Abstract
Purpose:
To evaluate the association between optical coherence tomography angiography (OCTA) features and prior visual field (VF) progression in primary angle closure glaucoma (PACG).
Methods:
In a cross-sectional study, 46 eyes of 31 PACG patients with 5 reliable VF examinations performed over ≥3 years of follow-up underwent OCTA imaging. Effect of clinical (age, gender, number of anti-glaucoma medications, mean and SD of intraocular pressure during follow-up), OCT (average retinal nerve fiber layer and ganglion cell complex thickness) and OCTA (whole enface vessel density of disc and macular scan, deep-layer microvascular dropout [MvD]) parameters on the rate of mean deviation (MD) change was evaluated using linear mixed models.
Results:
Average (± standard deviation) MD of the baseline VF was −7.4 ± 7.3 dB, and rate of MD change was −0.32±0.29 dB/year. Whole enface vessel density of disc and macular scans was 39.5%±8.1 and 38.7%±4.4, respectively. MvD was noted in 33.3% of the eyes. Multivariate mixed models showed that lower whole enface disc (coefficient: 0.02, p=0.03) and macular vessel densities (coefficient: 0.04, p=0.02) were significantly associated with faster rate of MD decline. Other factors significantly associated with faster progression in multivariate models were older age (coefficient: −0.02, p<0.05) and the presence of systemic hypertension (coefficient: −0.37, p=0.01) and diabetes (coefficient: −0.28, p=0.05).
Conclusions:
Lower superficial vessel density measured using OCTA was significantly associated with faster VF progression in PACG. In these eyes, OCTA parameters can serve as biomarker suggestive of past VF progression.
Keywords: primary angle closure glaucoma, progression, optical coherence tomography angiography, vessel density, microvascular dropout
Precis:
Lower whole enface disc (coefficient: 0.02, p=0.03) and macular vessel densities (coefficient: 0.04, p=0.02) on OCT angiography were significantly associated with faster rate of mean deviation decline.
INTRODUCTION
Optical coherence tomography angiography (OCTA) is a non-invasive, dye-free technology that can image large vessels as well as microvasculature of the retina, optic nerve head (ONH) and some part of the choriocapillaris by performing multiple OCT scans of the same region. The two most common OCTA features demonstrated in glaucoma are the reduction in vessel density in the superficial layers of the retina (in the peripapillary and macular region),1–6 and the presence of deep-layer microvasculature dropout (MvD) in the parapapillary region.7, 8 These OCTA changes have also shown a good correlation with the degree of glaucomatous damage. As the glaucoma severity increases, the reduction in vessel densities becomes more pronounced,3, 9–18 and the MvD become more prevalent and larger in size.7, 19–21
OCTA features provide useful information about the risk of glaucoma progression. In a longitudinal study of eyes with mild to moderate primary open-angle glaucoma (POAG), lower baseline macular and peripapillary vessel densities were associated with a faster rate of retinal nerve fiber layer (RNFL) thinning.22 In multiple studies, the presence of MvD was associated with a faster rate of RNFL thinning23, 24 and visual field (VF) progression.25 However, each of the studies assessing the association between OCTA changes and glaucoma progression was performed in POAG, and there is no literature on the utility of OCTA features in assessing the risk of progression in primary angle-closure glaucoma (PACG).
A systematic review and meta-analysis estimated that 64.3 million people aged between 40 and 80 years had glaucoma worldwide, of which 44.1 million had POAG and 20.2 million had PACG.26 Although the prevalence of PACG is half that of POAG globally, prevalence of blindness is reported to be higher in PACG than POAG.27 The purpose of the current study was to evaluate the association between OCTA features and VF progression in PACG.
METHODS
This was a cross-sectional, observational study conducted at Narayana Nethralaya, a tertiary eye care center in Bengaluru, South India between May 2019 and January 2020. The methodology adhered to the tenets of the Declaration of Helsinki for research involving human subjects. Written informed consent was obtained from all participants and the study was approved by the Ethics Committee of Narayana Nethralaya (EC number: C/2019/07/02).
The study included PACG patients with 5 reliable VF examinations performed over the past 3 or more years of follow-up (the last one done on the day of OCT examination). PACG patients had occludable anterior chamber angles on gonioscopy, and a history of intraocular pressure (IOP) >21 mm Hg. They also had glaucomatous optic nerve head changes on dilated fundus examination as documented by glaucoma specialists, and glaucomatous visual fields (defined below) that correlated with the changes in the optic nerve head. The anterior chamber angle was examined using indentation gonioscopy. The angle was considered occludable if the posterior trabecular meshwork was not seen in 3 or more quadrants in primary position without indentation.28 All patients had undergone a laser peripheral iridotomy prior to the date of first VF examination. Glaucomatous optic nerve head changes included focal or diffuse neuroretinal rim thinning, localized notching or RNFL defects. VF was considered glaucomatous if the glaucoma hemifield test result was outside normal limits, pattern standard deviation was abnormal at p<5% level, or ≥3 test points in a cluster on pattern deviation probability plot were abnormal at p<5% with at least one point abnormal at p<1%. Inclusion criteria for all patients were age ≥18 years, corrected distance visual acuity of 20/40 or better and refractive error within ±5 D sphere and ±3 D cylinder. Exclusion criteria were presence of any media opacities that prevented reliable VF tests (anytime during the follow-up) and good quality OCT scans, or any retinal or neurological disease other than glaucoma, which could confound the evaluation. Eyes with a history of trauma, inflammation or any incisional surgery in the past (cataract surgery or filtration surgery) were also excluded.
All eligible PACG patients underwent a comprehensive ocular examination, which included a detailed medical history, corrected distance visual acuity measurement, slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, dilated fundus examination, VF examination and OCT imaging with RTVue-XR SD-OCT (Optovue Inc., Fremont, CA). The mean IOP and standard deviation (SD) IOP during the entire follow-up period, beginning on the day of first VF, were calculated.
VF examinations were performed using a Humphrey Field analyzer II, model 720i (Zeiss Humphrey Systems, Dublin, CA), with the Swedish interactive threshold algorithm (SITA) standard 24–2 program. VFs were considered reliable if the fixation losses and false negative response rates were ≤33%, and the false positive response rates were ≤15%. Mean deviation (MD) of the reliable VFs were used to derive the rate of MD change per year.
OCTA imaging of the optic disc region and macula was performed using RTVue-XR SD-OCT (AngioVue, v2016.2.0.35). The procedure of OCTA imaging with RTVue-XR has been detailed previously.5 In brief, it uses an 840 nm diode laser source, with an A-scan rate of 70 kHz per second. Imaging is performed using a set of 2 scans; one vertical priority and one horizontal priority raster volumetric scan. The optic disc scan covers an area of 4.5 × 4.5 mm and the macular scan was performed using volumetric scans covering 6 × 6 mm. An orthogonal registration algorithm was used to produce merged 3-dimensional OCT angiograms.29 The software compares the consecutive B-scans at the same location to detect flow using motion contrast, thereby delineating blood vessels.30 Vessel density is defined as the percentage area occupied by the large vessels and microvasculature in a particular scan/sector. Vessel densities are calculated over the entire scan area, i.e. whole enface (image) vessel density (wiVD) disc and wiVD macula, as well as defined areas within each scan as described below. In addition, the software calculates vessel densities in various layers of the retina and the ONH. On the optic disc scan, the software automatically fits a 0.75 mm-wide elliptical annulus extending from the optic disc boundary, and the average vessel density within this region (called the average peripapillary vessel density) is calculated. The wiVD disc and peripapillary vessel densities were analyzed from the “Radial Peripapillary Capillary (RPC) segment” which extends from the ILM to the posterior boundary of the nerve fiber layer. On the macular scan, in addition to the wiVD macula, vessel densities were also analyzed over a 1 mm-wide parafoveal, circular annulus centered on the macula (called the parafoveal vessel density). Macular vessel densities analyzed in this study were of the superficial vascular plexus present in the inner layers of the retina (extending from the ILM to the inner plexiform layer).
Parapapillary deep-layer MvD was evaluated on the choroidal slab of the optic disc scan. MvD was defined as a focal, sectoral, capillary dropout, with no visible microvascular network identified on the choroidal enface image, the circumferential width of which was more than one half clock hour of the disc circumference.19 Two independent observers (TS and SS), masked to the clinical, VF and OCT details of the patients, evaluated the quality of the choroidal slabs and identified the presence of MvD. Disagreement between the observers were resolved by a third adjudicator (ZSP). Our method of detecting the presence of MvD has been described in detail previously.21, 31
The subjects also underwent the traditional peripapillary RNFL and macular ganglion cell complex (GCC) thickness measurements on RTVue-XR SD-OCT using the ONH and the GCC scans. These scan protocols have been explained in detail previously.32, 33 In brief, the ONH protocol consists of 12 radial scans 3.4 mm in length, and 6 concentric ring scans ranging from 2.5 to 4.0 mm in diameter all centered on the optic disc. ONH protocol generates a polar RNFL thickness map which is the RNFL thickness measured along a circle 3.45 mm in diameter centered on the optic disc. The GCC scan consists of one horizontal line scan 7 mm in length and 15 vertical line scans 7 mm in length and at 0.5 mm interval centered 1 mm temporal to the fovea. GCC scan measures the inner retinal thickness which includes the thickness of the nerve fiber layer, ganglion cell layer and the inner plexiform layer, collectively called the GCC thickness.
All the examinations for a particular subject (OCT, OCTA and the last VF of the series) were performed on the same day. Image quality was assessed for all OCTA and OCT scans. Poor quality scans, which were defined as those with a signal strength index (SSI) less than 35 or images with motion artifacts, local weak signal and segmentation errors were excluded from the analysis.
STATISTICAL ANALYSIS
Descriptive statistics included mean and standard deviation for continuous variables and percentages for categorical variables. The effect of clinical, OCT and OCTA parameters on rate of change of MD (MD slope) was evaluated using linear mixed models with random intercepts and random slopes.34, 35 In this model, the change in the outcome variable (MD) was explored using a linear function of time, and random intercepts and random slopes introduced patient- and eye-specific deviations from the average value. The model accounts for the fact that different eyes can have different MD slopes over the follow-up period, while accommodating correlations between both eyes of the same individual.34, 35
Because rate of MD change may depend on the disease severity, an unstructured covariance between random effects was assumed, allowing for correlation between intercepts and slopes of change.36 Effects of predictor variables were assessed on the baseline MD (baseline severity), and on the change in MD over time by introducing interaction terms between time and predictor variables. The clinical parameters (predictors) investigated for their association with baseline MD and rate of MD change were the age, gender, presence of hypertension, diabetes, central corneal thickness (CCT), follow-up duration, mean IOP and the SD of IOP during the follow-up, and the number of anti-glaucoma medications at the scanning visit. The OCT and OCTA predictors investigated were the average RNFL and GCC thickness, wiVD optic disc and macula scans, and the presence of MvD. Univariate models were constructed containing one predictor along with its interaction with time. Predictors associated with the rate of MD change at P<0.10 in univariate analysis were introduced into multivariate analysis. Collinearity between predictor variables were evaluated and predictors correlated with each other (correlation coefficient of >0.50) were evaluated using separate multivariate models. Rates of MD change were obtained from the linear mixed models using best linear unbiased prediction (BLUP).37, 38 Statistical analyses were performed using Stata software version 14.2 (StataCorp, College Station, TX). A p value of ≤0.05 was considered statistically significant for the final analysis.
RESULTS
Forty-six eyes of 31 PACG patients were recruited for the study. Table 1 shows the clinical, VF, OCT, OCTA features of the included patients. At the baseline visit (first VF visit), 27 eyes had a MD≥−6 dB, 7 eyes had MD between −6 dB and −12 dB, 8 eyes had MD between −12 and −18 dB, and 4 eyes had MD≤−18 dB. Mean follow-up duration over which the 5 VF examinations were done was 5.2 years. Mean and SD of IOP during the follow-up was 15.2 and 2.8 mm Hg respectively. Mean number of anti-glaucoma medications on the OCT scanning visit was 1.8. Thirteen eyes (28.3%) had poor quality optic disc OCTA scan and 8 eyes (17.4%) had poor quality macular OCTA scan. OCTA data of these eyes were excluded while the rest of the data from these eyes was used for the analysis. Choroidal slabs of 42 eyes were found to be of acceptable quality and MvD was found in 14 of these eyes (33.3%). Inter-examiner agreement in detection of the MvD was excellent (kappa: 0.89). Rate of MD change was −0.32 ± 0.29 dB/year in the cohort (range: −1.12 to 0.48). Disc hemorrhage was not present in any of the eyes at any of the follow-up visits. None of the eyes had a history of acute angle closure. At the OCT visit, the strength of association of MD, measured using coefficient of determination (R2), was 73.2% with average RNFL thickness, 49.2% with average GCC thickness, 79.6% with wiVD disc and 58.8% with wiVD macula.
Table 1.
Clinical features, visual field parameters and optical coherence tomography (OCT) measurements of the patients with primary angle closure glaucoma (46 eyes, 31 patients).
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 68.9 ± 9.4 | 48 to 84 |
| Gender (male:female) | 19:12 | |
| Hypertension (n, %) | 13 (41.9%) | |
| Diabetes mellitus (n, %) | 13 (41.9%) | |
| Intraocular pressure | ||
| Mean during follow-up (mm Hg) | 15.2 ± 3.0 | 9.2 to 21.5 |
| Fluctuation (SD) during follow-up (mm Hg) | 2.8 ± 1.4 | 0.8 to 8.1 |
| At the scanning visit (mm Hg) | 14.2 ± 3.3 | 8 to 20 |
| No. of glaucoma medications at OCTA visit | 1.8 ±1.1 | 0 to 4 |
| Central corneal thickness (μm) | 521 ± 43 | 418 to 588 |
| Follow-up duration (years) | 5.2 ±2.2 | 3.1 to 9.7 |
| Baseline mean deviation (dB) | −7.4 ± 7.3 | −25.8 to 1.3 |
| Baseline visual field index (%) | 85 ± 17 | 41 to 100 |
| Mean deviation slope (dB/year) | −0.32 ± 0.29 | −1.12 to 0.48 |
| Peripapillary RNFL thickness (μm) | 77.9 ± 15.9 | 42 to 97 |
| Average GCC thickness (μm) | 77.3 ± 14.2 | 51 to 105 |
| OCTA vessel density measurement | ||
| Whole enface disc (%), n=33 eyes | 39.5 ± 8.1 | 24.8 to 53.0 |
| Peripapillary (%) | 47.6 ± 10.4 | 28.5 to 68.4 |
| Whole enface macula (%), n=38 eyes | 38.7 ± 4.4 | 29.4 to 47.5 |
| Parafoveal (%) | 40.9 ± 4.2 | 32.9 to 48.3 |
| Choroidal microvascular dropout (n, %) | 14/42 (33.3%) |
dB: decibel; VF: visual field; RNFL: retinal nerve fiber layer; GCC: ganglion cell complex; OCTA: optical coherence tomography angiography.
Table 2 shows the effect of each predictor on the baseline MD in univariate analysis. Lower baseline MD value (greater severity of disease) was significantly associated with lower RNFL and GCC thickness, and lower wiVD disc and wiVD macula measurements. Lower baseline MD was also associated with the presence of MvD. Table 2 also shows the effect of each predictor on the MD slope. Faster rate of MD decline (more negative slope) was associated with greater age (coefficient=−0.02, p=0.04), presence of hypertension (coefficient=−0.44, p=0.01) and diabetes mellitus (coefficient=−0.44, p=0.01), greater number of anti-glaucoma medications (coefficient=−0.23, p=0.001), lower average GCC thickness (coefficient=0.01, p=0.06), lower wiVD disc (coefficient=0.03, p=0.02, Figure 1a) and lower wiVD macula (coefficient=0.05, p=0.02, Figure 1b).
Table 2.
Results of univariate analysis evaluating the effect of each predictive variable on visual field mean deviation (MD) measurement at baseline and MD change over time (slope) in primary angle closure glaucoma eyes
| Effect on Baseline MD | Effect on MD Slope | |||
|---|---|---|---|---|
| Coefficient ± SE | P | Coefficient ± SE | P | |
| Age | 0.10 ± 0.13 | 0.45 | −0.02 ± 0.01 | 0.04 |
| Gender (Male as ref) | 2.94 ± 2.47 | 0.23 | 0.25 ± 0.18 | 0.16 |
| Hypertension | 3.23 ± 2.47 | 0.19 | −0.44 ± 0.17 | 0.01 |
| Diabetes Mellitus | −0.42 ± 2.49 | 0.87 | −0.44 ± 0.16 | 0.01 |
| Central corneal thickness | −0.01 ± 0.03 | 0.84 | <0.001 ± 0.002 | 0.98 |
| Mean IOP during follow-up | 0.48 ± 0.36 | 0.19 | −0.01 ± 0.03 | 0.86 |
| IOP SD during follow-up | −0.82 ± 0.83 | 0.32 | −0.09 ± 0.06 | 0.18 |
| Number of anti-glaucoma medications | −1.15 ± 0.95 | 0.22 | −0.23 ± 0.07 | 0.001 |
| Follow-up duration | 0.79 ± 0.54 | 0.14 | −0.05 ± 0.04 | 0.15 |
| Retinal nerve fiber layer thickness | 0.40 ± 0.05 | <0.001 | 0.01 ± 0.01 | 0.14 |
| Ganglion cell complex thickness | 0.31 ± 0.06 | <0.001 | 0.01 ± 0.01 | 0.06 |
| wiVD disc | 0.76 ± 0.06 | <0.001 | 0.03 ± 0.01 | 0.02 |
| wiVD macula | 1.04 ± 0.20 | <0.001 | 0.05 ± 0.02 | 0.02 |
| Presence of MvD | −9.96 ± 1.60 | <0.001 | −0.21 ± 0.18 | 0.26 |
SE: standard error; IOP: intraocular pressure; SD: standard deviation; wiVD: whole image vessel density; MvD: Parapapillary deep-layer microvascular dropout. Optical coherence tomography examinations were performed at the last VF examination visit.
Figure 1.
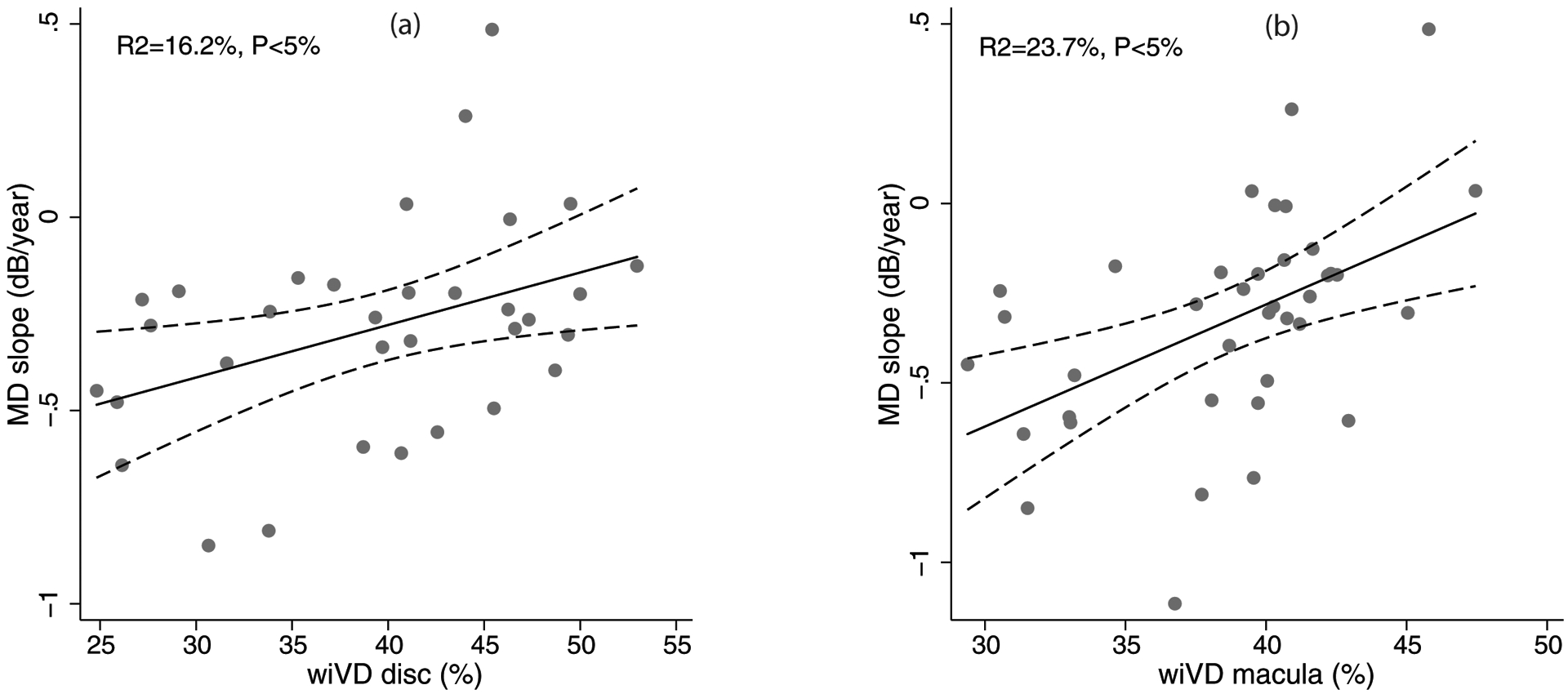
Scatterplots showing the relationship between rate of mean deviation (MD) change (MD slope) and whole enface vessel density of disc scan (wiVD disc, a), and whole enface vessel density of macular scan (wiVD macula, b). R2 represents the coefficient of determination of the relationship.
Table 3 shows the results of multivariate models incorporating the factors found to be significantly associated with rate of MD change in univariate analysis. As collinearity was noted between GCC thickness, wiVD disc and wiVD macula, separate multivariate models were built incorporating each of them with the other predictors found to significantly associated with rate of MD change on univariate analysis. Collinearity was also noted between hypertension and diabetes (10 patients had both hypertension and diabetes, another 3 had only hypertension and another 3 had only diabetes), and each of them were therefore evaluated in separate models. Each 1% reduction in wiVD disc was associated with a 0.02 dB/year faster rate of MD decline (p=0.03) and each 1% reduction in wiVD macula was associated with a 0.04 dB/year faster rate of MD decline (p=0.02). Similar results were found when average peripapillary vessel density was included in the model instead of wiVD disc (0.014 dB/year for every 1% reduction in peripapillary vessel density, P=0.03), and average parafoveal vessel density was included instead of wiVD macula (0.04 dB/year for every 1% reduction in parafoveal vessel density, P=0.01). An increase in age by 1 year was associated with a 0.02 dB/year faster MD decline (p<0.05). Rate of MD decline was 0.37 dB/year faster in patients with systemic hypertension (p=0.01). Rate of MD decline was 0.28 dB/year faster in patients with diabetes mellitus (p=0.05). Rate of MD decline was not associated with GCC thickness in multivariate analysis (p>0.05).
Table 3.
Results of multivariate mixed effect models showing the factors associated with the slope of mean deviation in primary angle closure glaucoma eyes
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Coefficient ± SE | P | Coefficient ± SE | P | Coefficient ± SE | P | |
| Age | −0.02 ± 0.01 | 0.10 | −0.02 ± 0.01 | 0.04 | −0.02 ± 0.01 | 0.01 |
| Hypertension | −0.34 ± 0.15 | 0.02 | −0.37 ± 0.14 | 0.01 | −0.38 ± 0.15 | 0.01 |
| Number of AGM | −0.20 ± 0.07 | 0.01 | −0.10 ± 0.07 | 0.16 | −0.09 ± 0.07 | 0.21 |
| GCC thickness | <0.001± 0.01 | 0.98 | ||||
| wiVD disc | 0.02± 0.01 | 0.03 | ||||
| wiVD macula | 0.04 ± 0.02 | 0.02 | ||||
SE: standard error; AGM: anti-glaucoma medications; GCC: ganglion cell complex; wiVD: whole image vessel density.
DISCUSSION
The current study found a significant association between past VF progression and superficial vessel density measurements in the optic disc and macular region measured using OCTA in PACG eyes. Each 1% reduction in wiVD disc and wiVD macula was associated with a 0.02 dB/year and 0.04 dB/year faster rate of MD decline, respectively. These findings suggest that macula and optic nerve head vessel density may serve as an important structural parameter associated with progressive damage of RGC function.
The association between superficial vessel density measurements of OCTA at baseline visit and future glaucoma progression has been evaluated in POAG eyes by Moghimi et al recently.22 In a prospective, longitudinal study including POAG eyes with mild to moderate severity of disease (MD better than −12 dB), they found that lower vessel density in the optic disc and macular region at baseline visit was associated with a faster rate of RNFL thinning when evaluated longitudinally. Each 1% lower wiVD disc and macula at baseline was associated with a 0.06 μm/year and 0.11μm/year faster rate of RNFL thinning, respectively. One of the reasons for the association between lower vessel density and faster progression is the possibility of more rapid retinal ganglion cell (RGC) death in eyes with lower vessel density. An alternative reason for such an association is the existence of dysfunctional RGCs that have lower metabolic demand causing reduction in vessel density.22 Another finding in the current study, which evaluated the OCTA measurement (at the final visit) and prior VF progression, is that the association of MD slope appeared stronger with macular compared to optic disc vessel density as seen by the coefficients of determination (23.7% vs. 16.2%). The study by Moghimi et al also found a stronger association between macular vessel density and RNFL slope (coefficient of determination, R2=12.5%) compared to optic disc vessel density (R2=3.3%).22 While previous studies report lower utility of macular (compared to peripapillary) vessel density in diagnosing glaucoma,5, 39 both the current study and the study by Moghimi et al show that macular vessel density seems to be better than optic disc vessel density in assessing the risk of glaucoma progression.
Deep-layer MvD in the current study was significantly associated with the severity of glaucoma (MD) at baseline, but was not associated with rate of MD change. Unlike the superficial retinal vessels which are branches of retinal artery, deep retinal and choroidal vessels (the absence of which in the parapapillary region forms the MvD) are branches of the short posterior ciliary artery that supply the prelaminar and laminar regions of the optic nerve head. Previous studies in POAG have shown a significant association of MvD with both structural progression (faster rate of RNFL thinning)23, 24 and functional (VF) progression.25 In a previous study, we found that the prevalence of MvD was significantly less (p<0.05) in PACG (35.7%) compared to POAG (58.3%) eyes.31 We hypothesized that the lower prevalence of MvD in PACG compared to POAG was possibly due to the differences in the pathogenesis of these 2 glaucoma subtypes, with non-IOP related (especially deep retinal and choroidal blood flow) factors playing a less important role in the pathogenesis of PACG. The lack of an association between MvD and rate of MD change in PACG eyes in the current study suggests that choroidal blood flow has a less important role in PACG progression, too. Future longitudinal studies should investigate this issue further.
VF progression was also found to be faster in older patients. A previous study by Verma et al has also reported VF progression to be faster in older PACG patients.40 Progression was also faster in patients with systemic hypertension and diabetes. These systemic conditions were self-reported by the patients and blood pressure or blood sugar of the patients was not measured in the current study. It is possible that the faster progression in patients with hypertension and diabetes may be due to reduced ocular blood flow. In the current study, the vessel densities in general appeared to be less in patients with hypertension and diabetes but the difference was small and statistically not significant. Mean wiVD disc in patients with and without hypertension (39.5% vs 39.5%, p=0.99), and in patients with and without diabetes (38.5% vs 40.2%, p=0.55) was not statistically different. Mean wiVD macula in patients with and without hypertension (37.7% vs 39.3%, p=0.30), and in patients with and without diabetes (37.6% vs 39.4%, p=0.20) was also not statistically different. Future studies are needed to explore this relationship further.
The other factor significantly associated with faster MD slope in the univariate analysis of the current study was the number of anti-glaucoma medications. The need for each additional anti-glaucoma medication was associated with 0.23 dB/year faster rate of MD decline. Contrary to expectation, mean and SD (fluctuation) of IOP during the follow-up were not associated with faster MD slope. However, it is important to note that all these (IOP and anti-glaucoma medications) are inter-related factors and the change in one would have affected (muted) the effect of other on VF progression in univariate analysis. This implies that it is difficult to evaluate the effect of each of these factors on progression without accounting for the interactions between them. However, the sample size of the current study was insufficient to investigate the interactions between these factors as well as the effects of these interactions on the MD slope.
RNFL and GCC thickness measurements in the current study were not associated with past rate of MD change in multivariate analysis, in spite of GCC thickness being associated with MD change in univariate analysis. A possible reason for not finding an association between OCT parameters and MD change is the inclusion of PACG eyes with severe VF loss (MD worse than −12 dB) at baseline. Due to the floor effect in structural measurements, eyes with advanced glaucoma are unlikely to show a relationship between OCT measurements and rates of MD change.41, 42 These findings also support the previous report that vascular parameters of OCTA are less affected by the floor effect and can used to monitor advanced glaucoma.43 When the analysis was repeated on PACG eyes with mild and moderate severity of glaucoma (MD better than −12 dB), both average RNFL thickness (coefficient=0.02, P=0.06) and GCC thickness (coefficient=0.02, P=0.02) were associated with MD slope; lower OCT measurements were associated with faster VF progression. wiVD disc (coefficient=0.06, P<0.001) and macula (coefficient=0.10, P<0.001) also were significantly associated with MD slope in this analysis of PACG eyes with mild and moderate severity. The strength of association of MD slope seemed to be stronger with optic disc (R2=27.2%) and macular vessel densities (R2=43.4%) compared to average RNFL (R2=15.8%) and GCC thickness (R2=21.7%) measurements in this subgroup analysis.
The rate of MD change found in the current study using BLUP was −0.32 dB/year. Previous studies have used other methods to estimate rate of MD change including pointwise linear regression, ordinary least square regression, etc. In spite of the differences in the method of estimation, the rate of MD change found in the current study is similar to the MD change reported in PACG eyes in previous studies.40, 44–46
The current study is limited by its cross-sectional design. VF progression was determined before OCTA imaging and the study found that lower disc and macular vessel density measurements were associated with prior VF progression. It is therefore still unclear if OCTA changes play a causative role in VF progression. Future prospective, longitudinal studies should evaluate if OCTA changes occur before VF progression. The other limitation of the study is that 5 VF examinations performed over a mean follow-up period of 5.2 years were used for the detection of the MD slope. A larger number of VF examinations and longer duration of follow-up may have provided a more robust estimate of MD change. Also, the interval between VF tests was not uniform. These may have affected the accuracy and precision of the MD slope estimation. However, MD slope in the current study was estimated using BLUP instead of the ordinary least square (OLS) regression. BLUPs are shrinkage estimates that take into account the results obtained by evaluating the whole sample of eyes.37 BLUPs give less weight to estimates obtained from eyes with fewer measurements and/or large intra-individual variability and have been shown to be more precise than OLS estimates in such situations.38 Therefore, the results of the study are less likely to be biased by the limited number and variable intervals between VF examinations.
In conclusion, there was a significant association between lower superficial vessel density measured using OCTA and prior VF progression in PACG in the current study. Future longitudinal studies are needed to elucidate the causative role of superficial vessel density reduction in PACG progression.
Financial disclosures:
Rao HL: Santen (C), Carl-Zeiss Meditec (C, S), Allergan (C); Thanemozhi S: none; Pradhan ZS: none; Sreenivasaiah S: none; Rao DAS: none; Puttaiah NK: none: Devi S: none; Moghimi S: none; Mansouri K: Santen (C), Allergan (S), ImplanData (C); Webers CAB: Alcon (S), Allergan (C), Pfizer (C), Santen (C); Weinreb RN: Optovue (S), Meditec-Zeiss (S), Heidelberg Engineering (S), Allergan (C), Bausch & Lomb (C), NiCox (C), Centervue (S), ImplanData (C). Supported in part by R01 EY029058 (RNW) for the National Eye Institute and an unrestricted grant from Research to Prevent Blindness (NY, New York)
REFERENCES
- 1.Jia Y, Morrison JC, Tokayer J, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express 2012;3:3127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014;121:1322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Jia Y, Takusagawa HL, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol 2015;133:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Invest Ophthalmol Vis Sci 2016;57:OCT451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao HL, Pradhan ZS, Weinreb RN, et al. Regional Comparisons of Optical Coherence Tomography Angiography Vessel Density in Primary Open-Angle Glaucoma. Am J Ophthalmol 2016;171:75–83. [DOI] [PubMed] [Google Scholar]
- 6.Rao HL, Pradhan ZS, Weinreb RN, et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS One 2017;12:e0173930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh MH, Zangwill LM, Manalastas PI, et al. Deep Retinal Layer Microvasculature Dropout Detected by the Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology 2016;123:2509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee EJ, Kim TW, Lee SH, Kim JA. Underlying Microstructure of Parapapillary Deep-Layer Capillary Dropout Identified by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2017;58:1621–7. [DOI] [PubMed] [Google Scholar]
- 9.Shin JW, Lee J, Kwon J, et al. Regional vascular density-visual field sensitivity relationship in glaucoma according to disease severity. Br J Ophthalmol 2017;101:1666–72. [DOI] [PubMed] [Google Scholar]
- 10.Mansoori T, Sivaswamy J, Gamalapati JS, Balakrishna N. Radial Peripapillary Capillary Density Measurement Using Optical Coherence Tomography Angiography in Early Glaucoma. J Glaucoma 2017;26:438–43. [DOI] [PubMed] [Google Scholar]
- 11.Geyman LS, Garg RA, Suwan Y, et al. Peripapillary perfused capillary density in primary open-angle glaucoma across disease stage: an optical coherence tomography angiography study. Br J Ophthalmol 2017;101:1261–8. [DOI] [PubMed] [Google Scholar]
- 12.Takusagawa HL, Liu L, Ma KN, et al. Projection-Resolved Optical Coherence Tomography Angiography of Macular Retinal Circulation in Glaucoma. Ophthalmology 2017;124:1589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akil H, Chopra V, Al-Sheikh M, et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. Br J Ophthalmol 2017. [DOI] [PubMed] [Google Scholar]
- 14.Hollo G Vessel density calculated from OCT angiography in 3 peripapillary sectors in normal, ocular hypertensive, and glaucoma eyes. Eur J Ophthalmol 2016;26:e42–5. [DOI] [PubMed] [Google Scholar]
- 15.Kumar RS, Anegondi N, Chandapura RS, et al. Discriminant Function of Optical Coherence Tomography Angiography to Determine Disease Severity in Glaucoma. Invest Ophthalmol Vis Sci 2016;57:6079–88. [DOI] [PubMed] [Google Scholar]
- 16.Akagi T, Iida Y, Nakanishi H, et al. Microvascular Density in Glaucomatous Eyes With Hemifield Visual Field Defects: An Optical Coherence Tomography Angiography Study. Am J Ophthalmol 2016;168:237–49. [DOI] [PubMed] [Google Scholar]
- 17.Ichiyama Y, Minamikawa T, Niwa Y, Ohji M. Capillary Dropout at the Retinal Nerve Fiber Layer Defect in Glaucoma: An Optical Coherence Tomography Angiography Study. J Glaucoma 2017;26:e142–e5. [DOI] [PubMed] [Google Scholar]
- 18.Yarmohammadi A, Zangwill LM, Manalastas PIC, et al. Peripapillary and Macular Vessel Density in Patients with Primary Open-Angle Glaucoma and Unilateral Visual Field Loss. Ophthalmology 2018;125:578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee EJ, Lee SH, Kim JA, Kim TW. Parapapillary Deep-Layer Microvasculature Dropout in Glaucoma: Topographic Association With Glaucomatous Damage. Invest Ophthalmol Vis Sci 2017;58:3004–10. [DOI] [PubMed] [Google Scholar]
- 20.Shin JW, Kwon J, Lee J, Kook MS. Choroidal Microvasculature Dropout is Not Associated With Myopia, But is Associated With Glaucoma. J Glaucoma 2018;27:189–96. [DOI] [PubMed] [Google Scholar]
- 21.Rao HL, Sreenivasaiah S, Dixit S, et al. Choroidal Microvascular Dropout in Primary Open-angle Glaucoma Eyes With Disc Hemorrhage. J Glaucoma 2019;28:181–7. [DOI] [PubMed] [Google Scholar]
- 22.Moghimi S, Zangwill LM, Penteado RC, et al. Macular and Optic Nerve Head Vessel Density and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. Ophthalmology 2018;125:1720–8. [DOI] [PubMed] [Google Scholar]
- 23.Park HL, Kim JW, Park CK. Choroidal Microvasculature Dropout Is Associated with Progressive Retinal Nerve Fiber Layer Thinning in Glaucoma with Disc Hemorrhage. Ophthalmology 2018;125:1003–13. [DOI] [PubMed] [Google Scholar]
- 24.Lin S, Cheng H, Zhang S, et al. Parapapillary Choroidal Microvasculature Dropout Is Associated With the Decrease in Retinal Nerve Fiber Layer Thickness: A Prospective Study. Invest Ophthalmol Vis Sci 2019;60:838–42. [DOI] [PubMed] [Google Scholar]
- 25.Kwon JM, Weinreb RN, Zangwill LM, Suh MH. Parapapillary Deep-Layer Microvasculature Dropout and Visual Field Progression in Glaucoma. Am J Ophthalmol 2019;200:65–75. [DOI] [PubMed] [Google Scholar]
- 26.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121:2081–90. [DOI] [PubMed] [Google Scholar]
- 27.Garudadri C, Senthil S, Khanna RC, et al. Prevalence and risk factors for primary glaucomas in adult urban and rural populations in the Andhra Pradesh Eye Disease Study. Ophthalmology 2010;117:1352–9. [DOI] [PubMed] [Google Scholar]
- 28.Weinreb RN, Friedman DS, ed. Angle closure and angle closure glaucoma, Reports and Consensus Statements of the 3rd Global AIGS Consensus Meeting on Angle Closure Glaucoma The Hague, The Netherlands: Kugler Publications, 2006; 1–20. [Google Scholar]
- 29.Kraus MF, Potsaid B, Mayer MA, et al. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express 2012;3:1182–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012;20:4710–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao HL, Sreenivasaiah S, Riyazuddin M, et al. Choroidal Microvascular Dropout in Primary Angle Closure Glaucoma. Am J Ophthalmol 2019;199:184–92. [DOI] [PubMed] [Google Scholar]
- 32.Rao HL, Zangwill LM, Weinreb RN, et al. Comparison of different spectral domain optical coherence tomography scanning areas for glaucoma diagnosis. Ophthalmology 2010;117:1692–9, 9 e1. [DOI] [PubMed] [Google Scholar]
- 33.Rao HL, Leite MT, Weinreb RN, et al. Effect of disease severity and optic disc size on diagnostic accuracy of RTVue spectral domain optical coherence tomograph in glaucoma. Invest Ophthalmol Vis Sci 2011;52:1290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–74. [PubMed] [Google Scholar]
- 35.Laird NM, Donnelly C, Ware JH. Longitudinal studies with continuous responses. Stat Methods Med Res 1992;1:225–47. [DOI] [PubMed] [Google Scholar]
- 36.Medeiros FA, Meira-Freitas D, Lisboa R, et al. Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology 2013;120:1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson GK. That BLUP is a good thing: the estimation of random effects. Stat Sci 1991;6:15–51. [Google Scholar]
- 38.Beckett LA, Tancredi DJ, Wilson RS. Multivariate longitudinal models for complex change processes. Stat Med 2004;23:231–9. [DOI] [PubMed] [Google Scholar]
- 39.Rao HL, Pradhan ZS, Weinreb RN, et al. Vessel Density and Structural Measurements of Optical Coherence Tomography in Primary Angle Closure and Primary Angle Closure Glaucoma. Am J Ophthalmol 2017;177:106–15. [DOI] [PubMed] [Google Scholar]
- 40.Verma S, Nongpiur ME, Atalay E, et al. Visual Field Progression in Patients with Primary Angle-Closure Glaucoma Using Pointwise Linear Regression Analysis. Ophthalmology 2017;124:1065–71. [DOI] [PubMed] [Google Scholar]
- 41.Mwanza JC, Kim HY, Budenz DL, et al. Residual and Dynamic Range of Retinal Nerve Fiber Layer Thickness in Glaucoma: Comparison of Three OCT Platforms. Invest Ophthalmol Vis Sci 2015;56:6344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowd C, Zangwill LM, Weinreb RN, et al. Estimating Optical Coherence Tomography Structural Measurement Floors to Improve Detection of Progression in Advanced Glaucoma. Am J Ophthalmol 2017;175:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moghimi S, Bowd C, Zangwill LM, et al. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology 2019;126:980–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Moraes CG, Liebmann JM, Liebmann CA, et al. Visual field progression outcomes in glaucoma subtypes. Acta Ophthalmol 2013;91:288–93. [DOI] [PubMed] [Google Scholar]
- 45.Yousefi S, Sakai H, Murata H, et al. Rates of Visual Field Loss in Primary Open-Angle Glaucoma and Primary Angle-Closure Glaucoma: Asymmetric Patterns. Invest Ophthalmol Vis Sci 2018;59:5717–25. [DOI] [PubMed] [Google Scholar]
- 46.Ballae Ganeshrao S, Senthil S, Choudhari N, et al. Comparison of Visual Field Progression Rates Among the High Tension Glaucoma, Primary Angle Closure Glaucoma, and Normal Tension Glaucoma. Invest Ophthalmol Vis Sci 2019;60:889–900. [DOI] [PubMed] [Google Scholar]


