Highlights
-
•
This review presents a conceptual framework linking motor control impairments after sport-related concussion (SRC) to elevated risk for lower extremity musculoskeletal injury.
-
•
Athletes with SRC demonstrate neuroanatomic and neurophysiologic changes relevant to motor control and altered motor function that support the conceptual framework.
-
•
Alterations in motor function after SRC include decreased muscle activation and force production, modified movement patterns, poor balance, and impaired motor task performance with or without a simultaneous cognitive task.
-
•
These deficits indicate a need to evaluate for and rehabilitate motor control impairments after SRC through the return to sport continuum to mitigate musculoskeletal injury risk.
Keywords: Clinical management, Concussion, Injury, Motor control, Rehabilitation
Abstract
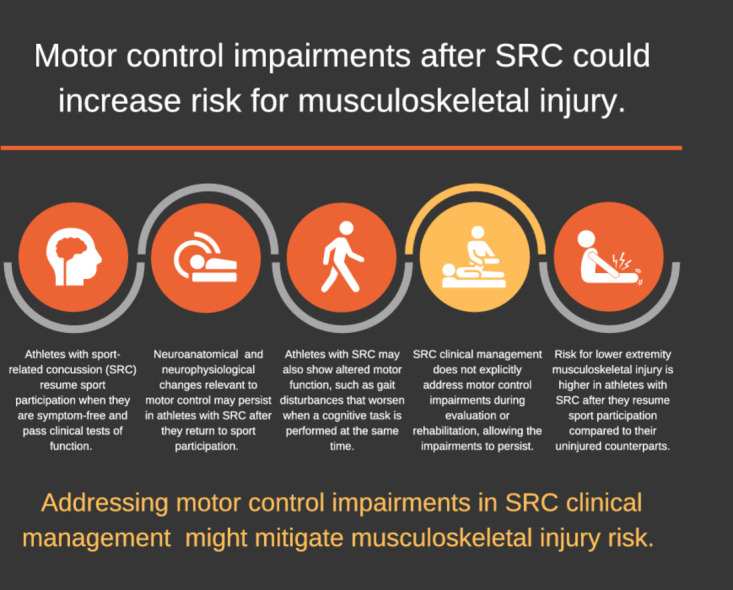
This review presents a conceptual framework and supporting evidence that links impaired motor control after sport-related concussion (SRC) to increased risk for musculoskeletal injury. Multiple studies have found that athletes who are post-SRC have higher risk for musculoskeletal injury compared to their counterparts. A small body of research suggests that impairments in motor control are associated with musculoskeletal injury risk. Motor control involves the perception and processing of sensory information and subsequent coordination of motor output within the central nervous system to perform a motor task. Motor control is inclusive of motor planning and motor learning. If sensory information is not accurately perceived or there is interference with sensory information processing and cognition, motor function will be altered, and an athlete may become vulnerable to injury during sport participation. Athletes with SRC show neuroanatomic and neurophysiological changes relevant to motor control even after meeting return to sport criteria, including a normal neurological examination, resolution of symptoms, and return to baseline function on traditional concussion testing. In conjunction, altered motor function is demonstrated after SRC in muscle activation and force production, movement patterns, balance/postural stability, and motor task performance, especially performance of a motor task paired with a cognitive task (i.e., dual-task condition). The clinical implications of this conceptual framework include a need to intentionally address motor control impairments after SRC to mitigate musculoskeletal injury risk and to monitor motor control as the athlete progresses through the return to sport continuum.
Graphical abstract
1. Introduction
Sport-related concussion (SRC) is induced by biomechanical forces to the head that result in a range of transient clinical signs, symptoms, and disturbances in function.1 Clinical examination for SRC includes an assessment of patient-reported symptomology and tests of neurologic, cognitive, vestibular, oculomotor, and postural function.1 After acute symptoms stabilize, the athlete is allowed to begin a graduated progression of physical activity and cognitive exertion, as long as symptoms are not exacerbated.1,2 Multidisciplinary, individualized care is recommended, which may involve treatment or rehabilitation to address persistent clinical impairments in vestibular, cervical, autonomic, and psychological domains.1,3, 4, 5 Clinical recovery after SRC is judged to be complete when the neurological examination is normal and post-concussion symptoms and the multifactorial assessments of function have returned to baseline status during daily activities including school, work, and sport.1,6 Most athletes meet these criteria within 21–30 days post-SRC.7 It is recommended that the return to sport participation after SRC follow a stepwise progression where participation in athletic activity without symptom recurrence is evident prior to sport participation at a competitive level.1
Despite advances in SRC clinical management protocols, a growing body of evidence has shown that athletes with SRC have double the odds of sustaining a musculoskeletal injury after they return to sport compared to athletes without SRC.8, 9, 10, 11, 12, 13 The relationship between SRC and musculoskeletal injury has been consistently observed among studies and in athletes of both sexes and across multiple sports and levels of play.10 Furthermore, the increased risk of musculoskeletal injury may persist for months or more past the time of return to sport participation.10 Previous reviews have discussed the increased risk of musculoskeletal injury after SRC.10,14, 15, 16, 17 The phenomenon has been theorized to result from persistent sensorimotor impairments,10 decreased neuromechanical responsiveness,16 altered perception–action coupling,15 or subtle neurocognitive and neuromuscular deficits.14,17 From a broader perspective, each of these theories relate to aspects of motor control. Previous reviews have also focused on a singular or narrow aspect of motor function, making it difficult to appreciate the many ways that motor function may change post-SRC.
Our review presents a conceptual framework and supporting evidence that link impaired motor control after SRC to musculoskeletal injury risk. In addition, potential changes in SRC clinical management and rehabilitation to mitigate injury risk are discussed.
2. A brief overview of motor control
Motor control refers to “how the nervous system interacts with the rest of the body and the environment to produce purposeful, coordinated movement” and is often used interchangeably with the term “neuromuscular control” in the rehabilitation literature.18,19 Motor control involves the processing of sensory information (e.g., somatosensory, visual, vestibular, and auditory) and coordination of motor output within the central nervous system. The relative weight of each component in producing a given movement varies by situation or task because motor control is context-dependent.18,20
Key aspects of motor control are motor planning and motor learning.21 Motor planning refers to the selection of a motor plan, which consists of the movement goals with respect to the muscles and joints.22 Motor planning starts with an awareness of sensory cues in the environment and requires appropriate processing of the sensory input in order to select an optimal motor plan.22 For movements that occur relatively slowly, the selected motor plan may be shaped by cognitive processing, or decision making, but when there is little time for cognitive processing (e.g., hitting a fastball in baseball), perceptual-motor routines and previously learned action sequences likely provide the basis for the motor plan.23 Motor learning refers to the experience-dependent acquisition of a motor skill or adaptation of a motor skill when task conditions change.24 Motor learning may be categorized as explicit or implicit. Explicit motor learning occurs with conscious, purposeful cognitive processing, whereas implicit motor learning occurs without conscious awareness of what is being learned and is thought to be a more automatic process.25, 26, 27 Most motor learning is neither purely explicit nor purely implicit.
The way feedback is given influences whether motor learning is explicit or implicit. Coaching and rehabilitation predominantly encourage explicit motor learning (principally, strategy-based learning) through verbal cues and visual feedback on motor skill performance.28,29 Feedback may be directed toward knowledge of results—that is, how well the movement achieved the goal of the performance—or knowledge of performance, which is about the movement characteristics that led to the performance.30 Implicit motor learning, and specifically sensorimotor adaptation, occurs in response to sensory prediction errors detected during motor task performance.31, 32, 33, 34 To illustrate these concepts, if an athlete is told to “bend your knee” during walking, this feedback on motor task performance initially requires cognitive processing and results in primarily explicit motor learning. In contrast, if an elastic band is used to resist knee flexion during gait, this initially results in less knee flexion during gait, causing a sensory error signal. The error signal results in the athlete producing greater knee flexion to overcome the resistance. By practicing this way, implicit learning through sensorimotor adaptation will occur so that when the elastic band is removed, the athlete will continue to produce greater knee flexion during gait.
3. Theoretical link between impaired motor control and musculoskeletal injury risk
Sport participation is inherently a series of motor tasks performed in a rapidly changing environment with additional cognitive demands, such as recalling plays. If an athlete cannot accurately perceive and process sensory cues in the athletic environment while simultaneously performing cognitive tasks related to the sport, motor plan selection, and thus motor function, could be negatively affected. It is conceivable that this could make the athlete vulnerable to musculoskeletal injury.
Preliminary studies in athletic populations support the proposition that impaired motor control is associated with musculoskeletal injury risk. Athletes with low perceptual and cognitive aptitude in domains such as reaction time, processing speed, and visual and verbal memory have increased risk for non-contact anterior cruciate ligament rupture and other lower extremity injuries.35, 36, 37 Additionally, athletes with a low aptitude in visual processing and memory domains demonstrate movement patterns during jump landing that are associated with a higher risk of knee injury.38,39 It is important to point out that mean neurocognitive test scores fall within acceptable limits in groups that sustain injury or demonstrate altered movement patterns. Furthermore, neurocognitive scores associated with elevated injury risk are not well-defined for athlete populations that vary by sport or other demographics. Despite this limitation, a consistent finding in the current literature is that reaction time scores on the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) of greater than 545–570 ms is associated with increased lower extremity injury risk in collegiate athletes.35, 36, 37 These data suggest that reaction time and speed of processing in completing cognitive tasks may be useful in evaluating athletes for elevated injury risk.
Research in the SRC population also supports the proposition that impaired motor control could increase the risk for musculoskeletal injury.14 For example, athletes with SRC who sustained a time-loss injury within 1 year demonstrated greater decrements in a gait speed differential (gait performed with or without an additional cognitive task) at testing between 21 days post-concussion and clearance for sport participation.40 In addition, athletes with SRC who sustained a lower extremity musculoskeletal injury within 1 year demonstrated at preseason testing and clearance for sport participation a slower speed and greater time in double-limb support during gait performance with or without a simultaneous cognitive task.41 Current research has not shown that athlete demographics and neurocognitive test scores at baseline or acutely following SRC are indicators of whether athletes are at risk of sustaining a musculoskeletal injury following SRC.42
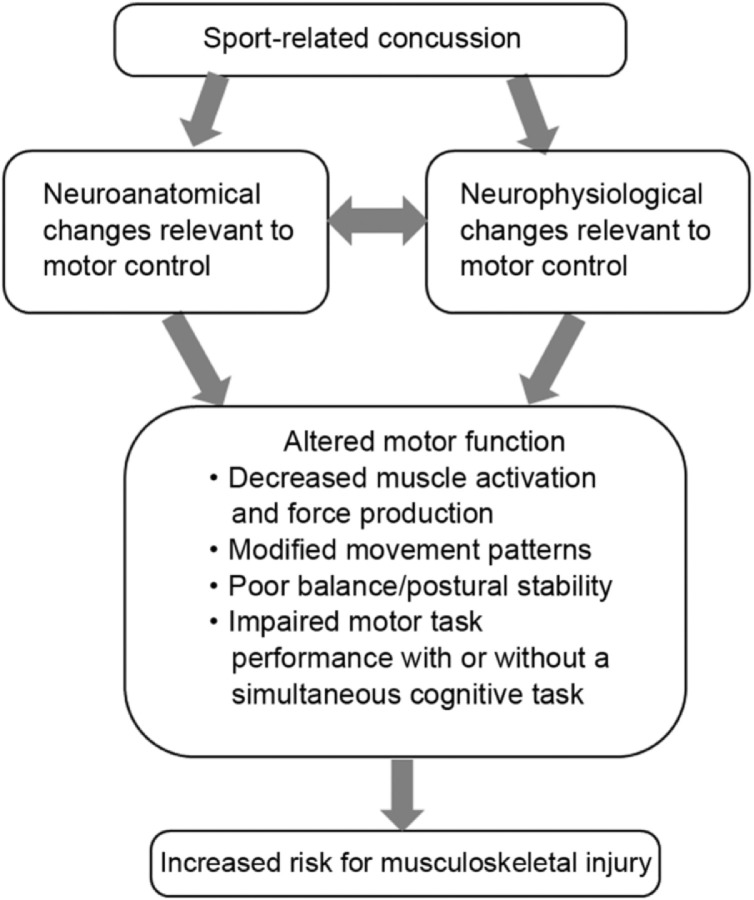
Current SRC clinical management attempts to normalize post-concussion impairments through return to sport criteria that cover a variety of functional domains.1 At this time, motor control is neither explicitly assessed nor targeted in interventions.6,17 However, the literature cited in the following sections provides evidence for the persistence of subtle changes in the central nervous system relevant to motor control and altered motor function even after return to sport criteria are fulfilled. Thus, we propose that impaired motor control after SRC, evidenced by neurobiological and motor function changes, leads to an increased risk for musculoskeletal injury (Fig. 1).
Fig. 1.
Conceptual framework that links unresolved impairments in motor control after sport-related concussion to an increased risk for musculoskeletal injury.
4. Evidence for subclinical changes in the central nervous system relevant to motor control after SRC
4.1. Neuroanatomic changes
Diffusion tensor magnetic resonance imaging has been used to evaluate white matter microstructure integrity after concussion, including SRC.43, 44, 45, 46, 47, 48, 49, 50, 51 Abnormalities in brain structures with a direct relationship to motor control, such as the internal capsule, cerebellar tracts, and corpus callosum,43, 44, 45, 46, 47, 48,51 have been revealed, although significant interindividual variability exists.49 The superior and inferior longitudinal fasciculi have also shown abnormalities that could affect connectivity between systems that provide critical information for motor planning, for example, visual and spatial attention, orientation, memory, and the motor system.44,50,51 While such imaging is not a standard component of SRC clinical management, the cited studies provide neuroanatomic evidence of impaired motor control after SRC.
4.2. Neurophysiological changes
Studies employing trans-cranial magnetic stimulation of the primary motor cortex have demonstrated increased cortical inhibition, increased motor activation threshold, and decreased intra-cortical facilitation after concussion.52 Non-motor areas, such as the somatosensory cortex, also undergo neurophysiological changes after SRC that can impair motor control.53, 54, 55 Changes in these areas may alter the somatosensory processing used to guide and modulate ongoing movement. It is of particular interest that neurophysiological changes in motor and non-motor areas of the brain appear to be dose-dependent, such that greater concussion severity or an increasing number of concussions results in greater alterations in neurophysiological function.56 Importantly, these studies have shown that neurophysiological abnormalities can persist for months or even years after the concussion event, well beyond the fulfillment of clinical return to sport criteria.52,54,57 Moreover, these neurophysiologic changes have been directly connected to alterations in motor function after SRC.56,58,59
5. Evidence for altered motor function after SRC
5.1. Muscle activation and force production
Voluntary muscle activation after SRC has only been examined in 1 study, which reported decreased activation of the first dorsal interosseous muscle.59 On the other hand, several studies have examined muscle force production after SRC, and the results have been inconclusive.59, 60, 61, 62, 63 For example, in one study no reduction was found in isometric force of the first dorsal interosseous muscles within 1 month post-SRC,59 whereas in another study, grip strength was reported to be reduced when concussion symptoms were elevated.60 Moreover, 1 study found that isometric strength of the anterolateral cervical musculature was reduced post-SRC,61 but a different study found no difference in isometric strength of the cervical musculature in subjects with or without a history of concussion.62 Finally, a study found no difference in isokinetic quadriceps and hamstrings strength between military personnel with or without a history of concussion; however, the same study found that the time to peak knee extension torque was slower in those with a history of concussion.63 It is unclear if decreased muscle strength is a persistent outcome after SRC. Because SRC often requires a period of rest and removal from sport participation, inactivity could contribute to acute decreases in strength. It may be that measures related to how muscle is activated are more sensitive to residual impairment. In support of this supposition, 1 study found that concussed football players tasked with holding a steady submaximal force showed faster and greater force decline over time, more force variability over time, and greater perceived sense of effort compared to their uninjured counterparts.59
5.2. Movement patterns
SRC might affect movement patterns, but the manner in which it does is not yet clear. Altered inter-joint coordination and larger changes in center of mass position have been found in gait after SRC,14 and it was found that leg stiffness during a single-legged jump-landing task decreased post-season in football players who sustained SRC.64 This could be problematic because insufficient joint stiffness is thought to increase the risk for soft tissue injury.65,66 Another study examined a two-legged jump-landing task and found no differences in lower extremity kinematics between subjects with a history of concussion and controls.67 Two studies have evaluated movement patterns during a jump-cut task.67,68 One of these studies reported that individuals with a concussion history displayed knee kinematics that could increase knee ligament injury risk (e.g., decreased knee varus and external rotation),68 while the other study found only slightly more trunk flexion during a cut to the non-dominant side in individuals with a concussion history.67
5.3. Balance and postural stability
Deficits in balance, or postural stability, are common after SRC and could result from either direct injury to vestibular apparatus or poor integration of sensory input.17 Balance is often assessed with tools such as the Balance Error Scoring System1,3 The Balance Error Scoring System has been extensively researched and holds good utility for acute balance assessment (sensitivity 34%; specificity 91%–96%),69 but is less able to identify deficits after 3–5 days post-concussion.70,71 Postural stability was examined during a single-legged hop from a box and a single-legged squat in subjects with a history of concussion and in subjects who served as controls.72 Time to stabilization after landing from the hop off a box was longer for the non-dominant limb in concussed subjects compared to controls, while no group differences were found for the dominant limb or in the squat task.72 At this time, it is unclear whether balance/postural stability tasks and measures can identify persistent motor control impairments after SRC.
5.4. Motor performance
Gait performance among post-SRC subjects shows slower speed and wider obstacle clearance compared to those without concussion.14 On a grooved pegboard test, performance was slower in adolescents with a recent concussion compared to controls when the non-dominant hand was used, while no group differences were found when the dominant hand was used.73 Finally, playing performance following SRC has been evaluated in hockey players with a modified plus–minus statistic that assigns points based on scoring and strength of schedule.74 Athletes with SRC showed an acute decline in playing performance that was similar to those with a lower extremity injury, but the decline was not long lasting.74 This small body of research suggests that the type of motor task or the way motor performance is assessed could determine if a deficit is identified after SRC.
5.5. Motor performance during dual-task conditions
Pairing a motor task with a cognitive task (e.g., counting by sevens) is known as a dual-task condition. Dual-task conditions require distribution of attention between the cognitive and motor tasks that can reveal or exacerbate poor motor task performance.75,76 Generally, as the cognitive task becomes more challenging, the magnitude of the motor control impairments tends to increase.14,76 Studies have shown that athletes with SRC demonstrate greater motor impairment, such as reduced speed and decreased postural stability, during dual-task gait compared to gait-only conditions.76,77 Athletes with a history of concussion also perform more poorly on reaction-time reaching and side-shuffling tasks performed in a dual-task condition.78 Recent work indicates that the risk of musculoskeletal injury after SRC may be directly related to the degree of motor control impairment during gait or side shuffling performed in a dual-task condition.40,79
Dual tasking is common in sport participation and could create the potential for musculoskeletal injury in an athlete with SRC in 2 ways. First, the athlete may place attention on the cognitive demands of the sport activity (e.g., recalling the play) at the expense of the motor task, potentially resulting in poor movement patterns or motor performance that put the athlete at risk for injury. Alternatively, the athlete may place attention on executing the motor task (e.g., running the play) instead of the other cognitive demands of the sport activity (e.g., observing location of other players and obstacles on the field), resulting in a missed opportunity to shape the motor plan appropriately to avoid injury.
6. Implications for SRC clinical management and rehabilitation
A key clinical issue to emerge from the conceptual model is how to ensure that motor control is sufficiently restored after SRC so that injury risk is mitigated. While motor control is not an explicit focus in current SRC clinical management, it could be argued that some tests indirectly (e.g., neuropsychological or vestibular-ocular tests) or directly (e.g., balance and gait tests) assess motor control. The evidence reviewed above suggests that these tests may not ensure that motor control is sufficiently restored when sport participation is resumed. For this reason, it is warranted to consider ways to evolve SRC clinical management to better address motor control.
A fundamental change in SRC clinical management would be to intentionally address motor control through targeted testing and rehabilitation interventions. Such a change aligns with recent concussion practice guidelines.80 Also, current SRC clinical management evaluates different types of function in isolation, but it is important to recognize that motor control requires that multiple systems (e.g., sensorimotor system, vestibular system, central nervous system, and musculoskeletal system) work together. Thus, an integrated systems approach is recommended for motor control assessment and treatment.
Motor control can be assessed through the performance of motor tasks and perceptual/cognitive challenges with increasing complexity. We recognize that no standard exists for evaluating motor task performance in relation to musculoskeletal injury risk. However, clinical judgments could be based on performance metrics, such as gait speed or cadence.40,41,81,82 Clinicians could also assess for abnormal movement patterns, including those known to increase musculoskeletal injury risk.83 Movement patterns can be analyzed during various motor tasks with visual observation or 2-dimensional techniques,84, 85, 86 which are more feasible in clinical and field settings compared to 3-dimensional analysis. To date, no movement variable has been sufficiently correlated with injury risk in the SRC population to justify the time and cost of 3-dimensional analysis. Performance on perceptual/cognitive tasks can be assessed by comparing performance under dual-task conditions to performance without a concurrent motor task or possibly to pre-SRC performance.
The selection and progression of motor tasks and perceptual/cognitive tasks should be given careful consideration. Motor task selection can be guided by general principles for therapeutic exercise and motor learning.87,88 Motor tasks with low physical demand may be presented before progressing to those with higher physical demand, and all motor tasks may first be presented in isolation before presenting with a perceptual/cognitive challenge (i.e., dual-task condition). It is best to select motor tasks with relevance to the athlete's activities of daily living and/or sports participation for better generalizability beyond the rehabilitation setting,87 and complex motor tasks may need to be practiced in parts for successful completion.22 The selection of cognitive/perceptual tasks should be guided by task complexity and knowledge of the athlete. Simple cognitive challenges, such as counting backwards by sevens, may be presented before progressing to those that involve greater cognitive processing, such as Stroop tasks. Some athletes might naturally have more difficulty with certain types of cognitive tasks, such as math-based challenges, and that should be considered when selecting the cognitive task.
An example of clinical application would be to first assess motor performance during low-demand motor tasks such as gait, static balance tests, or squatting. If an athlete demonstrates difficulty performing a motor task, then the motor task becomes a rehabilitation intervention by allowing practice with appropriate feedback. It may be advantageous in an early stage of rehabilitation to encourage implicit learning so as not to stress cognitive resources. Once the athlete demonstrates proficiency with the motor task, a perceptual/cognitive task can be added, starting with easier challenges such as calling out numbers on playing cards presented to the athlete, which acts as a visual distractor. Perceptual/cognitive tasks that involve visual and/or auditory stimuli should be considered because both types of sensory input are encountered during sport participation. Once the athlete demonstrates proficiency in low-demand motor tasks with low-demand perceptual/cognitive tasks, the athlete can be presented with high-demand motor tasks, such as jumping. When the motor task is performed appropriately, a perceptual/cognitive task may be added (e.g., the athlete adds the last 2 numbers seen in order to challenge working memory or calls out numbers written on balls tossed to the athlete). If intervention is needed, feedback to encourage explicit learning may be used in this advanced phase. The next progression includes sport-specific motor tasks (e.g., performing a lay-up) and perceptual/cognitive tasks (e.g., performing a lay-up with a defender) that are similar to what the athlete will encounter during sport participation.
It may also be advantageous to incorporate resistance training into rehabilitation to facilitate better motor output.89 This runs somewhat counter to current trends, which emphasize the use of aerobic activity.1 However, resistance training induces neuroplastic changes in the motor cortex, such as an increase in corticospinal excitability, a decrease in corticospinal inhibition, and an increase in intra-cortical facilitation,90,91 which could address cortical deficits after SRC. Moreover, having the athlete perform resistance training with auditory cues from a metronome could increase corticospinal excitability and decrease intra-cortical inhibition more than self-paced resistance training, thus possibly helping to improve motor skill performance over time.92 At this time, there is no guidance on appropriate timing and dosage for resistance training after SRC. It is reasonable that resistance training could be initiated in a controlled, graded fashion as the athlete approaches return to sport participation and can tolerate demanding physical and perceptual/cognitive challenges.
Once an athlete shows proficiency with motor tasks combined with perceptual/cognitive tasks in the clinical setting and fulfills other return to sport criteria, a return to sport continuum can be initiated.93 A return to sport continuum allows for gradual, progressive exposure to sport activities before participating in full competition.93 Limits are initially placed on the intensity, duration, and contact nature of sport participation. This may be particularly important for athletes with protracted recovery after SRC in which deconditioning could also contribute to the risk of musculoskeletal injury. If an athlete displays poor motor or perceptual/cognitive performance at any point during the return to sport continuum, the athlete would be given contextual drills to facilitate better motor control before continuing to progress toward the intensity and duration of sport participation.
7. Summary and future directions
This review presents a conceptual framework that links impairments in motor control after SRC to an increased risk for musculoskeletal injury. The evidence for motor control impairments after SRC includes neurobiological changes in the central nervous system and altered motor function that persist beyond the time of return to sport. A clinical implication of the conceptual framework is a need to intentionally assess for and treat motor control impairments after SRC to mitigate musculoskeletal injury risk. One way motor control could be assessed is by observing a motor task being performed with or without a perceptual/cognitive task. It is expected that many athletes with SRC will demonstrate motor task performance deficits that indicate a need for rehabilitation, and this may require a change in practice if supervised rehabilitation is currently offered only to athletes with persistent symptomology. In addition, motor control may need to be assessed even after the return to sport continuum is initiated because treatment clinics do not fully replicate the demands of sport participation and because motor learning can attenuate over time.94,95
Additional research is needed on the conceptual framework and SRC clinical management approach suggested in our review. Foremost, research is needed to directly confirm a link between motor control impairment and musculoskeletal injury in athletes with SRC. This may also include examining whether the motor control impairment results from an inability to perceive sensory cues, difficulty processing sensory cues, or challenges in motor planning or motor learning related to cognitive deficits, as demonstrated in a recent study.96 Since the suggested SRC clinical management approach is more resource intensive than current approaches, it would be advantageous to identify subgroups of athletes with SRC who are at the greatest risk for subsequent musculoskeletal injury. Similarly, further investigation is needed to identify which motor tasks and measures are predictive of musculoskeletal injury risk in order to give clinicians tools for monitoring rehabilitation progression, including return to sport clearance. It may be of benefit to explore motor tasks involving the upper extremities for athletes who participate in sports with high upper extremity demands. Finally, the risk-reductive and performance-enhancing effects of SRC rehabilitation targeting motor control need to be examined, as well as comparing motor control outcomes in athletes who do or do not participate in such rehabilitation.
Authors’ contributions
TLC contributed to the creation of the conceptual model, conception of the manuscript, literature review, and the writing of the manuscript; JT assisted with the conception of the manuscript, literature review, and writing of the manuscript; SS assisted with the literature review and writing of the manuscript; MH provided content expertise in athlete rehabilitation and return to sport decision-making and contributed to writing of the manuscript; DSR provided content expertise related to motor control and rehabilitation of motor control impairments and contributed to writing of the manuscript; RMB provided content expertise in neurophysiological testing and impairments after concussion and contributed to writing of the manuscript; JRC provided content expertise in concussion management and contributed to writing of the manuscript; DCH contributed to the creation of the conceptual model, conception of the manuscript, and writing of the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Appendix. Supplementary materials
References
- 1.McCrory P, Meeuwisse W, Dvořák J. Consensus statement on concussion in sport–the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 2.Harmon KG, Clugston JR, Dec K. American Medical Society for Sports Medicine position statement on concussion in sport. Br J Sports Med. 2019;53:213–225. doi: 10.1136/bjsports-2018-100338. [DOI] [PubMed] [Google Scholar]
- 3.Mucha A, Trbovich A. Considerations for diagnosis and management of concussion. J Orthop Sports Phys Ther. 2019;49:787–798. doi: 10.2519/jospt.2019.8855. [DOI] [PubMed] [Google Scholar]
- 4.Schneider KJ, Leddy JJ, Guskiewicz KM. Rest and treatment/rehabilitation following sport-related concussion: a systematic review. Br J Sports Med. 2017;51:930–934. doi: 10.1136/bjsports-2016-097475. [DOI] [PubMed] [Google Scholar]
- 5.Leddy JJ, Haider MN, Ellis MJ. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr. 2019;173:319–325. doi: 10.1001/jamapediatrics.2018.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haider MN, Leddy JJ, Pavlesen S. A systematic review of criteria used to define recovery from sport-related concussion in youth athletes. Br J Sports Med. 2018;52:1179–1190. doi: 10.1136/bjsports-2016-096551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kontos AP, Elbin RJ, Sufrinko A, Marchetti G, Holland CL, Collins MW. Recovery following sport-related concussion: integrating pre- and postinjury factors into multidisciplinary care. J Head Trauma Rehabil. 2019;34:394–401. doi: 10.1097/HTR.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 8.Lynall RC, Mauntel TC, Pohlig RT. Lower extremity musculoskeletal injury risk after concussion recovery in high school athletes. J Athl Train. 2017;52:1028–1034. doi: 10.4085/1062-6050-52.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman DC, Jones D, Harrison A. Concussion may increase the risk of subsequent lower extremity musculoskeletal injury in collegiate athletes. Sports Med. 2017;47:1003–1010. doi: 10.1007/s40279-016-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McPherson AL, Nagai T, Webster KE, Hewett TE. Musculoskeletal injury risk after sport-related concussion: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1754–1762. doi: 10.1177/0363546518785901. [DOI] [PubMed] [Google Scholar]
- 11.Brooks MA, Peterson K, Biese K, Sanfilippo J, Heiderscheit BC, Bell DR. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742–747. doi: 10.1177/0363546515622387. [DOI] [PubMed] [Google Scholar]
- 12.Fino PC, Becker LN, Fino NF, Griesemer B, Goforth M, Brolinson PG. Effects of recent concussion and injury history on instantaneous relative risk of lower extremity injury in Division I collegiate athletes. Clin J Sport Med. 2019;29:218–223. doi: 10.1097/JSM.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 13.Nordström A, Nordström P, Ekstrand J. Sports-related concussion increases the risk of subsequent injury by about 50% in elite male football players. Br J Sports Med. 2014;48:1447–1450. doi: 10.1136/bjsports-2013-093406. [DOI] [PubMed] [Google Scholar]
- 14.Howell DR, Lynall RC, Buckley TA, Herman DC. Neuromuscular control deficits and the risk of subsequent injury after a concussion: a scoping review. Sports Med. 2018;48:1097–1115. doi: 10.1007/s40279-018-0871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eagle SR, Kontos AP, Pepping GJ. Increased risk of musculoskeletal injury following sport-related concussion: a perception-action coupling approach. Sports Med. 2020;50:15–23. doi: 10.1007/s40279-019-01144-3. [DOI] [PubMed] [Google Scholar]
- 16.Wilkerson GB, Grooms DR, Acocello SN. Neuromechanical considerations for postconcussion musculoskeletal injury risk management. Curr Sports Med Rep. 2017;16:419–427. doi: 10.1249/JSR.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 17.Avedesian JM, Covassin T, Dufek JS. The influence of sport-related concussion on lower extremity injury risk: a review of current return-to-play practices and clinical implications. Int J Exer Sci. 2020;13:873–889. doi: 10.70252/WVYL1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Low M. A time to reflect on motor control in musculoskeletal physical therapy. J Orthop Sports Phys Ther. 2018;48:833–836. doi: 10.2519/jospt.2018.0614. [DOI] [PubMed] [Google Scholar]
- 19.Latash M. Elsevier; Amsterdam: 2012. Fundamentals of motor control. [Google Scholar]
- 20.Adams J. Historical review and appraisal of research on the learning, retention, and transfer of human motor skills. Psychol Bull. 1987;101:41–74. [Google Scholar]
- 21.Shumway-Cook A, Woollacott MH. 5th ed. Wolters Kluwer; Philadelphia, PA: 2017. Motor control: translating research into clinical practice. [Google Scholar]
- 22.Schmidt R, Lee T. 4th ed. Human Kinetics; Champaign, IL: 2005. Motor control and learning: a behavioral emphasis. [Google Scholar]
- 23.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- 24.Krakauer JW, Mazzoni P. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol. 2011;21:636–644. doi: 10.1016/j.conb.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Day KA, Leech KA, Roemmich RT, Bastian AJ. Accelerating locomotor savings in learning: compressing four training days to one. J Neurophysiol. 2018;119:2100–2113. doi: 10.1152/jn.00903.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDougle SD, Ivry RB, Taylor JA. Taking aim at the cognitive side of learning in sensorimotor adaptation tasks. Trends Cogn Sci. 2016;20:535–544. doi: 10.1016/j.tics.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleynen M, Braun SM, Bleijlevens MH. Using a Delphi technique to seek consensus regarding definitions, descriptions and classification of terms related to implicit and explicit forms of motor learning. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durham K, Van Vliet PM, Badger F, Sackley C. Use of information feedback and attentional focus of feedback in treating the person with a hemiplegic arm. Physiother Res Int. 2009;14:77–90. doi: 10.1002/pri.431. [DOI] [PubMed] [Google Scholar]
- 29.Urquhart JR, Skidmore ER. Guided and directed cues: developing a standardized coding scheme for clinical practice. OTJR (Thorofare N J) 2014;34:202–208. doi: 10.3928/15394492-20141006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma DA, Chevidikunnan MF, Khan FR, Gaowgzeh RA. Effectiveness of knowledge of result and knowledge of performance in the learning of a skilled motor activity by healthy young adults. J Phys Ther Sci. 2016;28:1482–1486. doi: 10.1589/jpts.28.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21:628–633. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor JA, Ivry RB. Cerebellar and prefrontal cortex contributions to adaptation, strategies, and reinforcement learning. Prog Brain Res. 2014;210:217–253. doi: 10.1016/B978-0-444-63356-9.00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haith AM, Krakauer JW. Model-based and model-free mechanisms of human motor learning. Adv Exp Med Biol. 2013;782:1–21. doi: 10.1007/978-1-4614-5465-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119:1199–1211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- 35.Swanik CB, Covassin T, Stearne DJ, Schatz P. The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. Am J Sports Med. 2007;35:943–948. doi: 10.1177/0363546507299532. [DOI] [PubMed] [Google Scholar]
- 36.Wilkerson G. Neurocognitive reaction time predicts lower extremity sprains and strains. Int J Athl Ther Trai. 2012;17:4–9. [Google Scholar]
- 37.McDonald AA, Wilkerson GB, McDermott BP, Bonacci JA. Risk factors for initial and subsequent core or lower extremity sprain or strain among collegiate football players. J Athl Train. 2019;54:489–496. doi: 10.4085/1062-6050-152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herman DC, Barth JT. Drop-jump landing varies with baseline neurocognition: implications for anterior cruciate ligament injury risk and prevention. Am J Sports Med. 2016;44:2347–2353. doi: 10.1177/0363546516657338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter K, Quintana C, Hoch M. The relationship between neurocognitive function and biomechanics: a critically appraised topic. J Sport Rehabil. 2021;30:327–332. doi: 10.1123/jsr.2020-0103. [DOI] [PubMed] [Google Scholar]
- 40.Howell DR, Buckley TA, Lynall RC, Meehan WP., 3rd Worsening dual-task gait costs after concussion and their association with subsequent sport-related injury. J Neurotrauma. 2018;35:1630–1636. doi: 10.1089/neu.2017.5570. [DOI] [PubMed] [Google Scholar]
- 41.Oldham JR, Howell DR, Knight CA, Crenshaw JR, Buckley TA. Gait performance is associated with subsequent lower extremity injury following concussion. Med Sci Sports Exerc. 2020;52:2279–2285. doi: 10.1249/MSS.0000000000002385. [DOI] [PubMed] [Google Scholar]
- 42.Buckley TA, Howard CM, Oldham JR, Lynall RC, Swanik CB, Getchell N. No clinical predictors of postconcussion musculoskeletal injury in college athletes. Med Sci Sports Exerc. 2020;52:1256–1262. doi: 10.1249/MSS.0000000000002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu YC, Harezlak J, Elsaid NMH. Longitudinal white-matter abnormalities in sports-related concussion: a diffusion MRI study. Neurology. 2020;95:e781–e792. doi: 10.1212/WNL.0000000000009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chamard E, Lassonde M, Henry L. Neurometabolic and microstructural alterations following a sports-related concussion in female athletes. Brain Inj. 2013;27:1038–1046. doi: 10.3109/02699052.2013.794968. [DOI] [PubMed] [Google Scholar]
- 45.Chamard E, Lefebvre G, Lassonde M, Theoret H. Long-term abnormalities in the corpus callosum of female concussed athletes. J Neurotrauma. 2016;33:1220–1226. doi: 10.1089/neu.2015.3948. [DOI] [PubMed] [Google Scholar]
- 46.McAllister TW, Ford JC, Flashman LA. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82:63–69. doi: 10.1212/01.wnl.0000438220.16190.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt J, Hayward KS, Brown KE. Imaging in pediatric concussion: a systematic review. Pediatrics. 2018;141 doi: 10.1542/peds.2017-3406. [DOI] [PubMed] [Google Scholar]
- 48.Khong E, Odenwald N, Hashim E, Cusimano MD. Diffusion tensor imaging findings in post-concussion syndrome patients after mild traumatic brain injury: a systematic review. Front Neurol. 2016;7:156. doi: 10.3389/fneur.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asken BM, DeKosky ST, Clugston JR, Jaffee MS, Bauer RM. Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport-related mild traumatic brain injury (mTBI): a systematic critical review. Brain Imaging Behav. 2018;12:585–612. doi: 10.1007/s11682-017-9708-9. [DOI] [PubMed] [Google Scholar]
- 50.Kinnunen KM, Greenwood R, Powell JH. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hellewell SC, Nguyen VPB, Jayasena RN, Welton T, Grieve SM. Characteristic patterns of white matter tract injury in sport-related concussion: an image based meta-analysis. Neuroimage Clin. 2020;26:102253. doi: 10.1016/j.nicl.2020.102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Major BP, Rogers MA, Pearce AJ. Using transcranial magnetic stimulation to quantify electrophysiological changes following concussive brain injury: a systematic review. Clin Exp Pharmacol Physiol. 2015;42:394–405. doi: 10.1111/1440-1681.12363. [DOI] [PubMed] [Google Scholar]
- 53.Ledwidge PS, Molfese DL. Long-term effects of concussion on electrophysiological indices of attention in varsity college athletes: an event-related potential and standardized low-resolution brain electromagnetic tomography approach. J Neurotrauma. 2016;33:2081–2090. doi: 10.1089/neu.2015.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore RD, Hillman CH, Broglio SP. The persistent influence of concussive injuries on cognitive control and neuroelectric function. J Athl Train. 2014;49:24–35. doi: 10.4085/1062-6050-49.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henry LC, Tremblay J, Tremblay S. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28:2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- 56.De Beaumont L, Lassonde M, Leclerc S, Théoret H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery. 2007;61:329–336. doi: 10.1227/01.NEU.0000280000.03578.B6. [DOI] [PubMed] [Google Scholar]
- 57.De Beaumont L, Mongeon D, Tremblay S. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011;46:234–240. doi: 10.4085/1062-6050-46.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearce AJ, Hoy K, Rogers MA. The long-term effects of sports concussion on retired Australian football players: a study using transcranial magnetic stimulation. J Neurotrauma. 2014;31:1139–1145. doi: 10.1089/neu.2013.3219. [DOI] [PubMed] [Google Scholar]
- 59.Powers KC, Cinelli ME, Kalmar JM. Cortical hypoexcitability persists beyond the symptomatic phase of a concussion. Brain Inj. 2014;28:465–471. doi: 10.3109/02699052.2014.888759. [DOI] [PubMed] [Google Scholar]
- 60.Reed N, Taha T, Monette G, Keightley M. A preliminary exploration of concussion and strength performance in youth ice hockey players. Int J Sports Med. 2016;37:708–713. doi: 10.1055/s-0042-104199. [DOI] [PubMed] [Google Scholar]
- 61.Schneider KJ, Meeuwisse WH, Palacios-Derflingher L, Emery CA. Changes in measures of cervical spine function, vestibulo-ocular reflex, dynamic balance, and divided attention following sport-related concussion in elite youth ice hockey players. J Orthop Sports Phys Ther. 2018;48:974–981. doi: 10.2519/jospt.2018.8258. [DOI] [PubMed] [Google Scholar]
- 62.Engelman G, Carry P, Sochanska A, Daoud AK, Wilson J, Provance A. Isometric cervical muscular strength in pediatric athletes with multiple concussions. Clin J Sport Med. 2021;36:36–41. doi: 10.1097/JSM.0000000000000681. [DOI] [PubMed] [Google Scholar]
- 63.Eagle SR, Kontos AP, Mi Q. Shared neuromuscular performance traits in military personnel with prior concussion. Med Sci Sports Exerc. 2019;51:1619–1625. doi: 10.1249/MSS.0000000000001974. [DOI] [PubMed] [Google Scholar]
- 64.Dubose DF, Herman DC, Jones DL. Lower extremity stiffness changes after concussion in collegiate football players. Med Sci Sports Exerc. 2017;49:167–172. doi: 10.1249/MSS.0000000000001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butler RJ, Crowell HP, 3rd, Davis IM. Lower extremity stiffness: implications for performance and injury. Clin Biomech (Bristol, Avon) 2003;18:511–517. doi: 10.1016/s0268-0033(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 66.Brazier J, Maloney S, Bishop C, Read PJ, Turner AN. Lower extremity stiffness: considerations for testing, performance enhancement, and injury risk. J Strength Cond Res. 2019;33:1156–1166. doi: 10.1519/JSC.0000000000002283. [DOI] [PubMed] [Google Scholar]
- 67.Lynall RC, Blackburn JT, Guskiewicz KM, Marshall SW, Plummer P, Mihalik JP. Reaction time and joint kinematics during functional movement in recently concussed individuals. Arch Phys Med Rehabil. 2018;99:880–886. doi: 10.1016/j.apmr.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Lapointe AP, Nolasco LA, Sosnowski A. Kinematic differences during a jump cut maneuver between individuals with and without a concussion history. Int J Psychophysiol. 2018;132:93–98. doi: 10.1016/j.ijpsycho.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Cavanaugh JT, Guskiewicz KM, Stergiou N. A nonlinear dynamic approach for evaluating postural control: new directions for the management of sport-related cerebral concussion. Sports Med. 2005;35:935–950. doi: 10.2165/00007256-200535110-00002. [DOI] [PubMed] [Google Scholar]
- 70.McCrea M, Barr WB, Guskiewicz K. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11:58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 71.Murray N, Salvatore A, Powell D, Reed-Jones R. Reliability and validity evidence of multiple balance assessments in athletes with a concussion. J Athl Train. 2014;49:540–549. doi: 10.4085/1062-6050-49.3.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lynall RC, Campbell KR, Mauntel TC, Blackburn JT, Mihalik JP. Single-legged hop and single-legged squat balance performance in recreational athletes with a history of concussion. J Athl Train. 2020;55:488–493. doi: 10.4085/1062-6050-185-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Servatius RJ, Spiegler KM, Handy JD, Pang KCH, Tsao JW, Mazzola CA. Neurocognitive and fine motor deficits in asymptomatic adolescents during the subacute period after concussion. J Neurotrauma. 2018;35:1008–1014. doi: 10.1089/neu.2017.5314. [DOI] [PubMed] [Google Scholar]
- 74.Van Pelt KL, Lapointe AP, Galdys MC, Dougherty LA, Buckley TA, Broglio SP. Evaluating performance of National Hockey League players after a concussion versus lower body injury. J Athl Train. 2019;54:534–540. doi: 10.4085/1062-6050-218-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parker TM, Osternig LR, Lee HJ, van Donkelaar P, Chou LS. The effect of divided attention on gait stability following concussion. Clin Biomech (Bristol, Avon) 2005;20:389–395. doi: 10.1016/j.clinbiomech.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Howell DR, Kirkwood MW, Provance A, Iverson GL, Meehan WP., 3rd Using concurrent gait and cognitive assessments to identify impairments after concussion: a narrative review. Concussion. 2018;3:CNC54. doi: 10.2217/cnc-2017-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fino PC, Parrington L, Pitt W. Detecting gait abnormalities after concussion or mild traumatic brain injury: a systematic review of single-task, dual-task, and complex gait. Gait Posture. 2018;62:157–166. doi: 10.1016/j.gaitpost.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 78.Wilkerson GB, Nabhan DC, Prusmack CJ, Moreau WJ. Detection of persisting concussion effects on neuromechanical responsiveness. Med Sci Sports Exerc. 2018;50:1750–1756. doi: 10.1249/MSS.0000000000001647. [DOI] [PubMed] [Google Scholar]
- 79.Wilkerson GB, Nabhan DC, Crane RT. Concussion history and neuromechanical responsiveness asymmetry. J Athl Train. 2020;55:594–600. doi: 10.4085/1062-6050-0401.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quatman-Yates CC, Hunter-Giordano A, Shimamura KK. Physical therapy evaluation and treatment after concussion/mild traumatic brain injury. J Orthop Sports Phys Ther. 2020;50:CPG1–CP73. doi: 10.2519/jospt.2020.0301. [DOI] [PubMed] [Google Scholar]
- 81.Howell DR, Buckley TA, Berkstresser B, Wang F, Meehan WP., 3rd Identification of post-concussion dual-task gait abnormalities using normative reference values. J Appl Biomech. 2019;35:290–296. doi: 10.1123/jab.2018-0454. [DOI] [PubMed] [Google Scholar]
- 82.Howell DR, Oldham JR, DiFabio M. Single-task and dual-task gait among collegiate athletes of different sport classifications: implications for concussion management. J Appl Biomech. 2017;33:24–31. doi: 10.1123/jab.2015-0323. [DOI] [PubMed] [Google Scholar]
- 83.Myer GD, Ford KR, Hewett TE. New method to identify athletes at high risk of ACL injury using clinic-based measurements and freeware computer analysis. Br J Sports Med. 2011;45:238–244. doi: 10.1136/bjsm.2010.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Padua DA, Marshall SW, Boling MC, Thigpen CA, Garrett WE, Jr, Beutler AI. The Landing Error Scoring System (LESS) is a valid and reliable clinical assessment tool of jump-landing biomechanics: the JUMP-ACL study. Am J Sports Med. 2009;37:1996–2002. doi: 10.1177/0363546509343200. [DOI] [PubMed] [Google Scholar]
- 85.Dos'Santos T, McBurnie A, Donelon T, Thomas C, Comfort P, Jones PA. A qualitative screening tool to identify athletes with “high-risk” movement mechanics during cutting: the Cutting Movement Assessment Score (CMAS) Phys Ther Sport. 2019;38:152–161. doi: 10.1016/j.ptsp.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Ressman J, Grooten WJA, Rasmussen Barr E. Visual assessment of movement quality in the single leg squat test: a review and meta-analysis of inter-rater and intrarater reliability. BMJ Open Sport Exerc Med. 2019;5 doi: 10.1136/bmjsem-2019-000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Magill R, Anderson D. McGraw-Hill Education; New York, NY: 2017. Motor learning and control: concepts and applications. [Google Scholar]
- 88.Kisner C, Colby LA. F.A. Davis & Company; Philadelphia, PA: 2012. Therapeutic exercise: foundations and techniques; pp. 157–232. [Google Scholar]
- 89.Griffin L, Cafarelli E. Resistance training: cortical, spinal, and motor unit adaptations. Can J Appl Physiol. 2005;30:328–340. doi: 10.1139/h05-125. [DOI] [PubMed] [Google Scholar]
- 90.Mason J, Frazer AK, Avela J, Pearce AJ, Howatson G, Kidgell DJ. Tracking the corticospinal responses to strength training. Eur J Appl Physiol. 2020;120:783–798. doi: 10.1007/s00421-020-04316-6. [DOI] [PubMed] [Google Scholar]
- 91.Siddique U, Rahman S, Frazer AK, Pearce AJ, Howatson G, Kidgell DJ. Determining the sites of neural adaptations to resistance training: a systematic review and meta-analysis. Sports Med. 2020;50:1107–1128. doi: 10.1007/s40279-020-01258-z. [DOI] [PubMed] [Google Scholar]
- 92.Leung M, Rantalainen T, Teo WP, Kidgell D. The corticospinal responses of metronome-paced, but not self-paced strength training are similar to motor skill training. Eur J Appl Physiol. 2017;117:2479–2492. doi: 10.1007/s00421-017-3736-4. [DOI] [PubMed] [Google Scholar]
- 93.Ardern CL, Glasgow P, Schneiders A. 2016 consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br J Sports Med. 2016;50:853–864. doi: 10.1136/bjsports-2016-096278. [DOI] [PubMed] [Google Scholar]
- 94.DiStefano LJ, Marshall SW, Padua DA. The effects of an injury prevention program on landing biomechanics over time. Am J Sports Med. 2016;44:767–776. doi: 10.1177/0363546515621270. [DOI] [PubMed] [Google Scholar]
- 95.Padua DA, DiStefano LJ, Marshall SW, Beutler AI, de la Motte SJ, DiStefano MJ. Retention of movement pattern changes after a lower extremity injury prevention program is affected by program duration. Am J Sports Med. 2012;40:300–306. doi: 10.1177/0363546511425474. [DOI] [PubMed] [Google Scholar]
- 96.Eagle SR, Kontos AP, Sinnott A. Utility of a novel perceptual-motor control test for identification of sport-related concussion beyond current clinical assessments. J Sports Sci. 2020;38:1799–1805. doi: 10.1080/02640414.2020.1756675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.