Abstract
Objective This study was aimed to review issues relating to the recognition, radiographic diagnosis, monitoring, and management of primary and secondary optic nerve sheath meningioma (ONSM).
Design This study is a review of peer-reviewed literature combined with illustrative case studies.
Participants and Methods A literature search was conducted via the PubMed database using pertinent search terms. Selected articles were limited to those written or translated into English. Additional works cited within articles were also included. Individual cases were drawn from the experience of a tertiary academic neuroophthalmic and orbital practice. Tables summarize radiotherapeutic and surgical studies, excluding single case reports and studies focusing on meningioma of intracranial origin.
Main Outcome Measurements Review of reported surgical and radiotherapeutic series is the primary measurement.
Results The natural history of optic nerve sheath meningiomas is primarily characterized by progressive ipsilateral vision loss. Diagnosis is typically based on radiographic imaging findings, with biopsy remaining indicated in some patients. Management strategies may include observation, radiation, and/or surgical intervention, or a combination of these approaches. The role of surgery, especially with respect to primary ONSM (pONSM), remains controversial. Advancement of radiotherapy techniques has shifted modern treatment paradigms in pONSM toward radiation as primary treatment, as surgical outcomes are inferior in major studies. Although radiation remains the treatment of choice in many cases, selected patients may benefit from surgery, especially in the setting of secondary ONSM (sONSM).
Conclusion A wide variety of radiotherapeutic and surgical treatment modalities for ONSM exist. The specific indications for each management strategy continue to be redefined.
Keywords: optic nerve sheath meningioma, optic nerve tumor, orbital meningioma, orbital tumor, radiation, radiotherapy, surgery
Introduction
Primary optic nerve sheath meningioma (pONSM) is the most common benign primary tumor affecting the optic nerve. Its site of origin at the neural arachnoid cap cells lends to unique characteristics that affect its clinical course and treatment consideration. Its management shares both commonality and difference with respect to other forms of meningioma that may primarily or secondarily affect the optic nerve (sONSM). Radiation treatment remains a mainstay in the treatment of primary lesions for which visual preservation is a goal. It may serve as a useful adjunct in tumors which secondarily involve the nerve, where surgery (including optic canal decompression) may also be appropriate. Timing of treatment remains a topic of debate and may require an individualized approach. Advances such as chemotherapeutic, immunologic, and biologic treatments remain limited and treatments with these agents are typically reserved for trial, rescue, or compassionate therapies. Significant improvements in radiation technology are some of the most important recent advances in care, and numerous articles describe results of these new highly conformal therapies.
Epidemiology
The pONSMs are rare tumors of the arachnoid cap cells within the optic nerve sheath, representing approximately 1 to 2% of all meningiomas and approximately 2% of all orbital tumors. 1 2 3 4 pONSMs are the most common benign tumor involving the optic nerve, accounting for approximately one-third of all primary tumors of the optic nerve, second to optic nerve glioma with respect to all primary intraorbital optic nerve tumors. 1 4 5 6 7 Meningiomas affecting the optic nerve may be divided into following two types: (1) pONSM, arising from either the intraorbital or intracanalicular portion of the arachnoid sheath surrounding the optic nerve and (2) secondary ONSM (sONSM) which typically originate intracranially and invade the orbit and optic canal secondarily. The sONSM may develop from the meninges overlying the sphenoid ridge, tuberculum sellae, olfactory groove, planum sphenoidale, pituitary fossa, clinoid, falciform ligament, frontoparietal region, basal-frontal region, or paranasal sinuses. 8 9 10 11 The concept of primary versus secondary origin of ONSM is an important one; pONSMs are generally considered to be poor candidates for resection when vision preservation is a primary goal due to the anatomic relationship of the tumor cells of origin and the delicate vasculature supplying the tumor. 1 11 12 13
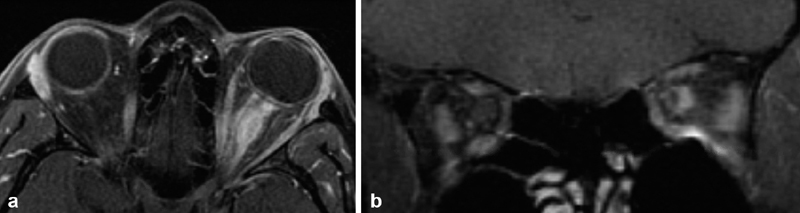
Overall, sONSMs are much more common than pONSMs. In reviewing 5,000 published cases of orbital meningioma, Dutton found that only 10% had originated from within the orbit, with the remaining 90% extending into the orbit from the intracranial space. Of those originating from the orbit, 96% arose from the optic nerve sheath itself, with 4% developing from an ectopic location inside the orbit. 1 6 11 The few cases of ectopic orbital meningioma that have been reported in the literature are exceedingly rare, with some authors questioning their true origin and existence. 14 15 In one such report, Tan et al described the case of what initially appeared to be an ectopic orbital meningioma based on magnetic resonance imaging (MRI) imaging findings. However, subsequent specialist's review of the MRI revealed the subtle finding of a dural tail connecting this lesion to an enhancing mass within the olfactory groove. 14 15 Among pONSMs, those that arise from the intraorbital portion of the optic nerve sheath are significantly more common than those arising from the intracanalicular portion. In the meta-analysis series by Dutton, only 8% were intracanalicular. 1 In addition, although ONSMs are typically unilateral, 5% may be bilateral. 1 16 ( Fig. 1A, B ). While bilateral ONSMs are often found in patients with neurofibromatosis type 2 (NF2), these lesions have been reported in patients without NF 2 in several studies. 1 6 16 17 18 19
Fig. 1.
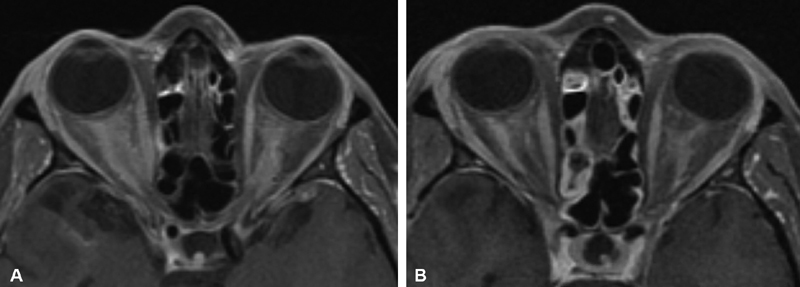
( A ) Axial T1-weighted, postcontrast MRI from a patient who presented with progressive bilateral visual loss over 2 years, and was found to have bilateral optic nerve edema and bilateral optic disc shunt vessels on exam. Symptoms were not responsive to a course of corticosteroids. ACE level and Gallium studies were normal without evidence of sarcoid. Right optic nerve biopsy via superior medial orbitotomy was nondiagnostic but showed no evidence of inflammation nor granuloma. The diagnosis of bilateral optic nerve sheath meningioma was therefore presumptive, and treated with 5,040 cGY IMRT radiation in 28 fractions of 1.8 Gy with significant improvement in vision after treatment. ( B ) 3 years later, with stable improved bilateral vision, axial T1-weighted, postcontrast MRI showed persistence of bilateral enhancement supporting the presumptive diagnosis of bilateral pONSM. ACE, angiotensin-converting enzyme; IMRT, intensity-modulated radiotherapy; MRI, magnetic resonance imaging; pONSM, primary optic nerve sheath meningioma.
Though the distinction between pONSM and sONSM carries important therapeutic implications, it must be noted that it may be difficult to clinically and radiographically differentiate between the two. 8 10 20 21 This shortfall is also apparent in the literature which skews some reports and further complicates our understanding of the differences between them. Just as sONSMs may originate intracranially and then spread within the subdural plane to involve the optic nerve sheath, pONSMs may originate within the intraorbital or intracanalicular portions of the optic nerve sheath and then spread proximally to involve the intracranial space. Thus, a given sONSM extending into the optic canal may be similar in appearance to an intracanalicular pONSM that extends intracranially. Similarly, the true pathological development of bilateral ONSMs is not entirely understood. Dutton also found that approximately 50% of patients with bilateral ONSMs displayed tumor extending across the planum sphenoidale, continuous with both ONSMs 1 ( Fig. 2A–C ) This finding calls into question the true origin of bilateral ONSM, and has led several authors to postulate that bilateral ONSMs may actually be the result of skull base meningiomas that have grown to involve the bilateral optic nerves. 6 7 16 17 22 23 24 In a series of 88 patients with ONSM, Saeed et al note that due to involvement of the planum sphenoidale in two of their total of four patients with bilateral ONSM, they were unable to determine if the tumor originated from one optic canal and extended into the other, or if the tumor actually developed at the planum and then extended into both canals from that point. 16 Furthermore, in the series by Dutton, 65% of the bilateral ONSMs were intracanalicular (and 38% of intracanalicular ONSMs were bilateral), while only 5.7% of the unilateral ONSMs involved the optic canal. 1 This further supports the possibility that at least some bilateral ONSM may represent intracranial meningiomas that invade the bilateral optic canals (sONSM), or ONSM with unilateral primary origin that subsequently spread to the contralateral optic nerve (pONSM); as opposed to those which originate bilaterally and separately. 1 6 16 17 These are important factors to consider, both when reviewing the literature, and when approaching an individual patient. Indeed, this distinction has treatment implications, as pONSMs are “true” ONSMs, originating from the cells surrounding the nerve and therefore generally unresectable without damage to the nerve.
Fig. 2.
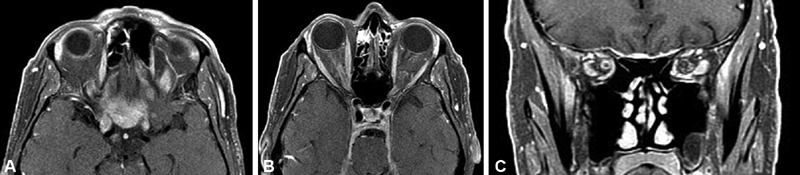
( A–C ) Axial ( A, B ) and coronal ( C ) T1-weighted postcontrast MRI images from 63-year-old male patient with 5-year history of no light perception in the right eye, and left eye visual acuity worsening from 20/20 to finger counting over the course of 1 year. Patient was found to have secondary intraorbital ONSM with extension from tuberculum sellae and planum sphenoidale affecting both optic nerve sheaths. Biopsy revealed lymphoplasmacyte-rich meningioma. He subsequently underwent modified orbitozygomatic, pretemporal transcavernous approach for resection of intradural anterior skull base tumor, and left optic nerve decompression. This rare form of meningioma may be confused with idiopathic hypertrophic pachymeningitis and other entities in which surgery may have a role as well as immunomodulators, and corticosteroids may have a role in long-term therapy. MRI, magnetic resonance imaging; ONSM, optic nerve sheath meningioma.
ONSMs occur more commonly in females (a finding common to meningiomas in general) and have been known to increase in size during pregnancy and during the menstrual cycle. 1 4 6 16 25 26 It is estimated that 55 to 85% of pONSMs occur in females and typically present in patients at approximately 40 years of age on average, generally between the ages of 30 and 50 years. 1 2 4 5 26 27 28 In the study by Dutton, 61% of patients were female, representing a female-to-male ratio of 3:2, comparable to the female preponderance known for intracranial meningioma, and the average at presentation was 40.8 years overall (42.5 years in females and 36.1 years in males, with a range of 3–80 years). 1 7 29 These figures are similar to those noted in other studies. 4 5 7 16 28 30 Notably, the age at presentation is slightly younger on average than that of intracranial meningioma, which generally present after the fifth decade of life. 4 6 25 31 Male patients may also present at younger ages; in a review of 50 patients, Wright et al found the median age at symptom onset to be 30 years of age for males and 42 years of age for females. 28 In addition, bilateral ONSM typically present at even younger ages, often in the first decade of life (mean age at symptom onset 12.8 years in Dutton). 1 6 25 Other risk factors for ONSM include history of NF2 and exposure to ionizing radiation. 11
Pediatric Optic Nerve Sheath Meningioma
Pediatric optic nerve sheath meningiomas represent approximately 2 to 4% of ONSMs overall. 1 2 ONSM is known to be associated with NF2, with an overall incidence of 2 to 8% in patients with NF2. 1 27 28 32 33 Approximately 28.3 to 33% of pediatric patients with pONSM are ultimately found to have NF2, and they present with ONSM at younger ages. 27 In reviewing 70 previously published cases of pediatric pONSM, combined with 8 of their own, Narayan et al found that 29% of patients had been diagnosed with NF2, and for many of them, their pONSM was the first manifestation of NF2. 34 The incidence of ONSM in NF2 patients, in screening 467 NF2 patients (children and adults) for tumors affecting vision, Li et al report that 3.6% of patients were found to have pONSM with a median age at diagnosis of 14. 33 Given the high prevalence of NF2 in children with ONSM, several authors recommend that any child found to have ONSM should be evaluated for NF2. 27 34
In addition, it is important to recognize that the natural history of pediatric pONSM may be more aggressive than the adult form, with an increased risk of progression, intracranial extension, and visual loss 27 35 36 37 ( Fig. 3A–H ). In a study of 88 patients, Saeed et al found that 53% of patients aged 30 or younger had intracranial disease, and the average volume of the intracranial component of the tumor was 829 mm 3 . By comparison, only 25% of patients aged 16–65 3 and 17% of those 61 and older had intracranial extension, with average tumor volumes of 344 and 41 mm, respectively. 16 In a study of eight pediatric patients by Narayan et al, out of six patients who were managed conservatively with observation, four (67%) demonstrated worsening of vision at follow-up. Only two patients had good vision at presentation and did not experience tumor progression. In addition, when combining their cases with those they reviewed from published literature, the authors note that 27% of patients (12 out of 44) had intracranial disease at the time of presentation, and 31% (5 out of 16) of patients treated with surgery who initially did not have intracranial disease later developed intracranial extension posttreatment. 34 By contrast, it is estimated that only 20% of adult patients with ONSM ultimately develop intracranial disease. 1 27 34 Historically, several authors have favored surgical resection of ONSM in children given this aggressive behavior, including those who recommended complete resection of tumor and optic nerve, as well as exenteration in cases of orbital spread. 27 38 However, there has been a shift away from surgery in recent years due to the high rate of surgical morbidity. 27 In addition, despite the fact that ONSM may behave more aggressively in children, there is an absence of evidence to suggest that age has any bearing on tumor-related mortality (which is negligible) or visual prognosis in the contralateral eye. 1 27 Narayan et al conclude that pediatric patients with good vision at presentation should be managed conservatively with observation as some pONSM in children may not progress. However, radiation treatment should be considered in those who do experience progression. 27 34 They also caution that greater research is needed to investigate the complications of radiation in children, as radiation treatment has been known to cause the development of additional benign or malignant tumors (including meningioma) that may present many years later and may be more aggressive than their sporadic counterparts. 34 39 40 In addition, the potential pituitary dysfunction that may result from radiation may cause additional problems unique to the pediatric population. 27
Fig. 3.
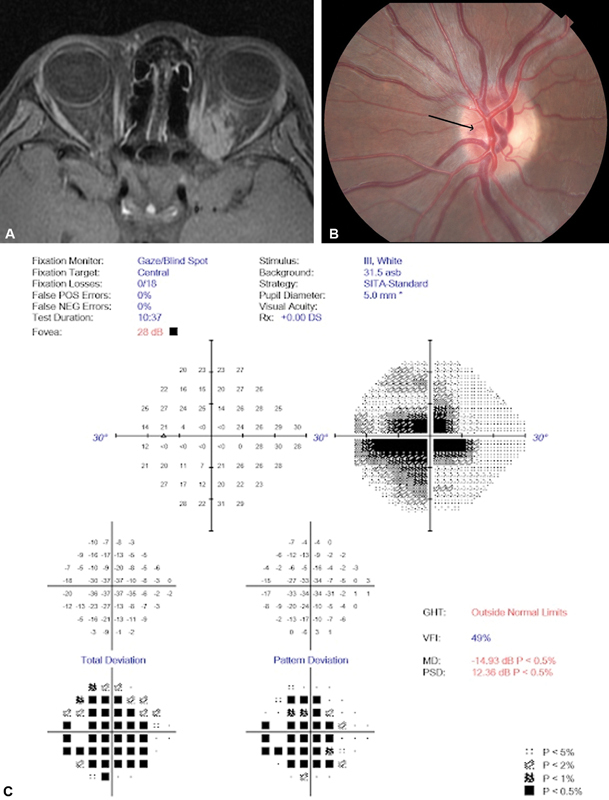
( A ) Axial T1 postcontrast fat-suppressed MRI, obtained at initial presentation at age 11 years, demonstrates a posterior orbital left enhancing mass originating from the optic nerve and infiltrating the orbit without significant prechiasmal involvement. ( B ) Fundus photograph of the involved left optic nerve demonstrating mild left disc swelling worse at the superior and nasal nerve, with deep nasal optociliary shunt vessel present (arrow). ( C ) A visual field prior to proton beam conformal therapy shows a dense cecocentral scotoma. ( D ) Axial T1 postcontrast fat-suppressed MRI obtained 4 years after initial presentation demonstrates significant tumor progression, extending intracranially through the left optic canal and orbital fissure, spreading along the falciform ligament and prechiasmal optic nerve. ( E ) Coronal T2 fat-suppressed MRI identifies the inferiorly displaced nerve as distinct from the surrounding tumor, albeit the CSF ring is absent on this cut but visible more anteriorly (not shown). ( F ) Axial fat suppressed T2 MRI, further revealing the optic nerve distinct from surrounding tumor, although with only a small CSF outline. ( G ) Axial T1 postcontrast fat-suppressed MRI postirradiation showing left central low signal, thought to represent radiation necrosis. ( H ) OCT shows an elevated nerve fiber layer and smaller optic cup seen with disc edema. The temporal nerve fiber layer appears pathologically thinned consistent with the central scotoma demonstrated by visual field testing. CSF, cerebrospinal fluid; GHT, glaucoma; MD, mean deviation; MRI, magnetic resonance imaging; NEG, negative; OCT, optical coherence tomography; OS, oculus sinister (left eye); OD, oculus dexter (right eye); PSD, pattern standard deviation; POS, positive; SITA, swedish interactive thresholding algorithm; VFI, visual field index.
Case Presentation: Progressive Intracranial and Orbital Extension in a Child Without NF2
An 11-year-old girl initially presented with an orbital mass causing 6 mm of proptosis, but preserved visual function without obvious optic nerve dysfunction, and was subsequently diagnosed with presumptive left-sided ONSM. Original MRI images were recovered ( Fig. 3A ). Lost to follow-up, she returned for medical attention 4 years later at the age 15 years, having progressed to 14 mm of left proptosis, mild optic disc edema with subtle cilioretinal shunt vessel of her optic nerve ( Fig. 3B ), ipsilateral cecocentral scotoma ( Fig. 3C ), depressed color vision, and 20/30 visual acuity in the affected eye. MRI at this time showed significant orbital and intracranial extension of her pONSM. The tumor had progressively extended intracranially through the left optic canal and orbital fissure and spread along the falciform ligament and prechiasmal optic nerve ( Fig. 3D , 3E , 3F ). Because of the rapid progression, the patient subsequently underwent anterior orbitotomy with biopsy of the orbital component of the deep orbital tumor. Histopathologic analysis confirmed meningothelial meningioma (grade I) with focal hypercellularity, with small cells and high nuclear–cytoplasmic ratio, immunoreactive to vimentin, and EMA (epithelial membrane antigen). K i -67 proliferative index was less than 1%.
After considerable multispecialty discussion which also involved neurosurgical consultation, the patient then underwent proton beam treatment rather than further orbital or intracranial debulking. Her course was complicated by 3 to 4 months of steroid-dependent painful increasing proptosis, related to radiographically diagnosed presumed intraneural necrosis, ( Fig. 3G ) with steroid responsive extraocular muscle myositis, as well as further increase in orbital tumor mass. Over the next 6 months, her course stabilized without further clinical or radiographic progression. Her visual acuity remains 20/25 approximately 1 year following radiotherapy (RT), with improved although asymmetric color vision, persistent central scotoma, persistent restricted extraocular motility, and resolved optic disc head swelling replaced by mild optic pallor. Optical coherence tomography (OCT) shows improved swelling of the left nerve head, with mild loss of the temporal nerve fiber layer bundle consistent with mild optic atrophy ( Fig. 3H ).
Unique Considerations in Children
With regard to NF2, one must consider that these patients may also carry an increased risk of radiation-induced malignancy, as well as the fact that individuals with childhood-onset NF2 may display a particularly aggressive form of disease. 32 41 42 Furthermore, the clinical and visual monitoring of pediatric patients including those with NF2 may present unique challenges, often requiring the need for serial MRI under anesthesia. A variety of electrophysiologic monitoring techniques, including flash and pattern visual-evoked potential (VEP), may be useful, especially in monitoring preverbal children. 43
While the utility of multifocal VEPs (mfVEPs) in monitoring ONSM has not been extensively studied, a recent study by Jayanetti et al reports that mfVEP was able to detect changes in optic nerve function that correlated with vision loss and preceded visible changes on MRI in three NF2 patients (five eyes) with ONSM. The authors suggest that mfVEP may represent another useful tool to monitor visual function in patients with NF2, particularly in children who may not tolerate frequent MRI. 44 Unfortunately, mfVEP remains limited in availability, compared with other more common forms of monitoring that are appropriate in children able to sit for the evaluations. Various forms of automated and manual static and kinetic perimetry, as well as OCT are standard tools in ophthalmic practice in the adult population, whereas in children, the visual field is less reliable, and the OCT has no well-established norms in this age group. Change from baseline may be helpful to judge clinical progression. Usually at the time of presentation of these tumors, unlike optic nerve glioma, the child is old enough to perform some of these tests.
Bilateral ONSM may present in the first decade of life or shortly thereafter (average was 12.8 years in Dutton's review). 1 6 25 As a result, some authors have recommended that bilateral ONSM to be considered in the differential diagnosis of children presenting with unexplained vision loss. Nickel et al presented a case of a child initially presenting at the age of 4 years whose vision had severely deteriorated by the time she was 10 years. Although MRI was initially normal, it eventually demonstrated bilateral optic nerve atrophy, and further MRIs were suggestive of bilateral pONSM. However, by the time the diagnosis was made, the patient had little vision left. 18 Because ONSM imaging and clinical presentation may mimic other lesions, and the clinical course may be particularly aggressive in children, children with unexplained visual loss attributed to unilateral or bilateral optic nerve disease require a thorough and prompt evaluation ( Fig. 4A–C ). 18 27 34 36 37 45 46 47
Fig. 4.
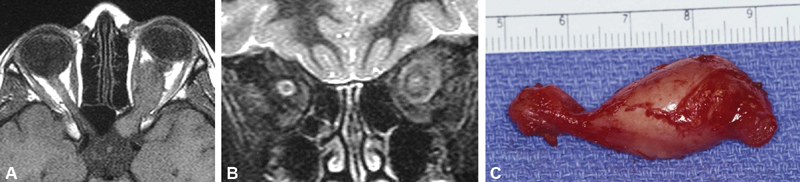
( A, B ) Axial ( A ) and coronal ( B ) T1-weighted MRI images, and intraoperative photograph ( C ) from a 12-year-old female patient with longstanding no light perception visual loss and proptosis of the left eye. Patient also had a history of NF1. The axial T1-weighted image demonstrates expansion and kinking of the intraorbital optic nerve extending to the left prechiasmal segment through an enlarged optic canal. The orbitocranial resection specimen demonstrates the entire nerve and prechiasmal area is expanded on the left. Histologic analysis confirmed meningothelial hyperplasia with microcalcification, (as demonstrated on the coronal T2 image), which may simulate meningeal involvement of an optic nerve sheath meningioma. In other imaging sections, one could confirm that the substance of the nerve was enlarged and expanded as well distinct from the arachnoid hyperplasia, which is an important finding for differentiation of meningioma from optic nerve glioma as in this juvenile pilocytic astrocytoma with Rosenthal fibers. Photo courtesy of Dr. Paul Langer and Dr. Neena Mirani. NF, neurofibromatosis; MRI, magnetic resonance imaging.
Clinical Findings
The classic presenting triad of progressive vision loss, optic nerve atrophy and presence of optociliary shunt vessels, with or without unilateral optic disc edema on fundus examination, has been described as pathognomonic for ONSM. While observation of this triad in an individual patient should prompt further evaluation for possible ONSM, few patients present with all three of these findings. 6 7 13 16 48 The most common presenting feature of ONSM is a progressive decline in both visual acuity and associated visual field. Visual field deficits and decreased color vision are also frequent symptoms. 1 6 10 16 26 28 30 In a study by Dutton, 96% of patients had decreased visual acuity. Visual field deficits were found in 83%, with peripheral constriction in 35%, central, centrocecal and paracentral scotomas in 29%, altitudinal defects in 16%, and increased size of blind spot in 13%. 1 Transient visual obscurations are also frequent findings, occurring in 13 to 23% of patients. 28 30 49 These may be spontaneous, gaze-evoked, and are occasionally precipitated by postural changes. 6 28 30 50 51 Gaze-evoked transient visual obscurations are thought to be ischemic in origin. 52 While orbital pain may occur, vision loss is typically painless. Visual decline occurs gradually over time; it is typically present for 1 to 5 years prior to presentation, and may progress to blindness in the affected eye. 1 6 7 25 26 28 In addition to visual symptoms, external signs such as proptosis and chemosis are frequently observed. 1 6 11 28 Proptosis is seen in 30 to 68% of patients and is typically mild, although it can become severe in some patients. 9 28 49 In a series of 50 patients, Wright et al found 36% to have proptosis, with a median of 2.0 mm, with a range of 0 to 11 mm. 28 In a series of 22 patients by Sibony et al, transient visual obscurations were the presenting symptom in 23% of cases. 30 Strabismus and ocular motility problems may also develop, and their origin is more often mechanical in nature than the result of cranial nerve dysfunction, especially in pONSM (although both may occur). 1 16 26 49 Less specific symptoms may include headache and seizure. 11
On examination, optic atrophy and/or edema of the optic disc are common; the result of compressive optic neuropathy caused by the tumor ( Fig. 5A ). Overall, the vast majority of patients (98% in the large series by Dutton) will show at least one of these findings. 1 7 Optic disc edema may progress to atrophy over time, and those with optic disc edema generally have better visual acuity than those with atrophy. 10 30 49 Afferent pupillary defect is also a common finding, observed in 86 to 93% of patients. 10 30 49 53 Optociliary shunt vessels have been historically known as a hallmark of ONSM, and are part of the classic presenting triad ( Fig. 5B ), though they are found in fewer than one-third of patients. These represent dilated collateral venous channels that connect the central retinal vein with the underlying choroidal circulation, and are caused by chronic compression of the central retinal vein, preventing venous return. 10 48 Notably, shunts are associated with other disease entities such as central retinal vein occlusion, pseudotumor cerebri, optic disc drusen, optic nerve arachnoid cyst, chronic glaucoma, and optic nerve glioma, as well as other causes of chronic papilledema, and have also been described as congenital in origin or as a possible normal finding in some patients. 54 55 56 Shunt vessels may also regress with treatment ( Fig. 6A–D ) and may also develop in the absence of observed optic disc edema ( Fig. 7 ).
Fig. 5.
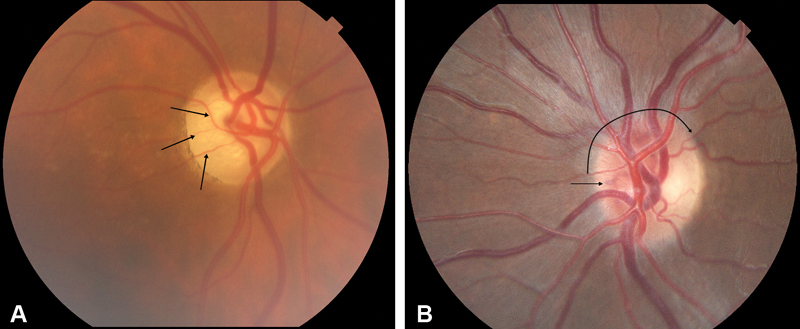
( A ) Right eye fundus photograph from patient with right-sided ONSM, demonstrating moderate temporal optic atrophy with narrowing of segmental components of retinal arteries on the disc (arrows), but no disc swelling nor optociliary shunt vessels seen in the classic triad. This optic atrophy is indistinguishable in this case from optic atrophy caused by other etiology. ( B ) Fundus photograph of left optic disc of a patient with pONSM, mild disc edema (curved arrow) and subtle nasal deep optociliary shunt vessel (arrow). ONSM, optic nerve sheath meningioma; pONSM, pONSM, primary ONSM.
Fig. 6.
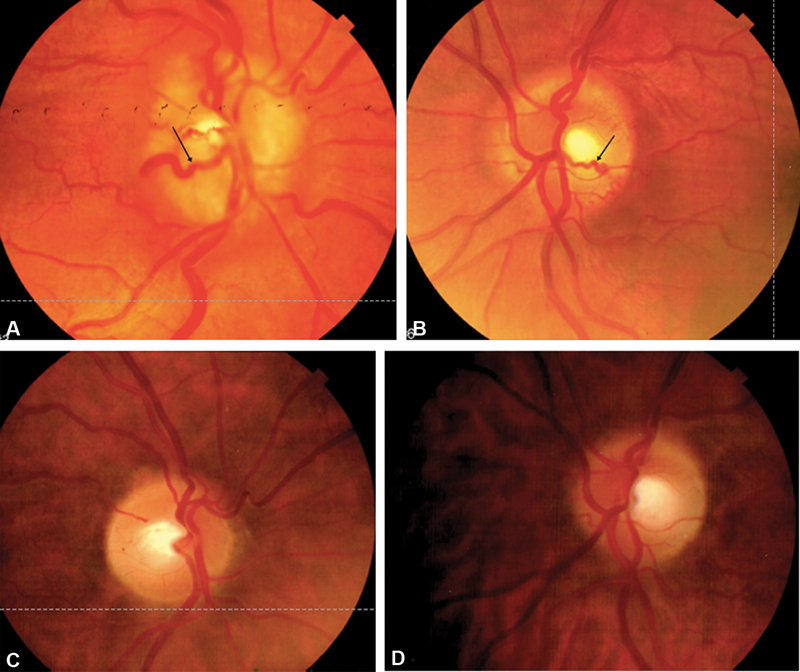
( A–D ) Fundus photographs (from same patient as in Fig. 1 ). Top images demonstrate low grade right ( A ) and left ( B ) optic disc edema and shunt vessels (arrows) supportive of bilateral ONSM. Bottom images demonstrate significant regression of right ( C ) and left ( D ) optic disc edema and previously dilated shunt vessels after radiation therapy. ONSM, optic nerve sheath meningioma.
Fig. 7.
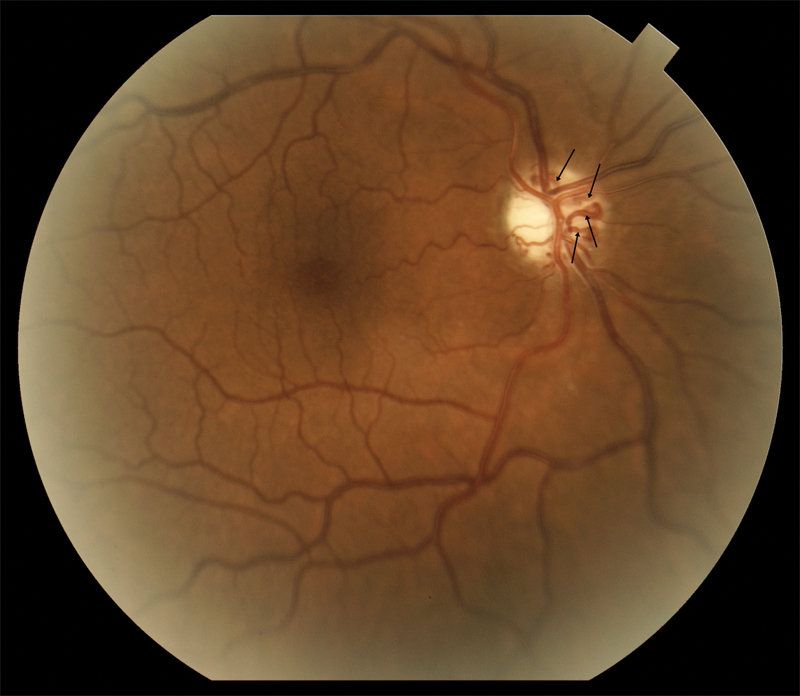
A fundus photograph of the right optic nerve in a patient with pONSM shows prominent optociliary shunt vessels (arrows) and optic atrophy without the presence of known optic disc edema. It is possible disc edema may have regressed prior to observation. pONSM, primary optic nerve sheath meningioma.
With regard to the classification of ONSMs, in 2003, Schick et al introduced a classification system based on tumor location. Type-I ONSM are pure intraorbital, with Ia lesions demonstrating flat extension around the optic nerve, Ib lesions taking the form of a mass growing concentrically around the nerve causing proptosis, and type Ic lesions demonstrating exophytic growth from the nerve. Type-II ONSM are located within the orbit and extend through the optic canal or superior orbital fissure. Type-IIa lesions are described as intraorbital tumors that grow through the optic canal, while type-IIb lesions involve the orbital apex and superior orbital fissure and may infiltrate the cavernous sinus in some cases. Type-III lesions originate intraorbitally and demonstrate >1 cm intracranial extension, with type-IIIa lesions extending to the chiasm, and type-IIIb lesions involving the chiasm extending to the contralateral optic nerve and planum sphenoidale. The pattern of intracranial extension may be described as diffuse or nodular. 57 58
Diagnosis
The diagnosis of ONSM is typically made on the basis of an appropriate clinical presentation supported by typical imaging findings, although biopsy is performed in some cases, some clinicians may discourage its use to prevent iatrogenic visual morbidity, as well as to avoid instances of intraorbital spread from the biopsy site which are occasionally reported. 6 38 45 53 59 Only atypical-appearing lesions need be biopsied.
MRI Imaging Techniques
High-resolution orbital and brain MRI is considered a standard technique in the radiologic evaluation for ONSM. 2 10 60 Identification of these tumors frequently requires modern high-magnetic field strength (at least 1.5 tesla), contrast-enhanced, and T1-weighted MRI with fat suppression of the orbit.
CT Imaging Techniques
Orbital computed tomography (CT) remains a readily available and useful tool with potential selected benefits over MRI in some scenarios. Orbital and brain CT are particularly sensitive to the detection of calcification, are excellent for defining optic canal and surrounding bone anatomy, and are of significantly shorter test duration than MRI imaging as well. 2 13 16 19
Nuclear Imaging
The role of nuclear studies including radiolabeled receptor ligand imaging remains in evolution, and various techniques may help differentiate meningioma from other inflammatory lesions in atypical cases, as well as to provide a gauge of relative clinical activity in treated patients. 18 61 62 63 64 65 66 Nuclear imaging techniques, such as positron emission tomography (PET) and single-photon emission computerized tomography (SPECT), may be of occasional utility. While several radiotracers have been studied in the imaging of intracranial meningiomas, radiolabeled somatostatin receptor ligands may be the best suited for evaluation of these tumors. 64 65 66 The most commonly used somatostatin receptor ligands used in PET imaging for meningiomas overall include 68 Ga-DOTANOC, 68 Ga-DOTATOC, and 68 Ga-DOTATATE (each labeled with Gallium-68; Fig. 8 ). 64 Due to the overexpression of somatostatin receptors (subtype 2, SSTR2) in meningiomas, the use of these ligands in PET imaging provides strong tumor-to-background contrast, resulting in high sensitivity for the detection of meningioma tissue. 65 67 68 The sensitivity of this imaging technique has been found to be higher than that of MRI in some studied; Rachinger et al found 68 Ga-DOTATATE to have a sensitivity of 90%, while MRI had 79%. 65 In addition, these ligands have demonstrated superior specificity as radiotracers by comparison to other somatostatin receptor ligands studied in meningioma, including 111 Indium-labeled octreotide. 68 69 Although more data are available regarding nuclear imaging for intracranial meningiomas, rather than ONSM specifically, several authors have reported on the use of somatostatin receptor analogs and PET imaging for tumors of the optic pathway. 68 70 71 72 Klingenstein et al reviewed the cases of 13 patients with ambiguous lesions of the optic pathway, and found that 68 Ga-DOTATATE PET/CT was able to identify 10 meningiomas, with 100% sensitivity and specificity, successfully differentiating these tumors from the remaining three lesions. 70 In a retrospective series of 26 patients with ONSM, somatostatin analog PET/CT was used in 85% of patients for RT planning. Interestingly, the authors note that out of five patients whose visual acuity worsened post-RT, two demonstrated low radiotracer uptake on PET imaging, and consider that these patients may have been misdiagnosed with ONSM. The authors thus recommend the use of PET imaging to confirm diagnosis of ONSM to ensure proper patient selection and suggest that when PET findings are atypical, biopsy may be considered. 72
Fig. 8.
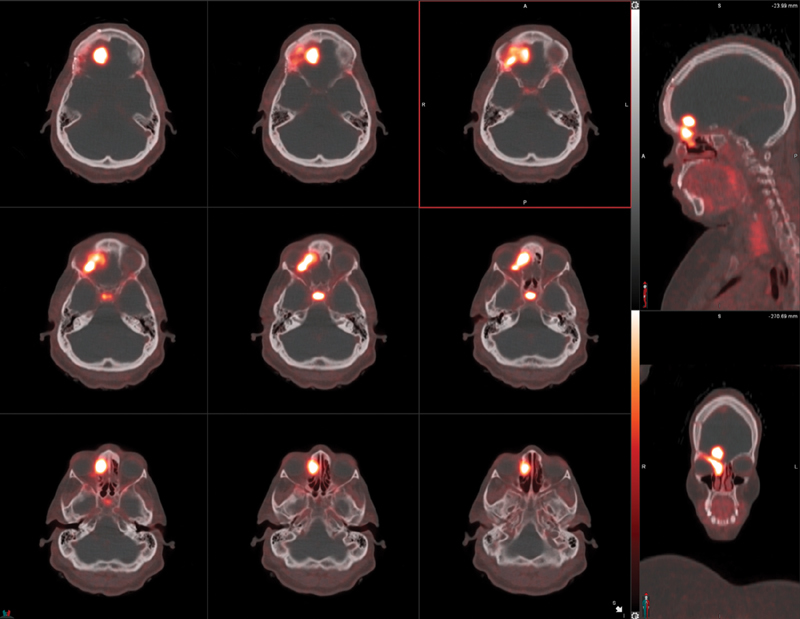
Avid radiotracer uptake in a biopsy proven incidental WHO grade-II planum sphenoidale meningioma, in a patient undergoing 68 Ga-DOTATATE PET study for metastatic neuroendocrine tumor. Image courtesy of Dr. Nasrin Ghesani.
In a prospective case series, Saeed et al evaluated the results of SPECT imaging with 111 Indium-labeled octreotide in 26 orbital meningiomas (including 14 ONSMs), reporting a sensitivity of 100% and specificity of 97.2% for ONSM. 73 The use of SPECT has also been described in several case reports in which the authors note that this imaging modality enabled them to confirm the diagnosis of ONSM when the etiology of vision loss was either unclear or previously misdiagnosed, allowing the patient to avoid biopsy. 74 75 SPECT/CT may also be used to track treatment response after radiation of ONSMs. 73 76 Andrews et al utilized 111 In-octreotide in SPECT/CT for six patients treated with radiation, observing decreased radiotracer uptake posttreatment in all patients. 76 Overall, while not intended to replace MRI as the primary imaging modality used in diagnosis of ONSM, nuclear imaging techniques may provide assistance in discriminating between ONSM and other types of lesions, perhaps avoiding a potentially vision-damaging biopsy. Nuclear imaging may also be used to assess tumor control.
Imaging Findings
Typically, ONSMs appear as a homogeneously enhancing mass surrounding the optic nerve in a sheath-like manner, globular, or alternatively, in an eccentric position to the nerve 10 16 72 76 ( Fig. 9A, B ). Five distinct patterns of optic nerve enlargement may be observed, the distribution of which are well illustrated in a retrospective series by Saeed et al; of 74 optic nerves, the tubular configuration was by far the most common (62%), followed by the globular form characterized by growth outside of the nerve sheath (23%). Less common was the fusiform pattern (11%) with focal enlargement of the optic nerve being the least common (4%).
Fig. 9.
Axial ( A ) and coronal ( B ) T1-weighted, fat-suppressed, contrast-enhanced MRI images from a patient with biopsy-proven left ONSM. Axial image shows the mass lesion of the intraorbital segment of the left optic nerve, which is hypointense on T1 sequence, enhances following contrast administration, and is isointense on T2 sequence (not shown). It measures approximately 1.2 cm in transverse and anteroposterior dimension. The coronal image of the posterior orbit shows the enhancing lateral tumor is separate from the medial compressed left optic nerve. MRI, magnetic resonance imaging; ONSM, optic nerve sheath meningioma.
Several classic imaging features associated with ONSM have been described, including the imaging sign known as “tram-tracking” ( Fig. 10 ). The meningioma appears as a thin medial and lateral line of bright soft tissue enhancing signal on CT or MRI. 2 6 13 78 79 80 Of historical interest, the bright signal in noncontrast-enhanced CT or tomograms represented linear calcium as a two-dimensional (2D) axial projection of calcium that may be circumferential in position. ( Fig. 11A, B ). Calcifications associated with ONSMs may also produce a tram-tracking appearance on either side of the optic nerve on CT imaging without contrast. 78 80 Tram-tracking has been described as a useful sign with regard to differentiating between ONSM and optic nerve glioma. The latter is associated with intrinsic enlargement of the glial elements of the nerve rather than the subdural elements of the meninges of the former. 78 80 81 However, this “classic tram track” finding is not observed in all patients with ONSM and may be present in other infiltrative optic nerve disease such as sarcoid 82 ( Fig. 12A , 12B ). Calcifications on CT are also considered as a common imaging finding in ONSM. In the aforementioned study by Saeed et al, calcifications were observed in 31%. Interestingly, the presence of calcifications was associated with slower tumor growth, although did not correlate with visual prognosis or intracranial disease. 16 In general, MRI with contrast is considered to be of greater utility than CT in evaluating ONSM due to its superior ability to resolve the soft tissues and is the best imaging study with regard to the identification and recognition of ONSM ( Fig. 13A ).
Fig. 10.
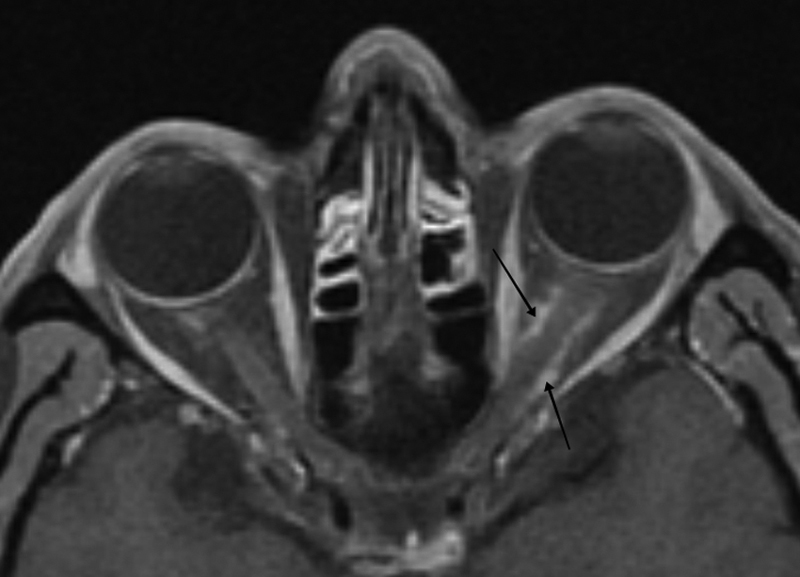
Axial T1-weighted, fat suppressed postcontrast MRI images of a left primary ONSM with tram-tracking enhancement in the mid and posterior segment (arrows) of the left intraorbital optic nerve. MRI, magnetic resonance imaging; ONSM, optic nerve sheath meningioma.
Fig. 11.
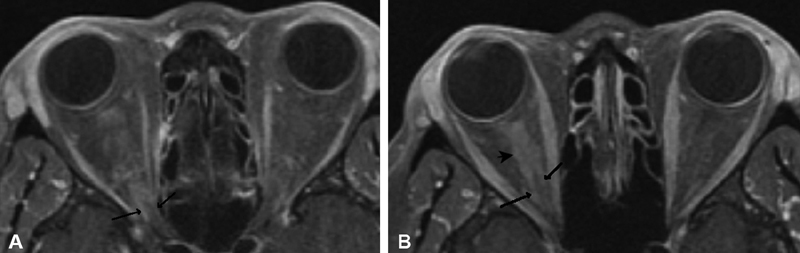
( A, B ) Axial T1-weighted, postcontrast MRI images of right biopsy proven primary ONSM with tram-tracking enhancement (arrows). The more diffuse complex enhancement (arrowhead) seen in the anterior right-sided aspect of the nerve in ( B ), if occurring at the correct angle, begins to suggest linear extension along the nerve. MRI, magnetic resonance imaging; ONSM, optic nerve sheath meningioma.
Fig. 12.
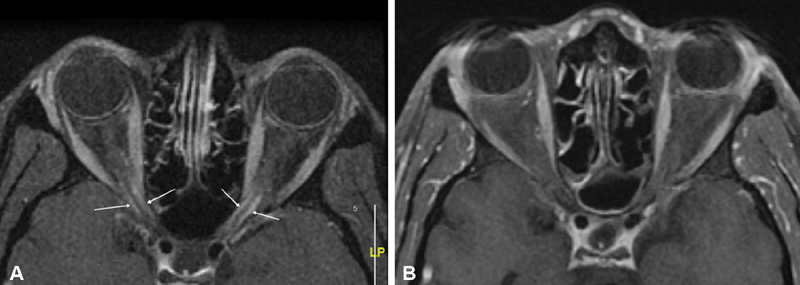
( A ) Axial T1-weighted, postcontrast MRI image shows bilateral “tram tracking” (arrows) of the intracanalicular segments of both optic nerves typical for intracanalicular ONSM. This case of biopsy proven neurosarcoid was associated with an elevated ACE level at 100, and lacrimal gland biopsy with noncaseating granulomas. ( B ) Axial T1-weighted, postcontrast MRI image of the same patient 14 years later shows durable complete resolution of the tram tracks after immunosuppressive treatment. ACE, angiotensin-converting enzyme; MRI, magnetic resonance imaging; ONSM, optic nerve sheath meningioma.
Fig. 13.
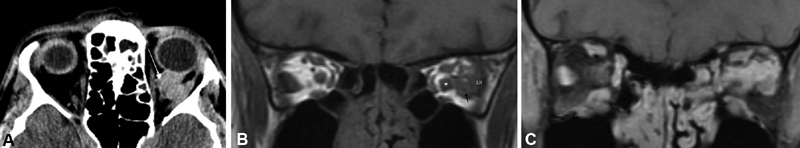
( A ) Noncontrast orbital axial CT image of the same lesion reveals scattered calcifications (arrow indicates large calcification) in the left retrobulbar space at the edge of the lesion, which may be seen in meningioma as well as venous orbital lesions such as this. ( B ) Noncontrast coronal T1 image without fat suppression demonstrates a left-sided lesion (arrow) in the posterior orbit distinct from the optic nerve, blending with the left lateral rectus (LR). In other areas of the orbit it was more difficult to separate the lesion from the optic nerve sheath complex. In this non-fat suppressed image, the bright signal of the fat serves to help delineate the nerve (asterisk) which is of normal size, separate from the lesion. ( C ) Coronal T1-weighted, postcontrast MRI images demonstrate a diffuse enhancing lesion extending from the optic nerve sheath complex, infiltrating the orbit between the nerve, superior rectus levator complex and lateral rectus. It would be too difficult to differentiate this vascular malformation from an ONSM on this image. CT, computed tomography; MRI, magnetic resonance imaging; ONSM, optic nerve sheath meningioma.
One rare imaging finding associated with ONSM (particularly intracanalicular ONSM) is that of pneumosinus dilatans, referring to the appearance of enlarged, pneumatized ethmoid, and/or sphenoid sinuses on imaging studies. 49 83 84 The exact cause of this phenomenon, as well as the mechanism by which it may cause vision loss, remain unknown. However, sinus obstruction, inflammation, and congenital abnormality have been suggested as possible reasons for this excess pneumatization, and it may potentially cause optic neuropathy due to compression on the nerve by a pneumatized cell containing air or mucosa. 85 Due to its association with ONSM, several authors recommend that patients with worsening vision loss who are found to have pneumosinus dilatans on imaging studies should be evaluated for possible ONSM, as this may be an “early sign” of the disease, occurring before the development of visible tumor growth. 83 84 85
Case Presentation: Chronic Orbital Vascular Lesion with Calcification Mimics ONSM
A 41-year-old man without visual complaint underwent CT and subsequent orbital MRI imaging at the time of a motor vehicle accident. A left orbital lesion was discovered and subsequent discussion with the patient suggested a many year history of intermittent episodes of transient left orbital congestion and proptosis. He had normal visual acuity of 20/20 and visual field testing. The lesion in the posterior orbit appeared distinct from the optic nerve, blending with the left lateral rectus. Although ONSM was a diagnostic consideration, his findings were more consistent with a chronic venous or mixed orbital vascular malformation with focal calcification ( Fig. 13A–C ).
Ophthalmic Monitoring
In most cases of pONSM and sONSM, preservation of visual function is a primary goal. Clinical progression should be measured with timely and repeated assessment of visual function by an ophthalmologist to follow chronic potentially progressive life-long optic neuropathy. The determination of best corrected visual acuity, color vision assessment and static or kinetic visual field monitoring are all considered standard in the long-term serial assessments necessary in the care of the ONSM patient. These various techniques are widely available and accepted but beyond the scope of this discussion. However, worth mentioning, an evolving and useful imaging modality in the evaluation and monitoring of the visual function in ONSM is OCT. Using OCT to measure the thickness of the retinal nerve fiber layer (RNFL) enables the clinician to quantify the extent of optic atrophy observed on examination which may assist in selecting treatment and predicting and monitoring response. 2 72 86 In a study of 12 patients (14 eyes) with anterior visual pathway meningiomas, Loo et al found that patients with normal pretreatment peripapillary retinal nerve fiber layer thickness (PRNFL) demonstrated significant improvement in visual acuity, color vision, and visual fields posttreatment, whereas those with thin pretreatment PRNFL did not improve; the prognostic value of OCT may be useful but we have also experienced patients who have improved despite thin RPNFL. Conversely, normal measurements of PRNFL may occur in the setting of anatomically thinned PRNFL with concurrent active swelling. Greater duration of symptoms prior to treatment was also noted to be an independent predictor of decreased improvement posttreatment. 86 This kind of prognostic information may guide choice of treatment, as significant loss of the RNFL and ganglion cell layer generally indicates likely irreversible damage. Eckert et al reported using OCT as part of their diagnostic workup for all patients in their series of 26 who were treated with RT for ONSM. The authors state that preserved RNFL loss in the absence of ganglion cell layer (GCL) loss on OCT supported the indication for radiation treatment in these patients, as this would indicate potential for functional improvement, even when associated with poor vision pretreatment. Patients with significant RNFL and GCL loss without useful vision in the affected eye underwent monitoring of visual function in the contralateral eye, only undergoing treatment if this was affected (note here we used the two terms peripapillary retinal nerve fiber layer thickness and retinal nerve fiber layer thickness as these authors used them in their original papers; however, these refer to the same structure). 72
Diagnostic Dilemmas
Multiple authors have illustrated similarities between ONSM and a wide variety of other radiographic mimics, with reports of ONSM most commonly misdiagnosed as optic neuritis (including acute papillitis and retrobulbar optic neuritis), perineuritis, 45 46 87 88 89 and infiltrating disease affecting the nerve sheath complex such as optic nerve sarcoid (which is also frequently misdiagnosed as ONSM), 82 90 91 92 93 94 lymphoma, and optic nerve glioma, 95 96 as well as other solitary orbital tumors such as cavernous hemangioma, 63 solitary fibrous tumor, and schwannoma. In addition, a variety of other causes of optic atrophy, optic disc vasculitis, retinal disease, optic nerve drusen, as well as ischemic optic neuropathy, are also clinical culprits. 45 46 Dilation of the optic nerve sheath, referred to as dural ectasia, optic hydrops, or patulous nerve sheath ( Fig. 14 ) may be a normal finding, may be seen in cases of increased intracranial pressure, as well as in cases harboring an occult compressive lesion, and lead to diagnostic confusion. 97 Furthermore, there have been reports of lesions including optic nerve glioma, 98 orbital melanoma, 99 metastatic squamous cell carcinoma from the uterine cervix, 100 metastatic breast carcinoma, 101 and immunoglobulin (Ig)-G4-related disease 102 that were initially diagnosed as ONSM. This diagnostic mimicry contributes to the frequent misdiagnosis of ONSM. In a retrospective chart review of 35 patients with ONSM seen at a single neuroophthalmology practice over a period of 15 years, 71% of patients were initially misdiagnosed, and did not receive a correct diagnosis for an average of 62.6 months (standard deviation [SD] = 89.25 months). Improper differential diagnosis was felt to play a role. Delay in diagnosis led to vision loss in 64%, as well as unnecessary laboratory tests in 48%, and unnecessary lumbar puncture in 20%. 45 Ineffective treatment with steroids (for presumed optic neuritis and temporal arteritis) occurred in 24% and has also been reported in other studies. 45 46 72 Notably, of 16 patients who ultimately received a correct diagnosis after being referred to the authors' institution, 11 patients had received misinterpreted MRI results (five due to interpretation by nonneuroradiologists and six due to lack of proper orbital and contrast enhanced sequences). 45 Failure to perform adequate imaging of the optic nerve has also been reported in other cases of misdiagnosis. 46 87 These studies highlight the importance of maintaining adequate clinical suspicion for ONSM when appropriate, as well as the importance of performing the correct diagnostic evaluation for ONSM. Failure to perform a thorough evaluation in a patient with ONSM may not only result in continued unexplained vision loss but also may progress to blindness; it may also result in unnecessary and potentially invasive testing, as well as the administration of ineffective treatment (such as corticosteroids). However, in the setting of atypical clinical presentation or progression, a brief corticosteroid trial may be warranted prior to consideration of biopsy. 21 103
Fig. 14.
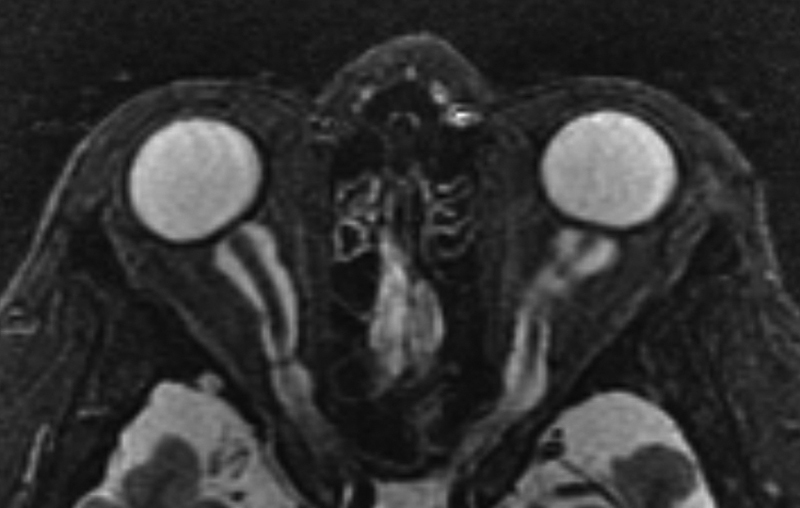
STIR axial MRI image in a patient with dural ectasia of the optic nerves, with severe bilateral optic nerve sheath dilatation/ectasia and flattening of the globes. STIR, Short-T1 Inversion Recovery; MRI, magnetic resonance imaging.
Case Presentation: Chronic Inflammatory Demyelinating Polyneuropathy–Mimicking ONSM
A 56-year-old woman was referred for evaluation of bilateral proptosis with resistance to digital globe retropulsion. She had a history of resection of multiple “Morton's neuromas” and suffered from a relatively severe distal motor and sensory peripheral neuropathy. MRI imaging of the brain and orbit revealed bilateral perineural enhancing lesions along the optic nerves simulating bilateral optic nerve meningiomas ( Fig. 15A ), bilateral enlarged lacrimal nerve cysts ( Fig. 15B ), as well as infiltration of both cavernous sinus, Meckel's caves, and adjacent structures ( Fig. 15C ). Despite the suggestion of potential NF2-related bilateral ONSM and bilateral lacrimal area schwannoma, subsequent multiple biopsies confirmed hypertrophic neuropathy of chronic inflammatory demyelinating polyneuropathy (CIDP) mimicking NF2 manifestations. Intraoperative intraorbital endoscopic photograph obtained via a Kronlein lateral orbitotomy after drainage of a large cyst associated with the supraorbital branches of the nerve ( Fig. 15D, E ), allowed for a biopsy of the tangles of enlarged posterior ciliary nerves simulating bilateral ONSM. Biopsy of the cystic lesion and ciliary nerve confirmed biopsy proven enlarged posterior ciliary nerves ( Fig. 15F ). Electron microscopy of a sural nerve biopsy showed findings of advanced CIDP ( Fig. 15G ). Case previously published in 2013. 104
Fig. 15.
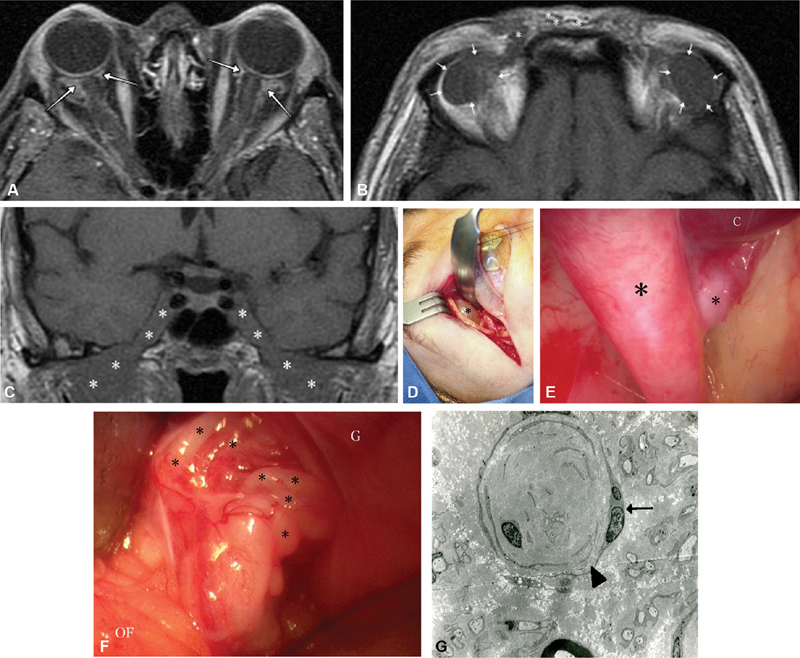
( A ) Axial T1 postcontrast fat-suppressed MRI demonstrating bilateral perineural enhancement (arrows) at the junction of both optic nerves and globes with subtle enhancement of the nerve sheaths suggestive of tram-tracking. In fact, this is a surgically confirmed mesh of enlarged ciliary nerves. ( B ) T1 axial noncontrast MRI demonstrating enlarged low signal lesions (outlined by arrows) against the background of orbital fat, later biopsy proven to be fluid filled cystic change associated with hypertrophic frontal nerve branches of CIDP. Bundles of enlarged, hypertrophic nerve branches (asterisks) are also visible within the subcutaneous tissue. ( C ) Coronal T1-weighted MRI revealing infiltration of bilateral cavernous sinuses, Meckel's caves and adjacent structures (asterisks indicate tumor). ( D ) Kronlein's lateral orbitotomy allowed access to the enlarged orbital cyst (asterisk) extending from underneath the orbital rim, which originated from a terminal branch of the frontal nerve, as well as the perineural tissue at the junction of the optic nerve and globe (see Fig. 15F ). ( E ) Endoscopic intraorbital photograph of the cyst (C, cyst) originating from the branching point (asterisks indicate nerve branches) of a hypertrophic supraorbital division of the frontal nerve, against the background of the orbital roof. ( F ) Intraoperative photograph shows tangles of enlarged posterior ciliary nerves (asterisks) at the junction of the optic nerve and globe (G, globe; OF, orbital fat). The optic nerve would be deep to these tangles; however their enlargement obscures the nerve (the ciliary nerves are not normally visible in this setting). Biopsy of a ciliary nerve confirmed hypertrophic neuropathy from CIDP. ( G ) Scanning electron microscopy of the same patient's sural nerve, which shows duplication of Schwann's cell process (arrowhead), multiple nuclei (arrow), and nearly complete loss of myelin with severe loss of axons. CIDP, chronic inflammatory demyelinating polyneuropathy; MRI, magnetic resonance imaging.
Case Presentation: Schwannoma-Mimicking OSNM
A 34-year-old woman complained of proptosis and blurry right vision. She was found to have normal visual acuity of 20/20 but a prominent afferent pupil defect consistent with an optic neuropathy. Orbital imaging revealed a solitary left orbital mass ( Fig. 16A ). Further inspection could delineate the lesion from the optic nerve ( Fig. 16B ) and T2 sequences showed areas with mixed signal ( Fig. 16C ). In addition, the tumor appeared to widen the superior orbital fissure rather than the optic nerve, supporting a peripheral nerve tumor rather than primary involvement of the optic nerve. A resection was planned, and subtotal resection of the tumor confirmed a schwannoma with s-100 positive spindle cells in a storiform pattern and verocay bodies consistent with schwannoma.
Fig. 16.
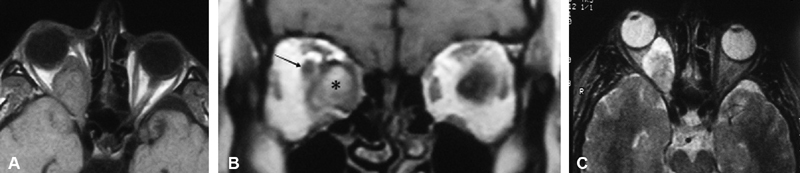
( A ) T1-weighted axial MRI without contrast reveals a large right intraconal mass largely replacing the orbital fat, with posterior extension initially believed to involve the optic canal. ( B ) T1-weighted coronal MRI with contrast without fat suppression delineates the tumor (asterisk) as being distinct from the optic nerve (arrow), which is displaced superolaterally. ( C ) Axial T2-weighted MRI images show a mixed tumor with hyper intense and hypointense signal with sparing at level of the right optic canal. MRI, magnetic resonance imaging.
Natural History, Goals of Treatment, and Indications
ONSM are generally benign, slow-growing tumors associated with ipsilateral visual loss. They arise from the arachnoid cap cells of the meninges surrounding the optic nerve (the optic nerve sheath) in pONSM and from the intracranial dura in sONSM. 1 6 10 These tumors invade the orbit and optic canal by spreading through the subdural space, frequently surrounding the optic nerve in a circumferential fashion. Growth may extend through the nerve sheath, obliterating the pial blood supply where compression may result in ischemia, disruption of axonal transport, and demyelination. 7 10 57 Additional ischemia results when an ONSM grows to obstruct the central retinal artery and/or vein. 8 In some cases, tumor infiltrates the substance of the nerve or optic disc. 57 Furthermore, some pONSMs may also extend intracranially, occasionally spreading to the optic chiasm, and contralateral optic nerve. 9 25 26
Progressive growth, causing direct neural compression with ensuing optic atrophy, vascular compromise, and/or direct infiltration of the nerve leads to progressive vision loss in most untreated cases. 53 105 Thus, the primary goal in treating optic nerve sheath meningioma is generally preservation of ipsilateral vision. 1 13 Secondary treatment goals include confirmation of histologic diagnosis or assessment of histologic tumor grade, as well as treatment or prevention of intracranial spread, contralateral vision loss, diplopia, disfiguring proptosis, ocular surface exposure, neurotrophic ocular ulceration, pain, and other cosmetic deformities.
Although some patients may maintain stable visual function without treatment, many will ultimately suffer worsening vision loss as time goes on. Dutton reported that 86% of patients whose tumors were simply observed over time (follow-up range: 1–7 years) ultimately suffered worsening vision loss. 1 In another major series, Saeed et al reviewed the cases of 88 patients (92 eyes), 39 of whom were managed with observation alone, 47 underwent surgery, and 6 were treated with RT. Overall, in monitoring patients' visual acuity changes either during their observation period or prior to treatment initiation (mean follow-up duration: 7.2 years), the authors found that of 45 eyes presenting with a visual acuity of 20/50 or better, 71% retained this at a mean follow-up of 5.2 years, while 29% worsened. Of those who worsened, 31% deteriorated to 20/50 to 20/200, 46% to 20/200 or less, and 23% to no light perception. These results suggest that a substantial proportion of individuals with good vision at presentation may remain stable for several years; however, the authors note that as the duration of observation increases, so does the number of patients who will ultimately decline. Notably, of patients followed for 5 years or more, only 29% were able to maintain visual acuity of 20/50 or better (compared with 71% overall). 16
With regard to radiation treatment, better outcomes have been associated with higher visual acuity at presentation, as well as the absence of optic disc abnormalities (such as optic atrophy) that may indicate irreversible damage. 72 106 107 108 109 110 111 112 This may influence the choice to initiate treatment versus management with observation. Seemingly a priori, visual outcomes are improved if ONSM can be stabilized before irreversible damage occurs. Timing of treatment, on the other hand, depends on a variety of factors including efficacy, durability, expense, and possible morbidity. Accordingly, patients with normal vision and those with mild deficits who have not shown clinical progression may be managed via careful observation for progression, with several authors recommending treatment initiation after there has been a documented decline in visual function. Several suggest a visual acuity of 20/40 or 20/50 as a threshold. 9 16 53 105 112 113 We prefer to depend on detection of clinically reproducible decline that correlates to a symptomatic decline in the vision or visual field as an early indication to treat in our practice. However, the timing of treatment initiation remains controversial, particularly with regard to radiation, as emerging evidence suggests initiating treatment prior to significant visual decline may result in better outcomes. 106 107 108 110 111 112 114 Due to the potential for rapid decline, close visual and radiographic follow-up is recommended. Jeremic and Pitz caution that while observation may be an appropriate approach for some patients, the longer one waits to initiate treatment, the greater risk there may be to those patients whose vision will ultimately worsen, with fewer chances to preserve any remaining vision with treatment. 115 There is also evidence indicating that certain prognostic factors such as preoperative visual acuity, symptom duration, and anatomical characteristics may influence surgical outcomes. 57 58 116 117 118 These may ultimately influence both surgical timing and the decision to recommend surgery in a given patient.
Several series comparing different treatment modalities have reported superior outcomes for patients treated with RT alone, in comparison to those treated with surgery, or a combination of surgery and RT. 53 113 119 120 Many cases of surgical resection of pONSM have been associated with immediate postoperative visual loss, generally attributed to iatrogenic injury to the pial blood supply of the optic nerve or vasospasm of the vasa nervorum caused by manipulation of the nerve. 1 7 9 10 12 13 Some patients who suffered fluctuating perioperative progressive visual loss may be responsive to corticosteroid and/or antivasospastic therapy. Given the overall high rate of surgical morbidity, combined with documented efficacy RT, there has been a paradigm shift away from surgery toward RT in the modern era, particularly for patients with good vision at presentation. As a result, surgical resection is generally reserved for patients with significant intracranial involvement that may impact the optic chiasm or contralateral optic nerve, which is more common in sONSM, patients with severe proptosis and/or ocular pain, or those that are already blind in the affected eye particularly in whom en bloc resection is considered. 1 2 13 28 121 122 However, there have been some recent reports indicating favorable outcomes after bony optic canal decompression and orbital decompression, with or without tumor resection. 57 58 116 117 118 121
It is important to note that surgical goals may vary between patients with useful vision and those who have progressed to blindness. Surgery in blind patients remains controversial, given that the mortality risk from ONSM is generally negligible; in the large series by Dutton et al, tumor-related mortality was 0% for ONSM. 1 28 While some authors have recommended resection for blind patients with the goal of preventing intracranial extension, intracranial disease does not develop in all patients, and modern imaging techniques are relatively sensitive to early detection. 9 13 28 Furthermore, it remains unclear how intracranial extension ultimately changes prognosis, particularly in the absence of reported mortality. 13 Surgical resection should be reserved for patients with severe disfigurement or clear evidence that intracranial extension is likely to affect the contralateral nerve, chiasm, or hypothalamus.
It is also important to note that while diagnosis of ONSM is generally based on clinical and imaging findings due to the morbidity associated with biopsy, not all ONSMs present in a typical fashion. 45 46 While biopsy of ONSMs does carry risk, including iatrogenic injury to the nerve, or orbital tumor spread, it may be necessary to obtain a tissue diagnosis. 22 28
Overall, given the clinical sequelae and multiple possible treatment options available for patients with ONSM, multiple specialties may be involved in the diagnosis and treatment of these patients. These tumors are optimally managed by an interdisciplinary team of ophthalmologists (including neuroophthalmologists and orbital specialists), radiation oncologists, neurosurgeons, medical oncologists, and neurologists. 29 72 103
Treatment
The Evolution of Radiotherapy
The acceptance of RT as the primary treatment for ONSM is a relatively new development. Prior to the 1980s, the development of radiation as a treatment modality in ONSM was hampered by concerns regarding potential radiation toxicity, as well as the historical widely held idea that meningiomas are radioresistant. 16 123 124 While the first report of radiation used as primary treatment for ONSM was by Byers in 1914 (who credited McReynolds), it was not commonly used until Smith et al reported it as effective in the treatment of pONSM in five patients. 51 124 125 Subsequently, other favorable reports on the use of conventional RT began to emerge. 1 51 113 124 In 1992, Dutton et al summarized the results of 12 published cases, reporting improvement of visual acuity in 75% patients, stability in 8%, and worsening in 17% (follow up range: 2–6 years). 1 Since that time, radiation therapy evolved into multiple modalities used in the treatment of ONSM, including three-dimensional (3D) conformal radiation therapy (CRT), intensity modulated radiation therapy (IMRT), stereotactic fractionated radiotherapy (SFRT), and radiosurgery; each of which offers its own advantages.
One study that played a critical role in demonstrating the superiority and durability of radiation therapy over surgical resection was published by Turbin et al in 2002. In this study of 64 patients, 18 were treated with radiation therapy alone (4,000–5,500 cGy conventional multiport or conformal external beam), 12 with surgery alone, 16 with a combination of radiation and resection, and 13 with observation alone. The authors found that 44.4% of patients treated with RT alone showed improved visual acuity, although 33.3% experienced radiation-related complications, albeit frequently mild and self-limited. However, most importantly, visual acuity declined and tended toward blindness in all groups except the RT-only treatment cohort. In addition, the complication rate for patients treated with RT alone was significantly lower than that of patients treated with RT plus surgical resection (62.5%), as well as those treated with resection alone (66.7%). This further illustrates the superiority of RT used in isolation without resection, as patients treated with both still ultimately did worse. Furthermore, the mean follow-up time was 150.2 months, supporting the long-term efficacy of radiation treatment. The authors recommend that radiation to be considered as initial treatment in selected cases, as soon as the patient demonstrates new visual deterioration. 53 Similar results have been reported in other studies. 16 113 119 120 In 2011, Adeberg et al followed-up a total of 40 patients prospectively, comparing those treated with SFRT alone (19 patients) and those treated with surgery plus SFRT postoperatively (21 patients). The authors found that of the 15 patients treated with RT plus surgery who had preexisting vision loss, 3 (20%) improved. By comparison, of the 12 patients treated with RT alone who had preexisting vision loss, 9 (75%) improved, further demonstrating that radiation treatment alone is likely the safest option for most patients. Notably, the authors also found that with regard to specific aspects of SFRT associated with better outcomes, patients treated with higher planning target volumes (PTV) were more likely to show improvement in visual function. 120 Table 1 summarizes studies published since 1988 that examine the safety and efficacy of RT for ONSM.
Table 1. Radiation treatment outcomes.
| Study (year) | RT modality | No. of patients | Radiation dose, number of fractions, follow-up | Functional outcome | Radiographic outcome | Toxicity | Conclusion |
|---|---|---|---|---|---|---|---|
| Kennerdell et al (1988) 113 | 2D-RT | 38 patients (39 eyes) total. RT alone: 6 patients RT + surgery: 5 patients Surgery alone: 10 patients Observation alone: 18 patients |
5,000–5,500 rad over 28–32 sessions RT only: mean 5.7 years (range: 30–84 months) RT + surgery: mean 6.4 years (range: 3–9 years) |
RT only: 6 patients (100%) had improved VA and VF RT + surgery: 1 patientimproved, 3 worsened, 1 patient NLP pre-treatment stable Surgery alone: 1 improved, 7 worsened, 2 stable (both NLP pre-op) Observation: 4 stable, remainder worsened |
Control rate 100% | Acute: transient dry eye in 1 patients Late: none |
Recommend close follow-up; if VA worsens to 20/40, early initiation of RT may preserve visual function. If blind at presentation and tumor extends intracranially, recommend resection; otherwise may observe. |
| Augspurger et al (1999; abstract only) 135 | IMRT | 14 patients (some post-op) | 49.3–50.4 Gy, 1.7–2 Gy fractions Median 20 months (range 2–51) |
Subjective visual improvement in 57% Objective improvement on VF evaluation in 50% Subjective and objective worsening in 14% |
Control rate 100%; regression in 7% | Acute: grade-2 toxicity in 2 patients, grade-3 toxicity in 1 patient Late: 1 patient (who had worsening vision after resection) became blind despite RT (considered grade 4 toxicity) |
IMRT safe and effective. |
| Tsao et al (1999; abstract only) 130 | 3D-CRT | 15 patients | 13 patients 5,400 cGy in 180 cGy daily fractions, 2 patients 5,040 cGy in 180 cGy daily fractions Median radiographic follow-up 24 months (range: 8–102 months) |
10 of 15 patients had improved VA and/or VF | Tumor progression in 2 patients | Acute: not reported (only abstract available) Late: radiation-induced retinopathy in 2 patients |
Recommend limiting retinal dose to 4,500 cGy to prevent radiation-induced retinopathy. Overall RT is reasonable as primary treatment. |
| Andrews et al (2002) 76 | SFRT | 30 patients, 33 optic nerves (pONSM); 14 patients with history of prior surgery. Compared with 33 historical control patients (10 observed, 23 post-op). |
Median 51 Gy (range: 50–54 Gy), median 28.5 fractions Median 89 weeks (range: 9–284 weeks) |
Of 24 optic nerves with visual function pre-RT, 92% stable or improved (42% improved, 50% stable, 8% worsened) By comparison, only 16% of historical controls maintained visual function; RT patients thus had 150% greater probability of improvement |
Control rate 100%; 13% decreased in size. Note: 6 patients monitored with 111 In- octreotide SPECT; all showed decreased tumor activity post-RT. Three of these patients had vision pre-RT, and all showed decreased tracer uptake correlating with improvement (2 patients) and stabilization (1 patient). |
Acute: none Late: radiation-induced optic neuritis: 1 patient Transient orbital pain: 1 patient Both resolved with steroids |
RT effective and well-tolerated; resulted in increased likelihood of vision preservation and decreased tumor recurrence rate when RT patients compared with historical controls managed with surgery or observation alone. Thus, recommend RT as primary treatment for most patients, with surgery and observation reserved for select cases. 111 In-octreotide SPECT may be useful for posttreatment monitoring. |
| Becker et al (2002) 140 | SFRT | 39 patients total; 15 patients, 16 eyes with pONSM. 3 history of biopsy, 1 history of subtotal resection 24 sONSM, 13 history of subtotal resection, 3 history of total resection |
54 Gy, 1.8 Gy fractions pONSM: median 39 months (range: 10–73 months) sONSM: median 32.5 months (range: 10–56 months) |
pONSM: VA: 1 eye improved, 15 stable. VF: 6 eyes improved, 8 stable (data available for only 14 of 16 eyes) sONSM: VA, 7 eyes improved, 19 stable. VF: 6 eyes improved, 17 stable, 1 worsened |
Control rate 100%; 1 patient decreased in size | Acute: pONSM: erythema: 33% alopecia: 73% new endocrine disturbance: 14% sONSM: erythema: 21% alopecia: 75% new endocrine disturbance: 8% Late: none |
SFRT is safe, controls tumor growth effectively, and has the potential to improve vision. Recommend early initiation of RT, before irreversible damage occurs. Improvement may also be observed early in RT course. |
| Liu et al (2002) 141 | SFRT | 5 patients, 6 eyes (all pONSM) | 45–54 Gy, 1.8 Gy fractions Median 3 years (range: 1–7 years) |
Overall, 4 patients had improved visual function, 1 patient remained stable VA: 3 patients improved, 2 patients stable VF: 4 patients improved, 1 patient stable |
Control rate 100% | Acute: none reported Late: none reported |
Recommend RT for patients with functional vision early in disease course. Notably, improvement seen within 3 months in this series. |
| Pitz et al (2002) 142 | SFRT | 15 patients, 16 optic nerves (all pONSM); 3 history of biopsy | 54 Gy, 1.8 Gy fractions Mean 37 months (range 12–71 months) |
Overall, all patients either stable or improved (43% of eyes improved, 57% stable) | Control rate 100% | Acute: focal erythema: 5 patients Focal alopecia: 11 patients Endocrine disturbance: 3 patients Late: none reported |
SFRT safe and effective; recommend initiation early in course prior to significant visual deterioration. |
| Turbin et al (2002) 53 | 2D-RT or 3D-CRT | 64 patients total (59 analyzed, 5 NLP patients excluded). RT alone: 18 patients Surgery alone: 12 patients Surgery + RT: 16 patients Observation alone: 13 patients |
4,000–5,500 cGy over 6 weeks Mean 150.2 months (range: 57–277 months) |
Overall, VA decreased significantly for all groups except RT alone. VA at follow-up: RT alone: 44.4% improved RT + surgery: 31.3% improved Of remaining patients (observation or surgery alone), only 8% improved |
32.8% with progression on imaging (4 patients observed, 7 surgery alone, 8 surgery + RT; 2 patients who had RT alone progressed before RT) | Acute: RT alone: none reported Late: RT alone: retinopathy or vascular occlusion: 4 patients Persistent iritis: 1 patient Temporal lobe atrophy: 1 patient Complication rates: RT alone: 33.3% RT + surgery: 62.5% Surgery alone: 66.7% |
Overall, patients treated with RT alone had superior visual outcome. Authors recommend RT as initial treatment in select cases when preservation of vision is the goal. |
| Narayan et al (2003) 35 | 3D-CRT | 14 patients (all pONSM), 2 post-op | 50.4–56 Gy, 1.8–2 Gy fractions Median 51.3 months (range: 8.9–80.9 months) |
VA: 5 patients (36%) improved, 7 patients (50%) stable, 2 patients (14%) worsened VF: of 9 patients with VF data, all improved |
Control rate 100%; 1 patient with slight decrease in size | Acute: temporary alopecia in most patients Temporary mild inflammation of the cornea: 1 patient Late: dry eye: 1 patient grade-2 radiation retinopathy: 1 patient (occurred 4 years post-RT, however VA remains improved) Iritis: 2 patients grade-2 orbital pain: 1 patient |
Supports RT as primary treatment for patients with functional vision. Consider RT early to preserve as much vision as possible. |
| Saeed et al (2003) 16 | 2D-RT alone: 5 patients SFRT alone: 1 patient |
88 patients (92 eyes) total. RT alone: 6 eyes Surgery: 47 eyes; 11 biopsy, 15 en bloc resection, 10 debulking, 1 complete resection, 10 ONS decompression Observation alone: 39 eyes |
2D: 50–55 Gy in 28–30 fractions SFRT: total 45 Gy Mean 7.2 years, median 4 years (range: 1–20 years) |
RT alone: VA: 3 improved, 3 stable. VF: all improved Surgery: 1 complete resection patient stable Of 10 ONS decompression patients, 1 improved, 2 stable, remainder worsened Note: detailed visual outcomes not reported for all surgical patients Observation: 5 NLP at presentation, 26 stable, 8 worsened |
Control rate 100% | Acute: RT alone: none reported Late: RT alone: cataract followed by macular degeneration in 1 patient (SFRT) |
Recommend conservative management for patients with stable vision 20/50 at least, RT indicated if visual function progressively worsens, surgery indicated in select cases. |
| Baumert et al (2004) 143 | SFRT | 23 patients (all pONSM); 1 patient history of biopsy, 1 patient history of partial resection | 45–54 Gy, 1.8–2.0 Gy fractions Median 20 months (range: 1–68 months) |
Overall, 16 of 22 patients with vision loss prior to RT improved, 5 stable, 1 patient worsened. 13 of 16 patients improved within 1–3 months post-RT. |
Control rate 100%, 1 decreased in size | Acute: eyelid edema: 8 patients Increased pain: 1 patient Focal alopecia: all patients Late: increased headaches: 1 patient Radiation retinopathy (with vitreous hemorrhage and cataract) 4 years post-RT: 1 patient |
RT safe and effective. Early improvement may be seen. Treatment should be initiated after beginning signs of visual decline, given that blind patients are unlikely to improve. |
| Schroeder et al (2004; abstract only) 138 | IMRT | 22 patients, some with history of prior surgery | 4930–5040 cGy, 160–200 cGy fractions Mean 20 months (range: 2–71 months) |
Subjective visual improvement in 71% overall (83% RT alone, 40% RT + surgery), 24% stable (17% RT alone, 40% RT + surgery). Objective VF improvement in 63% overall, (73% RT alone, 40% RT + surgery). One patient had worsening of vision subjectively and objectively (note patient had RT immediately post-op). |
Control rate 100%, regression in 17% | Acute: CSF leak at site of screw placement, contralateral retinal detachment Late: 1 patient with vision loss progressing to blindness (grade 4) |
Supports efficacy of IMRT alone; patients treated with surgery plus IMRT had worse outcomes. |
| Landert et al (2005) 144 | SFRT | 12 patients, (13 eyes) total; 7 eyes treated, compared with 6 eyes untreated due to patient or physician preference | 50–54 Gy, 1.7–1.8 Gy fractions Treated patients: mean 57 months (range 21–142) Untreated patients: mean 61 months (range: 16–118 months) |
Treated eyes: VA: 6 improved, 1 worsened. VF: 4 patients improved, 2 patients stable, 1 patient worsened. Untreated eyes: VA: 2 patients stable, 4 worsened; VF: 3 patients stable, 3 worsened. |
Treated eyes: 6 stable, 1 decreased in size Untreated eyes: 5 stable, 1 increased in size |
Acute: none Late: none |
SFRT is safe and effective; results support SFRT for ONSM patients with worsening visual function, with initiation of treatment prior to development of severe vision loss. |
| Richards et al (2005) 145 | SFRT | 4 patients | Mean 43.5 Gy in 26 fractions Mean 2 years |
VA: 100% stable or improved | Control rate 100% | Acute: transient hair loss in 1 patient Late: punctate microvascular changes evident on imaging in 1 patient |
Good results in four cases with SFRT, although long-term results unknown. |
| Sitathanee et al (2006) 146 | 11 SFRT, 1 SRS | 12 patients (all pONSM), 5 history of surgery | SFRT: mean 55.7 Gy, 180 cGy fractions. SRS: 15 Gy single session Median 34 months |
Of 7 patients with useful vision, 4 improved, 2 remained stable, 1 worsened 5 patients who were blind pretreatment remained unchanged |
Control rate 100% | Acute: none Late: vitreous hemorrhage 2 years posttreatment in 1 patient |
SFRT safe and effective in these patients, complications low risk. |
| Litré et al (2007) 147 | SFRT | 8 patients | 45 Gy, 25 fractions (5 fractions of 1.8 Gy each week) Mean 27 months |
100% with stable or improved vision | Control rate 100% | Acute: none reported Late: none |
SFRT safe and effective. |
| Arvold et al (2009) 161 | 13 SFRT, 9 proton beam, 3 SFRT + proton beam | 25 patients (24 primary, 1 recurrent), 3 history of subtotal resection, 5 history of biopsy | Median 50.4 GyE (range 45-59.4 GyE), 1.8 GyE fractions. Median 30 months (range: 3–168 months), 3 lost to follow-up |
VA: 95% stable or improved (14 improved, 7 stable, 1 worsened) VF: 9 improved Color vision: 8 improved |
Of 22 patients with follow-up, 95% remained stable, 1 patient (5%) recurred 11 years post-RT |
Acute: transient orbital pain: 1 patient Transient headaches: 1 patient Late: likely asymptomatic radiation retinopathy (based on exam): 3 patients |
PRT is as safe and effective as photon therapy, no significant difference between the two in terms of visual outcome, tumor control, or toxicity. |
| Milker-Zabel et al (2009) 148 | SFRT | 32 patients (all pONSM); 6 patients history of resection | 50.4–57.6 Gy, 1.8 Gy fractions Median 4.5 years |
VA: 97% stable or improved (1 patient worsened) | Control rate 100%, 6 patients decreased in size | Acute: Dizziness: 1 patient Most patients with temporary alopecia Late: hyperlacrimation: 3 patients |
SFRT safe and effective. |
| Smee et al (2009) 134 | 7 SFRT, 3 SRS (3 history of surgery), 3 2D-RT, 2 IMRT | 15 patients, 16 eyes (all pONSM) | SFRT: 50 Gy, 1.8–2 Gy fractions IMRT: 50 Gy, 1.8 Gy fractions SRS: 20 Gy single session Median 86.4 months (range: 5.5–157 months) |
VA: stable or improved in 14 patients (93%), worsened in 1 patient (7%) | Control rate 100%; 1 patient with recurrence outside treatment volume; no infield progression | Acute: Most patients with variable degree of fatigue Late: 1 patient with worsening vision due to tumor progression |
RT safe and effective. |
| Lesser et al (2010) 109 | 8 3D-CRT 2 SFRT 1 IMRT |
11 patients (all pONSM) | 45–54 Gy in 25–30 fractions Mean 89.6 months (range: 61–156 months) |
VA improved or stable in 91%. VF improved or stable in 82%. Note: of the 7/11 patients with final VA 20/20, 5 were 20/20 pre-RT; only 2 of those worse than 20/40 pre-RT improved to 20/20 |
Control 100%, 9 stable, 2 decreased in size | Acute: focal alopecia: 4 patients Fatigue: 7 patients Headache: 1 patient Late: none (1 patient with bilateral dry eye, not attributed to lacrimal gland irradiation) |
Good visual outcomes and tumor control Early treatment may result in better outcomes; RT should be initiated before severe visual deterioration, must balance against risk of toxicity (which is low). |
| Liu et al (2010) 154 | SRS (Gamma knife) | 30 patients (13 pONSM, 17 sONSM), 9 history of surgery | Mean cumulative peripheral dose: 13.3 Gy (range 10–17 Gy) Single session: 21 patients Two sessions: 9 patients Median 56 months (range: 38–108 months) |
VA: improved in 36.7%, stable in 43.3%, worsened in 20%. Of 18 patients with exophthalmos, 78% improved | Overall 5-year control rate 93.3%. Stable size in 26.7%, decreased size in 66.7%, increased size in 6.7% 2 patients with tumor progression received repeat SRS; then stabilized |
Acute: reversible conjunctival edema: 13.3% Transient headache: 3.3% Transient orbital pain: 3% Late: none reported |
Successful tumor control in the majority of patients. Supports use of SRS in ONSM Note: SRS separated into 2 sessions for patients with tumor enveloping nerve, whose visual acuity was at least 0.5. |
| Saeed et al (2010) 170 | 22 2D-RT, 12 SFRT |
34 patients, 8 history of biopsy | 45–54 Gy in 25–30 fractions Median 58 months (range: 51–156 months) |
VA: 14 patients (41%) improved, 17 patients (50%) stable, 3 patients (9%) worsened) Optic disc edema and optic atrophy identified as statistically significant prognostic factors, however visual acuity at presentation and timing of RT were not |
Not reported | Acute: temporary erythema at irradiation site: 16 patients (associated with temporary hair loss in 12 patients). Headache: 6 patients Late: dry eye: 5 patients Cataract: 3 patients Mild radiation retinopathy: 4 patients No signs/symptoms of pituitary dysfunction |
Primary RT is safe and effective. Note: VA outcome not associated with VA at presentation, and no association between timing of radiotherapy and outcome, no major difference in outcomes between the 2 modalities. Optic disc edema and atrophy associated with better response. |
| Adeberg et al (2011) 120 | SFRT | 40 patients (41 eyes). RT alone: 19 patients RT + surgery: 21 patients (9 directly post-op, 12 for progression post-op) |
54, 1.8 Gy fractions Median 60 months (range: 4–22 months) |
Of 27 patients with pre-RT vision loss, 12 (44%) improved, 3 worsened (1 also had worse VF, and 2 patients complained of decreased VA. Note: 75% of patients treated with RT only improved, while only 20% of patients treated with surgery + RT improved. Larger target volumes were associated with increased visual improvement. |
Control rate 100% | Acute: focal alopecia: majority of patients Fatigue: 20% of patients Xerophthalmia: 2 patients Conjunctivitis: 1 patient Headache: 1 patient Hyperlacrimation: 3 patients (irradiated eye) Taste change: 1 patient Late: none |
SFRT controlled tumor size with few side effects, however the fact that patients who had undergone surgery prior to RT improved significantly less than those treated with RT alone supports the use of RT as primary treatment. The prospective nature of this study lends strength to the authors findings. |
| Marchetti et al (2011) 152 | SRS (CyberKnife) | 21 patients (all pONSM), 13 history of surgery | 25 Gy, 5 fractions Mean 30 months (range: 11–68 months) |
Overall, visual function improved in 35%, and remained stable in 65%. Note: 17 assessed total, 4 not assessed due to blindness. VA: 27% with pre-RT vision loss improved VF: 11 patients stable, 6 patients improved |
Control rate 100%, 2 patients decreased in size | Acute: abnormal lacrimation: 2 patients Dizziness: 1 patient, resolved Late: mild optic neuropathy: 1 patient (improved with steroids) |
SRS is safe and effective; prospective study. |
| Metellus et al (2011) 119 | SFRT | 9 patients, 2 history of surgery | Median 50.4 Gy, 1.8 Gy fractions Mean 98 months, median 90 months (range: 61–151 months) |
Overall, 100% of patients stable or improved visual function (77.8% improved, 22.2% stable) Note: the 2 patients who remained stable had history of surgery and were NLP pre-RT |
Control rate 100%, 2 patients decreased in size | Acute: eyelid edema: 1 patient (resolved) Late: radiation retinopathy 2 years post-RT: 1 patient |
SFRT safe and effective. Better results seen in patients without history of prior surgery; RT effective as primary treatment. |
| Pacelli et al (2011) 149 | SFRT | 5 patients | 50.4 Gy, 1.8 Gy fractions Median 26 months (range: 9–37 months) |
VA: stable or improved in 100% of patients (2 patients improved, 3 patients stable) | Control rate 100% | Acute: orbital pain: 1 patient Mild conjunctivitis: 1 patient Late: none |
Authors note that although there was no late toxicity and mean dose to critical organs was kept within tolerance limits, as delayed-onset toxicity is known to occur, the follow-up is too short to make conclusions about late toxicity. |
| Romanelli et al (2011) 153 | SRS (CyberKnife) | 5 patients (all pONSM) | Maximum dose <30 Gy, 80% prescribed isodose of 20 Gy in 4 sessions of 5 Gy each Median 74 months (range: 36–88 months) |
VA and VF: 4 out of 5 patients showed improvement (note: at presentation, VA was 20/40 for 3 patients, 20/30 for 2 patients, all with visual field deficits) | Control rate 100% | Acute: none reported Late: none reported |
SRS safe and effective in this small series; a large prospective study evaluating staged SRS outcomes should be conducted. |
| Abouaf et al (2012) 112 | 5 3D-CRT 3 IMRT 2 2D-RT |
10 patients (8 pONSM, 2 sONSM) | Median total tumor dose 58 Gy (range: 50–64), 1.8–2.0 Gy fractions Median 51 months (range: 5–139 months) |
VA: 6 improved, 1 stable, 3 worsened VF: 6 improved, 2 stable, 2 worsened Better pre-RT VA associated with better outcomes. Longer time interval prior to RT (median 117 vs. 7 months) associated with worse outcome, but not statistically significant |
Control rate 100%, 2 decreased in size | Acute: conjunctivitis: 1 patient Fatigue: 2 patients Fatigue and alopecia: 1 patient Late: radiation-induced retinopathy: 2 patients Radiation-induced mature cataract: 1 patient |
RT is effective, however authors note that patients who experienced severe late toxicity (at least grade 2), received higher median mean eye dose (39.4 vs. 6.1 Gy); mean tolerable eye dose is thus a limiting factor in RT planning. Early treatment may be beneficial. |
| Paulsen et al (2012) 103 | SFRT | 109 patients (113 eyes). 37 pONSM: 2 history of biopsy, 8 history of partial resection, 10 history of total resection. 76 sONSM: 1 history of biopsy, 24 history of partial resection, 10 history of total resection. |
50.4–54 Gy, 1.8–2 Gy per fraction Mean 89.6 months, median 73 months (range: 61–156 months) |
VA (91 patients assessed): 12 eyes improved, 68 eyes stable, 11 eyes worsened. VA preserved in 94.8% at 3 years and in 90.9% at 5 years. Mean visual field defects (54 patients assessed): Ipsilateral eye: reduced from 33.6% ( n = 90) to 17.8% ( n = 56) Contralateral: reduced from 10% ( n = 94) to 6.7% ( n = 62). VF preserved in 93.7 at 3 years, and 90.9% at 5 years. Ocular motility: 23 eyes improved, 65 eyes stable, 3 eyes worsened. Note: no major difference in outcomes between patients treated with RT alone vs. RT + surgery. |
100% control at 3 years, 98% at 5 years Tumor regression in 5 patients Tumor progression in 4 patients |
Acute: alopecia: 46 patients grade 0, 43 patients grade 1, 23 patients grade 2 Erythema: 74 patients grade 0, 36 patients grade 1, 2 patients grade 2 Pain: 80 patients grade 0, 29 patients grade 1, 3 patients grade 2 Nausea: 101 patients grade 0, 11 patients grade 1, 0 patients grade 2 Note: 2 patients with obstructive hydrocephalus shortly after RT Late: worsening vision in 11 patients, “radiation neuropathy cannot be ruled out” Endocrine disturbance: 10 patients (was normal in 81.3% after 5 years) Alopecia: 8 patients Chronic toxicity: Pain: 26 patients Fatigue: 11 patients Sensory loss: 7 patients |
SFRT improved or stabilized vision in most patients Mild–moderate toxicity was observed. Can consider using radiation dose less than 54 Gy to minimize risk of visual deterioration post-RT. Recommend primary RT as treatment of choice, however finding that there was no difference between RT alone and RT + surgery cohorts emphasizes importance of interdisciplinary management. |
| Soldà et al (2012) 110 | SFRT | 45 patients (51 optic nerves), 6 history of surgery | 50 Gy, in 30-33 fractions Median 30 months (range: 1–13 years) |
Overall, of those with serviceable vision pre-RT, 89% remained stable or improved Of the 46 optic nerves that could be evaluated: 13 improved, 24 stable, 4 worsened; 5 blind pre-RT |
Tumor control rate 100% | Acute: fatigue and small areas of transient hair loss Late: radiation-induced retinopathy: 2 patients (1 asymptomatic, 1 with worsening vision 2 years post-RT) Central retinal artery occlusion 10 years posttreatment: 1patient |
Study supports efficacy of SFRT in patients with functional vision pre-RT. Patients with severe vision loss were less likely to improve post-RT; treatment should be initiated early, prior to significant deterioration. |
| Adams et al (2013) 107 | 3D-CRT | 17 patients (18 eyes), 2 history of biopsy | 46.8–55.8 Gy in 26–31 fractions Median radiographic follow-up 6.2 years (range: 0.9–13.3 years) Median clinical follow-up 5.3 years (range: 0.6–13.4 years) |
Overall, VA stable or improved vision in 89%, worsened in 11% Of 13 eyes with useful vision at presentation: 62% stable or improved, 38% worsened Median duration between symptom onset and treatment tended to be shorter for patients who had either stable or improved visual outcome (18 vs. 62 months); but not statistically significant |
Control rate 100%; 56% decreased in size | Acute: hair loss: 8 patients Headache: 2 patients Otitis externa: 1 patient Xerostomia: 1 patient Conjunctivitis: 1 patient Late: xerophthalmia: 5 patients Cataract: 4 patients Optic disc atrophy: 2 patients |
Overall, 3D-CRT safe and effective. Early treatment may be beneficial, even when presenting visual acuity is relatively good. |
| Brower et al (2013) 108 | 13 SFRT, 1 IMRT, 1 2D-RT | 15 patients, all history of surgery (one-third receiving RT for recurrence) | Median 50.4 Gy, 1.8 Gy fractions Median 12 years (range: 4–19 years) |
VA: 87% stable or improved (60% stable, 27% improved, 13% worsened). Overall, 86% of patients with useful vision pretreatment ultimately had useful vision at follow-up |
Control rate 100%; 93% stable, 7% decreased size | Acute: none reported (not reliably noted in medical record per authors) Late: radiation-induced retinopathy causing vision loss: 2 patients (13%) |
RT safe and effective. Study provides useful long-term results (long duration of follow-up). Majority of patients with useful vision pretreatment were able to retain useful vision; may support utility of earlier treatment initiation, prior to deterioration. |
| Hamilton et al (2018) 151 | SFRT | 41 patients (23 pONSM, 18 sONSM), 8 history of biopsy | 50–50.4 Gy, 28 fractions (except 1 patient 53 Gy, and 1 patient 54 Gy). Median visual follow-up 3.8 years Mean radiographic follow-up 4.4 years |
VA: 11 improved (27%), 26 stable (65%), 3 worsened (8%). VF: 7 improved (21%), 24 stable (70%), 3 worsened (9%). VF: 21% (7 patients) improved, 70% (24 patients) stable, 9% (3 patients) worsened |
Actuarial 5-year local control rate Primary: 100% Secondary: 86% |
Acute: headache: 13 patients (32%) Nausea: 6 patients (15%) Conjunctivitis: 3 patients (7%) Dry eye: 2 patients (5%) Eye discomfort: 1 patient (2%) Late: Pituitary dysfunction: 3 patients (13%) Radiation-induced Retinopathy: 3 patients (7%) Chronic eye pain: 2 patients (5%) Cataract: 1 patient (2%) |
SFRT generally safe and effective. Authors note that all patients who experienced hypopituitarism had tumors involving sella. |
| Inoue et al (2018) 114 | 4 IMRT, 1 SFRT | 5 patients (all pONSM) | Median 52.8 Gy (range: 46.0–59.4 Gy), median 25 fractions (range: 22–33) Median 36 months (range: 18–54 months) |
VA and VFs improved in all patients at median follow-up time, 4 patients (3 IMRT, 1 SFRT) experienced early improvement (within 2–4 weeks) | Control rate 100% Median tumor reduction 53% (range: 39–75%) |
Acute: none reported Late: radiation retinopathy with retinal bleeding 16 months post-RT: 1 patient |
Rapid improvement occurred after early treatment (RT started 1.5–7 months after visual deterioration). Support early RT as standard treatment for pONSM. |
| Jin et al (2018) 137 | 10 IMRT, 3 SRS (Gamma knife) | 13 patients (2 IMRT history of surgery, 1 SRS history of surgery) | IMRT: 9 patients 50 Gy, 25 fractions, 1 patient 46 Gy, 21 fractions. SRS: total dose 30 Gy, 39 Gy, and 24 Gy, respectively. Median 50 months (range: 12–133 months) |
Overall, VA preserved in 75% (9 of 12, 1 not evaluated) Visual outcome in ipsilateral eye: IMRT patients: 3 eyes improved, 4 stable, 3 worsened GKS patients: 1 eye improved, 1 stable, 1 not evaluated Visual outcome in contralateral eye: IMRT patients: 5 eyes improved, 3 stable, 2 worsened (note: in patients for whom contralateral eye worsened, 1 case not due to RT, other case unclear per authors. Both affected eyes improved) GKS patients: all 3 eyes stable |
Control rate 100%, decreased size in 2 patients | Acute: nausea: 3 patients Dry eye: 1 patient Headache: 2 patients Late: radiation-induced retinopathy 9 months post-RT: 1 patient |
IMRT and GKS effectively controlled tumor growth and preserved visual acuity. |
| Eckert et al (2019) 72 | IMRT | 26 patients, 1 history of surgery | 51–54 Gy, 1.7–1.8 Gy fractions Median 2.2 ± 0.5 years (range: 3 months–8.6 years) |
VA: 16 improved, 6 stable, 4 worsened VF: 14 improved, 8 stable, 2 worsened Overall visual function: 16 improved, 6 stable, 4 worsened Worsening visual function associated with increased age at start of RT, improvement associated with sheath like tumor growth (vs. fusiform). |
Control rate 100% | Acute: none reported Late: radiation-induced optic neuropathy: 1 patient Radiation-induced optic neuropathy: 1 patient |
IMRT is safe and effective, however patient selection and timing of treatment are highly important. Treatment planning requires an experienced team. |
| Kheir et al (2019) 150 | SFRT | 16 patients | 50.4, 1.8 Gy fractions Mean 31 months (range: 2–156 months) |
VA: improved or stable in 81.25% VF: mean defect improved in 86.66% Color vision: improved or stable in 73.33% |
Control rate 100% | Acute: none Late: none |
SFRT is safe and effective. |
| Pandit et al (2019) 129 | 3D-CRT | 16 pONSM, 2 history of biopsy | Mean 50 Gy (range: 36-54 Gy), mean 26.9 sessions (range: 18-30 sessions). 88% received total dose at least 50 Gy Mean 14.6 years (range 10.5-20.7 years) |
VA: 5 improved, 9 stable, 2 worsened VF: 100% stable (of 10 w/ available data) Of 7 patients with color vision deficit, 2 improved, 5 stable |
Control rate 100%; 11% decreased size, 89% stable size |
Acute: none Late: radiation retinopathy: 2 patients (13%), only 1 with worse VA as a result Dry eye: 1 patient Presumed radiation optic neuropathy: 1 patient |
Treatment is safe and effective in the long-term 3D-CRT demonstrates improvement upon conventional RT. |
| Ratnayake et al (2019) 111 | SFRT | 26 (all pONSM), 4 history of surgery | 76.9% of patients: 50 Gy, 28 fractions. 15.4% of patients: 54 Gy, 30 fractions. 7.7% of patients: 37.5 Gy, 15 fractions. Median 68 months (range: 20–134 months); first follow-up at 12 months |
10 improved (38.4%), 10 stable (38.4%), 6 worsened (23.1%). VA: Of patients with better pretreatment VA, 92.3% improved or stabilized. Of patients with worse pretreatment VA, 61.5% improved or stabilized. All post-op patients improved or stabilized |
Control rate 100%; 96.1% stable, 3.8% mildly reduced size (1 patient) |
Acute toxicity: 53.8% overall, all grade 1 Fatigue: 23.1% Headaches: 19.2% Alopecia: 3.8% Dizziness: 3.8%; Late toxicity: Grade 1 dry eye: 11.6% Grade 2 radiation retinopathy: 1 patient Multiple intracranial meningiomas found 10.9 years later: 1 patient |
Treatment safe and effective, earlier treatment may be superior. |
| Sasano et al (2019) 106 | IMRT | 15 patients | 50.4–54 Gy in 28–30 fractions Median 23 months (range: 8–57 months) |
VA: 7 improved, 4 stable, 4 worsened VF: 11 improved, 4 worsened, 1 patient not evaluable Eyes with fusiform and globular growth improved, while 2 of 5 eyes with diffuse pattern worsened. 5 eyes with normal optic disc improved, while 8 of 10 with edema/atrophy were stable or worsened. |
Control rate 100%; 2 decreased in size | Acute adverse events: Skin redness: 3 patients Lacrimation: 3 patients Eye pain: 4 patients Heaviness in eye: 2 patients Headache: 4 patients Heavy-handedness: 1 patient Dizziness: 2 patients Fatigue: 11 patients Dry nose: 1 patient Late: Acute case of ischemic optic neuropathy 127 days post-RT: 1 patient, Cataract 653 days post-RT: 1 patient No endocrine complications |
IMRT resulted in good outcomes, RT initiation prior to development of optic disc atrophy/edema may be superior |
Abbreviations: 2D, two-dimensional; 3D, three-dimensional; CRT, conformal radiation therapy; IMRT, intensity-modulated radiotherapy; NLP, no light perception; pONSM, primary optic nerve sheath meningioma; post-op, postoperative; pre-op, preoperative; RT, radiotherapy; SFRT, stereotactic fractionated radiotherapy; sONSM, secondary optic nerve sheath meningioma; SPECT, single-photon emission computerized tomography; SRS, stereotactic radiosurgery; VA, visual acuity; VF, visual field.
Note: Table includes studies published since 1988 evaluating outcomes of radiotherapy in the treatment of ONSM. Studies not specifically focused on ONSM, as well as single case reports were excluded.
3D Conformal Radiotherapy
Over the years, the use of conventional external 2D RT has given way to the development of CRT. 124 Types of CRT involved in treating ONSM include 3D-CRT, IMRT, and stereotactic fractionated radiotherapy (SFRT), the latter two of which developed from and built upon the advantages of 3D-CRT. 6 The major advantage of this RT modality is that it distributes its radiation dosage conformally over the target volume. This is accomplished via the utilization of radiation beams targeted at the lesion from multiple directions that reflect the lesion's shape. During treatment planning, critical structures are specifically outlined, so that they may be excluded from radiation as much as possible. This enables more complete tumor coverage and decreased radiation-related complications. 126 127
Studies examining the use of 3D-CRT have generally demonstrated favorable results in terms of visual outcome. Some cases of dramatic visual recovery after 3D-CRT have been reported; in one such case, although the patient's 3-mm proptosis and moderate ophthalmoplegia did not improve, her visual acuity improved from 20/200 to 20/30, and her visual field deficits resolved almost completely, remaining stable at 2-year follow-up. 128 In 2003, Narayan et al reviewed the outcomes of 14 patients treated with 3D-CRT, reporting that 86% patients had stable or improved visual acuity, and 14% (two patients) had worsening visual acuity. Tumor control rate was 100% and effects of late toxicity were acceptable and relatively minor. 35 Similarly, in 2013, Adams et al reported on the outcomes of 17 patients, 16 of whom underwent 3D-CRT (one remaining did FSRT). Vision either remained stable or improved in 89% of patients with a tumor control rate of 100%. Effects of acute and late toxicity were also relatively manageable. Interestingly, although not a statistically significant finding, the authors note that median time from onset of symptoms to start of RT tended to be shorter for those whose visual acuity remained stable or improved (18 months) by comparison to those whose vision worsened (62 months), suggesting that earlier treatment may be beneficial. The authors also note that while other authors have studied more recently developed treatment modalities due to their specific advantages, the study of 3D-CRT remains relevant, as technology such as IMRT/SFRT are not available at all institutions. 107
In a more recent study of 16 patients with pONSM across four centers, Pandit et al specifically evaluated the long-term treatment outcomes of fractionated 3D-CRT by analyzing data from patients with at least 10 years of follow-up. After receiving a mean cumulative radiation dose of 50 Gy over 26.9 sessions on average, 88% of patients showed improved or stabilized visual acuity. Notably, 69% had an acuity of at least 20/40 at final follow-up, including two patients who dramatically improved from a pretreatment visual acuity of 20/200 in one, and 20/300 in the other. Visual fields remained stable in all patients for whom this data were available (10 patients). Tumor control rate was 100%. Radiation-related complications were also relatively infrequent. Two patients had worsening vision, one caused by retrobulbar optic neuropathy attributable either to effects of radiation or lack of treatment response, and the other due to radiation-induced retinopathy. One other patient (total of two) developed radiation retinopathy; however, the patient did not suffer a change in visual acuity as a result. It is worth mentioning that the duration of follow-up in this study was particularly long (mean: 14.6 years, range: 10.5–20.7), lending strength to the authors' findings. 129
In general, 3D-CRT has been found to produce good outcomes in the majority of patients. In our review of the literature, we identified seven articles and one abstract containing patients treated with 3D-CRT, published between 1999 and 2019, excluding case reports ( Table 1 ). 35 53 107 109 112 129 130 Of these, four full-text articles focused primarily on 3D-CRT (three exclusively and one with 3D-CRT as the primary treatment modality). 35 107 109 129 These represented a total of 55 patients, including 58 eyes (38 specifically referred to as pONSM), with RT occurring postoperatively in two patients. Stable or improved visual acuity was reported in 86 to 91% of patients. Duration of follow-up ranged from 4.3 to 14.6 years (median or mean value). Tumor control rates were 100% in all patients, with no studies reporting radiographic progression, and some reporting decreases in tumor size. Rates of acute and late-onset radiation toxicity have been relatively low. Of late toxicity events, radiation-induced retinopathy occurred in a total of three patients (with only one experiencing worsening visual acuity), presumed radiation-induced optic neuropathy in one patient, dry eye in seven patients, cataract in four patients, orbital pain in one patient, and “optic disc atrophy” in two patients. 35 107 109 129 Pandit et al aptly point out in their study that while approximately one-third of patients in the 2002 study by Turbin et al experienced radiation-induced complications (the majority of whom were treated with conventional RT), the advent of 3D-CRT, particularly which is administered in multiple fractions, has greatly improved the safety of radiation treatment for ONSM and has decreased the frequency of radiation-induced complications. 53 129
Intensity-Modulated Radiation Therapy
Building upon the principles of 3D-CRT, IMRT allows for modulation of the dose delivered to different regions within the selected radiation field using a computer algorithm, thus improving errant dose to critical structures and increasing the delivered dose to targeted areas. In addition, it produces good conformality and allows for the use of a sharp dose gradient, thus further preventing radiation toxicity in surrounding tissues. 106 127 By contrast, SFRT administers a uniform dose to the selected field. 106 Various series have demonstrated the efficacy of IMRT in treating intracranial meningioma, some of which have included small numbers of ONSM cases. 131 132 133 Other studies focusing on ONSM have included small numbers of patients treated with IMRT and have shown promising results. 109 112 134 135
While studies dedicated to IMRT in ONSM are limited, there have been several recent studies supporting the efficacy of this treatment. 72 106 114 136 137 138 In a recent series from Germany, Eckert et al evaluated the outcomes of 26 patients treated with IMRT. At a median follow-up of 2.2 ± 0.5 years (range: 3 months–8.6 years), visual acuity improved in 9 patients (35%), stabilized in 12 patients (46%), and worsened in 5 patients (19%). Visual fields improved in 14 patients (54%), stabilized in 8 patients (31%), and worsened in 2 patients (8%); this information was unavailable for the remaining two patients. Overall, visual function was improved in 16 patients (62%), stable in 6 (23%), and worse in 4 (15%) at final follow-up. Increased age at treatment start time was associated with worse visual outcome; all patients who showed worsened visual acuity were older than the median age of 48.3 years in this group of patients. In addition, sheath-like tumor growth was associated with better visual field outcome than fusiform growth. Two patients (8%) experienced radiation-related toxicity; one with radiation-induced optic neuropathy and the other with retinopathy. No patients experienced tumor growth on follow-up imaging. Notably, seven patients with a pretreatment visual acuity of less than 0.1 (worse than 20/200) demonstrated statistically significant improvement, indicating that even patients with severe vision loss may benefit from this treatment modality. 72
Overall, our literature review identified eight articles and two abstracts containing patients treated with IMRT published between 1999 and 2019, excluding case reports ( Table 1 ). Of these, four of focused on IMRT (two exclusively and two with IMRT utilized in a majority of cases). 72 106 108 109 112 114 134 135 137 138 These studies comprised a total of total of 55 patients (55 eyes, 5 specifically referred to as pONSM), with RT-delivered postoperation in 1 patient. Objectively stable or improved visual function was reported in 70 to 100% of patients, with tumor control rates of 100%. Median duration of follow-up ranged from 23 months to 4.2 years. Patients with long-lasting damage due to radiation toxicity were few, with radiation-induced retinopathy occurring in three patients, radiation-induced optic neuropathy in one patient, ischemic optic neuropathy in one patient, and cataract in one patient. 72 106 114 137 In addition, several authors also emphasize the importance of the timing of RT, given emerging evidence to suggest that treatment initiation prior to the development of optic disc abnormalities may result in superior outcomes. 72 106 114
Stereotactic Fractionated Radiotherapy
SFRT has emerged as the most frequently studied form of radiotherapy in the treatment of pONSM and is considered the treatment of choice for ONSM at many institutions. In SFRT, the target lesion undergoes stereotactic localization, enabling the highly precise delivery of the prescribed radiation dose to the target volume, while avoiding critical structures. In general, this is of great utility when treating pathologies, such as ONSM, in which critical structures are in very close proximity to the tumor. 127 139
In one of the largest series on SFRT, including 109 patients (113 eyes, 37 pONSM, and 76 sONSM), Paulsen et al reported that after 5 years of follow-up, visual acuity was preserved in 90.9%, visual fields were preserved in 90.9%, and ocular motility was preserved in 94.9%. The tumor control rate was 98%. Statistical analysis indicated that radiation dose may predict tumor control (54 vs. <54 Gy) but does not predict visual acuity, visual fields, ocular motility, or pituitary function outcomes. Interestingly, outcomes did not vary significantly between those with pONSM versus those with sONSM. Given the excellent visual outcomes in these patients, the authors recommended SFRT as standard treatment. 103 With regard to long-term treatment outcomes of SFRT for pONSM, Brower et al reported on 15 cases of ONSM, the majority of which were treated with SFRT (13 SFRT, 1 IMRT, and 1 2D 1–2 fields), with a median follow-up of 12 years, the longest for all studies on SFRT identified in our literature review. Visual acuity was stable or improved in 87% of patients, and tumor control rate was 100% (93% stable in size and 7% decreased). Two patients (13%) had worsening vision due to radiation-induced retinopathy. 108 Broadly, these studies support the safety and efficacy of SFRT.
To summarize, our literature review identified a total of 25 articles (case reports excluded) containing patients treated with SFRT with 19 focusing on SFRT (17 exclusively, 2 with a majority of patients receiving SFRT). 76 103 108 110 111 119 120 140 141 142 143 144 145 146 147 148 149 150 151 These comprised a total of 484 patients, including 497 eyes (230 pONSMs and 118 sONSMs), 142 of which had a history of surgery (not including biopsy) during their treatment course. Stable or improved visual function was reported in 76.8 to 100% of patients, with 12 out of 19 studies reporting improvement rates of over 90%. Tumor control rates approached 100%, with several authors reporting decreased tumor size. Follow-up duration ranged from 20 months to 12 years (mean or median value), with five studies reporting at least 5 years of follow-up. Toxicity rates were acceptable in the majority of studies. Late toxicity events included radiation retinopathy in 10 patients. 76 103 108 110 111 119 120 140 141 142 143 144 145 146 147 148 149 150 151 Additionally, one study stated that radiation-induced optic neuropathy could not be ruled out in 11 patients who had worsening vision. 103 Endocrine disturbance was reported in a total of 13 patients from two studies; one of which noted that their three patients with resulting endocrinopathy ended up receiving doses of >45 Gy to the sellar region (above which pituitary dysfunction may occur). 148 In the other study, the authors noted that of their 10 patients with endocrine disturbance, 81.3% had normal endocrine function after 5 years. 103 151 Other significant late events included pain in 29 patients, fatigue in 11, alopecia in 8, sensory loss in 7, hyperlacrimation in 3, dry eye in 3, vitreous hemorrhage in 2, cataract in 2, and optic neuritis in 1. 76 103 108 110 111 119 120 140 141 142 143 144 145 146 147 148 149 150 151 One series included a patient who developed multiple meningiomas 10.9 years post-RT. 111 Overall, while radiation toxicity remains of significant concern, the majority of patients in these studies demonstrated visual improvement after SFRT. The safety and efficacy of this treatment modality is further supported by the large number of patients collectively represented in these studies, as well as the long follow-up duration of several.
Stereotactic Radiosurgery
Stereotactic radiosurgery (SRS) has also emerged as a treatment option for ONSM in recent years, with Gamma knife and CyberKnife among the most commonly used systems. In general, SRS has been avoided as an RT modality for ONSM due to concern that the higher radiation doses required for a given fraction may cause injury to the optic pathway. As a result, some have considered SRS as a treatment modality only to be used in patients with very severe vision loss and/or postoperative patients. 2 6 12 However, several recent reports have included patients receiving SRS as primary treatment in the absence of severe vision loss. 152 153 154 Advantages of SRS include the fact that it offers very strong conformality and tight dose distribution, potentially minimizing irradiation of surrounding critical structures to a greater extent than other modalities. It can also be delivered in a single session or a small number of fractions (note treatment can have up to a maximum of five sessions to still be considered SRS). 6 While Gamma knife and CyberKnife may be comparable in terms of dose coverage and level of conformality, there is some evidence to suggest that Gamma knife may offer a steeper dose gradient, making it potentially superior in terms of sparing of the surrounding tissues. 155 However, CyberKnife offers the advantage of real-time image guidance, and the absence of the rigid head frame used during Gamma knife allows for a greater number of possible trajectories. 155 156
In one of the largest series to date, Liu et al. evaluated treatment outcomes for 30 patients treated with Gamma knife radiosurgery, 9 of whom had had prior resection. Mean tumor volume was 3.6 cm 3 (1.4–9.7 cm 3 ), and the mean prescription peripheral dose was 13.3 Gy (range: 10–17 Gy). After a median follow-up of 56 months, visual acuity improved in 11 patients (37%), stabilized in 13 patients (43%), and worsened in 6 (20%). Follow-up imaging demonstrated tumor progression in two patients; the remainder showed either stable size or decrease in size. No late radiation-toxicity events were reported. Given the long duration of follow-up for these patients, this study supports the relative long-term efficacy in treating ONSM. 154 Other small series and case reports have demonstrated relatively comparable results, several patients demonstrating significant improvement. 152 153 154 157
Overall, our literature search identified five articles containing ONSM patients treated with SRS, with three focused exclusively on SRS (excluding case reports, as well as one study reporting on three patients who were also later included in case series by Romanelli et al). 134 146 152 153 154 156 These three studies represented a total of 56 patients (including 39 pONSMs), 22 of whom had a history of prior surgery. Visual function was reported as stable or improved in 80 to 100% of patients. Duration of follow-up ranged from 30 to 74 months (mean/median values). With regard to late toxicity, one study reported mild optic neuropathy that improved with steroids in one patient; the others did not report any late radiation-induced complications. 152 153 154 While these studies have supported the benefit of SRS for ONSM, this treatment modality has been less extensively investigated than others, and further study will be required to further evaluate its safety and efficacy.
Proton Beam Radiotherapy
Proton beam radiotherapy (PBRT) has been used to treat a variety of intracranial tumors, including meningioma. 158 159 This modality produces highly conformal radiation; studies on the use of PBRT in the treatment of intracranial meningioma suggest that by comparison to 3D-CRT and IMRT, the use of protons may improve target coverage, as well as decrease the integral radiation dose to nontarget tissues. This may be beneficial with regard to decreasing the risk of radiation-induced toxicity, as well as the risk of developing secondary radiation-induced tumors; however, this potential clinical benefit will require further investigation. 158 160
Few studies have focused on examining outcomes of PBRT in treating ONSM. In 2009, Arvold et al reviewed the cases of 25 patients, 9 of whom were treated with PBRT, 13 of whom were treated with SFRT, and 3 of whom were treated with a combination of both modalities. Overall, of 22 patients for whom data were available, 21 (95%) had stable or improved visual acuity at a median follow-up of 30 months (range: 3–168 months), with 1 patient worsening. All demonstrated radiographic control. Notably, no difference in outcome was found between those treated with proton RT and those treated with photon RT. These results thus suggest that PBRT is safe and effective, and produces comparable results to photon therapy. 161 Outside of this study, there have been a few reports with variable results. 162 It appears that while PBRT has been demonstrated to be a relatively effective treatment for meningioma in general, further studies will be necessary to determine both the safety and efficacy of this radiation modality with regard to ONSM.
Radiation Dose and Timing
One of the major challenges in the development of radiation treatment for ONSM has been determining the optimal radiation dose, due to the high-radiation sensitivity of the optic nerve. In a study of 1,388 patients with pituitary adenoma, Becker et al determined the rate of brain necrosis was rare (0.2%) when radiation doses do not exceed 54 Gy given in 1.8 Gy daily fractions. 140 163 Furthermore, in the absence of prior surgical trauma to the optic nerves, the risk of radiation-induced damage to the visual pathways has been found to be less than 2% when total dose remains between 45 and 50 Gy delivered in fractions of less than 2 Gy, and less than 5% with total doses up to 54 Gy. 140 163 164 165 166 167 As a result, for fractionated radiotherapy, the most commonly used radiation dose is 50.4 to 54 Gy, given in 1.8 daily fractions, with doses within this range considered appropriate by several authors. 107 108 126
Other more recent studies have commented on radiation dosage as well. In a 2012 study, Abouaf et al analyzed visual outcomes in 10 patients (five treated with 3D-CRT, three with IMRT, and two with conventional 2D-RT), 3 of whom experienced visual decline due to radiation-related toxicity after 3D-CRT. Interestingly, the authors found toxicity to be correlated with the mean dose to the eye and not the total tumor dose. In those patients who improved, the median mean eye dose was 6.1 Gy, whereas it was 39.4 Gy in those who deteriorated. In addition, although not a statistically significant finding, the authors found that the median distance between the tumor and the posterior pole of the eye was 14.6 mm in those who improved, while those who worsened had tumors that approached the posterior pole (median distance: 0 mm). The authors thus note that mean eye dose and tumor location may play a role in predicting long-term visual outcome, and must be taken into account when planning treatment. 112 Somewhat similarly, in the study by Arvold et al, 3 out of 25 patients (13 treated with SFRT, 9 with proton beam, and 3 with SFRT + proton beam) developed asymptomatic radiation-induced retinopathy. For these three patients, the authors reported median values of 28.4 GyE for mean dose to the retina, and 54.9 GyE for maximum dose to the retina. 161
While SRS has been used less frequently in the treatment of ONSM, certain studies have indicated that the higher radiation dosages required for this treatment modality may indeed be tolerable. For example, in a study of 218 patients with sellar/parasellar tumors, Stafford et al found that the risk of clinically significant radiation-induced optic neuropathy was approximately 1.1% for patients who received less than 12 Gy to a portion of the anterior optic apparatus. 168 However, in a series of 50 patients receiving Gamma knife SRS for benign skull base lesions, Leber et al reported an optic neuropathy incidence rate of 0% for those receiving less than 10 Gy but 26.7% for those receiving 10 to 15 Gy and 77.8% for those receiving at least 15 Gy. 169 The dosages used in published studies including ONSMs treated with SRS have been variable ( Table 1 ; individual SRS doses varied from those used in fractionated SRS, as low as 5 Gy per fraction, given sequentially for a total of 25 Gy, to those used in single-session SRS; as high as 20 Gy given in a single fraction). Further research will be needed to determine the optimal dose.
In general, the timing of radiation therapy remains controversial, and there is evidence to suggest that earlier treatment, prior to the onset of irreversible severe optic atrophy, may lead to superior outcomes. 106 107 108 109 110 111 112 Both Adams et al and Abouaf et al found that the duration elapsed between onset of symptoms and start of RT tended to be shorter for patients who remained stable or improved in comparison to those who worsened. Although this finding was not statistically significant in either study, this remains an interesting trend. Furthermore, in the study by Abouaf et al, higher pretreatment visual acuity was significantly associated with better outcomes. 107 112 The authors note that although it is commonly recommended to reserve treatment until a patient's vision has demonstrably worsened to an acuity of 20/40 or less, they caution that if treatment is delayed, vision may deteriorate to a point where it is ultimately less likely to ever improve. Other authors have found that higher pretreatment visual acuity to be associated with better outcomes as well. In a recent series of 26 patients treated with SFRT, Ratnayake et al found that 92.3% of patients with good vision, pretreatment ultimately had improved or stabilized visual acuity after treatment with SFRT, compared with only 61.5% of patients with poor vision pretreatment. 111 In addition, Sasano et al found that all five eyes (out of 15) that did not have optic disc abnormalities prior to treatment demonstrated improved visual acuity and fields immediately after treatment, with only one showing worsened visual acuity at final follow-up, and thus recommend that RT be initiated before the development of such changes. 106 Similar results have been reported in other studies. 72 106 107 108 109 110 112 Saeed et al also found optic atrophy to be associated with worse visual outcomes than optic nerve edema, although the timing of RT and visual acuity at presentation did not predict outcome. 170 Overall, the optimal timing of radiation treatment has yet to be determined, and bears further study; however, the possible benefits of early RT must not be underestimated.
It is also worth noting that several studies have reported rapid improvement after RT. 114 136 140 141 143 167 In one study by Inoue et al containing four patients treated with IMRT, three experienced improvement early on in their treatment course, within 2 to 4 weeks; a finding consistent with other studies examining other RT treatment modalities. 114 140 141 143 167 Other authors have also reported rapid improvement after SFRT. 141 143 163 167 Additional study will be needed to determine the typical time course of visual improvement, as well as whether or not it has any prognostic value, and if the total radiation dose or duration of treatment should be adjusted to provide further protection against potential toxicity.
In summary, outcomes have been comparable between the major available radiation treatment modalities, although no comparative randomized controlled trials have been conducted. It is worth mentioning that in a study of 34 patients, 22 of whom were treated with conventional fractionated RT and 12 of whom were treated with SFRT, Saeed et al found no significant difference in treatment outcomes was identified between the two modalities. 170 In their study of 25 patients, 13 treated with SFRT, 9 with PBRT, and 3 with both, Arvold et al also found no difference in outcomes between the two modalities. 161 However, more sophisticated forms of radiation treatment such as 3D-CRT, IMRT, SFRT, PBRT, and SRS offer distinct advantages over conventional RT that make them more desirable and safer treatment options, including the benefit of conformal dose distribution that spares critical structures. 126 153 Overall, radiation remains the treatment of choice for the majority of patients with pONSM, and most would extend that statement to many intracranial and orbital of sONSM, with treatment of both forms resulting in stable to improved vision in the majority of patients, and excellent tumor control rates of 90 to 100% (as illustrated in Table 1 ). 108
Surgery
Surgical resection of pONSMs has historically been associated with poor functional outcomes in patients with pONSM, and has generally been avoided as a primary vision preserving therapy in recent years. 1 2 6 16 53 171 172 173 While radiation is likely to remain the mainstay of treatment, with surgery being indicated in only select cases, there have been some recent reports demonstrating acceptable outcomes after various types of surgical intervention. Here, we will discuss surgical interventions including biopsy, optic nerve sheath fenestration, open microsurgical resection, endoscopic endonasal resection, and prechiasmatic optic nerve transection. Table 2 summarizes findings from studies published since 1988 that report on surgical outcomes for ONSM, focusing primarily on pONSM.
Table 2. Surgical outcomes.
| Article | Number of patients and pre-op visual function | Follow-up | Type of surgery | Functional outcomes | Conclusion |
|---|---|---|---|---|---|
| Ito et al (1988) 189 | 11 patients, 7 pONSM, 4 sONSM. pONSM: Surgery alone: 5 patients RT alone: 2 patients sONSM: Surgery alone: 1 patient Surgery + RT: 3 patients Pre-op VA (pONSM): Blind: 3 patients 20/1000: 1 patient 20/500: 1 patient 20/300: 2 patients 20/100: 1 patient Pre-op VF (pONSM): All with VF defects |
pONSM: mean 5 years (range: 2–9 years) | Frontal craniotomy, extradural unroofing of the orbit, removal of supraorbital rim, resection of tumor, opening of optic canal. After intraconal tumor removal, Teflon prosthesis was placed within muscle cone (for prevention of enophthalmos post-operatively). In 3 patients NLP at presentation, the nerve and tumor were excised together. | Visual outcome: improved: 3 patients Stable: 3 patients (all blind pre-op) Worsened: 1 patient Complications: central retinal artery occlusion in 1 patient (with worsening vision) |
It is difficult to avoid injuring the central retinal artery when resecting apical pONSMs. Otherwise, if the tumor is small and located anteriorly, resection with adequate outcome is possible. Recommend excision of tumor with nerve when patient is blind pre-op. If pre-op vision is good, consider observation; however, if vision loss is progressing, consider radiation |
| Kennerdell et al (1988) 113 | 38 patients (39 eyes). RT alone: 6 patients RT + surgery: 5 patients Surgery alone: 10 patients Observation alone: 18 patients Surgery pre-op: 2 patients NLP 1 patients light perception 1 finger fountain 1 20/400 1 20/70 1 patient 20/50 2 patients 20/30 1 patient 20/15 |
At least 3 years in all patients | Subtotal resection in patients with adequate vision. In patients with poor vision, tumor and nerve were excised via lateral orbitotomy |
Surgery group: improved: 1 patient Stable: 2 patients (both NLP pre-op) Worsened: 7 patients Note: optic nerve excised with tumor in 4 patients, including 2 NLP patients) Complications: central retinal artery occlusion in 2 patients, partial central retinal artery occlusion in 1 patient |
Recommend observation for patients with good vision with close follow-up If patient is blind and tumor is contained only within the orbit, recommend observation; however, if it is extending intracranially, recommend complete resection of tumor and nerve |
| Clark et al (1989) 173 | 9 patients (all pONSM) Pre-op vision loss in all patients; 3 blind. |
Median 36.2 months (longest follow-up 8 years) | Lateral orbitotomy, microsurgical exploration of the orbit. If anterior tumor, resection with attempt preserve vision; if posterior, biopsy is obtained to confirm diagnosis (as patient is less likely to improve). In patients with poor vision, optic nerve is excised. Craniotomy done in 8 patients due to intracranial extension of tumor | Vision improved in one patient | Authors state that unless patient has a small, anteriorly located tumor, vision is unlikely to be maintained post-op. In patients with adequate vision and posteriorly located tumors, recommend observation; if vision deteriorates, then resect nerve and tumor together. If intracranial extension occurs, recommend orbitotomy with craniotomy, with optic nerve resection (for complete tumor removal) |
| Kuroda et al (1990) 190 | 8 patients (all pONSM) Pre-op initial symptom: vision loss: 6 patients Proptosis: 2 patients |
Mean 42.8 months (range: 1 month–13 years) | Orbital approaches: transcranial transorbital approach: 2 patients Transcranial transorbital approach with supraorbital rim resection: 5 patients Transcranial supraorbital approach: 1 patient Lateral orbitotomy: 2 patients Craniotomy (orbital approach not described): 1 patient (3 patients underwent 2 surgeries with orbital approach, thus 11 total listed here) |
All patients without useful vision pre-op (surgery done once patients developed blindness, proptosis and/or intracranial extension) Complications: extraocular muscle disturbance: 7 patients, partial in 1 Ptosis: 6 patients, transient in 2 Wound infection: 1 patient Phthisis bulbi: 1 patient Cerebellar hemorrhage: 1 patient Tumor recurrence: 3 patients |
Emphasize importance of early diagnosis and treatment Recommend early surgery for anterior tumors; in cases of blindness, recommend complete resection once diagnosis has been determined |
| Yüceer et al (1994) 172 | 7 patients (all pONSM) Pre-op vision loss in all patients |
Median 22.23 months, up to 4 years | Craniotomy, orbital unroofing, microsurgical exploration | Visual outcome: stable: 3 patients Worsened: 4 patients |
In general, only anteriorly located, small tumors can be resected without causing severe vision loss. This is highly unlikely with posterior tumors; surgery often yields no benefit. Recommend orbitotomy and craniotomy for excision of tumor with nerve in cases of intracranial extension. |
| Cristante (1994) 171 | 57 patients (15 pONSM, including 3 ectopic orbital meningioma, 42 sONSM) Pre-op symptoms (pONSM): Vision loss: 12 patients (80%) VF defect: 13 patients (87%) Optic atrophy: 4 patients (27%) Proptosis: 9 patients (60%) Trigeminal neuralgia/retrobulbar pain: 2 patients (13%). |
Mean 6 years (range: 18 months–10 years) | Craniotomy, when tumor involved optic canal, performed bony decompression of optic canal beginning in the extradural and finishing in the intradural plane via pterional approach. Radical resection in 9 patients; 5 including resection of nerve, tumor and dura (in patients blind pre-op), 3 patients partial resection. In 3 patients with vision pre-op, canal was decompressed and sheath was incised | Visual outcome (pONSM): Improved: 1 patient Stable: 7 patient Worsened: 2 patient Note: improved patient had ectopic ONSM. 1 pONSM recurred, 5 progressed Complications (pONSM) Amaurosis: 1 patient Permanent ocular motility defect: 1 patient Transient ocular motility defect: 6 patients CSF fistula: 1 patient Note: Postoperative visual outcome correlated with preoperative vision loss |
Surgery for pONSM remains more controversial than that for sONS. For pONSM patients, palliation was accomplished in that pre-op VA was maintained for 21–28 months; given the natural history, the utility of surgery is questionable. Visual outcome correlated with pre-op vision loss; patients with poor VA unlikely to improve post-op. |
| Saeed et al (2003) 16 | 88 patients (92 eyes). RT alone: 6 patients Surgery: 47 patients Observation alone: 39 patients En bloc resection patients: 15 total Subtotal resection patients: 10 patients Complete resection: 1 patient Biopsy: 11 patients ONS decompression: 10 patients (Detailed visual data only presented for ONS decompression patients) Pre-op VA: 20/30: 2 patients 20/50: 3 patients 20/60: 1 patient 20/80: 1 patient 20/200 1 patient Finger counting: 2 patients |
ONS decompression patients: Mean 8.1 years (range: 6–10 years) |
Lateral ONS decompression | ONS decompression patients: VA: stable: 2 patients (1 at 20/30, 1 at 20/50) Worsened: 8 patients (5 to NLP, 1 from finger counting to light perception, 1 from 20/50 to hand motion, 1 from 20/60 to finger counting) VF: Improved: 1 patient (VA also stable in this patient) Stable: 4 patients Worsened: 5 patients Tumor progression in 2 patients |
Given that 8 out of 10 patients had poor visual outcome after ONS decompression, would not recommend this procedure as primary treatment for vision preservation. Overall, authors state surgery may be indicated in cases with significant intracranial extension approaching the chiasm, or in blind patients with proptosis. Recommend biopsy if patient has rapid visual decline or atypical imaging features. |
| Schick et al (2004) 57 | 73 patients (7 primary bilateral, 3 with spread to contralateral side) Pre-op symptoms: VA: “good” (Snellen equivalent ≥ 0.5): 34 patients “fair” (< 0.5 to > 0.1): 17 patients “poor (≤ 0.1 to 0): 22 patients VF: deficits present in 32 patients with useful vision. Proptosis: 29 patients Tumors located within the optic canal were associated with worse pretreatment vision loss |
Median 45.4 months (range: 6–144 months) | Pterional craniotomy, decompression of the optic canal via intradural approach in 54 patients, extradural approach via posterior orbitotomy in 10, combined approach in 7, lateral orbitotomy for resection of exophytic tumor in 2. 10 patients received post-op RT |
Initial post-op VA: 35 patients good, 10 patients fair, 28 patients poor. VA at follow-up: of 35 with good VA, 27 stable, 8 worsened Of 10 with fair VA, 1 improved, 5 stable, 4 worsened. Of 28 with poor VA, 1 improved, 27 stable. Note: postoperative visual outcome correlated with preoperative vision loss. Proptosis resolved in 4 patients, partially resolved in 13, stable in 12. Morbidity: Transient: 12.3% Permanent: 2.7% Stroke: 2 patients CSF-fistula: 3 patients (treated with lumbar drain) Subdural hygroma: 2 patients Epidural hematoma: 1 patient Meningitis: 1 patient Seizure: 2 patients 17.8% of patients experienced tumor progression |
Recommendations: For purely intraorbital ONSM: if type Ia or Ib, observe; initiate RT once vision loss begins. Surgery for Ib tumors if mass causes pain and vision is very poor. If type Ic ONSM with large exophytic aspect, treat surgically. For ONSM involving optic canal: If type IIa with vision loss and possible intracranial extension, explore intradural space, decompress canal and resect intracranial portion; can use RT for remaining tumor. If type IIb, obtain biopsy, decompress canal and superior orbital fissure within the extradural plane, RT for intracavernous portion. For ONSM with intracranial extension (type III): resect intracranial portion, decompress canal, RT for intraorbital portion when vision loss develops. |
| Turbin et al (2006) 179 | 2 patients Pre-op VA: Patient 1: 20/200 (note: history of SFRT) Patient 2: NLP |
Patient 1: 7 years, patient 2: 3.5 years | Patient 1: left lateral orbitotomy for biopsy and optic nerve sheath decompression Patient 2: superior-medial eyelid crease orbitotomy, left optic nerve sheath biopsy with creation of dural window. Received SFRT post-op |
Patient 1: VA improved to 20/25 by post-op week 5; stable at final follow up Patient 2: improved to 20/200 by post-op week 4; stable at final follow up Neither patient developed tumor progression |
Optic nerve decompression via nerve sheath fenestration may allow select patients to improve (those with rapidly progressive, severe vision loss and disc edema). |
| Roser et al (2006) 117 | 24 patients (all pONSM), 22 treated with surgery. Pre-op symptoms: VA: normal: 3 patients 0.8: 4 patients 0.6: 3 patients 0.3: 3 patients light perception: 5 patients blind: 6 patients VF: all patients with deficits except 2 Proptosis: 10 patients Eye pain: 7 patients Diplopia: 5 patients Increased pretreatment symptom duration correlated with worse pre-op visual acuity |
Median 45.4 months (range: 6–144 months) | Pterional craniotomy with subtotal resection of tumor (intradural/extradural approach), bony decompression of optic canal and orbit. If patient blind with proptosis, can transect optic nerve and resect intraorbital tumor |
8 out of 16 nonblind patients either remained stable or improved. Only patients with rapid decline pre-op improved. Morbidity: Frontal hygroma: 1 patient CSF fistula: 2 patients (treated with lumbar drain) Oculomotor nerve dysfunction/weakness of levator palpebrae: 5 patients; resolved in 4 out of 5 within 2 months 4 patients experienced tumor progression, causing vision loss in 2 (remaining 2 patients blind pre-op). No blind patients who had total resection and optic nerve transection recurred. SRS used to treat recurrence in 3 patients. |
Surgery may be valuable in cases with rapid visual deterioration, as RT does not cause rapid decrease in tumor size. In these cases, the goal of surgery is to quickly relieve pressure from the optic apparatus. Any remaining tumor may be irradiated, and these remaining targets may be safer due to smaller size Authors recommend surgery for type-IIa ONSM with rapid visual loss. Surgery also indicated for intracranial extension. |
| Schick et al (2010) 58 (includes patients from 2004 study 57 ) | 90 patients Surgery alone: 65 patients RT alone: 5 patients Surgery + RT: 18 patients Observation: 2 patients Pre-op VA (of 83 total patients who had surgery): “good” (≥ 0.5): 40 patients “fair” (<0.5 to >0.1): 12 patients “poor” (≤0.1-0): 31 patients |
Median 45.8 months (range: 6–220 months) | Frontotemporal craniotomy, intradural (56 patients) vs. combined intradural and extradural (18 patients) approach, optic canal decompression, resection (resect intracranial portion, do not resect intraorbital portion). For blind patients with severe proptosis, prechiasmatic transection of nerve was done. In the absence of intracranial extension, extradural pterional approach was preferred for posterior orbitotomy and canal decompression and superior orbital fissure (7 patients) | Of 40 patients with good pre-op vision: 9 worsened, 31 stable. Of 12 patients with fair pre-op vision: 2 improved, 4 worsened, 6 were stable. Of 31 patients with poor vision, 2 improved, 29 stable. Complications: Stroke: 2 patients CSF fistula: 3 patients Epidural hematoma: 2 patients Subdural hygroma: 2 patients Seizure: 2 patients Meningitis: 1 patient Transient morbidity: 13.3% Permanent morbidity: 2.4% |
Surgery including resection of intracranial tumor and optic canal decompression is preferred for cases with intracranial and intracanalicular extension. Surgical goals include resection of tumor/relief of mass effect, and optic canal decompression, with attention paid to pial blood vessels. Radiation can be used to treat residual or recurrent tumor. Authors do not recommend radiation in patients with rapid visual decline; surgery is indicated in these cases as it provides immediate decompression of visual structures. |
| Zweckberger et al (2013) 196 | 12 patients Pre-op: all patients blind in ipsilateral eye |
Mean 50.6 months | Prechiasmatic transection of optic nerve via pterional craniotomy, with intradural or combined extradural and intradural approach, and resection of tumor Gross total resection accomplished in 58.3% |
Vision in contralateral eye was stable in all patients. Overall, 67% of patients showed no tumor recurrence during follow-up period, but 4 ultimately demonstrated delayed recurrence; treated with RT. |
Prechiasmatic transection of the optic nerve may prevent spread of ONSM to the contralateral optic nerve, and thus may aid in vision preservation in the contralateral eye, when ipsilateral eye has already progressed to blindness. |
| Rassi et al (2018) 116 | 8 patients (all pONSM), 1 patient hx prior subtotal resection and RT Pre-op VA: “good” (≥0.5): 6 patients “fair” <0.5 to >0.1): 2 patients. Tumor involved posterior 3rd of optic nerve in all patients, middle 3rd in 4 patients, anterior 3rd in 2 patients, 1 patient had bilateral ONSM across planum sphenoidale |
Mean 38.9 months (range: 3–88 months) | Supraorbital craniotomy (with anterior clinoidectomy if necessary), bony optic canal decompression, resection of tumor. Gross total resection was accomplished in 50% | Post-op visual function: 4 improved, 1 stable, 3 worsened. All patients with good VA pre-op retained good VA. Morbidity: binocular diplopia in 4 patients, resolved. No evidence of tumor recurrence. Patients with good pre-op VA had better outcomes. Patients with tumors involving anterior third of optic nerve had worse outcomes, possibly secondary to involvement of central retinal artery and optociliary arteries in this area. |
Surgery as primary treatment may have successful visual outcomes in select cases. Pre-op VA and tumor location may be prognostic factors for surgical outcome. Tumors restricted to the middle and posterior thirds of the optic nerve, those within which an arachnoidal plane is preserved between tumor and nerve, and patients with better pre-op visual acuity may be the best surgical candidates. Total resection may indeed be possible and may result in improved or stabilized vision. |
| Furdová et al (2018) 197 | 3 patients with orbital meningioma (1 pONSM). [insert paragraph] Pre-op symptoms: VA very poor, all with primary complaint of severe exophthalmos |
Not reported | Enucleation and partial exenteration of ipsilateral eye | Primary outcome: relief of painful exophthalmos in all patients | This procedure is useful for the treatment of patients with severe tumor infiltration into the orbit, in the absence of functional vision. |
| Maza et al (2019) 118 | 4 patients (all pONSM) Pre-op symptoms: VA: Respectively for each patient: 20/30, 20/70, 20/20–2, and “functional blindness” VF: deficits in all patients Proptosis: 2 patients Eye pain: 3 patients Diplopia: 2 patients |
Mean 14 months | Endoscopic endonasal optic canal decompression, orbital decompression. Surgery included complete removal of medial aspect of canal, without incision of optic nerve sheath. Periorbita incised in 2 patients due to intraconal extension of tumor into orbital apex. Decompression extended to superior orbital fissure and cavernous sinus in 1 patient. | VA and VF: 3 improved, 1 stable Proptosis: 1 improved, 1 stable Eye pain: 2 improved, 1 not reported Diplopia: 1 patient partially improved, 1 patient stable No evidence of radiographic progression. Note all patients refused post-op radiation or additional surgery due to symptom improvement vs. personal preference. |
This procedure could be a potentially viable primary treatment option for patients with pONSM. Improvement appears more common in those with rapid vision loss and overall less pre-op visual deficit. Authors note that while incision of optic nerve sheath is often performed in pONSM, this may not always be necessary given these results, and avoiding it may prevent CSF leaks, seeding of tumor, and ophthalmic artery lesions; may reserve this as secondary option. |
Abbreviations: CSF, cerebrospinal fluid; NLP, no light perception; ONSM, optic nerve sheath meningioma; pONSM, primary ONSM; post-op, postoperative; pre-op, preoperative; RT, radiotherapy; SFRT, stereotactic fractionated radiotherapy; sONSM, secondary optic nerve sheath meningioma; SRS, stereotactic radiosurgery; VA, visual acuity; VF, visual field.
Note: Table includes studies published since 1988 evaluating surgical treatment outcomes for ONSM. Studies discussing surgical outcomes for pONSM were included. Studies primarily discussing meningiomas of intracranial origin were excluded, as well as studies combining surgical outcomes for multiple tumor types. Single case reports were also excluded. Two articles also discussed in Table 1 were included again here, to discuss their specific results with regard to surgical intervention.
When reviewing these studies, a few important distinctions must be taken into account, such as the degree of decompression and/or resection, as well as the exact pathology treated. Some centers perform optic canal decompression only, with some studies suggesting that this may be sufficient to treat visual symptoms while minimizing complications. 118 Other studies have reported on outcomes of orbital wall decompression, with some authors performing this procedure in conjunction with canal decompression for the treatment of compressive optic neuropathy, and others utilizing this procedure specifically for the purpose of treating proptosis. 174 175 Lastly, several studies do not specifically distinguish between pONSM and sONSM. As these two entities behave quite differently from a surgical perspective, this complicates the reader's interpretation studies on ONSM.
Biopsy
Biopsy of the optic nerve or its sheath may be accomplished via medial transconjunctival orbitotomy, superior medial transcutaneous orbitotomy, lateral orbitotomy, endoscopic endonasal approach, or transcranial approach. 176 While reported by some to cause significant morbidity, a few recent reports have indicated positive outcomes. 177 178 Biopsy may be of utility in selected cases when imaging results are inconclusive. In a retrospective chart review, Parlin et al compared preoperative diagnoses of cases of intraconal orbital lymphoma and ONSM, two well-known retrobulbar optic nerve space-occupying lesions. The authors found in 2 out of 5 cases, the clinical diagnosis did not match the biopsy result, and note that although both tumors may be treated with radiation, the radiation dosage, as well as disease prognosis, may vary between the two. 95
Optic Nerve Sheath Fenestration
Some studies have focused on the role of optic nerve sheath fenestration (ONSF) in treating ONSM. ONSF has been shown to be of great utility in treating papilledema, particularly idiopathic intracranial hypertension. 176 Previous reported outcomes for ONSF in the treatment of ONSM have been very poor, causing this procedure to be largely abandoned. 16 115 176 However, some recent reports have indicated favorable outcomes. 178 179 180 Turbin et al reported on the cases of two patients, both with significant edema of the optic disc on examination; the first patient with severe vision loss to 20/200, and the second with no light perception. Both underwent orbitotomy and ONSF primarily for biopsy with the secondary result of decompression of the optic nerve sheath. In the first patient, who notably had a history of prior RT, visual acuity decreased from 20/200 preoperatively to no light perception at the immediate postoperative examination, but subsequently improved to 20/25 by 5 weeks later and remained stable at final follow-up after 7 years. The second patient regained light perception after 2 weeks (before starting RT), and then eventually improved to 20/200 after 4 weeks, remaining stable at final follow-up 8 years later. 179 While concern has persisted that ONSF may promote the spread of tumor into the orbit, neither patient demonstrated orbital spread of tumor at follow-up. 22 28 179 The authors conclude that ONSF as an adjuvant form of treatment may provide partial decompression of the optic nerve, potentially benefiting selected patients with severe vision loss and disc edema, and may occur either before or after RT. In addition, the authors also note that none of the patients treated with ONSF described by Saeed et al was reported to receive adjuvant RT, possibly influencing the poor outcomes that were observed. 179 In another report, Meeker et al presented a case of a patient with worsening visual acuity and disc edema, who subsequently underwent ONSF with biopsy and was found to have ONSM. Interestingly, the patient's vision demonstrated improvement after the surgical decompression itself, prior to the initiation of planned RT. After a follow-up of 3 years, he had experienced no complications of this treatment. 178 There have also been reports of ONSF being used to treat other space-occupying lesions of the optic nerve, including metastatic lesions and optic nerve glioma. 176 While only limited data support the therapeutic role of ONSF, these reports suggest a role for ONSF in selected patients, particularly those with significant visual deterioration due to compressive optic neuropathy from sONSM, or compartment syndrome within the orbit.
Case Presentation: Improvement and Stabilization after Biopsy and Radiotherapy
A 32-year-old woman developed worsening of the visual acuity in her left eye rapidly from 20/20 to 20/300 over 5 months and was offered a course of radiation therapy. Her evaluation was notable for 1 mm of left eye proptosis and a prominently swollen optic nerve. Her vision subsequently worsened to no light perception, and this typical progression in the absence of pregnancy or radiographic worsening ( Fig. 17A , 17B ) lead us to pursue an optic nerve sheath biopsy with decompression ( Fig. 17C-E ). Shortly after surgery, she realized visual improvement to 10/400 which continued to improve to 20/200 after LINAC (linear accelerator) based radiotherapy. Her postoperative scan revealed no significant progression and her visual recovery to 20/200 remained durable 8 years after treatment ( Fig. 17F ). Case previously published in 2006. 179
Fig. 17.
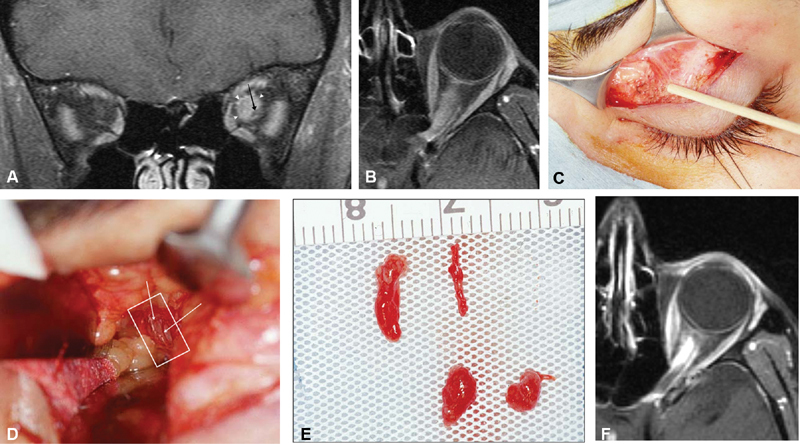
( A ) Coronal T1-weighted, postcontrast MRI image shows a hypointense laterally displaced atrophic left optic nerve (arrow) within a dilated and enhancing optic nerve sheath complex (outlined by arrowheads). ( B ) Axial T1-weighted, postcontrast MRI image shows an enhancing lesion extending the length of the nerve, from the anterior optic nerve through the orbital apex to the prechiasmal left optic nerve. ( C ) The lesion underwent biopsy through a superior medial eyelid crease incision. In this intraoperative photo, the wood applicator points to the medial edge of the levator superioris. The fat plane medial to this edge provides the corridor to access the anterior optic nerve. ( D ) Orbital retractors expose the white optic nerve sheath complex (outlined) surrounded by a plexus of short posterior ciliary arteries (arrows), which must be avoided to potentially preserve vision. ( E ) After opening an approximately 3 mm × 5 mm window, small fragments which extruded from the open sheath were removed piecemeal, with care not to disturb the pial circulation. ( F ) Axial T1-weighted, postcontrast MRI image 5 years after the biopsy and subsequent LINAC radiotherapy. The lesion remained stable and vision remained stable, having recovered to 20/200 from no light perception preoperatively. MRI, magnetic resonance imaging.
Optic Canal Decompression, Orbital Decompression, and Tumor Resection: Surgical Approaches
Endoscopic Approaches
As ONSM is known to cause compressive optic neuropathy, endoscopic endonasal decompression of the optic canal may be able to reverse some of the damage caused by compression of the optic nerve. It may be performed with or without resection of tumor, and with or without opening of the optic nerve sheath itself. 118 121 181 182 183 184 In addition, it may be performed in conjunction with orbital wall decompression to achieve further optic nerve decompression, as reported by some authors. 118 Orbital wall decompression alone may also be performed for the treatment of compressive optic neuropathy, as well as with the specific goal of treating proptosis due to excessive tumor growth. 174 175
Several studies have demonstrated strong visual outcomes for patients undergoing endoscopic endonasal (and transnasal) decompression of the canal for the treatment of compressive optic neuropathy caused by a variety of etiologies, including ONSM. 118 121 182 183 184 Furthermore, endoscopic endonasal approaches have been shown to be safe and effective in the resection of meningiomas involving the skull base. 185 In a recent report, Maza et al commented on the potential utility of endoscopic endonasal optic nerve decompression as primary treatment (without radiation), specifically for pONSM. In this series of four patients, surgery consisted of a unilateral endoscopic endonasal approach, including both optic canal decompression and orbital decompression, without resection of tumor. In two patients with intraconal extension of tumor into the orbital apex, the periorbita was incised, and in one patient, decompression was extended to the cavernous sinus and superior orbital fissure. At follow-up, three out of four patients displayed improved visual acuity and visual fields; the one remaining patient was noted to have optic nerve atrophy at baseline. No patients demonstrated any evidence of tumor progression on repeat imaging or physical exam. 118
Similarly, Hunt et al reported on the use of optic canal decompression with resection of tumor in a patient with intracanalicular ONSM. The patient's visual acuity had deteriorated to 20/40 in the affected eye at time of treatment, with decreased color vision (7/12), and a worsening superior arcuate defect on visual field testing. As imaging revealed a focal lesion within the left optic canal, inferomedial to the optic nerve, an endoscopic endonasal approach was chosen to resect the tumor with the intention of minimizing manipulation of the nerve and promoting direct visualization of involved blood vessels. Two weeks postoperatively, her visual acuity recovered to 20/20, her color vision returned to normal, and her visual field deficit improved; at 1 year, she had a normal ophthalmologic examination. 121
The endonasal approach may also be useful in performing orbital decompression alone (without canal decompression), with or without resection of tumor. While this procedure may be used to treat compressive optic neuropathy via expansion of the orbital apex, it is also used to treat proptosis. 174 This procedure has been shown to have good outcomes in the treatment of proptosis due to other types of pathology. 174 181 In 2018, Zoia et al reported a case in which an endoscopic endonasal approach was used to perform both orbital decompression and resection of tumor in a patient who was already blind in the affected eye but who primarily complained of exophthalmos and conjunctival irritation. In comparison to transcranial approaches, an endoscopic approach is less invasive, and may offer superior orbital decompression. 175
It must be noted that incision of the optic nerve sheath remains controversial, with several authors recommending avoiding this to prevent complications such as injury to the ophthalmic artery and cerebrospinal fluid leak. 118 184 However, other authors report performing a more extensive opening of the optic nerve sheath, as well as opening the annulus of zinn. 57 116 117 The extent of bony decompression may also vary, from simple unroofing of the canal to extensive bony decompression with detethering of the falciform ligament. 186 187 Overall, the results of these studies indicate relatively positive outcomes, suggesting that the aforementioned procedures may be useful in selected cases.
Transcranial Approaches
While certain studies have demonstrated the safety and efficacy of transcranial approaches to the orbit for the surgical treatment of varying types of intraorbital pathology, fewer studies focusing on ONSM have demonstrated positive results. 177 188 Historically, surgery was more commonly recommended for the treatment of ONSM, although outcomes were relatively poor. However, some more recent reports have provided evidence supporting the possible utility of these approaches in treatment of ONSM. As with the endoscopic approaches previously discussed, transcranial approaches may be used to perform optic canal and/or orbital decompression, with or without resection of tumor. Indications and outcomes for different types of surgical procedures may vary.
In 2004, Schick et al reviewed the cases of 73 patients treated with transcranial surgery, including optic canal decompression and resection of tumor. This set of patients included both primary and secondary optic nerve sheath meningiomas, though most fell into the latter classification. At the time of hospital discharge, no significant difference between preoperative and postoperative visual acuity was detected. After extended follow-up (mean, 45.4 months), some patients worsened; however, the majority remained stable within each of these three categories; of 35 patients with good initial postoperative vision, 8 worsened; and of 10 patients with fair postoperative vision, 1 improved, 5 remained stable, and 4 worsened. Unsurprisingly, of the 28 patients with poor initial postoperative vision, 27 remained poor. Overall, rate of transient morbidity was 12.3% and permanent morbidity was 2.7%. Thirteen patients (17.8%) later experienced growth of residual tumor. Although visual acuity worsened with increasing duration of follow-up, given the fact that visual acuity was preserved in the majority of patients in the initial postoperative period, the authors conclude that surgery can indeed be performed safely, with preservation of visual function. These authors came to a similar conclusion as most others: in pONSM (those originating from the orbital or canalicular portion of the optic nerve), radiation is preferred but with sONSM, surgery to resect the tumor is reasonable. 57
In a similar study, Roser et al reviewed the cases of 24 patients with pONSM, 22 of whom were treated with surgery, including optic canal decompression, orbital decompression, and tumor resection. After pterional craniotomy, the authors performed epidural dissection of the orbital roof and removal of the lateral orbital wall, followed by bony decompression of the optic canal and optic nerve sheath. The authors state that patients with type-Ib and-Ic tumors may undergo tumor debulking within the orbit. If intracranial extension is present, an intradural approach for resection of tumor around the optic nerve should be performed first. The authors also emphasize the importance of preserving feeder vessels between the carotid and the optic nerve, as well as respecting the border between the nerve and tumor during resection. In addition, they state that canal decompression is mandatory for type-II and -III tumors. For patients without useful vision, the optic nerve may be transected, with removal of the intraorbital portion. Resection was otherwise subtotal in patients with useful vision. After a mean follow-up of 90.3 months, of the 16 patients who were not blind preoperatively, 8 had either stable or improved visual function. A total of four patients experienced tumor progression postoperatively, resulting in worsening vision in two (remaining two were blind preoperatively). Three patients underwent SRS due to tumor progression postoperatively, all of whom were blind prior to RT. Of the blind patients who underwent total resection and transection of the optic nerve, none demonstrated tumor recurrence. Morbidity rates were acceptable and relatively low. Notably, visual outcomes were inversely related to duration of symptoms; only patients who had experienced rapid preoperative visual deterioration ultimately improved. 117 The authors concluded that surgery may be valuable in cases of rapid visual decline, as surgery can provide expeditious relief of pressure, particularly for patients with type-IIa tumors. Patients with type-Ia and -IIa tumors without vision loss may be managed conservatively or with RT as primary treatment. Treatment of type-Ib tumors may include surgical debulking followed by RT for the remaining tumor. In addition, surgery remains indicated in patients with intracranial extension, in cases when tumor growth may progress to involve the optic chiasm or carotid artery, again with RT for treatment of any residual tumor. 117
In 2018, Rassi et al reviewed the cases of eight patients with intracanalicular pONSM who underwent canal decompression with resection of tumor via supraorbital craniotomy. During surgery, a supraorbital craniotomy is performed with the inclusion of anterior clinoidectomy when needed, for exposure of tumors that extend inferiorly. Subsequently, bony decompression of the optic canal is performed, followed by opening of the annulus of Zinn to release the optic nerve, and then resection of tumor. In this study, gross total resection was achieved in 50%. Overall, after a mean follow-up of 38.9 months, four patients improved, one remained stable, and three declined. There were no instances of tumor recurrence and no long-lasting complications. Notably, patients with good preoperative visual function were able to retain this postoperatively. In addition, the authors found that patients with tumors involving the middle and posterior thirds of the intraorbital optic nerve were better suited for gross total resection and ended up with better visual outcomes which the authors suggest may be due to possible involvement of the optociliary arteries and central retinal arteries anteriorly. Preoperative visual acuity and tumor location may influence the choice to recommend surgery, as well as the timing of surgery. 116
In summary, despite the fact that surgical outcomes for ONSM have been historically poor, emerging evidence suggests that there may still be a role for surgery in selected cases. Various recent studies have indicated acceptable outcomes for optic nerve decompression, orbital decompression, and tumor resection (in various combinations), performed via endoscopic and transcranial approaches. As is the case with several studies on RT, certain surgical series indicate that longer and worse preoperative visual dysfunction predict worse postoperative visual function. 57 116 117 171 Tumor location may also impact surgical outcome as well. 116 172 173 189 190
Optic Canal Surgery and Secondary Optic Nerve Sheath Meningiomas
Both pONSM and sONSM may evolve into a progressive compartment syndrome at the level of the involved optic canal. 180 191 Although pONSM and sONSM may be difficult to distinguish and are frequently discussed in conjunction with one another by many authors, there are significant differences between the two that are worth noting with regard to surgical management. As alluded to in the discussion above, however, both the safety and efficacy of surgery remain controversial subjects and should be assessed relative to the distinction of secondary from primary ONSM which is often not the case in published series. In our opinion, resection of a pONSM is almost never warranted unless a patient has failed or has an absolute contraindication to radiotherapy, or in cases of disfigurement or pain. 1 7 10 12 13 57 Because they originate intracranially, many sONSM spread along the optic nerve preserving a potential dissection plane, rather than infiltrating the fragile pial blood supply of the intraorbital optic nerve 192 193 ( Figs. 18 , 19 ). Tumors that preserve this arachnoidal plane may lend themselves to safe microsurgical dissection of the tumor from nerve without causing ischemia. In a systematic review of surgical outcome data for meningiomas affecting the optic nerve, Hénaux et al stratified cases into those causing isolated compression at the intraorbital, canalicular, and intracranial aspects of the optic nerve. The authors noted that when the optic nerve is compressed only in its cisternal portion, approximately 50% of patients improved after resection. Of cases involving compression within the optic canal, 31% improved, and of cases involving compression of the intraorbital aspect of the nerve, only 11% improved. The authors attribute these stark differences in improvement rates partially to the arachnoid plane that is more consistently observed in association with the intracranial portion of the nerve, and the lack thereof with regard to intraorbital ONSM. 192
Fig. 18.
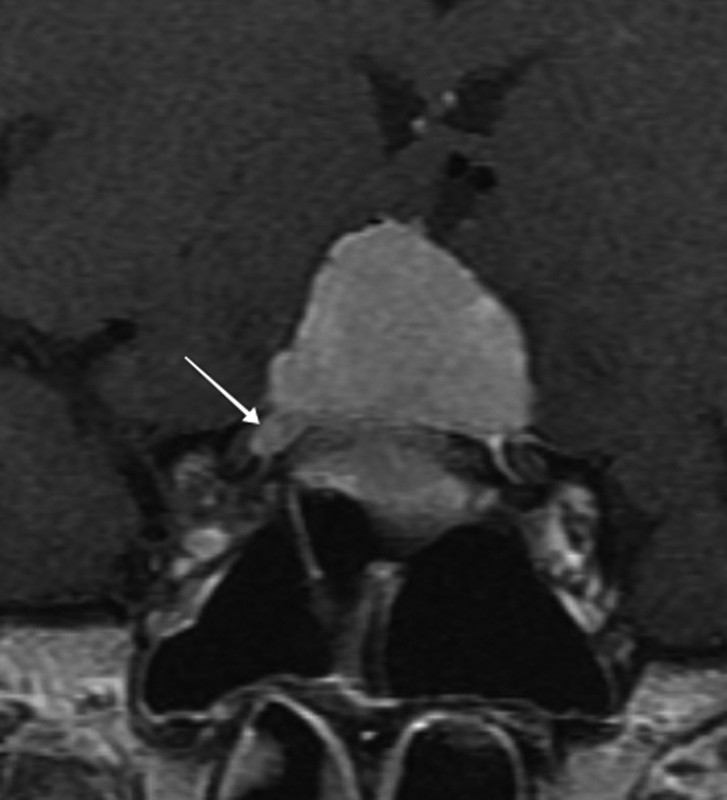
Coronal T1-weighted contrast-enhanced MRI image shows a large dural based planum sphenoidale lesion consistent with a meningioma extending into the right optic canal (arrow). In the setting of visual loss from bilateral optic nerve compression, the patient would benefit from surgical debulking, potentially with a right optic canal decompression and likely subsequent radiotherapy. MRI, magnetic resonance imaging.
Fig. 19.
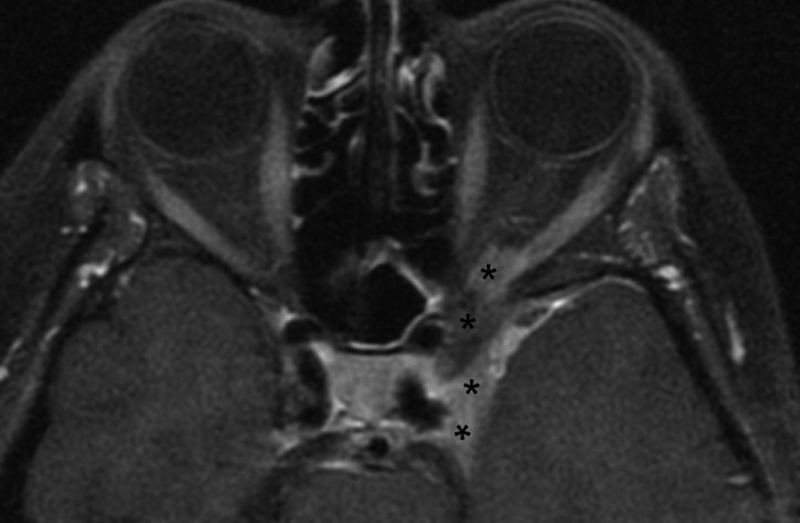
Axial T1-weighted contrast enhanced MRI showing a secondary ONSM, most likely originating from the left cavernous sinus with extension into the left optic canal (asterisks). Classically this type of case would benefit from primary radiotherapy, albeit some highly skilled skull base surgeons have experience with intracavernous resection, and optic canal decompression. The latter remains a high-risk procedure for visual loss, and would have been shown to be less advantageous than primary radiotherapy in some studies. 53 MRI, magnetic resonance imaging; ONSM, optic nerve sheath meningioma.
As previously discussed, canal decompression and partial resection may still result in positive outcomes, particularly in patients with sONSM. 194 To this point, Lehmberg and colleagues reviewed a sequential case series of 46 skull base meningiomas involving the optic nerve, operated on by three experienced surgeons from 2006 to 2011. All of their patients would likely be classified as sONSM; 39% had meningiomas of the anterior clinoid process, 17% sphenoid wing, 13% sphenoorbital, 9% cavernous sinus, 9% sphenoid plane, 9% tuberculum sellae, and 4% petroclival. The study compares outcomes among patients undergoing tumor resection without decompression (26%), with intradural optic canal unroofing (22%), and with wide bony decompression (typically extradural) and anterior clinoidectomy (52%). Gross total resection occurred in 46% of cases, with subtotal resection in the remaining 54%. Preoperative visual data were somewhat limited in this report, but authors described that overall, 57% of patients had a preoperative deficit in visual acuity. Of those receiving anterior clinoidectomy, 61% improved, and 39% remained stable. By comparison, in patients who did not receive anterior clinoidectomy, only 25% improved, 70% remained stable, and 5% worsened. 186 Overall, the majority of patients in this study demonstrated improvement postoperatively.
In another study of 63 patients, 16 with pONSM (five with recurrence after prior surgery), and 47 with sONSM (either sphenoid ridge or tuberculum sellae meningioma, 9 with history of prior surgery, outcomes may be summarized as follows: symptom improvement in sONSM cases was observed in 91% overall, with 65% improving fully and without complication. By contrast, although pONSM patients in this series did not undergo surgery until they lost useful vision, complications included oculomotor palsy in three patients, and two developed recurrence with intracranial extension. The authors note that while surgical outcomes were very strong for patients with sONSM, pONSM patients experienced less favorable outcomes. 195
Overall, the results of these studies suggest that good visual outcomes may be achieved in the surgical treatment of meningiomas that secondarily invade the canal.
Case Presentation: Secondary ONSM Treated with Partial Resection of Intracranial Component and Optic Canal Decompression Followed by Proton Beam Divided Dose Radiotherapy
A 70-year-old woman presented with transient visual loss, mild right optic disc edema, a moderate partial ipsilateral third nerve palsy, and compressive optic neuropathy with a dense afferent, as well as efferent pupil defect, and ipsilateral visual field cut despite relatively preserved visual acuity of 20/25. A discussion of primary RT versus combined approach with surgery and postoperative RT ensued at the multispecialty skull base board. The neurosurgical consultant recommended a decompression of the optic canal and third nerve in the context of sONSM, and associated hyperostosis. The patient underwent a right-sided one-piece orbitozygomatic transcavernous approach to the parasellar skull base, resection of intradural cavernous sinus meningioma with extradural peeling of the lateral wall of the cavernous sinus, and right-sided optic nerve decompression using optic roof decompression and anterior clinoidectomy, oculomotor nerve decompression. Her disc edema, vision loss, and third nerve palsy responded to the operative approach which was subsequently augmented with proton beam divided dose radiotherapy. Her vision returned to 20/20, visual field cut regressed, and third nerve palsy resolved. She remains stable 4 years after the treatments. ( Fig. 20A–C )
Fig. 20.
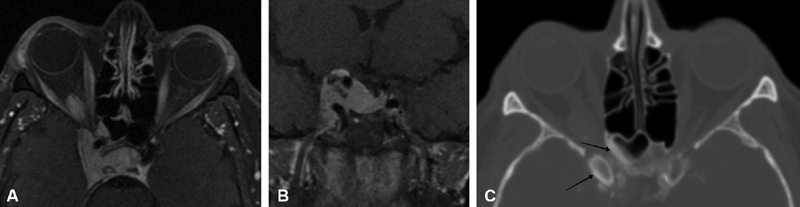
( A–C ) Axial ( A ) and coronal ( B ) T1-weighted, postcontrast MRI image show a clinoidal and cavernous sinus meningioma surrounding the right carotid with invasion of the sella turcica and right orbit, probably through both the superior orbital fissure and optic canal. Axial bone windowed CT ( C ) with contrast shows a narrowing right optic canal with hyperostosis (arrows) of the sphenoid sinus wall and right anterior clinoid, as well as intraorbital soft-tissue extension to the right optic nerve. CT, computed tomography; MRI, magnetic resonance imaging. Case courtesy of Dr. James K. Liu.
Surgery in Cases of Severe Vision Loss
As previously discussed, indications for surgery in patients without useful vision remain controversial. Surgical treatment to prevent or correct disfiguring proptosis is a reasonable goal. Similarly, surgery to prevent contralateral visual loss is a reasonable goal but not always clinically necessary. For instance, some authors have reported the use of prechiasmatic transection of the affected optic nerve to prevent tumor progression to the contralateral nerve and subsequent bilateral vision loss, with several routinely transecting the nerve when performing resection in blind patients. 196 In our experience, in the setting of ONSM, intracranial nerve transection and extirpation has rarely been necessary. Given that these patients require continued clinical and radiographic follow-up, this aggressive approach could be offered for cases with documented inexorable progression despite more conservative measures. There are numerous cases of patients with severe visual loss that have enjoyed meaningful and durable recovery of visual acuity after radiation therapy, and we routinely prescribe a course of radiotherapy in these situations despite severe visual loss.
In patients with severe proptosis, more limited tumor debulking including orbital bone decompression may obviate the need for more extended cranial or craniofacial approaches in select cases. Similarly, enucleation or the removal of the globe after detachment of the extraocular muscles typically followed by placement of an indwelling orbital implant and secondary cosmetic ocular shell, may benefit patients with a blind and painful degenerating globe. Exenteration of the eye and orbit is a more radical procedure that adds resection of additional soft tissue and may be performed from an anterior limited approach or combined with more comprehensive craniofacial resection of periorbita and bone in the most severe cases. 57 190 197 198 In our experience, exenteration is rarely utilized for pONSM and sONSM and its use is limited to cases of advanced primary or secondary orbital malignancy.
Medical Therapy
Historically, medical therapy has not played a role in the treatment of intracranial meningiomas, as drug trials have remained relatively unsuccessful. As meningiomas are known to express estrogen and progesterone receptors, hormone-directed therapies including tamoxifen (an antiestrogen agent) and mifepristone (an antiprogesterone agent) were investigated in clinical trials; however, these agents proved to be of little to no benefit. 29 199 200 Recently, other pharmacotherapies including peptide receptor radionuclide therapy (consisting of radiolabeled somatostatin receptor ligands), antiangiogenic targeted therapies (mainly the vascular endothelial growth factor [VEGF] inhibitor bevacizumab, and the multitargeted tyrosine kinase inhibitors vatalanib and sunitinib with both anti-VEGF and anti-PDGF properties), and trabectedin (a tetrahydroisoquinoline with antineoplastic properties) have shown relatively promising results. 200 201 In addition, immunomodulation with α-interferon treatment has shown limited results. 202 At this time, the only medical therapies that have been recommended by the National Comprehensive Cancer Network (NCCN) include α-interferon, somatostatin receptor agonists, and antiangiogenic agents. 203 204 Treatment with hydroxyurea, a ribonucleotide reductase inhibitor known to induce apoptosis in meningioma cell culture, has also shown modest results with regard to intracranial meningioma, and there has been one reported case of ONSM treated with hydroxyurea resulting in improved visual acuity and visual fields. 29 200 205 206 207 However, for the most part, there has been relatively little investigation into the potential utility of chemotherapeutic agents in the treatment of ONSM specifically. 29
Recently, two studies have explored the potential therapeutic utility of chemotherapeutic agents in ONSM. Investigation of the antitumor effects of farnesol (a plant and fungal-derived compound that has been found to have chemotherapeutic potential) and diosgenin (a steroidal saponin compound derived from fenugreek seeds) on an ONSM cell line (HBL-52) in vitro have met with some success. 208 209 210 There have also been some reports on the use of mitomycin C to treat and/or prevent tumor recurrence. 211 212 Overall, there is very little evidence to support the current use of chemotherapeutic agents in the treatment of ONSM. The development and common use of chemotherapeutic or other medical agents to treat ONSM will require additional investigation.
Conclusion
ONSMs are rare tumors of the optic nerve sheath that frequently present a diagnostic challenge. Although several classic clinical and radiographic features have been described, many ONSMs do not present in a typical fashion, and may mimic (or be mimicked by) a variety of other inflammatory or infiltrative clinical entities, including optic neuritis/perineuritis, optic nerve sarcoid, and lymphoma. In the setting of significant diagnostic uncertainty despite thorough evaluation, a corticosteroid trial may aid in ruling out potential entities that respond to steroids. Ultimately, some cases may require biopsy to confirm a diagnosis. In addition, while many patients display slowly progressive vision loss, allowing sufficient time to establish the diagnosis, the rapidity of diagnosis may be of greater importance in selected patients such as pregnant females who may experience increased tumor growth due to hormonal changes. Treatment planning requires an individualized approach. Patients with pONSM, including those with tumor confined to the optic canal, may benefit from radiation therapy as primary treatment. There may be a role for surgery in selected patients, such as those with significant intracranial extension or severe proptosis (especially when vision loss has already become severe and preservation of vision is of less concern), or in patients with sONSM or those secondarily invading the optic canal. Historically, surgery for resection has been associated with poor functional outcomes, generally attributed to the anatomical relationship of the blood supply to the tumor infiltrating the optic nerve sheath complex. This has resulted in a shift toward decompression alone. However, surgical practices vary institutionally and regionally and outcomes may vary based on the skill of the surgeon. Several cases of patients have been reported, demonstrating improved or stable vision postoperatively, highlighting the importance of treatment at a center with a multidisciplinary skull base surgery team.
Acknowledgments
We would like to acknowledge Drs. Paul Langer, Neena Mirani, James K. Liu and Nasrin Ghesani for their case contributions.
Funding Statement
Financial Support This study received fund from Gene C. Coppa Memorial Endowment, Newark, New Jersey; the organization had no role in the design or conduct of this manuscript.
Footnotes
Conflict of Interest None declared.
Pearls and Tips.
Conformal divided dose radiation therapy is a mainstay of treatment when the treatment goal is preservation of vision. It is commonly initiated in the setting of documented worsening visual function or radiographic progression.
Visual function may deteriorate in the absence of radiographic progression and may be related to isovolemic compression of the nerve. Therefore, serial ophthalmic evaluation is a critical component of the long-term care of patients with ONSM.
Identification of pONSM and differentiation from other lesions is aided by recognizing the delineation of optic nerve tissue from surrounding tumor, with the nerve typically of low signal and potentially displaced or compressed eccentrically within an extrinsic enhancing tumor mass. Similarly, pONSMs tend to extend into the optic canal, rather than the superior and inferior orbital fissures which is a more common finding in secondary tumors affecting the optic nerve. It may be difficult to differentiate some secondary types from primary lesions with extraorbital origin. The latter limitations should be taken into account when reviewing case series reported in the literature.
A small ring or rim of cerebrospinal fluid (CSF) on high-resolution images such as constructive interference in steady state (CISS) and other steady-state free precession (SSPF) images may indicate a dissection plane in secondary tumors affecting the optic nerve amenable to surgery in contrast to a tumor that infiltrates the pial vasculature, the nerve tissue itself, or that which arises as primary ONSM.
A limited corticosteroid trial may occasionally be necessary and appropriate to differentiate ONSM tumor from other infiltrative or inflammatory lesions.
Biopsy may be necessary in lesions with atypical radiology or clinical course, and the initial decompressive effect may improve vision but should be supplemented with adjuvant radiotherapy in many ONSM.
It may be difficult to determine if some tumors, especially those arising from the intracanalicular segment, are primary or secondarily affecting the optic nerve. There may be a surgical role in these cases, but radiotherapy should be regarded as a primary therapy for intracanalicular and intraorbital segments.
References
- 1.Dutton J J. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37(03):167–183. doi: 10.1016/0039-6257(92)90135-g. [DOI] [PubMed] [Google Scholar]
- 2.Parker R T, Ovens C A, Fraser C L, Samarawickrama C. Optic nerve sheath meningiomas: prevalence, impact, and management strategies. Eye Brain. 2018;10:85–99. doi: 10.2147/EB.S144345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohtsuka K, Hashimoto M, Suzuki Y. A review of 244 orbital tumors in Japanese patients during a 21-year period: origins and locations. Jpn J Ophthalmol. 2005;49(01):49–55. doi: 10.1007/s10384-004-0147-y. [DOI] [PubMed] [Google Scholar]
- 4.Shields J A, Shields C L, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111(05):997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Lindegaard J, Heegaard S, Prause J U. Histopathologically verified non-vascular optic nerve lesions in Denmark 1940-99. Acta Ophthalmol Scand. 2002;80(01):32–37. doi: 10.1034/j.1600-0420.2002.800107.x. [DOI] [PubMed] [Google Scholar]
- 6.Shapey J, Sabin H I, Danesh-Meyer H V, Kaye A H. Diagnosis and management of optic nerve sheath meningiomas. J Clin Neurosci. 2013;20(08):1045–1056. doi: 10.1016/j.jocn.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Eddleman C S, Liu J K. Optic nerve sheath meningioma: current diagnosis and treatment. Neurosurg Focus. 2007;23(05):E4. doi: 10.3171/FOC-07/11/E4. [DOI] [PubMed] [Google Scholar]
- 8.Jeremic B, Pitz S. Berlin, Germany: Springer-Verlag; 2008. Overall introduction, problem definition, incidence; pp. 1–6. [Google Scholar]
- 9.Miller N R. Primary tumours of the optic nerve and its sheath. Eye (Lond) 2004;18(11):1026–1037. doi: 10.1038/sj.eye.6701592. [DOI] [PubMed] [Google Scholar]
- 10.Turbin R E, Pokorny K. Diagnosis and treatment of orbital optic nerve sheath meningioma. Cancer Contr. 2004;11(05):334–341. doi: 10.1177/107327480401100508. [DOI] [PubMed] [Google Scholar]
- 11.Hwang C J, Patel B CK, Singh A eds. Switzerland: Springer; 2019. Clinical Ophthalmic Oncology: Orbital Tumors. [Google Scholar]
- 12.Bloch O, Sun M, Kaur G, Barani I J, Parsa A T. Fractionated radiotherapy for optic nerve sheath meningiomas. J Clin Neurosci. 2012;19(09):1210–1215. doi: 10.1016/j.jocn.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Kim J W, Rizzo J F, Lessell S. Controversies in the management of optic nerve sheath meningiomas. Int Ophthalmol Clin. 2005;45(04):15–23. doi: 10.1097/01.iio.0000176367.16758.f4. [DOI] [PubMed] [Google Scholar]
- 14.Gündüz K, Kurt R A, Erden E. Ectopic orbital meningioma: report of two cases and literature review. Surv Ophthalmol. 2014;59(06):643–648. doi: 10.1016/j.survophthal.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Tan L T, Stewart C M, Sheerin F, MacDonald B, Silva P, Norris J H. Ectopic orbital meningioma: fact or fiction? Orbit. 2017;36(03):144–146. doi: 10.1080/01676830.2017.1279663. [DOI] [PubMed] [Google Scholar]
- 16.Saeed P, Rootman J, Nugent R A, White V A, Mackenzie I R, Koornneef L. Optic nerve sheath meningiomas. Ophthalmology. 2003;110(10):2019–2030. doi: 10.1016/S0161-6420(03)00787-5. [DOI] [PubMed] [Google Scholar]
- 17.Misra S, Misra N, Gogri P, Mehta R. A rare case of bilateral optic nerve sheath meningioma. Indian J Ophthalmol. 2014;62(06):728–730. doi: 10.4103/0301-4738.136238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nickel M, Löbel U, Holst B. Unexplained loss of vision in a child: consider bilateral primary optic nerve sheath meningioma. Neuropediatrics. 2014;45(05):321–324. doi: 10.1055/s-0034-1372303. [DOI] [PubMed] [Google Scholar]
- 19.Cohn E M. Optic nerve sheath meningioma. Neuroradiologic findings. J Clin Neuroophthalmol. 1983;3(02):85–89. [PubMed] [Google Scholar]
- 20.Craig W M, Gogela L J.Intraorbital meningiomas; a clinicopathologic study Am J Ophthalmol 194932121663–1680., illust15399170 [Google Scholar]
- 21.Turbin R E, Kennerdell J S. Berlin, Germany: Springer-Verlag; 2008. Clinical evaluation of primary optic nerve sheath meningioma; pp. 17–37. [Google Scholar]
- 22.Wright J E, Call N B, Liaricos S. Primary optic nerve meningioma. Br J Ophthalmol. 1980;64(08):553–558. doi: 10.1136/bjo.64.8.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trobe J D, Glaser J S, Post J D, Page L K. Bilateral optic canal meningiomas: a case report. Neurosurgery. 1978;3(01):68–74. doi: 10.1227/00006123-197807000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Lewis T, Kingsley D, Moseley I. Do bilateral optic nerve sheath meningiomas exist? Br J Neurosurg. 1991;5(01):13–18. doi: 10.3109/02688699108998441. [DOI] [PubMed] [Google Scholar]
- 25.Carrasco J R, Penne R B. Optic nerve sheath meningiomas and advanced treatment options. Curr Opin Ophthalmol. 2004;15(05):406–410. doi: 10.1097/01.icu.0000138617.53435.d9. [DOI] [PubMed] [Google Scholar]
- 26.Wilson W B. Meningiomas of the anterior visual system. Surv Ophthalmol. 1981;26(03):109–127. doi: 10.1016/0039-6257(81)90060-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee H B, Garrity J A, Cameron J D, Strianese D, Bonavolontà G, Patrinely J R. Primary optic nerve sheath meningioma in children. Surv Ophthalmol. 2008;53(06):543–558. doi: 10.1016/j.survophthal.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Wright J E, McNab A A, McDonald W I. Primary optic nerve sheath meningioma. Br J Ophthalmol. 1989;73(12):960–966. doi: 10.1136/bjo.73.12.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller N R. New concepts in the diagnosis and management of optic nerve sheath meningioma. J Neuroophthalmol. 2006;26(03):200–208. doi: 10.1097/01.wno.0000235569.19131.ac. [DOI] [PubMed] [Google Scholar]
- 30.Sibony P A, Krauss H R, Kennerdell J S, Maroon J C, Slamovits T L. Optic nerve sheath meningiomas. Clinical manifestations. Ophthalmology. 1984;91(11):1313–1326. doi: 10.1016/s0161-6420(84)34148-3. [DOI] [PubMed] [Google Scholar]
- 31.Whittle I R, Smith C, Navoo P, Collie D.Meningiomas Lancet 2004363(9420):1535–1543. [DOI] [PubMed] [Google Scholar]
- 32.Bosch M M, Wichmann W W, Boltshauser E, Landau K. Optic nerve sheath meningiomas in patients with neurofibromatosis type 2. Arch Ophthalmol. 2006;124(03):379–385. doi: 10.1001/archopht.124.3.379. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Wang Z, Zhou Q. A retrospective analysis of vision-impairing tumors among 467 patients with neurofibromatosis type 2. World Neurosurg. 2017;97:557–564. doi: 10.1016/j.wneu.2016.10.080. [DOI] [PubMed] [Google Scholar]
- 34.Narayan D S, Traber G L, Figueira E. Natural history of primary paediatric optic nerve sheath meningioma: case series and review. Br J Ophthalmol. 2018;102(08):1147–1153. doi: 10.1136/bjophthalmol-2017-310672. [DOI] [PubMed] [Google Scholar]
- 35.Narayan S, Cornblath W T, Sandler H M, Elner V, Hayman J A. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003;56(02):537–543. doi: 10.1016/s0360-3016(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 36.Monteiro M LR, Gonçalves A CP, Siqueira S AC, Gebrim E MM. Optic nerve sheath meningioma in the first decade of life: case report and review of the literature. Case Rep Ophthalmol. 2012;3(02):270–276. doi: 10.1159/000342261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin L A, Jakobiec F A. Optic nerve tumors of childhood: a decision-analytical approach to their diagnosis. Int Ophthalmol Clin. 1992;32(01):223–240. doi: 10.1097/00004397-199203210-00017. [DOI] [PubMed] [Google Scholar]
- 38.Alper M G. Management of primary optic nerve meningiomas. Current status--therapy in controversy. J Clin Neuroophthalmol. 1981;1(02):101–117. [PubMed] [Google Scholar]
- 39.Friedman D L, Whitton J, Leisenring W. Subsequent neoplasms in 5-year survivors of childhood cancer: the childhood cancer survivor study. J Natl Cancer Inst. 2010;102(14):1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godlewski B, Drummond K J, Kaye A H. Radiation-induced meningiomas after high-dose cranial irradiation. J Clin Neurosci. 2012;19(12):1627–1635. doi: 10.1016/j.jocn.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Bosch M M, Boltshauser E, Harpes P, Landau K. Ophthalmologic findings and long-term course in patients with neurofibromatosis type 2. Am J Ophthalmol. 2006;141(06):1068–1077. doi: 10.1016/j.ajo.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 42.Baser M E, Evans D G, Jackler R K, Sujansky E, Rubenstein A. Neurofibromatosis 2, radiosurgery and malignant nervous system tumours. Br J Cancer. 2000;82(04):998. doi: 10.1054/bjoc.1999.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan A CS, Chen L, Png R, Chia A. The diagnosis and assessment of visual function in Singaporean children with electrophysiology: 10-year results. Doc Ophthalmol. 2016;132(03):189–199. doi: 10.1007/s10633-016-9539-9. [DOI] [PubMed] [Google Scholar]
- 44.Jayanetti V, Klistorner A I, Graham S L. Monitoring of optic nerve function in neurofibromatosis 2 children with optic nerve sheath meningiomas using multifocal visual evoked potentials. J Clin Neurosci. 2018;50:262–267. doi: 10.1016/j.jocn.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Kahraman-Koytak P, Bruce B B, Peragallo J H, Newman N J, Biousse V. Diagnostic errors in initial misdiagnosis of optic nerve sheath meningiomas. JAMA Neurol. 2019;76(03):326–332. doi: 10.1001/jamaneurol.2018.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao J-F, Xia X-B, Tang X-B, Zhang X-Y, Wen D. Analyses on the misdiagnoses of 25 patients with unilateral optic nerve sheath meningioma. Int J Ophthalmol. 2016;9(09):1315–1319. doi: 10.18240/ijo.2016.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalen B D, Hess R A, Abi-Aad K R. Addressing misdiagnosis of optic nerve sheath meningiomas. World Neurosurg. 2020;133:419–420. doi: 10.1016/j.wneu.2019.10.122. [DOI] [PubMed] [Google Scholar]
- 48.Ling J D, Chao D, Al Zubidi N, Lee A G. Big red flags in neuro-ophthalmology. Can J Ophthalmol. 2013;48(01):3–7. doi: 10.1016/j.jcjo.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 49.Moster M L. Detection and treatment of optic nerve sheath meningioma. Curr Neurol Neurosci Rep. 2005;5(05):367–375. doi: 10.1007/s11910-005-0060-x. [DOI] [PubMed] [Google Scholar]
- 50.Orcutt J C, Tucker W M, Mills R P, Smith C H. Gaze-evoked amaurosis. Ophthalmology. 1987;94(03):213–218. doi: 10.1016/s0161-6420(87)33471-2. [DOI] [PubMed] [Google Scholar]
- 51.Smith J L, Vuksanovic M M, Yates B M, Bienfang D C. Radiation therapy for primary optic nerve meningiomas. J Clin Neuroophthalmol. 1981;1(02):85–99. [PubMed] [Google Scholar]
- 52.Bradbury P G, Levy I S, McDonald W I. Transient uniocular visual loss on deviation of the eye in association with intraorbital tumours. J Neurol Neurosurg Psychiatry. 1987;50(05):615–619. doi: 10.1136/jnnp.50.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turbin R E, Thompson C R, Kennerdell J S, Cockerham K P, Kupersmith M J.A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy Ophthalmology 200210905890–899., discussion 899–900 [DOI] [PubMed] [Google Scholar]
- 54.Masuyama Y, Kodama Y, Matsuura Y, Sawada A, Harada K, Tsuchiya T. Clinical studies on the occurrence and the pathogenesis of optociliary veins. J Clin Neuroophthalmol. 1990;10(01):1–8. doi: 10.3109/01658109008997254. [DOI] [PubMed] [Google Scholar]
- 55.Perlmutter J C, Klingele T G, Hart W M, Jr., Burde R M. Disappearing opticociliary shunt vessels and pseudotumor cerebri. Am J Ophthalmol. 1980;89(05):703–707. doi: 10.1016/0002-9394(80)90291-3. [DOI] [PubMed] [Google Scholar]
- 56.Okamoto N, Suzuki A, Ohnishi M, Fukuda M. The formation and involution of optociliary veins during the course of central retinal vein occlusion. Jpn J Ophthalmol. 2000;44(03):312–313. doi: 10.1016/s0021-5155(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 57.Schick U, Dott U, Hassler W. Surgical management of meningiomas involving the optic nerve sheath. J Neurosurg. 2004;101(06):951–959. doi: 10.3171/jns.2004.101.6.0951. [DOI] [PubMed] [Google Scholar]
- 58.Schick U, Jung C, Hassler W E. Primary optic nerve sheath meningiomas: a follow-up study. Cent Eur Neurosurg. 2010;71(03):126–133. doi: 10.1055/s-0029-1246136. [DOI] [PubMed] [Google Scholar]
- 59.Karp L A, Zimmerman L E, Borit A, Spencer W. Primary intraorbital meningiomas. Arch Ophthalmol. 1974;91(01):24–28. doi: 10.1001/archopht.1974.03900060028007. [DOI] [PubMed] [Google Scholar]
- 60.Mafee M F, Goodwin J, Dorodi S.Optic nerve sheath meningiomas. Role of MR imaging Radiol Clin North Am 1999370137–58., ix ix [DOI] [PubMed] [Google Scholar]
- 61.Samarawickrama C, Frydenberg E, Wells M, Steel T, Ghabrial R. An unusual radiological presentation of optic nerve sheath meningioma. Saudi J Ophthalmol. 2016;30(02):137–139. doi: 10.1016/j.sjopt.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nabavizadeh S A, Santi M, Belasco J B, Zimmerman R A. Primary atypical optic nerve sheath meningioma in a child with restricted diffusion on magnetic resonance imaging. J Neuroophthalmol. 2014;34(02):173–176. doi: 10.1097/WNO.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 63.Savignac A, Lecler A. Optic nerve meningioma mimicking cavernous hemangioma. World Neurosurg. 2018;110:301–302. doi: 10.1016/j.wneu.2017.11.107. [DOI] [PubMed] [Google Scholar]
- 64.Galldiks N, Albert N L, Sommerauer M. PET imaging in patients with meningioma-report of the RANO/PET group. Neuro-oncol. 2017;19(12):1576–1587. doi: 10.1093/neuonc/nox112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rachinger W, Stoecklein V M, Terpolilli N A. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015;56(03):347–353. doi: 10.2967/jnumed.114.149120. [DOI] [PubMed] [Google Scholar]
- 66.Gehler B, Paulsen F, Oksüz MÖ. [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. 2009;4(01):56. doi: 10.1186/1748-717X-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dutour A, Kumar U, Panetta R. Expression of somatostatin receptor subtypes in human brain tumors. Int J Cancer. 1998;76(05):620–627. doi: 10.1002/(sici)1097-0215(19980529)76:5<620::aid-ijc2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 68.Al Feghali K A, Yeboa D N, Chasen B, Gule M K, Johnson J M, Chung C. The use of 68 Ga-DOTATATE PET/CT in the non-invasive diagnosis of optic nerve sheath meningioma: a case report . Front Oncol. 2018;8:454. doi: 10.3389/fonc.2018.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antunes P, Ginj M, Zhang H. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34(07):982–993. doi: 10.1007/s00259-006-0317-x. [DOI] [PubMed] [Google Scholar]
- 70.Klingenstein A, Haug A R, Miller C, Hintschich C. Ga-68-DOTA-TATE PET/CT for discrimination of tumors of the optic pathway. Orbit. 2015;34(01):16–22. doi: 10.3109/01676830.2014.959185. [DOI] [PubMed] [Google Scholar]
- 71.Mokhtarzadeh A, Maltry A, McClelland C. Waiting to deliver a final diagnosis. Surv Ophthalmol. 2017;62(04):583–586. doi: 10.1016/j.survophthal.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Eckert F, Clasen K, Kelbsch C. Retrospective analysis of fractionated intensity-modulated radiotherapy (IMRT) in the interdisciplinary management of primary optic nerve sheath meningiomas. Radiat Oncol. 2019;14(01):240. doi: 10.1186/s13014-019-1438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saeed P, Tanck M WT, Freling N, Baldeschi L, Mourits M P, Bennink R J. Somatostatin receptor scintigraphy for optic nerve sheath meningiomas. Ophthalmology. 2009;116(08):1581–1586. doi: 10.1016/j.ophtha.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 74.Chandra P, Purandare N, Shah S, Agrawal A, Rangarajan V. Somatostatin receptor SPECT/CT using 99m Tc Labeled HYNIC-TOC aids in diagnosis of primary optic nerve sheath meningioma . Indian J Nucl Med. 2017;32(01):63–65. doi: 10.4103/0972-3919.198487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nussbaum-Hermassi L, Ahle G, Zaenker C, Duca C, Namer I J. Optic nerve sheath meningioma detected by single- photon emission computed tomography/computed tomography somatostatin receptor scintigraphy: a case report. J Med Case Reports. 2016;10(01):96. doi: 10.1186/s13256-016-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andrews D W, Faroozan R, Yang B P.Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery Neurosurgery 20025104890–902., discussion 903–904 [DOI] [PubMed] [Google Scholar]
- 77.Khan S N, Sepahdari A R. Orbital masses: CT and MRI of common vascular lesions, benign tumors, and malignancies. Saudi J Ophthalmol. 2012;26(04):373–383. doi: 10.1016/j.sjopt.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanamalla U S. The optic nerve tram-track sign. Radiology. 2003;227(03):718–719. doi: 10.1148/radiol.2273010758. [DOI] [PubMed] [Google Scholar]
- 79.Johns T T, Citrin C M, Black J Sherman JL. CT evaluation of perineural orbital lesions: evaluation of the “tram-track” sign. AJNR Am J Neuroradiol. 1984;5(05):587–590. [PMC free article] [PubMed] [Google Scholar]
- 80.Peyster R G, Hoover E D, Hershey B L, Haskin M E. High-resolution CT of lesions of the optic nerve. AJR Am J Roentgenol. 1983;140(05):869–874. doi: 10.2214/ajr.140.5.869. [DOI] [PubMed] [Google Scholar]
- 81.Daniels D L, Williams A L, Syvertsen A, Gager W E, Harris G J. CT recognition of optic nerve sheath meningioma: abnormal sheath visualization. AJNR Am J Neuroradiol. 1982;3(02):181–183. [PMC free article] [PubMed] [Google Scholar]
- 82.Ing E B, Garrity J A, Cross S A, Ebersold M J. Sarcoid masquerading as optic nerve sheath meningioma. Mayo Clin Proc. 1997;72(01):38–43. doi: 10.4065/72.1.38. [DOI] [PubMed] [Google Scholar]
- 83.Nair A, Behari S, Jain M, Jaiswal A K. Bilateral primary optic nerve sheath meningiomas with pneumosinus dilatans. Acta Neurochir (Wien) 2011;153(12):2495–2497. doi: 10.1007/s00701-011-1140-0. [DOI] [PubMed] [Google Scholar]
- 84.Hirst L W, Miller N R, Hodges F J, III, Corbett J J, Thomspson S. Sphenoid pneumosinus dilatans. A sign of meningioma originating in the optic canal. Neuroradiology. 1982;22(04):207–210. doi: 10.1007/BF00341251. [DOI] [PubMed] [Google Scholar]
- 85.Skolnick C A, Mafee M F, Goodwin J A. Pneumosinus dilatans of the sphenoid sinus presenting with visual loss. J Neuroophthalmol. 2000;20(04):259–263. [PubMed] [Google Scholar]
- 86.Loo J-L, Tian J, Miller N R, Subramanian P S. Use of optical coherence tomography in predicting post-treatment visual outcome in anterior visual pathway meningiomas. Br J Ophthalmol. 2013;97(11):1455–1458. doi: 10.1136/bjophthalmol-2013-303449. [DOI] [PubMed] [Google Scholar]
- 87.Alroughani R, Behbehani R. Optic nerve sheath meningioma masquerading as optic neuritis. Case Rep Neurol Med. 2016;2016:5.419432E6. doi: 10.1155/2016/5419432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sawaya R A, Sidani C, Farah N, Hourani-Risk R. Presumed bilateral optic nerve sheath meningiomas presenting as optic neuritis. J Neuroophthalmol. 2008;28(01):55–57. doi: 10.1097/WNO.0b013e3181674331. [DOI] [PubMed] [Google Scholar]
- 89.Charpentier P, Mouriaux F. Total recovery of optic nerve sheath meningioma. BMJ Case Rep. 2016;2016:bcr2016215532. doi: 10.1136/bcr-2016-215532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seltzer S, Mark A S, Atlas S W. CNS sarcoidosis: evaluation with contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 1991;12(06):1227–1233. [PMC free article] [PubMed] [Google Scholar]
- 91.Nowak D A, Gumprecht H, Widenka D C, Stölzle A, Lumenta C B. Solitary sarcoid granulomatosis mimicking meningioma. J Neurosurg. 2000;93(05):897–897. doi: 10.3171/jns.2000.93.5.0897. [DOI] [PubMed] [Google Scholar]
- 92.Jackson A, Patankar T, Laitt R D. Intracanalicular optic nerve meningioma: a serious diagnostic pitfall. AJNR Am J Neuroradiol. 2003;24(06):1167–1170. [PMC free article] [PubMed] [Google Scholar]
- 93.Roberti F, Lee H H, Caputy A J, Katz B.“Shave” biopsy of the optic nerve in isolated neurosarcoidosis J Neurosurg Sci 2005490259–63., discussion 63 [PubMed] [Google Scholar]
- 94.Micieli J A, Streutker C J, McIntyre K. Isolated optic perineuritis as the presenting sign of sarcoidosis. J Neuroophthalmol. 2020;40(02):255–257. doi: 10.1097/WNO.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 95.Parlin A, Dumitrescu A, Nunery W T, Timoney P J, Sokol J A. Retrospective chart review of the use of imaging and biopsy in the diagnosis of optic nerve sheath meningiomas and intra-conal orbital lymphomas at a single institution. Orbit. 2017;36(06):392–396. doi: 10.1080/01676830.2017.1337198. [DOI] [PubMed] [Google Scholar]
- 96.Islam M N, Amin M S, Dipi R M, Khan N A. Comparison of computed tomographic and cytopathological findings in the evaluation of adult orbital mass. Mymensingh Med J. 2013;22(01):75–79. [PubMed] [Google Scholar]
- 97.Bakbak B, Dönmez H, Kansu T, Kiratli H. Dural ectasia of the optic nerve sheath: is it always benign? Eye Brain. 2009;1:5–7. doi: 10.2147/eb.s7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liauw L, Vielvoye G J, de Keizer R J, van Duinen S G. Optic nerve glioma mimicking an optic nerve meningioma. Clin Neurol Neurosurg. 1996;98(03):258–261. doi: 10.1016/0303-8467(96)00022-4. [DOI] [PubMed] [Google Scholar]
- 99.Bains S, Kim U, Shanti R. Orbital melanoma with calcification: a diagnostic dilemma. Indian J Ophthalmol. 2016;64(12):932–934. doi: 10.4103/0301-4738.198849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tunio M A, AlAsiri M, Riaz K, Abdulmoniem R. Optic nerve metastasis from squamous cell carcinoma of the uterine cervix. J Coll Physicians Surg Pak. 2015;25(09):694–695. [PubMed] [Google Scholar]
- 101.Cherekaev V A, Lasunin N V, Stepanian M A.[Breast carcinoma metastasis to the optic nerve: case report and review of literature] (in Russian) Vopr Neirokhir 2013770342–48., discussion 48 [PubMed] [Google Scholar]
- 102.Noshiro S, Wanibuchi M, Akiyama Y. IgG4-related disease initially presented as an orbital mass lesion mimicking optic nerve sheath meningioma. Brain Tumor Pathol. 2015;32(04):286–290. doi: 10.1007/s10014-015-0223-7. [DOI] [PubMed] [Google Scholar]
- 103.Paulsen F, Doerr S, Wilhelm H, Becker G, Bamberg M, Classen J. Fractionated stereotactic radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2012;82(02):773–778. doi: 10.1016/j.ijrobp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 104.Hickman S J, Allen J A, Baisre A. Neuro-ophthalmological complications of chronic inflammatory demyelinating polyradiculoneuropathy. Neuroophthalmology. 2013;37(04):146–156. doi: 10.3109/01658107.2013.809459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Egan R A, Lessell S. A contribution to the natural history of optic nerve sheath meningiomas. Arch Ophthalmol. 2002;120(11):1505–1508. doi: 10.1001/archopht.120.11.1505. [DOI] [PubMed] [Google Scholar]
- 106.Sasano H, Shikishima K, Aoki M, Sakai T, Tsutsumi Y, Nakano T. Efficacy of intensity-modulated radiation therapy for optic nerve sheath meningioma. Graefes Arch Clin Exp Ophthalmol. 2019;257(10):2297–2306. doi: 10.1007/s00417-019-04424-w. [DOI] [PubMed] [Google Scholar]
- 107.Adams G, Roos D E, Crompton J L. Radiotherapy for optic nerve sheath meningioma: a case for earlier intervention? Clin Oncol (R Coll Radiol) 2013;25(06):356–361. doi: 10.1016/j.clon.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 108.Brower J V, Amdur R J, Kirwan J, Mendenhall W M, Friedman W. Radiation therapy for optic nerve sheath meningioma. Pract Radiat Oncol. 2013;3(03):223–228. doi: 10.1016/j.prro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 109.Lesser R L, Knisely J PS, Wang S L, Yu J B, Kupersmith M J. Long-term response to fractionated radiotherapy of presumed optic nerve sheath meningioma. Br J Ophthalmol. 2010;94(05):559–563. doi: 10.1136/bjo.2009.167346. [DOI] [PubMed] [Google Scholar]
- 110.Soldà F, Wharram B, Gunapala R, Brada M. Fractionated stereotactic conformal radiotherapy for optic nerve sheath meningiomas. Clin Oncol (R Coll Radiol) 2012;24(08):e106–e112. doi: 10.1016/j.clon.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 111.Ratnayake G, Oh T, Mehta R. Long-term treatment outcomes of patients with primary optic nerve sheath meningioma treated with stereotactic radiotherapy. J Clin Neurosci. 2019;68:162–167. doi: 10.1016/j.jocn.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Abouaf L, Girard N, Lefort T. Standard-fractionated radiotherapy for optic nerve sheath meningioma: visual outcome is predicted by mean eye dose. Int J Radiat Oncol Biol Phys. 2012;82(03):1268–1277. doi: 10.1016/j.ijrobp.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 113.Kennerdell J S, Maroon J C, Malton M, Warren F A. The management of optic nerve sheath meningiomas. Am J Ophthalmol. 1988;106(04):450–457. doi: 10.1016/0002-9394(88)90882-3. [DOI] [PubMed] [Google Scholar]
- 114.Inoue T, Okuno Y, Nishiguchi I, Ikenaga K, Mimura O. Rapid recovery of vision following early intervention with fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Int Med Case Rep J. 2018;11:17–22. doi: 10.2147/IMCRJ.S149592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeremic B, Pitz S. Primary optic nerve sheath meningioma: stereotactic fractionated radiation therapy as an emerging treatment of choice. Cancer. 2007;110(04):714–722. doi: 10.1002/cncr.22859. [DOI] [PubMed] [Google Scholar]
- 116.Rassi M S, Prasad S, Can A, Pravdenkova S, Almefty R, Al-Mefty O. Prognostic factors in the surgical treatment of intracanalicular primary optic nerve sheath meningiomas. J Neurosurg. 2018;131(02):481–488. doi: 10.3171/2018.4.JNS173080. [DOI] [PubMed] [Google Scholar]
- 117.Roser F, Nakamura M, Martini-Thomas R, Samii M, Tatagiba M. The role of surgery in meningiomas involving the optic nerve sheath. Clin Neurol Neurosurg. 2006;108(05):470–476. doi: 10.1016/j.clineuro.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 118.Maza G, Subramaniam S, Yanez-Siller J C, Otto B A, Prevedello D M, Carrau R L. The role of endonasal endoscopic optic nerve decompression as the initial management of primary optic nerve sheath meningiomas. J Neurol Surg B Skull Base. 2019;80(06):568–576. doi: 10.1055/s-0039-1677689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Metellus P, Kapoor S, Kharkar S. Fractionated conformal radiotherapy for management of optic nerve sheath meningiomas: long-term outcomes of tumor control and visual function at a single institution. Int J Radiat Oncol Biol Phys. 2011;80(01):185–192. doi: 10.1016/j.ijrobp.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Adeberg S, Welzel T, Rieken S, Debus J, Combs S E. Prior surgical intervention and tumor size impact clinical outcome after precision radiotherapy for the treatment of optic nerve sheath meningiomas (ONSM) Radiat Oncol. 2011;6:117. doi: 10.1186/1748-717X-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hunt P J, DeMonte F, Tang R A, Su S Y, Raza S M. Surgical resection of an optic nerve sheath meningioma: relevance of endoscopic endonasal approaches to the optic canal. J Neurol Surg Rep. 2017;78(02):e81–e85. doi: 10.1055/s-0037-1600897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu J K, Forman S, Moorthy C R, Benzil D L. Update on treatment modalities for optic nerve sheath meningiomas. Neurosurg Focus. 2003;14(05):e7. [PubMed] [Google Scholar]
- 123.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(01):22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeremic B. Berlin, Germany: Springer-Verlag; 2008. Conventional radiation therapy in primary optic nerve sheath meningioma; pp. 77–83. [Google Scholar]
- 125.Byers W GM. Tumors of the optic nerve. JAMA. 1914;63:20–24. [Google Scholar]
- 126.Lo S S, Chao S T, Suh J H. Berlin, Germany: Springer-Verlag; 2008. 3D conformal RT; pp. 86–94. [Google Scholar]
- 127.Stieber V W. Radiation therapy for visual pathway tumors. J Neuroophthalmol. 2008;28(03):222–230. doi: 10.1097/WNO.0b013e318177ee9d. [DOI] [PubMed] [Google Scholar]
- 128.Moyer P D, Golnik K C, Breneman J. Treatment of optic nerve sheath meningioma with three-dimensional conformal radiation. Am J Ophthalmol. 2000;129(05):694–696. doi: 10.1016/s0002-9394(99)00477-8. [DOI] [PubMed] [Google Scholar]
- 129.Pandit R, Paris L, Rudich D S, Lesser R L, Kupersmith M J, Miller N R. Long-term efficacy of fractionated conformal radiotherapy for the management of primary optic nerve sheath meningioma. Br J Ophthalmol. 2019;103(10):1436–1440. doi: 10.1136/bjophthalmol-2018-313135. [DOI] [PubMed] [Google Scholar]
- 130.Tsao M N, Hoyt W F, Horton J. Improved visual outcome with definitive radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 1991;45S:324–325. [Google Scholar]
- 131.Uy N W, Woo S Y, Teh B S. Intensity-modulated radiation therapy (IMRT) for meningioma. Int J Radiat Oncol Biol Phys. 2002;53(05):1265–1270. doi: 10.1016/s0360-3016(02)02823-7. [DOI] [PubMed] [Google Scholar]
- 132.Grant W, III, Cain R B. Intensity modulated conformal therapy for intracranial lesions. Med Dosim. 1998;23(03):237–241. doi: 10.1016/s0958-3947(98)00015-6. [DOI] [PubMed] [Google Scholar]
- 133.Maclean J, Fersht N, Bremner F, Stacey C, Sivabalasingham S, Short S. Meningioma causing visual impairment: outcomes and toxicity after intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(04):e179–e186. doi: 10.1016/j.ijrobp.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 134.Smee R I, Schneider M, Williams J R. Optic nerve sheath meningiomas--non-surgical treatment. Clin Oncol (R Coll Radiol) 2009;21(01):8–13. doi: 10.1016/j.clon.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 135.Augspurger M E, Teh B S, Uhl B M. Conformal intensity modulated radiation therapy for the treatment of optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 1999;45(03):324–324. [Google Scholar]
- 136.Inoue T, Mimura O, Ikenaga K, Okuno Y, Nishiguchi I. The rapid improvement in visual field defect observed with weekly perimetry during intensity-modulated radiotherapy for optic nerve sheath meningioma. Int Cancer Conf J. 2019;8(03):136–140. doi: 10.1007/s13691-019-00371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jin J, Joo J D, Han J H. Optic nerve sheath meningioma: preliminary analysis of the role of radiation therapy. Brain Tumor Res Treat. 2018;6(01):8–12. doi: 10.14791/btrt.2018.6.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schroeder T M, Yogeswaren S T, Augspurger M E. Intensity modulated radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2004;60(01):S315. [Google Scholar]
- 139.Jeremic B, Wasik M W, Villa S. Berlin, Germany: Springer-Verlag; 2008. Stereotactic radiation therapy in primary optic nerve sheath meningioma; pp. 105–125. [Google Scholar]
- 140.Becker G, Jeremic B, Pitz S. Stereotactic fractionated radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2002;54(05):1422–1429. doi: 10.1016/s0360-3016(02)03753-7. [DOI] [PubMed] [Google Scholar]
- 141.Liu J K, Forman S, Hershewe G L, Moorthy C R, Benzil D L.Optic nerve sheath meningiomas: visual improvement after stereotactic radiotherapy Neurosurgery 20025005950–955., discussion 955–957 [DOI] [PubMed] [Google Scholar]
- 142.Pitz S, Becker G, Schiefer U. Stereotactic fractionated irradiation of optic nerve sheath meningioma: a new treatment alternative. Br J Ophthalmol. 2002;86(11):1265–1268. doi: 10.1136/bjo.86.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baumert B G, Villà S, Studer G. Early improvements in vision after fractionated stereotactic radiotherapy for primary optic nerve sheath meningioma. Radiother Oncol. 2004;72(02):169–174. doi: 10.1016/j.radonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 144.Landert M, Baumert B G, Bosch M M, Lütolf U M, Landau K. The visual impact of fractionated stereotactic conformal radiotherapy on seven eyes with optic nerve sheath meningiomas. J Neuroophthalmol. 2005;25(02):86–91. doi: 10.1097/01.wno.0000165105.78365.22. [DOI] [PubMed] [Google Scholar]
- 145.Richards J C, Roden D, Harper C S. Management of sight-threatening optic nerve sheath meningioma with fractionated stereotactic radiotherapy. Clin Exp Ophthalmol. 2005;33(02):137–141. doi: 10.1111/j.1442-9071.2005.00973.x. [DOI] [PubMed] [Google Scholar]
- 146.Sitathanee C, Dhanachai M, Poonyathalang A, Tuntiyatorn L, Theerapancharoen V. Stereotactic radiation therapy for optic nerve sheath meningioma; an experience at Ramathibodi Hospital. J Med Assoc Thai. 2006;89(10):1665–1669. [PubMed] [Google Scholar]
- 147.Litré C F, Noudel R, Colin P, Sherpereel B, Peruzzi P, Rousseaux P. [Fractionated stereotactic radiotherapy for optic nerve sheath meningioma: eight cases] (in French) Neurochirurgie. 2007;53(05):333–338. doi: 10.1016/j.neuchi.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 148.Milker-Zabel S, Huber P, Schlegel W, Debus J, Zabel-du Bois A. Fractionated stereotactic radiation therapy in the management of primary optic nerve sheath meningiomas. J Neurooncol. 2009;94(03):419–424. doi: 10.1007/s11060-009-9874-8. [DOI] [PubMed] [Google Scholar]
- 149.Pacelli R, Cella L, Conson M. Fractionated stereotactic radiation therapy for orbital optic nerve sheath meningioma - a single institution experience and a short review of the literature. J Radiat Res (Tokyo) 2011;52(01):82–87. doi: 10.1269/jrr.10139. [DOI] [PubMed] [Google Scholar]
- 150.Kheir V, Faouzi M, Borruat F-X. Visual outcomes of fractionated radiotherapy in optic nerve sheath meningioma: a retrospective study. Klin Monatsbl Augenheilkd. 2019;236(04):526–529. doi: 10.1055/a-0828-7335. [DOI] [PubMed] [Google Scholar]
- 151.Hamilton S N, Nichol A, Truong P. Visual outcomes and local control after fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Ophthal Plast Reconstr Surg. 2018;34(03):217–221. doi: 10.1097/IOP.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 152.Marchetti M, Bianchi S, Milanesi I.Multisession radiosurgery for optic nerve sheath meningiomas--an effective option: preliminary results of a single-center experience Neurosurgery 201169051116–1122., discussion 1122–1123 [DOI] [PubMed] [Google Scholar]
- 153.Romanelli P, Bianchi L, Muacevic A, Beltramo G. Staged image guided robotic radiosurgery for optic nerve sheath meningiomas. Comput Aided Surg. 2011;16(06):257–266. doi: 10.3109/10929088.2011.622615. [DOI] [PubMed] [Google Scholar]
- 154.Liu D, Xu D, Zhang Z.Long-term results of Gamma knife surgery for optic nerve sheath meningioma J Neurosurg 2010113(suppl):28–33. [DOI] [PubMed] [Google Scholar]
- 155.Kaul D, Badakhshi H, Gevaert T.Dosimetric comparison of different treatment modalities for stereotactic radiosurgery of meningioma Acta Neurochir (Wien) 201515704559–563., discussion 563–564 [DOI] [PubMed] [Google Scholar]
- 156.Romanelli P, Wowra B, Muacevic A. Multisession CyberKnife radiosurgery for optic nerve sheath meningiomas. Neurosurg Focus. 2007;23(06):E11. doi: 10.3171/FOC-07/12/E11. [DOI] [PubMed] [Google Scholar]
- 157.Kooshkabadi A, Elchin I, Kano H, Lunsford L D. Prolonged vision return after radiosurgery for an optic nerve-sheath meningioma. J Radiosurg SBRT. 2012;2(01):73–77. [PMC free article] [PubMed] [Google Scholar]
- 158.Swiss Proton Users Group . Weber D C, Lomax A J, Rutz H P. Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother Oncol. 2004;71(03):251–258. doi: 10.1016/j.radonc.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 159.Lesueur P, Calugaru V, Nauraye C. Proton therapy for treatment of intracranial benign tumors in adults: a systematic review. Cancer Treat Rev. 2019;72:56–64. doi: 10.1016/j.ctrv.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 160.Lomax A J, Bortfeld T, Goitein G. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother Oncol. 1999;51(03):257–271. doi: 10.1016/s0167-8140(99)00036-5. [DOI] [PubMed] [Google Scholar]
- 161.Arvold N D, Lessell S, Bussiere M. Visual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2009;75(04):1166–1172. doi: 10.1016/j.ijrobp.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 162.Redjal N, Agarwalla P K, Dietrich J. Remote acute demyelination after focal proton radiation therapy for optic nerve meningioma. J Clin Neurosci. 2015;22(08):1367–1369. doi: 10.1016/j.jocn.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 163.Becker G, Kocher M, Kortmann R-D. Radiation therapy in the multimodal treatment approach of pituitary adenoma. Strahlenther Onkol. 2002;178(04):173–186. doi: 10.1007/s00066-002-0826-x. [DOI] [PubMed] [Google Scholar]
- 164.Brada M, Rajan B, Traish D. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf) 1993;38(06):571–578. doi: 10.1111/j.1365-2265.1993.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 165.Parsons J T, Bova F J, Fitzgerald C R, Mendenhall W M, Million R R. Radiation optic neuropathy after megavoltage external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30(04):755–763. doi: 10.1016/0360-3016(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 166.Goldsmith B J, Rosenthal S A, Wara W M, Larson D A. Optic neuropathy after irradiation of meningioma. Radiology. 1992;185(01):71–76. doi: 10.1148/radiology.185.1.1523337. [DOI] [PubMed] [Google Scholar]
- 167.Vagefi M R, Larson D A, Horton J C. Optic nerve sheath meningioma: visual improvement during radiation treatment. Am J Ophthalmol. 2006;142(02):343–344. doi: 10.1016/j.ajo.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 168.Stafford S L, Pollock B E, Leavitt J A. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55(05):1177–1181. doi: 10.1016/s0360-3016(02)04380-8. [DOI] [PubMed] [Google Scholar]
- 169.Leber K A, Berglöff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88(01):43–50. doi: 10.3171/jns.1998.88.1.0043. [DOI] [PubMed] [Google Scholar]
- 170.Saeed P, Blank L, Selva D. Primary radiotherapy in progressive optic nerve sheath meningiomas: a long-term follow-up study. Br J Ophthalmol. 2010;94(05):564–568. doi: 10.1136/bjo.2009.166793. [DOI] [PubMed] [Google Scholar]
- 171.Cristante L. Surgical treatment of meningiomas of the orbit and optic canal: a retrospective study with particular attention to the visual outcome. Acta Neurochir (Wien) 1994;126(01):27–32. doi: 10.1007/BF01476490. [DOI] [PubMed] [Google Scholar]
- 172.Yüceer N, Erdogan A, Ziya H. Primary optic nerve sheath meningiomas. Report of seven cases (clinical neuroradiological, pathological and surgical considerations in seven cases) J Neurosurg Sci. 1994;38(03):155–159. [PubMed] [Google Scholar]
- 173.Clark W C, Theofilos C S, Fleming J C. Primary optic nerve sheath meningiomas. Report of nine cases. J Neurosurg. 1989;70(01):37–40. doi: 10.3171/jns.1989.70.1.0037. [DOI] [PubMed] [Google Scholar]
- 174.Juniat V, McGilligan J A, Curragh D, Selva D, Rajak S. Endoscopic orbital decompression for proptosis in non-thyroid eye disease. Oral Maxillofac Surg. 2020;24(01):85–91. doi: 10.1007/s10006-019-00826-6. [DOI] [PubMed] [Google Scholar]
- 175.Zoia C, Bongetta D, Pagella F, Antoniazzi E R, Gaetani P. New surgical option for optic nerve sheath meningiomas: fully endoscopic transnasal approach. Can J Ophthalmol. 2018;53(04):e142–e144. doi: 10.1016/j.jcjo.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 176.Chen H, Zhang Q, Tan S, Fu H, Farris B K, Yang Z. Update on the application of optic nerve sheath fenestration. Restor Neurol Neurosci. 2017;35(03):275–286. doi: 10.3233/rnn-160693. [DOI] [PubMed] [Google Scholar]
- 177.Gündüz K, Catak E, Erden E. Optic nerve biopsy via a medial transconjunctival orbitotomy approach in the diagnosis of optic nerve and sheath tumors. Orbit. 2010;29(04):190–193. doi: 10.3109/01676831003664368. [DOI] [PubMed] [Google Scholar]
- 178.Meeker A R, Ko M W, Carruth B P, Strumpf K B, Bersani T A. Diagnosis of optic nerve sheath meningioma during optic nerve sheath decompression. Orbit. 2017;36(01):35–38. doi: 10.1080/01676830.2017.1279648. [DOI] [PubMed] [Google Scholar]
- 179.Turbin R E, Wladis E J, Frohman L P, Langer P D, Kennerdell J S. Role for surgery as adjuvant therapy in optic nerve sheath meningioma. Ophthal Plast Reconstr Surg. 2006;22(04):278–282. doi: 10.1097/01.iop.0000225420.06323.76. [DOI] [PubMed] [Google Scholar]
- 180.Jaggi G P, Mironov A, Huber A R, Killer H E. Optic nerve compartment syndrome in a patient with optic nerve sheath meningioma. Eur J Ophthalmol. 2007;17(03):454–458. doi: 10.1177/112067210701700334. [DOI] [PubMed] [Google Scholar]
- 181.Pletcher S D, Sindwani R, Metson R.Endoscopic orbital and optic nerve decompression Otolaryngol Clin North Am 20063905943–958., vi vi [DOI] [PubMed] [Google Scholar]
- 182.Pletcher S D, Metson R. Endoscopic optic nerve decompression for nontraumatic optic neuropathy. Arch Otolaryngol Head Neck Surg. 2007;133(08):780–783. doi: 10.1001/archotol.133.8.780. [DOI] [PubMed] [Google Scholar]
- 183.Shin M, Kondo K, Hanakita S. Endoscopic transnasal approach for resection of locally aggressive tumors in the orbit. J Neurosurg. 2015;123(03):748–759. doi: 10.3171/2014.11.JNS141921. [DOI] [PubMed] [Google Scholar]
- 184.Berhouma M, Jacquesson T, Abouaf L, Vighetto A, Jouanneau E. Endoscopic endonasal optic nerve and orbital apex decompression for nontraumatic optic neuropathy: surgical nuances and review of the literature. Neurosurg Focus. 2014;37(04):E19. doi: 10.3171/2014.7.FOCUS14303. [DOI] [PubMed] [Google Scholar]
- 185.Hayhurst C, Sughrue M E, Gore P A, Bonney P A, Burks J D, Teo C. Results with expanded endonasal resection of skull base meningiomas technical nuances and approach selection based on an early experience. Turk Neurosurg. 2016;26(05):662–670. doi: 10.5137/1019-5149.JTN.16105-15.3. [DOI] [PubMed] [Google Scholar]
- 186.Lehmberg J, Krieg S M, Mueller B, Meyer B. Impact of anterior clinoidectomy on visual function after resection of meningiomas in and around the optic canal. Acta Neurochir (Wien) 2013;155(07):1293–1299. doi: 10.1007/s00701-013-1741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Abhinav K, Acosta Y, Wang W-H.Endoscopic endonasal approach to the optic canal: anatomic considerations and surgical relevance Neurosurgery 20151103431–445., discussion 445–446 [DOI] [PubMed] [Google Scholar]
- 188.Abuzayed B, Kucukyuruk B, Tanriover N.Transcranial superior orbitotomy for the treatment of intraorbital intraconal tumors: surgical technique and long-term results in single institute Neurosurg Rev 20123504573–582., discussion 582 [DOI] [PubMed] [Google Scholar]
- 189.Ito M, Ishizawa A, Miyaoka M, Sato K, Ishii S. Intraorbital meningiomas. Surgical management and role of radiation therapy. Surg Neurol. 1988;29(06):448–453. doi: 10.1016/0090-3019(88)90139-5. [DOI] [PubMed] [Google Scholar]
- 190.Kuroda R, Nakatani J, Yorimae A, Nakao Y, Ohtori T. Clinical experience of intraorbital optic nerve sheath meningioma--report of eight cases. Neurol Med Chir (Tokyo) 1990;30(07):468–475. doi: 10.2176/nmc.30.468. [DOI] [PubMed] [Google Scholar]
- 191.Hao J, Pircher A, Miller N R, Hsieh J, Remonda L, Killer H E. Cerebrospinal fluid and optic nerve sheath compartment syndrome: A common pathophysiological mechanism in five different cases? Clin Exp Ophthalmol. 2020;48(02):212–219. doi: 10.1111/ceo.13663. [DOI] [PubMed] [Google Scholar]
- 192.Hénaux P-L, Bretonnier M, Le Reste P-J, Morandi X. Modern management of meningiomas compressing the optic nerve: a systematic review. World Neurosurg. 2018;118:e677–e686. doi: 10.1016/j.wneu.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 193.Carlson A, Stippler M, Morley S, Myers O. Predictive factors for vision recovery after optic nerve decompression for chronic compressive neuropathy: systematic review and meta-analysis. J Neurol Surg B Skull Base. 2013;74(01):20–38. doi: 10.1055/s-0032-1329624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Turbin R E, Kennerdell J S. Berlin, Germany: Springer-Verlag; 2008. Surgery in primary optic nerve sheath meningioma; pp. 67–76. [Google Scholar]
- 195.Mourits M P, van der Sprenkel J WB. Orbital meningioma, the Utrecht experience. Orbit. 2001;20(01):25–33. doi: 10.1076/orbi.20.1.25.2640. [DOI] [PubMed] [Google Scholar]
- 196.Zweckberger K, Unterberg A W, Schick U. Pre-chiasmatic transection of the optic nerve can save contralateral vision in patients with optic nerve sheath meningioms. Clin Neurol Neurosurg. 2013;115(12):2426–2431. doi: 10.1016/j.clineuro.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 197.Furdová A, Babál P, Kobzová D. Optic nerve orbital meningioma. Cesk Slov Oftalmol. 2018;74(01):23–30. [PubMed] [Google Scholar]
- 198.Samples J R, Robertson D M, Taylor J Z, Waller R R. Optic nerve meningioma. Ophthalmology. 1983;90(12):1591–1594. doi: 10.1016/s0161-6420(83)34346-3. [DOI] [PubMed] [Google Scholar]
- 199.Thom M, Martinian L. Progesterone receptors are expressed with higher frequency by optic nerve sheath meningiomas. Clin Neuropathol. 2002;21(01):5–8. [PubMed] [Google Scholar]
- 200.Gupta S, Bi W L, Dunn I F. Medical management of meningioma in the era of precision medicine. Neurosurg Focus. 2018;44(04):E3. doi: 10.3171/2018.1.FOCUS17754. [DOI] [PubMed] [Google Scholar]
- 201.Goldbrunner R, Minniti G, Preusser M. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(09):e383–e391. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- 202.Chamberlain M C. IFN-α for recurrent surgery- and radiation-refractory high-grade meningioma: a retrospective case series. CNS Oncol. 2013;2(03):227–235. doi: 10.2217/cns.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Karsy M, Guan J, Cohen A, Colman H, Jensen R L. Medical management of meningiomas: current status, failed treatments, and promising horizons. Neurosurg Clin N Am. 2016;27(02):249–260. doi: 10.1016/j.nec.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 204.Kaley T, Nabors L B.Management of central nervous system tumors J Natl Compr Canc Netw 201917(5.5):579–582. [DOI] [PubMed] [Google Scholar]
- 205.Newton H B, Scott S R, Volpi C. Hydroxyurea chemotherapy for meningiomas: enlarged cohort with extended follow-up. Br J Neurosurg. 2004;18(05):495–499. doi: 10.1080/02688690400012392. [DOI] [PubMed] [Google Scholar]
- 206.Saraf S, McCarthy B J, Villano J L. Update on meningiomas. Oncologist. 2011;16(11):1604–1613. doi: 10.1634/theoncologist.2011-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Paus S, Klockgether T, Urbach H, Schlegel U. Meningioma of the optic nerve sheath: treatment with hydroxyurea. J Neurol Neurosurg Psychiatry. 2003;74(09):1348–1350. doi: 10.1136/jnnp.74.9.1348-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zeng C, Guo B, Chen J, He W. Antitumor effects of Farnesol in optic nerve sheath meningioma cell line and its effects on cell cycle progression, autophagy, cell migration and invasion. J BUON. 2019;24(05):2168–2172. [PubMed] [Google Scholar]
- 209.Chen Y, Tang Y-M, Yu S-L. Advances in the pharmacological activities and mechanisms of diosgenin. Chin J Nat Med. 2015;13(08):578–587. doi: 10.1016/S1875-5364(15)30053-4. [DOI] [PubMed] [Google Scholar]
- 210.Zhu X, Chen Z, Li X. Diosgenin inhibits the proliferation, migration and invasion of the optic nerve sheath meningioma cells via induction of mitochondrial-mediated apoptosis, autophagy and G0/G1 cell cycle arrest. J BUON. 2020;25(01):508–513. [PubMed] [Google Scholar]
- 211.Mearza A A, Aslanides I M. Uses and complications of mitomycin C in ophthalmology. Expert Opin Drug Saf. 2007;6(01):27–32. doi: 10.1517/14740338.6.1.27. [DOI] [PubMed] [Google Scholar]
- 212.Dhoot D S, Shults W T, Ng J D. Successful use of mitomycin C to prevent recurrence of the cystic component of an optic nerve sheath meningioma. Ophthal Plast Reconstr Surg. 2008;24(03):235–236. doi: 10.1097/IOP.0b013e3181706d39. [DOI] [PubMed] [Google Scholar]


