Abstract
This systematic review summarizes the current evidence related to the reliability of toenail total arsenic concentrations (thereafter “arsenic”) as a biomarker of long-term exposure. Specifically, we reviewed literature on consistency of repeated measures over time, association with other biomarkers and metal concentrations, factors influencing concentrations, and associations with health effects. We identified 129 papers containing quantitative original data on arsenic in toenail samples covering populations from 29 different countries. We observed geographic differences in toenail arsenic concentrations, with highest median or mean concentrations in Asian countries. Arsenic-contaminated drinking water, occupational exposure or living in specific industrial areas were associated with an increased toenail arsenic content. The effects of other potential determinants and sources of arsenic exposure including diet, gender and age on the concentrations in toenails need further investigations. Toenail arsenic was correlated with the concentrations in hair and fingernails, and with urine arsenic mainly among highly exposed populations with a toenail mean or median ≥1 μg/g. Overall, there is a growing body of evidence suggesting that arsenic content from a single toenail sample may reflect long-term internal dose-exposure. Toenail arsenic can serve as a reliable measure of toxic inorganic arsenic exposure in chronic disease research, particularly promising for cancer and cardiovascular conditions.
Keywords: toenail, exposure, biomonitoring, biomarker, arsenic
1. Introduction
Arsenic is a metalloid ubiquitous in the environment found in different forms (inorganic and organic) and oxidation states (−3, 0, +3, +5). Inorganic arsenic is a known cause of multiple cancer and non-cancer health outcomes (IARC, 2012). For non-occupationally exposed individuals, drinking water and food intake are considered among the major arsenic exposure sources. People may also be exposed by breathing air or by skin contact with polluted soil and water (Davis et al., 2017; Marchiset-Ferlay et al., 2012). Inorganic arsenic predominates in drinking water; whereas both inorganic and organic arsenic forms are found in food (Carbonell-Barrachina et al., 2009; Cubadda et al., 2016).
Ingested inorganic arsenic is absorbed through the gastro-intestinal tract into the blood stream, taken up by the liver and transformed to monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) through a methylation process catalyzed by the enzyme arsenic (+3) methyltransferase (AS3MT) with S-adenosylmethionine as the methyl group donor (Agusa et al., 2011; Antonelli et al., 2014; Jansen et al., 2016; Tseng, 2009). The majority of absorbed arsenic is eliminated in urine within 3–5 days (Buchet et al., 1981; Marchiset-Ferlay et al., 2012; Meharg et al., 2014). The methylation process is usually considered a mechanism for detoxification however this process is incomplete; therefore, unmetabolized inorganic arsenic including arsenite and arsenate along with organic forms such as DMA and MMA are detectable in urine (Signes-Pastor et al., 2017). During the inorganic arsenic methylation process intermediate trivalent metabolites are generated (e.g., MMAIII, and DMAIII), and they may be highly reactive (Tseng, 2009). Inorganic arsenic has high affinity to keratin-rich tissues, and thus a fraction of the inorganic arsenic from the blood flow binds to the sulfhydryl-groups of hair and nails and is accumulated mainly at lower oxidation states (Marchiset-Ferlay et al., 2012; Pearce et al., 2010). On the contrary, evidence suggests that ingestion of organic forms of arsenic including arsenobetaine and other complex organosenicals associated with marine products consumption are excreted in the urine unchanged or after metabolism and are not accumulated in the human body (Cubadda et al., 2016; Navas-Acien et al., 2011).
Biomarkers of inorganic arsenic exposure are necessary to understand the mechanism of toxicity, and to assess the health impacts. Blood, urine, hair, and nails are the most common biological substrates used in epidemiological studies. Toenail samples are advantageous for large epidemiological studies due to the ease of collection and storage, and because they are considered less susceptible to external contamination compared to hair (Esteban and Castaño, 2009; Karagas et al., 2000; Mandal et al., 2003; Marchiset-Ferlay et al., 2012). Toenails have a slow rate of growth and their clippings reflect several weeks of growth that occurred 5 to 18 months ago. Thus, toenail clipping total arsenic concentrations (thereafter “arsenic”) provide a measurement of internal exposure to inorganic arsenic from the previous 5 to 18 months (Goullé et al., 2009; Slotnick, 2011). In light of the growing use of toenails as a biomarker of arsenic exposure, we systematically reviewed and summarized the available data on toenail arsenic as exposure biomarker in exposure science and epidemiology research. We specifically focused on 1) reliability of repeated measures over time; 2) their relation with other toenail metals and with other commonly used arsenic biomarkers; 3) potential factors influencing toenail arsenic concentrations; and 4) associations with health effects.
2. Material and methods
This review is reported according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) publication standards (Moher et al., 2009). The protocol was registered in PROSPERO, the international prospective register of systematic reviews (registration number CRD42019129138) (University of York, 2019).
2.1. Search strategy
We performed a systematic literature search for peer-reviewed papers with original data on arsenic concentrations in human toenail samples following the approach described previously (Gutiérrez-González et al., 2019). We searched for all available studies published before December 31, 2017 in three different databases: PubMed/MEDLINE, Web of Science, and Scopus using the following search strategy: 1) toenail OR nail; 2) exposure OR biomonitoring OR biomarker; 3) arsenic. This strategy retrieved 573 references. Additionally, another 19 papers were identified through the reference lists in the papers initially found. Five reviewers (AS, EG, MG, FR and JL) screened abstracts, reviewed full-text papers and performed quality assessments using the STROBE checklists. Only reports written in English were considered, and only those that contained quantitative original data on human toenail arsenic levels were included. Questions about the type of nails or other selection criteria were clarified by directly asking the authors before inclusion when needed (Ali et al., 2010; Farzan et al., 2017; Hossain et al., 2012; Huda et al., 2014; Islam et al., 2015; Watts et al., 2009). After removing duplicates, 306 articles were recruited for title and abstract screening. From these, 259 articles were selected for full text assessment for eligibility. Finally, for the purpose of this review, 129 articles met the inclusion criteria. Among them, there were reports focused on the same study population that potentially reported arsenic concentrations in the same toenail samples. The flow chart of the study selection process is shown in Figure S1.
2.2. Data collection
Data from selected studies were extracted and compiled into a customized spreadsheet by eight investigators (EG, MG, FR, JL, EV, RP, MP, and AN) and reviewed for accuracy and completeness by a ninth investigator (AS). According to a purpose-designed protocol, the following information was extracted from each paper: 1) basic information including first author, country, year of publication, research project, source of arsenic exposure, and health effects; 2) study design, main objective and conclusions, sample size, population sampling method, participant characteristics, informed consent request, and ethical committee approval; 3) sample and analytical information including toenail type such as big toe or all toes, sample preparation, analytical method, quality control measures, and limits of detection (LOD); 4) arsenic concentrations including range, percentiles, interquartile range (IQR), median, arithmetic mean, and geometric mean when available; and 5) association with other biomarkers, other toenail metal concentrations, personal characteristics, environmental data or with previous toenail sample measurements. The units of measurement were heterogeneous across studies. In order to facilitate comparisons among studies, all arsenic concentrations were converted to μg/g or parts per million (Kato et al., 2013; Maity et al., 2012; Middleton et al., 2016; Otto et al., 2007; Punshon et al., 2015; Saat et al., 2013; Watts et al., 2009). The information extracted from the selected studies is described below and provided in the Supplemental Information.
3. Results
3.1. Study characteristics
We identified 129 studies with quantitative data on arsenic content in toenail samples published before December 31, 2017. The largest number of studies were from the United States of America (US) (number of studies (n) = 49) and Bangladesh (n = 25) followed by China (n = 7) and Canada (n = 7). Other countries with multiple studies included Australia (n = 4), United Kingdom (n = 3), Thailand (n = 3), Poland (n = 3) and Pakistan (n = 3). The oldest study was published in 1993 (Garland et al., 1993), and the number of manuscripts began to increase from 2004 with over half of the included studies being published since 2012 (Figure S2).
Near to 70% of the included studies focused on arsenic as a single agent. However, several studies also reported toenail concentrations of other elements. Cadmium, manganese and lead were the most common elements assessed after arsenic and their toenail contents were reported in 29 studies (Table S1).
The number of participants in each investigation was highly variable: 31% of the studies included less than 100 individuals, 57% between 100 and 1,000 and 10% more than 1,000 participants (range: 7–4,257). The largest sample size was the New Hampshire Skin Cancer Study with 2,881 cases and 1,376 controls (Farzan et al., 2015). Sample size was unspecified in 2% of the reports (Abdulrahman et al., 2012; Nath et al., 2008; Watts et al., 2009) (Table S1).
A cross-sectional study design was performed in 62% of the included studies, followed by case-control for 24% and cohort studies for 13%. Additionally, our search identified an intervention study designed to assess the effectiveness of consumption of bottle water in reducing arsenic exposure (Josyula et al., 2006).
3.2. Analytical methodology: sample collection, toenail preparation, analysis and quality control.
Toenail clippings, the last end fraction of the nail, were usually obtained using stainless-steel clippers or scissors and frequently stored in paper envelops or plastic bags/vials at room temperature until analysis.
Many studies (n = 81) did not identify whether the toenail samples were collected from all toes or only from big toes. Among those that did, clippings of all toes (n = 47) or big toe only (n = 1) (Lampron-Goulet et al., 2017) were collected. Some studies collected fingernails in addition to toenails samples (Coelho et al., 2014; Phan et al., 2011; Schmitt et al., 2005; Wade et al., 2015) and they were analyzed for arsenic content separately.
The amount of collected toenail samples used for analysis (i.e., mean or range) was reported in only 14% of the studies. In these studies, toenail mass ranged from 0.001 g to 0.855 g (Button et al., 2009; Freeman et al., 2004; Karagas et al., 1996; Martin et al., 2013; Nichols et al., 1998; Nygaard et al., 2017; Pearce et al., 2010; Raińska et al., 2007). In a few studies, a minimum weight of toenail sample was noted to be necessary to measure the arsenic content (e.g., >0.05 g (Rahman et al., 2017; Tsuji et al., 2005)) as the LOD is affected by toenail mass (Nygaard et al., 2017).
The reported methods for processing toenail samples including cleaning prior to analysis differed across studies. However, the following steps were usually identified (Table S1). If samples contained nail polish this was first removed with acetone and visible dirt was manually cleaned (Farzan et al., 2016, 2017; Martin et al., 2013; Normandin et al., 2014; Nygaard et al., 2017; Tsuji et al., 2005). Then, toenail samples were washed with detergent, deionized water, methanol, Triton solution, or acetone in a sonicator or in an ultrasound bath (Aguiar and Saiki, 2001; Chanpiwat et al., 2015; Hossain et al., 2012; Huyck et al., 2007; Islam et al., 2015; Karim et al., 2010; MacIntosh et al., 1997; Nath et al., 2008; Rahman et al., 2017; Rodrigues et al., 2015; Saat et al., 2013). Finally, samples were dried in an oven, air-dried or freeze-dried before analysis (Abdulrahman et al., 2012; Alamdar et al., 2016; Cottingham et al., 2013; Farzan et al., 2015; Gruber et al., 2012; Karagas, et al., 2001a, 2001b, 2002, 2004; Lee et al., 2016). A digestion process prior analysis was performed when arsenic content was measured using inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectrometry (AAS) or atomic fluorescence spectrometry (AFS), which are among the most common techniques for measuring toenail arsenic concentrations (Table S1). The digestion process usually involved strong acids (e.g., nitric acid) and hydrogen peroxide as a powerful oxidizing agent, and generally took place in a microwave digestion system (Chiou et al., 1997; Coelho et al., 2014, 2012; Intarasunanont et al., 2012; Kile et al., 2016; Lewińska et al., 2007; Lin et al., 2017; Maity et al., 2012; Rahman et al., 2017; Subhani et al., 2015).
Only one study described randomizing samples before analysis to reduce uncertainty from the artifacts related to injection order and instrumental sensitivity during the entire sequence (Subhani et al., 2015). Overall, 100 studies described quality control procedures including the use of study specific nail reference material, recovery analysis, procedural blanks, duplicate samples, and spike samples. The LOD was usually calculated as the mean blank signal plus 3 standard deviations (Button et al., 2009; Middleton et al., 2016) and was reported in 49 studies. Among these, 44 reported the LOD multiplied by the dilution factor and given in μg of arsenic per g of toenail. In the remaining 5 studies the LOD was reported in μg/L (Beamer et al., 2016; Freeman et al., 2004; Kuiper et al., 2014; Maity et al., 2012; Rahman et al., 2015). The reported LODs showed a wide variability ranging from ≤0.001 μg/g (Johnson et al., 2011; Kile et al., 2016, 2007; Lambrou et al., 2012; Nygaard et al., 2017) to 1.23 μg/g (Farzan et al., 2017). The percentage of samples with arsenic content below the LOD was not always reported. Toenail arsenic concentrations below the LOD were either excluded (Adair et al., 2006; Phan et al., 2011) or a value was imputed before statistical analysis. The imputed value was usually one-half the LOD value; however, the LOD divided by the square root of 2 was also used (Beamer et al., 2016; Gagnon et al., 2016; Lampron-Goulet et al., 2017; Rivera-Núñez et al., 2011; Wade et al., 2015; Farzan et al., 2017).
3.3. Arsenic concentrations in toenails across populations.
Descriptive measures of toenail arsenic concentrations were reported differently across studies (e.g., arithmetic mean, median, geometric mean, minimum, maximum, etc.) (Table S2). The most common measures presented were arithmetic means (n = 74) and medians (n = 54) (Figure 1).
Figure 1:
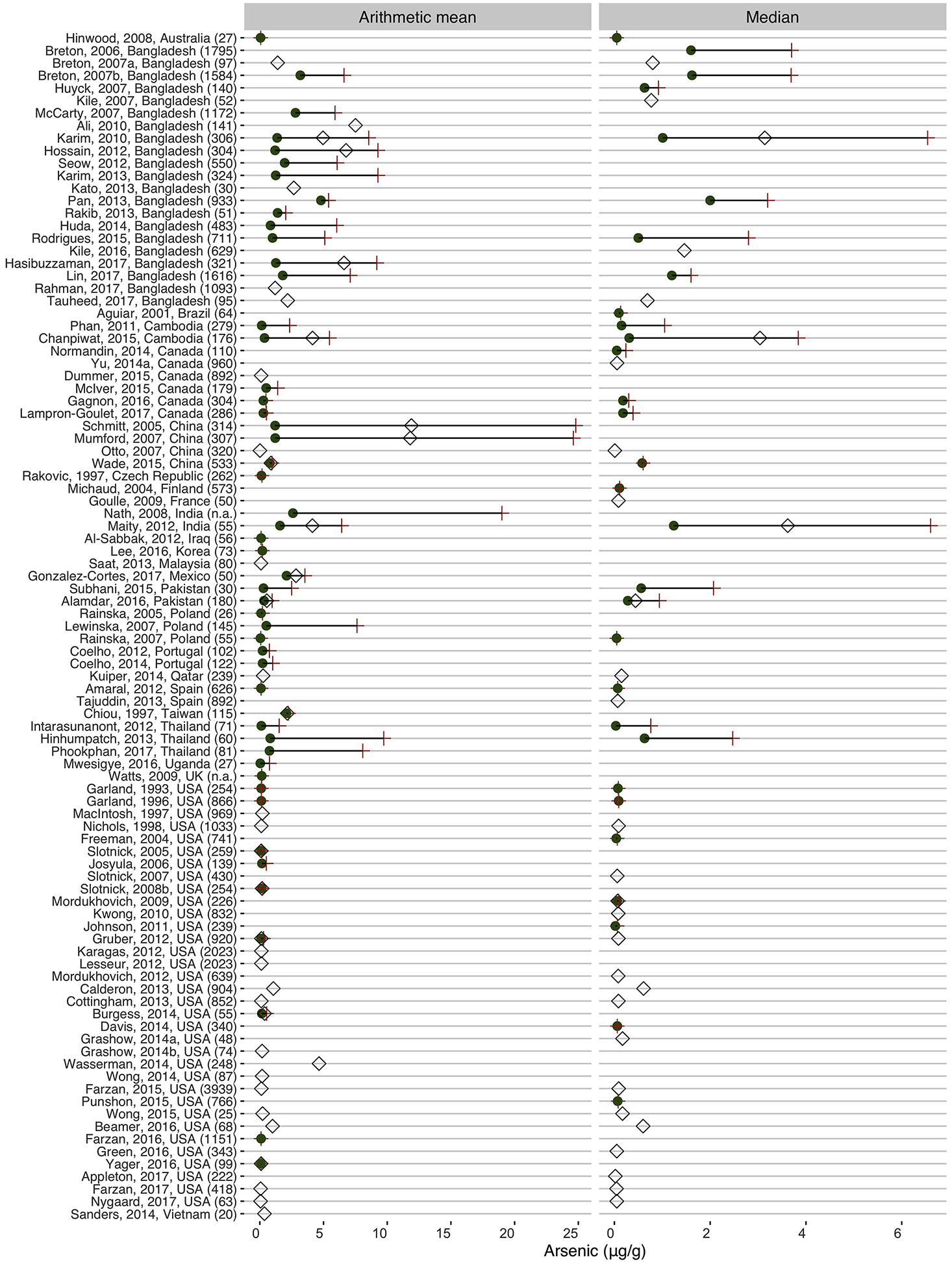
Arsenic concentrations (arithmetic mean or median, μg/g) in human toenails (1993 – 2017).
The green circles and red crosses refer to the minimum and maximum values of toenail arsenic in population subgroups assessed in the reviewed study. The empty rhombus refers to the overall value of toenail arsenic reported in the reviewed study. The number within brackets refers to the overall size of the study population, and when this information is not available (n.a.). The studies are sorted by country, year, and first author’s name. UK = United Kingdom; USA = United States of America.
The selected studies provided data on toenail arsenic concentrations from populations living in 29 different countries. The highest levels were found in those corresponding to Asian countries, particularly Bangladesh, China, and India (Figure 2). There were particularly high median concentrations (≥6.0 μg/g) in populations from India and Bangladesh (Karim et al., 2010; Maity et al., 2012), while the highest arithmetic mean concentration of 24 μg/g was found in adult populations living in Inner Mongolia, China consuming highly arsenic-contaminated water (i.e., 430–690 μg/L) (Mumford et al., 2007; Schmitt et al., 2005). In regard to other areas, relatively high concentrations were also observed among children living in rural areas of Mexico reaching an arithmetic mean of 3.54 μg/g (Gonzalez-Cortes et al., 2017). An arithmetic mean and median toenail arsenic concentrations ≤0.05 μg/g were noted in several studies in populations living in the US (Appleton et al., 2017; Freeman et al., 2004; Green et al., 2016; Johnson et al., 2011; Nygaard et al., 2017), Australia (Hinwood et al., 2008), Canada (Normandin et al., 2014), China (Otto et al., 2007), Poland (Raińska et al., 2007), Thailand (Intarasunanont et al., 2012), and Uganda (Mwesigye et al., 2016).
Figure 2:
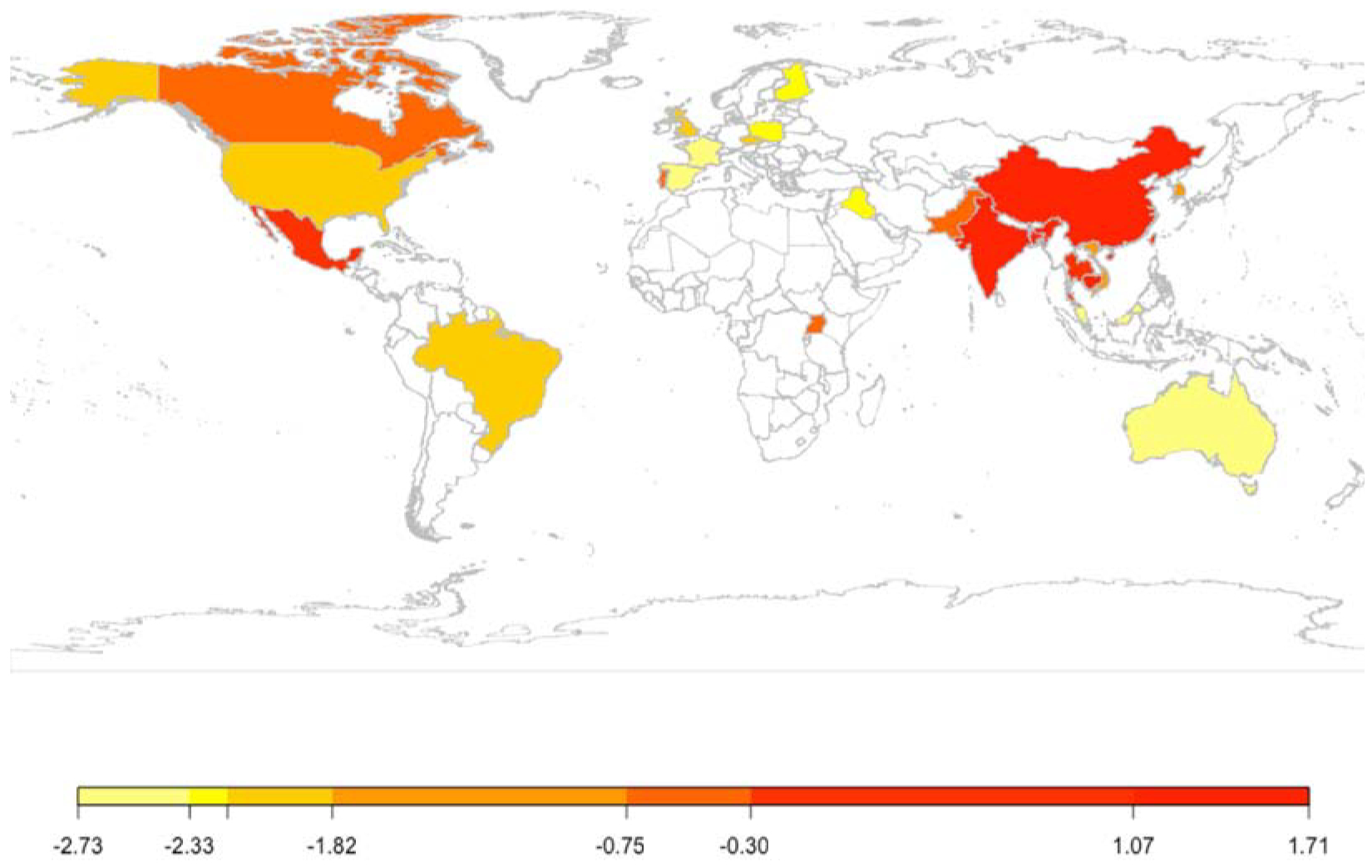
Median arsenic content natural log transformed in toenails according to country based on the reported arithmetic mean/median value.
The colors from yellow to red refer to the log-transformed median toenail arsenic concentrations calculated using the overall mean/median value reported in the included studies. The mean toenail arsenic concentrations were available for Australia (Swab - coastal Plain) (Hinwood et al., 2008), Bangladesh (Sirajdikhan and Pabna regions; Marua in Jessore; Dutpatila and Vultie in Chuadanga; Bheramara in Kushtia; Chowkoli in Naogaon; Communities served by Dhaka Community Hospital; Achintanagar in Jhenaidah) (Ali et al., 2010; Breton et al., 2007a; 2007b; Hasibuzzaman et al., 2017; Hossain et al., 2012; Huda et al., 2014; Karim et al., 2013, 2010; Kato et al., 2013; Lin et al., 2017; McCarty et al., 2007; Pan et al., 2013; Rahman et al., 2017; Rakib et al., 2013; Rodrigues et al., 2015; Seow et al., 2012; Tauheed et al., 2017), Cambodia (Sambour, Preak Chrov, Prey Veng, Chang Kaoh and Kampong Toul in Kandal; Mekong River basin) (Chanpiwat et al., 2015; Phan et al., 2011), Canada (Nova Scotia; Québec) (Dummer et al., 2015; Gagnon et al., 2016; Lampron-Goulet et al., 2017; McIver et al., 2015), China (Inner Mongolia; Ba Men region) (Mumford et al., 2007; Otto et al., 2007; Schmitt et al., 2005; Wade et al., 2015), Czech Republic (City of Prague) (Rakovic et al., 1997), India (West Bengal; 20 km south of Calcutta) (Maity et al., 2012; Nath et al., 2008), Iraq (Communities served by Fallujah Central Hospital)(Al-Sabbak et al., 2012), Korea (Seoul; Kyunggido) (Lee et al., 2016), Malaysia (Saat et al., 2013), Mexico (Comarca Lagunera) (Gonzalez-Cortes et al., 2017), Pakistan (Lahore and Sargodha of Punjab province; Northern Frozen Mountainous; Lower Himalyian Wet Mountainous; Alluvial Riverine; Low lying zone) (Alamdar et al., 2016; Subhani et al., 2015), Poland (Gdansk; Southwestern part of Poland) (Lewińska et al., 2007; Raińska et al., 2007, 2005), Portugal (Panasqueira Mine Area) (Coelho et al., 2014, 2012), Qatar (Kuiper et al., 2014), Spain (Mediterranean coast) (Amaral et al., 2012), Taiwan (Meicheng, and Meifu Village in Lanyang Basin) (Chiou et al., 1997), Thailand (Ron Phibul District, Nakhon Sri Thammarat Province) (Hinhumpatch et al., 2013; Intarasunanont et al., 2012; Phookphan et al., 2017), Uganda (Kilembe mine located 10 km of Kasese town) (Mwesigye et al., 2016), United Kingdom (Nottingham area) (Watts et al., 2009), United States of America (Arizona; 11 states; Across US; Michigan; Massachusetts; New Hampshire; Arizona; Nevada; Maine; New Mexico) (Beamer et al., 2016; Burgess et al., 2014; Calderon et al., 2013; Cottingham et al., 2013; Farzan et al., 2015, 2016, 2017; Garland et al., 1993; Grashow et al., 2014b; Gruber et al., 2012; Josyula et al., 2006; Karagas et al., 2012; Lesseur et al., 2012; MacIntosh et al., 1997; Nichols et al., 1998; Nygaard et al., 2017; Slotnick et al., 2008b, 2005; Wasserman et al., 2014; Wong et al., 2014, 2015; Yager et al., 2016), and Vietnam (Nghia Lo village) (Sanders et al., 2014). For the following countries the mean toenail arsenic concentrations were not available, thus the median concentrations were used: Brazil (São Paulo City) (Aguiar and Saiki, 2001), Finland (Michaud et al., 2004), and France (Goullé et al., 2009).
3.4. Reliability studies of toenail arsenic and correlations with other biological matrices
Within person variability of toenail arsenic concentrations over time was evaluated in two studies from the US (Garland et al., 1993; Karagas, et al., 2001a) and one from Bangladesh (Huyck et al., 2007) (Table 1). The reproducibility over a 6-year period of toenail arsenic was assessed by comparing the concentrations in paired specimens collected in 1982–83 and 1988 from 127 women registered nurses aged 30–55 years living in the US. Average toenail arsenic was 0.11 μg/g, with the spearman correlation coefficient of 0.54 over the 6-year period (Garland et al., 1993). A later US study with a geometric mean of 0.12 μg/g, reported an intraclass correlation of 0.60 calculated for toenail arsenic concentrations collected 3 to 5 years apart among controls of a non-melanoma skin case-control study (Karagas, et al., 2001a). Among 52 women of 18–38 years from Bangladesh with a median toenail arsenic of 0.76 μg/g followed-up multiple times during pregnancy, the correlation coefficient for repeated toenail arsenic concentrations was 0.49 (Huyck et al., 2007).
Table 1:
Correlation coefficients between arsenic in toenails and concentrations in other biological specimens.
| Reference | n | Toenail reproducibility overtime | Infants’ toenails | Urine | Hair | Fingernails | Blood | Saliva | Placenta |
|---|---|---|---|---|---|---|---|---|---|
| Hinwood et al., 2003, Australia | 153 | 0.530 | |||||||
| Huyck et al., 2007, Bangladesh | 52 | 0.490b | 0.630 | ||||||
| Rakib et al., 2013, Bangladesh | 51 | 0.615 (males), and 0.728 (females) | |||||||
| Rodrigues et al., 2015, Bangladesh | 711 | 0.520a | 0.680 | 0.550d | |||||
| Phan et al., 2011, Cambodia | 279 | 0.830 | 0.930 | ||||||
| Chanpiwat et al., 2015, Cambodia | 176 | 0.297 | 0.721 | ||||||
| Normandin et al., 2014, Canada | 110 | 0.610, and 0.604 | 0.594, and 0.619 | ||||||
| Gagnon et al., 2016, Canada | 304 | 0.340 | |||||||
| Maity et al., 2012, India | 55 | 0.710 | 0.730 | ||||||
| Anwar 2005, Pakistan | 155 | 0.270 | |||||||
| Subhani et al., 2015, Pakistan | 30 | (+) | |||||||
| Coelho et al., 2012, Portugal | 102 | 0.750 | 0.850 | ||||||
| Coelho et al., 2014, Portugal | 122 | 0.220 | 0.216 | 0.487 | 0.282 | ||||
| Chiou et al. 1997, Taiwan | 115 | (+) | (+) | ||||||
| Hinhumpatch et al., 2013, Thailand | 60 | 0.510 | |||||||
| Garland et al. 1993, USA | 254 | 0.540 (6-year) | |||||||
| Karagas et al., 2001a, USA | 195 | 0.60 (3/5-year) | 0.420 | ||||||
| Karagas et al., 2002, USA | 1395 | 0.360 | |||||||
| Adair et al., 2006, USA | 95 | 0.301 | |||||||
| Josyula et al., 2006, USA | 139 | 0.344 | |||||||
| Calderon et al., 2013, USA | 904 | 0.450 | |||||||
| Burgess et al., 2014, USA | 55 | 0.161 (AsIII), 0.410 (AsV), 0.203 (MMA), 0.233 (DMA), and 0.289 (ΣAs) | |||||||
| Davis et al., 2014, USA | 340 | 0.340a | 0.190c | ||||||
| Punshon et al., 2015, USA | 766 | 0.300/0.400 | |||||||
| Yager et al., 2016, USA | 99 | 0.360 (iAs), 0.290 (MMA), and 0.290 (DMA) | |||||||
| Farzan et al., 2016, USA | 1151 | 0.180 | |||||||
| Green et al., 2016, USA | 343 | 0.190 | 0.030 | ||||||
| Loh et al., 2016, USA | 70 | 0.330 |
Arsenic correlation between maternal and infant toenails.
Arsenic correlation between prenatal visit and birth.
Arsenic correlation between infant toenails and maternal urine.
Cord blood.
AsIII = Arsenite. AsV = Arsenate. iAs = Inorganic arsenic. MMA = Monomethylarsonic acid. DMA = Dimethylarsinic acid. ∑As = AsIII + AsV + MMA + DMA. + = Positive association. USA = United States of America. The studies are sorted by country, year, and first author’s name.
A total of 28 studies evaluated the correlation of toenail arsenic with other biological matrices (i.e., urine, hair, fingernail, blood, placenta, and saliva), from various parts of the world, and a range of sample sizes from 30 to 1,151 (Table 1). Urinary arsenic is a biomarker of short-term exposure and was the biological matrix more often compared with that in toenail samples (n = 17). Among studies including populations highly exposed from drinking water or with urinary arsenic speciation analysis (n = 14), the positive correlation coefficients ranged from 0.18 to 0.71 with a median of 0.34. The strongest correlation (r = 0.71) was reported from a population with high water arsenic exposure in West Bengal, India, based on 239 adults belonging to 52 different families (Maity et al., 2012), and the lowest coefficients (i.e., <0.20) were observed among US populations, with generally lower water arsenic concentrations (Burgess et al., 2014; Farzan et al., 2016; Green et al., 2016).
Hair has a similar keratin composition to toenails, and our search identified 13 studies that assessed the association between toenail and hair arsenic concentrations. The correlation coefficients ranged from 0.22 to 0.83, and the strongest correlations (r >0.70) were found in studies from Bangladesh, Cambodia, and India with known drinking water arsenic contaminations, and in a mining area in Portugal (Chanpiwat et al., 2015; Coelho et al., 2012; Maity et al., 2012; Phan et al., 2011; Rakib et al., 2013). Three studies evaluated the correlation between arsenic concentrations in toenails and fingernails with moderate (i.e., ranging from >0.3 to <0.7) (Coelho et al., 2014), strong (i.e., >0.7) (Coelho et al., 2012; Phan et al., 2011), and very strong (i.e., >0.9) correlation coefficients (Phan et al., 2011). Only two studies evaluated the correlation between toenail and blood total arsenic content and found moderate correlation coefficients (i.e., 0.28 and 0.55) among populations living in a mining or a water arsenic-contaminated area (Coelho et al., 2014; Rodrigues et al., 2015). Two studies reported low (i.e., ≤0.3) to moderate (i.e., ranging from >0.3 to <0.7) associations between toenail and placenta arsenic concentrations derived from the same study populations in the US (Green et al., 2016; Punshon et al., 2015). The associations between maternal and infants’ arsenic concentrations in toenail samples collected at 4–8 weeks of birth were investigated in two studies with a correlation of 0.34 and 0.42 in mother-infant pairs from US and Bangladesh, respectively (Davis et al., 2014; Rodrigues et al., 2015). Our search identified only one study that investigated the association between toenail and saliva arsenic concentrations. It included children living in an arsenic-contaminated area of Thailand and found a correlation coefficient of 0.51 (Hinhumpatch et al., 2013).
3.5. Correlation of arsenic with other metals in toenails.
The correlation between toenail arsenic and other toenail metal concentrations was explored in 8 studies (Table 2). The correlations between arsenic and toenail content of cadmium (n = 7), manganese (n = 7), and lead (n = 6) were the most frequently investigated. Generally, a modest positive correlation was observed between arsenic and cadmium ranging from 0.25 to 0.59 (Coelho et al., 2012; Grashow et al., 2014a; Mordukhovich et al., 2012; Sanders et al., 2014; Slotnick et al., 2005; Wong et al., 2015), similar to that with lead, with correlations ranging from 0.27 to 0.39 (Grashow et al., 2014a; Mordukhovich et al., 2012; Sanders et al., 2014; Slotnick et al., 2005; Wong et al., 2015). A study from a mining area in Portugal reported a strong positive correlation between arsenic and manganese with a correlation of 0.71 (Coelho et al., 2012). Other studies reported moderately positive correlations between arsenic and manganese with coefficients ranging from 0.27 to 0.58 (Grashow et al., 2014a; Mordukhovich et al., 2012; Sanders et al., 2014; Slotnick et al., 2005; Wong et al., 2015). A study from Qatar of farm workers did not find clear correlations with toenail concentrations of arsenic and cadmium, manganese, or lead. Likewise, they did not observe correlations between arsenic and the concentrations of copper, barium, molybdenum, and uranium in toenail samples (Kuiper et al., 2014). Only one study examined the association between arsenic and aluminum, cobalt and vanadium, and reported moderately positive correlations of 0.34, 0.23 and 0.44, respectively (Slotnick et al., 2005). Two studies found weak correlation coefficients between arsenic and nickel (i.e., 0.20 and 0.26) (Grashow et al, 2014a; Wong et al., 2015). Finally, two studies detected weak inverse correlations (i.e., 0.09 and −0.10) between toenail arsenic and mercury concentrations (Mordukhovich et al., 2012; Sanders et al., 2014).
Table 2:
Correlation coefficients between arsenic and other elements concentrations in human toenails.
| Reference | n | Cd | Mn | Pb | Se | Cr | Cu | Hg | Ni | Al | Ba | Co | Mo | U | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coelho et al., 2012, Portugal | 102 | 0.431 | 0.714 | ||||||||||||
| Kuiper et al., 2014, Qatar | 239 | −0.021 | −0.057 | −0.024 | 0.036 | −0.051 | −0.029 | −0.080 | −0.036 | ||||||
| Slotnick et al., 2005, USA | 259 | 0.242 | 0.298 | 0.397 | 0.274 | 0.407 | 0.177 | 0.343 | 0.226 | 0.441 | |||||
| Mordukhovich et al., 2012, USA | 639 | 0.390 | 0.560 | 0.490 | 0.090 | ||||||||||
| Burgess et al., 2014, USA | 55 | −0.547 | |||||||||||||
| Wong et al., 2015, USA | 25 | 0.250 | 0.270 | 0.270 | 0.260 | ||||||||||
| Grashow et al.,2014a, USA | 48 | 0.370 | 0.370 | 0.490 | 0.200 | ||||||||||
| Sanders et al., 2014, Vietnam | 20 | 0.590 | 0.580 | 0.420 | 0.070 | −0.100 |
The studies are sorted by country, year, and first author’s name. USA = United States of America.
3.6. Sources of exposure
Drinking water is one of the main sources of inorganic arsenic for humans throughout the world, and, consequently, it has also been the main exposure investigated in studies of toenail arsenic concentrations. Other possible sources of arsenic that have been evaluated include diet (Lambrou et al., 2012; Tabata et al., 2006), smoking (Tabata et al., 2006), and occupation such as farming (Kuiper et al., 2014; Saat et al., 2013), galvanizing (Menezes et al., 2004), fertilizing (Raińska et al., 2007, 2005), smelting (Lewińska et al., 2007; Sanders et al., 2014) and mining activities or living in a mining area (Button et al., 2009; Coelho et al., 2014, 2012; Loh et al., 2016; Martin et al., 2013; Ndilila et al., 2014; Pearce et al., 2010; Watts et al., 2009), and other environmental factors such as exposure to dust (Alamdar et al., 2016; Subhani et al., 2015) or soil (Hinwood et al., 2003).
Consumption of water with arsenic level ≥1 μg/L was associated with increased levels of toenail arsenic concentrations (Calderon et al., 2013; Cottingham et al., 2013; Hinwood et al., 2003; Rodrigues et al., 2015; Yu et al., 2014a). Mainly all the studies that explicitly reported drinking water to be among the major source of arsenic exposure came from the US or from populations living in Asian arsenic-contaminated areas, in which sometimes concentrations reached levels >500 μg/L (Breton et al., 2007a; Kile et al., 2005; Maity et al., 2012; Mumford et al., 2007). Drinking water was also identified as an important source of arsenic exposure among populations living in Canada (Lampron-Goulet et al., 2017; McIver et al., 2015; Normandin et al., 2014; Yu et al., 2014b), rural communities in Mexico (Gonzalez-Cortes et al., 2017), and in a southwestern part of England in the United Kingdom (Middleton et al., 2016).
Diet is another main source of exposure, especially among populations with access to low-arsenic drinking water and not occupationally exposed, but available information in regard to toenail arsenic levels is still unclear. Some US studies with modest sample sizes (n >400) have found that toenail arsenic is related to marine products consumption, including fish and seafood intake assessed using food frequency questionnaires (FFQs) (Cottingham et al., 2013; Heck et al., 2009; Slotnick et al., 2007). High toenail arsenic concentrations were also related to consumption of white wine, beer, brussels sprouts, and foods cooked with arsenic contaminated water (Cottingham et al., 2013); in contrast, several dietary lipids (e.g., total fat, total animal fat, total vegetable fat, total monounsaturated fat, total polyunsaturated fat, and total saturated fat), also assessed with information gathered from a FFQ administered to a study population >800 participants, were inversely related to toenail arsenic concentrations (Gruber et al., 2012). Consumption of brown rice, yellow squash, and hot dogs were among the food items identified through a FFQ associated with toenail arsenic in the Nurses’ Health and Health Professional Follow-up study including 969 participants (MacIntosh et al., 1997). Among 1,616 pregnant women in Bangladesh, toenail arsenic was positively associated with consumption of several vegetables, fish, and meat items; however, it was negatively associated with consumption of rice, cereals, fruits, and milk-based items assessed using a semi-quantitative FFQ (Lin et al., 2017). On the contrary, a study from Japan including 159 participants found increased toenail arsenic concentrations associated with daily rice intake in an elderly population, and the authors did not observe an association with alcohol intake, fish or seaweed consumption (Tabata et al., 2006). A lack of association between marine products consumption assessed using a FFQ and toenail arsenic was also reported in a study from Nevada including 95 participants (Adair et al., 2006), and an inverse association was found in a study from Pakistan with 155 participants with available toenail arsenic concentrations (Anwar, 2005).
Some tobacco contains trace amounts of arsenic (Lazarević et al., 2012). Toenail arsenic levels were elevated among elderly smokers (n = 13) with a geometric mean of 0.65 μg/g compared to 0.40 μg/g for non-smokers (n = 110) in a study from Japan with a p for trend obtained from a linear regression adjusted for sex and age of 0.051 (Tabata et al., 2006). On the contrary, in US populations with smoking average prevalence of 15% and toenail arsenic ranging from <0.01 μg/g to 1.26 μg/g no association was found between smoking status and toenail arsenic concentrations (Calderon et al., 2013; Karagas et al., 2000; Slotnick et al., 2007). Similarly, null findings were reported from Australia and China among populations with average smoking prevalence of about 50% and an order of magnitude higher toenail arsenic content than that in the aforementioned US studies (Hinwood et al., 2003; Schmitt et al., 2005).
Occupation and community exposure from industrial sources may lead to higher arsenic exposure. Elevated toenail arsenic concentrations were found among smelters in Poland (mean of 7.63 μg/g) compared to controls (mean of 0.51 μg/g) (Lewińska et al., 2007). Similar findings were reported among workers of galvanizing factories in Brazil (Menezes et al., 2004) and mine workers in Portugal (Coelho et al., 2014, 2012). Also, phosphatic fertilizer production workers in Poland had twice the toenail arsenic concentrations of controls (Raińska et al., 2005); these findings however were not supported in a later study (Raińska et al., 2007). Residents of near former mines or exposed to soil dust also have been associated with an increase of toenail arsenic concentrations in Portugal (Coelho et al., 2012, 2014), Australia (Martin et al., 2013; Pearce et al., 2010), Pakistan (Alamdar et al., 2016; Subhani et al., 2015), and the United Kingdom (Button et al., 2009).
3.7. Personal characteristics
A study from Czech Republic investigated toenail arsenic concentrations in 188 women and 74 men from the Capital City of Prague and found higher arsenic content in women’s versus men’s toenail samples (Rakovic et al., 1997). Increased toenail arsenic concentrations have been found among Bangladeshi women compared to men (Rakib et al., 2013). On the contrary, other studies did not note toenail arsenic gender-related differences (Anwar, 2005; Tabata et al., 2006) or find a reduced toenail arsenic content among women study participants compared to men (Abdulrahman et al., 2012; Schmitt et al., 2005; Yu et al., 2014a).
Changes in toenail arsenic concentrations with age are inconsistent. A population living in West Bengal, India, showed an increased arsenic content in toenail samples with age with median concentrations of 1.24 μg/g, 5.24 μg/g, 6.32 μg/g, 6.60 μg/g, and 5.89 μg/g among ≤14, 14–28, 29–42, 43–56, ≥57 years old age groups, respectively (Maity et al., 2012). Conversely, a US study of New Hampshire residents aged 25–74 reported a decline of −1.49% in toenail arsenic concentrations with age in multiple log-linear regression analysis (Karagas et al., 2000). Toenail concentrations did not differ by age in other studies (Calderon et al., 2013; Coelho et al., 2014; Tabata et al., 2006).
Generally, monthly income and higher education were associated with a decrease in toenail arsenic content (Rodrigues et al., 2015; Yu et al., 2014a). Also, obesity-related dietary patterns and body mass index were found to be inversely related with toenail arsenic levels (Grashow, et al., 2014b; Yu et al., 2014a; Yu et al., 2014b); however, that trend was not identified among participants from the Veterans Administration Normative Aging Study. Indeed, a median toenail arsenic concentration of 0.07 μg/g, 0.07 μg/g, and 0.08 μg/g was reported among participants with a body mass index <25, 25–29, and ≥30, respectively (Mordukhovich et al., 2009).
3.8. Health outcomes/biologic response markers
Overall, 60 studies investigated the association between toenail arsenic concentrations and health outcomes or disease-related biologic responses (Table 3).
Table 3:
Health effects associated with toenail concentrations of arsenic.
| Reference | Cancer | Cardiovascular effects | Other health effects |
|---|---|---|---|
| Breton et al., 2006, Bangladesh | (∅) Hemoglobin level | ||
| Breton et al., 2007b, Bangladesh | (↑) Skin lesions | ||
| Huyck et al., 2007, Bangladesh | ∅ Birth weight | ||
| Ali et al., 2010, Bangladesh | (↓) Plasma cholinesterase | ||
| Karim et al., 2010, Bangladesh | (↑) Activity of lactate dehydrogenase | ||
| Hossain et al., 2012, Bangladesh | (↑) Big endothelin-1 | ||
| Seow et al., 2012, Bangladesh | (↑) Skin lesions | ||
| Karim et al., 2013, Bangladesh | (↑) Oxidized low-density lipoprotein and other inflammatory and adhesion molecules | ||
| Pan et al., 2013, Bangladesh | (↑) Risk of diabetes | ||
| Huda et al., 2014, Bangladesh | (↑) Hypertension | ||
| Islam et al., 2015, Bangladesh | (↑) Matrix metalloproteinase-2 and 9 | ||
| Rahman et al., 2015, Bangladesh | (↑) Vascular endothelial growth factor | ||
| Kile et al., 2016, Bangladesh | (↓) Birth weight | ||
| Hasibuzzaman et al., 2017, Bangladesh | (↑) Soluble thrombomodulin | ||
| Hossain et al., 2017, Bangladesh | (↓) Long interspersed nuclear element-1 (LINE-1) | ||
| Rahman et al., 2017, Bangladesh | (↓) Birth weight | ||
| Tauheed et al., 2017, Bangladesh | (↑) Risk of myelomeningocele | ||
| Aguiar et al., 2001, Brazil | (∅) Cystic fibrosis. | ||
| Yu et al., 2014a, Canada | (↓) Obesity | ||
| Yu et al., 2014b, Canada | (↓) Obesity | ||
| Lampron-Goulet et al., 2017, Canada | (↑) Risk of diabetes | ||
| Otto et al., 2007, China | (↑) Neurosensory effects | ||
| Mumford et al., 2007, China | (↑) Risk of arrhythmia | ||
| Mo et al., 2009a, China | (↑) mRNA levels of ERCC1 expression | ||
| Mo et al., 2009b, China | (↑) hTERT mRNA expression levels | ||
| Wade et al., 2015, China | (↑) Risk of cardiovascular disease | ||
| Maity et al., 2012, India | (↑) Skin lesions | ||
| Al-Sabbak et al., 2012, Iraq | (↑) Birth defects | ||
| Lee et al., 2016, Korea | (↑) Serum triglyceride and total cholesterol level | ||
| Saat et al., 2013, Malaysia | (↑) Hypertension | ||
| Yager et al., 2016, Mexico | (∅) Risk of melanoma | ||
| Gonzalez-Cortes et al., 2017, Mexico | (↑) MMP9 methylaFon | ||
| Amaral et al., 2012, Spain | (↑) Risk of pancreatic cancer | ||
| Tajuddin et al., 2013, Spain | (↓) LINE-1 methylation | ||
| Intarasunanont et al., 2012, Thailand | (↑) p53 methylaFon. | ||
| Phookphan et al., 2017, Thailand | (↓) Gene hypomethylaFon (↑) 8-nitroguanine level | ||
| Garland et al., 1996, USA | (∅) Breast cancer | ||
| Nichols et al., 1998, USA | (↑) Risk of skin cancer | ||
| Karagas et al., 2001b, USA | (↑) Risk of skin cancer | ||
| Freeman et al., 2004, USA | (↑) Risk melanoma | ||
| Karagas et al., 2004, USA | (↑) Risk of bladder cancer among smokers | ||
| Marsit et al., 2006, USA | (↑) Tumor suppressor genes (RASSF1A and PRSS3) (∅) p16INK4A | ||
| Andrew et al., 2009, USA | (↑) Risk of lung cancer | ||
| Heck et al., 2009, USA | (↑) Risk of lung cancer | ||
| Mordukhovich et al., 2009, USA | (↑) Risk factor for arrhythmia and sudden cardiac death | ||
| Kwong et al., 2010, USA | (↓) Survival hazard raFo bladder cancer | ||
| Johnson et al., 2011, USA | (↑) Colorectal cancer and lung cancer | ||
| Karagas et al., 2012, USA | (↑) Risk of bladder cancer | ||
| Lambrou et al., 2012, USA | (↓) Decreasing LINE-1 DNA methylation | ||
| Lesseur et al., 2012, USA | (↑) Risk of bladder cancer | ||
| Mordukhovich et al., 2012, USA | (↑) Hypertension | ||
| Burgess et al., 2014, USA | (↓) Alpha 1-Antitrypsin | ||
| Farzan et al., 2015, USA | (↑) Risk of ischemic heart disease mortality among smokers | ||
| Wong et al., 2015, USA | (∅) Arterial compliance (augmentation index). | ||
| Beamer et al., 2016, USA | (↓) Lung funcFon | ||
| Farzan et al., 2016, USA | (↑) GestaFonal diabetes | ||
| Green et al., 2016, USA | (↑) Placental DNA methylaFon | ||
| Appleton et al, 2017, USA | (↑) Placental NR3C1 methylaFon | ||
| Farzan et al., 2017, USA | (↑) Cellular adhesion molecules (↓) matrix metalloproteinase-9 | ||
| Nygaard et al., 2017, USA | (↓) T cell populaFon at birth |
↓ = Decreased effect. ↑ = Increased effect. ∅ = Null effect. USA = United States of America. The studies are sorted by country, year, and first author’s name.
We identified 14 studies that evaluated the association between toenail arsenic and cancer. A positive association was found between toenail arsenic content and several types of cancers including squamous cell skin cancer (Karagas, et al., 2001b; Nichols et al., 1998), melanoma (Freeman et al., 2004), lung cancer (Andrew et al., 2009; Heck et al., 2009; Johnson et al., 2011), bladder cancer (Karagas et al., 2012, 2004; Lesseur et al., 2012), and cancer of pancreas (Amaral et al., 2012). Among individuals from the State of New Hampshire with toenail arsenic concentrations above the 97th percentile (0.34 μg/g), the adjusted odds ratios (OR) were 2.07 (95% confidence interval (CI): 0.92, 4.66) for squamous cell carcinoma and 1.44 (95% CI: 0.74, 2.81) for basal cell carcinoma (Karagas, et al., 2001b). Also, in a population from Iowa, an OR equal to 2.1 (95% CI: 1.4, 3.3) for risk of melanoma was calculated among participants with toenail arsenic ≥0.08 μg/g in relation to ≤0.02 μg/g (Freeman et al., 2004). In contrast, in a New Mexico population-based study toenail arsenic was not associated with cutaneous melanoma (Yager et al., 2016). Elevated toenail arsenic also appears to be associated with an increased risk of lung and bladder cancer among US populations (Andrew et al., 2009; Heck et al., 2009; Johnson et al., 2011; Karagas et al., 2012, 2004; Lesseur et al., 2012). For example, an increased risk of small-cell and squamous-cell carcinoma of the lung (OR = 2.75; 95% CI: 1.00, 7.57) was associated with toenail arsenic concentrations ≥0.11 μg/g (Heck et al., 2009). Likewise, an elevated risk for bladder cancer was observed (OR = 2.17, 95% CI: 0.92, 5.11) for participants with toenail arsenic >0.33 μg/g; however, the association was lost among never smokers (Karagas et al., 2004). Toenail arsenic was inversely associated with bladder cancer (hazard ratio (HR) = 0.5 (95% CI: 0.4, 0.8) in participants with toenail arsenic >0.12 μg/g against <0.06 μg/g (Kwong et al., 2010). In Spain, the highest quartile toenail arsenic concentrations (>0.11 μg/g) were related to an increased risk of pancreatic cancer (OR = 2.02, 95% CI: 1.08, 3.78) compared to concentrations within the first quartile (<0.05 μg/L) (Amaral et al., 2012). There was no evidence of an association between toenail arsenic concentrations and the risk of breast cancer (Garland et al., 1996).
We identified 14 studies that investigated the association between toenail arsenic concentrations and cardiovascular outcomes. Several studies from Bangladesh, China, Korea, Malaysia, and US found an increased risk of cardiovascular diseases associated with toenail arsenic (Farzan et al., 2015, 2017; Hasibuzzaman et al., 2017; Hinhumpatch et al., 2013; Huda et al., 2014; Karim et al., 2013; Lee et al., 2016; Mordukhovich et al., 2009, 2012; Mumford et al., 2007; Pan et al., 2013; Rivera-Núñez et al., 2011; Saat et al., 2013; Wade et al., 2015). For example, among participants from the Normative Aging Study, an IQR in toenail arsenic (0.06 μg/g) was associated with higher systolic blood pressure (ß = 1.43 mmHg; 95% CI: 0.34, 2.51) in the fully adjusted linear regression model (Mordukhovich et al., 2012). Higher toenail arsenic concentrations with an average of 0.07 μg/g were observed among Malaysian farmers with hypertension compared to participants with normal blood pressure, whose average toenail arsenic was 0.06 μg/g (Saat et al., 2013). In China, the prevalence rates of cardiac repolarization abnormalities with corrected QT interval prolongation were 3.9%, 11.1%, 20.6% among participants exposed to low, medium, and high arsenic with average toenail concentrations of 1.21 μg/g, 9.79 μg/g, and 24.61 μg/g, respectively (Mumford et al., 2007). These findings are in agreement with a later US study, which reported that an IQR increase in toenail arsenic (0.06 μg/g) was associated with a 2.5-millisecond increase in corrected QT (95% CI: 0.11, 4.9) in a linear regression model (Mordukhovich et al., 2009). In contrast, a study of US welders found no association with arterial compliance, an indicator of the extent to which arteries distend and constrict following systole (Wong et al., 2015).
Additional studies have evaluated the associations with diabetes. Compared with participants exposed to the lowest quartile of toenail arsenic (≤0.93 μg/g), the adjusted OR for type 2 diabetes was 3.34 (95% CI: 1.16, 5.31) for those in the second quartile (0.94–2.12 μg/g), 3.40 (95% CI: 1.04, 4.87) for those in the third quartile (2.13–6.18 μg/g), and 6.22 (95% CI: 2.63, 14.69) for those in the fourth quartile (≥6.19 μg/g) in a study from Bangladesh (Pan et al., 2013), and that trend appeared consistent among US and Canada populations (Farzan et al., 2016; Lampron-Goulet et al., 2017).
Further studies evaluated the association between toenail arsenic concentrations and other health outcomes. In arsenic endemic areas in Bangladesh and India, higher toenail arsenic concentrations were related to an increased risk of melanosis/keratosis (Breton et al., 2007b; Maity et al., 2012; Seow et al., 2012). A case-control study including participants served by the Pabna Community Clinic in Bangladesh found that cases with skin lesions had 2-fold higher mean toenail concentrations (6.61 μg/g) compared to controls (3.18 μg/g) (Breton et al., 2007b). Also, a follow-up study showed that participants with skin lesions had a mean toenail arsenic concentration 3-fold higher (6.07 μg/g) compared to recovered cases with no skin lesions (1.95 μg/g) (Seow et al., 2012). Also in Bangladesh, an increase in maternal natural log toenail arsenic was associated with decreased birth weight (β = −15.72 g, 95% CI: −24.52, −6.91) in a structural equation model mediated through gestational age and maternal gain during pregnancy (Kile et al., 2016), which was in agreement with a later study (Rahman et al., 2017); however, the association was not noticed in another study (Huyck et al., 2007). Toenail arsenic was also associated with an increased risk of myelomeningocele in Bangladesh (Tauheed et al., 2017) and birth defects in Iraq (Al-Sabbak et al., 2012). Among immune markers investigated, an inverse association was found in regression analysis between maternal toenail arsenic and the T cells population at birth (ß = −21%, 95% CI: −36%, −3%) (Nygaard et al., 2017). Several studies investigated the association between a wide range of toenail arsenic concentrations and different indicators of DNA methylation and gene expression with inconsistent results. For example, an increase in toenail arsenic was positively associated with placental DNA methylation (Appleton et al., 2017; Green et al., 2016), p53 methylation (Intarasunanont et al., 2012) and mRNA levels of ERCC1 expression (Mo et al., 2009a). Toenail arsenic was inversely associated with LINE-1 methylation (Hossain et al., 2017; Lambrou et al., 2012; Tajuddin et al., 2013), and alpha 1-antitrypsin (Burgess et al., 2014).
4. Discussion
In this systematic review, we summarized available data related to toenail arsenic content across various populations around the world and assessed the validity of toenail arsenic concentrations as an exposure biomarker and its use in chronic disease research. Toenail samples are gaining attention as useful biomarkers for large-scaled epidemiological studies because of their ability to accumulate arsenic, slow growth rate, and being potentially less prone to exogenous contamination compared to fingernail and hair samples. Furthermore, they have logistical advantages such as non-invasive collection, easy transport and conservation (Ab Razak et al., 2015; Adair et al., 2006; Button et al., 2009; Gutiérrez-González et al., 2019; Marchiset-Ferlay et al., 2012; Slotnick et al., 2008a). Additionally, advanced technologies now permit trace and even ultra-trace detection levels. Nevertheless, there are still uncertainties regarding the accuracy of a single toenail sample arsenic content as a long-term exposure biomarker. Also, two relevant issues that may limit its use and its interpretation is the lack of toenail arsenic reference material, as well as the absence of a standardized toenail arsenic processing protocol, which should, among other factors, address the impact of the cleaning procedure and the effect of toenail weights in the results obtained. The development of standardized toenail preparation and analysis protocols would be useful to support a routine use of toenail arsenic concentrations as an exposure biomarker.
Toenail keratin matrix accumulates arsenic from the blood flow during growth (Marchiset-Ferlay et al., 2012). It is estimated that toenails follow a linear growth of 0.03–0.05 mm per day (Goullé et al., 2009; Slotnick, 2011), and thus, depending on individuals’ growth rate and clipping length, a toenail sample is expected to reflect exposures occurring from 5 to 18 months before collection. However, the impact of unknown factors on toenail growth make it difficult to identify the exact time window of exposure (Adair et al., 2006; Esteban and Castaño, 2009; Garland et al., 1993; Goullé et al., 2009; Slotnick, 2011). Currently, growth rate is not easily measured and it is not accounted for in association analysis. Growth factors could be related to subclinical disease, which would reversely influence the association with health outcomes. In regard to the use of a point measurement as a proxy of longer term exposure, some authors have reported high reproducibility of arsenic levels among women from a highly arsenic-contaminated region in Bangladesh in different toenail samples collected throughout the pregnancy period (Huyck et al., 2007), and similar results have been found in the US (Signes-Pastor et al., 2019). Further, evidence among US adult populations suggests that toenail arsenic content may reflect exposures occurring over even longer periods of time ranging from 3 to 6 years (Garland et al., 1993; Karagas, et al., 2001a). These findings provide an optimistic degree of confidence in the use of toenail levels as a reflection of inorganic arsenic exposure over long periods of time, even at relatively low exposure levels, and particularly among populations with consistent patterns of exposure. However, additional studies with the assessment of repeated measures of toenail arsenic concentrations over time are needed.
Toenails are also capable of accumulating other trace elements (Gutiérrez-González et al., 2019) that may correlate with toenail arsenic concentrations suggesting common exposure sources or metabolic pathways (Signes-Pastor et al., 2019; Stafoggia et al., 2017). Associations have been observed between toenail arsenic and the concentrations of cadmium, manganese, and lead (Coelho et al., 2012; Grashow et al, 2014a; Mordukhovich et al., 2012; Sanders et al., 2014; Slotnick et al., 2005; Wong et al., 2015) in line with later findings (Signes-Pastor et al., 2019).
Accumulated toenail arsenic is expected to represent levels of human exposure over long periods of time. In this sense, results from chronic highly exposed populations provide evidence of a strong consistency of arsenic content in toenail samples with those of other long-term exposure biomarkers (i.e., hair and nails). Hair and fingernails share similar features with toenails in terms of slow rate of growth, similar composition and ability to accumulate arsenic; however, hair and fingernails are considered more susceptible to external contamination (e.g., from dust, shampoos, and cosmetic procedures), which may limit their use as long-term arsenic exposure biomarkers (Esteban and Castaño, 2009; Karagas et al., 2000; Marchiset-Ferlay et al., 2012). Nevertheless, among highly exposed populations with high toenail arsenic concentrations (i.e., arithmetic mean or median ≥1 μg/g), concurrence was observed between toenail arsenic and concentrations in hair and fingernails (Chanpiwat et al., 2015; Maity et al., 2012; Phan et al., 2011; Rakib et al., 2013; Rodrigues et al., 2015).
Toenails are considered to have more stable concentrations than those biomarkers of recent exposures such as urine, which may vary between and within days (Adair et al., 2006; Esteban and Castaño, 2009; Garland et al., 1993; Goullé et al., 2009; Meharg et al., 2014; Slotnick, 2011). Furthermore, data suggest that toenail arsenic only reflects exposure to inorganic arsenic. Thus, toenail concentrations are less susceptible to inorganic arsenic exposure misclassification compared to urine or blood arsenic that reflects exposure to both organic and inorganic arsenic making the latter suitable for arsenic metabolism studies (Cubadda et al., 2016; Jones et al., 2016; Marchiset-Ferlay et al., 2012; Navas-Acien et al., 2011). However, lack of arsenic speciation analysis in urine and blood may leave open the likelihood of inorganic arsenic exposure misclassification due to ingestion of organic forms such as that from marine products consumption (Matoušek et al., 2017; Navas-Acien et al., 2011; Taylor et al., 2016), which may compromise the validity of the associations between urine/blood and toenail arsenic concentrations. Even though a strong positive correlation between toenail arsenic and urinary concentrations was reported from a population consuming arsenic-contaminated water in India (Maity et al., 2012), this association was not consistent across studies (Adair et al., 2006; Calderon et al., 2013; Chanpiwat et al., 2015; Coelho et al., 2014, 2012; Gagnon et al., 2016). Genetic variants in AS3MT may influence an individual’s arsenic methylation ability (Agusa et al., 2011; Antonelli et al., 2014). Thus, higher methylation capacity could alter toenail arsenic concentrations although there are limited data on whether this occurs. Correlation between toenail arsenic and other biomarkers among less exposed populations may also be hard to evaluate due to the imprecision of the measures at the lower end of exposure, therefore, additional data at low arsenic exposure levels are needed using rigorous advanced methods.
The studies included in this review provided quantitative evidence on toenail arsenic from populations residing in several areas around the world; but these were not evenly distributed across countries. Based on the reported literature, there were clear geographic differences in toenail arsenic, that likely reflect population differences in the magnitude and sources of arsenic exposure. We identified several populations with high toenail arsenic concentrations, which include those with geogenic arsenic contaminated water in Bangladesh (Ali et al., 2010; Breton et al., 2007a, 2007b; Hasibuzzaman et al., 2017; Hossain et al., 2012; Huda et al., 2014; Karim et al., 2013, 2010; Kato et al., 2013; Lin et al., 2017; McCarty et al., 2007; Pan et al., 2013; Rahman et al., 2017; Rakib et al., 2013; Rodrigues et al., 2015; Seow et al., 2012; Tauheed et al., 2017) but also from other Asian countries (i.e., Cambodia, China, India, Taiwan, and Thailand) (Chanpiwat et al., 2015; Chiou et al., 1997; Hinhumpatch et al., 2013; Intarasunanont et al., 2012; Maity et al., 2012; Nath et al., 2008; Phan et al., 2011; Phookphan et al., 2017; Schmitt et al., 2005; Subhani et al., 2015). In America, levels were also high in Canada (McIver et al., 2015), Mexico (Gonzalez-Cortes et al., 2017), and the US (Beamer et al., 2016; Calderon et al., 2013; Wasserman et al., 2014).
As expected, toenail arsenic concentrations were associated with higher amounts of arsenic in drinking water where inorganic arsenic predominates (Calderon et al., 2013; Cottingham et al., 2013; Hinwood et al., 2003; Rodrigues et al., 2015; Yu et al., 2014b). Arsenic-contaminated drinking water is the main exposure source for arsenic-endemic areas worldwide (Breton et al., 2007a; Kile et al., 2005; Maity et al., 2012; Mumford et al., 2007; Podgorski and Berg, 2020). Diet is a major exposure source where drinking water contains relatively low levels of arsenic (Nachman et al., 2018). However, there remain uncertainties regarding the specific foods such as marine products or beverages that affect arsenic levels in toenails. This may be due to misclassification of the foods and beverages consumed or the time period for which the dietary information represents when assessed using FFQs (Adair et al., 2006; Anwar, 2005; Cottingham et al., 2013; Heck et al., 2009; Lin et al., 2017; MacIntosh et al., 1997; Slotnick, 2007, 2011; Tabata et al., 2006). Also, the reported negative associations between toenail arsenic and consumption of rice, cereals, fruits, and milk-based items could be related to low arsenic levels in these food items as well as lower consumption of rice relative to that of grains, cereals and bread (Lin et al., 2017). Clearly, additional studies are needed in this area. There are also inconsistent findings regarding the association between smoking and arsenic levels in toenail samples (Calderon et al., 2013; Hinwood et al., 2003; Schmitt et al., 2005; Slotnick et al., 2007; Tabata et al., 2006). In regard to occupational exposures, there is some evidence of increased toenail arsenic concentrations in relation to residing in specific industrial areas and with exposure in the workplace, which appears to affect mainly men employed in mines or smelting operations (Coelho et al., 2014; Lewińska et al., 2007; Subhani et al., 2015). However, we did not find clear evidence of toenail arsenic gender-related differences (Abdulrahman et al., 2012; Anwar, 2005; Rakovic et al., 1997; Schmitt et al., 2005; Tabata et al., 2006; Yu et al., 2014a) or variations according to age (Calderon et al., 2013; Coelho et al., 2014; Maity et al., 2012; Tabata et al., 2006) including among children (Appleton et al., 2017; Hinhumpatch et al., 2013; Mwesigye et al., 2016). On the contrary, higher socioeconomic status was associated with a decreased toenail arsenic content, which could be related to an increased likelihood for consuming low-arsenic drinking water and nutritious food among populations with high economic and social status (Rodrigues et al., 2015; Yu et al., 2014a) but this will need to be explored further.
Mechanism of arsenic carcinogenesis include both genotoxic and non-genotoxic processes, which may differ according to the arsenic exposure level (Rossman, 2003; Rossman and Klein, 2011). Nevertheless, we observed a consistent increased risk of skin cancer with toenail arsenic concentrations (Freeman et al., 2004; Karagas, et al., 2001b; Nichols et al., 1998). An increased risk of pancreas, bladder and lung cancer was identified across various studies (Amaral et al., 2012; Andrew et al., 2009; Heck et al., 2009; Johnson et al., 2011; Karagas et al., 2012, 2004; Lesseur et al., 2012). Regardless of the arsenic exposure level, we found evidence of an increased risk of several cardiovascular adverse health effects with toenail arsenic concentrations (Farzan et al., 2015; Hasibuzzaman et al., 2017; Huda et al., 2014; Islam et al., 2015; Karim et al., 2013; Lee et al., 2016; Mordukhovich et al., 2009, 2012; Mumford et al., 2007; Pan et al., 2013; Saat et al., 2013; Wade et al., 2015). In aggregate, findings support toenail arsenic concentrations as a biomarker to asses chronic disease research including cancer, and cardiovascular adverse health effects even at relatively low arsenic exposure levels. Evidence is growing on an association between toenail arsenic concentrations and risk of diabetes (Farzan et al., 2016; Lampron-Goulet et al., 2017; Pan et al., 2013). Still, further research is needed to support the association between toenail arsenic and additional adverse health and mechanistic effects, especially for the general populations exposed at relatively low levels of inorganic arsenic.
We observed great heterogeneity in terms of baseline characteristics dependent on the aims of each investigation, which challenge our ability to carry out quantitative pooling and comparisons. It should be noted too that various studies also failed in providing, or in taking into account in their analyses toenail weights, which may largely bias toenail arsenic concentrations, quality control parameters and LOD. In addition, our review only indexed reports written in English, and several studies were excluded because the origin of the nail samples could not be determined (i.e., fingernails or toenails). However, the reviewed studies allowed analysis of the available evidence on the role of toenails as a biological matrix of arsenic exposure. We identified several uncertainties on the potential role of diet, gender, age, lifestyle-related and other factors on arsenic levels in toenails. Nevertheless, we found evidence that a single toenail sample may reflect long-term internal dose of inorganic arsenic exposure enabling toenail arsenic as an adequate biomarker to assess chronic disease research such as cancer and cardiovascular conditions and potentially others. Still, more research is needed particularly among populations exposed at relatively low levels of arsenic using rigorous advanced methods.
Supplementary Material
HIGHLIGHTS.
Data suggests that a single toenail arsenic content indicates long-term exposure
Available data show that toenail arsenic reflects a wide window of exposure
Toenail arsenic correlates with concentrations in other exposure biomarkers
A validated toenail processing protocol for toenail arsenic determination is needed
Toenail arsenic may be an adequate exposure biomarker in chronic disease research
Funding sources
AS and MK are funded in part by the following projects P01ES022832, RD83544201, R25CA134286, P42ES007373, and 5UH3OD023275. MG is funded by CIBERESP (PhD-employment-contract and fellowship for short stays abroad-2019). BP is partially funded by PI17CIII/00034. AN is funded by the following projects P42ES010349, P30ES009089, R01ES028758.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no competing financial interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdulrahman F, Akan J, Chellube Z, Waziri M, 2012. Levels of Heavy Metals in Human Hair and Nail Samples from Maiduguri Metropolis, Borno State, Nigeria. World Environment 2, 81–89. 10.5923/j.env.20120204.05 [DOI] [Google Scholar]
- Ab Razak NH, Praveena SM, Hashim Z, 2015. Toenail as a biomarker of heavy metal exposure via drinking water: A systematic review. 10.1515/reveh-2014-0063 [DOI] [PubMed]
- Adair BM, Hudgens EE, Schmitt MT, Calderon RL, Thomas DJ, 2006. Total arsenic concentrations in toenails quantified by two techniques provide a useful biomarker of chronic arsenic exposure in drinking water. Environmental Research 101, 213–220. 10.1016/j.envres.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Aguiar AR, Saiki M, 2001. Determination of trace elements in human nail clippings by neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry 249, 413–416. 10.1023/A:1013235123875 [DOI] [Google Scholar]
- Agusa T, Fujihara J, Takeshita H, Iwata H, 2011. Individual variations in inorganic arsenic metabolism associated with AS3MT genetic polymorphisms. 10.3390/ijms12042351 [DOI] [PMC free article] [PubMed]
- Alamdar A, Musstjab Akber Shah Eqani S, Waqar Ali S, Sohail M, Bhowmik AK, Cincinelli A, Subhani M, Ghaffar B, Ullah R, Huang Q, Shen H, 2016. Human Arsenic exposure via dust across the different ecological zones of Pakistan. Ecotoxicology and Environmental Safety 126, 219–227. 10.1016/j.ecoenv.2015.12.044 [DOI] [PubMed] [Google Scholar]
- Ali N, Hoque MA, Haque A, Salam KA, Karim MR, Rahman A, Islam K, Saud ZA, Khalek MA, Akhand AA, Hossain M, Mandal A, Karim MR, Miyataka H, Himeno S, Hossain K, 2010. Association between arsenic exposure and plasma cholinesterase activity: a population-based study in Bangladesh. Environmental health : a global access science source 9, 36. 10.1186/1476-069X-9-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sabbak M, Ali SS, Savabi O, Savabi G, Dastgiri S, Savabieasfahani M, 2012. Metal contamination and the epidemic of congenital birth defects in Iraqi cities. Bulletin of Environmental Contamination and Toxicology 89, 937–944. 10.1007/s00128-012-0817-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral AFS, Porta M, Silverman DT, Milne RL, Kogevinas M, Rothman N, Cantor KP, Jackson BP, Pumarega JA, López T, Carrato A, Guarner L, Real FX, Malats N, 2012. Pancreatic cancer risk and levels of trace elements. Gut 61, 1583–1588. 10.1136/gutjnl-2011-301086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew AS, Mason RA, Memoli V, Duell EJ, 2009. Arsenic activates EGFR pathway signaling in the lung. Toxicological Sciences 109, 350–357. 10.1093/toxsci/kfp015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli R, Shao K, Thomas DJ, Sams R, Cowden J, 2014. AS3MT, GSTO, and PNP polymorphisms: Impact on arsenic methylation and implications for disease susceptibility. Environmental Research 132, 156–167. 10.1016/j.envres.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Anwar M, 2005. Arsenic, Cadmium and Lead Levels in Hair and Toenail Samples in Pakistan. Environmental Sciences 2, 71–86. [PubMed] [Google Scholar]
- Appleton AA, Jackson BP, Karagas M, Marsit CJ, 2017. Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics 12, 607–615. 10.1080/15592294.2017.1320637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer PI, Klimecki WT, Loh M, Van Horne YO, Sugeng AJ, Lothrop N, Billheimer D, Guerra S, Lantz RC, Canales RA, Martinez FD, 2016. Association of children’s urinary CC16 levels with arsenic concentrations in multiple environmental media. International Journal of Environmental Research and Public Health 13, 1–16. 10.3390/ijerph13050521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Houseman EA, Kile ML, Quamruzzaman Q, Rahman M, Mahiuddin G, Christian DC, 2006. Gender-specific protective effect of hemoglobin on arsenic-induced skin lesions. Cancer Epidemiology Biomarkers and Prevention 15, 902–907. 10.1158/1055-9965.EPI-05-0859 [DOI] [PubMed] [Google Scholar]
- Breton CV, Kile ML, Catalano PJ, Hoffman E, Quamruzzaman Q, Rahman M, Mahiuddin G, Christiani DC, 2007a. GSTM1 and APE1 genotypes affect arsenic-induced oxidative stress: A repeated measures study. Environmental Health: A Global Access Science Source 6, 1–9. 10.1186/1476-069X-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Zhou W, Kile ML, Houseman EA, Quamruzzaman Q, Rahman M, Mahiuddin G, Christiani DC, 2007b. Susceptibility to arsenic-induced skin lesions from polymorphisms in base excision repair genes. Carcinogenesis 28, 1520–1525. 10.1093/carcin/bgm063 [DOI] [PubMed] [Google Scholar]
- Buchet JP, Lauwerys R, Roels H, 1981. Comparison of the Urinary Excretion of Arsenic Metabolites After a Single Oral Dose of Sodium Arsenite, Monomethylarsonate, or Dimethylarsinate in Man. Intemational Archives of Int Arch Occup Environ Health 48, 71–79. 10.1007/BF00405933 [DOI] [PubMed] [Google Scholar]
- Burgess JL, Kurzius-Spencer M, Poplin GS, Littau SR, Kopplin MJ, Stürup S, Boitano S, Clark Lantz R, 2014. Environmental arsenic exposure, selenium and sputum alpha-1 antitrypsin. Journal of Exposure Science and Environmental Epidemiology 24, 150–155. 10.1038/jes.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button M, Jenkin GRT, Harrington CF, Watts MJ, 2009. Human toenails as a biomarker of exposure to elevated environmental arsenic. Journal of Environmental Monitoring 11, 610–617. 10.1039/b817097e [DOI] [PubMed] [Google Scholar]
- Calderon RL, Hudgens EE, Carty C, He B, Le XC, Rogers J, Thomas DJ, 2013. Biological and behavioral factors modify biomarkers of arsenic exposure in a U.S. population. Environmental Research 126, 134–144. 10.1016/j.envres.2013.04.004 [DOI] [PubMed] [Google Scholar]
- Carbonell-Barrachina AA, Signes-Pastor AJ, Vázquez-Araújo L, Burló F, Sengupta B, 2009. Presence of arsenic in agricultural products from arsenic-endemic areas and strategies to reduce arsenic intake in rural villages. Molecular Nutrition & Food Research 53, 531–541. 10.1002/mnfr.200900038 [DOI] [PubMed] [Google Scholar]
- Chanpiwat P, Himeno S, Sthiannopkao S, 2015. Arsenic and other metals’ presence in biomarkers of cambodians in arsenic contaminated areas. International Journal of Environmental Research and Public Health 12, 14285–14300. 10.3390/ijerph121114285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou HY, Hsueh YM, Hsieh LL, Hsu LI, Yi-Hsiang H, Hsieh FI, Wei ML, Chen HC, Yang HT, Leu LC, Chu TH, Chen-Wu C, Yang MH, Chen CJ, 1997. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutation Research - Reviews in Mutation Research 386, 197–207. 10.1016/S1383-5742(97)00005-7 [DOI] [PubMed] [Google Scholar]
- Coelho P, Costa S, Costa C, Silva S, Walter A, Ranville J, Pastorinho MR, Harrington C, Taylor A, Dall’Armi V, Zoffoli R, Candeias C, Silva E.F. da, Bonassi S, Laffon B, Teixeira JP, 2014. Biomonitoring of several toxic metal(loid)s in different biological matrices from environmentally and occupationally exposed populations from Panasqueira mine area, Portugal. Environmental Geochemistry and Health 36, 255–269. 10.1007/s10653-013-9562-7 [DOI] [PubMed] [Google Scholar]
- Coelho P, Costa S, Silva S, Walter A, Ranville J, Sousa AC, Costa C, Coelho M, García-Lestón J, Pastorinho MR, Laffon B, Pásaro E, Harrington C, Taylor A, Teixeira JP, 2012. Metal(Loid) levels in biological matrices from human populations exposed to mining contamination-panasqueira mine (Portugal). Journal of Toxicology and Environmental Health - Part A: Current Issues 75, 893–908. 10.1080/15287394.2012.690705 [DOI] [PubMed] [Google Scholar]
- Cottingham KL, Karimi R, Gruber JF, Zens MS, Sayarath V, Folt CL, Punshon T, Morris JS, Karagas MR, 2013. Diet and toenail arsenic concentrations in a New Hampshire population with arsenic-containing water. Nutrition journal 12, 149. 10.1186/1475-2891-12-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubadda F, Jackson BP, Cottingham KL, Van Horne YO, Kurzius-Spencer M, Ornelas Y, Horne V, Kurzius-Spencer M, 2016. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Science of The Total Environment 579, 1228–1239. 10.1016/j.scitotenv.2016.11.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Li Z, Gilbert-Diamond D, Mackenzie TA, Cottingham KL, Jackson BP, Lee JS, Baker ER, Marsit CJ, Karagas MR, 2014. Infant toenails as a biomarker of in utero arsenic exposure. Journal of exposure science & environmental epidemiology 24, 467–73. 10.1038/jes.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Signes-Pastor AJ, Argos M, Slaughter F, Pendergrast C, Punshon T, Gossai A, Ahsan H, Karagas MR, 2017. Assessment of Human Dietary Exposure to Arsenic through Rice. Science of The Total Environment 586, 1237–1244. 10.1016/j.scitotenv.2017.02.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer TJB, Yu ZM, Nauta L, Murimboh JD, Parker L, 2015. Geostatistical modelling of arsenic in drinking water wells and related toenail arsenic concentrations across Nova Scotia, Canada. Science of the Total Environment 505, 1248–1258. 10.1016/j.scitotenv.2014.02.055 [DOI] [PubMed] [Google Scholar]
- Esteban M, Castaño A, 2009. Non-invasive matrices in human biomonitoring: A review. Environmental International 35, 438–449. 10.1016/j.envint.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Farzan SF, Chen Y, Rees JR, Zens MS, Karagas MR, 2015. Risk of death from cardiovascular disease associated with low-level arsenic exposure among long-term smokers in a US population-based study. Toxicology and Applied Pharmacology 287, 93–97. 10.1016/j.taap.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Gossai A, Chen Y, Chasan-Taber L, Baker E, Karagas MR, 2016. Maternal arsenic exposure and gestational diabetes and glucose intolerance in the New Hampshire birth cohort study. Environmental Health: A Global Access Science Source 15, 1–8. 10.1186/s12940-016-0194-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Howe CG, Zens MS, Palys T, Channon JY, Li Z, Chen Y, Karagas MR, 2017. Urine Arsenic and Arsenic Metabolites in U.S. Adults and Biomarkers of Inflammation, Oxidative Stress, and Endothelial Dysfunction: A Cross-Sectional Study. Environmental health perspectives 125, 127002. 10.1289/EHP2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LEB, Dennis LK, Lynch CF, Thorne PS, Just CL, 2004. Toenail arsenic content and cutaneous melanoma in Iowa. American Journal of Epidemiology 160, 679–687. 10.1093/aje/kwh267 [DOI] [PubMed] [Google Scholar]
- Gagnon F, Lampron-Goulet E, Normandin L, Langlois M-F, 2016. Measurements of Arsenic in the Urine and Nails of Individuals Exposed to Low Concentrations of Arsenic in Drinking Water From Private Wells in a Rural Region of Quebec, Canada. Journal of environmental health 78, 76–83. [PubMed] [Google Scholar]
- Garland M, Morris JS, Colditz G. a, Stampfer MJ, Spate VL, Baskett CK, Rosner B, Speizer FE, Willett WC, Hunter DJ, 1996. Toenail trace element levels and breast cancer: a prospective study. American journal of epidemiology 144, 653–660. 10.1093/oxfordjournals.aje.a008977 [DOI] [PubMed] [Google Scholar]
- Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, Willett WC, Hunter DJ, 1993. Toenail trace element levels as biomarkers: reproducibility over a 6- year period. Cancer Epidemiol Biomarkers Prev 2, 493–497. [PubMed] [Google Scholar]
- Gonzalez-Cortes T, Recio-Vega R, Lantz RC, Chau BT, 2017. DNA methylation of extracellular matrix remodeling genes in children exposed to arsenic. Toxicology and Applied Pharmacology 329, 140–147. 10.1016/j.taap.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goullé JP, Saussereau E, Mahieu L, Bouige D, Groenwont S, Guerbet M, Lacroix C, 2009. Application of inductively coupled plasma mass spectrometry multielement analysis in fingernail and toenail as a biomarker of metal exposure. Journal of Analytical Toxicology 33, 92–98. 10.1093/jat/33.2.92 [DOI] [PubMed] [Google Scholar]
- Grashow R, Zhang J, Fang SC, Weisskopf MG, Christiani DC, Cavallari JM, 2014a. Toenail metal concentration as a biomarker of occupational welding fume exposure. Journal of Occupational and Environmental Hygiene 11, 397–405. 10.1080/15459624.2013.875182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashow R, Zhang J, Fang SC, Weisskopf MG, Christiani DC, Kile ML, Cavallari JM, 2014b. Inverse association between toenail arsenic and body mass index in a population of welders. Environmental Research 131, 131–133. 10.1016/j.envres.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Karagas MR, Punshon T, Jackson BP, Robbins DJ, Houseman EA, Marsit CJ, 2016. Epigenome-Wide Assessment of DNA Methylation in the Placenta and Arsenic Exposure in the New Hampshire Birth Cohort Study (USA). Environmental Health Perspectives. 10.1289/ehp.1510437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber JF, Karagas MR, Gilbert-Diamond D, Bagley PJ, Zens MS, Sayarath V, Punshon T, Morris JS, Cottingham KL, 2012. Associations between toenail arsenic concentration and dietary factors in a New Hampshire population. Nutrition Journal 11, 1. 10.1186/1475-2891-11-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-González E, García-Esquinas E, Larrea-Baz N.F. de, Salcedo-Bellido I, Navas-Acien A, Lope V, Gómez-Ariza JL, Pastor R, Pollán M, Pérez-Gómez B, 2019. Toenails as biomarker of exposure to essential trace metals: A review. Environmental Research 179, 108787. 10.1016/j.envres.2019.108787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasibuzzaman MM, Hossain S, Islam S, Rahman A, Anjum A, Hossain F, Mohanto NC, Karim R, Hoque M, Saud ZA, Miyataka H, Himeno S, Hossain K, 2017. Association between arsenic exposure and soluble thrombomodulin: A cross sectional study in Bangladesh. PLoS ONE 12, 1–16. 10.1371/journal.pone.0175154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck J, Andrew A, Onega T, Rigas J, Jackson B, Karagas MR, Duell E, 2009. Lung Cancer in a US Population with Low to Moderate Arsenic Exposure. Environmental Health Perspectives 117, 1718–1723. 10.1289/ehp.0900566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinhumpatch P, Navasumrit P, Chaisatra K, Promvijit J, Mahidol C, Ruchirawat M, 2013. Oxidative DNA damage and repair in children exposed to low levels of arsenic in utero and during early childhood: Application of salivary and urinary biomarkers. Toxicology and Applied Pharmacology 273, 569–579. 10.1016/j.taap.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Hinwood A, Horwitz P, Rogan R, 2008. Human exposure to metals in groundwater affected by acid sulfate soil disturbance. Archives of Environmental Contamination and Toxicology 55, 538–545. 10.1007/s00244-007-9076-3 [DOI] [PubMed] [Google Scholar]
- Hinwood AL, Sim MR, Jolley D, Klerk N. de, Bastone EB, Gerostamoulos J, Drummer OH, 2003. Hair and toenail arsenic concentrations of residents living in areas with high environmental arsenic concentrations. Environmental Health Perspectives 111, 187–193. 10.1289/ehp.5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain E, Islam K, Yeasmin F, Karim MR, Rahman M, Agarwal S, Hossain S, Aziz A, Al Mamun A, Sheikh A, Haque A, Hossain MT, Hossain M, Haris PI, Ikemura N, Inoue K, Miyataka H, Himeno S, Hossain K, 2012. Elevated levels of plasma Big endothelin-1 and its relation to hypertension and skin lesions in individuals exposed to arsenic. Toxicology and Applied Pharmacology 259, 187–194. 10.1016/j.taap.2011.12.023 [DOI] [PubMed] [Google Scholar]
- Hossain K, Suzuki T, Hasibuzzaman MM, Islam MS, Rahman A, Paul SK, Tanu T, Hossain S, Saud ZA, Rahman M, Nikkon F, Miyataka H, Himeno S, Nohara K, 2017. Chronic exposure to arsenic, LINE-1 hypomethylation, and blood pressure: a cross-sectional study in Bangladesh. Environmental Health 16, 20. 10.1186/s12940-017-0231-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda N, Hossain S, Rahman M, Karim MR, Islam K, Mamun AA, Hossain MI, Mohanto NC, Alam S, Aktar S, Arefin A, Ali N, Salam KA, Aziz A, Saud ZA, Miyataka H, Himeno S, Hossain K, 2014. Elevated levels of plasma uric acid and its relation to hypertension in arsenic-endemic human individuals in Bangladesh. Toxicology and Applied Pharmacology 281, 11–18. 10.1016/j.taap.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, Dobson CB, Frelich J, Hoffman E, Yousuf J, Afroz S, Islam S, Christiani DC, 2007. Maternal arsenic exposure associated with low birth weight in Bangladesh. Journal of Occupational and Environmental Medicine 49, 1097–1104. 10.1097/JOM.0b013e3181566ba0 [DOI] [PubMed] [Google Scholar]
- IARC, 2012. Arsenic, Metals, Fibers and Dusts. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans. 100C, 527. [PMC free article] [PubMed] [Google Scholar]
- Intarasunanont P, Navasumrit P, Waraprasit S, Chaisatra K, Suk WA, Mahidol C, Ruchirawat M, 2012. Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environmental Health: A Global Access Science Source 11, 1. 10.1186/1476-069X-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Mohanto NC, Karim MR, Aktar S, Hoque MM, Rahman A, Jahan M, Khatun R, Aziz A, Salam KA, Saud ZA, Hossain M, Rahman A, Mandal A, Haque A, Miyataka H, Himeno S, Hossain K, 2015. Elevated concentrations of serum matrix metalloproteinase-2 and −9 and their associations with circulating markers of cardiovascular diseases in chronic arsenic-exposed individuals. Environmental Health: A Global Access Science Source 14, 1–12. 10.1186/s12940-015-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RJ, Argos M, Tong L, Li J, Rakibuz-Zaman M, Islam MT, Slavkovich V, Ahmed A, Navas-Acien A, Parvez F, Chen Y, Gamble MV, Graziano JH, Pierce BL, Ahsan H, 2016. Determinants and consequences of arsenic metabolism efficiency among 4,794 individuals: Demographics, lifestyle, genetics, and toxicity. Cancer Epidemiology Biomarkers and Prevention 25, 381–390. 10.1158/1055-9965.EPI-15-0718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N, Shelton BJ, Hopenhayn C, Tucker TT, Unrine JM, Huang B, Huang B, Christian WJ, Zhang Z, Shi X, Li L, Johnson N, Shelton BJ, Hopenhayn C, Tucker TT, Unrine JM, Huang B, Christian WJ, Zhang Z, Li L, 2011. Concentrations of Arsenic, Chromium, and Nickel in Toenail Samples From Appalachian Kentucky Residents. Journal of Environmental Pathology, Toxicology and Oncology 30, 213–223. 10.1615/JEnvironPatholToxicolOncol.v30.i3.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MR, Tellez-Plaza M, Vaidya D, Grau M, Francesconi KA, Goessler W, Guallar E, Post WS, Kaufman JD, Navas-Acien A, 2016. Estimation of Inorganic Arsenic Exposure in Populations with Frequent Seafood Intake: Evidence from MESA and NHANES. American Journal of Epidemiology 184, 590–602. 10.1093/aje/kww097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josyula AB, McClellen H, Hysong TA, Kurzius-Spencer M, Poplin GS, Stürup S, Burgess JL, 2006. Reduction in urinary arsenic with bottled-water intervention. Journal of Health, Population and Nutrition 24, 298–304. [PMC free article] [PubMed] [Google Scholar]
- Karagas MR, Andrew AS, Nelson HH, Li Z, Punshon T, Schned A, Marsit CJ, Morris JS, Moore JH, Tyler AL, Gilbert-Diamond D, Guerinot ML, Kelsey KT, 2012. SLC39A2 and FSIP1 polymorphisms as potential modifiers of arsenic-related bladder cancer. Human Genetics 131, 453–461. 10.1007/s00439-011-1090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas MR, Le CX, Morris S, Blum J, Lu X, Spate V, Carey M, Stannard V, Klaue B, Tosteson TD, 2001a. Markers of low level arsenic exposure for evaluating human cancer risks in a US population. Int J Occup Med Environ Health 14, 171–175. [PubMed] [Google Scholar]
- Karagas MR, Morris JS, Weiss JE, Spate V, Baskett C, Greenberg ER, 1996. Toenail samples as an indicator of drinking water arsenic exposure. Cancer Epidemiology Biomarkers and Prevention 5, 849–852. [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Morris JS, Tosteson TD, Weiss JE, Spencer SK, Greenberg ER, 2001b. Skin cancer risk in relation to toenail arsenic concentrations in a US population-based case-control study. American journal of epidemiology 153, 559–65. 10.1093/aje/153.6.559 [DOI] [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Tosteson TD, 2002. Assessment of cancer risk and environmental levels of arsenic in New Hampshire. Int J Hyg Environ Health 205, 85–94. 10.1078/1438-4639-00133 [DOI] [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Blum J, Klaue B, Weiss JE, Stannard V, Spate V, Morris JS, 2000. Measurement of Low Levels of Arsenic Exposure: A Comparison of Water and Toenail Concentrations. American Journal of Epidemiology 152, 84–90. 10.1093/aje/152.1.84 [DOI] [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Morris JS, Demidenko E, Mott L, Heaney J, Schned A, 2004. Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer causes & control : CCC 15, 465–472. 10.1023/B:CACO.0000036452.55199.a3 [DOI] [PubMed] [Google Scholar]
- Karim MR, Rahman M, Islam K, Mamun AA, Hossain S, Hossain E, Aziz A, Yeasmin F, Agarwal S, Hossain MI, Saud ZA, Nikkon F, Hossain M, Mandal A, Jenkins RO, Haris PI, Miyataka H, Himeno S, Hossain K, 2013. Increases in oxidized low-density lipoprotein and other inflammatory and adhesion molecules with a concomitant decrease in high-density lipoprotein in the individuals exposed to arsenic in Bangladesh. Toxicological Sciences 135, 17–25. 10.1093/toxsci/kft130 [DOI] [PubMed] [Google Scholar]
- Karim MR, Salam KA, Hossain E, Islam K, Ali N, Haque A, Saud ZA, Yeasmin T, Hossain M, Miyataka H, Himeno S, Hossain K, 2010. Interaction between chronic arsenic exposure via drinking water and plasma lactate dehydrogenase activity. Science of the Total Environment 409, 278–283. 10.1016/j.scitotenv.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Kato M, Kumasaka MY, Ohnuma S, Furuta A, Kato Y, Shekhar HU, Kojima M, Koike Y, Dinh Thang N, Ohgami N, Ly TB, Jia X, Yetti H, Naito H, Ichihara G, Yajima I, 2013. Comparison of Barium and Arsenic Concentrations in Well Drinking Water and in Human Body Samples and a Novel Remediation System for These Elements in Well Drinking Water. PLoS ONE 8, 4–9. 10.1371/journal.pone.0066681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, Quamruzzaman Q, Rahman M, Christiani DC, 2016. Estimating effects of arsenic exposure during pregnancy on perinatal outcomes in a bangladeshi cohort. Epidemiology 27, 173–181. 10.1097/EDE.0000000000000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Houseman EA, Breton CV, Quamruzzaman Q, Rahman M, Mahiuddin G, Christiani DC, 2007. Association between total ingested arsenic and toenail arsenic concentrations. Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering 42, 1827–1834. 10.1080/10934520701566819 [DOI] [PubMed] [Google Scholar]
- Kile ML, Houseman EA, Rodrigues E, Smith TJ, Quamruzzaman Q, Rahman M, Mahiuddin G, Su L, Christiani DC, 2005. Toenail arsenic concentrations, GSTT1 gene polymorphisms, and arsenic exposure from drinking water. Cancer Epidemiology Biomarkers and Prevention 14, 2419–2426. 10.1158/1055-9965.EPI-05-0306 [DOI] [PubMed] [Google Scholar]
- Kuiper N, Rowell C, Nriagu J, Shomar B, 2014. What do the trace metal contents of urine and toenail samples from Qatar[U+05F3]s farm workers bioindicate? Environmental Research 131, 86–94. 10.1016/j.envres.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Kwong RC, Karagas MR, Kelsey KT, Mason RA, Tanyos SA, Schned AR, Marsit CJ, Andrew AS, Kwong RC, Mason RA, Tanyos SA, Andrew AS, Biostatistics S, 2010. Arsenic exposure predicts bladder cancer survival in a US population 28, 487–492. 10.1007/s00345-009-0477-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrou A, Baccarelli A, Wright RO, Weisskopf M, Bollati V, Amarasiriwardena C, Vokonas P, Schwartza J, 2012. Arsenic exposure and DNA methylation among elderly men. Epidemiology 23, 668–676. 10.1097/EDE.0b013e31825afb0b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampron-Goulet É, Gagnon F, Langlois MF, 2017. Association between consumption of private well water contaminated by low levels of arsenic and dysglycemia in a rural region of Quebec, Canada. Environmental Research 159, 232–238. 10.1016/j.envres.2017.07.049 [DOI] [PubMed] [Google Scholar]
- Lazarević K, Nikolić D, Stosić L, Milutinović S, Videnović J, Bogdanović D, 2012. Determination of lead and arsenic in tobacco and cigarettes: an important issue of public health. Central European journal of public health 20, 62–66. 10.21101/cejph.a3728 [DOI] [PubMed] [Google Scholar]
- Lee O, Moon JH, Kim SH, Chung YS, Sun GM, 2016. Toenail elemental analysis of Korean young adults by instrumental neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry 307, 2571–2575. 10.1007/s10967-015-4638-8 [DOI] [Google Scholar]
- Lesseur C, Gilbert-Diamond D, Andrew AS, M. Ekstrom R, Li Z, Kelsey KT, Marsit CJ, Karagas MR, 2012. A case-control study of polymorphisms in xenobiotic and arsenic metabolism genes and arsenic-related bladder cancer in New Hampshire. Toxicology Letters 210, 100–106. 10.1016/j.toxlet.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewińska D, Palus J, Stepnik M, Dziubaítowska E, Beck J, Rydzyński K, Natarajan AT, Nilsson R, 2007. Micronucleus frequency in peripheral blood lymphocytes and buccal mucosa cells of copper smelter workers, with special regard to arsenic exposure. International Archives of Occupational and Environmental Health 80, 371–380. 10.1007/s00420-006-0130-7 [DOI] [PubMed] [Google Scholar]
- Lin PI, Bromage S, Mostofa MG, Allen J, Oken E, Kile ML, Christiani DC, 2017. Associations between diet and toenail arsenic concentration among pregnant women in bangladesh: A prospective study. Nutrients 9. 10.3390/nu9040420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh MM, Sugeng A, Lothrop N, Klimecki W, Cox M, Wilkinson ST, Lu Z, Beamer PI, 2016. Multimedia exposures to arsenic and lead for children near an inactive mine tailings and smelter site. Environmental Research 146, 331–339. 10.1016/j.envres.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh D, Williams P, Hunter D, Sampson L, Morris S, Willett W, Rimm E, 1997. Evaluation Approach of a Food Frequency for Estimating Dietary Intake of Inorganic and Composition Arsenic. Cancer Epidemiology Biomarkers and Prevention 6, 1043–1050. [PubMed] [Google Scholar]
- Maity JP, Nath B, Kar S, Chen CY, Banerjee S, Jean JS, Liu MY, Centeno JA, Bhattacharya P, Chang CL, Santra SC, 2012. Arsenic-induced health crisis in peri-urban Moyna and Ardebok villages, West Bengal, India: An exposure assessment study. Environmental Geochemistry and Health 34, 563–574. 10.1007/s10653-012-9458-y [DOI] [PubMed] [Google Scholar]
- Mandal BK, Ogra Y, Suzuki KT, 2003. Speciation of arsenic in human nail and hair from arsenic-affected area by HPLC-inductively coupled argon plasma mass spectrometry. Toxicology and Applied Pharmacology 189, 73–83. 10.1016/S0041-008X(03)00088-7 [DOI] [PubMed] [Google Scholar]
- Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat MP, 2012. What is the best biomarker to assess arsenic exposure via drinking water? Environment International 39, 150–171. 10.1016/j.envint.2011.07.015 [DOI] [PubMed] [Google Scholar]
- Marsit CJ, Karagas MR, Danaee H, Liu M, Andrew A, Schned A, Nelson HH, Kelsey KT, 2006. Carcinogen exposure and gene promoter hypermethylation in bladder cancer. Carcinogenesis 27, 112–116. 10.1093/carcin/bgi172 [DOI] [PubMed] [Google Scholar]
- Martin R, Dowling K, Pearce D, Bennett J, Stopic A, 2013. Ongoing soil arsenic exposure of children living in an historical gold mining area in regional Victoria, Australia: Identifying risk factors associated with uptake. Journal of Asian Earth Sciences 77, 256–261. 10.1016/j.jseaes.2013.03.026 [DOI] [Google Scholar]
- Matoušek T, Wang Z, Douillet C, Musil S, Stýblo M, 2017. Direct Speciation Analysis of Arsenic in Whole Blood and Blood Plasma at Low Exposure Levels by Hydride Generation-Cryotrapping-Inductively Coupled Plasma Mass Spectrometry. Analytical Chemistry 89, 9633–9637. 10.1021/acs.analchem.7b01868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty KM, Chen YC, Quamruzzaman Q, Rahman M, Mahiuddin G, Hsueh YM, Su L, Smith T, Ryan L, Christiani DC, 2007. Arsenic methylation, GSTT1, GSTM1, GSTP1 polymorphisms, and skin lesions. Environmental Health Perspectives 115, 341–345. 10.1289/ehp.9152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver DJ, Vanleeuwen JA, Knafla AL, Campbell JA, Alexander KM, Gherase MR, Guernsey JR, Fleming DE, 2015. Evaluation of a novel portable x-ray fluorescence screening tool for detection of arsenic exposure. Physiological Measurement 36, 2443–2459. 10.1088/0967-3334/36/12/2443 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Williams PN, Deacon CM, Norton GJ, Hossain M, Louhing D, Marwa E, Lawgalwi Y, Taggart M, Cascio C, Haris P, 2014. Urinary excretion of arsenic following rice consumption. Environmental pollution 194, 181–7. 10.1016/j.envpol.2014.07.031 [DOI] [PubMed] [Google Scholar]
- Menezes MÂB, Maia EC, Albinati CC, Sabino CV, Batista JR, 2004. How suitable are scalp hair and toenail as biomonitors? Journal of Radioanalytical and Nuclear Chemistry 259, 81–86. 10.1023/B:JRNC.0000015810.22775.72 [DOI] [Google Scholar]
- Michaud DS, Wright ME, Cantor KP, Taylor PR, Virtamo J, Albanes D, 2004. Arsenic concentrations in prediagnostic toenails and the risk of bladder cancer in a cohort study of male smokers. American Journal of Epidemiology 160, 853–859. 10.1093/aje/kwh295 [DOI] [PubMed] [Google Scholar]
- Middleton DRS, Watts MJ, Hamilton EM, Fletcher T, Leonardi GS, Close RM, Exley KS, Crabbe H, Polya DA, 2016. Prolonged exposure to arsenic in UK private water supplies: toenail, hair and drinking water concentrations. Environ. Sci.: Processes Impacts 18, 562–574. 10.1039/C6EM00072J [DOI] [PubMed] [Google Scholar]
- Mo J, Xia Y, Ning Z, Wade TJ, Mumford JL, 2009a. Elevated ERCC1 Gene Expression in Blood Cells Associated with Exposure to Arsenic from Drinking Water in Inner Mongolia. Anticancer Res 29, 3253–3259. [PubMed] [Google Scholar]
- Mo J, Xia Y, Ning Z, Wade TJ, Mumford JL, 2009b. Elevated human telomerase reverse transcriptase gene expression in blood cells associated with chronic arsenic exposure in inner Mongolia, China. Environmental Health Perspectives 117, 354–360. 10.1289/ehp.11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP, 2009. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine 6, e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordukhovich I, Wright RO, Amarasiriwardena C, Baja E, Baccarelli A, Suh H, Sparrow D, Vokonas P, Schwartz J, 2009. Association between low-level environmental arsenic exposure and qt interval duration in a general population study. American Journal of Epidemiology 170, 739–746. 10.1093/aje/kwp191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, Sparrow D, Vokonas P, Schwartz J, 2012. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the Normative aging study. Environmental Health Perspectives 120, 98–104. 10.1289/ehp.1002805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JL, Wu K, Xia Y, Kwok R, Yang Z, Foster J, Sanders WE, 2007. Chronic arsenic exposure and cardiac repolarization abnormalities with QT interval prolongation in a population-based study. Environmental Health Perspectives 115, 690–694. 10.1289/ehp.9686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwesigye AR, Young SD, Bailey EH, Tumwebaze SB, 2016. Population exposure to trace elements in the Kilembe copper mine area, Western Uganda: A pilot study. Science of the Total Environment 573, 366–375. 10.1016/j.scitotenv.2016.08.125 [DOI] [PubMed] [Google Scholar]
- Nachman KE, Punshon T, Rardin L, Signes-pastor AJ, Murray CJ, Jackson BP, Guerinot ML, Burke TA, Chen CY, Ahsan H, Argos M, Cottingham KL, Cubadda F, Ginsberg GL, Goodale BC, Kurzius-spencer M, Meharg AA, Miller MD, Nigra AE, Pendergrast CB, Raab A, Reimer K, Scheckel KG, Schwerdtle T, Taylor VF, Tokar EJ, Warczak TM, Karagas MR, 2018. Opportunities and Challenges for Dietary Arsenic Intervention. Environmental Health Perspectives 126, 6–11. 10.1289/EHP3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath B, Sahu SJ, Jana J, Mukherjee-Goswami A, Roy S, Sarkar MJ, Chatterjee D, 2008. Hydrochemistry of arsenic-enriched aquifer from rural West Bengal, India: A study of the arsenic exposure and mitigation option. Water, Air, and Soil Pollution 190, 95–113. 10.1007/s11270-007-9583-x [DOI] [Google Scholar]
- Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E, 2011. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environmental Research 111, 110–118. 10.1016/j.envres.2010.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndilila W, Callan AC, McGregor LA, Kalin RM, Hinwood AL, 2014. Environmental and toenail metals concentrations in copper mining and non mining communities in Zambia. International Journal of Hygiene and Environmental Health 217, 62–69. 10.1016/j.ijheh.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Nichols TA, Morris JS, Mason MM, Spate VL, Baskett CK, Cheng TP, Tharp CJ, Scott JA, Horsman TL, Colbert JW, Rawson AE, Karagas MR, Stannard V, 1998. The study of human nails as an intake monitor for arsenic using neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry 236, 51–56. 10.1007/BF02386317 [DOI] [Google Scholar]
- Normandin L, Ayotte P, Levallois P, Ibanez Y, Courteau M, Kennedy G, Chen L, Le XC, Bouchard M, 2014. Biomarkers of arsenic exposure and effects in a Canadian rural population exposed through groundwater consumption. Journal of Exposure Science and Environmental Epidemiology 24, 127–134. 10.1038/jes.2013.80 [DOI] [PubMed] [Google Scholar]
- Nygaard UC, Li Z, Palys T, Jackson B, Subbiah M, Malipatlolla M, Sampath V, Maecker H, Karagas MR, Nadeau KC, 2017. Cord blood T cell subpopulations and associations with maternal cadmium and arsenic exposures. PLoS ONE 12, 1–13. 10.1371/journal.pone.0179606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto D, Xia Y, Li Y, Wu K, He L, Telech J, Hundell H, Prah J, Mumford J, Wade T, 2007. Neurosensory effects of chronic human exposure to arsenic associated with body burden and environmental measures. Human & experimental toxicology 26, 169–177. 10.1177/0960327107070561 [DOI] [PubMed] [Google Scholar]
- Pan W-C, Seow WJ, Kile ML, Hoffman EB, Quamruzzaman Q, Rahman M, Mahiuddin G, Mostofa G, Lu Q, Christiani DC, 2013. Association of Low to Moderate Levels of Arsenic Exposure With Risk of Type 2 Diabetes in Bangladesh. American Journal of Epidemiology 178, 1563–1570. 10.1093/aje/kwt195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce DC, Dowling K, Gerson AR, Sim MR, Sutton SR, Newville M, Russell R, McOrist G, 2010. Arsenic microdistribution and speciation in toenail clippings of children living in a historic gold mining area. Science of the Total Environment 408, 2590–2599. 10.1016/j.scitotenv.2009.12.039 [DOI] [PubMed] [Google Scholar]
- Phan K, Sthiannopkao S, Kim KW, 2011. Surveillance on chronic arsenic exposure in the Mekong River basin of Cambodia using different biomarkers. International Journal of Hygiene and Environmental Health 215, 51–58. 10.1016/j.ijheh.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Phookphan P, Navasumrit P, Waraprasit S, Promvijit J, Chaisatra K, Ngaotepprutaram T, Ruchirawat M, 2017. Hypomethylation of inflammatory genes (COX2, EGR1, and SOCS3) and increased urinary 8-nitroguanine in arsenic-exposed newborns and children. Toxicology and Applied Pharmacology 316, 36–47. 10.1016/j.taap.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Podgorski J, Berg M, 2020. Global threat of arsenic in groundwater Science 868, 845–850. 10.1126/science.aba1510 [DOI] [PubMed] [Google Scholar]
- Punshon T, Davis MA, Marsit CJ, Theiler SK, Baker ER, Jackson BP, Conway DC, Karagas MR, 2015. Placental arsenic concentrations in relation to both maternal and infant biomarkers of exposure in a US cohort. Journal of Exposure Science and Environmental Epidemiology 25, 599–603. 10.1038/jes.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Mamun AA, Karim MR, Islam K, Amin HA, Hossain S, Hossain MI, Saud ZA, Noman ASM, Miyataka H, Himeno S, Hossain K, 2015. Associations of total arsenic in drinking water, hair and nails with serum vascular endothelial growth factor in arsenic-endemic individuals in Bangladesh. Chemosphere 120, 336–342. 10.1016/j.chemosphere.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Rahman MML, Valeri L, Kile ML, Mazumdar M, Mostofa G, Qamruzzaman Q, Rahman MML, Baccarelli A, Liang L, Hauser R, Christiani DC, 2017. Investigating causal relation between prenatal arsenic exposure and birthweight: Are smaller infants more susceptible? Environment International 108, 32–40. 10.1016/j.envint.2017.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raińska E, Biziuk M, Bode P, Długołecki P, Astel K, 2007. An evaluation of endemic exposure of citizens living in near a Gdańsk Phosphatic Fertilizer Plant. Polish Journal of Environmental Studies 16, 243–250. [Google Scholar]
- Raińska E, Biziuk M, Sarbu C, Szczepaniak K, Frontasyeva MV, Culicov O, Bode P, Astel A, 2005. Assessment of phosphatic fertilizer production impact on occupational staff based on NAA of hair, nails, and inhald particles. Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering 40, 2137–2152. 10.1080/10934520500234635 [DOI] [PubMed] [Google Scholar]
- Rakib MA, Huda ME, Hossain SM, Naher K, Khan R, 2013. Arsenic Content in Inactive Tissue: Human Hair and Nail 2, 522–535. [Google Scholar]
- Rakovic M, Foltynová V, Pilecká N, Glagolicová A, Kucera J, 1997. Assessment of metals and metalloids in skin derivatives of volunteers from capital city of Prague, Czech Repoblic 98, 107–114. [PubMed] [Google Scholar]
- Rivera-Núñez Z, Meliker JR, Meeker JD, Slotnick MJ, Nriagu JO, 2011. Urinary arsenic species, toenail arsenic, and arsenic intake estimates in a Michigan population with low levels of arsenic in drinking water. Journal of Exposure Science and Environmental Epidemiology 22, 182–190. 10.1038/jes.2011.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues EG, Kile M, Dobson C, Amarasiriwardene CJ, Quamruzzaman Q, Rahman M, Golam M, Christiani DC, 2015. Maternal–infant biomarkers of prenatal exposure to arsenic and manganese. Journal of Exposure Science and Environmental Epidemiology 25, 639–648. 10.1038/jes.2015.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG, 2003. Mechanism of arsenic carcinogenesis: An integrated approach. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 533, 37–65. 10.1016/j.mrfmmm.2003.07.009 [DOI] [PubMed] [Google Scholar]
- Rossman TG, Klein CB, 2011. Genetic and epigenetic effects of environmental arsenicals. Metallomics 3, 1135–1141. 10.1039/c1mt00074h [DOI] [PubMed] [Google Scholar]
- Saat N, Chow S, Ghazali A, Hamid Z, Lubis S, Mohamed N, Ishak I, Othman H, 2013. Study of heavy metal levels in nails and hairs among vegetable farmers in Malaysia. 10.3923/rjasci.2013.449.455 [DOI]
- Sanders AP, Miller SK, Nguyen V, Kotch JB, Fry RC, 2014. Toxic metal levels in children residing in a smelting craft village in Vietnam: A pilot biomonitoring study. BMC Public Health 14, 1–8. 10.1186/1471-2458-14-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MT, Schreinemachers D, Wu K, Ning Z, Zhao B, Le ZC, Mumford JL, 2005. Human nails as a biomarker of arsenic exposure from well water in Inner Mongolia: Comparing atomic flourescence spectrometry and neutron activation analysis. Biomarkers 10, 95–104. 10.1080/13547500500087913 [DOI] [PubMed] [Google Scholar]
- Seow WJ, Pan WC, Kile ML, Baccarelli AA, Quamruzzaman Q, Rahman M, Mahiuddin G, Mostofa G, Lin X, Christiani DC, 2012. Arsenic reduction in drinking water and improvement in skin lesions: A follow-up study in Bangladesh. Environmental Health Perspectives 120, 1733–1738. 10.1289/ehp.1205381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Carey M, Vioque J, Navarrete-Muñoz E, Rodríguez-Dehli C, Tardón A, Begoña-Zubero M, Santa-Marina L, Vrijheid M, Casas M, Llop S, Gonzalez-Palacios S, Meharg AAA, 2017. Urinary Arsenic Speciation in Children and Pregnant Women from Spain. Exposure and Health 9, 105–111. 10.1007/s12403-016-0225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Doherty BT, Romano ME, Gleason KM, Gui J, Baker E, Karagas MR, 2019. Prenatal exposure to metal mixture and sex-specific birth outcomes in the New Hampshire Birth Cohort Study. Environmental Epidemiology 3, e068. 10.1097/ee9.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick MJ, 2011. Toenails for Biomonitoring of Environmental Exposures, 2nd ed. Elsevier Inc. 10.1016/B978-0-444-52272-6.00370-6 [DOI] [Google Scholar]
- Slotnick MJ, Meliker JR, AvRuskin GA, Ghosh D, Nriagu JO, 2007. Toenails as a biomarker of inorganic arsenic intake from drinking water and foods. Journal of toxicology and environmental health. Part A 70, 148–58. 10.1080/15287390600755232 [DOI] [PubMed] [Google Scholar]
- Slotnick MJ, Meliker JR, Kannan S, Nriagu JO, 2008a. Effects of nutritional measures on toenail arsenic concentration as a biomarker of arsenic exposure. Biomarkers 13, 451–466. 10.1080/13547500802029050 [DOI] [PubMed] [Google Scholar]
- Slotnick MJ, Meliker JR, Nriagu JO, 2008b. Intra-individual variability in toenail arsenic concentrations in a Michigan population, USA. Journal of Exposure Science and Environmental Epidemiology 18, 149–157. 10.1038/sj.jes.7500569 [DOI] [PubMed] [Google Scholar]
- Slotnick MJ, Nriagu JO, Johnson MM, Linder AM, Savoie KL, Jamil HJ, Hammad AS, 2005. Profiles of trace elements in toenails of Arab-Americans in the Detroit Area, Michigan. Biological Trace Element Research 107, 113–126. 10.1385/BTER:107:2:113 [DOI] [PubMed] [Google Scholar]
- Stafoggia M, Breitner S, Hampel R, Basagaña X, 2017. Statistical Approaches to Address Multi-Pollutant Mixtures and Multiple Exposures: the State of the Science. Current environmental health reports 4, 481–490. 10.1007/s40572-017-0162-z [DOI] [PubMed] [Google Scholar]
- Subhani M, Mustafa I, Alamdar A, Katsoyiannis IA, Ali N, Huang Q, Peng S, Shen H, Eqani SAMAS, 2015. Arsenic levels from different land-use settings in Pakistan: Bio-accumulation and estimation of potential human health risk via dust exposure. Ecotoxicology and Environmental Safety 115, 187–194. 10.1016/j.ecoenv.2015.02.019 [DOI] [PubMed] [Google Scholar]
- Tabata H, Anwar M, Horai S, Ando T, Nakano A, Wakamiya J, Koriyama C, Nakagawa M, Yamada K, Akiba S, 2006. Toenail arsenic levels among residents in Amami-Oshima Island, Japan. Environmental sciences: an international journal of environmental physiology and toxicology 13, 149–60. [PubMed] [Google Scholar]
- Tajuddin SM, Amaral AF, Fernández AF, Rodríguez-Rodero S, Rodríguez RM, Moore LE, Tardón A, Carrato A, García-Closas M, Silverman DT, Jackson BP, García-Closas R, Cook AL, Cantor KP, Chanock S, Kogevinas M, Rothman N, Real FX, Fraga MF, Malats N, 2013. Genetic and non-genetic predictors of LINE-1 methylation in leukocyte DNA. Environmental Health Perspectives 121, 650–656. 10.1289/ehp.1206068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauheed J, Sanchez-Guerra M, Lee JJ, Paul L, Ibne Hasan MOS, Quamruzzaman Q, Selhub J, Wright RO, Christiani DC, Coull BA, Baccarelli AA, Mazumdar M, 2017. Associations between post translational histone modifications, myelomeningocele risk, environmental arsenic exposure, and folate deficiency among participants in a case control study in Bangladesh. Epigenetics 12, 484–491. 10.1080/15592294.2017.1312238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, Karagas MR, Francesconi KA, 2016. Human exposure to organic arsenic species from seafood. Science of The Total Environment. 10.1016/j.scitotenv.2016.12.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.-h.H., 2009. A review on environmental factors regulating arsenic methylation in humans. Toxicology and Applied Pharmacology 235, 338–350. 10.1016/j.taap.2008.12.016 [DOI] [PubMed] [Google Scholar]
- Tsuji JS, Van Kerkhove MD, Kaetzel RS, Scrafford CG, Mink PJ, Barraj LM, Crecelius EA, Goodman M, 2005. Evaluation of exposure to arsenic in residential soil. Environmental Health Perspectives 113, 1735–1740. 10.1289/ehp.8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of York, 2019. PROSPERO. https://www.crd.york.ac.uk/prospero/
- Wade TJ, Xia Y, Mumford J, Wu K, Le XC, Sams E, Sanders WE, 2015. Cardiovascular disease and arsenic exposure in Inner Mongolia, China: A case control study. Environmental Health: A Global Access Science Source 14, 1–10. 10.1186/s12940-015-0022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, LoIacono NJ, Kline J, Factor-Litvak P, Van Geen A, Mey JL, Levy D, Abramson R, Schwartz A, Graziano JH, 2014. A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environmental Health: A Global Access Science Source 13, 1–10. 10.1186/1476-069X-13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts M, Button M, Jenkin G, 2009. Arsenic speciation: A tool for assessing the environmental toxicology of arsenic using earthworms and toenails as biomarkers. Spectroscopy Europe 21, 17–19. [Google Scholar]
- Wong J, De Vivo I, Lin X, Grashow R, Cavallari J, Christiani D, 2014. The Association Between Global DNA Methylation and Telomere Length in a Longitudinal Study of Boilermakers 38, 254–264. 10.1002/gepi.21796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JY, Fang SC, Grashow R, Fan T, Christiani DC, 2015. The Relationship Between Occupational Metal Exposure and Arterial Compliance. Journal of Occupational and Environmental Medicine 57, 355–360. 10.1097/JOM.0000000000000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JW, Erdei E, Myers O, Siegel M, Berwick M, 2016. Arsenic and ultraviolet radiation exposure: melanoma in a New Mexico non-Hispanic white population. Environmental Geochemistry and Health 38, 897–910. 10.1007/s10653-015-9770-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZM, Dummer TJB, Adams A, Murimboh JD, Parker L, 2014a. Relationship between drinking water and toenail arsenic concentrations among a cohort of Nova Scotians. Journal of Exposure Science and Environmental Epidemiology 24, 135–144. 10.1038/jes.2013.88 [DOI] [PubMed] [Google Scholar]
- Yu ZM, Fung B, Murimboh JD, Parker L, Dummer TJB, 2014b. What is the role of obesity in the aetiology of arsenic-related disease? Environment International 66, 115–123. 10.1016/j.envint.2014.01.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


