Abstract
Background
COVID-19 is a potentially critical infectious disease. Inflammatory response and disease severity may vary according to immune system status. The aim of this case series is to investigate different presentation of COVID-19 in immunocompromised patients.
Methods
this is a single centre case series about 17 immunocompromised patients admitted to our respiratory department during the recent COVID-19 pandemic. White blood cell count, C reactive protein, interleukin 6, lymphocytic subpopulation count (CD4+, CD8+, CD20+) and immunoglobulin count (IgG, IgM, IgA) were measured at hospitalization.
Results
the most common causes of immunosuppression observed in our severe COVID-19 population are hematological malignancies, immunosuppressant drugs for transplant, primary immunodeficiency and inflammatory bowel disease. Onset symptoms were fever (88%), cough (53%), dyspnoea (24%), asthenia (35%), anosmia and/or ageusia (17%), expectoration (12%). Compared to benign conditions, patients with malignancies show a lower lymphocytic count (490 vs 1100 cells/uL) and higher interleukin 6 (33 vs 13 pg/mL).
Conclusions
immunocompromised patients are at risk of adverse outcome from COVID-19. Hematological malignancies and anti-CD20 therapies induce a high risk. Primary immunodeficiency and classical immunosuppressant such as calcineurin inhibitors and antimetabolites share an intermediate risk.
Keywords: Lymphoma, Immunodeficiency, Immunosuppressant, Anti-CD20, SARS-CoV2 persistence, SARS-CoV2 relapse
1. Background
Coronavirus disease 2019 (COVID-19) is an emergent infectious disease caused by a novel coronavirus named SARS-CoV2. Clinical manifestations can widely range between mild respiratory symptoms and severe acute respiratory distress syndrome (ARDS). In early 2021 more than 80 million cases have been confirmed worldwide, with 1.7 million of deaths [1]. Certain medical conditions are at risk of severe COVID-19. Older age, chronic cardiovascular and pulmonary diseases, diabetes mellitus are commonly observed in critical cases. Tumors and antitumoral therapy potentially compromise the immune system thus influencing the disease severity and prognosis. It has been reported that hematological malignancies have higher mortality for COVID-19 than general population [2]. A decrease in the innate antiviral response and chronic lymphopenia are common in neoplastic patients. Nevertheless an immune dysregulation can be observed in many other conditions such as primary and acquired immunodeficiency and immune diseases. Since the underlying mechanisms and potential therapies are controversial, the aim of this case series is to investigate different presentation of COVID-19 in immunocompromised patients.
2. Methods
This is a single centre case series involving immunocompromised patients admitted to our respiratory department during the recent COVID-19 pandemic. We admitted patients affected by respiratory failure and severe illness. SARS-CoV-2 infection was confirmed by reverse transcriptase polymerase chain reaction (PCR) on nasopharyngeal swab. Data collection at admission included clinical history, previous therapy, onset time and symptoms. At hospitalization all patients underwent blood gas analysis to determine PaO2/FiO2 (PF), and high resolution chest tomography (HRCT) with assessment of total severity score (TSS) from 1 to 20 sec. Chung. We also evaluated white blood cell count (WBC), lymphocyte count, C reactive protein (CRP), interleukin 6 (IL6), lymphocytic subpopulation count (CD4+, CD8+, CD20+) and immunoglobulin count (IgG, IgM, IgA).
3. Results
Data from 17 patients were collected. Age range was 29–83 years, 14 were males and 3 females. All patients were considered immunocompromised hosts due to a previous diagnosis. The most commonly represented causes were hematological malignancies such as non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia and myelodysplastic syndrome (9 patients). Immunosuppressant drugs were also recorded; 3 patients received immunosuppressants after transplant, 4 received antiCD-20 drugs for NHL and 1 for vasculitis. Primary (2) and secondary (1) immunodeficiency was observed. Furthermore, we included chronic inflammatory bowel disease (2). The estimated prognosis for survival before SARS-CoV-2 infection was >12 months for all patients. Baseline characteristics are reported in Table 1.
Table 1.
Baseline characteristics. CVID: common variable immunodeficiency; HIV: human immunodeficiency virus; HBV: hepatitis B virus; DM: diabetes mellitus; DCM: dilated cardiomyopathy; AF: atrial fibrillation; hypertension: chronic systemic hypertension; PTE: pulmonary thromboembolism; COPD: chronic obstructive pulmonary disease; CAD: coronary artery disease.
| age | cause of immunosuppression | years | other conditions | PF | TSS | WBC (10^3/μL) | LYMP (cells/uL) | CRP (mg/dL) | IL6 (pg/ml) | CD4/CD8/B (%) | Ig M/G/A (mg/dL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | Non Hodgkin lymphoma | 9 | / | 90 | 15 | 9.19 | 200 | 20.4 | 311 | 4/50/1.5 | 23/540/67 |
| 2 | 51 | Non Hodgkin lymphoma | 11 | Parkinson | 250 | 8 | 5.45 | 350 | 1.9 | 13.2 | 37/52/0.2 | <21/772/67 |
| 3 | 75 | Non Hodgkin lymphoma | 10 | HBV hepatitis, hypertension, renal failure | 162 | 17 | 3.16 | 520 | 8.1 | 33 | 7.6/14/17 | >640/554/10 |
| 4 | 77 | Chronic lymphocytic leukemia | 5 | hypertension | 103 | 15 | 56.6 | 5173 | 7.6 | 24 | 0.8/1.7/89 | <21/524/96 |
| 5 | 77 | Chronic lymphocytic leukemia | 3 | prostatic cancer, obesity | 90 | 16 | 19.62 | 11580 | 1.6 | 10.6 | 2.3/6/66 | 91/692/255 |
| 6 | 67 | Non Hodgkin lymphoma | 8 | / | 144 | 14 | 5.56 | 410 | 9.1 | 70.7 | 5.5/32/2.6 | <21/700/15 |
| 7 | 57 | Non Hodgkin lymphoma | 5 | DM | 280 | 8 | 56 | 100 | 3.7 | 27.5 | 14/49/1 | 35/974/193 |
| 8 | 79 | myelodysplasia | 6 | DCM, AF, hypertension | 70 | 17 | 11.02 | 490 | 20.1 | 90 | 0.9/23/3 | 105/823/300 |
| 9 | 83 | myelodysplasia | 8 | DM, AF, hypertension | 83 | 9 | 10.54 | 510 | 24.9 | 116 | 18/42/4.1 | 227/1600/336 |
| 10 | 57 | Ciclosporin + everolimus | 15 | DM, AF, previous PTE, renal failure | 300 | 5 | 7.19 | 1580 | 1.9 | 19 | 22/45/3 | 74/823/230 |
| 11 | 72 | Ciclosporin + micofenolate | 7 | AF, COPD | 140 | 7 | 5.1 | 960 | 6 | 6.6 | 18/43/3 | 101/850/310 |
| 12 | 53 | Rituximab for vasculitis | 6 | Emphysema | 80 | 15 | 6.54 | 150 | 13 | 67.6 | 27 12 | 30/282/47 |
| 13 | 41 | CVID | 8 | Bronchiectasis | 83 | 9 | 4.49 | 1270 | 11.1 | 6.2 | 26/65/0.1 | <21/<35/<7.8 |
| 14 | 29 | CVID | 2 | / | 300 | 5 | 5.47 | 1410 | 13 | 6 | 43/35/4.5 | <31/399/9 |
| 15 | 58 | HIV | 3 | obesity, lung nodule, CAD, hypertension | 90 | 14 | 13.43 | 540 | 6.8 | 8.2 | 3.5/32/3.7 | 164/928/315 |
| 16 | 72 | Chron's disease | 8 | COPD | 83 | 18 | 21.3 | 700 | 6.4 | 273 | 18/46/3.5 | 162/1120/729 |
| 17 | 62 | Ulcerative rectocolitis | 9 | hypertension, asthma | 80 | 12 | 21.46 | 1240 | 1 | 33.7 | 19/50/2.9 | 107/1058/630 |
The most common onset symptoms were fever (88%), cough (53%), dyspnoea (24%), asthenia (35%), anosmia and/or ageusia (17%), expectoration (12%).
At hospitalization, patients with malignancies (group M) showed a median PF of 103 (86.5–206) and a median TSS of 15 (8.5–16.5), while patients affected by benign diseases (group B) had a median PF of 86.5 (81–220) and a TSS of 10.5 (6–14.5). Median lymphocytes were 490 cells/uL (275–2846) in group M and 1100 cells/uL (620–1340) in group B. Median CRP and IL6 were respectively 8.1 mg/dL (2.8–20) and 33 pg/mL (18.6–103) in group M, while 6.6 mg/dL (3.95–12) and 13.6 pg/mL (6.4–50.65) in group B.
Administered therapies, outcomes and complications are reported in Table 2.
Table 2.
Administered therapies and outcomes. IVIG: intravenous immunoglobulin; HXC: hydroxycloroquine; AZI: azithromycin. SCS: systemic corticosteroid. LMWH: low molecular weight heparin; ICU: intensive care unit admission; PTE: pulmonary thromboembolism.
| COVID-19 therapy | Seroconversion (yes/no) | viral persistence (days) | outcome | complication | |
|---|---|---|---|---|---|
| 1 | IVIG 5 days, HXC, AZI | yes | 15 | death | multiple organ failure |
| 2 | Tocilizumab, HXC, AZI, IVIG 2 days, Remdesivir 10 days | no | 230 | death | Bacterial and fungal sovrainfection |
| 3 | IVIG 5 days, SCS 1 mg/kg, fondaparinux | no | 40 | death | Pneumomediastinum. Major bleeding |
| 4 | IVIG 3 days, SCS 1 mg/kg, fondaparinux | no | 44 | death | Bacterial and fungal sovrainfection |
| 5 | SCS 1 mg/kg, fondaparinux, AZI | no | 32 | death | multiple organ failure |
| 6 | IVIG 5 days, Remdesivir, convalescent plasma, SCS 2 mg/kg | no | 43 | death | Pneumomediastinum. ICU |
| 7 | IVIG 3 days, SCS 2 mg/kg, LMWH | no | 84 | discharged | |
| 8 | SCS 2 mg/kg, fondaparinux | no | 10 | death | ICU |
| 9 | AZI, LMWH, Remdesivir 5 days, SCS 1 mg/kg | yes | 39 | discharged | Bacterial sovrainfection |
| 10 | AZI, LMWH, SCS 1 mg/kg | yes | 24 | discharged | Bacterial sovrainfection |
| 11 | AZI, LMWH, Remdesivir 5 days, SCS 1 mg/kg | yes | 26 | discharged | |
| 12 | IVIG 5 days, SCS 2 mg/kg, fondaparinux | no | 33 | death | ICU |
| 13 | IVIG 5 days, SCS 1 mg/kg, fondaparinux | yes | 29 | discharged | Bacterial sovrainfection |
| 14 | IVIG 5 days, LMWH | yes | 31 | discharged | |
| 15 | SCS 2 mg/kg fondaparinux | no | 21 | death | ICU |
| 16 | Remdesivir 5 days, LMWH, SCS 1 mg/kg | yes | 42 | discharged | |
| 17 | Remdesivir 5 days, IVIG 3 days, fondaparinux, SCS 1 mg/kg | no | 32 | death | PTE. Major bleeding. ICU |
3.1. Therapeutic approach and case description
Reduction of at least 2 classes of immunoglobulin was very common in our cohort. 5 out of 9 patients in group M. It was less common in Group B, affecting 2 patients with primary immunodeficiency and 1 patient previously treated with Rituximab. A reduction of B lymphocytes was very common affecting 6 patients in group M, and 7 patients in group B. Subsequently, intravenous immunoglobulin (IVIG) was administered at the dose of 5 mL/kg for 3 consecutive days, or 5 days in case of Ig reduction at baseline.
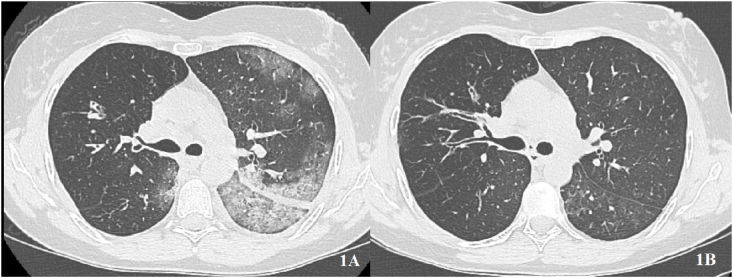
A 41-years old female affected by common variable immunodeficiency, bronchiectasis and recurrent respiratory infections received IVIG for 5 days, low dose systemic corticosteroid (SCS) and prophylactic low molecular weight heparin (LMWH). Oxygen support with high flow nasal cannula (HFNC) was also required for a severe ARDS. The patient showed a complete remission of symptoms and seroconversion at day 26 of disease. She was then discharged without oxygen supply and with a negative PCR for SARS-CoV2. HRCT showed a complete resolution of pneumonia (Fig. 1).
Fig. 1.
Young female with primary immunodeficiency. A: asymmetrical interstitial pneumonia at baseline. B: complete resolution after immunoglobulin.
Among hematological malignancies, 6 patients out of 9 received IVIG but only 3 subjects obtained seroconversion despite normalization of Ig classes after treatment. 4 cases of malignancies were previously treated with anti-CD20 drugs such as Rituximab and Obinutuzumab. None of them obtained seroconversion over a follow up of 6 months.
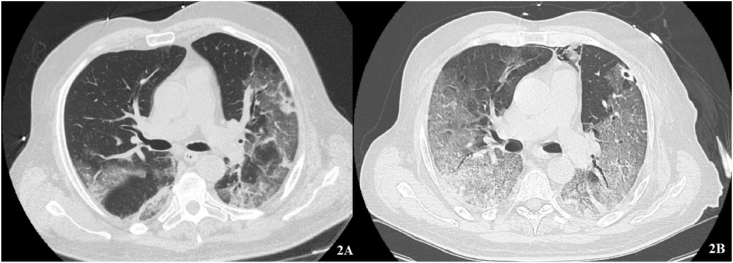
This is the case of a 67-years old man affected by non-Hodgkin lymphoma and humoral deficiency who experienced fever, dyspnoea and asthenia. At hospital admission a moderate ARDS and extensive interstitial pneumonia were observed. CRP was 9.1 mg/dL and IL6 was 70.7 pg/mL. The subject underwent continuous positive airway pressure (CPAP) via Helmet (PEEP: 10 cmH2O and FiO2 70%) and a combination of IVIG and Remdesivir for 5 days. Since persistence of symptoms and respiratory failure, convalescent plasma was administered for 2 days. Nevertheless clinical conditions rapidly deteriorated and the patient was intubated, then died at day 45 of disease (Fig. 2).
Fig. 2.
Non-Hodgkin lymphoma. A: ground glass opacities at baseline. B: after convalescent plasma, confluent ground glass and crazy paving with associated small pneumomediastinum.
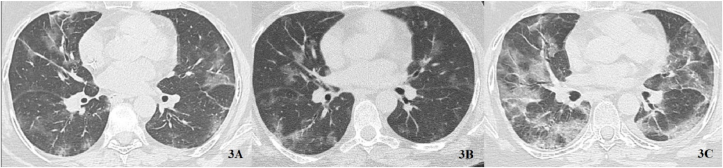
On the other side SARS-CoV2 infection can also determine a slow progression and an initially indolent clinical manifestation in certain subjects, especially those with a significant impairment of B lymphocytes induced by Rituximab. A 51-years old woman affected by non-Hodgkin lymphoma had quite subtle symptoms at onset of disease, with relatively mild radiologic extension of disease (TSS 8/20). CRP was 1.9 mg/dL and IL6 was 13.2 pg/mL. The subject firstly received Tocilizumab, hydroxycloroquine, azithromycin and low dose SCS. Secondly, she was treated with IVIG for only 2 days because of an allergic adverse event. Finally she also received a 10 days course of intravenous Remdesivir. After every of the 3 drug regimens a clinical and radiological improvement was observed, but no antibody response followed and SARS-CoV2 was persistently detected by PCR. A COVID-19 relapse occurred with even more severely compromised conditions (Fig. 3) and finally the patient died after 230 days of viral persistence.
Fig. 3.
Young female with non-Hodgkin lymphoma. A: interstitial pneumonia at baseline. B: partial resolution borne by left and right lower lobes after Remdesivir. C: relapsed SARS-CoV2 pneumonia.
Immunosuppressant drugs have a variable effect on COVID-19 progression. Classical immunosuppressant such as calcineurin inhibitors and antimetabolites seemed to have a little influence on the interaction between virus and host. Here we report 2 cases of heart transplant receiving ciclosporin plus everolimus and ciclosporin plus micofenolate. Independently from the severity of the disease at baseline as reported in Table 1, both were discharged without oxygen support. Viral persistence was 24 and 26 days, similarly to otherwise healthy subjects.
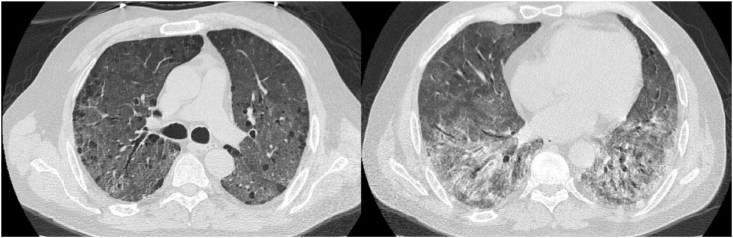
Contrastingly, anti-CD20 drugs such as Rituximab and Obinutuzumab, significantly impair host defence against viruses even in benign conditions. A 53-years old man affected by cryoglobulinemic vasculitis cured with Rituximab, showed a severe ARDS and COVID-19. He was hospitalized at day 14 of disease, presenting fever and cough. CRP was 13 mg/dL and IL6 67.6 pg/mL. B lymphocytes and Ig were reduced. This patient received Helmet CPAP (PEEP: 10 cmH2O and FiO2 80%) as well as a 5 days course IVIG, fondaparinux and high dose SCS. After IVIG no improvement was observed on blood gases and HRCT (Fig. 4). The patient was intubated at day 32 of disease.
Fig. 4.
Progression of SARS-CoV2 pneumonia in a young man affected by vasculitis and receiving Rituximab.
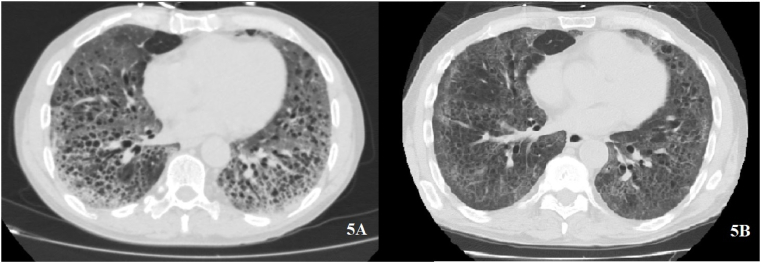
Chronic inflammatory bowel disease is the result of an immune imbalance of the intestinal mucosa. A 72-years old man affected by Chron's disease was hospitalized during the hyperinflammatory stage of COVID-19. Severe ARDS and a massive lung involvement at HRCT were observed. IL6 was 273 pg/mL and lymphocytes were 700 cells/uL. IgM and IgG were within normal ranges, while IgA was overexpressed. This patient received Remdesivir for 5 days, LMWH and low dose SCS until he gradually recovered after 42 days (Fig. 5).
Fig. 5.
Chron's disease. A: massive confluent ground glass with associated emphysema at baseline. B: partial resolution after Remdesivir.
4. Discussion
IVIG is made up of human immunoglobulins, mostly IgM and IgG. It is not routinely recommended for COVID-19 treatment and many countries are currently experimenting IVIG efficacy by randomized controlled trials. The mechanism of action of the drug remains unclear. Based on previous evidences, IVGV inhibit neutrophils and monocytes degranulation while activating phagocytes to internalize viruses [3]. In addition it has been suggested that IVIG can counterbalance the cytokine storm that typically characterizes severe COVID-19 [4]. Single cases of successful IVIG for severe COVID-19 have been reported in immunocompetent patients [5].
Based on our experience, the early reduction of B lymphocytes observed during SARS-CoV2 infection may lead to a less efficient humoral immunity. It is a threatening condition, therefore indicators such as Ig classes and B lymphocytes should be routinely measured at hospitalization for SARS-CoV2 infection. Since IVIG has immunomodulatory effects, we suggest its use in case of multiple Ig classes reduction or B lymphocytes decrease.
The overall prognosis for immunocompromised patients is worse than other subjects. Particularly, hematological malignancies and anti-CD20 drugs induce a lower survival rate and a higher risk of complications. On the other hand, patients receiving classical immunosuppressant drugs and patients affected by primary immunodeficiency share a better prognosis. In our case series 5 patients underwent endotracheal intubation and died. Intensive care showed no improvement of prognosis neither in malignant nor in benign conditions. This observation leads in favour of a conservative care for immunocompromised patients especially in the elder population.
Declaration of competing interest
All authors declare they have no conflict of interest.
Acknowledgements
All authors have given final approval of the version to be published. Other assistance with the article: none. Financial support and sponsorship: no financial support was received for this study. Conflicts of interest: authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. Presentation: none.
References
- 1.https://covid19.who.int/
- 2.Wood W.A., Neuberg D.S., Thompson J.C., Tallman M.S., Sekeres M.A., Sehn L.H., Anderson K.C., Goldberg A.D., Pennell N.A., Niemeyer C.M., Tucker E., Hewitt K., Plovnick R.M., Hicks L.K. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH research collaborative data hub. Blood Adv. 2020 Dec 8;4(23):5966–5975. doi: 10.1182/bloodadvances.2020003170. PMID: 33278301; PMCID: PMC7724912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moradimajd P., Samaee H., Sedigh-Maroufi S., Kourosh-Aami M., Mohsenzadagan M. Administration of intravenous immunoglobulin in the treatment of COVID-19: a review of available evidence. J. Med. Virol. 2020 Dec 12 doi: 10.1002/jmv.26727. Epub ahead of print. PMID: 33314173. [DOI] [PubMed] [Google Scholar]
- 4.Fathi N., Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol. Int. 2020 Sep;44(9):1792–1797. doi: 10.1002/cbin.11403. Epub 2020 Jun 3. PMID: 32458561; PMCID: PMC7283672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanza M., Polistina G.E., Imitazione P., Annunziata A., Di Spirito V., Novella C., Fiorentino G. Successful intravenous immunoglobulin treatment in severe COVID-19 pneumonia. IDCases. 2020 May 16;21 doi: 10.1016/j.idcr.2020.e00794. PMID: 32426229; PMCID: PMC7229478. [DOI] [PMC free article] [PubMed] [Google Scholar]