Abstract
This article traces the development of orbital surgery and its subsequent modifications. It also points out the importance of defining one’s goal before embarking on orbital surgery. Although generally considered part of ophthalmology, surgery on the orbit has been relatively neglected and not routinely practiced. This article reviews the history of development of orbital surgery, both the revolutionary ideas and the evolutionary changes. There are multiple orbital lesions that do not need to be treated with surgery at all. These days chemotherapy, radation therapy, or even immunotherapy may be more appropriate. The most common orbital pathology, that is thyroid orbitopathy, the physician needs to decide whether or not the orbit needs to be decompressed or whether there are problems related to motility that can be dealt with by eye muscle surgery.
Keywords: orbitototomy, fine needle aspiration biopsy, computerized axial topography, transconjunctival incisions, Krönlein's orbitotomy, orbital deconstruction
Introduction
Sir Isaac Newton has been quoted as having said that if he could see further, it was because he was able to stand on the shoulders of giants. One of the advantages of an interest in history is our understanding of how our history determines our future.
In one of his early monographs, Harvey Cushing, while discussing neurosurgery, the newest surgical subspecialty, referred to ophthalmology as the “oldest surgical subspecialty.” In Hirschberg's definitive work on the history of ophthalmology, 50% of medical practitioners in ancient Greece and Egypt considered ophthalmic problems as one of their primary concerns. It is not a coincidence that while most of medicine shares Latin roots, ophthalmology derives many of its words from the preceding Greek. Lacrimal surgery, in particular, was practiced in Egypt and Rome, and the suturing of wounds dates back to Babylon and the early Indian tradition. The orbit, however, was considered as Terra incognita . “Derangements of structures within the eye socket, in particular, received scant attention in the early literature. This is logical considering the infrequency of orbital disease and the inaccessibility of the socket in ancient times to all except anatomists and dissectors.” 1 During most of the Golden Era of Greek and Roman medicine, protrusions of the orbit, as with many other diseases, were treated by venesection and purging. As early as 1583, cupping as therapy found its way into illustrations.
In the Arabian and Muslim cultures, as recorded in the 10th century, local or topical treatment of the affected orbit was particularly risky because such administrations, if detrimental, might result in equivalent judgment being directed against an ocular appendage of the well meaning but unfortunate healer, Hammurabi's “an eye for an eye” ( Fig. 1 ).
Fig. 1.

Stele illustrating the Code of Hamurabi; copy at the Library of the History of Medicine, Kansas City.
Although proptosis was recognized in the Muslim literature as early as the 10th century as translated by Casey Wood, the treatments were somewhat wanting. “The face of the affected eye of the patient should be bathed with cold saltwater. If this treatment succeeds, well and good, if not, adjust the lid plate to the affected eye and leave the rest to God.” The earliest orbital procedures were enucleation and modified exenteration as illustrated by Bartish in 1583, 2 passing pieces of silk through the globe ( Fig. 2 ). It is not surprising, however, that limitations were substantial considering the lack of anesthesia ( Fig. 3 ).
Fig. 2.
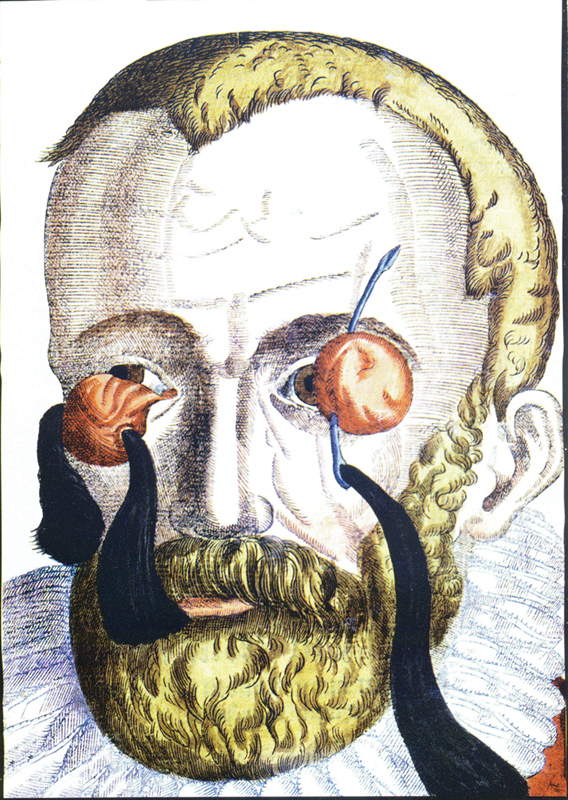
Enucleation as illustrated by Bartisch. 2
Fig. 3.
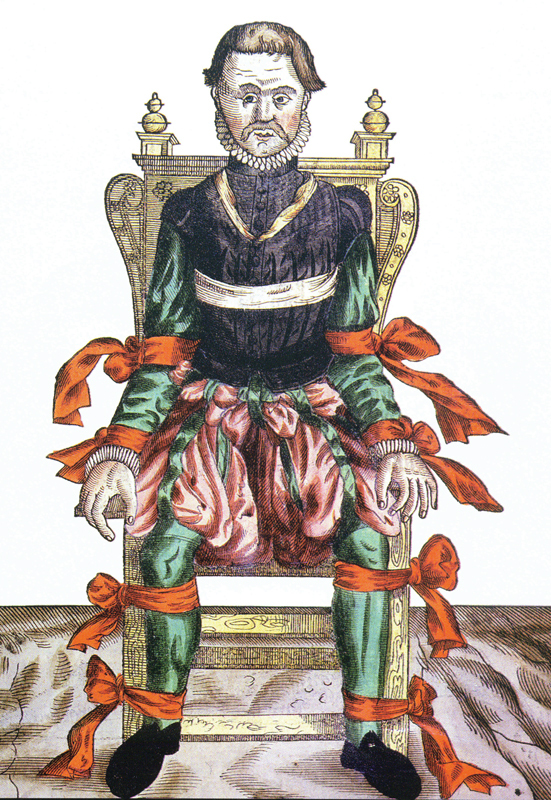
“Anesthesia” cica 1583. 2
It was not until the middle of the 19th century when Crawford Long ( Fig. 4 ) in March of 1842 and William Morton ( Fig. 5 ) in 1846 demonstrated the effects of ether in surgery. In 1884, Koller ( Fig. 6 ), on a suggestion from his roommate Freud, investigated the use of a derivative from South America, cocaine, which was found to be an effective topical anesthetic. Even more than the ophthalmoscope, the tremendous implications of this discovery was immediately recognized. 3 Shortly thereafter, regional injections of cocaine and later procaine permitted surgery in and around the eye without pain. 4 5
Fig. 4.

Crawford Long.
Fig. 5.

William Morton.
Fig. 6.
Karl Koller.
Symptoms of orbital pathology were well recognized even before the 19th century. While patients with orbital pathology could present with decreased vision, double vision, pain, and tearing, proptosis was the most important sign of orbital involvement ( Fig. 7 ). By the end of the 19th century, the signs and symptoms of orbital pathology could be summarized in texts. “The symptoms are more or less pronounced exophthalmos, paralysis of one or more of the ocular muscles from pressure on their nerves …, edema of the conjunctiva and eyelids, sometimes extending down upon the cheek or upon the temple, more or less immobility of the eye, intense pain in the ophthalmic branch of the fifth nerve …, mydriasis, engorgement of the retinal veins, papillitis, impaired vision, anesthesia of the cornea …, edema of the mastoid region, delirium, coma, and death.” 6 These symptoms could well be recognized in an orbital clinic today, although hopefully with less mortality. Signs of orbital pathology could be quantitated by the 19th century inventions, the Hertel or the Luedde exophthalmometer ( Fig. 8 ), measuring the extent of displacement of the globe from the lateral orbital rim.
Fig. 7.

Proptosis (and probable buphthalmos. 2
Fig. 8.
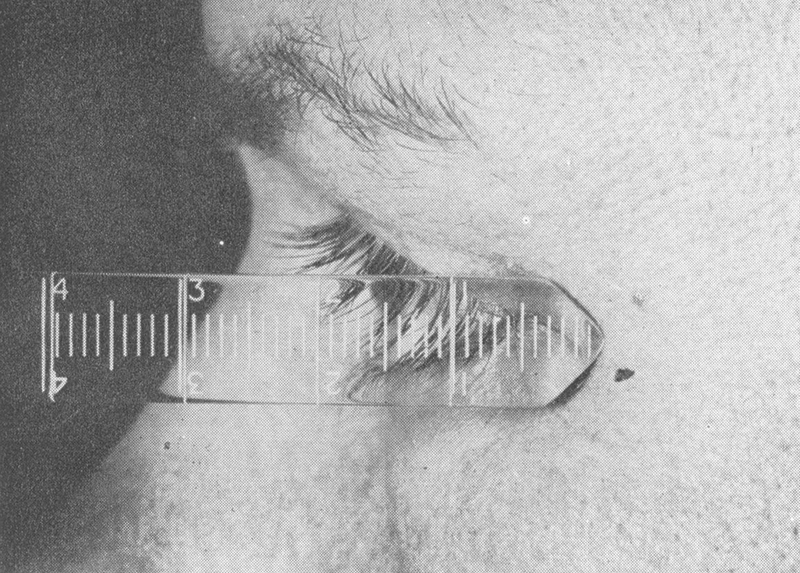
Luedde exophthalmometer. 7
Dystopia could be recognized in the displacement of the globe superiorly or inferiorly. 7 Acuity could be quantitated by Snellen's 1862 optotypes. Motility disturbance could be measured with Steven's ophthalmometer, and later with Hess' 1909 quantitative tangent screen.
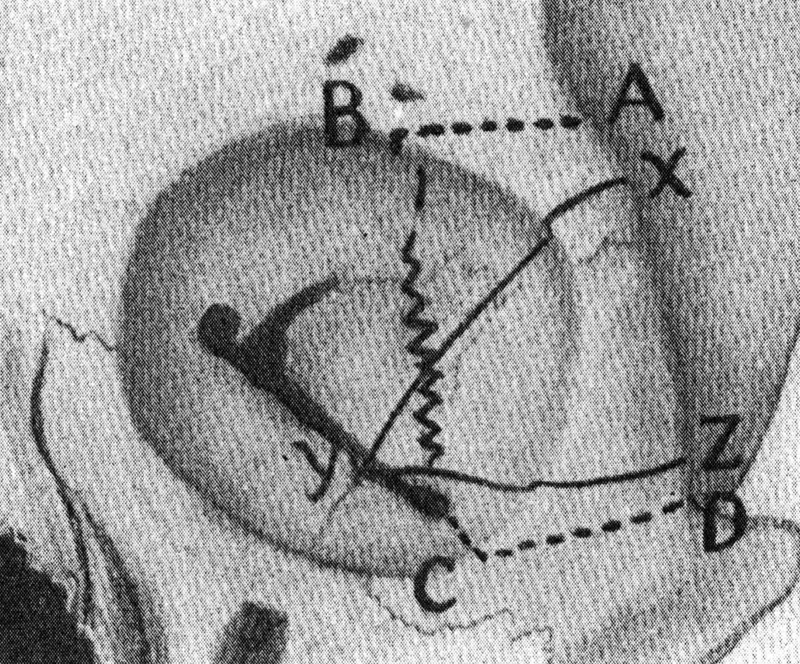
Diagnosis of orbital pathology ca. 1918 was summarized by Casey Wood in volume 12 of his American Encyclopedia and Dictionary of Ophthalmology, 8 indicating the importance of “history and inspection, palpation, percussion, transillumination, focal lighting, fluoroscopy, skiagraphy,” and, even then, the potential for an aspirating needle. Early attempts at surgery included Thomas Hope's transcutaneous operation in 1744, 9 John Jeffries' comment about transconjunctival operations in his lectures of 1831 10 and in an article by Khan and Albert in the Survey of Ophthalmology in 1988 on Willard Parker (the first to perform an appendectomy in the United States) who reported a transconjunctival operation that was complicated by symblepharon. 11 Despite these early attempts at surgery as summarized by Henry Stallard in his 1973 presidential address on the evolution of the lateral orbitotomy, 12 “before 1886 orbital surgery amounted to anterior incisions and crude exenteration for new formations, the cavity being packed with pieces of lint and cotton wool, impregnated with zinc chloride paste and nothing of reconstructive value was done for fractures.” It was the Swiss ophthalmologist Krönlein in 1886 (published in 1888 13 ) who described the use of a lateral orbitotomy for a patient with a dermoid cyst.
Krönlein's curvilinear incision proved to produce unacceptable scarring ( Fig. 9 ). Looking at the bony outline of the dermoid, Krönlein was able to connect the inferior orbital fissure with a Gigli's saw to the temporal fossa by removing the lateral orbital rim ( Fig. 10 ). Other seminal descriptions of orbital pathology and approaches included Louis de Wecker's descriptions of a transconjunctival approach for optic disc edema, and Herman Knapp's original 1874 description of a transconjunctival approach to an optic nerve tumor. 14 Five years later, he published this report in the Transaction of the American Ophthalmological Society using his finger to dissect out the tumor through a transconjunctival approach. 15
Fig. 9.
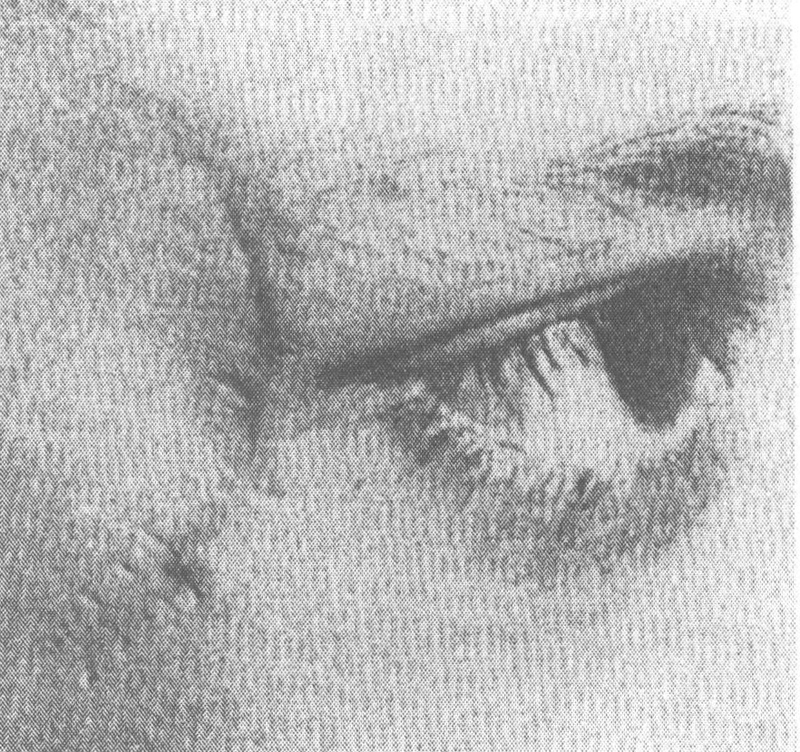
The Krönlein skin incision. 23
Fig. 10.
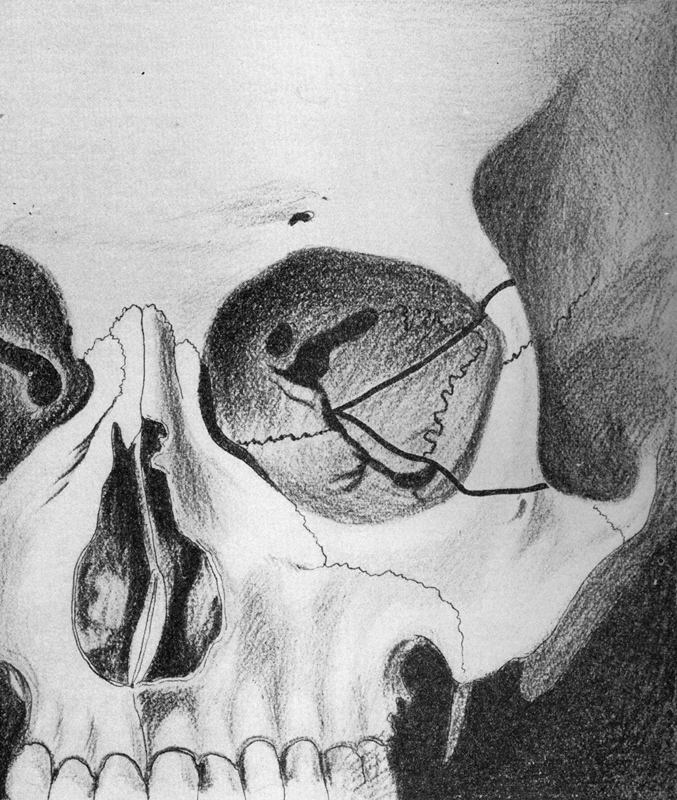
The Krönlein bone incisions. 36
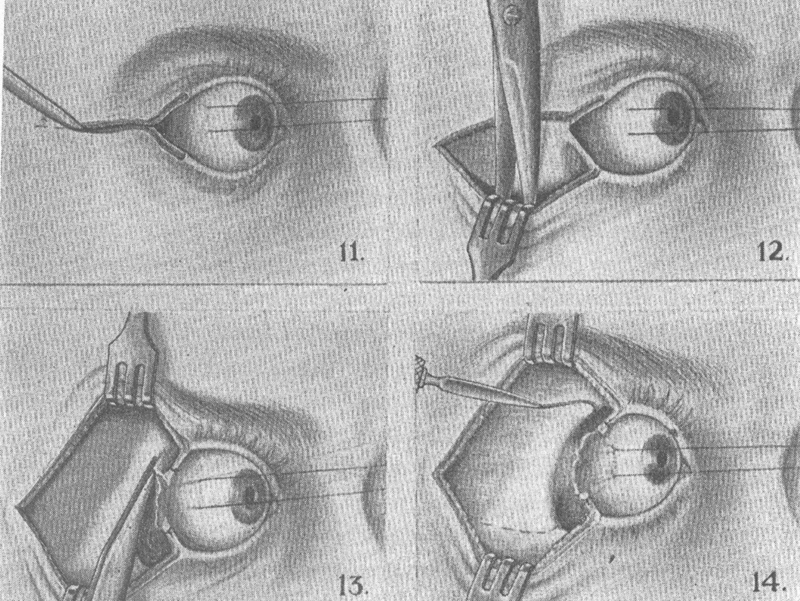
In 1941, Walter Dandy 16 reported his series of transcranial orbitotomies starting with the first case done in 1921. Twenty-four cases underwent surgery and an additional seven cases were observed. The spectrum of pathology in these patients, however, was unique, with nine patients having meningiomas, five diagnosed with Schüller Christian Disease (many of whom probably represented idiopathic orbital inflammatory disease), and only rare cases of primary orbital tumors. His approach involved a craniotomy with removal of the frontal bone, followed by an extradural dissection. The dura was subsequently opened to decompress the anterior cranial fossa by releasing cerebrospinal fluid from the parachiasmatic cistern. By removing the orbital roof, Dandy discovered that he had an unprecedented panoramic view of the orbital contents ( Fig. 11 ). In particular, the orbital apex and the course of the optic nerve could be easily traced.
Fig. 11.
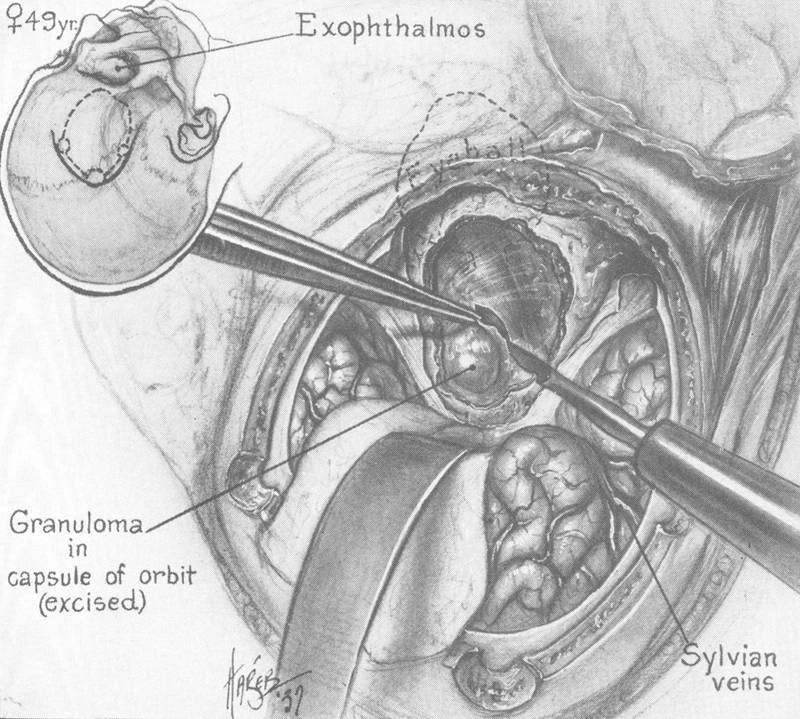
Transcranial orbitotomy. 16
Another seminal approach was summarized in a 1957 paper by Walsh and Ogura, 17 documenting the use of a transantral orbital decompression for malignant exophthalmos. This was not only useful for decompressing the orbit in cases of severe proptosis but would also allow access to lesions that were located in the inferior and medial orbit. An additional combination of an inferior approach through the sinuses was described by Conley in 1962, 18 involving a medial and inferior approach with a swinging cheek flap released by the Weber Fergusson incision. By removing the orbital floor, one could have complete access to the inferior orbit. Maxillectomy and ethmoidectomy could thus be combined with exenteration if necessary ( Fig. 12 ).
Fig. 12.
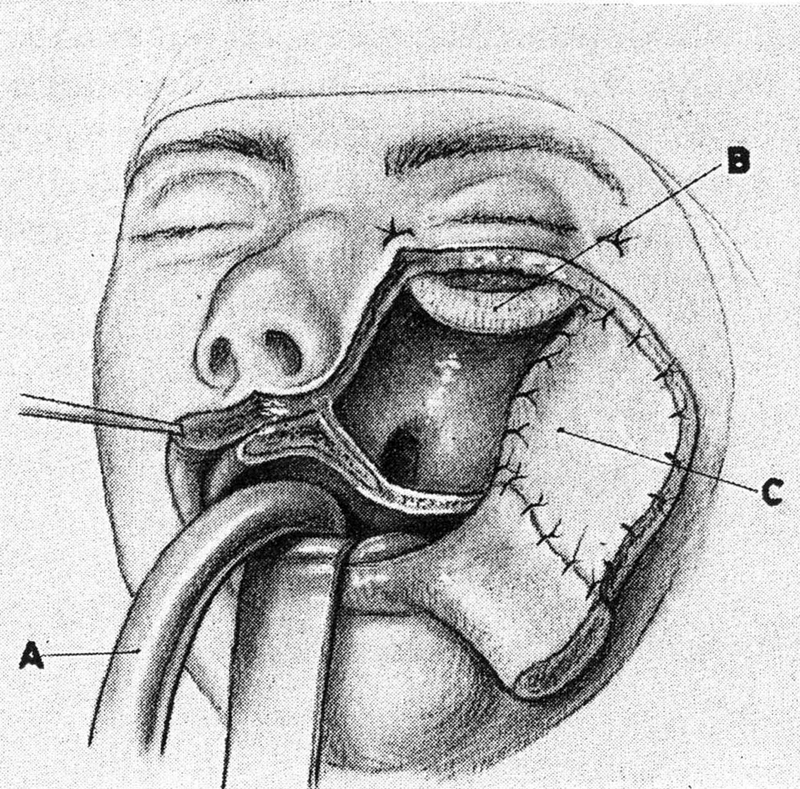
Transfacial Weber–Fergusson approach. 18
The lateral orbitotomy as described by Krönlein was gradually modified by several important players including Benedict, 19 20 who, following study at the Mayo clinic, spent most of his academic career in Minneapolis. There he described the use of an incision through the brow which allowed direct access to the superior and medial orbital rim ( Fig. 13 ).
Fig. 13.
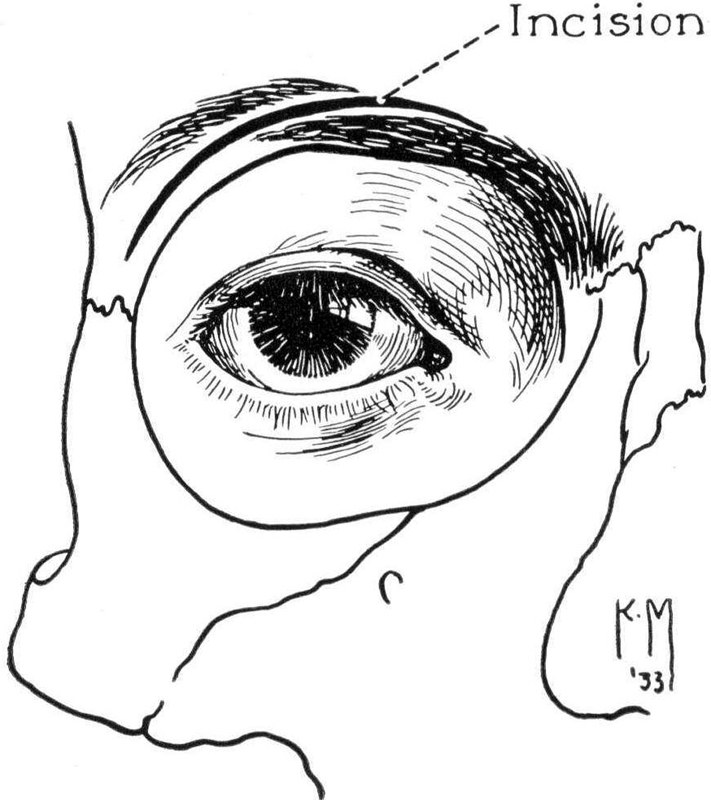
Benedict's brow incision. 29
F.A. Davis in 1940 described a similar inferior orbital rim approach 21 ( Fig. 14 ). The disadvantage of this approach, lymphedema due to interruption of drainage, led to movement of the skin incision to a subciliary location. 22
Fig. 14.
Davis' inferior rim approach. 29
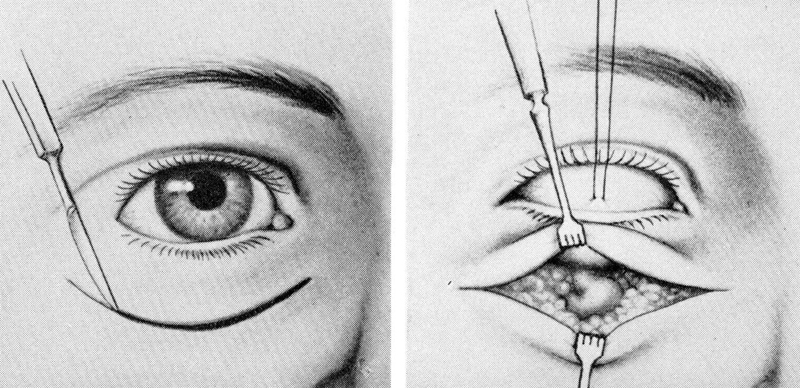
In his 1953 report in the Transactions of the American Ophthalmological Society, Berke described some of the most important techniques that are still employed in orbital surgery today. 23 These included the use of a linear lateral canthal incision with release of the superior and inferior heads of the lateral canthal tendon ( Fig. 15 ) combined with a change in the bony incision to two horizontal incisions through the rim ( Fig. 16 ). This permitted removal of a larger portion of the lateral orbital wall. Czermak, in 1905, 24 had discussed modifications to the lateral orbitotomy of Krönlein, extending the bony removal to include portions of the zygomatic arch and frontal bone ( Fig. 17 ).
Fig. 15.
The Berke lateral orbitotomy. 23
Fig. 16.
The Berke bone incision modifications. 23
Fig. 17.
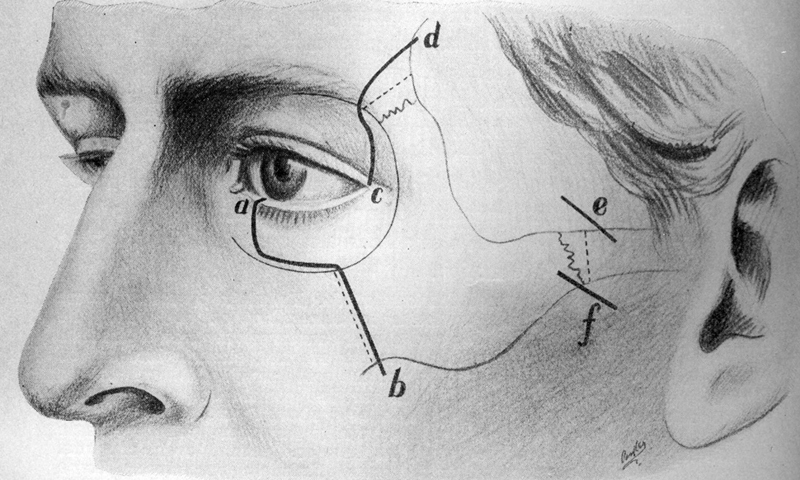
The Czermak bone modifications. 36
Although Stallard was to describe an S -shaped incision predating the later Wright incision in his initial textbook on eye surgery in 1947, 25 he still was using the more classical Berke incision. John Wright described shaving the brow and including an S -shaped incision from the lateral brow over the zygomatic arch extending 4 cm in length ( Fig. 18 ), expediting full access to the entire lateral orbital rim and hiding the skin incision within the skin natural creases around the orbit. 26 Interestingly, John Wright, as with others, did not close the posteriosteum, but did wire the bone flap with suturing of periosteum over the rim. Other investigators have used sutures, more recently plates, and some simply closed the periosteum leaving the bone in place without firmly fixing it. 27
Fig. 18.

Wright's S -shaped lateral orbitotomy incision. 26
The transcranial approach has also undergone evolution with John Jane's description of removing the orbital rim using a Gigli's saw which permitted a more basilar and thus direct approach 28 ( Fig. 19 ). Maroon and Kennerdell, in an article in Journal of Neurosurgery in 1984 described the use of advanced neurosurgical instruments for debulking the tumor, including the use of the cavitron ultrasonic surgical aspirator (CUSA) (an enlarged phacoemulsification unit), and the CO 2 laser. 27 They also designed a self-retaining retractor to assist in orbital surgery.
Fig. 19.
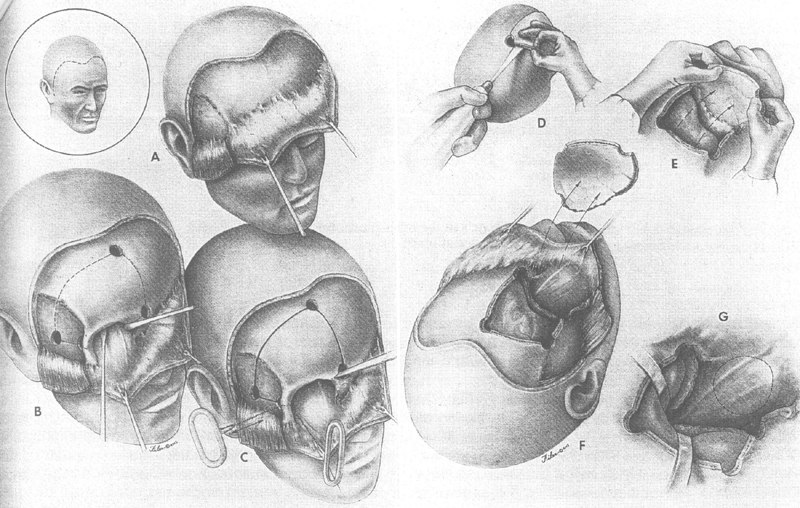
John Jane's orbital rim modification of transcranial approach. 27
It was Dandy's 1941 monograph that initiated a significant period of controversy. Dandy summarized his experience, “It was rarely possible for the operation to be certain whether or not the tumor also lies within the cranial chamber,” as so many of them, at least in his series, did. “Where tumor is confined to the orbit, this operation offered a far better exposure than the original Krönlein description. 16 ” Based on this and his improved visualization, he suggested that all orbital tumors be treated transcranially.
Stallard, in 1973, 12 mentioned the undue influence of Dandy. Because of his “dogmatic statement,” there followed a “dark period for the last 30 years in which neurosurgeons have staked a dominating claim in favor of the transcranial access to the orbit. The arguments that Stallard made against transcranial surgery including the potential for missing cases 25 as reported by him and also by Algernon Reese. In studying the pathology of tumors affecting the orbit and those lesions causing proptosis, Reese reported, in his 1951 monograph, on orbital tumors 29 that “the commonest cause for unilateral exophthalmos was endocrine ophthalmopathy,” which is well recognized today. Reese suggested that in the absence of evidence of intracranial extension or intracranial bone involvement, a transcranial operation should be avoided, arguing that proper treatment cannot be established without knowing the diagnosis. Reese, in a meeting, discussed the status of orbital surgery in 1964, 30 stating that “because the patient has unilateral exophthalmos, one cannot assume that it is due to a tumor, or to a tumor that lends itself to excision. I estimate that only one in six patients operated on has a localized encapsulated tumor that can be excised. On this premise then, five out of six operations by way of the cranium would be unnecessary except to obtain biopsy material.” Stallard and Reese 31 argued about the potential of a “stormy postoperative course with subsequent ptosis and damage to the superior rectus.” Worries about possible meningitis and rhinorrhea if the “sinus was entered” are rare.
Subsequent studies included those by Reese in 1951 29 which included 355 tumors of which 14% were hemangiomas, 9.6% pseudotumors, and only 5.6% meningiomas. Love's series in 1962 32 (from the department of neurosurgery at Mayo Clinic) included 30% meningiomas and 9% cavernomas, supporting the referral bias. Other authors have confirmed the relatively low incidence of intracranial lesions presenting as orbital expansile pathology. 33
The most recent series by Rootman published in 2003 34 included over 4,000 cases. Among the cases, 60% were related not to neoplasia but rather to inflammation with over half of them being secondary to thyroid orbitopathy. Tumors made up only 18% of cases with 2.2% meningiomas, 2.4% lymphomas, 1.2% hemangiomas, and 2.3% with secondary invasion.
Stallard 12 had pointed out that prior to 1940, lateral orbitotomy was rarely done in England. The orbit was clearly regarded as a surgical no man's land. Reese, writing in 1971, had suggested that no orbital surgery was done in New York in the 1920's other than exenteration, and it was not until 1940 that Krönlein's operation was done in New York hospitals. 12 28
Nevertheless, there remained champions of ophthalmic approaches to these tumors. Arnold Knapp, Herman Knapp's son, wrote in 1912 that the lateral orbitotomy was the best way of approaching orbital lesions because of its diagnostic ability, thus permitting the determination of the extent of orbital tumors before embarking on a full-scale attempt at removing them. 35 If a preliminary biopsy at the time of the original approach showed evidence of malignancy, then the procedure could be abandoned or exenteration could be performed. Stallard in 1973 12 felt that it was essential for any surgical operation to have adequate exposure, generally by the shortest and most direct route. He would have argued that the lateral wall was the most direct approach to all intraorbital lesions, minimizing the chance of “tissue damage and avoidance of injury to important structures.” One could easily, however, agree with Dandy, that the exposure from above was often far more expansive.
As early as 1911, in his two-volume textbook on ocular surgery, Casey Wood suggested that “in the removal of growths in this situation one should bear in mind their size, their situation (whether they rest in the anterior or posterior segment of the cavity, whether they are within or without the muscle cone), their extent (whether they involve or have spread from other cavities), their boundaries (whether circumscribed or diffuse), their character and their origin.” 36 Another important question raised by Casey Wood 8 was whether or not the tumor could be extirpated without removal of other tissues, and whether the eyeball itself may not also require enucleation. Reese in 1951 29 stated that he “could not recall a single incident in which an orbit has been explored with benefit merely because there was an unexplained unilateral exophthalmos. In these cases, temporizing is in order and an unabated effort should be made at regular intervals to determine the cause of exophthalmos. The exception to this rule is some deep nonpalpable orbital lesion that is jeopardizing the function of the eye by pressure or by its effect on the optic nerve.” He went on to state “in some incidences in which the diagnosis is obscure and biopsy material is needed, a surgical biopsy could be avoided by employing the so-called needle aspiration biopsy.” Stallard, for his part, had strongly argued against this approach as it could result in spreading the tumor or its recurrence. By 1964, Byron Smith 22 was able to recognize that a lateral canthotomy would improve the ability to palpate and possibly biopsy an orbital lesion before embarking on a full lateral orbitotomy.
The real revolution in the history of “this dark bloody hole” was the advent of radiography. By early 1896, less than 6 months after Roentgen's report of X-rays in Wurtzburg, Burry had used this technology to locate buck shot in a hand in Chicago, and Francis Williams in the spring of 1896 had used radiography to localize an intraocular copper foreign body in a patient in Boston. 37
By 1896, Thompson was using stereoscopic methods for localization within the orbit, particularly to decide whether or not the pathology was located within the globe. This was further refined by William Sweet in 1898 38 and Comberg in 1927 39 using geometric methods that would find its way to the battlefields of World War I. Vogt used bone free dental films to look for small metallic objects. 40 Caldwell introduced a view useful for the paranasal sinuses 41 which was further refined by Waters to look at the ethmoid sinuses. 42 Marked improvements in the use of radiography came with the replacement of the Crookes tube by Coolidge in 1913, allowing for far shorter exposures. Reese introduced the optic canal view in 1917 and tomography was introduced by Cone, Moore, and Dean in 1939. 43
In spite of these advances, X-rays were extremely crude in dealing with the orbit, unless the bone was affected. Additional advances in radiographic techniques when dealing with the orbit were instituted by Gasteiger and Grauer in 1929 44 with the injection of air. Offret, Gillies, and Blanchot suggested the use of higher volumes of air combined with tomography in 1954. 45
To obtain increased soft tissue delineation, oil-based and later water-soluble contrast were introduced ( Fig. 20 ). Another major advance was Moniz's 1927 introduction of angiography. 46 While this had limited use in orbital disease, Silva 47 suggested the use of intravenous injection of contrast administered through a frontal vein injection ( Fig. 21 ).
Fig. 20.
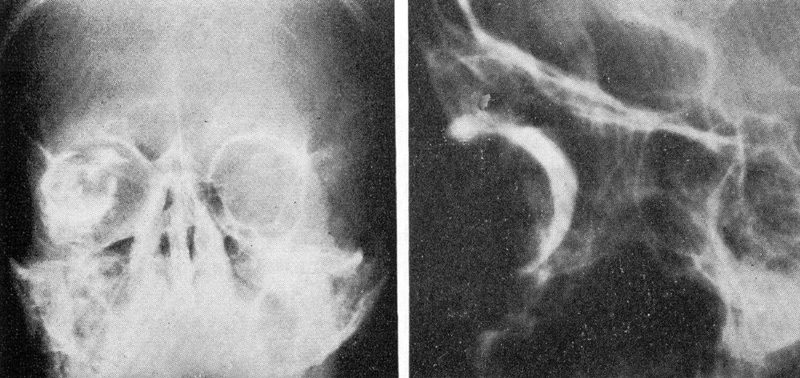
Orbital contrast study. 7
Fig. 21.
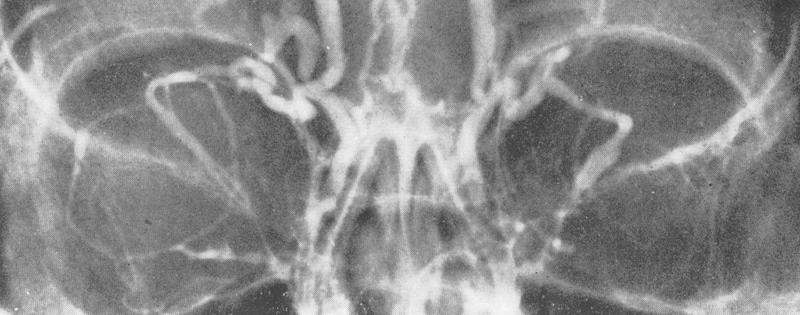
Orbital venogram. 7
In his 1943 article on 200 cases of exophthalmos, 48 Pfeiffer was able to demonstrate radiographic changes in 138 patients, with a specific diagnosis suggested in 84 of these, or approximately 42% overall. Changes were seen in soft tissue and bone, as well as evidence of increased intracranial pressure.
Another diagnostic technique, introduced somewhat later, was that of ultrasound. In 1967, Purnell 49 suggested the use of ultrasound in radiology and Coleman 50 and Dallow and Coleman 51 were able to outline the ultrasonic classification of orbital tumors in 1977. Ossoinig, 52 in Iowa, pioneered the use of quantitative A-scan revealing specific patterns based on anterior spike, the amount of decay, and whether or not there was a distinct terminal aspect.
Of all the changes in our ability to approach orbital tumors, nothing matches Hounsfield's introduction of computed tomography (CT) neuroimaging studies, first imported to the United States in 1973. 53 The first clinical prototype produced by Electrical and Music Industries (EMI) (which also produced the Beatles albums), consisting of an 80 × 80 matrix requiring a Leucite filled water bath, was installed at the Atkinson Morley Hospital, outside of London, 54 and the first reports of its use in orbital disease came from Gawler et al 55 and Wright 56 in England. The first three units in the United States were installed at the Mayo Clinic, Mass General, and the George Washington University Hospital. Alper immediately recognized the dramatic improvement in ability to visualize lesions within the orbit. 57
CT in its early iterations, had marked limitations. 58 The Leucite box kept one from obtaining direct coronals, the matrix was extremely limited ( Fig. 22 ), and prior to the advent of contrast material, there was poor tissue specificity (especially of meningiomas). Although thyroid patients showed more fat and inflammation demonstrated low density, there was very poor resolution at the orbital apex. The equal density of the soft tissue made it very difficult to determine whether in fact there was a meningioma present.
Fig. 22.
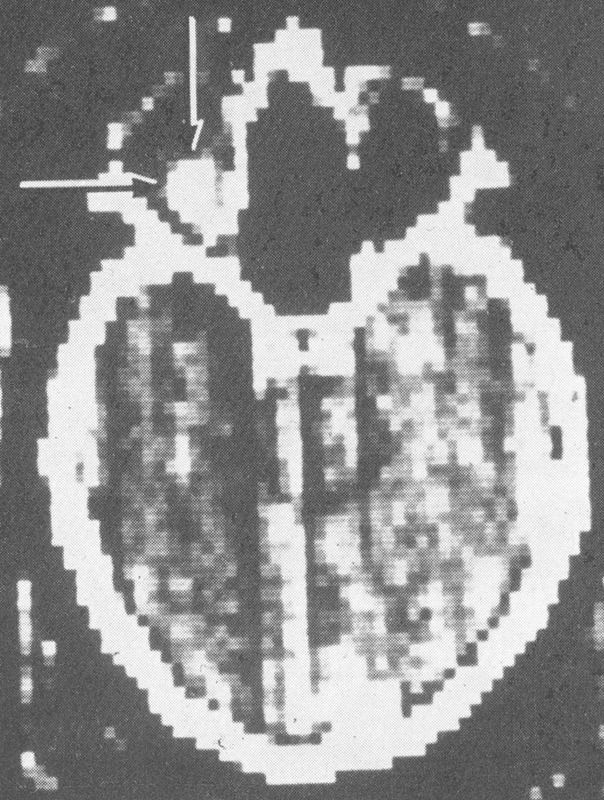
Early computed tomography (CT) scan orbit demonstrating a hemangioma. 57
Other early case series include reports from Boston by Grove in 1975 59 summarizing the evaluation of 50 cases of exophthalmos collected over 12 months. 60 Within a year of its introduction, more advanced machines designed for whole body studies did away with the water bath. This permitted positioning that would provide direct coronal images of the orbit, first described in 1978. 61
By 1980, the critical importance of determining the location of the lesion with regard to the optic nerve became a deciding factor in planning an orbital approach. For apex and canal lesions, a transcranial frontal approach was thought to be the best. For lesions located in the anterior two-thirds of the orbit, an anterior transcutaneous approach was more appropriate (interestingly, the transconjunctival approach was not really emphasized). Of course, for lesions located lateral to the optic nerve, a lateral orbitotomy with the traditional Krönlein procedure as modified by Berke 23 and others were appropriate. “Frequently a team approach working with neurosurgeons, ear, nose and throat surgeons is necessary because the mass is inaccessible or involves contiguous areas.”
This team approach was further emphasized by Maroon, a neurosurgeon, and Kennerdell, a neuroophthalmologist and orbital plastic surgeon, in a review article in 1984 published in the Journal of Neurosurgery . 27 Several modifications were made in the lateral orbitotomy including recognition that opening the canthal tendon was probably not necessary (palpation deeper in the orbit was now obviated by the results of the CT scan). Their self-retaining retractor combined with the addition of the operating microscope improved visualization. A more rigorous approach could thus be made to lesions within the orbit. They suggested the choice of approach depends on “the following criteria: (1) the location of the tumor relative to the optic nerve; (2) the size of the lesion; (3) the vascularity and ultrasonic characteristics of the tumor; and (4) the probable pathology anticipated.” These two authors further identified the potential use of ultrasonic and laser excision of tumors to improve hemostasis. Although other authors were starting to use plates and screws to refixate the lateral rim, Maroon and Kennerdell felt that simply by placing the bone in place and closing the periosteum over it, 27 the bone fragment would be held adequately in place. This would have the added advantage, although unstated, of not producing artifact on subsequent magnetic resonance imaging (MRI) testing.
The effect of CT scanning on orbital surgery was dramatic. Writing in 1978, Trokel (a neuroophthalmologist who pioneered the use of the Excimer laser in refractive surgery) and Hilal pointed out the dramatic diminution in the number of lateral orbitotomies because of alternative approaches, once the location of the tumor was known. 62 But, they pointed out the obvious that ultimately, only a biopsy would provide diagnostic certainty. In spite of the advances in imaging provided by CT (and subsequently by MRI), tissue specificity was still not possible.
The advantages of having tissue for diagnosis had been appreciated as early as 1898 when Bull, writing in his summary chapter on Orbital Disease in Norris and Oliver, 6 had suggested that a potential “puncture with needle or trocar” could provide a specific diagnosis by providing tissue to the pathologist. Wood, in the American Encyclopedia and Dictionary of Ophthalmology 8 had recommended that “before a serious operation is undertaken upon any orbital growth, every possible effort should be made to place its character beyond doubt by tuberculin and Wasserman tests, examination of the nose, and accessory cavities, and finally, in every case in which syphilis could possibly be suspected, by vigorous treatment with mercury and iodine. When all of these resources have been exhausted, an excised piece of tissue should be histologically studied.” It was Kennerdell et al, however, who reported on the routine use of fine needle aspiration biopsy in making specific orbital diagnosis. 63 After the success of their first 15 cases reported in 1979, their series was up to 150 cases with an 85% diagnostic response by 1985. 63 By combining needle aspiration with CT 64 or ultrasound guidance, Kennerdell et al were able to further access lesions that might be otherwise too small or difficult to find within the orbit.
What became apparent with the advances in localization and preoperative planning (ultrasound, CT, MRI, and biopsy) was, the answer to the orbital approach controversy of the three decades between 1940 and 1970 was that there was no the “one best orbital approach.” The surgical approach needs to be tailored to the location and the expected pathology. In addition, optimal results could be obtained by a team approach bringing experts in from multiple disciplines. The introduction to Dandy's monograph was written by Alan Wood who was the Chairman of the Department of Ophthalmology at the Wilmer Eye Institute. He pointed out that joint conferences were held to assist in planning approach to lesions within the orbit. 16 Stallard suggested in 1973 12 that it was “ideal when the eye surgeon works with them in operations, specifically including neurosurgeons and otolaryngologists and head and neck surgeons.” This was more emphatically stated by Maroon and Kennerdell, 27 “the most direct surgical approach to the lesion is then planned.” The transcranial approach was used for tumors within the orbital apex and medial to the optic nerve and the optic canal, or for those with intracranial extension. For tumors located superiorly and temporally, a lateral orbitotomy could be tailored and for those tumors medial to the optic nerve, a transcutaneous or transconjunctival approach could be used. “By thus approaching tumors directly optimal exposure is obtained and functional deficits are minimized. 27 ”
It is critical to recognize that there is no operation that cannot potentially make a patient worse. In 1892, Osler published his Principles and Practice of Medicine, it was the last time that a single author attempted to take on the daunting task of covering all of medicine by himself. By utilizing specialists within particular areas (head and neck surgery, neurosurgery, etc.) the ophthalmologist could team up to optimize the approach to any lesion within the orbit. 56 65
So where are we now? A 1995 Atlas published by Rootman et al 66 emphasized the myriad options that we have in approaching lesions within the orbit. Cutaneous incisions include rim incisions, subciliary incisions, the Berke lateral canthotomy incision, or facial crease (including upper lid crease) incisions to produce the best cosmetic result following surgery while giving adequate access ( Fig. 23 ).
Fig. 23.
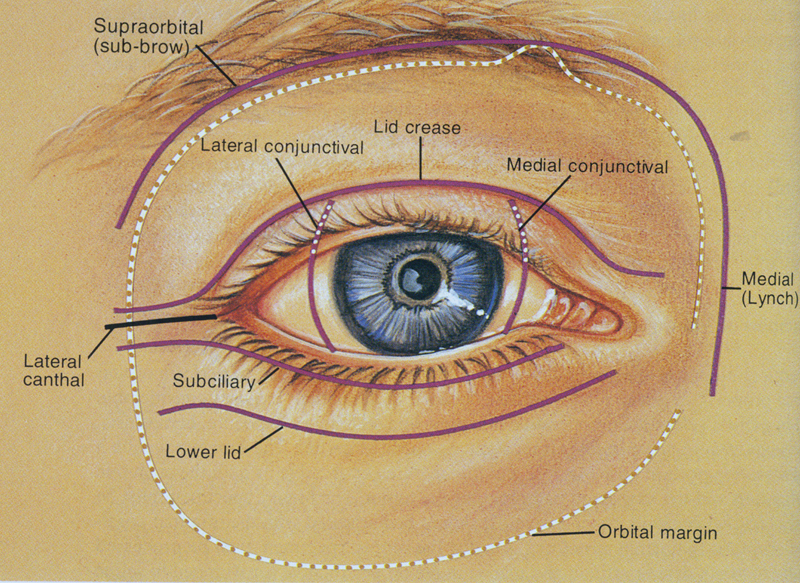
Transcutaneous approaches to the orbit. 66
Transconjunctival approaches permit access to anterior aspects of the orbit. There are multiple options in terms of bone disassembly based on the traditional Krönlein lateral orbitotomy, including the ability to remove additional sections of the orbital rim, superiorly and inferiorly, including portions of the zygoma ( Fig. 24 ). More recently lesions in the inferomedial orbit have been approached transnasally by endoscopic techniques pioneered in Germany by Wolfgang, and then imported into the United States by Dr. Kennedy, 67 especially after he moved to the University of Pennsylvania.
Fig. 24.
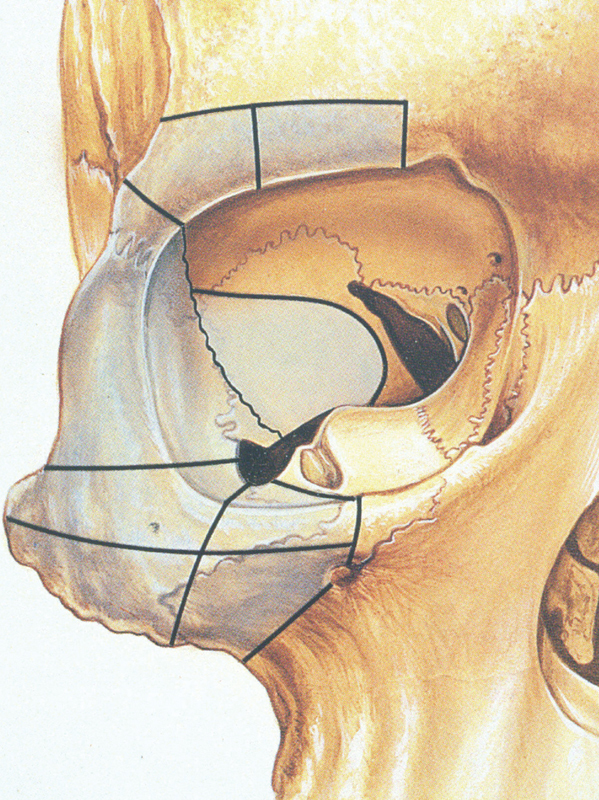
Bony disassembly of the orbit. 66
Finally, however, the most recent piece to our evolutionary puzzle has been provided by the recognition of identifying goals of operating on any orbital lesion. In 1912, Harvey Cushing pointed out while discussing pituitary tumors that perhaps ultimately surgery would come to play a less, not more, important role in dealing with lesions of the sellar region. Similarly, as we are better able to recognize the specific nature of lesions within the orbit, we can determine what alternative/adjuvant therapies may serve the patient better. This is perhaps best illustrated by our changing approach to optic nerve sheath meningiomas based on the retrospective study by Turbin et al, 68 which demonstrated that patients treated with radiation therapy did far better than those patients treated with surgery, observation, or surgery combined with radiation. Similarly, some of the lesions that we enjoy taking out, such as hemangiomas or gliomas, may not need to be treated at all. Hoyt and Baghdassarian in 1969 69 suggested that often optic nerve gliomas in childhood did not grow at all (or if they did, they did so early 70 ), and therefore could simply be monitored. More recent studies by Roger Packer suggest that even with growth, often these lesions could be better treated with chemotherapy. Even more importantly, as demonstrated by Orcutt et al, 71 most hemangiomas, often now picked up serendipitously by imaging studies done for other reasons, may not grow, and simply can be followed.
Conclusion
In conclusion, the history of orbital surgery follows the same paradigm as all of surgery. The presence of a lesion is not an indication for surgical intervention. One needs to define surgical goals based on a thorough knowledge of the location of the lesion, its imaging characteristics, and ultimately if necessary, a specific biopsy. 56 Is our goal complete excision, decompression, or simply to identify and confirm the histopathology? One size does not fit all. A team utilizing the expertise of radiologists, ultrasonographers, pathologists, otolaryngologists, head and neck surgeons, plastic surgeons, and neurosurgeons complements the orbital surgeon in planning the safest, lowest morbidity approach. This is dependent on the relationship of the pathology to the optic nerve 56 and, ultimately, the specific goals of the patient. As this evolution shows, we can truly see farther by standing on the shoulders of those who came before us.
Footnotes
Conflict of Interest None declared.
Pearls and Tips.
Not all orbital pathology needs to be operated upon. Some may be followed because it does not change. Some may be treated with chemotherapy or radiation therapy.
Knowing the nature of the lesion, is often helpful. Imaging studies may be helpful in this, but sometimes a needle aspiration biopsy may be necessary.
-
Surgical approach depends on:
The goal of surgery, whether complete excision or simply biopsy.
The location of the lesion and its relationship to the optic nerve.
References
- 1.Henderson J W, Farrow G M. Philadelphia, PA: WB Saunders; 1973. Orbital Tumors; pp. 1–8. [Google Scholar]
- 2.Bartisch G.OphthalmodouleiaDresden, Matthes Stöckel,1583 [Google Scholar]
- 3.Knapp H. On cocaine and its use in ophthalmic and general surgery. Arch Ophthalmol. 1884;13:402–408. [Google Scholar]
- 4.Atkinson W S. Retrobulbar injection of anesthetic within the muscular cone. Arch Ophthalmol. 1936;16:494. [Google Scholar]
- 5.Swan K C. New drugs and techniques for ocular anesthesia. Trans Am Acad Ophthalmol Otolaryngol. 1956;60(03):368–375. [PubMed] [Google Scholar]
- 6.Bull C S. Vol. III. Philadelphia, PA: J.B. Lippincott Co.; 1898. Diseases of the orbit; pp. 3–62. [Google Scholar]
- 7.Duke-Elder S, McFall P A. Vol. XIII. London, United Kingdom: Kimpton; 1974. General considerations; pp. 773–810. [Google Scholar]
- 8.Wood C A.Operations on the orbit Chicago, IL: Cleveland Press; 19189147–9184.; 9187–9190 [Google Scholar]
- 9.Hope T. An account of a remarkable cure performed on the eye of a young woman in Scotland. Philos Trans R Soc Lond B Biol Sci. 1744;43:193–200. [Google Scholar]
- 10.Bartley G B. Early American oculoplastic surgery: the lectures of Dr. John Jeffries. Ophthal Plast Reconstr Surg. 2001;17(03):157–160. doi: 10.1097/00002341-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Khan J A, Albert D M. Willard Parker's 1841 orbital operation. Surv Ophthalmol. 1988;33(02):117–120. doi: 10.1016/0039-6257(88)90163-4. [DOI] [PubMed] [Google Scholar]
- 12.Stallard H B. The presidential address. The evolution of lateral orbitotomy. Trans Ophthalmol Soc U K. 1973;93(00):3–17. [PubMed] [Google Scholar]
- 13.Krönlein R U. Zur Pathologie und operativen Berhandlung der Dermoidcystern der Orbita. Beitr Klin Chir. 1888;4:149–163. [Google Scholar]
- 14.Knapp H. A case of carcinoma of the outer sheath of the optic nerve, removed with preservation of the eyeball. Arch Ophthalmol. 1874;4:323–354. [Google Scholar]
- 15.Knapp H. Tumor of the optic nerve. Trans Am Ophthalmol Soc. 1879;2:557–560. [PMC free article] [PubMed] [Google Scholar]
- 16.Dandy W E. New York, NY: Oskar Piest; 1941. Orbital Tumors. Results Following the Transcranial Operative Attack. [Google Scholar]
- 17.Walsh T E, Ogura J H. Transantral orbital decompression for malignant exophthalmos. Laryngoscope. 1957;67(06):544–568. doi: 10.1288/00005537-195706000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Conley J J. Washington, DC: Butterworth; 1962. Medial and inferior approach to orbital tumors; pp. 85–89. [Google Scholar]
- 19.Benedict W L. Removal of orbital tumors. Surg Gynecol Obstet. 1934;58:383–389. [Google Scholar]
- 20.Benedict W L.Surgical treatment of tumors and cysts of the orbit Am J Ophthalmol 194932(Pt.1):763–773. [DOI] [PubMed] [Google Scholar]
- 21.Davis F A.Primary tumors of the optic nerve Arch Ophthalmol 194023735–821., 957–1018 [Google Scholar]
- 22.Smith B. St. Louis, MO: C.V. Mosby; 1964. Surgical approach to the orbit. New and Controversial Aspects. [Google Scholar]
- 23.Berke R N. A modified Krönlein operation. Trans Am Ophthalmol Soc. 1953;51:193–231. [PMC free article] [PubMed] [Google Scholar]
- 24.Czermak W. Zur osteoplastinischen Resektion der äusseren Augenhöhlenwand. Dtsch med Wshr. 1905;31:1549–1589. [Google Scholar]
- 25.Stallard H B.A plea for lateral orbitotomy (Krönlein's operation) BMJ 19471(4499):408–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callahan A. Birmingham, England: Aesculapius Publishing; 1978. Wright's lateral orbitotomy; pp. 893–897. [Google Scholar]
- 27.Maroon J C, Kennerdell J S. Surgical approaches to the orbit. Indications and techniques. J Neurosurg. 1984;60(06):1226–1235. doi: 10.3171/jns.1984.60.6.1226. [DOI] [PubMed] [Google Scholar]
- 28.Jane J A, Park T S, Pobereskin L H, Winn H R, Butler A B. The supraorbital approach: technical note. Neurosurgery. 1982;11(04):537–542. [PubMed] [Google Scholar]
- 29.Reese A B. New York, NY: Paul B. Hoeber; 1951. Tumors of the Eye; p. 539. [Google Scholar]
- 30.Reese A B. St Louis, MO: C.V. Mosby; 1964. Incidence and management of unilateral proptosis. New and Controversial Aspects; pp. 389–394. [Google Scholar]
- 31.Reese A B. Washington, DC: Butterworth; 1962. The temporal approach to orbital tumors; pp. 77–80. [Google Scholar]
- 32.Love J G. Washington, DC: Butterworth; 1962. Transcranial removal of intra-orbital tumors; pp. 81–85. [Google Scholar]
- 33.Moss H M. Expanding lesions of the orbit. A clinical study of 230 consecutive cases. Am J Ophthalmol. 1962;54:761–770. doi: 10.1016/0002-9394(62)94157-0. [DOI] [PubMed] [Google Scholar]
- 34.Rootman J. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. Diseases of the Orbit. [Google Scholar]
- 35.Knapp A. The Krönlein operation as an exploratory procedure in affections of the orbit. JAMA. 1912;LIX:988–989. [Google Scholar]
- 36.Wood C A. Chicago, IL: Cleveland Press; 1911. Some operations on the orbital walls and contents; pp. 813–860. [Google Scholar]
- 37.Taveras J L, Haik B G. Radiography of the eye and orbit: a historical overview. Surv Ophthalmol. 1988;32(05):361–368. doi: 10.1016/0039-6257(88)90098-7. [DOI] [PubMed] [Google Scholar]
- 38.Sweet W M. The roentgen rays in ophthalmic surgery. JAMA. 1898;30:5–10. [Google Scholar]
- 39.Comberg W. Ein neues Verfahren zur roentgenlokalization am Augapfel. Arch Ophthalmol. 1927;118:175. [Google Scholar]
- 40.Vogt A. Skelettfrie Rontgenaufnahme des vorderen Bulbusabschnittes. Schweiz Med Wochenschr. 1921;2:145. [Google Scholar]
- 41.Caldwell E W. Skiagraphy of the accessory sinuses of the nose. Am Q Roentgenol. 1906;1:27–35. [Google Scholar]
- 42.Waters C A, Waldron C W. Roentgenology of the accessory nasal sinuses. AJR Am J Roentgenol. 1915;2:634–639. [Google Scholar]
- 43.Cone A J, Moore S, Dean L W. Relationship of paranasal sinus disease to ocular disorders. Laryngoscope. 1939;49:374–393. [Google Scholar]
- 44.Gasteiger H, Grauer S. Zur Diagnose der Doppelperforation des Augapfels mit hilfe von Lufteinblasung in den Tenonschen Raum. Fortschr Geb Rontgenstr Nuklearmed Erganzungsband. 1929;40:272–278. [Google Scholar]
- 45.Offret G, Gilles E, Blanchot F.De l'intérêt des tomographies orbitaires avec injection d'air en ophtalmologie Arch Ophtalmol Rev Gen Ophtalmol 19541403259–273., 79 [PubMed] [Google Scholar]
- 46.Moniz E. L'encéphalographie artérielle, son importance dans la localisation des tumeurs cérébrales. Rev Neurol (Paris) 1927;2:72–90. [Google Scholar]
- 47.Silva D. Exoftalmos postural. Arch Assoc Ceguera (Mex) 1947;5:109–127. [PubMed] [Google Scholar]
- 48.Pfeiffer R L.Roentgenography of exophthalmos, with notes on the roentgen ray in ophthalmology Am J Ophthalmol 194326724–741., 816, 33,901–11 [PMC free article] [PubMed] [Google Scholar]
- 49.Purnell E W. St. Louis, MO: CV Mosby; 1969. Ultrasonic interpretation of orbital disease; pp. 249–255. [Google Scholar]
- 50.Coleman D J. Reliability of ocular and orbital diagnosis with B-scan ultrasound. 2. Orbital diagnosis. Am J Ophthalmol. 1972;74(04):704–718. doi: 10.1016/0002-9394(72)90833-1. [DOI] [PubMed] [Google Scholar]
- 51.Dallow R L, Coleman D J. Birmingham, England: Aesculapius Publishing; 1978. Ultrasonic evaluation of orbital disorders; pp. 311–339. [Google Scholar]
- 52.Ossoinig K C. Standardized echography: basic principles, clinical applications, and results. Int Ophthalmol Clin. 1979;19(04):127–210. [PubMed] [Google Scholar]
- 53.Hounsfield G N. Computerized transverse axial scanning (tomography). 1. Description of system. Br J Radiol. 1973;46(552):1016–1022. doi: 10.1259/0007-1285-46-552-1016. [DOI] [PubMed] [Google Scholar]
- 54.Ambrose J. Computerized transverse axial scanning (tomography). 2. Clinical application. Br J Radiol. 1973;46(552):1023–1047. doi: 10.1259/0007-1285-46-552-1023. [DOI] [PubMed] [Google Scholar]
- 55.Gawler J, Sanders M D, Bull J WD, du Boulay G, Marshall J. Computer assisted tomography in orbital disease. Br J Ophthalmol. 1974;58(06):571–587. doi: 10.1136/bjo.58.6.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright J E. The role of surgery in the management of orbital tumours. Mod Probl Ophthalmol. 1975;14:553–556. [PubMed] [Google Scholar]
- 57.Alper M G, Davis D O, Pressman B D. Use of computerized axial tomography (EMI scanner) in diagnosis of exophthalmos. Trans Am Acad Ophthalmol Otolaryngol. 1975;79:150–165. [Google Scholar]
- 58.Wright J E, Lloyd G AS, Ambrose J. Computerized axial tomography in the detection of orbital space-occupying lesions. Am J Ophthalmol. 1975;80(01):78–84. doi: 10.1016/0002-9394(75)90873-9. [DOI] [PubMed] [Google Scholar]
- 59.Grove A S, Jr., New P FJ, Momose K J. Computed tomographic (CT) scanning for orbital evaluation. Trans Am Acad Ophthalmol Otolaryngol. 1975;79:137–149. [Google Scholar]
- 60.Grove A S., Jr Evaluation of exophthalmos. N Engl J Med. 1975;292(19):1005–1013. doi: 10.1056/NEJM197505082921905. [DOI] [PubMed] [Google Scholar]
- 61.Grove A S., Jr . Birmingham, England: Aesculapius Publishing; 1978. Coronal computed tomography (CCT) of the orbit; pp. 271–280. [Google Scholar]
- 62.Trokel S L, Hilal S K. Birmingham, England: Aesculapius Publishing; 1978. Computed tomography of orbital and ocular tumors; pp. 257–270. [Google Scholar]
- 63.Kennerdell J S, Slamovits T L, Dekker A, Johnson B L. Orbital fine-needle aspiration biopsy. Am J Ophthalmol. 1985;99(05):547–551. doi: 10.1016/s0002-9394(14)77955-3. [DOI] [PubMed] [Google Scholar]
- 64.Kennerdell J S, Dubois P J, Dekker A, Johnson B L. CT-guided fine needle aspiration biopsy of orbital optic nerve tumors. Ophthalmology. 1980;87(06):491–496. doi: 10.1016/s0161-6420(80)35204-4. [DOI] [PubMed] [Google Scholar]
- 65.Kennerdell J S. Vol 2. St. Louis, MO: C.V. Mosby; 1987. Orbital diagnosis; pp. 1008–1024. [Google Scholar]
- 66.Rootman J, Stewart B, Goldberg R A. Philadelphia, PA: Lippincott-Raven; 1995. Orbital Surgery. A Conceptual Approach. [Google Scholar]
- 67.Kennedy D W, Goodstein M L, Miller N R, Zinreich S J. Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg. 1990;116(03):275–282. doi: 10.1001/archotol.1990.01870030039006. [DOI] [PubMed] [Google Scholar]
- 68.Turbin R E, Thompson C R, Kennerdell J S, Cockerham K P, Kupersmith M J.A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy Ophthalmology 200210905890–899., discussion 899–900 [DOI] [PubMed] [Google Scholar]
- 69.Hoyt W F, Baghdassarian S A. Optic glioma of childhood. Natural history and rationale for conservative management. Br J Ophthalmol. 1969;53(12):793–798. doi: 10.1136/bjo.53.12.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imes R K, Hoyt W F. Childhood chiasmal gliomas: update on the fate of patients in the 1969 San Francisco Study. Br J Ophthalmol. 1986;70(03):179–182. doi: 10.1136/bjo.70.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orcutt J C, Wulc A E, Mills R P, Smith C H. Asymptomatic orbital cavernous hemangiomas. Ophthalmology. 1991;98(08):1257–1260. doi: 10.1016/s0161-6420(91)32146-8. [DOI] [PubMed] [Google Scholar]


