Abstract
Objective This study was aimed to illustrate the features and complexities of nonspecific orbital inflammation via discussion of two representative cases.
Design Present study is a retrospective case review.
Setting The study was conducted at a tertiary care medical center.
Participants Two patients with nonspecific orbital inflammation were participants of this retrospective study.
Main Outcome Measures Outcome of the study was disease-free patients and off all medications.
Results At follow-up, both patients are disease free and off all medications.
Conclusion Surgery plays a diagnostic and therapeutic role. While the clinical subtype is important for differential diagnosis and symptomatic treatment, the histologic subtype is similarly important. For inflammatory dacryoadenitis, surgery can be therapeutic. For extensive granulomatosis with polyangiitis, debulking surgery may allow better penetration of medications, especially rituximab.
Keywords: orbit, inflammation, orbital pseudotumor, dacryoadenitis, granulomatosis with polyangiitis
Introduction
Orbital inflammation, especially if associated with formation of a fibroinflammatory mass, is capable of simulating the symptoms and findings of an orbital tumor. Birch-Hirschfeld introduced the term orbital pseudotumor in 1930 to describe this scenario where orbital inflammation produced proptosis. 1 Through the years, virtually all types of inflammation were included under this all-encompassing term such that it really had no pathologic meaning. Over time, however, various types of inflammation were excluded 2 to the point where “inflammatory orbital pseudotumor” has now come to be defined by what it is not. For example, in the early 1960's thyroid orbitopathy given its own category, as were cases with granulomatous inflammation. In the mid 1960's vasculitic inflammation was excluded. Lymphocytic phenotyping introduced in the 1970's laid the foundation for the exclusion of clonal lymphoid lesions in the 1980's. This period also saw introduction of better imaging which permitted anatomic and subsequent clinical classification of the various orbital inflammations into the following seven subtypes: myositis, dacryoadenitis, diffuse, anterior, perioptic neuritis, apical, and extra orbital extension. 3 4 5 Further pathologic evaluation in the 1990's gave cases with sclerosing orbital inflammation 6 7 their own diagnosis, followed by specification of immunoglobulin (Ig)-G4–related orbital inflammation 8 9 in the 2000's. What used to be very nonspecific in terms of orbital inflammation has now, by exclusion, become more specific, although the term, “nonspecific orbital inflammation” (NSOI) persists.
Clinical Evaluation and Treatment
Orbital inflammations are named for the predominant histologic component ( Table 1 ). This underscores the importance of a biopsy. 10 There are two roles for surgery in this setting. The first and most obvious is the diagnostic function. Subsequent therapeutic decisions can be based upon these results. 11 12 13 14 In our experience, rituximab gives a favorable response with IgG4 and lesions with a vasculitic component, while the anti–tumor necrosis factor (TNF) agents work well for with granulomatous disease.
Table 1. Histologic subtypes of orbital inflammation a .
| Histologic subtypes of orbital inflammation a |
|---|
| Acute inflammation |
| Fibroblastic proliferative |
| Fibrotic |
| Foamy histiocytic |
| Granulomatous |
| Lymphocytic |
| Lymphoplasmacytic |
| Lymphoplasmacytic (IgG4) |
| Necrotic |
| Vasculitic |
Courtesy: Diva Salomao, MD.
The other role for surgery is therapeutic; patients may be able to reduce or eliminate their systemic medications following surgery. This has been described in patients with inflammatory dacryoadenitis 15 16 and our unpublished data on 12 patients with severe granulomatosis with polyangiitis (GPA) who were debulked and subsequently treated with rituximab, all with favorable results. The following two cases illustrate these principles.
Case 1: Recalcitrant Inflammatory Dacryoadenitis
In 2004, a 24-year-old female developed pain and swelling over the superior temporal portion of her right orbit. There were no comorbid issues. She was diagnosed with inflammatory dacryoadenitis and treated with oral steroids but became steroid dependent. Methotrexate was introduced with temporary improvement. She was treated with a course of oral steroids and orbital radiation therapy (2,000 cGy), all with no improvement. When seen at Mayo in January 2007, she was steroid dependent (20 mg) and complaining of pain over the right lacrimal gland. Abnormalities on examination revealed a dusky, swollen right upper lid with tenderness over the lacrimal gland. There were 3-mm proptosis, and orbital magnetic resonance imaging (MRI) showed an enhancing lesion centered on the lacrimal gland with enhancement spilling over to surround the mildly enlarged lateral and superior rectus muscles ( Fig. 1 ). A lateral orbitotomy was done and all the palpable fibrous tissue was removed followed by a retrobulbar injection of 80-mg triamcinolone. Histopathologic examination showed fibrosis with chronic, nonspecific inflammation without evidence of granulomas or vasculitis. She was able to taper-off prednisone and with 8 years of follow-up has had no recurrences.
Fig. 1.
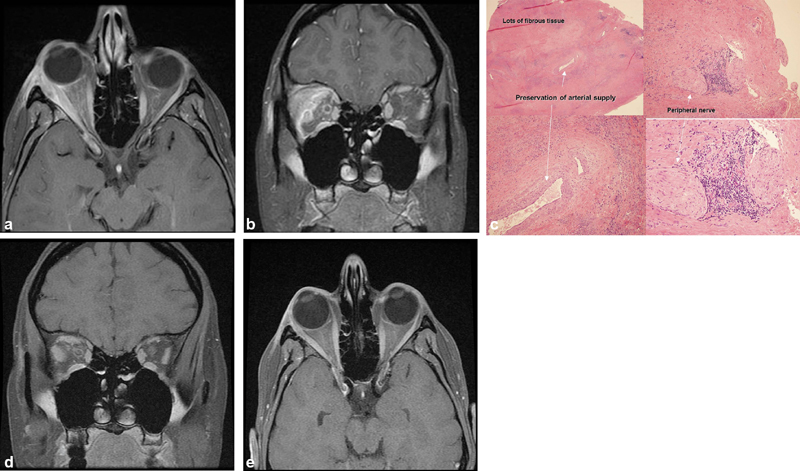
( A, B ) Axial and coronal magnetic resonance imaging (MRI), T1 with contrast: the right lacrimal gland and lateral rectus are enlarged and enhance as do the lateral/superior periorbita and optic nerve sheath; stranding of the adjacent orbital fat. ( B ) Coronal MRI, T1 with contrast: the right lacrimal gland and lateral rectus are enlarged and enhance as does the lateral/superior periorbita and the optic nerve sheath. There is also stranding of the adjacent orbital fat. ( C ) Right lacrimal gland. Hematoxylin and eosin (H&E) × 20 upper left, × 100 lower left and upper right, ×200 lower right: note the abundance of fibrous tissue and loss of lacrimal gland architecture with preservation of arterial supply. There is a peripheral nerve with adjacent inflammation which may account for some of the discomfort associated with this condition. ( D, E ) Axial and coronal MRI, T1 with contrast: postoperative scan at 6 months shows mild residual enlargement of right superior and lateral rectus muscles and resection of lacrimal gland mass.
Case 2: Progressive Orbital Granulomatosis with Polyangiitis
A 21-year-old male noted recurrent sinusitis in January 2009; biopsy was nondiagnostic. He had a poor response to medical therapy. Five months later, right orbital inflammation developed, and biopsy was consistent with GPA. Serologic studies were positive for proteinase-3 and Centrally Accentuated Antineutrophil Cytoplasmic Antibody Test (C-ANCA). There was no other evidence of systemic involvement. Prednisone and cyclophosphamide were used for induction therapy, followed by maintenance with prednisone and methotrexate. Despite this, evidence of orbital progression was apparent by December 2010. When seen at Mayo Clinic in March 2011, he reported episodic 5/10 orbital pain with diplopia at extremes of gaze. Visual acuity was 20/30 right eye and 20/20 left eye. The remainder of his examination was notable for a right globe that was mildly injected and chemotic with 11-mm proptosis and restricted motility. There was corneal scarring, and the right optic disc was swollen. Orbital imaging showed that the right orbit was virtually filled in with fibrous tissue, while there was minimal medial infiltrate on the left ( Fig. 2 ). The right orbit was debulked of all the palpable fibrous tissue and 80-mg triamcinolone was given as a retrobulbar injection. Postoperatively, the vision improved to 20/20, the pain resolved as did the restricted motility and disc edema. Histopathologic examination showed a very active vasculitis and granulomas. He was given rituximab (375 mg/m 2 ) weekly × 4 plus 60-mg prednisone for induction therapy. Over the ensuing 4 years of follow-up, he has remained asymptomatic without treatment.
Fig. 2.
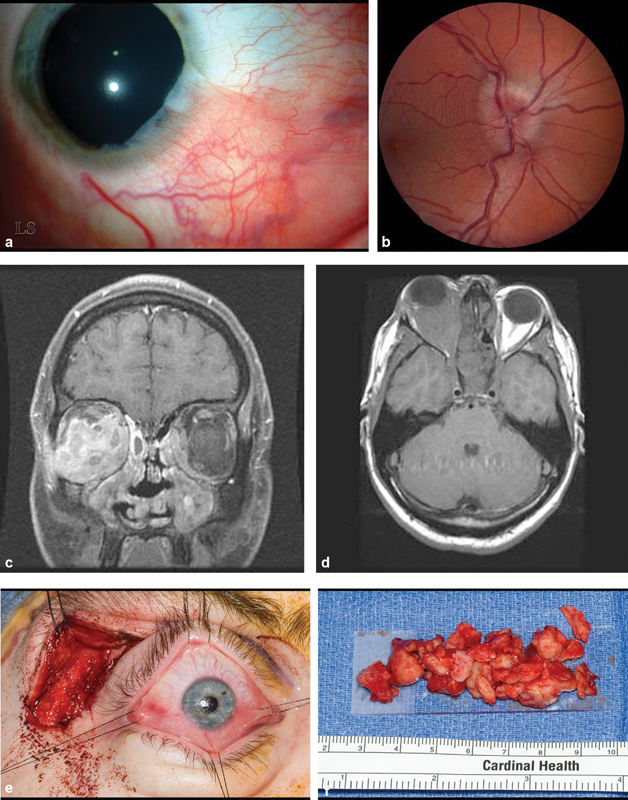
( A ) Slit lamp photograph of right cornea showing a pseudopterygium characteristic of healed marginal keratitis from 3:00 through 6:00. ( B ) Fundus photograph of right eye demonstrates swollen optic disk. This resolved following debulking surgery. ( C ) Axial magnetic resonance imaging (MRI) T1 without contrast shows that the right orbit is virtually filled with fibrous tissue. ( D ) Coronal MRI, T1 with contrast: the right medial, inferior and lateral rectus muscles are mildly enlarged and a contrast enhancing infiltrate surrounds these muscles and the optic nerve. There is a small infiltrate in the medial portion of the left orbit. ( E ) Intraoperative photography of a no-bone flap lateral orbitotomy with sutures around each rectus muscle insertion. Gentle traction on suture is transmitted to corresponding muscle belly allowing identification of each encased muscle. All of the palpable retrobulbar tissue was removed through this incision. ( F ) Gross pathology of debulked orbital fibrous tissue.
Conclusion
There are numerous subtypes of inflammation affecting the orbit, all of which can produce signs and symptoms of mass effect consistent with “orbital pseudotumor.” A biopsy can establish the histopathologic diagnosis which can then direct therapy. The role of surgery is both diagnostic, and, sometimes, therapeutic in selected cases. The mechanism of this response is unknown, although it may be postulated that a smaller mass may permit better penetration/higher local concentration of drug.
Footnotes
Conflict of Interest None declared.
Pearls and Tips.
Nonspecific orbital inflammation is a diagnosis of exclusion. There are many subtypes of inflammation, and appropriate use of biopsy procedures is important to identify the various subtypes to treat the underlying inflammation. Optimal treatment can be based on the type of inflammation.
References
- 1.Birch-Hirschfeld A. 2nd ed. Berlin, Germany: Springer-Verlag; 1930. Handbuch der Gesamten Augenheilkunden: Die Krankheiten der Orbita. [Google Scholar]
- 2.Jakobiec F A, Font R L. Philadelphia, PA: W.B. Saunders; 1986. Orbit; pp. 2777–2795. [Google Scholar]
- 3.Nugent R A, Rootman J, Robertson W D, Lapointe J S, Harrison P B. Acute orbital pseudotumors: classification and CT features. AJR Am J Roentgenol. 1981;137(05):957–962. doi: 10.2214/ajr.137.5.957. [DOI] [PubMed] [Google Scholar]
- 4.Kennerdell J S, Dresner S C. The nonspecific orbital inflammatory syndromes. Surv Ophthalmol. 1984;29(02):93–103. doi: 10.1016/0039-6257(84)90166-8. [DOI] [PubMed] [Google Scholar]
- 5.Mahr M A, Salomao D R, Garrity J A. Inflammatory orbital pseudotumor with extension beyond the orbit. Am J Ophthalmol. 2004;138(03):396–400. doi: 10.1016/j.ajo.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy J M, White V A, Harris G, Simons K B, Kennerdell J, Rootman J. Idiopathic sclerosing inflammation of the orbit: immunohistologic analysis and comparison with retroperitoneal fibrosis. Mod Pathol. 1993;6(05):581–587. [PubMed] [Google Scholar]
- 7.Rootman J, McCarthy M, White V, Harris G, Kennerdell J. Idiopathic sclerosing inflammation of the orbit. A distinct clinicopathologic entity. Ophthalmology. 1994;101(03):570–584. doi: 10.1016/s0161-6420(94)31298-x. [DOI] [PubMed] [Google Scholar]
- 8.Plaza J A, Garrity J A, Dogan A, Ananthamurthy A, Witzig T E, Salomão D R. Orbital inflammation with IgG4-positive plasma cells: manifestation of IgG4 systemic disease. Arch Ophthalmol. 2011;129(04):421–428. doi: 10.1001/archophthalmol.2011.16. [DOI] [PubMed] [Google Scholar]
- 9.Stone J H, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366(06):539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 10.Mombaerts I, Rose G E, Garrity J A. Orbital inflammation: Biopsy first. Surv Ophthalmol. 2016;61(05):664–669. doi: 10.1016/j.survophthal.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Carruth B P, Wladis E J. Inflammatory modulators and biologic agents in the treatment of idiopathic orbital inflammation. Curr Opin Ophthalmol. 2012;23(05):420–426. doi: 10.1097/ICU.0b013e328355715e. [DOI] [PubMed] [Google Scholar]
- 12.Suhler E B, Lim L L, Beardsley R M. Rituximab therapy for refractory orbital inflammation: results of a phase 1/2, dose-ranging, randomized clinical trial. JAMA Ophthalmol. 2014;132(05):572–578. doi: 10.1001/jamaophthalmol.2013.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrity J A, Coleman A W, Matteson E L, Eggenberger E R, Waitzman D M. Treatment of recalcitrant idiopathic orbital inflammation (chronic orbital myositis) with infliximab. Am J Ophthalmol. 2004;138(06):925–930. doi: 10.1016/j.ajo.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 14.Garrity J A, Matteson E L. Biologic response modifiers for ophthalmologists. Ophthal Plast Reconstr Surg. 2008;24(05):345–347. doi: 10.1097/IOP.0b013e3181829f7b. [DOI] [PubMed] [Google Scholar]
- 15.Mombaerts I, Cameron J D, Chanlalit W, Garrity J A. Surgical debulking for idiopathic dacryoadenitis: a diagnosis and a cure. Ophthalmology. 2014;121(02):603–609. doi: 10.1016/j.ophtha.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Mombaerts I, Schlingemann R O, Goldschmeding R, Noorduyn L A, Koornneef L. The surgical management of lacrimal gland pseudotumors. Ophthalmology. 1996;103(10):1619–1627. doi: 10.1016/s0161-6420(96)30454-5. [DOI] [PubMed] [Google Scholar]


