Abstract
Introduction
Levels of medical mistrust have historically been higher among racial/ethnic minority patients compared to whites, largely owing to societal and health system inequities and history of discrimination or experimentation. However, recently trust in physicians has declined in the United States in general. We investigated trust in physicians among a large cohort of cancer patients residing in Texas.
Methods
A sample of recently diagnosed cancer patients in Texas were identified from the Texas Cancer Registry with 1344 patients returning surveys between March 2017 and March 2020. The multi-scale inventory was mailed to each individual and included the Trust in the Medical Profession Scale which assesses levels of agreement with 11 trust-related statements. Multivariable linear regression models were constructed to assess the adjusted relationship between trust in medical profession aggregate score and socio-demographic and clinical factors.
Results
A total of 1250 surveys were evaluable for trust in medical profession. The mean aggregate trust score for all patients was 37.3 (95% CI 36.8–37.7). Unadjusted trust scores were higher for Hispanic (40.5) and black (38.2) respondents compared to white (36.4) (p<.001). Multivariable analyses showed white, younger, more-educated, or those with lower levels of self-reported health estimated toward lower adjusted scores for trust in medical profession.
Conclusion
We observed relatively higher levels of medical mistrust among white, younger, more educated individuals with cancer or those with poorer health. While the relatively higher trust among minority individuals is encouraging, these findings raise the possibility that recent societal trends toward mistrust in science may have implications for cancer care.
Keywords: Mistrust, trust, cancer, race
Introduction
Trust is the cornerstone of a healthy patient-physician relationship. A patient trusts that a physician works with competence, integrity, and with the patient’s best interest in mind, while a physician trusts their recommendations will be followed1,2. Trust in intrinsically linked to vulnerability, and becomes increasingly important in moments of greatest vulnerability2,3. And few medical events evoke more vulnerability—and thus a greater opportunity for trust—than a diagnosis of cancer, where patients are often faced with sudden life-altering news, physical or emotional pain, complex treatment recommendations, and an uncertain prognosis4.
Trust in physicians has dropped dramatically in the United States (US) over the last several decades, with one poll showing 80% of patients trusting medical institutions in 1975 compared to just 37% in 20155. With emerging alternative sources of authority and information from the internet or social media, feelings of disconnect with the corporatization and growth of large medical institutions, and transition to electronic health platforms, patients may be left wondering in whom or what they should place their trust6–8. Mistrust in medical institutions may lead to less continuity of primary care, more emergency room visits, and increase cost and resource utilization9,10, may stifle innovation through decreased clinical trial enrollment11 and, most importantly, is associated with poor adherence to physician recommendations and worse health outcomes12–14.
Medical mistrust varies by race and ethnicity, with numerous studies demonstrating higher levels of mistrust among black and Hispanic patients compared to whites14–17. This mistrust has been associated with poor adherence to cancer screening guidelines in breast18, colorectal19,20, and prostate cancer21, the underuse of recommended adjuvant breast radiation therapy22, and may contribute to disproportionally high rates of late-stage cancer diagnoses and cancer mortality among racial and ethnic minority groups23–25. Less is known about how medical mistrust varies by education level, patient age, patient health, or socioeconomic status.
Here we sought to evaluate trust in physicians generally (as opposed to trust in a specific physician) among a large and diverse cohort of cancer patients residing in the state of Texas. We explore variation in trust by various patient characteristics, and hypothesize that trust will vary by age, education level, and rurality. Moreover, we hypothesized that, in line with prior studies, trust in medical providers would be lower among non-white racial and ethnic minority groups.
Methods
Data source and study sample, and survey procedure
This study recruited individuals from the state-wide population-based Texas Cancer Registry (TCR) (https://www.dshs.state.tx.us/tcr/). TCR is the 4th largest cancer registry in the United states and meets the data standards of the National Program of the Central Cancer Registries and the Centers for Disease Control and is also Gold Certified by the North American Association of Central Cancer Registries. In 2020 the registry was also accepted as a member of the Surveillance, Epidemiology, and End Results program. We obtained a random sample of 6222 Texas residents ≥ 18 years of age within 12 months of their diagnosis of any solid tumor malignancy, of which 6203 were deemed eligible and to whom a survey packet (described below) was mailed. Eighty-two individuals were subsequently found to be deceased, and 516 surveys were undeliverable. Of the remaining located 5605 individuals, 1344 returned the survey packet for a response rate of 24%. Attempts to follow up by mail and/or telephone were made for all non-responders. We included follow-up telephone call early in the study, however the yield was very low (<10%). Initially (through the first approximately 2300 potential participants sent from TCR), if no response was received from an individual to whom a survey was mailed, we sent 3 follow-up reminders (one at 2 weeks, 4–6 weeks, and 8–10 weeks, respectively. During the course of this project’s data collection efforts, the Texas Cancer Registry/Texas Department of State Health Services changed their policy regarding the number of attempted contacts that could be made to potential study participants and we limited contacts accordingly to a single follow-up reminder at 8–10 weeks. The response rates as approximately 25% with either 1 versus 3 mail-out follow up attempts.
This survey study is part (Project 4) of the larger multi-year CERCIT (Comparative Effectiveness Research on Cancer in Texas) (https://www.utmb.edu/scoa/research/supported-research-programs/comparative-effectiveness-research-on-cancer-in-texas/current-projects) program, funded by the Cancer Prevention and Research Institute of Texas (CPRIT). The CERCIT Project 4 survey packet included a multi-scale, 117-item inventory including: medical mistrust, end of life preferences, decision self-efficacy, health literacy, functional health status, and self-reported socio-demographic items. The focus of this investigation is the medical mistrust scale among respondents who filled out that section of the survey (n=1250). The mailed survey itself was available only in English. Individuals with Spanish surnames were sent a recruitment cover letter in both English and Spanish and it was indicated in the letter that a family member could translate the English survey to the respondent and return the survey. Prior to mailing the survey packet, we contacted the patients’ physicians to ensure no objections to their respective patient’s participation in the study. No objections from patients’ physicians were received. All data were collected between March 2017 and March 2020 and study procedures were approved by both the Institutional Review Boards of both MD Anderson and the Texas Department of State Health Services.
Measurement of medical mistrust
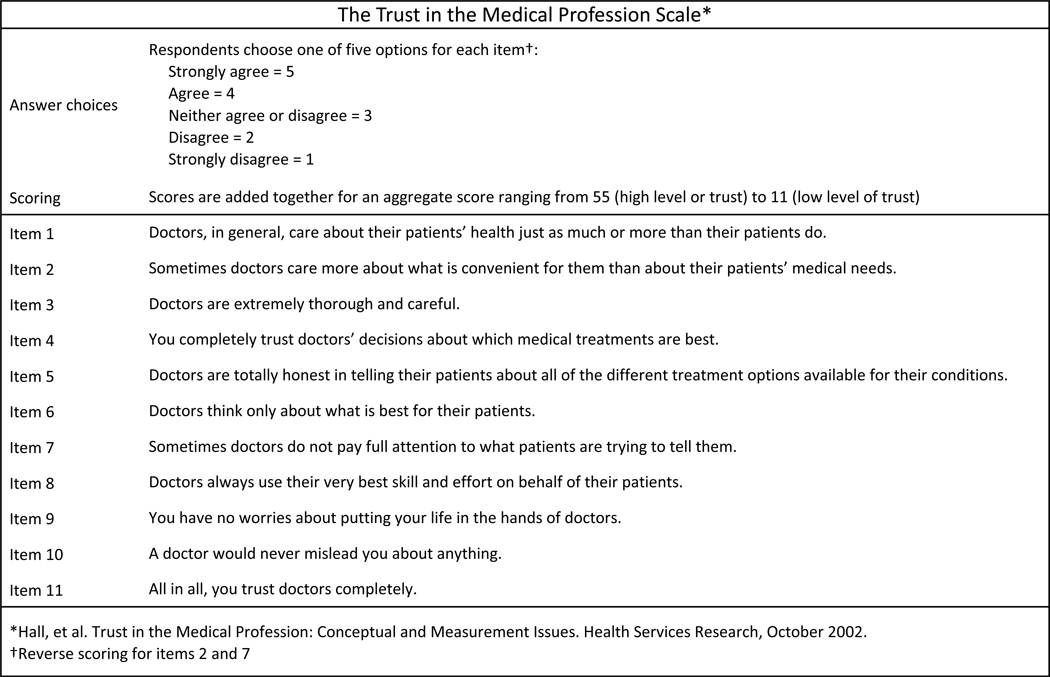
Trust in physicians was measured using the Trust in the Medical Profession Scale. Designed and validated by Hall and colleagues26, this survey uses a 5-level Likert scale to assess levels of agreement with 11 trust-related statements (Figure 1). An aggregate score is obtained by summing the 11 individual scores. The survey aims to assess general trust in physicians/medical professionals (versus trust in a specific physician) and represents four domains of general trust (fidelity, competence, honesty, and global trust). It has shown high reliability (Cronbach’s alpha co-efficient 0.89) and response variability (range 11–54, mean 33.5, standard deviation 6.9)26. Other measures of medical mistrust exist, one common example being the Group Based Medical Mistrust Scale (GBMMS)27. The GBMMS assesses the tendency to mistrust those outside of one’s own ethnic group and includes several items prefaced as “People of my ethnic group…”18. Here, however, we sought to assess an individual’s trust in physicians generally and did not want to attune the respondent to race or ethnicity as the perspective from which to respond to our trust measure. Of the 1272 returned surveys, 1217 (95.7%) had completed all 11 items of the Trust in the Medical Profession Scale while 29 (2.3%) were missing 1 item, 4 (0.3%) were missing 2 items, and 22 (1.7%) were missing 3 or more items. The aggregate Trust in the Medical Profession Scale score allows for 1 or 2 missing items (with average score imputed for missing values), but not for 3 or more26. As such, a total of 1250 surveys were evaluable for level of medical mistrust.
Figure 1:
Trust in the Medical Profession Scale is an 11-item assessment of trust in physicians.
Sociodemographic, clinical and health status variables
Sociodemographic and cancer clinical characteristics were obtained from both TCR registry data as well as self-reported information. Race/ethnicity was self-reported or, in cases of missing self-reported values (63 of 1250 individuals), obtained from TCR registry. Only 63 of 1250 individuals (5%) had not self-reported race/ethnicity, and were thus identified by TCR data. Agreement between TCR race/ethnicity data for remaining 1187 was high with 94% agreement on ethnicity and 98% agreement on race. Race and ethnicity groups are non-Hispanic white, non-Hispanic black, and Hispanic. The term “non-Hispanic” designation is dropped for remainder of manuscript for simplicity. Items were included for age, gender primary language spoken in the home, income, marital status, whether the individual lived alone or with others, and highest attained education level. Health-related quality of life measures were collected with the Medicare Health Outcomes Survey,28 which assesses self-reported health by asking respondents to rate their health on a 5-point scale from poor to excellent. Rurality and Texas Health Service Region (Supplemental Figure 1) were derived by zip code at the time of diagnosis from cancer. Rurality was defined as per the US Department of Agriculture 2013 Rural/Urban Continuum Codes (http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx), with codes 1–3 considered urban and codes 4–9 considered rural. Cancer type and stage at diagnosis were obtained from the TCR database.
Statistical Analysis
We conducted one-way analysis of variance (ANOVA) tests to examine the significance of association of Trust in Medical the Profession aggregate score with respondent sociodemographic and clinical variables (Table 1). We included 95% confidence intervals (CI) and standard deviations (SD) to facilitate comparisons of differences among the groups in the unadjusted analyses. Multivariable linear regression models were constructed to assess the adjusted relationship between trust in physicians aggregate score and socio-demographic and clinical factors. Linear regression assumptions were checked and verified not to be violated for all multiple linear regression analyses. Variance inflation factors were checked for collinearity. Durbin-Watson test was conducted for testing correlated residuals. Residual analyses were conducted for testing normality and heteroscedasity. In order to attempt to address potential selection bias in our findings, we performed an inverse probability weighting analysis.29,30 For each observed case (n=1250), we computed the probability of return of the survey from a pool of the 5605 eligible cases. We determined weights based on five factors: age, gender, race/ethnicity, cancer site, health services area in Texas. The inverse probability of response was derived to weight each observation attempting to balance the selection bias due to non-response. Finally, normalized inversed probability (inversed probability divided by the mean) was used in the final weighting analysis. Normalized inverse probability was used to reduce weight loading because weights can increase the standard errors of estimates and introduce instability in the data. Data analyses were performed using SAS (version 9.4 SAS Institute Inc., Cary, NC, USA).
Table 1:
Mean Aggregate Trust Score by Patient Characteristics
| Unweighted | Weighted | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | # pts (%) | Trust score (mean) | SD | p value | Trust score (mean) | SD | p value |
| All cases | 1250 (100) | 37.3 | 8.14 | N/A | 37.4 | 12.17 | N/A |
| Age at diagnosis | 0.027 | 0.006 | |||||
| 40 and under | 80 (6) | 35.5 | 8.08 | 35.4 | 12.98 | ||
| 41–64 | 615 (49) | 37.0 | 8.22 | 37.0 | 12.24 | ||
| 65–79 | 489 (39) | 37.8 | 8.14 | 38.1 | 12.00 | ||
| 80+ | 66 (5) | 38.8 | 6.95 | 39.2 | 10.64 | ||
| Gender | 0.017 | 0.008 | |||||
| Female | 823 (66) | 36.9 | 8.17 | 36.9 | 11.91 | ||
| Male | 427 (34) | 38.1 | 8.03 | 38.2 | 12.57 | ||
| Race/Ethnicity | <0.001 | <.0001 | |||||
| White | 887 (71) | 36.4 | 7.95 | 36.5 | 11.12 | ||
| Black | 134 (11) | 38.2 | 8.98 | 37.5 | 15.29 | ||
| Hispanic | 192 (15) | 40.5 | 7.54 | 40.2 | 12.47 | ||
| Other | 37 (3) | 38.1 | 8.03 | 38.0 | 15.99 | ||
| Language at home | <0.001 | <.0001 | |||||
| English | 1,110 (89) | 36.9 | 8.06 | 36.9 | 11.86 | ||
| Other | 96 (8) | 41.3 | 7.83 | 40.4 | 13.63 | ||
| Unknown | 44 (4) | 39.6 | 8.31 | 40.4 | 12.77 | ||
| Education | <0.001 | <.0001 | |||||
| Some high school or less | 102 (8) | 41.4 | 7.99 | 41.0 | 12.81 | ||
| High school | 221 (18) | 37.3 | 8.65 | 37.2 | 13.64 | ||
| Some college and above | 898 (72) | 36.7 | 7.86 | 36.8 | 11.44 | ||
| Unknown | 29 (2) | 40.9 | 8.49 | 41.8 | 12.47 | ||
| Marital status | 0.479 | 0.081 | |||||
| Married | 820 (66) | 37.5 | 7.94 | 37.8 | 11.78 | ||
| Not married | 407 (33) | 36.9 | 8.47 | 36.7 | 12.83 | ||
| Unknown | 23 (2) | 37.8 | 9.28 | 38.0 | 13.00 | ||
| Living arrangements | 0.171 | 0.021 | |||||
| Alone | 222 (18) | 36.6 | 9.01 | 36.2 | 13.36 | ||
| Not alone | 1010 (81) | 37.4 | 7.93 | 37.6 | 11.85 | ||
| Unknown | 18 (1) | 39.7 | 8.31 | 40.2 | 12.27 | ||
| Income | 0.071 | 0.189 | |||||
| Less than $19,999 | 175 (14) | 38.8 | 8.66 | 38.5 | 13.39 | ||
| $20,000 – $39,999 | 154 (12) | 37.3 | 8.17 | 37.1 | 12.67 | ||
| $40,000 – $99,999 | 398 (32) | 36.7 | 8.05 | 36.9 | 11.74 | ||
| $100,000 or more | 319 (26) | 37.2 | 7.75 | 37.4 | 11.29 | ||
| Unknown | 204 (16) | 37.4 | 8.34 | 37.8 | 12.76 | ||
| Residence | 0.149 | 0.096 | |||||
| Urban | 1080 (86) | 37.1 | 8.2 | 37.3 | 12.24 | ||
| Rural | 170 (14) | 38.1 | 7.75 | 38.4 | 11.61 | ||
| Self-reported health | <0.001 | <.0001 | |||||
| Excellent or very good | 406 (32) | 38.5 | 7.58 | 39.1 | 11.07 | ||
| Good | 515 (41) | 37.6 | 8.07 | 37.6 | 11.94 | ||
| Fair | 252 (20) | 36.0 | 8.19 | 36.0 | 12.58 | ||
| Poor | 70 (6) | 33.5 | 9.91 | 33.7 | 15.04 | ||
Results
A total of 1,250 surveys were evaluable for valid trust in physician aggregate score and are included in the analyses. Respondents were more commonly female (66%), age 41–64 (49%), married (66%), college-educated (72%) and living in an urban location (86%). A total of 71% of respondents were white compared to 11% black and 15% Hispanic (Table 1). This differed from the population make-up of Texas in that the state is 41.2% non-Hispanic white, 12.9% non-Hispanic black, and 39.7% Hispanic. Additionally, 29.3% of Texans have a Bachelor’s degree or higher, and 35.5% of households report a language other than English is spoken at home. 31 We tested for interactions among socio-demographic factors and race/ethnicity to see if there were significant differences among the racial/ethnic subgroups with respect to other characteristics such as age, education level, or self-reported health and found no differences in ethnicity/race by age (p=0.31), by education (p=0.17) or by self-reported health status (p=0.46). The most common primary disease sites were breast (40%) and colorectal (16%), with 58% of respondents having cancer localized to the primary site compared to 24% who had regional extension of their tumor and 7% with distant spread of their disease (Supplemental Table 1).
The mean aggregate trust score for all patients was 37.3 (95% CI: 36.8–37.7, SD: 8.14). Unadjusted analyses showed trust score was significantly higher for older, male, or less-educated respondents, as well as for those with better levels of self-reported health (Table 1). Trust score was also higher for Hispanic (40.5) and black (38.2) respondents compared to white (36.4) (p value < 0.001), as well as for those who primarily spoke a non-English language in the home. Weighted analysis of univariate associations showed similar results to the unweighted analysis with the exception of a change in the significance of association between trust score and status of living alone, not-alone or unknown (Table 1). There was no significant difference in trust score by marital status, income level, rurality, Texas Health Service Region, or stage of disease (Table 1 and Supplemental Table 1). The mean aggregate trust score for sociodemographic factors shown by racial/ethnic subgroup is shown in Supplemental Table 2.
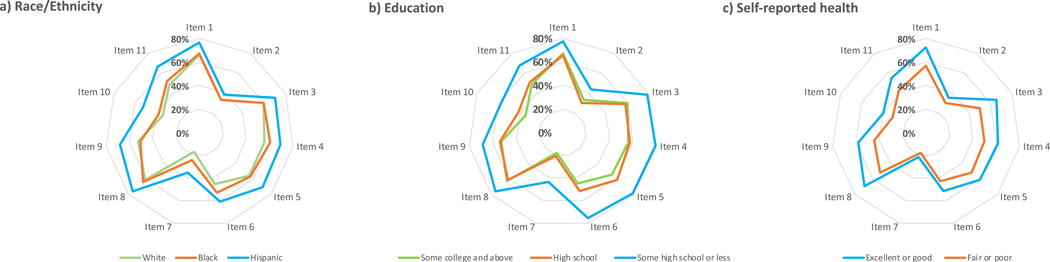
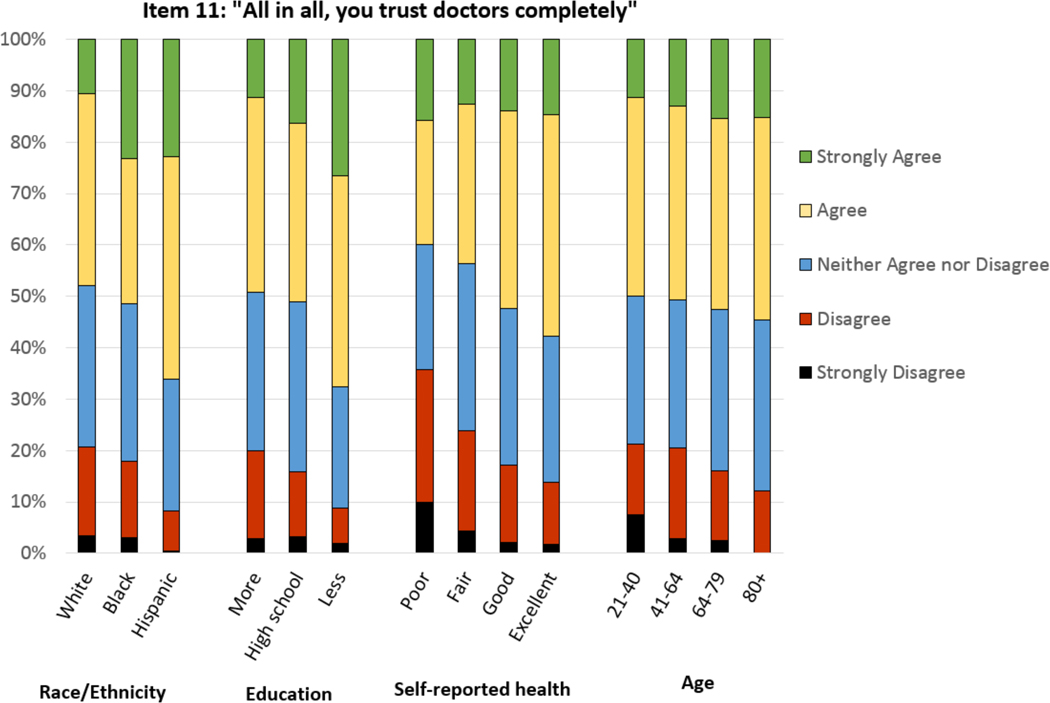
Percent of respondents who agree or strongly agree with each item of the trust survey (items 2 and 7 are reverse-scored) are depicted by radar plot in Figure 2, showing consistently high levels of trust on individual items for Hispanics, for those with less than a high school diploma, and for those with good or excellent self-reported health. A 5-level categorical breakdown for the final item on the Trust in the Medical Profession Scale (“All in all, you trust doctors completely”) again demonstrates higher levels of trust among those who are black or Hispanic, have lower levels of education, are older, and have better self-reported health (Figure 3). We performed an analysis to identify interactions between independent variables and their significance of impact on mean trust score. We did not identify significant interactions between race/ethnicity by age; race/ethnicity by educational level; nor race/ethnicity by self-reported health.
Figure 2:
Radar plots comparing levels of trust by a) race/ethnicity, b) level of education, and c) self-reported health. The radar axis represents percent of respondents who “agree” or “strongly agree” with each item of the Trust in the Medical Profession Scale. Refer to Figure 1 for full description of items 1–11. As items 2 and 7, however, are reverse-scored, these were calculated as percent of respondents who “disagree” or “strongly disagree”. Further out on radar plot thus indicates a higher level of trust.
Figure 3:
Stacked bar chart demonstrating categorical scoring of item 11 of the Trust in the Medical Profession Scale (“All in all, you trust doctors completely”), stratified by respondent race/ethnicity, level of education, self-reported health, and age.
Multivariable analysis showed significant differences in adjusted estimates for trust score for age, race/ethnicity, education, and self-reported health remaining significant in the unweighted multiple linear regression model (Table 2). Specifically, younger patients, white patients, those with poorer self-reported health status, and those with higher levels of education estimated toward lower adjusted aggregate trust scores. Hispanic cancer patients exhibited high levels of trust in the medical profession in the adjusted analysis. In the weighted multiple linear regression analysis (Table 2), the aggregate trust score was not significantly different between white and Black respondents, but Hispanic respondents continued to exhibit significantly higher aggregate trust score than other racial/ethnic groups. Also in the weighted analysis, the group who completed high school had similar aggregate trust score as those with some high school or less, but those with some college or more showed persistent significantly lower trust aggregate score than those with lower levels of educational attainment. The significance of difference of aggregate trust score by income level was not observed in the weighted multiple linear regression analyses.
Table 2:
Multiple linear regression model for aggregate trust score
| Unweighted | Weighted | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Coefficient | SE | 95% CI | p-value | Coefficient | SE | 95% CI | p-value |
| Age at diagnosis | ||||||||
| 40 and under | Ref. | Ref. | ||||||
| 41–64 | 1.42 | 0.94 | (−0.4 , 3.3) | 0.114 | 1.57 | 0.87 | (−0.1 , 3.3) | 0.095 |
| 65–79 | 2.38 | 0.96 | (0.5 , 4.3) | 0.011 | 2.63 | 0.90 | (0.9 , 4.4) | 0.008 |
| 80+ | 3.70 | 1.33 | (1.1 , 6.3) | 0.002 | 4.16 | 1.26 | (1.7 , 6.6) | 0.001 |
| Gender | ||||||||
| Male | Ref. | Ref. | ||||||
| Female | −0.66 | 0.48 | (−1.6 , 0.3) | 0.163 | −0.71 | 0.47 | (−1.6 , 0.2) | 0.177 |
| Race/Ethnicity | ||||||||
| White | Ref. | Ref. | ||||||
| Black | 1.87 | 0.75 | (0.4 , 3.3) | 0.019 | 1.26 | 0.69 | (−0.1 , 2.6) | 0.137 |
| Hispanic | 2.93 | 0.78 | (1.4 , 4.5) | <.0001 | 2.39 | 0.72 | (1.0 , 3.8) | 0.002 |
| Other | 1.25 | 1.39 | (−1.5 , 4.0) | 0.300 | 0.99 | 1.07 | (−1.1 , 3.1) | 0.419 |
| Language at home | ||||||||
| English | Ref. | Ref. | ||||||
| Other | 1.11 | 1.06 | (−1.0 , 3.2) | 0.277 | 0.68 | 0.93 | (−1.2 , 2.5) | 0.529 |
| Unknown | 0.39 | 1.53 | (−2.6 , 3.4) | 0.797 | 1.33 | 1.39 | (−1.4 , 4.1) | 0.368 |
| Education | ||||||||
| Some high school or less | Ref. | Ref. | ||||||
| High school | −2.22 | 1.00 | (−4.2 , −0.3) | 0.025 | −2.07 | 0.94 | (−3.9 , −0.2) | 0.060 |
| Some college and above | −2.79 | 0.94 | (−4.6 , −1.0) | 0.003 | −2.50 | 0.89 | (−4.3 , −0.8) | 0.013 |
| Unknown | 3.30 | 2.36 | (−1.3 , 7.9) | 0.147 | 5.14 | 2.42 | (0.4 , 9.9) | 0.041 |
| Marital status | ||||||||
| Married | Ref. | Ref. | ||||||
| Not married | 0.31 | 0.64 | (−0.9 , 1.6) | 0.624 | 0.14 | 0.64 | (−1.1 , 1.4) | 0.840 |
| Unknown | −4.14 | 2.67 | (−9.4 , 1.1) | 0.111 | −4.19 | 2.53 | (−9.1 , 0.8) | 0.046 |
| Living arrangements | ||||||||
| Alone | Ref. | Ref. | ||||||
| Not alone | 1.05 | 0.77 | (−0.5 , 2.6) | 0.195 | 1.70 | 0.78 | (0.2 , 3.2) | 0.056 |
| Unknown | 1.77 | 3.11 | (−4.3 , 7.9) | 0.589 | −0.11 | 3.14 | (−6.3 , 6.1) | 0.972 |
| Income | ||||||||
| Less than $19,999 | Ref. | Ref. | ||||||
| $20,000 – $39,999 | −1.71 | 0.89 | (−3.5 , 0.0) | 0.055 | −1.68 | 0.86 | (−3.4 , 0.0) | 0.106 |
| $40,000 – $99,999 | −2.02 | 0.79 | (−3.6 , −0.5) | 0.010 | −1.70 | 0.78 | (−3.2 , −0.2) | 0.053 |
| $100,000 or more | −1.46 | 0.89 | (−3.2 , 0.3) | 0.092 | −1.23 | 0.89 | (−3.0 , 0.5) | 0.207 |
| Unknown | −2.01 | 0.87 | (−3.7 , −0.3) | 0.021 | −1.49 | 0.85 | (−3.2 , 0.2) | 0.141 |
| Residence | ||||||||
| Metropolitan | Ref. | Ref. | ||||||
| Non-metropolitan | 0.78 | 0.66 | (−0.5 , 2.1) | 0.210 | 0.69 | 0.65 | (−0.6 , 2.0) | 0.367 |
| Self-reported health | ||||||||
| Excellent or very good | Ref. | Ref. | ||||||
| Good | −1.31 | 0.53 | (−2.3 , −0.3) | 0.010 | −1.79 | 0.54 | (−2.8 , −0.7) | 0.001 |
| Fair | −3.09 | 0.65 | (−4.4 , −1.8) | <.0001 | −3.76 | 0.65 | (−5.0 , −2.5) | <.0001 |
| Poor | −5.90 | 1.04 | (−7.9 , −3.9) | <.0001 | −6.10 | 0.98 | (−8.0 , −4.2) | <.0001 |
| Unknown | −5.05 | 2.99 | (−10.9 , 0.8) | 0.015 | −5.18 | 3.40 | (−11.9 , 1.5) | 0.013 |
Discussion
In this study we sought to assess trust in the medical profession generally, as contrasted with trust in one’s own physician or health system specifically, among a large cohort of Texas residents recently diagnosed with cancer. In contrast to other studies that have shown higher levels of mistrust in the medical profession among non-whites, our study revealed that trust in medical professionals was relatively lower among non-Hispanic white individuals. Even in analyses performed to adjust for selection bias in this convenience sample, we did not observe that trust in medical professionals was lower among minority populations. Additionally, we also showed that mistrust was relatively higher among younger and relatively educated individuals as well as those with poorer self-reported health status.
Until recently, most studies that evaluated racial differences in medical mistrust, whether focusing on physicians or the health care system in general, demonstrated that non-whites exhibited greater levels of medical mistrust.32–37 Higher medical mistrust has also been reported among minority cancer patients specifically. Hughes Halbert and colleagues38 also showed African American men with prostate cancer surveyed in Pennsylvania in the mid-2000s exhibited higher levels of medical mistrust than white patients. While few published studies specifically examine medical mistrust among Hispanics, 36 Bustillo and colleagues39 published a survey study in 2017, of a diverse cohort of men in South Florida with localized prostate cancer and found higher levels of medical mistrust among both Hispanic and black patients compared to non-Hispanic whites. Disparate health outcomes for minority populations stem not only from steep societal structural inequities, but also from systemic racism and inequities in the US health care system itself.40 Therefore, the findings of these studies were unsurprising.
Our multi-year research program was devised after identification of disparities in quality of end of life care among minority cancer patients in Texas41 and multiple aims of the overarching project are to better understand needs and characteristics of the diverse population of cancer patients in Texas. Our finding that non-Hispanic white individuals reported relatively lower trust in the medical profession was unexpected. Furthermore, the largest effect size association for race was among Hispanic cancer patients who showed the highest levels of trust in the medical profession both on weighted and unweighted analyses. This finding stands in contrast to previous studies as described above, and could reflect differences in attitudes among cancer patients in Texas versus the broader US population. Alternatively, the findings here may suggest changing societal trends in attitudes about science and expertise and patient/physician relationships as experienced by minority populations. Further qualitative research could clarify underlying factors that may drive the unexpected trends in medical mistrust observed in this sample.
While we did not observe a consistent signal in the association between income and levels of trust in the medical profession, Hughes Halbert and colleagues38 showed more medical mistrust among low income individuals newly diagnosed with prostate cancer surveyed from 2003–2007. We observed that trust in the medical profession was lower among those with poorer health status. This finding is consistent with that of others such as Armstrong and colleagues42 who also reported that distrust in the health care system was correlated to poorer self-reported health. Bustillo and colleagues, reported a similar finding that higher levels of medical mistrust were associated with poorer physical well-being. The explanation for this association remains unclear. LaVeist and colleagues have reported that higher medical mistrust is associated with underutilization of health care services,33 and perhaps higher mistrust translates to forgoing needed health interventions. However, it is also plausible that this association may be the result of individuals with poorer health status having more contact with the health system and consequently more potential for negative interactions or disappointing health care episodes.
Another somewhat surprising observation in our study was that higher education levels were significantly associated with lower levels of trust in the medical profession. This raises the question of why a population that is presumably better able to consume scientific and health-related information would exhibit relatively lower levels of trust in the medical profession. The answer may lie in our current socio-political environment which has engendered a mistrust in science.6,43,44 The underpinnings of this mistrust in science may arise from popular disillusionment with commercialization of medicine or profit-motive in health care.45,46 Furthermore, over a third of Americans agreed with medical conspiracy theories specifically related to cancer in a recent survey study of “medical conspiracism”.47 A recognizable instance of mistrust in science translating to health-impactful behaviors relates to the anti-vaccination movement, which tends to be comprised of young, white, more educated individuals in the US, a profile not unlike those we have identified here in our cohort as showing relatively lower levels of trust in the medical profession. In fact, Baron and Berinsky6 publicly commented recently that this mistrust in institutions and science in the US likely undermines the physician-patient trust relationship. Our data as well as recent investigative journalism suggest that these sentiments could also be at play among current individuals with cancer in Texas.44
We acknowledge that there are limitations to this study. Chief among these is the low overall response rate among those mailed a survey, and the relatively low proportion of Hispanic patients compared to the population of Texas31.Due to limitations in study resources, we used an English language-only version of the survey and thus likely under-sampled data from non-English speaking patients. This would have provided valuable information, particularly in Texas where approximately 40% of residents are Hispanic and 36% speak a language other than English at home. Non-Hispanic white individuals are thus overrepresented in our study, as are those reporting high-levels of education, when compared to the population composition of Texas31. The associations reported between demographic factors and trust in medical profession may thus have limited generalizability even in Texas, much less beyond the state. Furthermore, the instrument used to assess trust was developed and validated among a predominantly non-Hispanic white cohort of patients, and failed to find any relationship between trust and race26. The appropriateness of this instrument to assess differences in trust between racial and ethnic subgroups has thus not been demonstrated, and may be a limitation of this analysis. However, we selected this instrument versus other mentioned in the methods section above because it assessed an individual’s trust in providers generally and was suitable for a diverse population-based sample. We did not want to attune the respondent to racial/ethnic concerns specifically and thus risk alienating potential respondents. Furthermore, the unexplained variation in our adjusted models was high, suggesting that there are factors that we did not measure that explain different levels of trust in the medical profession. A low R2 value is not uncommon in studies attempting to explain human behavior and may reflect that we did not specifically ask about other social/behavioral domains such as religiosity, political affiliation,43 time with physician, gender or racial concordance with current physicians, etc. However, even with low R2, it is still valid to draw some conclusions where the relationship differences between independent variables is statistically significant. The survey was a written-only data collection approach so no further verbal clarifying information was sought or obtained to illuminate our findings. The instrument assesses all domains of trust with the exception of confidentiality (i.e. trust that there will be proper use of sensitive information), which may be particularly relevant to health care consumers in an era of data breeches and concerns about data marketing.26 While many patient and disease factors were included in this analysis, others were not including disease severity and expected longevity and may be confounding factors. Lastly, we cannot know from the data we collected at what point a statistically significant difference in trust scores has a negative impact on patient communication or care. However, while the absolute thresholds for trust in the medical profession remain uncertain, the relative values and associations contribute to our understanding of trust in the medical profession among this large cohort of cancer patients in the second most populous state in the US.
In conclusion, we observed relatively lower general trust in the medical profession among younger, more educated individuals recently diagnosed with cancer in Texas. In the unweighted analysis we observed that the lowest trust levels were among white individuals. While this observation did not persist in a weighted analysis, we did not observe lower levels of trust among racial/ethnic minorities as had been shown previously by other investigators. This suggests that trends in trust in medical professionals may be changing. Trust is the linchpin for high quality health care delivery. These findings may have implications across the cancer care spectrum spanning from eagerness to engage in screening behaviors pre-diagnosis to efficacy of critical patient-physician communication regarding end of life care. Larger socio-cultural sentiments may be affecting trust in the medical profession in ways that may not have been previously well-appreciated and this may impact cancer care delivery.
Supplementary Material
Acknowledgments
Funding sources/disclaimers: This research was supported by a grant from the Cancer Prevention Research Institute of Texas (RP160674, Guadagnolo co-PI). The funding source was uninvolved in the conduct of the research and the interpretation of results. The authors declare no conflicts of interest with the funder and none with other entities related to the research. The authors have full control of the data which were obtained under a Data Use Agreement from the Texas Cancer Registry and the Centers for Medicare and Medicaid Services.
Footnotes
Conflicts of interest: The authors report no conflicts of interest related to this work.
References
- 1.Pearson SD, Raeke LH. Patients’ trust in physicians: many theories, few measures, and little data. J Gen Intern Med. 2000;15(7):509–513. doi: 10.1046/j.1525-1497.2000.11002.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall MA, Dugan E, Zheng B, Mishra AK. Trust in physicians and medical institutions: what is it, can it be measured, and does it matter? Milbank Q. 2001;79(4):613–639, v. doi: 10.1111/1468-0009.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall MA. Law, Medicine, and Trust. Stanford Law Rev. 2002;55(2):463. doi: 10.2307/1229596 [DOI] [PubMed] [Google Scholar]
- 4.Hillen MA, de Haes HCJM, Smets EMA. Cancer patients’ trust in their physician-a review. Psychooncology. 2011;20(3):227–241. doi: 10.1002/pon.1745 [DOI] [PubMed] [Google Scholar]
- 5.Confidence in Institutions, Gallup Poll 2016, Gallup Canada Inc. Accessed May 28, 2020. https://news.gallup.com/poll/1597/confidence-institutions.aspx [Google Scholar]
- 6.Baron RJ, Berinsky AJ. Mistrust in Science - A Threat to the Patient-Physician Relationship. N Engl J Med. 2019;381(2):182–185. doi: 10.1056/NEJMms1813043 [DOI] [PubMed] [Google Scholar]
- 7.Walker DM, Johnson T, Ford EW, Huerta TR. Trust Me, I’m a Doctor: Examining Changes in How Privacy Concerns Affect Patient Withholding Behavior. J Med Internet Res. 2017;19(1):e2. doi: 10.2196/jmir.6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall JL, McGraw D. For telehealth to succeed, privacy and security risks must be identified and addressed. Health Aff Proj Hope. 2014;33(2):216–221. doi: 10.1377/hlthaff.2013.0997 [DOI] [PubMed] [Google Scholar]
- 9.Gill JM, Mainous AG, Nsereko M. The effect of continuity of care on emergency department use. Arch Fam Med. 2000;9(4):333–338. doi: 10.1001/archfami.9.4.333 [DOI] [PubMed] [Google Scholar]
- 10.Arnold LD, McGilvray MM, Kyle Cooper J, James AS. Inadequate Cancer Screening: Lack of Provider Continuity is a Greater Obstacle than Medical Mistrust. J Health Care Poor Underserved. 2017;28(1):362–377. doi: 10.1353/hpu.2017.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penman DT, Holland JC, Bahna GF, et al. Informed consent for investigational chemotherapy: patients’ and physicians’ perceptions. J Clin Oncol Off J Am Soc Clin Oncol. 1984;2(7):849–855. doi: 10.1200/JCO.1984.2.7.849 [DOI] [PubMed] [Google Scholar]
- 12.Thom DH, Hall MA, Pawlson LG. Measuring patients’ trust in physicians when assessing quality of care. Health Aff Proj Hope. 2004;23(4):124–132. doi: 10.1377/hlthaff.23.4.124 [DOI] [PubMed] [Google Scholar]
- 13.Blair RA, Morse BS, Tsai LL. Public health and public trust: Survey evidence from the Ebola Virus Disease epidemic in Liberia. Soc Sci Med 1982. 2017;172:89–97. doi: 10.1016/j.socscimed.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. (Smedley BD, Stith AY, Nelson AR, eds.). National Academies Press (US); 2003. Accessed May 28, 2020. http://www.ncbi.nlm.nih.gov/books/NBK220358/ [PubMed] [Google Scholar]
- 15.Sewell AA. Disaggregating ethnoracial disparities in physician trust. Soc Sci Res. 2015;54:1–20. doi: 10.1016/j.ssresearch.2015.06.020 [DOI] [PubMed] [Google Scholar]
- 16.López-Cevallos DF, Harvey SM, Warren JT. Medical mistrust, perceived discrimination, and satisfaction with health care among young-adult rural latinos. J Rural Health Off J Am Rural Health Assoc Natl Rural Health Care Assoc. 2014;30(4):344–351. doi: 10.1111/jrh.12063 [DOI] [PubMed] [Google Scholar]
- 17.Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Rep Wash DC 1974. 2003;118(4):358–365. doi: 10.1093/phr/118.4.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson HS, Valdimarsdottir HB, Winkel G, Jandorf L, Redd W. The Group-Based Medical Mistrust Scale: psychometric properties and association with breast cancer screening. Prev Med. 2004;38(2):209–218. doi: 10.1016/j.ypmed.2003.09.041 [DOI] [PubMed] [Google Scholar]
- 19.Bynum SA, Davis JL, Green BL, Katz RV. Unwillingness to participate in colorectal cancer screening: examining fears, attitudes, and medical mistrust in an ethnically diverse sample of adults 50 years and older. Am J Health Promot AJHP. 2012;26(5):295–300. doi: 10.4278/ajhp.110113-QUAN-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams LB, Richmond J, Corbie-Smith G, Powell W. Medical Mistrust and Colorectal Cancer Screening Among African Americans. J Community Health. 2017;42(5):1044–1061. doi: 10.1007/s10900-017-0339-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter WR, Godley PA, Clark JA, et al. Racial differences in trust and regular source of patient care and the implications for prostate cancer screening use. Cancer. 2009;115(21):5048–5059. doi: 10.1002/cncr.24539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bickell NA, Weidmann J, Fei K, Lin JJ, Leventhal H. Underuse of breast cancer adjuvant treatment: patient knowledge, beliefs, and medical mistrust. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(31):5160–5167. doi: 10.1200/JCO.2009.22.9773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deshpande AD, Jeffe DB, Gnerlich J, Iqbal AZ, Thummalakunta A, Margenthaler JA. Racial disparities in breast cancer survival: an analysis by age and stage. J Surg Res. 2009;153(1):105–113. doi: 10.1016/j.jss.2008.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai Y, Wang C, Civan JM, et al. Effects of Cancer Stage and Treatment Differences on Racial Disparities in Survival From Colon Cancer: A United States Population-Based Study. Gastroenterology. 2016;150(5):1135–1146. doi: 10.1053/j.gastro.2016.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66(4):290–308. doi: 10.3322/caac.21340 [DOI] [PubMed] [Google Scholar]
- 26.Hall MA, Camacho F, Dugan E, Balkrishnan R. Trust in the medical profession: conceptual and measurement issues. Health Serv Res. 2002;37(5):1419–1439. doi: 10.1111/1475-6773.01070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson LD, Bigman CA. A systematic review of medical mistrust measures. Patient Educ Couns. 2018;101(10):1786–1794. doi: 10.1016/j.pec.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 28.Medicare Health Outcomes Survey, Centers for Medicare & Medicaid Services. https://www.hosonline.org/en/survey-instrument/
- 29.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 30.Skinner CJ, D’arrigo. Inverse probability weighting for clustered nonresponse. Biometrika. 2011;98(4):953–966. doi: 10.1093/biomet/asr058 [DOI] [Google Scholar]
- 31.Census Bureau QuickFacts US: Texas. Accessed June 4, 2020. https://www.census.gov/quickfacts/TX
- 32.Corbie-Smith G, Thomas SB, St George DMM. Distrust, race, and research. Arch Intern Med. 2002;162(21):2458–2463. doi: 10.1001/archinte.162.21.2458 [DOI] [PubMed] [Google Scholar]
- 33.LaVeist TA, Isaac LA, Williams KP. Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res. 2009;44(6):2093–2105. doi: 10.1111/j.1475-6773.2009.01017.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev MCRR. 2000;57 Suppl 1:146–161. doi: 10.1177/1077558700057001S07 [DOI] [PubMed] [Google Scholar]
- 35.Armstrong K, McMurphy S, Dean LT, et al. Differences in the patterns of health care system distrust between blacks and whites. J Gen Intern Med. 2008;23(6):827–833. doi: 10.1007/s11606-008-0561-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benkert R, Cuevas A, Thompson HS, Dove-Meadows E, Knuckles D. Ubiquitous Yet Unclear: A Systematic Review of Medical Mistrust. Behav Med Wash DC. 2019;45(2):86–101. doi: 10.1080/08964289.2019.1588220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guadagnolo BA, Cina K, Helbig P, et al. Medical mistrust and less satisfaction with health care among Native Americans presenting for cancer treatment. J Health Care Poor Underserved. 2009;20(1):210–226. doi: 10.1353/hpu.0.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halbert CH, Weathers B, Delmoor E, et al. Racial Differences in Medical Mistrust among Men Diagnosed with Prostate Cancer. Cancer. 2009;115(11):2553–2561. doi: 10.1002/cncr.24249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bustillo NE, McGinty HL, Dahn JR, et al. Fatalism, medical mistrust, and pretreatment health-related quality of life in ethnically diverse prostate cancer patients. Psychooncology. 2017;26(3):323–329. doi: 10.1002/pon.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feagin J, Bennefield Z. Systemic racism and U.S. health care. Soc Sci Med 1982. 2014;103:7–14. doi: 10.1016/j.socscimed.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 41.Guadagnolo BA, Liao K-P, Giordano SH, Elting LS, Shih Y-CT. Variation in intensity and costs of care by payer and race for patients dying of cancer in Texas: an analysis of registry-linked Medicaid, Medicare, and dually eligible claims data. Med Care. 2015;53(7):591–598. doi:doi: 10.1097/MLR.0000000000000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong K, Rose A, Peters N, Long JA, McMurphy S, Shea JA. Distrust of the health care system and self-reported health in the United States. J Gen Intern Med. 2006;21(4):292–297. doi: 10.1111/j.1525-1497.2006.00396.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler K. The anti-vax movement’s radical shift from crunchy granola purists to far-right crusaders. Mother Jones. Accessed July 5, 2020. https://www.motherjones.com/politics/2020/06/the-anti-vax-movements-radical-shift-from-crunchy-granola-purists-to-far-right-crusaders/ [Google Scholar]
- 44.Texas Anti-Vaxxers Fear Mandatory COVID-19 Vaccines More Than the Virus Itself. Texas Monthly. Published March 18, 2020. Accessed July 5, 2020. https://www.texasmonthly.com/news/texas-anti-vaxxers-fear-mandatory-coronavirus-vaccines/ [Google Scholar]
- 45.Camargo K, Grant R. Public health, science, and policy debate: being right is not enough. Am J Public Health. 2015;105(2):232–235. doi: 10.2105/AJPH.2014.302241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins H. Rejecting knowledge claims inside and outside science. Soc Stud Sci. 2014;44(5):722–735. doi: 10.1177/0306312714536011 [DOI] [PubMed] [Google Scholar]
- 47.Oliver JE, Wood T. Medical conspiracy theories and health behaviors in the United States. JAMA Intern Med. 2014;174(5):817–818. doi: 10.1001/jamainternmed.2014.190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.