Abstract
Endovascular thrombectomy (EVT) has played a major role in advancing adult stroke care and may serve a similar role in pediatric stroke care. However, there is a need to develop better evidence and infrastructure for pediatric stroke care. In this work, we review two experienced pediatric EVT programs and examine key design features in both care environments, including a formalized protocol and workflow, integration with an adult EVT workflow, simplification and automation of workflow steps, pediatric adaptations of stroke imaging, advocacy of pediatric stroke care, and collaboration between providers, among others. These essential features transcend any single hospital environment and may provide an important foundation for other pediatric centers that aim to enhance the care of children with stroke.
Keywords: Acute ischemic stroke, pediatric stroke, stroke workflow, Acute stroke, Pediatrics, Thrombectomy
BACKGROUND
Endovascular thrombectomy (EVT) has proven remarkably effective in treating adults suffering from acute ischemic stroke (AIS) caused by large vessel occlusion (LVO)1–7. As a result, considerable effort has been directed toward improving patient triage, facilitating ambulance transport, and optimizing in-hospital clinical workflows to enhance the accessibility and efficacy of EVT8–12. These process improvements have dramatically increased the number of EVT procedures performed, reducing the overall population burden of disability caused by AIS13,14.
As many as 70% of children with AIS experience persistent neurologic deficits, with impact felt over decades15. Unfortunately, a randomized trial of EVT in childhood AIS is unlikely given the lower incidence of AIS in children compared to adults, relative lack of concentrated pediatric AIS expertise, and perceived loss of equipoise given the strength of adult EVT data. Thus, despite promising outcomes in case reports and case series16–18 and the development of informal criteria for primary pediatric stroke center designation19, EVT in children remains an off-label procedure without established guidelines20,21.
There is a clear need to collect prospective outcome data for EVT in children. In tandem with these efforts, there is a strong operational need to bolster pediatric stroke expertise and pediatric stroke care infrastructure in a variety of settings. While every health system with a pediatric hospital is unique, stroke care can be broadly classified based on the geographical relationship of the pediatric hospital with a partnered adult stroke hospital with EVT experience. Specifically, sites may be considered co-located if they are sufficiently close that vehicular patient transport is not needed between sites, or separated if vehicular transport is needed.
Here, we describe two successful pediatric thrombectomy programs, one co-located and one separated, and review key design features that are likely to be important for other qualified centers that aspire to implement pediatric EVT capability and reduce the long-term harms of pediatric AIS.
THROMBECTOMY PROGRAM ORGANIZATION
Organization of centers reviewed in this work are outlined here and serve as prototypical examples of pediatric EVT programs. Local institutional review board approval was not required for this study.
Co-Located Hospitals
St. Louis Children’s Hospital (SLCH) is a free-standing pediatric hospital that is physically connected by enclosed walkways to Barnes-Jewish Hospital, an adult comprehensive stroke center that accepts acute neurovascular referrals from over 40 surrounding hospitals. Both hospitals are part of a large, university-affiliated academic health system centered in the metropolitan area of St. Louis, Missouri. Physicians at both hospitals belong to the same academic physician group and may have privileges at one or both hospitals. Non-physician staff and resources at these two hospitals are administratively separate.
Separated Hospitals
Seattle Children’s Hospital (SCH) is a free-standing pediatric hospital located 6 miles from the University of Washington Medicine Stroke Center at Harborview Medical Center, a comprehensive stroke center that accepts acute neurovascular referrals from a multi-state region. Both hospitals are part of a large, university-affiliated academic health system centered in the metropolitan area of Seattle, Washington. Physicians at both hospitals belong to the same academic physician group and may have privileges at one or both hospitals. Non-physician staff and resources at these two hospitals are administratively separate.
EXAMPLE CLINICAL WORKFLOW
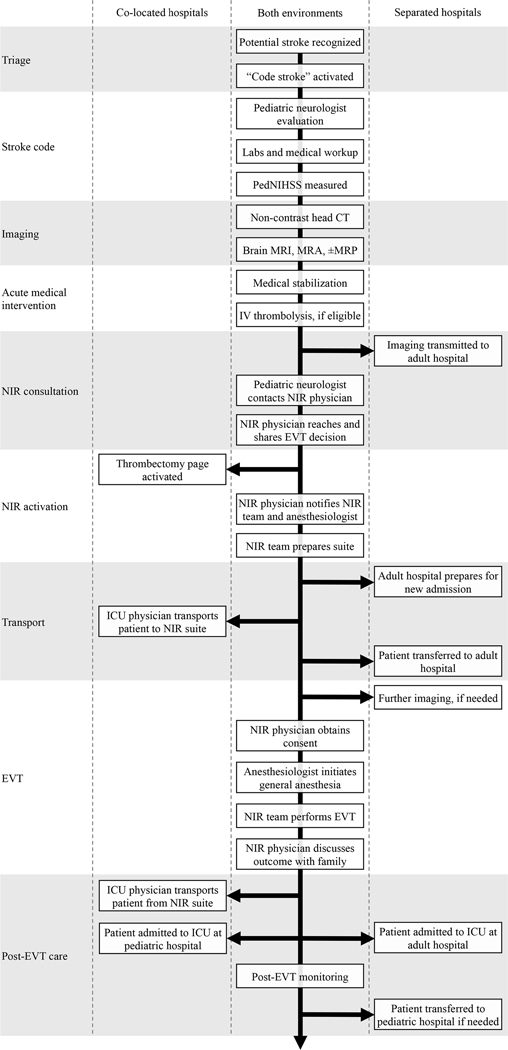
Specific workflow steps inevitably vary based on hospital organization and resource availability. However, key components of any thrombectomy workflow include triage, stroke code, imaging, acute medical intervention, neurointerventional radiology (NIR) consultation, NIR activation, transport, endovascular treatment, and post-EVT care (Figure 1). Here, we describe major workflow steps alongside specific implementation at SLCH, which is similar to SCH.
Figure 1:
Pediatric AIS workflows at co-located and separated hospitals. Pediatric neurology physician may refer to a pediatric vascular neurology attending physician or a pediatric neurology resident. NIR physician may refer to an interventional neuroradiology attending physician or an interventional neuroradiology fellow. PedNIHSS = Pediatric NIH stroke scale. MRP = Magnetic resonance perfusion. MRA = Magnetic resonance angiography.
Triage and Stroke Code
Upon recognition of possible AIS in a child, a pediatric stroke code is activated from anywhere in the hospital or via a centralized hospital operator for a patient en route to the emergency department. The hospital operator then immediately sends a page to the pediatric neurology resident physician and pediatric vascular neurology attending physician with the patient’s name, location, and contact phone number.
Simultaneous with stroke code, staff already with the patient obtain pre-specified labs for medical workup, ECG, and non-contrast head CT. The pediatric neurology resident evaluates the patient as soon as possible, focusing on obtaining history, time last seen well, and neurological examination including pediatric NIH Stroke Scale (PedNIHSS). Labs typically include CBC with differential, electrolytes, glucose, prothrombin time, partial thromboplastin time, erythrocyte sedimentation rate, basic toxicology screen, and in females of childbearing age, beta human chorionic gonadotropin. Blood gases and blood coagulation assays such as INR are tested if needed.
Imaging
If initial evaluation suggests the possibility of AIS due to LVO and the patient may be a candidate for EVT, additional neuroimaging is performed using a hyperacute stroke MRI/MRA protocol comprising diffusion-weighted imaging (DWI), time-of-flight magnetic resonance angiography (MRA), fluid-attenuated inversion recovery (FLAIR), along with optional susceptibility-weighted imaging and dynamic susceptibility contrast-enhanced perfusion-weighted imaging (PWI). Required sequences collectively require about 15 minutes to prepare and acquire on a 1.5T system (Siemens Aera, Erlangen, Germany).
A neurologist (resident, fellow, and/or attending) is present in the control room during the scan and a diagnostic neuroradiologist (fellow or attending) is either in the control room or remotely reviewing images in real time. The protocol intentionally prioritizes DWI and MRA sequences that allow identification of infarct and large vessel occlusion, respectively, but excludes T1- or T2-weighted imaging to expedite imaging.
DWI and MRA (and PWI, if performed) sequences are sent for automated processing by RAPID software (iSchemaView, Menlo Park, CA). If a patient cannot safely undergo MRI, CT angiography can be performed instead. CT perfusion is not performed in children in consideration of radiation dose.
Acute Medical Intervention
In concert with this evaluation, acute medical intervention is undertaken to maintain brain perfusion by initiating blood pressure control, hemodynamic stabilization, and antipyretic measures. Intravenous alteplase is considered based on pre-specified criteria22 and non-contrast head CT or, ideally, brain MRI. If eligible, consent is obtained and intravenous alteplase is administered.
NIR Consultation
If neuroimaging reveals LVO and other clinical and radiological selection criteria (Table 1) are met, the pediatric neurology resident pages the NIR physician to discuss possible EVT. This discussion follows a pre-specified script (Figure 2) to ensure that necessary information is conveyed with high fidelity. This script is distributed to pediatric neurology residents in the form of an “on-call card” that each resident carries at all times.
Table 1.
Sample inclusion and exclusion criteria for pediatric EVT. Treatment decisions in individual patients may deviate from these general criteria based on expert consensus. For instance, patients younger than the specified inclusion criteria may be considered.
| SLCH | SCH | |
|---|---|---|
| Inclusion criteria | • Age ≥13 years. | • Age ≥5 years. |
| • Time last seen well <24 hours. | • Time last seen well <24 hours (ages 13–17 years), <6 hours (ages 5–12 years). | |
| • Radiological evidence of LVO. | • Radiologic evidence of LVO. | |
| • ASPECTS ≥6. | • ASPECTS ≥6. | |
| • Debilitating symptoms consistent with AIS. | • PedNIHSS >6 with non-improving symptoms. | |
| Exclusion criteria | • Intracranial hemorrhage, mass, or mass effect generating stroke symptoms. | • Intracranial hemorrhage, mass, or mass effect generating stroke symptoms. • ASPECTS <6. |
| • Pre-morbid mRS ≥2 or comorbidities that impact recovery potential. | ||
| • Severe uncontrolled hypertension (SBP >185 mmHg or DBP >110 mmHg). | ||
| • Bleeding diathesis (platelet <30,000/microliter, INR >30, or use of direct oral anticoagulants with evident therapeutic effect based on PT, PTT, TT, or anti Factor Xa level). | ||
ASPECTS = Alberta Stroke Program Early CT Score, which can be assessed using non-contrast CT or diffusion weighted MRI.
Figure 2:
Scripted communication between pediatric neurologist and neurointerventionalist during pediatric EVT consultation. PACS = Picture Archiving and Communication System; MRN= medical record number; rt-PA = recombinant tissue plasminogen activator; mRS = modified Rankin Scale.
NIR Activation
If the neurologist and NIR physician agree that EVT is indicated, the pediatric neurologist will contact the hospital operator to activate the “thrombectomy pager” (Figure 3). Activating the thrombectomy pager leads to immediate recruitment of personnel relevant for pediatric AIS care (Table 2).
Figure 3:
Sample page following thrombectomy pager activation.
Table 2.
Responsibilities of personnel activated by thrombectomy page.
| Personnel | Responsibility |
|---|---|
| NIR physician | Confirm page was received, prepare for procedure. |
| Pediatric anesthesia attending physician | Go to NIR procedure area, prepare for case. |
| Pediatric anesthesia resident physician | Assess patient and obtain consent for anesthesia. |
| Operating room nurse | Schedule case if presenting from off-site. |
| Adult anesthesia attending physician | Aware of case, assist with anesthesia resource allocation. |
| Pediatric ICU attending physician | Transport patient to NIR procedure suite. |
| Medical Transporter | Assist as needed with patient transport to NIR suite. |
| Pediatric ICU fellow | Be aware of possible admission for stroke. |
| Pediatric ICU charge nurse | Be aware of possible admission for stroke. |
| Pediatric neurology resident physician | Notify providers, escort family to appropriate location. |
| Pediatric neurology attending physician | Notify providers, escort family to appropriate location. |
| Diagnostic neuroradiology physician | Immediate review of neuroimaging as it is acquired. |
Transport
The patient is physically transported from the pediatric hospital to the adult hospital under the supervision of a pediatric ICU physician. Upon arrival to the NIR suite but before the start of EVT, the pediatric ICU attending and anesthesiologist engage in another scripted handoff.
Endovascular Treatment
Pediatric EVT is performed under general anesthesia with close attention to intraoperative blood pressure. Pediatric EVT technique is similar to adult EVT technique and generally involves either aspiration or stent-retriever thrombectomy, typically from a right femoral arterial approach. Intra-arterial thrombolytics are available but rarely used. Hemostasis at the arterial access site is achieved with manual compression, after which the NIR physician discusses the outcome of the case with the neurologist as well as the patient’s family.
Post-EVT Care
Following EVT, the patient is transported back to either a general or cardiac ICU at the pediatric hospital for close neurological monitoring and frequent vascular assessment. The duration of post-procedure extremity immobilization is recommended by the neurointerventionalist. Because seizures after AIS are common in children23,24, electroencephalography (EEG) lead placement is considered to monitor for seizures and evolving background asymmetry that may prompt acute head imaging. All involved teams evaluate the patient’s condition on the day following the thrombectomy procedure.
Alternate Modes of Presentation
The specified clinical workflow is flexible and can accommodate children who present for EVT from settings other than the emergency department. For inpatients, the overall protocol remains similar aside from slight modifications to accommodate the different physical path used to transport patients to the NIR suite. For patients transferred from other centers, the protocol allows bypass of evaluation steps (e.g., neuroimaging) that have been completed elsewhere.
KEY ELEMENTS OF PROGRAMMATIC SUCCESS
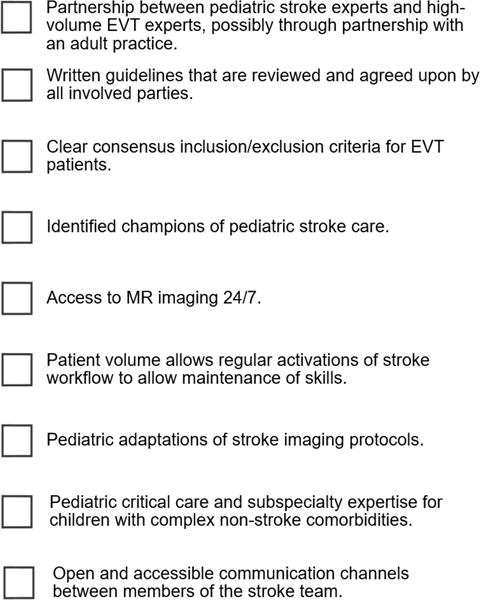
While protocol and workflow details differ between our two centers, there are numerous shared design elements that have been key to success. These elements may be foundational design considerations for centers that aim to develop and implement pediatric EVT protocols. A suggested checklist for centers seeking to implement pediatric EVT is provided in Figure 4.
Figure 4:
Suggested criteria for offering pediatric EVT.
Formalized Protocol and Workflow
A clinical protocol for pediatric EVT was first implemented at SCH in August 2015 and SLCH in May 2018. At both centers, the protocol was developed collaboratively with a pediatric vascular neurologist, neurointerventional radiologist, pediatric anesthesiologist, and diagnostic neuroradiologist, with additional key input from pediatric neurosurgeons, pediatric intensivists, adult anesthesiologists, and pediatric cardiologists. While specific steps and criteria are tailored to account for each hospital’s individual strengths, the existence of a formalized protocol that has been developed by a multidisciplinary team of physicians and approved by appropriate hospital leadership is crucial to define and encourage adherence to expected care pathways.
Among the most important elements of a formalized protocol are key inclusion and exclusion criteria for pediatric EVT. While these criteria vary between centers based on the judgment and capability of local experts, and children outside of these criteria can be considered on a case-by-case basis, the process of establishing interdisciplinary consensus is critical for program cohesion and dissemination.
Integration with Adult EVT Workflow
Our protocols take advantage of the close affiliation our pediatric hospitals have with high-volume adult EVT centers. At each site, the pediatric protocol closely mirrors the adult protocol to harness the greater familiarity that stroke physicians have with more commonly used adult workflows. In this manner, potential confusion arising from infrequent activation of the pediatric EVT protocol is mitigated by the large number of adult EVT cases. The parallels between pediatric and adult EVT workflows go beyond superficial similarities. For example, the name and function of the thrombectomy pager are identical in both workflows. This mimicry also allows the pediatric stroke workflow to benefit from improvements in adult stroke workflow25.
Simplicity and Automation
Our adult EVT protocols are streamlined to achieve better clinical outcomes via reduced door-to-puncture times8,26,27, and our pediatric EVT protocol is similarly simplified and automated. For example, pre-EVT imaging loads automatically to a mobile viewer. Similarly, the thrombectomy pager notifies key personnel of impending EVT using a single point of contact. The implementation of a consultation script ensures that key information is consistently obtained and that effort is not expended gathering superfluous information.
Pediatric Adaptation of Stroke Imaging
Imaging should be adapted to the particular needs and challenges of imaging young patients. Evaluation of suspected LVO using MRI rather than CT may be particularly beneficial in children. MRI can confirm stroke, which is important due to the high incidence of stroke mimics in childhood28,29. It is also much more sensitive than CT to posterior circulation stroke30, which accounts for up to half of all childhood stroke31,32.
MRI also avoids the use of ionizing radiation. Because children are more susceptible to long-term carcinogenic effects of ionizing radiation33, CT imaging should be used judiciously34,35. CT perfusion, in particular, delivers high radiation doses and should generally be avoided in pediatric patients36, which can impede measurement of ischemic volumes that is central to patient evaluation for extended time window EVT.
The principal disadvantages to using MRI over CT are the need for metal implant screening, rapid availability of an MRI technologist, and potentially greater time required for imaging, though the latter is mitigated with dedicated hyperacute stroke MRI protocols37. At centers where timely MRI is not available or contraindicated, non-contrast head CT and CT angiography are reasonable when performed with low-dose protocols tailored towards minimizing radiation dose to children.
Pediatric Stroke Care Advocacy
Development of infrastructure to provide high quality care to children with AIS requires time, energy, and resources from physicians and hospital administrators. Centers looking to implement pediatric stroke care infrastructure would do well to identify physicians and nurses who will enthusiastically champion these efforts, actively engage in ongoing program maintenance, and view pediatric AIS as a professional priority.
Though pediatric neurologists and neurointerventionalists are the most visible members of the stroke care team, our programs conspicuously involve pediatric providers in neuroradiology, emergency medicine, intensive care, anesthesiology, and cardiology. Early and robust engagement of physicians across disciplines has reduced barriers to engaging these experts when needed. For example, early participation of the pediatric anesthesiology team in protocol development facilitated routine inclusion of the on-call pediatric anesthesiologist at the time of thrombectomy pager activation in spite of the fact that a different anesthesiologist may ultimately care for the patient.
Comparative Advantage
Centers caring for adults typically see a greater volume of EVT than pediatric-only centers, and this greater experience is known to enhance outcomes38. On the other hand, pediatric neurologists provide content expertise pertaining to conditions that increase stroke risk in children, common pediatric stroke mimics, and the pediatric neurological examination. Thus, there is enormous comparative advantage to efficiently incorporating both pools of expertise into the stroke care model.
Both of our centers feature close collaboration between a high-volume adult EVT center and a subspecialized pediatric center. This arrangement allows pediatric and adult providers to bring their respective strengths to the care of children with AIS. Our groups have recognized the comparative advantage created by this arrangement based on the strengths of our centers, but other centers may benefit from alternative arrangements. For example, a minority of pediatric centers do offer pediatric EVT within their own walls, but even these centers often rely on neurointerventionalists that also provide care at nearby adult hospitals.
Trust and Collaboration
Children experiencing AIS are a uniquely challenging population of patients due to complex etiologies, comorbidities, and lack of clinical outcome data. Consequently, optimal management of pediatric AIS is rarely clear-cut and judgment calls are often required. The inherent challenges of making judgment calls in critically ill patients can be mitigated by mutual trust, goodwill, and shared team identity within a multidisciplinary stroke team. Specifically, these considerations can dampen concerns about potential reputational harm arising from decisive calls in high-risk cases.
Routinely including pediatric stroke experts in discussion of adult stroke processes, case review, and stroke education efforts is an effective method of building trust, goodwill, and team identity. This approach allows all team members to witness repeated thoughtful discussion of actual stroke cases, philosophical and technical approaches, and underlying clinical evidence.
ONGOING CHALLENGES
Lack of Pediatric Stroke Outcome Data
The lack of prospective data for EVT in children experiencing AIS pose major challenges39,40. Low per-hospital incidence of pediatric AIS was a major factor contributing to the early termination of a prospective trial of tPA in children28, and similar issues plague the study of pediatric EVT41. As a result, EVT efficacy and safety remain largely undefined, which confounds decision-making and warrants discussion of the unproven nature of pediatric EVT prior to intervention. Lack of pediatric data also interferes with pediatric-specific optimizations of workup (e.g., imaging selection thresholds) and EVT itself (e.g., device selection) to account for differences between pediatric and adult stroke. In this context, even perfectly delivered adult EVT may not be tailored to the needs of children. Thus, separate from the need to develop infrastructure to deliver high quality pediatric stroke care, there is a pressing need for a prospective trial or registry to capture key metrics of EVT safety and efficacy in children.
Lack of Formal Pediatric Stroke Center Accreditation
The development of primary and comprehensive stroke center designations for adult hospitals occurred alongside advances in adult AIS treatment, ultimately producing standardized best practices that result in improved performance and superior outcomes42–44. While criteria for pediatric stroke center certification have been suggested19,45, there is currently no formal accrediting body to manage pediatric stroke certification and facilitate adoption of best practices.
Delays to Presentation
Workflows for pediatric stroke must account for greater delays in stroke recognition and workup46–48. Stroke symptoms in children are often underrecognized, impeding what should be rapid progression towards treatment. Aside from diminishing benefit of treatment with longer time delays, such delays also reduce eligibility for intravenous thrombolysis, increase reliance on neurovascular imaging, and may negatively bias outcomes in these patients.
Pediatric Comorbidities
Children experiencing stroke may have very different underlying comorbidities than adults with stroke39,49,50. For example, children with congenital heart disease have an elevated risk of AIS and are overrepresented amongst patients being considered for AIS treatment51. Similarly, arteriopathies are found in nearly 80% of previously healthy pediatric AIS patients52. In children with these or other stroke-related co-morbidities, cardiovascular fragility, altered post-surgical vascular anatomy, and potential thrombotic propensity can alter the safety and efficacy of EVT. Likewise, these co-morbidities may influence choice of treatment, post-treatment prognosis, and the need for long-term antiplatelet or anticoagulation therapy53. Indeed, the presence of such comorbidities also complicates efforts to follow evidence-based practice, as the limited pediatric data that is currently available may translate poorly to children with underlying rare conditions. These comorbidities also present practical barriers to follow-up care and rehabilitation.
Economic Considerations
Due to relatively low patient volume for pediatric EVT procedures, recouping fixed costs related to program maintenance, staffing, and equipment may be difficult. For example, the substantial cost of dedicated perfusion imaging software or an overnight MRI technologist in an adult center can be amortized over a large number of patients, but these same costs may present significant barriers for pediatric centers.
The most obvious route to reduce cost is partnership with a high-volume adult center. In the same manner that patient outcomes may be maximized by calling upon the experience of neurointerventionalists at high-volume EVT centers, hospital costs may be minimized by calling upon the specialized stroke care infrastructure that already exists at adult centers. For costs that cannot be reduced, broad involvement of all affected departments in program development can promote a holistic view of program-related costs and facilitate acceptance as a necessary part of patient care.
CONCLUSION
As in adults, the delivery of high-quality care for children with AIS depends on the presence of well-designed infrastructure. Using two prototypical examples of pediatric EVT programs, we highlight common design features that may provide a foundation to develop such infrastructure in a variety of settings. This guidance may be useful to pediatric centers that aim to bolster their ability to care for children with AIS.
Besides prospective outcome data, the other necessary element for advancing safe, responsible, and high quality AIS care for children is to implement appropriate stroke care infrastructure. We review two successful pediatric EVT programs and identify key design features that may be useful for other centers that aim to bring the advances in adult stroke treatment to children under their care.
ACKNOWLEDGEMENTS
None
SOURCES OF FUNDING
KG was supported by National Institute for Neurological Disorders and Stroke: K23NS099472
DISCLOSURES
AK is a consultant for Microvention, Penumbra, and ISchemaView.
MG has stock in IBM, and received personal fees and non-financial support from Shandong Madic Technology Co, Capital Medical University, and Tancheng Talent Office for an educational trip in 2019.
Non-standard abbreviations and acronyms
- EVT:
Endovascular thrombectomy
- AIS:
Acute ischemic stroke
- LVO:
Large vessel occlusion
- SLCH:
St. Louis Children’s Hospital
- SCH:
Seattle Children’s Hospital
- NIR:
Neurointerventional radiology
- PedNIHSS:
Pediatric NIH stroke scale
- DWI:
Diffusion weighted imaging
- PWI:
Perfusion weighted imaging
- PACS:
Picture archiving and communication system
- rt-PA:
Recombinant tissue plasminogen activator
- MRN:
Medical record number
- mRS:
Modified Rankin scale
- DOB:
Date of birth
- PMH:
Past medical history
- NIHSS:
NIH stroke scale
- ASPECTS:
Alberta stroke program early CT score
- SBP:
Systolic blood pressure
- DBP:
Diastolic blood pressure
- MRP:
Magnetic resonance perfusion
- MRA:
Magnetic resonance angiography
REFERENCES
- 1.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Lansberg MG, Kemp S, Tsai JP, Lavori P, Christensen S, Mlynash M, Kim S, Hamilton S, Yeatts SD, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke. 2017;12:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kansagra AP, Wallace AN, Curfman DR, McEachern JD, Moran CJ, Cross DT, Lee JM, Ford AL, Manu SG, Panagos PD, et al. Streamlined triage and transfer protocols improve door-to-puncture time for endovascular thrombectomy in acute ischemic stroke. Clin Neurol Neurosurg. 2018;166:71–75. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Thevathasan A, Dowling R, Bush S, Mitchell P, Yan B. Streamlining Workflow for Endovascular Mechanical Thrombectomy: Lessons Learned from a Comprehensive Stroke Center. J Stroke Cerebrovasc Dis. 2017;26:1655–1662. [DOI] [PubMed] [Google Scholar]
- 10.McTaggart RA, Moldovan K, Oliver LA, Dibiasio EL, Baird GL, Hemendinger ML, Haas RA, Goyal M, Wang TY, Jayaraman MV. Door-in-Door-Out Time at Primary Stroke Centers May Predict Outcome for Emergent Large Vessel Occlusion Patients. Stroke. 2018;49:2969–2974. [DOI] [PubMed] [Google Scholar]
- 11.Schonenberger S, Weber D, Ungerer MN, Pfaff J, Schieber S, Uhlmann L, Heidenreich P, Bendszus M, Kieser M, Wick W, et al. The KEEP SIMPLEST Study: Improving In-House Delays and Periinterventional Management in Stroke Thrombectomy-A Matched Pair Analysis. Neurocrit Care. 2019;31:46–55. [DOI] [PubMed] [Google Scholar]
- 12.Kansagra AP, Meyers GC, Kruzich MS, Cross DT 3rd, Moran CJ Wide Variability in Prethrombectomy Workflow Practices in the United States: A Multicenter Survey. AJNR Am J Neuroradiol. 2017;38:2238–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rumalla K, Ottenhausen M, Kan P, Burkhardt JK. Recent Nationwide Impact of Mechanical Thrombectomy on Decompressive Hemicraniectomy for Acute Ischemic Stroke. Stroke. 2019;50:2133–2139. [DOI] [PubMed] [Google Scholar]
- 14.Saber H, Navi BB, Grotta JC, Kamel H, Bambhroliya A, Vahidy FS, Chen PR, Blackburn S, Savitz SI, McCullough L, et al. Real-World Treatment Trends in Endovascular Stroke Therapy. Stroke. 2019;50:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.deVeber GA, Kirton A, Booth FA, Yager JY, Wirrell EC, Wood E, Shevell M, Surmava AM, McCusker P, Massicotte MP, et al. Epidemiology and Outcomes of Arterial Ischemic Stroke in Children: The Canadian Pediatric Ischemic Stroke Registry. Pediatr Neurol. 2017;69:58–70. [DOI] [PubMed] [Google Scholar]
- 16.Shoirah H, Shallwani H, Siddiqui AH, Levy EI, Kenmuir CL, Jovin TG, Levitt MR, Kim LJ, Griauzde J, Pandey AS, et al. Endovascular thrombectomy in pediatric patients with large vessel occlusion. J Neurointerv Surg. 2019;11:729–732. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia K, Kortman H, Blair C, Parker G, Brunacci D, Ang T, Worthington J, Muthusami P, Shoirah H, Mocco J, et al. Mechanical thrombectomy in pediatric stroke: systematic review, individual patient data meta-analysis, and case series. J Neurosurg Pediatr. 2019:1–14. [DOI] [PubMed] [Google Scholar]
- 18.Madaelil TP, Kansagra AP, Cross DT, Moran CJ, Derdeyn CP. Mechanical thrombectomy in pediatric acute ischemic stroke: Clinical outcomes and literature review. Interv Neuroradiol. 2016;22:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard TJ, Rivkin MJ, Scholz K, deVeber G, Kirton A, Gill JC, Chan AK, Hovinga CA, Ichord RN, Grotta JC, et al. Emergence of the primary pediatric stroke center: impact of the thrombolysis in pediatric stroke trial. Stroke. 2014;45:2018–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sporns PB, Sträter R, Minnerup J, Wiendl H, Hanning U, Chapot R, Henkes H, Henkes E, Grams A, Dorn F, et al. Feasibility, Safety, and Outcome of Endovascular Recanalization in Childhood Stroke: The Save ChildS Study. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhogal P, Hellstern V, AlMatter M, Ganslandt O, Bazner H, Aguilar Perez M, Henkes H. Mechanical thrombectomy in children and adolescents: report of five cases and literature review. Stroke Vasc Neurol. 2018;3:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amlie-Lefond C, Shaw DWW, Cooper A, Wainwright MS, Kirton A, Felling RJ, Abraham MG, Mackay MT, Dowling MM, Torres M, et al. Risk of Intracranial Hemorrhage Following Intravenous tPA (Tissue-Type Plasminogen Activator) for Acute Stroke Is Low in Children. Stroke. 2020;51:542–548. [DOI] [PubMed] [Google Scholar]
- 23.Singh RK, Zecavati N, Singh J, Kaulas H, Nelson KB, Dean NP, Gaillard WD, Carpenter J. Seizures in acute childhood stroke. J Pediatr. 2012;160:291–296. [DOI] [PubMed] [Google Scholar]
- 24.Fox CK, Mackay MT, Dowling MM, Pergami P, Titomanlio L, Deveber G, Investigators S. Prolonged or recurrent acute seizures after pediatric arterial ischemic stroke are associated with increasing epilepsy risk. Dev Med Child Neurol. 2017;59:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajani NK, Pearce K, Campion T, Salpietro V, Planells M, Chong W, Patankar T, Mankad K. Pediatric stroke: current diagnostic and management challenges. Quant Imaging Med Surg. 2018;8:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schregel K, Behme D, Tsogkas I, Knauth M, Maier I, Karch A, Mikolajczyk R, Hinz J, Liman J, Psychogios MN. Effects of Workflow Optimization in Endovascularly Treated Stroke Patients - A Pre-Post Effectiveness Study. PLoS One. 2016;11:e0169192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurav SK, Zirpe KG, Wadia RS, Naniwadekar A, Pote PU, Tungenwar A, Deshmukh AM, Mohopatra S, Nimavat B, Surywanshi P. Impact of “Stroke Code”-Rapid Response Team: An Attempt to Improve Intravenous Thrombolysis Rate and to Shorten Door-to-Needle Time in Acute Ischemic Stroke. Indian J Crit Care Med. 2018;22:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivkin MJ, deVeber G, Ichord RN, Kirton A, Chan AK, Hovinga CA, Gill JC, Szabo A, Hill MD, Scholz K, et al. Thrombolysis in pediatric stroke study. Stroke. 2015;46:880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wharton JD, Barry MM, Lee CA, Massey K, Ladner TR, Jordan LC. Pediatric Acute Stroke Protocol Implementation and Utilization Over 7 Years. J Pediatr. 2020;220:214–220 e211. [DOI] [PubMed] [Google Scholar]
- 30.Hwang DY, Silva GS, Furie KL, Greer DM. Comparative sensitivity of computed tomography vs. magnetic resonance imaging for detecting acute posterior fossa infarct. J Emerg Med. 2012;42:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goeggel Simonetti B, Rafay MF, Chung M, Lo WD, Beslow LA, Billinghurst LL, Fox CK, Pagnamenta A, Steinlin M, Mackay MT, et al. Comparative study of posterior and anterior circulation stroke in childhood: Results from the International Pediatric Stroke Study. Neurology. 2020;94:e337–e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rai AT, Seldon AE, Boo S, Link PS, Domico JR, Tarabishy AR, Lucke-Wold N, Carpenter JS. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg. 2017;9:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutanzi KR, Lumen A, Koturbash I, Miousse IR. Pediatric Exposures to Ionizing Radiation: Carcinogenic Considerations. Int J Environ Res Public Health. 2016;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goske MJ, Applegate KE, Boylan J, Butler PF, Callahan MJ, Coley BD, Farley S, Frush DP, Hernanz-Schulman M, Jaramillo D, et al. The ‘Image Gently’ campaign: increasing CT radiation dose awareness through a national education and awareness program. Pediatr Radiol. 2008;38:265–269. [DOI] [PubMed] [Google Scholar]
- 35.Slovis TL. The ALARA concept in pediatric CT: myth or reality? Radiology. 2002;223:5–6. [DOI] [PubMed] [Google Scholar]
- 36.Sabarudin A, Yusof MZ, Mohamad M, Sun Z. Radiation dose associated with cerebral CT angiography and CT perfusion: an experimental phantom study. Radiat Prot Dosimetry. 2014;162:316–321. [DOI] [PubMed] [Google Scholar]
- 37.Goyal MS, Hoff BG, Williams J, Khoury N, Wiesehan R, Heitsch L, Panagos P, Vo KD, Benzinger T, Derdeyn CP, et al. Streamlined Hyperacute Magnetic Resonance Imaging Protocol Identifies Tissue-Type Plasminogen Activator-Eligible Stroke Patients When Clinical Impression Is Stroke Mimic. Stroke. 2016;47:1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svendsen ML, Ehlers LH, Ingeman A, Johnsen SP. Higher stroke unit volume associated with improved quality of early stroke care and reduced length of stay. Stroke. 2012;43:3041–3045. [DOI] [PubMed] [Google Scholar]
- 39.Tsze DS, Valente JH. Pediatric stroke: a review. Emerg Med Int. 2011;2011:734506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenham M, Gordon A, Anderson V, Mackay MT. Outcome in Childhood Stroke. Stroke. 2016;47:1159–1164. [DOI] [PubMed] [Google Scholar]
- 41.Ferriero DM, Fullerton HJ, Bernard TJ, Billinghurst L, Daniels SR, DeBaun MR, deVeber G, Ichord RN, Jordan LC, Massicotte P, et al. Management of Stroke in Neonates and Children: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke. 2019;50:e51–e96. [DOI] [PubMed] [Google Scholar]
- 42.Man S, Zhao X, Uchino K, Hussain MS, Smith EE, Bhatt DL, Xian Y, Schwamm LH, Shah S, Khan Y, et al. Comparison of Acute Ischemic Stroke Care and Outcomes Between Comprehensive Stroke Centers and Primary Stroke Centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston Alberts M,, Does Stroke Center Designation Improve Patient Outcomes? ACEP Now. 2011. [Google Scholar]
- 44.Shen YC, Chen G, Hsia RY. Community and Hospital Factors Associated With Stroke Center Certification in the United States, 2009 to 2017. JAMA Netw Open. 2019;2:e197855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernard TJ, Friedman NR, Stence NV, Jones W, Ichord R, Amlie-Lefond C, Dowling MM, Rivkin MJ. Preparing for a “Pediatric Stroke Alert”. Pediatr Neurol. 2016;56:18–24. [DOI] [PubMed] [Google Scholar]
- 46.Rivkin MJ, Bernard TJ, Dowling MM, Amlie-Lefond C. Guidelines for Urgent Management of Stroke in Children. Pediatr Neurol. 2016;56:8–17. [DOI] [PubMed] [Google Scholar]
- 47.Saver JL. Time is brain--quantified. Stroke. 2006;37:263–266. [DOI] [PubMed] [Google Scholar]
- 48.Rafay MF, Pontigon AM, Chiang J, Adams M, Jarvis DA, Silver F, Macgregor D, Deveber GA. Delay to diagnosis in acute pediatric arterial ischemic stroke. Stroke. 2009;40:58–64. [DOI] [PubMed] [Google Scholar]
- 49.Chiang K-L, Cheng C- Y. Epidemiology, risk factors and characteristics of pediatric stroke: a nationwide population-based study. QJM: An International Journal of Medicine. 2018;111:445–454. [DOI] [PubMed] [Google Scholar]
- 50.Numis AL, Fox CK. Arterial ischemic stroke in children: risk factors and etiologies. Curr Neurol Neurosci Rep. 2014;14:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ducharme-Crevier L, Wainwright MS. Childhood Stroke and Congenital Heart Disease. Pediatr Neurol Briefs. 2015;29:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyle CA, Bernard TJ, Goldenberg NA. Childhood arterial ischemic stroke: a review of etiologies, antithrombotic treatments, prognostic factors, and priorities for future research. Semin Thromb Hemost. 2011;37:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eleftheriou D, Ganesan V, Hong Y, Klein NJ, Brogan PA. Endothelial injury in childhood stroke with cerebral arteriopathy: a cross-sectional study. Neurology. 2012;79:2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]