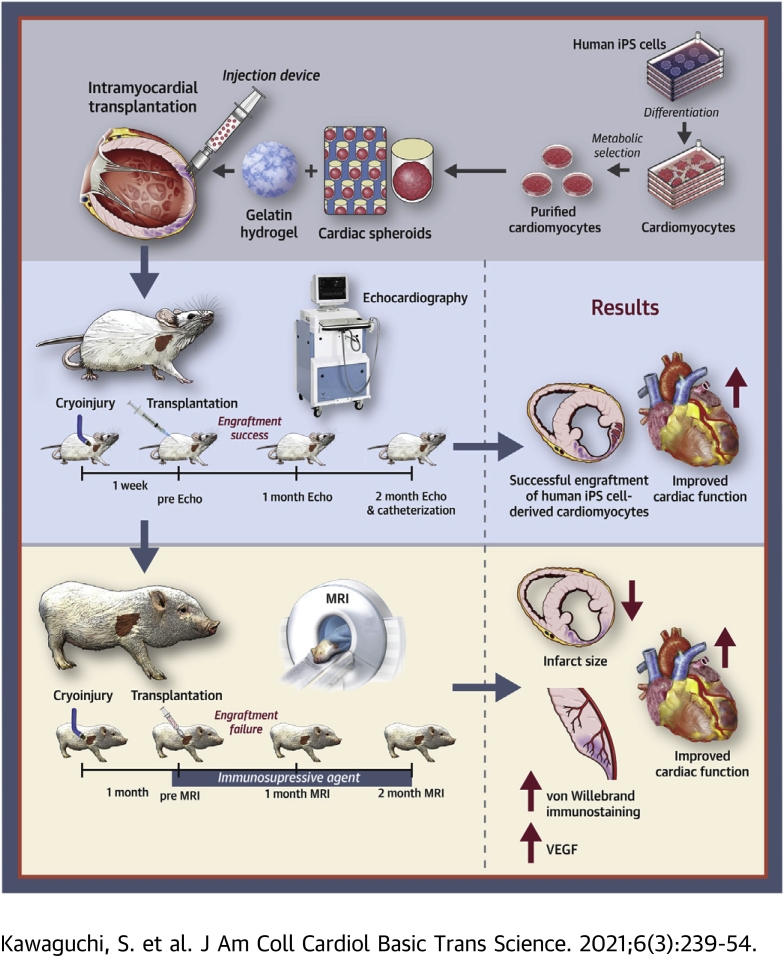
Visual Abstract
Key Words: cardiac spheroids, cardiomyocyte, cell transplantation, heart failure, human iPS cells
Abbreviations and Acronyms: CM, cardiomyocyte; CMR, cardiac magnetic resonance; CS, cardiac spheroid; dp/dtmax, maximum rate of left ventricular pressure rise; ECG, electrocardiogram; EF, ejection fraction; FAC, fractional area change; GH, gelatin hydrogel; HF, heart failure; hiPSC, human induced pluripotent stem cell; hPSC, human pluripotent stem cell; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; sCM, single cardiomyocyte; VEGF, vascular endothelial growth factor
Highlights
-
•
hiPSCs are differentiated into CMs with large-scale 2-dimensional culture system, and refined by metabolic purification.
-
•
hiPSC-derived CMs are developed into CSs in special microwell plates.
-
•
Intramyocardial transplantation of CSs and GH improves cardiac function in small and large animal models.
-
•
Engraftment of CMs and angiogenesis are mechanisms for improvement of cardiac function.
-
•
Intramyocardial transplantation of CSs with a transplant injection device is a safe, effective, and feasible strategy for the treatment of HF.
Summary
The severe shortage of donor hearts hampered the cardiac transplantation to patients with advanced heart failure. Therefore, cardiac regenerative therapies are eagerly awaited as a substitution. Human induced pluripotent stem cells (hiPSCs) are realistic cell source for regenerative cardiomyocytes. The hiPSC-derived cardiomyocytes are highly expected to help the recovery of heart. Avoidance of teratoma formation and large-scale culture of cardiomyocytes are definitely necessary for clinical setting. The combination of pure cardiac spheroids and gelatin hydrogel succeeded to recover reduced ejection fraction. The feasible transplantation strategy including transplantation device for regenerative cardiomyocytes are established in this study.
Heart failure (HF) is a global pandemic disease, especially in developed countries. Although several therapies have been proposed to improve its prognosis, HF patient numbers have increased in the 21st century; ∼26 million people suffer from HF (1). Cardiac transplantation is the last resort for severe HF patients (2); however, shortage of heart donors is a critical problem (3). Thus, cardiac regenerative therapies emerged decades ago, and many clinical trials were performed with bone marrow cells, myoblasts, and cardiac spheres to promote cardiac repair (4). Although some studies showed beneficial effects, they are yet to be standardized as feasible therapies. Therefore, the direct transplantation of cardiomyocytes (CMs) themselves has attracted considerable attention.
Human pluripotent stem cells (hPSCs), such as human embryonic stem cells and human induced pluripotent stem cells (hiPSCs), have been the ideal in vitro sources of CMs since their discovery (5,6). For clinical applications of hPSCs for HF treatment, 2 major issues must be overcome: 1) the culture strategy to obtain large amounts of hPSCs and hPSC-derived CMs. Cardiac repair demands huge amounts of CMs, and the proliferative potential of terminally differentiated CMs is very limited, even though they exhibit fetal phenotype; and 2) their tumorigenicity, which is caused by their pluripotency. An efficient 2-dimensional culture system for large amounts of hPSCs and CMs was invented to overcome the former issue (7). Moreover, the metabolic selection system, glucose- and glutamine-deprived and lactate supplemented media, enabled us to eliminate non-CMs, including undifferentiated stem cells (8,9). A combination of the massive 2-dimensional culture system and metabolic selection protocol produced a high number of purified CMs. Furthermore, a majority of purified CMs after metabolic selection showed the ventricular phenotype to be most appropriate for cell therapies (8).
To improve the engraftment ratio of directly transplanted CMs, extracellular matrix might be useful. Transplantation of CMs with gelatin hydrogel (GH) significantly improves cardiac function in rodents (10). Aggregations of CMs, termed cardiac spheroids (CSs), have also been developed to improve engraftment (11). Furthermore, a combination of CSs and a transplantation device significantly improves the effectiveness of cell retention in swine HF models (12).
Although the patch of human embryonic stem cell–derived cardiac progenitors showed clinical feasibility (13), intramyocardial transplantation of hPSC-derived CMs has not reached the clinical application stage. The safety and effectiveness of cell transplantation with purified CMs should be investigated in both small (exploratory studies) and large animal models (confirmatory studies) as translational research for regenerative therapies before clinical trials (14). Here, the feasibility of CS transplantation therapy with purified CMs was investigated in preclinical HF models.
Methods
hiPSC-derived CSs
hiPSCs (253G4) were maintained on growth factor-reduced Matrigel-coated culture plates using modified Stem Fit media (Ajinomoto, Tokyo, Japan), as reported previously (7). hiPSCs were differentiated into CMs and non-CMs using 4-layer culture plates with active gas ventilation (7). After cardiac differentiation, the CMs were metabolically selected using glucose- and glutamine-depleted Dulbecco’s modified Eagle’s medium supplemented with 4 mM L-lactic acid (Wako Pure Chemical Industries, Tokyo Japan) (7, 8, 9). Differentiation efficiency and purity were evaluated by flow cytometry analysis of cardiac troponin T levels (troponin T antibody [clone: REA400, Miltenyi Biotec, Auburn, California]). CSs were constituted with pure CMs in microwell plates as previously reported (12). One CS contains ∼1,000 CMs, and its size is ∼200 μm. Contraction and relaxation patterning of CSs were detected and visualized by Cell Motion Imaging System SI8000 (Sony Imaging Products & Solutions Inc., Tokyo, Japan). CSs were stimulated by 0.5-μM isoproterenol, and beating rate was measured.
Animals
The experimental protocol was approved by the Sub-committee on Animal Care and the Institutional Review Board of Keio University and Jichi Medical University according to Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions (Ministry of Education, Culture, Sports, Science and Technology). All animals received humane care in accordance with the Guide for the Care and Use of Laboratory Animals. Adult female F344-Il2rgem7Kyo XSCID rats (n = 18; National BioResource Project, Rat No. 0694, Kyoto University, Kyoto, Japan) and adult female micro-miniature pigs (n = 30; Fuji Micra, Shizuoka, Japan) were used (15,16).
Cardiac functional assessment with echocardiography in rats
Transthoracic echocardiography was performed before and 4 and 8 weeks after cell transplantation (n = 15; 5 each from the GH, single CM [sCM], and CS groups). Rats were anesthetized with low-dose isoflurane for echocardiographic examination. Two-dimensional targeted M-mode traces were obtained at the papillary muscle level using echocardiography (Vevo 2100, Visual Sonics, Toronto, Ontario, Canada). Left ventricular (LV) internal diameter in diastole and LV internal diameter in systole were measured in at least 3 consecutive cardiac cycles. Ejection fraction (EF), fractional shortening, and fractional area change (FAC) were calculated with the Teichholz formula.
Cardiac hemodynamic assessment in rats
Cardiac hemodynamics was assessed at 8 weeks after cell transplantation (n = 15; 5 each from GH, sCM, and CS groups). Rats were anesthetized with low-dose isoflurane and mechanically ventilated. A 1.4-F conductance-micromanometer catheter (SPR 839; Millar Instruments, Houston, Texas) was inserted via the right carotid artery across aortic valve and into the LV chamber. LV pressure maximum rise rate (+dp/dtmax) and LV maximum drop rate (–dp/dtmax) were obtained.
Cardiac functional assessment with cardiac magnetic resonance imaging in swine
Cardiac function was evaluated by using 1.5-T magnetic resonance imaging (Magnetom Essenza; Siemens Healthcare Sector, Erlangen, Germany) with a phased-array cardiac coil. Cardiac magnetic resonance (CMR) was performed before and 4 and 8 weeks after cell transplantation (n = 20; 7, 5, and 8 from GH, sham, and CS groups, respectively). All images were acquired under apnea with continuous electrocardiogram (ECG) gating, and enhanced by gadolinium enhancement. LVEF, LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV) were assessed and compared between the groups. Myocardial scar area and normal myocardial area were evaluated by gadolinium contrast injection.
Pathological and morphometric analysis in swine
Eight weeks after transplantation, the animals were sacrificed by intravenous transfusion of potassium chloride (20 mEq/body) and their hearts were explanted. LV was sectioned into 1-cm-thick short-axis slices to measure infarct area. Transverse ventricular slices were then incubated with 2% TTC (2,3,5-triphenyl tetrazolium chloride) for 20 min at 37°C to stain viable myocardium. Each slice was imaged digitally to measure the infarct size. Infarct size was expressed as a percentage of LV surface area by an image-analysis system Image J version: 2.0.0-rc-43/1.51w (National Institutes of Health, Bethesda, Maryland).
Statistics
IBM SPSS Advanced Statistics 23 (IBM, Armonk, New York) was used for statistical analyses. Error bars represented the SD. Continuous variables were evaluated by F test. The significance of differences between 3 groups in terms of hemodynamic data, scar area, and cell size were analyzed using a 1-way analysis of variance. The significance of differences among 3 groups in terms of cardiac function by cardiac echocardiography and CMR, and infarct area by CMR were analyzed using a 2-way repeated-measures analysis of variance. The post hoc multiple comparison analysis was performed by Tukey's test at 8 weeks after transplantation. The significance of differences between 2 groups in beating rate of CSs and pathological evaluation for angiogenesis was analyzed by Student’s t test. A p value < 0.05 was considered statistically significant.
An expanded Methods section is provided in the Supplemental Appendix.
Results
Purification of hiPSC-derived CMs constituted CSs
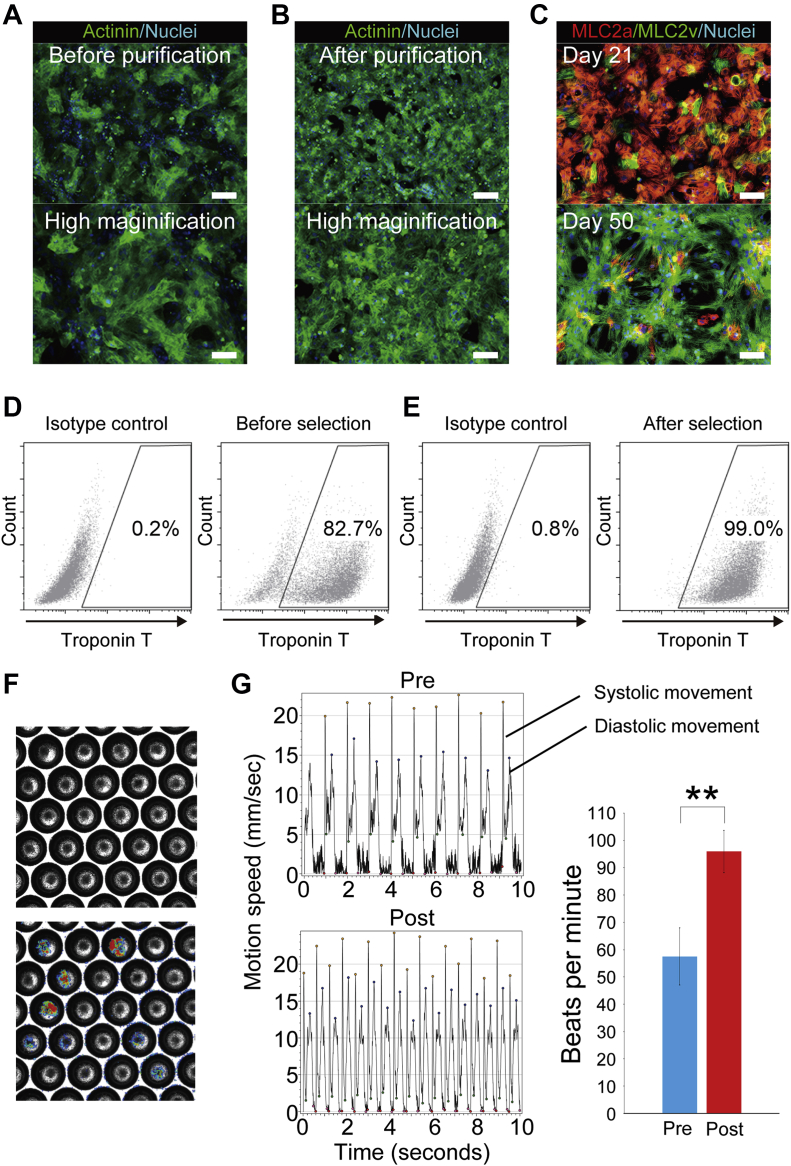
hiPSCs (253G4) directly differentiated into CMs with BMP4 (bone morphogenic protein 4), GSK3β (CHIR99021), and Wnt inhibitors (IWR-1) using monolayer culture (Supplemental Figure 1); they contained non-CMs before purification (Figure 1A). To prevent tumor formation clinically, undifferentiated hiPSCs and non-CMs were eliminated by metabolic selection, glucose and glutamine depletion, and lactate-supplemented medium (Figure 1B) (8). The cardiac phenotype of purified CMs was analyzed by immunofluorostaining. After cardiac purification, CMs were MLC2a (myosin light chain 2a) positive, indicating their immaturity. However, most of CMs became MLCv positive after long-term culture. These data indicated purified CMs possessed the ventricular phenotype (Figure 1C). Flow cytometry analysis showed ∼80% differentiation efficiency (Figure 1D). The proportion of cardiac troponin T was >98% in purified CMs (Figure 1E). CMs aggregated to form CSs in a microwell plate, leading to improved cell retention (Figure 1F) (12). The beating of CSs was analyzed by Cell Motion Imaging System SI8000, indicating that they were functional CMs (Figure 1G). CSs were stimulated by β adrenoreceptor agonist (isoproterenol) in order to assess drug response. The beating rate of CSs significantly increased (p < 0.001) (Figure 1G).
Figure 1.
hIPSC-Derived CSs
(A) Immunostained images of differentiated human induced pluripotent stem sell (hiPSC)–derived cardiomyocytes (CMs) before metabolic selection. (B) Immunostained images of differentiated hiPSC-derived CMs after metabolic selection. Note that most of the cells are α-actinin–positive CMs. Scale bars represent 200 μm in upper panels and 100 μm in lower panels. (C) hiPSC-derived CMs are positive for MLC2a (myosin light chain 2a), indicating immature CMs, at day 21 (upper panel). Most of purified hiPSC-derived CMs become positive for MLC2v, indicating ventricular CMs, at 50 days (lower panel). Scale bars represent 100 μm. (D) Differentiation efficiency is ∼80%. (E) After purification, the proportion of cardiac troponin T–positive CMs is >98%. (F) Cardiac spheroids (CSs) are generated from hiPSC-derived CMs in microwell plates. (G) Systolic and diastolic movements of beating CMs are analyzed by Cell Motion Imaging System SI8000. Stimulation of β-adrenoreceptor significantly increased the beats rate of hiPSC-derived CSs (p < 0.001) (n = 5). ∗∗ p < 0.01.
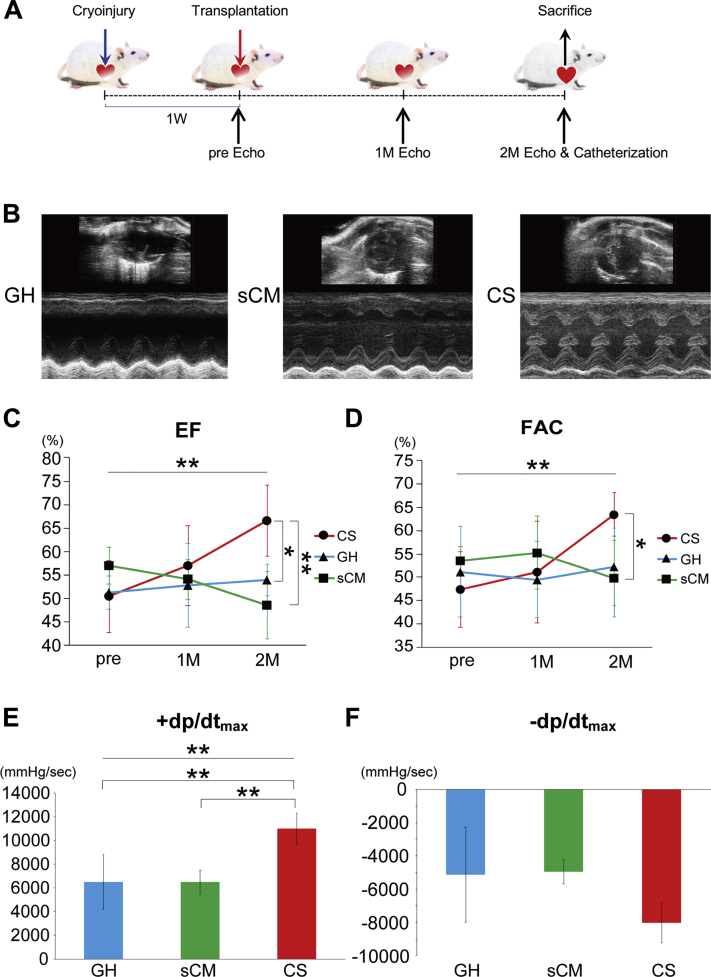
Transplantation of CSs with GH improves cardiac function in rodent HF model
Transplantation protocol is shown in Figure 2A. CSs (n = 3,000; 3 • 106 CMs), or 3 • 106 sCMs, were transplanted into a cryoinjured heart with GH. Only GH was transplanted in the control group. Cardiac function was evaluated by echocardiography before and 4 and 8 weeks after cell transplantation. The representative short-axis and M-mode images are shown in Figure 2B. Both cardiac EF and FAC did not significantly differ at 4 weeks after transplantation in any group. However, only CS-transplanted rats exhibited improved cardiac EF and FAC at 8 weeks (for EF, p < 0.001 [CS vs. GH: p = 0.022, CS vs. sCM; p = 0.002]; for FAC, p = 0.005 [CS vs. GH: p = 0.073, CS vs. sCM: p = 0.029]) (Figures 2C and 2D and Supplemental Table 1). The functional data are summarized in Table 1. Moreover, pressure study revealed improvement of systolic function (+dp/dtmax) in CS group (p = 0.003 [CS vs. GH: p = 0.003, CS vs. sCM: p = 0.009]) (Figure 2E and Supplemental Table 2). Diastolic function (–dp/dtmax) tended to improve in CS group (p = 0.129) (Figure 2F and Supplemental Table 2). Hence, transplantation of hiPSC-derived CSs contributes to cardiac repair in small animal HF models.
Figure 2.
hiPSC-Derived CSs Significantly Improved Cardiac Function in Immunocompromised Rats With Heart Failure
(A) Transplantation protocol for immunocompromised rats is shown. (B) Representative short-axis and M-mode images of hearts in the control (gelatin hydrogel [GH]), single CM (sCM), and CS groups. (C) Cardiac ejection fraction (EF) significantly improved in the CS group (p < 0.001). Post hoc analysis also showed the significant improvement in the CS group at 2 months (CS vs. GH; p = 0.022 CS vs. sCM; p = 0.002). (D) Cardiac fractional area change (FAC) significantly improved in the CS group (p = 0.005). Post hoc analysis showed that the CS group significantly improved at 2 months in comparison with the sCM group, and tended to improve in comparison with the GH group (CS vs. GH: p = 0.073; CS vs. sCM: p = 0.029). (E) Hemodynamic data showed that the +dp/dtmax significantly improved in the CS group (p = 0.003). Post hoc analysis showed that the CS group significantly improved +dp/dtmax at 2 months (CS vs. GH: p = 0.003; CS vs. sCM: p = 0.009). (F) Diastolic function (–dp/dtmax) tended to improve in the CS group (p = 0.129). ∗p < 0.05; ∗∗p < 0.01. M = month; W = week.
Table 1.
Cardiac Function in the Rat Heart Failure Model
| Group | GH |
sCM |
CS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 Month | 2 Months | Baseline | 1 Month | 2 Months | Baseline | 1 Month | 2 Months | |
| EF, % | 51.2 ± 3.6 | 52.8 ± 8.8 | 53.9 ± 3.3 | 57.0 ± 3.9 | 54.1 ± 4.3 | 48.6 ± 7.2 | 50.5 ± 7.7 | 57.0 ± 8.5 | 66.5 ± 7.6 |
| FS, % | 26.8 ± 2.3 | 28.1 ± 5.8 | 28.6 ± 2.2 | 30.8 ± 2.9 | 28.8 ± 3.0 | 25.4 ± 4.4 | 26.4 ± 4.7 | 31.0 ± 5.8 | 37.9 ± 5.7 |
| FAC, % | 51.1 ± 9.7 | 49.5 ± 8.2 | 52.2 ± 8.3 | 53.6 ± 3.1 | 55.2 ± 7.8 | 49.8 ± 8.2 | 47.3 ± 8.0 | 51.1 ± 11.0 | 63.5 ± 4.7 |
Values are mean ± SD.
CS = cardiac spheroid; EF = ejection fraction; FAC = fractional area change; FS = fractional shortening; GH = gelatin hydrogel; sCM = single cardiomyocyte.
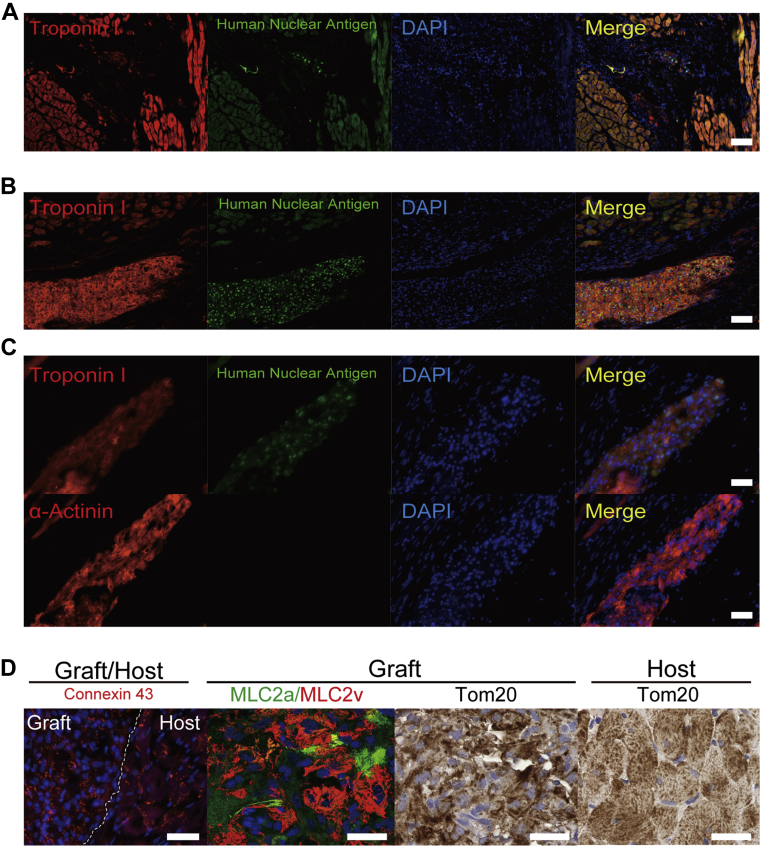
CSs efficiently engrafted in rodents’ hearts
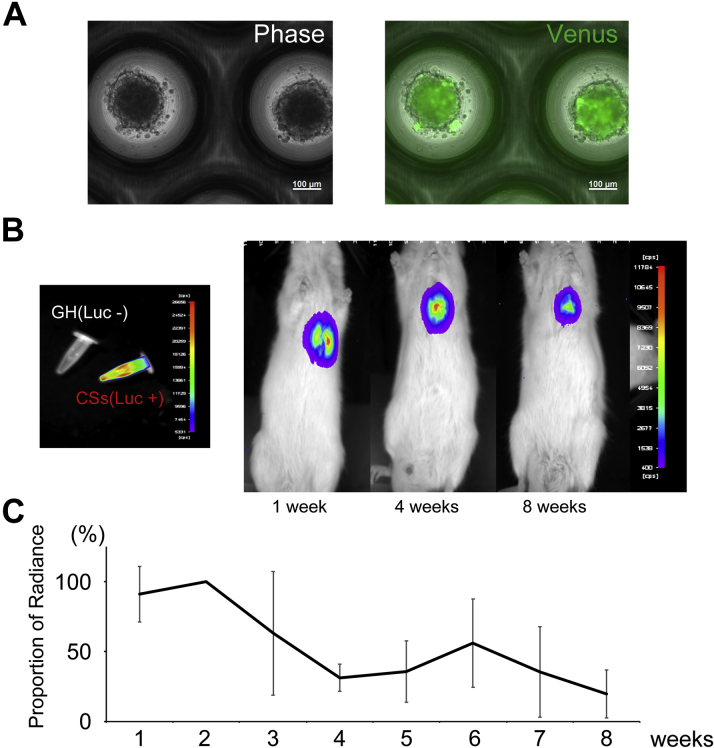
To assess the engraftment of hiPSC-derived CMs, immunofluorostaining was performed for cardiac troponin I and human nuclear antigen. The representative pathological image in the sCM group is shown in Figure 3A. Human nuclear antigen indicated transplantation of hiPSC-derived CMs. Only a few CMs engrafted in a recipient’s heart in sCM group. Numerous transplanted CMs engrafted in a recipient’s heart in CS groups (Figures 3B and 3C). They were positive for human nuclear antigen and cardiac troponin I (Figure 3B). Cardiac troponin I–positive hiPSC-derived CMs were also positive for α-actinin in a serial section (Figure 3C). The transplanted hiPSC-derived CMs clearly expressed connexin 43, but their expression pattern did not align and look immature in comparison with host CMs (Figure 3D). Most of them were MLC2v-positive CMs, which indicated that they had a ventricular phenotype (Figure 3D). Moreover, mitochondrial import receptor subunit Tom20 staining showed that their mitochondria were still immature, compared with those of host CMs, which had a mesh pattern–like morphology. These data revealed that the transplanted hiPSC-derived CMs were still immature; however, they became more mature than their phenotype at transplantation. No teratoma was induced by pure hiPSC-derived CMs. In order to confirm the engraftment of CSs, CSs were infected by lentivirus with Venus (modified GFP [green fluorescent protein]) and luciferase (Figure 4A). The signal of luciferase decreased at 1 month after transplantation, but there were still significant signals remained in the recipient heart at 2 months (Figures 4B and 4C).
Figure 3.
hiPSC-Derived CSs Strongly Engrafted in Immunocompromised Rats With Heart Failure
(A) sCMs engrafted rarely in a recipient’s heart. (B) Large amount of transplanted CMs, which is positive for both human nuclear antigen and cardiac troponin I, strongly engrafted in recipients’ hearts in the CS group. (C) Cardiac troponin I–positive CMs are also positive for α-actinin. (D) Connexin43 was expressed in transplanted cardiomyocytes. MLC2v was more dominant than MLC2a in hiPSC-derived CMs. Tom20 staining indicated that the transplanted CMs had immature mitochondria in comparison with those of adult CMs. Scale bars represent 100 μm in A and B and 50 μm in C and D. DAPI = 4′,6-diamidino-2-phenylindole; other abbreviations as in Figures 1 and 2.
Figure 4.
hiPSC-Derived CSs Were Transduced by a Lentiviral Vector Encoding Venus and Luciferase
(A) Venus was expressed in hiPSC-derived CSs by transduction of a lentiviral vector encoding Venus and luciferase. (B) Luciferin-added CSs express bioluminescence signal. The representative bioluminescence signal in the XSCID rat's heart. (C) The bioluminescence signal decreased 1 month after cell transplantation; however, significant signals remained at 2 months (n = 3). Abbreviations as in Figure 1.
Transplantation of CSs with GH into swine HF model
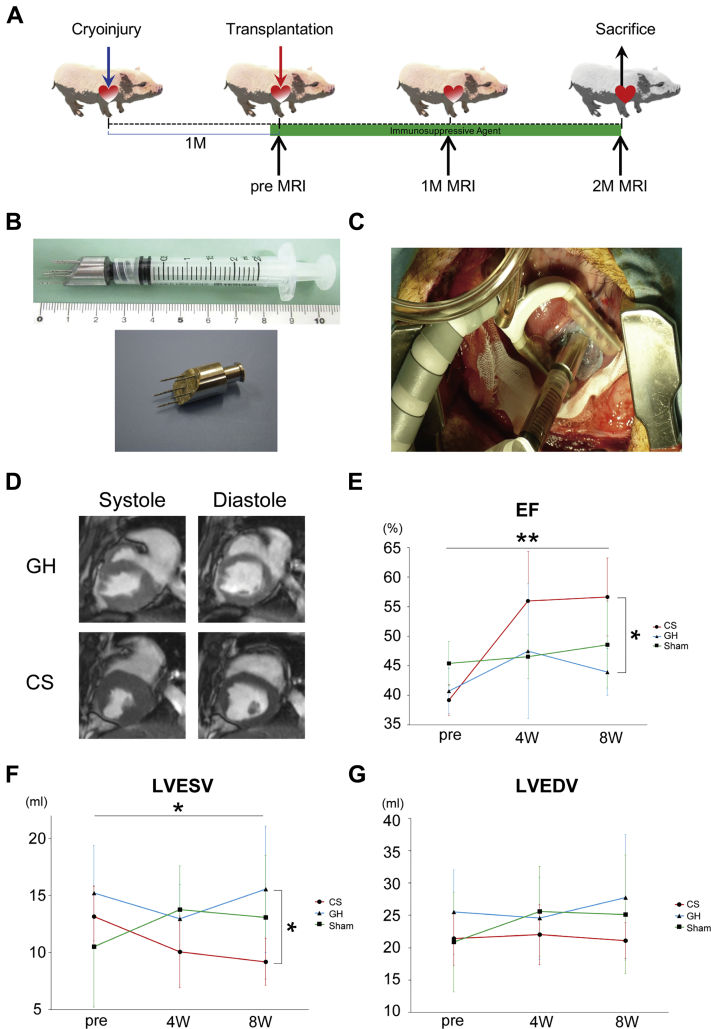
Functional relevance of CSs in large animal models was assessed using swine (Supplemental Table 3). The transplantation protocol is shown in Figure 5A. The immunosuppressive therapy started at 5 days before transplantation. In total, 1 × 105 CSs (1 × 108 CMs) with GH were transplanted. A transplantation device with 6 needles was used for safe and effective cell retention (Figure 5B) Each needle had a blind edge and side holes (12). Anterior wall was fixed with a stabilizer, and CSs were injected into anterior free wall by the transplantation device. Polyglycolic acid felt coated by factor XIII with fibrinogen was used on transplantation site (Figure 5C, Video 1). To avoid perforation, the edge of the device needle was carefully inserted under observation of ultrasonography (Figure 5C, Video 2). No swine died during experimental period.
Figure 5.
hiPSC-Derived CSs Significantly Improved Swine Cardiac Function in Swine Heart Failure
(A) Transplantation protocol. (B) An injection device has 6 needles with a blind tip and side holes. (C) CSs are transplanted by the injection device to anterior wall, which is stabilized by a stabilizer (Videos 1 and 2). (D) Representative images of systolic and diastolic phase of hearts in the GH and CS groups (Videos 3 and 4). (E) EF significantly improved in the CS group (p = 0.001). Post hoc analysis showed that the CS group significantly improved at 2 months in comparison with the GH group, and tended to improve in comparison with sham group (CS vs. GH: p = 0.002; CS vs. sham: p = 0.074). (F) Left ventricular end-systolic volume significantly improved in CS group (p = 0.023). Post hoc analysis showed that the CS group significantly improved at 2 months in comparison with the GH group, and tended to improve in comparison with the sham group (CS vs. GH: p = 0.037; CS vs. sham: p = 0.262). (G) Left ventricular end-diastolic volume remained unaltered between the control and CS groups (p = 0.192). ∗p < 0.05. ∗∗p < 0.01. MRI = magnetic resonance imaging; other abbreviations as in Figures 1, 2 and 3.
hiPSC-derived CSs improved cardiac function 4 and 8 weeks after transplantation in swine HF model
Cardiac function was evaluated by CMR (Figure 5D, Videos 3 and 4). Cardiac EF improved at 4 weeks after transplantation in both groups. However, EF decreased at 8 weeks in the control group. Only CS-transplanted swine exhibited improved EF (p = 0.001 [CS vs. GH: p = 0.002, CS vs. sham: p = 0.074]) (Figure 5E). LVESV, LVEDV, and stroke volume were also assessed by CMR. Similar to EF, LVESV improved at 8 weeks after cell transplantation (p = 0.023 [CS vs. GH: p = 0.037, CS vs. sham: p = 0.262]) (Figure 5F). LVEDV remained unaltered between the control and treatment groups (p = 0.192) (Figure 5G). The functional data are summarized in Table 2 and Supplemental Table 4. Thus, transplantation of CSs contributed to functional cardiac repair in large animal model at 2 months.
Table 2.
Cardiac Function in the Swine Heart Failure Model
| Group | Sham |
GH |
CS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 Month | 2 Months | Baseline | 1 Month | 2 Months | Baseline | 1 Month | 2 Months | |
| EF, % | 45.4 ± 3.7 | 46.5 ± 3.7 | 48.6 ± 7.3 | 40.7 ± 3.8 | 47.5 ± 11.4 | 43.9 ± 3.9 | 39.2 ± 2.6 | 56.0 ± 8.4 | 56.6 ± 6.6 |
| LVESV, ml | 10.5 ± 5.3 | 13.7 ± 3.9 | 13.1 ± 5.4 | 15.2 ± 4.2 | 13.0 ± 3.0 | 15.5 ± 5.5 | 13.2 ± 2.7 | 10.1 ± 3.1 | 9.2 ± 2.1 |
| LVEDV, ml | 20.9 ± 7.7 | 25.6 ± 7.0 | 25.1 ± 9.2 | 25.5 ± 6.5 | 24.6 ± 6.4 | 27.8 ± 9.7 | 21.5 ± 4.2 | 22.0 ± 4.6 | 21.1 ± 2.8 |
| LVSV, ml | 10.3 ± 3.1 | 11.9 ± 3.3 | 12.1 ± 4.1 | 10.3 ± 2.5 | 11.7 ± 5.4 | 12.2 ± 4.4 | 8.6 ± 2.2 | 12.2 ± 2.8 | 11.9 ± 1.8 |
Values are mean ± SD.
LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; LVSV = left ventricular stroke volume; other abbreviations as in Table 1.
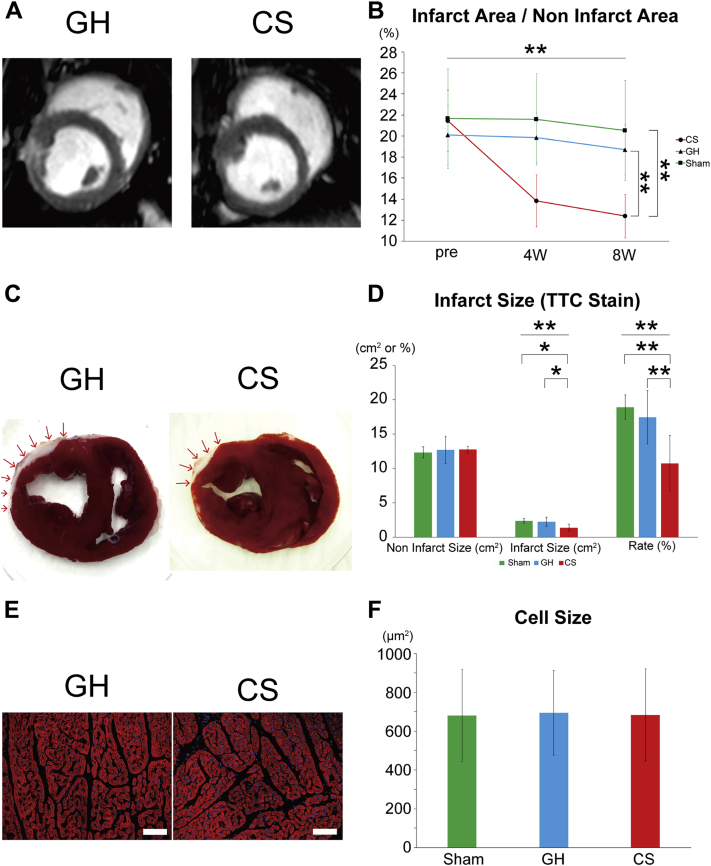
hiPSC-derived CSs reduced infarcted area without cardiac hypertrophy in swine HF model
Late gadolinium enhancement of CMR was used to confirm the scar area in the LV (Figure 6A and Supplemental Table 5). The proportion of scar area in the CS group was 12.4 ± 2.1% (Figure 6B). It was much smaller in the CS group compared with the control groups (p < 0.001, [CS vs. GH; p < 0.001 CS vs. sham; p < 0.001]) (Figure 6B and Supplemental Table 5). Cardiac sections were stained with TTC for pathological evaluation of the infarcted lesions, and morphometric analysis was performed in both models. The left anterior myocardium was ablated from the epicardium to the inner layers, which resulted in a thinner scar area in both groups (Figure 6C). The proportion of scar area in the control was 17.4 ± 3.9%, as determined by TTC staining. Corroborating the results of late gadolinium–enhanced area in CMR, the proportion of the scar area was the smallest in the CS group (infarct size: p = 0.006 [CS vs. GH: p = 0.016, CS vs. sham: p = 0.013]; rate: p = 0.001 [CS vs. GH: p = 0.006, CS vs. sham: p = 0.002]) (Figure 6D and Supplemental Table 6). The CM size in remote area was evaluated by pathological analysis (Figure 6E). We measured 250 cells in each group (n = 15; 5 each from the CS, GH, and sham groups). There was no significant difference between the control and CS groups (p = 0.769) (Figure 6F). Thus, hiPSC-derived CSs reduced the scar area and improved cardiac function without the enhancement of cardiac hypertrophy.
Figure 6.
Morphometric Analysis of Infarction Area in Swine Hearts
(A) Representative late gadolinium enhancement images of cardiac magnetic resonance in the control and CS groups. (B) Infarcted area was assessed by late gadolinium enhancement. The proportion of infarcted area remarkably decreased in the CS group (p < 0.001). Post hoc analysis showed that the CS group significantly decreased the proportion of infarcted area at 2 months in comparison with the GH and sham groups (CS vs. GH: p < 0.001; CS vs. sham: p < 0.001). (C) Representative short-axis figures of TTC (2,3,5-triphenyl tetrazolium chloride)-stained hearts. (D) Infarcted area was assessed by TTC staining. These data confirmed that infarcted area in CS group decreased significantly (infarct size: p = 0.006; rate: p = 0.001). Post hoc analysis showed that the CS group significantly improved at 2 months in comparison with the GH and sham groups (for infarct size, CS vs. GH: p = 0.016; CS vs. sham: p = 0.013; for rate, CS vs. GH: p = 0.006; CS vs. sham: p = 0.002) (E) Representative α-actinin staining of cardiac tissues in remote area. Scale bars represent 100 μm. (F) The average size of CMs in the CS group is not different from that of the control and sham groups (p = 0.769). ∗p < 0.05. ∗∗p < 0.01. Abbreviations as in Figures 1 and 2.
The engraftment of transplanted CMs was very rare 8 weeks after transplantation in swine
The mechanism of cardiac function improvement by CSs was evaluated. At first, the contribution of engrafted CMs was assessed. Iron-loaded CSs were transplanted into a swine, and the transplanted CMs were clearly detected in intramyocardium with T2∗-weighted images immediately after transplantation (Supplemental Figure 2A). Pathologically, numerous CMs still remained in swine hearts for 2 weeks after transplantation (Supplemental Figures 2B and 2C); however, most of them disappeared at 8 weeks (data not shown). To assess immunological reaction between host hearts and grafted cells, far-red stained CSs were transplanted into normal hearts under immunosuppressive therapies. The signal intensity remarkably reduced by 4 weeks after transplantation (Supplemental Figure 2D). Several inflammatory cells (leucocytes and T lymphocytes) invaded surrounding transplanted CMs at 2 weeks after transplantation (Supplemental Figure 2E). Hence, xenotransplantation of CSs engrafted rarely in swine hearts even after strong immunosuppressive therapies.
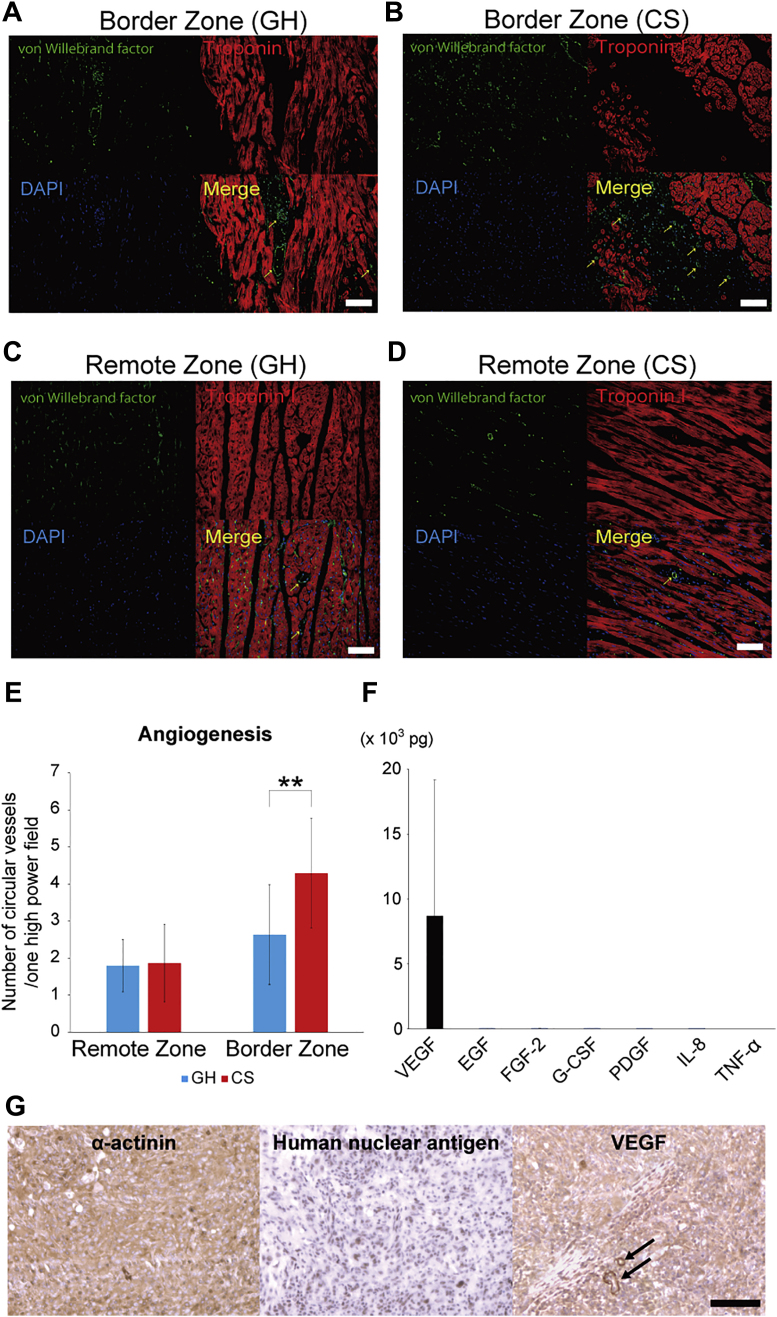
Angiogenesis was strongly promoted in the hiPSC-derived CS group in swine HF model
Next, we focused on angiogenesis, because it is well known that CMs secrete various angiogenic factors (10,17, 18, 19). Heart sections were stained with anti-von Willebrand factor antibody, which indicated endothelial cells (Figures 7A to 7D). The number of anti-von Willebrand factor–positive cells in border zone and remote area was counted in the GH and CS groups, and the CS groups exhibited markedly increased number of vascular cells in the border zone (p < 0.001) (Figure 7E). In order to evaluate the angiogenic potential for CSs, angiogenic cytokines were analyzed by cytokine array. CSs remarkably produced the angiogenic cytokine, vascular endothelial growth factor (VEGF) (Figure 7F). Other cytokines were released in much smaller volume than VEGF (Figure 7F). We confirmed that hiPSC-derived CMs expressed VEGF in vivo at 2 weeks after transplantation (Figure 7G). Note that vascular cells also expressed VEGF (arrows). These results proved that CSs strongly promoted angiogenesis after transplantation.
Figure 7.
Human CSs Strongly Induced Angiogenesis in Swine Hearts
(A, B) Representative figures of von Willebrand Factor–stained endothelial cells and troponin I–stained CMs at border zone in control and CS groups. Scale bars represent 100 μm. (C, D) Representative images of endothelial cells and CMs at the remote zone in the control and CS groups. (E) Angiogenesis was significantly promoted at border zone in the CS group (p < 0.001). ∗∗p < 0.01. (F) Angiogenic and inflammatory cytokines were measured by multiplex cytokine assay. Vascular endothelial growth factor (VEGF) was remarkably released from CSs. Only a small amount of cytokines were released from hiPSC-derived CSs except VEGF. (G) Transplanted hiPSC-derived CMs expressed VEGF in vivo. Vascular cells also expressed VEGF (arrows). The scale bar represents 100 μm. EGF = epidermal growth factor; FGF = fibroblast growth factor; G-CSF = granulocyte colony-stimulating factor; PDGF = platelet-derived growth factor-AB/BB; IL = interleukin; TNF = tumor necrosis factor; other abbreviations as in Figures 1, 2 and 3.
Ventricular arrhythmia was induced in correlation with tachycardia in hiPSC-derived CS group in the swine HF model
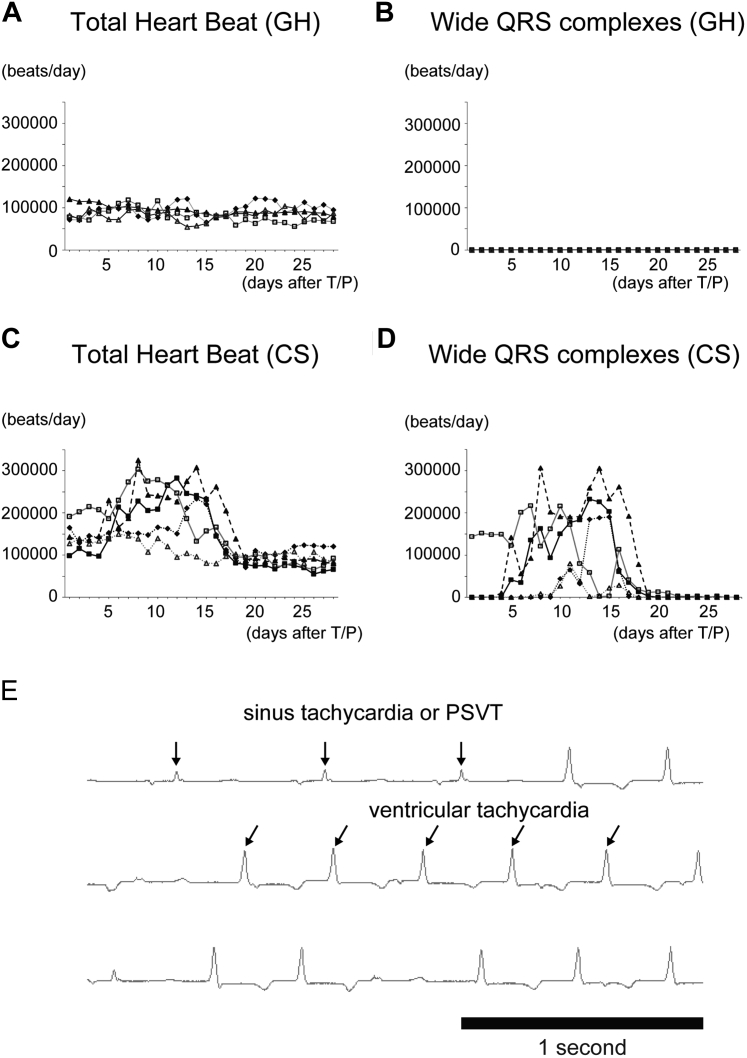
The arrhythmogenicity of hiPSC-derived CSs was evaluated by implantation of telemonitor ECG. During the transplantation surgery of CSs, no significant ventricular arrhythmia was detected in the ECG monitor (data not shown). Control swine showed stable heart rate (61.0 ± 10.8 beats/min) at days 5 to 20 and no significant ventricular arrhythmia (Figures 8A and 8B). In the CS group, heart rate increased after transplantation of CSs. The average heart rate was 119.1 ± 47.3 beats/min at days 5 to 20. Sinus or paroxysmal supraventricular tachycardia continued for 20 days after the transplantation of CSs (Figure 8C). Ventricular tachycardia also emerged at the same period, but no fatal arrhythmia had happened (Figures 8D and 8E).
Figure 8.
Ventricular Arrhythmia Was Induced in the hiPSC-Derived CS Group in the Swine Heart Failure Model
(A, B) No control swine showed either tachycardia or ventricular arrhythmia. (C, D) hiPSC-derived CS-transplanted swine increased their heart rate and the number of wide QRS complex for 20 days after cell transplantation. (E) Ventricular tachycardia emerged in correlation with the emergence of sinus or paroxysmal supraventricular tachycardia (PSVT), but no fatal arrhythmia happened. T/P = transplantation; other abbreviations as in Figures 1 and 2.
Discussion
Purified hiPSC-derived CSs improved cardiac function in rat HF models. They successfully engrafted at cryoinjured hearts at 2 months. Preclinical study with a swine HF model was performed for clinical application of CSs to severe HF. Although the transplanted CMs could hardly remain in host swine hearts due to inflammation resulting from xenotransplantation, they successfully improved cardiac function at 8 weeks after transplantation. Pathological studies proved that CSs significantly enhanced angiogenesis.
The transplanted hiPSC-derived CSs exhibited strong potential to engraft and form regenerated myocardium in immunocompromised recipients’ hearts. Teratoma formation due to residual PSCs and highly proliferating immature cells is an issue in the transplantation of hPSC derivatives (20,21). hiPSC-derived CMs were purified and enriched with metabolic selection in this study; thus, the transplanted pure hiPSC-derived CSs did not generate teratoma despite their efficient engraftment. These data confirmed the high feasibility of cardiac regenerative therapies with hiPSC-derived CSs to patients with severe HF.
Even though stem cell therapies improve cardiac function in small animal models, clinical studies often show no or small clinical benefits in stem cell research (22,23). Therefore, large animal models are strongly recommended to assess the preclinical effects before translational studies (14). To evaluate surgical devices and transplantation strategies, a swine is an ideal large animal model, as its anatomical structure resembles that of a human body. Thus, xenotransplantation of human embryonic stem cell or hiPSC derivatives is necessary for translational research. Contractile function (LVEF) of swine improved at 1 month in both groups, as shown previously (24). However, only the CS group exhibited improvement in LVEF at 2 months. To enhance the engraftment of CMs, strong immunosuppressive therapies were applied to swine. However, a lot of CMs still existed in the recipient myocardium at 2 weeks; their abundance diminished after 1 month. Contrastingly, the number of vessels significantly increased in the CS group at 2 months. These data strongly indicated that transplanted CSs improved cardiac function with pleiotropic effects at chronic phase. In particular, hiPSC-derived CSs released vascular endothelial growth factor to promote angiogenesis. This effect may be enhanced by co-transplanted GH, which can augment sustained release of CM-derived cytokines (10).
In this study, ventricular arrhythmia was induced by the transplantation of hiPSC-derived CSs in HF swine. Interestingly, wide QRS complexes started to appear a few days after cell transplantation in correlation with the emergence of sinus or paroxysmal supraventricular tachycardia. The arrhythmia continued for 2 to 3 weeks. This period matched with the peak of immunological rejection to the surviving transplanted cells. After most of transplanted cells were gone 1 month after cell transplantation, wide QRS complexes and tachycardia disappeared. Actually, inflammatory cells invaded into myocardium in pathological studies. Therefore, on the one hand, arrhythmogenicity in this study could be induced by the inflammation due to immunological rejection. On the other hand, monkeys, such as Macaca fascicularis and Macaca nemestrina, are another feasible choice for preclinical trials (25,26) and cell engraftment studies. Human embryonic stem cell–derived CMs engrafted for approximately 3 months in monkeys’ hearts (25). HLA-matched monkey iPSC-derived CMs also show improvement of cardiac function and engraftment of CMs (26). However, both studies revealed strong arrhythmogenicity of transplanted CMs at approximately 1 month after transplantation, even though transplanted CMs exhibited the potential to beat in synchronization with host myocardium (25). The mixed non-CMs or various phenotypes of CMs, such as pacemaker cells, may have triggered ventricular arrhythmia in these studies, because the differentiation ratio of CMs was ∼80% to 90%. Therefore, conquest of arrhythmogenicity must be the last hurdle for clinical application of hPSC-derived CMs.
Study limitations
At first, hiPSC-derived CMs generally have immature fetal phenotype. In comparison with adult myocardium, they are quite immature. Second, the current intramyocardial transplantation needs open-chest surgery. Less invasive transplant methods are preferred for patients with severe HF; thus, catheter delivery methods should be explored in the future. Third, we evaluated the relatively short-term recovery of cardiac function in swine HF models but could not assess long-term function due to xenotransplantation. After 2 months, the immunosuppressive therapies caused severe infection, and it was difficult to observe their cardiac function precisely. Thus, further exploration of HF treatment in cardiac regeneration therapy is warranted.
Conclusions
Transplantation of hiPSC-derived CSs is safe and effective to improve cardiac function in rat and swine HF models. Transplanted CSs can engraft and constitute cardiac tissues in immunocompromised recipients. Therefore, inflammatory and immunological rejection must be controlled for the long-term engraftment of transplanted CMs after cell transplantation.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: hiPSCs have been considered ideal sources for regenerative CMs since their discovery. Although the transplantation of hiPSC-derived CMs is expected to reach the clinical application stage soon, transplantation strategy must be validated in preclinical trials. Here, we demonstrated the clinical concept for the transplantation of CSs as miniature cardiac tissues inside host myocardium in small and large animal HF models.
TRANSLATIONAL OUTLOOK: This study proved that the transplantation of hiPSC-derived CSs is a feasible technique to improve cardiac function in HF patients.
Funding Support And Author Disclosures
This work was supported by the Highway Program for Realization of Regenerative Medicine (17bm054006h0007 [to Dr. Fukuda]) and the Research Project for Practical Applications of Regenerative Medicine (17bk010462h0001 [to Dr. Fukuda]) from the Japan Agency for Medical Research and Development, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (nos. 15K09098 [to Dr. Kanazawa], 16K09507 [to Dr. Fujita], 19H03660 [to Dr. Fujita], 17H05067 [to Dr. Tohyama], 18K15903 [to Dr. Nakajima]). Drs. Kanazawa, Tohyama, Fukuda, and Fujita have patents related to this work. Drs. Tohyama, Shimizu, Kanazawa, Fukuda, and Fujita own equity in Heartseed, Inc. Dr. Tohyama is an advisor of Heartseed, Inc. Dr. Fukuda is a co-founder and CEO of Heartseed, Inc.; and receives a salary from Heartseed, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Miho Yamaguchi, Rei Ohno, Chihana Fujita, Sayaka Kanaami, Yoshiko Miyake, and Tomoko Haruna for technical assistance. They also thank the National BioResource Project - Rat for providing the rat strain, F344-Il2rgem7Kyo. The authors thank Astellas Pharma Inc. for the gift of tacrolimus. They also thank Profs. Hideyuki Okano (Department of Physiology, Keio University) and Masaya Nakamura (Department of Orthopaedic Surgery, Keio University) for providing the lentiviral vector (CSII-EF-dVenus-Luc2).
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods and References sections as well as supplemental figures, videos, and tables, please see the online version of this paper.
Appendix
Transplantation of hiPSC-derived CSs
Echocardiography of a swine heart transplanted with CSs
MRI of control swine heart
MRI of CSs-transplanted swine heart
References
- 1.Ponikowski P., Anker S.D., AlHabib K.F. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Khush K.K., Cherikh W.S., Chambers D.C. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37:1155–1168. doi: 10.1016/j.healun.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Behfar A., Crespo-Diaz R., Terzic A., Gersh B.J. Cell therapy for cardiac repair--lessons from clinical trials. Nat Rev Cardiol. 2014;11:232–246. doi: 10.1038/nrcardio.2014.9. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K., Tanabe K., Ohnuki M. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 7.Tohyama S., Fujita J., Fujita C. Efficient large-scale 2D culture system for human induced pluripotent stem cells and differentiated cardiomyocytes. Stem Cell Rep. 2017;9:1406–1414. doi: 10.1016/j.stemcr.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tohyama S., Fujita J., Hishiki T. Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab. 2016;23:663–674. doi: 10.1016/j.cmet.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Tohyama S., Hattori F., Sano M. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima K., Fujita J., Matsui M. Gelatin hydrogel enhances the engraftment of transplanted cardiomyocytes and angiogenesis to ameliorate cardiac function after myocardial infarction. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hattori F., Chen H., Yamashita H. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 12.Tabei R., Kawaguchi S., Kanazawa H. Development of a transplant injection device for optimal distribution and retention of human induced pluripotent stem cellderived cardiomyocytes. J Heart Lung Transplant. 2019;38:203–214. doi: 10.1016/j.healun.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Menasche P., Vanneaux V., Hagege A. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. 2018;71:429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 14.Chamuleau S.A.J., van der Naald M., Climent A.M. Translational research in cardiovascular repair: a call for a paradigm shift. Circ Res. 2018;122:310–318. doi: 10.1161/CIRCRESAHA.117.311565. [DOI] [PubMed] [Google Scholar]
- 15.Hirano A., Fujita J., Kanazawa H. Cryoinjury-induced acute myocardial infarction model and ameroid constrictor-induced ischemic heart disease model in adult micro-mini pigs for preclinical studies. Transl Med Commun. 2017;2:1. [Google Scholar]
- 16.Mashimo T., Takizawa A., Voigt B. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giordano F.J., Gerber H.P., Williams S.P. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kardami E., Detillieux K., Ma X. Fibroblast growth factor-2 and cardioprotection. Heart Fail Rev. 2007;12:267–277. doi: 10.1007/s10741-007-9027-0. [DOI] [PubMed] [Google Scholar]
- 19.Lancaster J.J., Sanchez P., Repetti G. Human induced pluripotent stem cell derived cardiomyocyte patch in rats with heart failure. Ann Thorac Surg. 2019;108:1169–1177. doi: 10.1016/j.athoracsur.2019.03.099. [DOI] [PubMed] [Google Scholar]
- 20.Miura K., Okada Y., Aoi T. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 21.Nori S., Okada Y., Nishimura S. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015;4:360–373. doi: 10.1016/j.stemcr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyongyosi M., Wojakowski W., Lemarchand P. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346–1360. doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher S.A., Doree C., Mathur A., Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res. 2015;116:1361–1377. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 24.Romagnuolo R., Masoudpour H., Porta-Sanchez A. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Rep. 2019;12:967–981. doi: 10.1016/j.stemcr.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong J.J., Yang X., Don C.W. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiba Y., Gomibuchi T., Seto T. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transplantation of hiPSC-derived CSs
Echocardiography of a swine heart transplanted with CSs
MRI of control swine heart
MRI of CSs-transplanted swine heart