voids. ( C ) T1-weighted MRI with contrast demonstrates diffuse contrast enhancement. ( D ) In the cranial projection of the left external carotid artery angiogram, the AVM's primary supply arises from the ophthalmic artery, superficial temporal artery and facial artery (angular branch).
AVMs can be classified as diffuse or localized based on the pattern of arterial inflow. Localized lesions are characterized by a few well-defined inflow vessels, while more diffuse lesions are fed by widespread, indistinct vascular networks. This distinction has implications for therapy, whether endovascular or surgical.
Management Strategies
Management of orbital AVMs can be challenging due to the threat of hemorrhage, vascular occlusion, damage to surrounding structures, and tissue necrosis. These considerations loom large in management decisions and fortunately many cases can be followed without any intervention. Progressive disfiguring and/or debilitating proptosis, pain, or visual compromise are indications for intervention. AVMs are typically managed with endovascular embolization and/or surgery, although newer biological therapies hold some promise.
Selective embolization can be a primary management strategy or utilized to augment surgical resection. Embolization aims to close-off arterial inflow, and thereby block the abnormal vascular diversion. The dual challenges of endovascular therapy are to deliver embolic material into the lesion while avoiding embolization of normal downstream structures, and to place it in such a manner as to completely block all vascular inflow. This can be precarious in the periocular region, as unpredictable flow and anastomotic irregularities can permit flow through to critical structures, such as the eye and extraocular muscles, with obvious dysfunctional consequences. Additionally, lesions fed primarily via internal carotid artery (ICA) circulation that will often require canalization of the ophthalmic artery and thrombosis of this vessel is a persistent risk.
Rarely is complete endovascular eradication of flow into the nidus possible, as these lesions are typically fed from a range of sources derived from both ICA and external carotid artery (ECA). Partial embolization alone is generally followed by long-term recurrence, with development of new feeding vessels of a smaller caliber and from more sources, thus converting a potentially localized lesion into a more diffuse AVM. Diffuse AVMs, whether primary or recurrent, are typically more difficult to manage. This is at least partially due to the impracticality of closing-off all source vessels, as well as the caliber of the vessels prohibiting endovascular access.
Surgical resection can be considered a primary therapy or utilized in conjunction with preoperative embolization. Embolization can improve hemostasis during the procedure and assist in identifying selective feeding vessels during surgery. Similar to embolization, better outcomes are associated with localized lesions. Diffuse AVM resection in the periorbital region is typically associated with significant morbidity related to disfigurement and dysfunction due to involvement of critical aesthetic and functional structures. Again, as with embolization, partial treatment often results in further angiogenesis and recurrence. 14 The first attempt is often the best opportunity for long-term management, and utilizing a combined approach with preoperative embolization and wide, en bloc resection may be the best strategy if safely possible. 6 Due to the rarity of these lesions, most recommendations for treatment of AVM in the orbital region are not evidence based.
The use of antiangiogenic drugs, such as bevacizumab, has emerged as a promising systemic therapy. The appeal of such a strategy is clear and based on the pathophysiology involving recruitment and expansion of the lesions through neovascular processes.
The broadest available literature on the use of antivascular endothelial growth factor (anti-VEGF) agents in AVM is related to bevacizumab treatment in HHT. HHT is a multisystemic autosomal dominant vascular dysplasia characterized by AVMs in the lungs, liver, and brain. 15 16 17 Research suggests that VEGF is overexpressed in this population, 18 and clinical studies have shown bevacizumab to be useful in reducing the need for transfusions due to epistaxis and gastrointestinal bleeding, 16 19 20 21 22 as well as in mitigating high-output cardiac failure. 23 This strategy may be useful in periorbital AVM that are unrelated to known genetic disease. Further research in this exciting area of therapeutics is clearly required.
Lymphatic Malformations
When considering lymphatic malformations, morphology of the lesion becomes important in understanding the etiology, pathophysiology, and management. Lymphatic malformations can be classified morphologically into macrocystic and microcystic forms. Systemic classifications for macrocystic malformations typically cite 1 cm 3 as being the size threshold for a cyst to be “macro.” In the orbit, it may be useful to rather consider macrocystic to be any cyst that can be individually penetrated by a needle. Microcystic lesions would be below this threshold. Most lesions will contain elements of both. For the purposes of discussion, these entities will be described separately, as they tend to present different clinical challenges and management strategies.
Macrocystic Malformations
Clinical Presentation
Macrocystic lesions act in a similar manner to an encapsulated mass in the orbit, they tend to produce proptosis with minimal infiltrative features ( Fig. 2A ). Patients often present in childhood with proptosis expanding with age. If anteriorly located, a blue hue can be visualized through the skin. Conjunctival lymphangiectasis may be present. Sudden onset of painful proptosis in the context of a known or unknown lesion can be a dramatic presentation due to spontaneous intralesional hemorrhage. This event may lead to orbital dysfunction and optic neuropathy.
Fig. 2.

( A ) A 17-year-old man with a left orbital macrocystic lymphaticovenous malformation. Axial ( B ), coronal ( C ) and sagittal ( D ) T1-weighted FS postcontrast magnetic resonance imaging (MRI) depicts a macrocystic multilobuled intraconal lesion surrounding the optic nerve. ( E ) Histopathology of a different patient affected by a microcystic lymphatic malformation demonstrating serous and venous spaces with vascularized stromal follicles and inflammatory fibrosis (hematoxylin and eosin [H&E] staining ×4). ( F ) Extensive vascular endothelial growth factor (VEGF) stromal staining and of a budding element (arrow; VEGF staining × 4). ( G ) Neovascularization in lumen and stroma. ( H ) Direct puncture contrast injection of a different patient's macrocystic malformation demonstrates a component with distinct internal lymphatic network.
Imaging features are characteristic. MRI is the preferred imaging modality as the various components of the lesions can be well characterized ( Fig. 2B–D ). 24 Lesions are typically isointense to brain on T1 and highly hyperintense on T2. 25 Rim enhancement is common, although septations can enhance as well, creating a patchy pattern. 26 Layering within the cystic cavity may be present and represents blood products of various ages. Ultrasound can also be useful for more anterior lesions, demonstrating the cystic nature of the mass.
Pathophysiology
Pathologically, macrocytic malformations are characterized by large luminal spaces lined by mature lymphatic endothelium with relatively sparse stromal tissues. Larger vessel spaces may be surrounded by disorganized smooth muscle tissue. Lymphoid aggregates and adipocytes are commonly noted. Neovascular fronds can be found within luminal spaces rarely. The lesions are typically thin-walled and can be pseudoencapsulated. This may account for the relatively good orbital function often noted in patients with predominantly macrocystic lesions, despite widespread involvement of the orbit and often significant proptosis.
Management
Management is directed symptomatically. In many cases, observation is reasonable if orbital and visual dysfunction are absent. Periodic episodes of intralesional bleeding can be dramatic, with rapid increases in the volume of the lesion and associated proptosis, pain, and orbital dysfunction. These may be associated with upper respiratory infection. In such cases, emergency treatment may be warranted and may involve percutaneous or open surgical drainage of the so called “chocolate cyst.” This is usually a temporizing measure, as the cyst may return to prehemorrhagic size rapidly, and recurrent episodes of bleeding may become a chronic issue. Additional indications for management include excessive proptosis, cosmetic deformity, visual changes, or other functional problems.
There is a paucity of literature specifically focused on management of macrocytic lymphatic malformations of the orbit, as most series combine microcystic and macrocystic lesions. Discussion regarding treatment recommendations remains somewhat anecdotal for orbital disease.
Intervention may be surgical or nonsurgical. Surgery is long established as a challenging endeavor due to the thin walled, easily collapsible nature of the malformation. This approach is associated with high rates of both recurrence and collateral damage to associated structures. 27 Augmenting surgery by injecting fibrin or another glue intraluminally has been proposed as a strategy to ease identification and dissection of the lesion; however, this approach has not gained widespread adoption. 28
Conceptually the primary clinical problem relates to mass effect from the large luminal spaces, as motility and other orbital functions can be relatively preserved. Therapy directed at closing the fluid-filled spaces and reducing the lumen-to-stroma ratio could be expected to reduce symptomatology without subjecting the orbit to the risks of direct surgical intervention. Various sclerotherapy agents have been utilized for this purpose including sodium tetradecyl sulfate, sodium morrhuate, ethanol, picibanil (OK-432), bleomycin, and doxycycline. Selecting the appropriate medication involves balancing endothelial toxicity of the material and secondary inflammation may spawn. More toxic chemicals are more inflammatory. A more pronounced inflammatory response has the potential for spillover toxicity to critical structures (optic nerve, muscles, and globe) and the development of an acute postprocedure compartment syndrome. For these reasons, peripheral and smaller lesions may be more appropriately treated with more toxic materials, such as ethanol, while deeper orbital lesions may be better approached with less inflammatory substances such as bleomycin.
Repeated cycles of expression and irrigation are ideal when possible, as this allows a greater intralesional concentration of active ingredients (undiluted by lesional fluid content). 29 This of course is not always possible, as the lumen can collapse and block outflow and/or the fluid can be turbid and difficult to express. If very little outflow occurs from the lesion, leaving sclerosant within it can have lasting effect over hours or even days.
Percutaneous therapy is ideal, allowing for preservation of normal orbital anatomic planes and minimizing collateral surgical damage. This can be performed unguided or with ultrasound or fluoroscopic guidance. Fluoroscopy is our preferred method, as this provides an opportunity to confirm lesion filling and flow of sclerosant within the vascular spaces. Additionally, intralesional connection between cystic pockets can be investigated, and fully separate sacs can be treated individually ( Fig. 2B–D ).
Microcystic Malformations
Clinical Presentation
The presentation of predominantly microcystic lesions tends to be more akin to an infiltrative process, as opposed to the mass effect in macrocystic lesions. In this way, orbital dysfunction is a more prominent aspect of the clinical manifestation. Slowly increasing proptosis with elements of motility restriction, strabismus, pain, and vision loss are commonly evident at presentation. 27 29 As lesions typically present in childhood, amblyopia can be an issue as well. Additionally, due to the presence of lymphoid tissue in stroma-rich microcystic lesions, expansion of the mass can occur in the context of lymphatic proliferation, for instance in the setting of a viral infection. Again, it is notable that most microcystic lesions do contain macrocystic components. Thus, clinical presentation can overlap, for instance with noninfiltrative mass effect or intralesional hemorrhage.
Microcystic lesions can extend beyond the orbit and often involve structures, such as the eyelid and periorbital fossae (pterygopalatine, infratemporal, etc.). Associated intracranial vascular anomalies are not uncommon. 30 Examination of periorbital structures is critical in both clinical and radiologic evaluation, and in many cases, patients may present to the head and neck and/or neurologic services primarily.
Pathophysiology
Microcystic lymphaticovenous malformation (LVM) are indistinguishable pathological malformation from macrocystic lesions in terms of the stromal and endothelial cell populations. 31 The main differences are related to the relative preponderance of stromal and associated cellular elements in the microcystic lesions, and conversely, the relative paucity of luminal fluid components. This ratio is central in defining the clinical behavior, biological processes responsible for growth, and management options. Macrocystic elements tend to expand by increasing luminal volume. Although they do contain cellular components, the processes that drive growth in these regions are proportionally less important, simply due to the relative paucity of such elements. The opposite is true of microcystic lesions, in which growth is related to cellular proliferation, differentiation, and cytokine milieu. 32
The development of lymphatic vessels is thought to be regulated by Prox1, a transcription factor that determines vascular endothelial differentiation to the lymphatic vascular fate. 33 34 VEGF receptor-3 (VEGFR-3) is upregulated in Prox1 positive cells, and interactions between VEGFR-3 and VEGF-capillary (VEGF-C) are critical in the development of lymphatic systems. These systems are also known to be critical in tumor lymphangiogenesis 35 36 and are likely to be important for growth and differentiation of lymphatic malformations. 37 This pathway may be involved in the growth of microcystic malformations. It is known that VEGF and VEGFR are expressed extensively in these lesions 38 ( Fig. 2E–G ), particularly in recurrences, 39 and that such interactions can lead to differentiation of local progenitor cells and/or blood-derived pluripotent elements. 40 This may explain the infiltrative nature of microcystic malformations. As the new cellular elements become integrated into the lesion, there may be overlap within the local tissues from which the cells are derived.
It is also interesting to note that mechanical forces can play a role in lymphatic tissue maintenance. Specifically, in the presence of flow, the mechanical forces can cause lymphatic endothelium to differentiate into a venous phenotype. 41 This dedifferentiation may explain the common finding of venous components being evident in drainage basins (typically apical in the orbit), later in the life span of lymphatic malformations ( Fig. 3 ). All of these cellular and physiologic processes provide targets for management.
Fig. 3.

A 63-year-old woman with left orbital lymphaticovenous malformation. ( A ) Axial computed tomography (CT) with contrast at 30 seconds demonstrates deeper vascular elements, and ( B ) Valsalva augmented contrast enhanced sequence of the same orbit at 70 seconds demonstrates distensibility of deep venous elements in the apex (circles). Incidental phleboliths (arrows).
Management
A range of surgical and nonsurgical options have been proposed for the management of microcystic lymphatic malformations. Observation is a potential strategy, particularly in smaller and minimally symptomatic lesions. This is particularly appealing in the context of surgical options, which have not proven to be highly efficacious and can be associated with functional complications.
In general, as lymphatic malformations tend to be unencapsulated and infiltrative, it is technically challenging to completely excise these lesions without significant collateral damage to locally involved tissues. 42 43 Recurrence is common and related to regrowth from residual elements. Some recommend periodic debulking of symptomatic components over time, and this may be a reasonable strategy. 44 Extreme cases with persistent pain in the context of a blind eye have been managed with exenteration. If complete resection is to be a realistic goal, the lesion would ideally be small and anterior, and in these cases, the first opportunity at intervention is likely the best opportunity. 45
Percutaneous sclerosing techniques have classically aimed to shrink the vascular lumen likely the same manner as with macrocystic malformations. 46 However, this strategy can be challenging due to the morphologic characteristics of the lesion, as the vascular spaces make up a relatively small proportion of the malformation, rendering full penetration of all luminal components difficult to ensure. Some lesions do carry an internal vascular network, which can be accessed from a single point ( Fig. 2H ). However, in most cases flow is so low and indirect that a single-puncture position is unlikely to fill all vascular spaces. Some have suggested using long infusion times, 47 or gravity-dependent instillation to presume penetration. 48 Utilizing multiple puncture sites may also be an appropriate approach. Regardless of the strategy utilized, often multiple sessions are required over time. 29
Conceptually, the sclerosis approach may address flow-mediated dedifferentiation and some of the endothelial signaling aspects of lesion growth; however, this does not directly address the biological signals for cellular recruitment and growth. Targeting the VEGF pathway has several theoretical advantages related to both the pathophysiology of lymphatic malformation formation and the relative availability and safety of anti-VEGF medications. Bevacizumab is a recombinant humanized monoclonal antibody binding VEGFA that has been used widely in oncology and in the treatment of retinal vascular disorders. This medicine primarily targets VEGFR-1–VEGFA pathways, although there is some crossover efficacy in VEGFR-3 signaling. 49 Combination therapy with both bleomycin and bevacizumab has shown some promise in local treatment of recalcitrant microcystic and combined lymphatic malformations in the orbit. 48 50 There may also be a role for systemic bevacizumab therapy in these disorders; however, this has been sparsely investigated. VEGF-C targeting medications could theoretically also be useful for lymphatic malformation management and some are in early stages of preclinical development.
Few cases will require an open approach. Treatment can be performed utilizing ultrasound guidance, 29 or simply based on feel and knowledge of the local anatomy in larger, more anterior lesions. 46 The use of fluoroscopy has many advantages. It allows for precise targeting of lesion components in real time, the distribution of medication throughout the lesion can be assessed directly, and multiple puncture sites can be utilized to progressively fill the lesion. Further, higher flow regions can be identified and avoided, thus limiting endovascular downstream collateral damage due to unintended sclerosis.
Due to the challenges in treating these lesions locally with surgery and/or highly inflammatory localized therapies, a purely systemic approach for management is attractive. One intriguing therapy is sirolimus. Research has emerged suggesting that the mechanistic target of rapamycin (mTOR) pathway may play a significant role in lymphatic malformation pathogenesis. 51 Sirolimus is thought to have an effect on lymphatic malformation maintenance and growth by inhibiting this pathway. 52 This medication has been utilized to manage a range of lymphatic malformations in the systemic vascular literature, 53 54 and a few case reports of its use in orbital malformations have shown promising results. 55 56 More research is clearly required, as it is an appealing therapy in that it would remove the risk of orbital complications related to localized treatments.
Venous Malformation
Venous malformations of the orbit present unique challenges from those affecting other areas of the body. Due to the confined space of the orbit and the unidirectional option for volume expansion, the characteristics of distensibility and flow become more critical in understanding both the symptomatology and strategies for management in orbital venous disease. A distensible venous malformation expands in size during periods of increased venous pressure. Clinically, this is demonstrated by a Valsalva's maneuver; however, a patient perspective may manifest during exercise, bending over, or most concerningly when supine during sleep. The degree, speed, and frequency of distension-emptying cycles will determine patient symptoms and guide treatment decision-making.
Clinical Presentation
In normal, resting, and upright positions, patients may be asymptomatic. However, with increased venous pressure, the lesion can expand, compressing, and distorting local structures, leading to symptoms such as diplopia and vision loss. Further, distension can be painful, likely due to a combination of nervi vasorum stimulation, increased orbital pressure, and direct compression of local structures. Repeated cycles of expansion and regression over many years can further lead to fat atrophy and bony remodeling. These changes simultaneously increase orbital bony volume and decrease the volume of the orbital contents leading to enophthalmos which can be pronounced.
Diagnosis can be made clinically in most cases; however, imaging is typically required to confirm diagnosis, characterize the components of the lesion, define spatial relationships, and understand connections to periorbital structures. MRI imaging can be useful in defining the soft tissue components of the lesion, particularly if there are lymphatic elements ( Fig. 4A ). Noncontrast computed tomography (CT) can provide some additional information regarding bony remodeling and potential phlebolith formation. As the central characteristics defining these lesions are flow and distensibility, time resolved sequencing with Valsalva's augmentation is extremely useful, particularly in treatment planning.
Fig. 4.
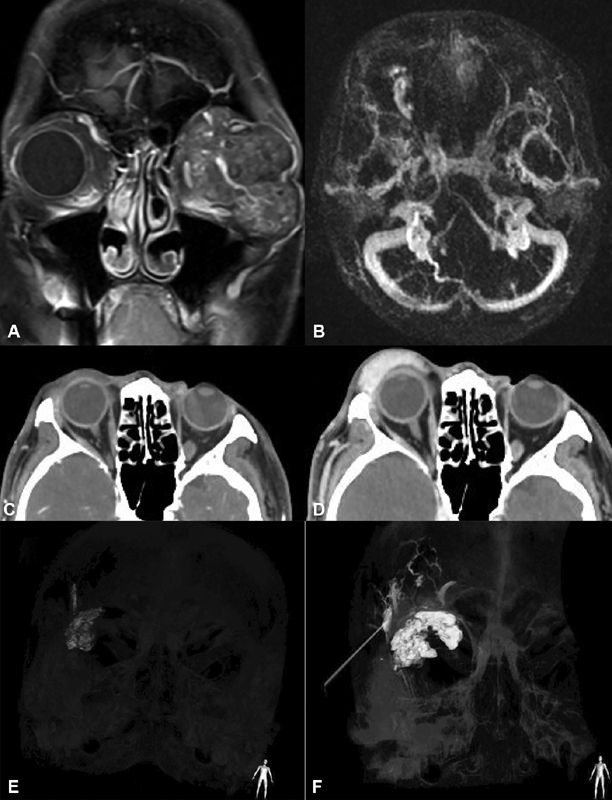
( A ) Left orbital lymphaticovenous malformation with extensive lymphatic elements. ( B ) Valsalva's augmented time-resolved angiography with interleaved stochastic trajectories (TWIST) of a different patient demonstrating right orbital venous malformation filling in the early venous phase. ( C–F ) Images of a different patient with right orbital venous malformation extending into the temporalis fossa. ( C ) Dual-phase axial CTA with Valsalva's maneuver at 30 seconds demonstrating minimal thickening of the eyelid. ( D ) Dual-phase computed tomography angiography (CTA) with Valsalva at 70 seconds demonstrating massive expansion of anterior orbital lesion. ( E ) Three-dimensional (3D) reconstruction after the first layer of deep glue placed percutaneously; ( F ) after second layer of glue application demonstrating expansion of the anterior and posterior orbital components, in addition to intraosseus and temporalis components.
Time-resolved MRI sequences with spatial resolution in the range of 1 to 1.5 seconds can provide information regarding inflow 57 ; however, as these sequences obtain full-head volumes with short acquisition time, the spatial resolution is generally poor ( Fig. 4B ). Additionally, the gadolinium does not wash out during the sequences and thus outflow is difficult to define.
Dual phase CT angiography (CTA) with Valsalva's maneuver is a useful sequence in which CT images are obtained after contrast infusion and again at 70 seconds during the venous phase with the patient performing the Valsalva maneuver. 58 These sequences have adequate time resolution for the venous structures of interest and provide excellent spatial resolution during Valsalva's maneuver. Lesions unidentifiable on nonaugmented sequences can appear much larger on Valsalva's augmentation ( Fig. 4C,D ). This can be useful in planning for invasive angiography which is the gold standard for defining flow characteristics. Having a good sense of the spatial distribution for these lesions is ideal prior to interventional assessment to plan for single-session investigation and intravascular management, followed, if needed, by surgery either in the same anesthesia session or soon thereafter.
Management
Management is generally focused on restricting cycles of distension. Although sclerotherapy has been utilized systemically for this purpose, 59 due to the larger doses required and risk to surrounding structures precluding the use of the more effective (and inflammatory) agents, this strategy has not proven to be highly effective in the orbit. In our series, we have noted that all orbital venous malformation cases treated with sclerotherapy have eventually recurred, requiring further therapy. The most effective therapy to date is surgical excision.
Unlike lymphatic malformations, isolated venous malformations typically do not invade local structures and can be separated bluntly from orbital tissues. The vein wall acts as a capsule in a sense. There are, of course, exceptions to this characterization, particularly in ectatic venous networks (type-IV malformations) 60 that may not have a defined saccular component and in cases of combined microcystic LVMs. However, in most cases, a central distensible component can be treated as a potential mass. In most circumstances, it is deflated and does not act mass like, unless filled either with blood or another substance.
The central challenge to surgical excision is, of course, bleeding risk. Although unlikely to be explosive, in the context of a confined, fat-filled space, moderate bleeding can make surgical manipulation in the orbit challenging. Further, the thin-walled vessels often respond poorly to cautery, tearing with energy application and leading to further bleeding. Filling the luminal space with a hemostatic substance can be an effective strategy. 61
Endovascular embolization of various vascular anomalies is a well-known treatment for vascular diseases of many types. Filling of the endovascular space is accomplished most commonly utilizing one of two substances, n-butyl cyanoacrylate (nBCA) glue, or onyx. Both are effective in filling the space, reducing flow, and leading to clot formation within the vascular sac. In the context of surgical planning, nBCA may be preferred as onyx contains tantalum which can spark when coming in contact with monopolar electrocautery during surgery. Both have been used widely in the endovascular literature. 62 63 The lesion, once filled, will act as a mass and can then be surgically excised.
There are several technical advantages to this “glue and excise” approach. Under normal circumstances, venous malformations may be flat and flaccid. Intraluminal glue expands the lesion, making it easier to identify in the orbit, and allows for stability when dissecting free of orbital attachments ( Figs. 5 , 6 ). Additionally, the lack of flow within the lesion is forgiving in case small tears in the fragile wall are made. Without bleeding risk, these lesions can be segmented or decapitated safely to access deeper components in the orbit, where space is at a premium. This is often necessary as the lesion can expand quite significantly with glue ( Fig. 4E, F ) and may be much larger at surgery than on imaging. Additionally, as lesions expand with gluing, additional components may be identified and controlled. This is particularly important for lesions with intraosseous components, as these spaces can present challenges for traditional hemostasis due to the rigid bony channels surrounding the ectatic vessels.
Fig. 5.

( A ) Noncontrast T2-weighted magnetic resonance imaging (MRI) demonstrating a tear shaped thrombosed venous malformation in the right medial orbit and ( B ) surgical view in which the lesion presents as a large, smooth red mass (star).
Fig. 6.

Transcutaneous upper eyelid orbitotomy for excision of a previously glued right vascular malformation. Lesion is ( A ) decapitated and ( B ) segmented.
Safely introducing embolic substances into venous lesions while minimizing inadvertent flow of embolic material to downstream structures can be achieved percutaneously in most cases by working in conjunction with a skilled and creative interventionalist. A range of strategies can be employed. The selection of appropriate methodology depends mainly on the physiology and flow characteristics of the lesion.
Low flow lesions in which Valsalva's maneuver reveals a slow fill and empty cycle can often be managed with direct puncture techniques. Intraoperative Valsalva's maneuver can be achieved by increasing positive end-expiratory pressure (PEEP), thus raising the intrathoracic pressure and decreasing venous return. This leads to expansion of the malformation and a larger target for percutaneous puncture. Superficial lesions can often be accessed with butterfly needles, while deeper lesions can be reached with spinal-type needles that can be custom shaped based on location ( Fig. 7A–D ).
Fig. 7.
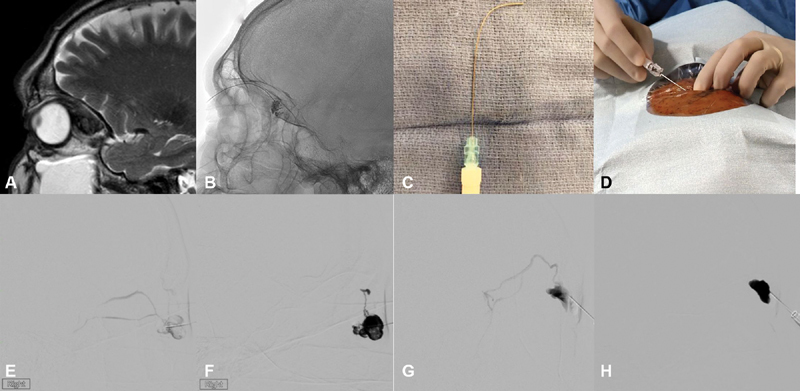
( A ) Sagittal T2-weighted magnetic resonance imaging (MRI) depicting a localized intraconal vascular malformation in the superior orbit. ( B ) Percutaneous sclerotherapy was accessed from a single point using a curved needle ( C ) . ( D ) External photograph of orbital percutaneous sclerotherapy. A different patient with ( E ) early outflow managed with rapidly polymerizing glue; ( F ) with n-butyl cyanoacrylate (nBCA) extending into one of the outflow vessels proximally. A different patient with ( G ) filling of an outflow vessel and slowly polymerizing glue application; ( H ) later filling of the lesion without extension into proximal outflow.
Lesions in which outflow is early after dye injection may be suited to a faster polymerizing mixture of nBCA and lipiodol (1:1). In this case, although outflow may fill with glue, it will rapidly polymerize in the proximal outflow and avoid inadvertent flow of embolic material downstream ( Fig. 7E, F ). The remainder of the lesion can then be backfilled. Lesions with slower outflow can be managed with a more slowly polymerizing mixture (3:1 or 4:1), allowing for a longer working time and more complete filling ( Fig. 7G, H ).
Lesions with faster outflow may require specific outflow management, as polymerization may not be fast enough to occur in the proximal outflow before the embolic material is carried downstream. In these cases, transvenous access can be useful. Retrograde endovascular canalization can access the outflow without passage through the lesion, thus allowing the outflow to be treated primarily. This can be followed by direct percutaneous perforation and glue application proximal to that site. Outflow control can be achieved by temporarily inflating an endovascular balloon and then introducing embolic material through the central microcatheter ( Fig. 8 ). This is appropriate for large and fast outflow systems. It is important to note that nBCA can stick to the plastic balloons, and onyx may be more appropriate for at least the outflow embolization component. In other cases in which the outflow is slower and more focal, the embolic material can be introduced into the lesion directly from the microcatheter in a retrograde fashion.
Fig. 8.
Cranial projections of a balloon-assisted embolization at the venous outlet of a right orbital venous malformation. ( A ) Venography of the venous malformation outflow channel through the cavernous sinus. ( B ) Sagittal X-ray demonstrating inflated balloon (arrow). ( C ) Percutaneous direct puncture embolization of the venous malformation with onyx (arrow) obstructing outflow during anterior n-butyl cyanoacrylate (nBCA) application.
It should be noted that in cases of significant enophthalmos in the context of a venous malformation, it may be appropriate to leave the embolic material in situ and not to remove the mass surgically. This strategy would achieve the dual goals of reducing Valsalva's maneuver associated symptomatology and providing volume to a contracted orbit ( Fig. 9 ). Due to the pre-existing enophthalmos, the risk of orbital compartment syndrome would be extremely low.
Fig. 9.

Patient with left orbital venous malformation. ( A ) Preoperative external photograph demonstrates significant left enophthalmos. The patient underwent embolization with n-butyl cyanoacrylate (nBCA), and the glue was left in situ. ( B ) 2-month postembolization shows improvement in enophthalmos. The patient reported improvement in Valsalva's maneuver–induced pain and proptosis. Dual-phase computed tomography angiography (CTA) preembolization illustrates lesion expansion before ( C, D ) and during ( E, F ) Valsalva's maneuver.
Lymphaticovenous Malformations
Development of the venous and lymphatic systems are intimately connected. The chemical and mechanical microenvironment is critical in the determination of venous and lymphatic fate during vascular development, and as noted above, this cross-differentiation process may proceed throughout life, particularly in the context of a vascular malformation. It is thus not surprising that venous and lymphatic malformations occur as a spectrum of disease, an observation emphasized in the ISSVA classification. The divisions presented in this discussion may not accurately represent real world presentations, as microcystic, macrocystic and venous components often coexist in a single lesion.
For these cases, it may appropriate to consider the different aspects of the lesions as each relates to symptomatology, and then manage specific lesion components separately. Combination therapy is common in such circumstances and staging of therapeutics is often prudent.
Conclusion
Orbital vascular disease presents unique challenges to the skull base surgeon due to the confined bony space, proximity to critical structures with shared circulation, and fat-filled operative field. Through an understanding of lesion physiology and morphology, specific therapeutic approaches can be utilized to effectively manage symptomatology, while minimizing collateral damage. This goal can best be achieved in a multidisciplinary team environment by utilizing a range of systemic, minimally invasive, and surgical techniques.
Footnotes
Conflict of Interest None declared.
Pearls and Tips.
Arteriovenous malformations are classically managed via endovascular embolization of inflow vessels with or without surgery.
Arteriovenous malformations with diffuse inflow tend to recur and systemic antiangiogenic therapy may be a consideration.
Lymphatic malformations are distinguished morphologically as macrocystic and microcystic.
Macrocystic malformations maintain a large lumen-to-stroma ratio with minimal invasive features and can be treated with luminal sclerotherapy.
Microcystic lesions are characterized by greater cellular elements with a small lumen-to-stroma ratio and can be approached with multimodality management utilizing sclerotherapy and antiangiogenic substances. Rarely surgery is indicated in emergent conditions of rapid growth (hemorrhage and thrombosis).
Venous malformations are distinguished by distensibility that can be demonstrated both clinically and radiologically with Valsalva's maneuver.
Various endovascular techniques can be utilized to safely fill the lesion with embolic material, making subsequent surgical excision safer and more effective.
References
- 1.International Society for the Study of Vascular Anomalies ISSVA classificationAccessed May 5, 2020 at:issva.org/classification
- 2.Mulliken J B, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69(03):412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Garzon M C, Huang J T, Enjolras O, Frieden I J.Vascular malformations: part I J Am Acad Dermatol 20075603353–370., quiz 371–374 [DOI] [PubMed] [Google Scholar]
- 4.Jackson I T, Carreño R, Potparic Z, Hussain K. Hemangiomas, vascular malformations, and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg. 1993;91(07):1216–1230. doi: 10.1097/00006534-199306000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kim A W, Kosmorsky G S. Arteriovenous communication in the orbit. J Neuroophthalmol. 2000;20(01):17–19. doi: 10.1097/00041327-200020010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Warrier S, Prabhakaran V C, Valenzuela A, Sullivan T J, Davis G, Selva D. Orbital arteriovenous malformations. Arch Ophthalmol. 2008;126(12):1669–1675. doi: 10.1001/archophthalmol.2008.501. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell E L, Taylor G I, Houseman N D, Mitchell P J, Breidahl A, Ribuffo D. The angiosome concept applied to arteriovenous malformations of the head and neck. Plast Reconstr Surg. 2001;107(03):633–646. doi: 10.1097/00006534-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Qiao C, Richter G T, Pan W, Jin Y, Lin X. Extracranial arteriovenous malformations: from bedside to bench. Mutagenesis. 2019;34(04):299–306. doi: 10.1093/mutage/gez028. [DOI] [PubMed] [Google Scholar]
- 9.Pardali E, Goumans M J, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20(09):556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Govani F S, Shovlin C L. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet. 2009;17(07):860–871. doi: 10.1038/ejhg.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Olabi L, Polubothu S, Dowsett K. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest. 2018;128(04):1496–1508. doi: 10.1172/JCI98589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu J, Li Y, Hu Z. Notch1 and 4 signaling responds to an increasing vascular wall shear stress in a rat model of arteriovenous malformations. BioMed Res Int. 2014;2014:368082. doi: 10.1155/2014/368082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei T, Zhang H, Cetin N. Elevated expression of matrix metalloproteinase-9 not matrix metalloproteinase-2 contributes to progression of extracranial arteriovenous malformation. Sci Rep. 2016;6:24378. doi: 10.1038/srep24378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flye M W, Jordan B P, Schwartz M Z. Management of congenital arteriovenous malformations. Surgery. 1983;94(05):740–747. [PubMed] [Google Scholar]
- 15.Guilhem A, Fargeton A E, Simon A C. Intra-venous bevacizumab in hereditary hemorrhagic telangiectasia (HHT): a retrospective study of 46 patients. PLoS One. 2017;12(11):e0188943. doi: 10.1371/journal.pone.0188943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epperla N, Hocking W. Blessing for the bleeder: bevacizumab in hereditary hemorrhagic telangiectasia. Clin Med Res. 2015;13(01):32–35. doi: 10.3121/cmr.2013.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou G, Galorport C, Enns R. Bevacizumab and gastrointestinal bleeding in hereditary hemorrhagic telangiectasia. World J Gastrointest Surg. 2016;8(12):792–795. doi: 10.4240/wjgs.v8.i12.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadick H, Sadick M, Götte K.Hereditary hemorrhagic telangiectasia: an update on clinical manifestations and diagnostic measures Wien Klin Wochenschr 2006118(3-4):72–80. [DOI] [PubMed] [Google Scholar]
- 19.Al-Samkari H, Kasthuri R S, Parambil J G.An international, multicenter study of intravenous bevacizumab for bleeding in hereditary hemorrhagic telangiectasia: the InHIBIT-Bleed study Haematologica 2020(e-pub ahead of print) 10.3324/haematol.2020.261859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavan A, Schumann-Binarsch S, Luthe L. Systemic therapy with bevacizumab in patients with hereditary hemorrhagic telangiectasia (HHT) Vasa. 2013;42(02):106–110. doi: 10.1024/0301-1526/a000253. [DOI] [PubMed] [Google Scholar]
- 21.Iyer V N, Apala D R, Pannu B S. Intravenous bevacizumab for refractory hereditary hemorrhagic telangiectasia-related epistaxis and gastrointestinal bleeding. Mayo Clin Proc. 2018;93(02):155–166. doi: 10.1016/j.mayocp.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Lupu A, Stefanescu C, Treton X, Attar A, Corcos O, Bouhnik Y. Bevacizumab as rescue treatment for severe recurrent gastrointestinal bleeding in hereditary hemorrhagic telangiectasia. J Clin Gastroenterol. 2013;47(03):256–257. doi: 10.1097/MCG.0b013e3182688d49. [DOI] [PubMed] [Google Scholar]
- 23.Dupuis-Girod S, Ginon I, Saurin J C. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA. 2012;307(09):948–955. doi: 10.1001/jama.2012.250. [DOI] [PubMed] [Google Scholar]
- 24.Grob S R, Bokman C, Nathe C, Rootman D B, Feldman K A. Diagnosis and management of acute thrombosis in venous dominant orbital venolymphatic malformations. Ophthal Plast Reconstr Surg. 2020;36(04):359–364. doi: 10.1097/IOP.0000000000001553. [DOI] [PubMed] [Google Scholar]
- 25.Khan S N, Sepahdari A R. Orbital masses: CT and MRI of common vascular lesions, benign tumors, and malignancies. Saudi J Ophthalmol. 2012;26(04):373–383. doi: 10.1016/j.sjopt.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung E M, Smirniotopoulos J G, Specht C S, Schroeder J W, Cube R. From the archives of the AFIP: Pediatric orbit tumors and tumorlike lesions: nonosseous lesions of the extraocular orbit. Radiographics. 2007;27(06):1777–1799. doi: 10.1148/rg.276075138. [DOI] [PubMed] [Google Scholar]
- 27.Harris G J, Sakol P J, Bonavolontà G, De Conciliis C. An analysis of thirty cases of orbital lymphangioma. Pathophysiologic considerations and management recommendations. Ophthalmology. 1990;97(12):1583–1592. doi: 10.1016/s0161-6420(90)32370-9. [DOI] [PubMed] [Google Scholar]
- 28.Boulos P R, Harissi-Dagher M, Kavalec C, Hardy I, Codère F. Intralesional injection of Tisseel fibrin glue for resection of lymphangiomas and other thin-walled orbital cysts. Ophthal Plast Reconstr Surg. 2005;21(03):171–176. doi: 10.1097/01.iop.0000160594.06829.00. [DOI] [PubMed] [Google Scholar]
- 29.Hill R H, III, Shiels W E, II, Foster J A. Percutaneous drainage and ablation as first line therapy for macrocystic and microcystic orbital lymphatic malformations. Ophthal Plast Reconstr Surg. 2012;28(02):119–125. doi: 10.1097/IOP.0b013e318242ab0f. [DOI] [PubMed] [Google Scholar]
- 30.Bisdorff A, Mulliken J B, Carrico J, Robertson R L, Burrows P E. Intracranial vascular anomalies in patients with periorbital lymphatic and lymphaticovenous malformations. AJNR Am J Neuroradiol. 2007;28(02):335–341. [PMC free article] [PubMed] [Google Scholar]
- 31.Chen E Y, Hostikka S L, Oliaei S, Duke W, Schwartz S M, Perkins J A. Similar histologic features and immunohistochemical staining in microcystic and macrocystic lymphatic malformations. Lymphat Res Biol. 2009;7(02):75–80. doi: 10.1089/lrb.2009.0003. [DOI] [PubMed] [Google Scholar]
- 32.Nassiri N, Rootman J, Rootman D B, Goldberg R A. Orbital lymphaticovenous malformations: current and future treatments. Surv Ophthalmol. 2015;60(05):383–405. doi: 10.1016/j.survophthal.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Wigle J T, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98(06):769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 34.Wigle J T, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21(03):318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- 35.Skobe M, Hawighorst T, Jackson D G. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7(02):192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 36.Mandriota S J, Jussila L, Jeltsch M. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20(04):672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Corral I, Zhang Y, Petkova M. Blockade of VEGF-C signaling inhibits lymphatic malformations driven by oncogenic PIK3CA mutation. Nat Commun. 2020;11(01):2869. doi: 10.1038/s41467-020-16496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itakura E, Yamamoto H, Oda Y, Furue M, Tsuneyoshi M. VEGF-C and VEGFR-3 in a series of lymphangiomas: is superficial lymphangioma a true lymphangioma? Virchows Arch. 2009;454(03):317–325. doi: 10.1007/s00428-008-0720-8. [DOI] [PubMed] [Google Scholar]
- 39.Sidle D M, Maddalozzo J, Meier J D, Cornwell M, Stellmach V, Crawford S E. Altered pigment epithelium-derived factor and vascular endothelial growth factor levels in lymphangioma pathogenesis and clinical recurrence. Arch Otolaryngol Head Neck Surg. 2005;131(11):990–995. doi: 10.1001/archotol.131.11.990. [DOI] [PubMed] [Google Scholar]
- 40.Wu J K, Kitajewski C, Reiley M. Aberrant lymphatic endothelial progenitors in lymphatic malformation development. PLoS One. 2015;10(02):e0117352. doi: 10.1371/journal.pone.0117352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C Y, Bertozzi C, Zou Z. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest. 2012;122(06):2006–2017. doi: 10.1172/JCI57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schick U, Hassler W. Treatment of deep vascular orbital malformations. Clin Neurol Neurosurg. 2009;111(10):801–807. doi: 10.1016/j.clineuro.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Parentin F, Borzaghini L, Perissutti P. The role of ultrasonography in the diagnosis of orbital lymphangiomas. Ophthalmologica. 2001;215(03):238–240. doi: 10.1159/000050866. [DOI] [PubMed] [Google Scholar]
- 44.Tunç M, Sadri E, Char D H. Orbital lymphangioma: an analysis of 26 patients. Br J Ophthalmol. 1999;83(01):76–80. doi: 10.1136/bjo.83.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rootman J, Kao S C, Graeb D A. Multidisciplinary approaches to complicated vascular lesions of the orbit. Ophthalmology. 1992;99(09):1440–1446. doi: 10.1016/s0161-6420(92)31786-5. [DOI] [PubMed] [Google Scholar]
- 46.Schwarcz R M, Ben Simon G J, Cook T, Goldberg R A. Sclerosing therapy as first line treatment for low flow vascular lesions of the orbit. Am J Ophthalmol. 2006;141(02):333–339. doi: 10.1016/j.ajo.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 47.Da Ros V, Iacobucci M, Puccinelli F, Spelle L, Saliou G. Lymphographic-like technique for the treatment of microcystic lymphatic malformation components of <3 mm. AJNR Am J Neuroradiol. 2018;39(02):350–354. doi: 10.3174/ajnr.A5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdelaziz O, Hassan F, Elessawy K, Emad-Eldin S, Essawy R E. Image-guided percutaneous bleomycin and bevacizumab sclerotherapy of orbital lymphatic malformations in children. Cardiovasc Intervent Radiol. 2019;42(03):433–440. doi: 10.1007/s00270-018-2128-4. [DOI] [PubMed] [Google Scholar]
- 49.Liang W C, Wu X, Peale F V. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281(02):951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 50.Mustak H, Ugradar S, Goldberg R, Rootman D. Bevacizumab and Bleomycin combination for treatment of orbital lymphatico-venous malformation recalcitrant to sclerosing therapy alone. Clin Exp Ophthalmol. 2018;46(07):815–816. doi: 10.1111/ceo.13159. [DOI] [PubMed] [Google Scholar]
- 51.Luks V L, Kamitaki N, Vivero M P. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr. 2015;166(04):1048–1054.e1–5. doi: 10.1016/j.jpeds.2014.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo Y, Liu L, Rogers D. Rapamycin inhibits lymphatic endothelial cell tube formation by downregulating vascular endothelial growth factor receptor 3 protein expression. Neoplasia. 2012;14(03):228–237. doi: 10.1593/neo.111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams D M, Trenor C C, III, Hammill A M. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics. 2016;137(02):e20153257. doi: 10.1542/peds.2015-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lackner H, Karastaneva A, Schwinger W. Sirolimus for the treatment of children with various complicated vascular anomalies. Eur J Pediatr. 2015;174(12):1579–1584. doi: 10.1007/s00431-015-2572-y. [DOI] [PubMed] [Google Scholar]
- 55.Gildener-Leapman J R, Rosenberg J B, Barmettler A.Proptosis reduction using sirolimus in a child with an orbital vascular malformation and blue rubber bleb nevus syndrome Ophthalmic Plast Reconstr Surg 201733(3S suppl 1):S143–S146. [DOI] [PubMed] [Google Scholar]
- 56.Lagrèze W A, Joachimsen L, Gross N, Taschner C, Rössler J. Sirolimus-induced regression of a large orbital lymphangioma. Orbit. 2019;38(01):79–80. doi: 10.1080/01676830.2018.1436569. [DOI] [PubMed] [Google Scholar]
- 57.Kahana A, Lucarelli M J, Grayev A M, Van Buren J J, Burkat C N, Gentry L R. Noninvasive dynamic magnetic resonance angiography with time-resolved imaging of contrast kinetics (TRICKS) in the evaluation of orbital vascular lesions. Arch Ophthalmol. 2007;125(12):1635–1642. doi: 10.1001/archopht.125.12.1635. [DOI] [PubMed] [Google Scholar]
- 58.Heran M KS, Rootman J, Sangha B S, Yeo J M. Dynamic arterial and Valsalva-augmented venous phase multi-detector computed tomography for orbital vascular lesions: a pictorial review. Ophthal Plast Reconstr Surg. 2014;30(02):180–185. doi: 10.1097/IOP.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 59.De Maria L, De Sanctis P, Tollefson M. Sclerotherapy for low-flow vascular malformations of the orbital and periocular regions: systematic review and meta-analysis. Surv Ophthalmol. 2020;65(01):41–47. doi: 10.1016/j.survophthal.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Rootman J, Heran M K, Graeb D A. Vascular malformations of the orbit: classification and the role of imaging in diagnosis and treatment strategies*. Ophthal Plast Reconstr Surg. 2014;30(02):91–104. doi: 10.1097/IOP.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 61.Ramesh S, Duckwiler G, Goldberg R A, Rootman D B. Multimodality management of complex periorbital venolymphatic malformations. Ophthal Plast Reconstr Surg. 2019;35(04):387–398. doi: 10.1097/IOP.0000000000001294. [DOI] [PubMed] [Google Scholar]
- 62.Wu E M, El Ahmadieh T Y, McDougall C M. Embolization of brain arteriovenous malformations with intent to cure: a systematic review. J Neurosurg. 2019;132(02):388–399. doi: 10.3171/2018.10.JNS181791. [DOI] [PubMed] [Google Scholar]
- 63.Elsenousi A, Aletich V A, Alaraj A. Neurological outcomes and cure rates of embolization of brain arteriovenous malformations with n-butyl cyanoacrylate or Onyx: a meta-analysis. J Neurointerv Surg. 2016;8(03):265–272. doi: 10.1136/neurintsurg-2014-011427. [DOI] [PubMed] [Google Scholar]



