Abstract
SARS-CoV-2 is responsible for the ongoing world-wide pandemic which has already taken more than two million lives. Effective treatments are urgently needed. The enzymatic activity of the HECT-E3 ligase family members has been implicated in the cell egression phase of deadly RNA viruses such as Ebola through direct interaction of its VP40 Protein. Here we report that HECT-E3 ligase family members such as NEDD4 and WWP1 interact with and ubiquitylate the SARS-CoV-2 Spike protein. Furthermore, we find that HECT family members are overexpressed in primary samples derived from COVID-19 infected patients and COVID-19 mouse models. Importantly, rare germline activating variants in the NEDD4 and WWP1 genes are associated with severe COVID-19 cases. Critically, I3C, a natural NEDD4 and WWP1 inhibitor from Brassicaceae, displays potent antiviral effects and inhibits viral egression. In conclusion, we identify the HECT family members of E3 ligases as likely novel biomarkers for COVID-19, as well as new potential targets of therapeutic strategy easily testable in clinical trials in view of the established well-tolerated nature of the Brassicaceae natural compounds.
Subject terms: Infection, Medical genomics
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) associated with the emerging disease (COVID-19) has resulted in an unprecedented global health and economic crisis1,2. To date (March, 9, 2021), there are at least 24 putative drug treatments for the disease. However, most are still at early stages of research. The focus has been on the development of new and repositioned vaccines, monoclonal antibodies, and drugs3–7. Another treatment option involves passive antibody administration via convalescent plasma transfusion. Convalescent plasma has been successfully used in the past as post-exposure prophylaxis and in the therapeutic treatment of other coronavirus outbreaks (e.g., SARS-1 and Middle East respiratory syndrome [MERS]) and other autoimmune and chronic inflammatory diseases. Although some promising results have been initially reported8, no significant clinical efficacy has been documented in treated patients9. Several repurposed drugs have been tested with disappointing results and many other promising ones are undergoing clinical experimentation10–13. However, to date there is no effective specific target drug against COVID-19. The unavailability of selective and effective antiviral drugs is probably due to the poor knowledge of the pharmacological targets of the host cell necessary for the virus replication and/or for the egress of new virions. Thus, a deeper knowledge of SARS-CoV-2 virus-host interaction for is fundamental to understand the molecular mechanisms that underly the life cycle of COVID-19 in order to develop treatments worthy of a clinical trial assesement14.
The enzymatic activity of the HECT-E3 ligases has been implicated in the cell egression phase of some RNA viruses possibly highjacking the endosomal sorting complexes required for transport (ESCRT) machinery15–17, and specifically members of a subgroup of HECT-E3 ligases, known as C2-WW-HECT (NEDD4-like) comprising at least nine members in humans (NEDD4, NEDD4L, ITCH, SMURF1, SMURF2, WWP1, WWP2, HECW1, and HECW2). This subgroup is characterized by a common modular architecture composed of a C2 domain related to N-terminal C protein kinase, two to four domains with central tryptophan–tryptophan (WW), and a C-terminal HECT domain18. The C2 domain is a Ca2+-dependent binding domain and is mainly involved in targeting these enzymes to membrane compartments such as the plasma membrane, Golgi apparatus, endosomes, and lysosomes18. WW domains mediate protein– protein interactions through the recognition of Pro-rich motifs (PPxY, LPxY or related sequences) and phosphorylated Ser/Thr-Pro19,20. These domains provide a scaffold for recruiting protein substrates and regulators. Several viral proteins have been shown to recruit WW-domain host cell proteins of the NEDD4 family through PPxY motifs to facilitate their egression and diffusion21,22. Among them, WWP1 was found to interact with Ebola Virus VP40 to regulate egression suggesting that viral PPxY-host WW domain-mediated interaction could represent a potential new target for host-oriented inhibitors of EBOV and other virus egression23. Several studies have shown that the HECT family members not only physically interact with specific viral proteins to regulate the release of mature viral particles through the ESCRT machine, but to regulate endocytosis through ubiquitination24.
Here, we investigated the involvement of HECT family of E3 ligases in COVID-19 patients and their possible involvement in SARS-CoV-2 infection. We found that WWP1, WWP2, SMURF1, and NEDD4 mRNA are overexpressed in COVID-19 vs. SARS-CoV-2 negative patients in nasopharyngeal and oropharyngeal swab cells, as well as in the lung of affected patients and in mouse models of COVID-19. We also identified a subset of rare allelic variants in these genes and studied their distribution in a large cohort of patients (COVID Human Genetic Effort (https://www.covidhge.com) severely affected by COVID-19 vs. asymptomatic or paucisymptomatic infected subjects. We showed that some of the identified variants display gain of function and aberrant activity. We finally evaluated whether selective inhibition of HECT proteins by a natural NEDD4 and WWP1 inhibitor from Brassicaceae displayed anti-SARS-CoV-2 activity, thus providing preclinical support for the possible development of clinical trials using this natural inhibitor in COVID-19 patients.
Results
HECT family members interact and ubiquitinate the SARS-CoV-2 Spike protein
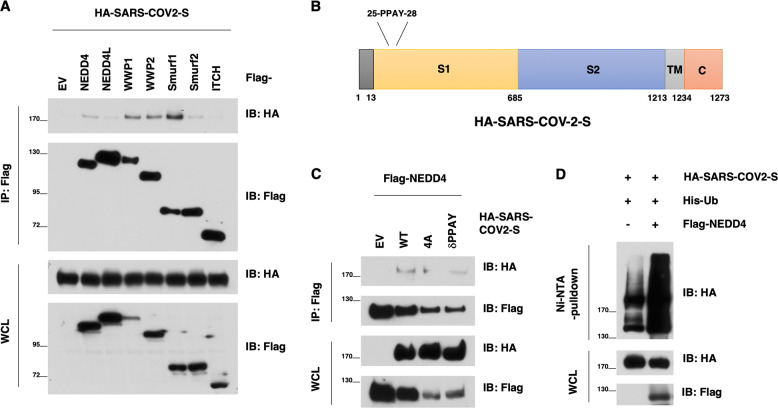
To determine whether and how HECT type of E3 ligase family members are involved in SARS-CoV-2 pathology, we at first focused on exploring how HECT E3 ligase might interact with the SARS-CoV-2 Spike protein, which plays a critical role for the virus infection and egression processes, and encode a PPxY motif (25-PPAY-28 in Spike protein)25. Notably, we found that the SARS-CoV-2 S protein could interact with several HECT-E3 family members, including NEDD4, WWP1, WWP2, SMURF1, and SMURF2 (Fig. 1A). Given that the PPxY motif is known to mediate the binding with NEDD4 family members23, we next mutated the PPAY motif to Alanine (4A) or deleted this motif (delta-PPAY) altogether (Fig. 1B). We found that these mutants reduced the binding with NEDD4 while not abrogating it entirely (Fig. 1C). Importantly and in support of a putative role of NEDD4 in regulating SARS-CoV-2 viral life cycle, we found that ectopic expression of NEDD4 could promote the ubiquitination of the SARS-CoV-2 S protein in cells (Fig. 1D).
Fig. 1. NEDD4 binds and ubiquitinates the SARS-CoV-2 S protein.
A Immunoblotting (IB) of flag-immunoprecipates (IP) and whole cell lysis (WCL) derived from HEK293T cells that were transfected with HA-SARS-CoV-2 S and indicated NEDD4 family members. B A schematic diagram to show the PPxY motif in the SARS-CoV-2 S protein. C IB of flag-immunoprecipates and WCL derived from HEK293T cells that were transfected with NEDD4 and SARS-CoV-2 S WT or mutants. D IB of Ni-NTA pulldown and WCL derived from HEK293T cells that were transfected with SARS-CoV-2 S and NEDD4 WT (or EV as a negative control).
HECT genes and proteins expression in SARS-CoV-2 patients and mouse models
We first analyzed the gene expression levels of the nine HECT family members by qRT-PCR using specific primer pairs (Table 2) on cDNA from residual unidentified nasopharyngeal and oropharyngeal swabs of 37 COVID-19 patients with severe respiratory symptoms and 25 patients negative for the detection of SARS-CoV-2.
Table 2.
Real-Time PCR primer sequences.
| Gene | Accession number | Sequence (5’→3’) | Product size (bp) | |
|---|---|---|---|---|
| WWP1 | NM_007013.4 | Fw | TGTAAATGTTACGCCACAGACT | 105 |
| Rv | GCTTGTTTCAAATCTATCGTTGC | |||
| WWP2 | NM_007014.5 | Fw | GAAAGTGGTGTCCGCAAAGC | 175 |
| Rv | ATGACTCTGTGCCGTGACATT | |||
| NEDD4 | NM_006154.4 | Fw | CTGCTACGGACAATTATACCCTA | 129 |
| Rv | CATCCAACAGTTTGCCATGATA | |||
| NEDD4L | NM_001144967.3 | Fw | ACGTAGCGGATGAGAATAGAGAAC | 115 |
| Rv | CTGTGATTAGATGGGTTTACCCTGA | |||
| ITCH | NM_031483.7 | Fw | GGTTCAGTATTTCCGGTTCTGGT | 118 |
| Rv | GGGACTGAAGCTCATTATCTGTTG | |||
| SMURF1 | NM_020429.3 | Fw | CCGCTCCAAGGCTTCAAGG | 125 |
| Rv | ATCCGGTTAAAGCAGGTATGGG | |||
| SMURF2 | NM_022739.4 | Fw | GCAAATGGATCAGGAAGTCGGAAA | 100 |
| Rv | CCGGAGGCCGGAGGA | |||
| HECW1 | NM_015052.5 | Fw | CGAGCAACCACCCCCAGTGT | 136 |
| Rv | CCATGGCTTGGAAATCTGAGAGA | |||
| HECW2 | NM_001348768.2 | Fw | CTACCAGCATAACCGCGACC | 112 |
| Rv | AAAGAATGCCTTGCCCTGGT | |||
| GAPDH | NM_002046 | Fw | AAGGTCGGAGTCAACGGATTT | 100 |
| Rv | TGAAGGGGTCATTGATGGCA | |||
| ACTB | NM_001101 | Fw | ATTGCCGACAGGATGCAGAA | 150 |
| Rv | GCTGATCCACATCTGCTGGAA | |||
| RPLP0 | NM_001002 | Fw | ACCCAGCTCTGGAGAAACT | 198 |
| Rv | AAAAGGAGGTCTTCTCGGG | |||
WWP1 WW domain containing E3 ubiquitin protein ligase 1, WWP2 WW domain containing E3 ubiquitin protein ligase 2, NEDD4 neural precursor cell expressed, developmentally downregulated 4, E3 ubiquitin protein ligase, NEDD4L neural precursor cell expressed, developmentally downregulated 4-like, E3 ubiquitin protein ligase, ITCH HECT-type E3 ubiquitin transferase itchy homolog, SMURF1 SMAD specific E3 ubiquitin protein ligase 1, SMURF2 SMAD specific E3 ubiquitin protein ligase 2, HECW1 HECT, C2 and WW domain containing E3 ubiquitin protein ligase 1, HECW2 HECT, C2 and WW domain containing E3 ubiquitin protein ligase 2, GAPDH glyceraldehyde-3-phosphate dehydrogenase, ACTB β-actin, RPLP0 ribosomal protein, large, P0, Fw forward, Rev reverse. PCR polymerase chain reaction.
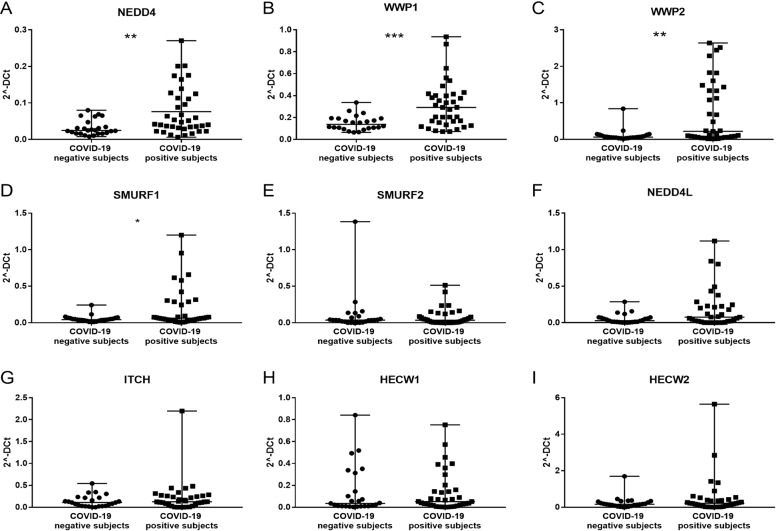
NEDD4 (FC = + 2.06, p ≤ 0.005), WWP1 (FC = + 1.85, p ≤ 0.0005), WWP2 (FC = + 4.11; p < 0.005) and SMURF1 (FC = + 1.7, p ≤ 0.05) showed a significant overexpression in nasopharyngeal and oropharyngeal swabs of COVID-19 positive patients compared to negative patients (Fig. 2A–D). No significant differences were observed in the other analyzed genes (Fig. 2E–I).
Fig. 2. HECT E3 ubiquitin ligase gene expression level in SARS-CoV-2 positive and negative groups of subjects.
A NEDD4 expression level in SARS-CoV-2 positive and negative groups of subjects, Mann–Whitney test, exact p value p = 0.0016, **; B WWP1 expression level in SARS-CoV-2 positive and negative groups of subjects, Mann–Whitney test, exact p value p = 0.0005, ***; C WWP2 expression level in SARS-CoV-2 positive and negative groups of subjects, Mann–Whitney test, exact p value p = 0.0038, **; D SMURF1 expression level in SARS-CoV-2 positive and negative groups of subjects, Mann–Whitney test, exact p value p = 0.044, *; E SMURF2 expression level in SARS-CoV-2 positive and negative groups of subjects, Mann–Whitney test, non-significant p value; F NEDD4L expression level in SARS-CoV-2 positive and negative groups of subjects, Mann–Whitney test, non-significant p value; G ITCH expression level in SARS-CoV-2 positive and negative groups of subjects, Mann–Whitney test, non-significant p value; H HECW1 expression level in SARS-CoV-2 positive and negative groups of subjects, Mann–Whitney test, non-significant p value; I HECW2 expression level in SARS-CoV-2 positive and negative groups of subjects, Mann–Whitney test, non-significant p value.
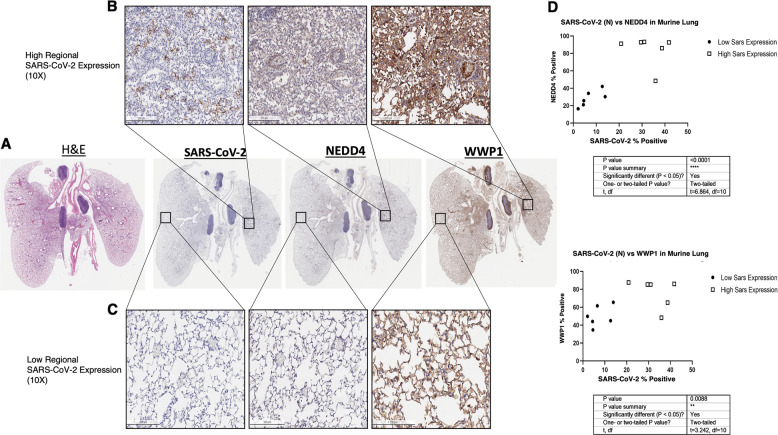
We then studied the expression of NEDD4 and WWP1 at protein level taking advantage of a COVID-19 mouse model and of available human lung specimens from infected patients necropsy. Overexpression at the protein level was observed for both WWP1 and NEDD4. However, WWP1 protein levels were more increased than those of NEDD4 in the SARS-CoV-2-infected human lung tissue. In PCR negative COVID-19 human lungs, the basal expression of NEDD4 and WWP1 was high. Interestingly, however, in PCR positive COVID-19 human lungs, NEDD4 and WWP1 were downregulated everywhere except in regions that expressed SARS-CoV-2 proteins (Supplementary Fig. 1). In keeping with the human data, both NEDD4 (p < 0,0001) and WWP1 (p < 0,01) proteins were significantly increased in mouse lungs overexpressing SARS-CoV-2 (Fig. 3). Thus, SARS-CoV-2 infection sustains and increases the expression levels of HECT Family members.
Fig. 3. Mouse lungs (n = 3) expressing higher SARS-CoV-2 nucleocapsid protein express significantly higher NEDD4 (p < 0.0001*****) and WWP1 (p < 0.01**) in consecutive sections.
Mouse lungs (n = 3) expressing higher SARS-CoV-2 nucleocapsid protein express significantly higher NEDD4 (p < 0.0001*****) and WWP1 (p < 0.01**) in consecutive sections (10X mag). Regions qualitatively defined as having high (>20% positive cells) Sars-CoV-2 expression (top row) and low (<20% positive cells) SARS-CoV-2 expression (bottom row), show with the corresponding regions in WWP1 and NEDD4 stained sections.
HECT germline allelic variants in critical/life-threatening COVID-19 patients
We hypothesized that if high levels and sustained expression of specific HECT-E3 ligase family members are triggered by the SARS-CoV-2 infection, allelic variants that would affect their function could dictate the outcome and natural history of the disease. To test this hypothesis, we initially collected a cohort of 130 unrelated Italian SARS-CoV-2-positive patients, showing respiratory distress, Acute Respiratory Disease Syndrome (ARDS) or requiring invasive ventilation and Intensive Care Unit (ICU) admission. We identified a total of 408 HECT different pLOF, missense and in-frame monoallelic germline DNA variants. Data were extrapolated from previous studies performing WES analysis described in previous publications26–28. Introducing a cut-off at MAF < 0.01, we found 21 missense and 5 splice-region variants in NEDD4, NEDD4L, SMURF1, SMURF2, HECW1, HECW2, WWP1, and WWP2 genes, in a total of 24 patients (Table 1). No variant was detected in the ITCH gene. The allelic frequencies of 12 genetic variants identified were significantly higher, when compared with those reported in GnomAD database for the EUR reference population (Table 1). One variant, M114I in WWP2 gene, was never detected before (Table 1). Interestingly, five of the twelve variants observed with a higher frequency than that reported in GnomAD database are located in the NEDD4 gene.
Table 1.
HECT genes variants in a cohort of 130 SARS-CoV-2 positive patients (MAF < 0.01 in GnomAD v2.1.1; in bold p < 0.05).
| Gene | Genetic form | Genotype | dbSNP | Consequence | AF GnomAD | Gender | Age [years] | p-value | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| HECW1 | Known | A1332T/WT | rs200973212 | missense | 0.0000161 | M | 54 | 0.0114 | Survived |
| Known | E502Q/WT | rs61756576 | missense | 0.0025630 | M | 83 | 0.5801 | Deceased | |
| Known | N1265S/WT | rs200912368 | missense | 0.0017820 | F | 50 | 0.4656 | Survived | |
| HECW2 | Known | S559G/WT | rs779373864 | missense | 0.0000085 | M | 59 | 0.0072 | Survived |
| Known | N417S/WT | rs138998510 | missense | 0.0005529 | M | 54 | 0.0791 | Survived | |
| Known | A537P/WT | rs750339715 | missense | 0.0000121 | F | 93 | 0.0092 | Deceased | |
| NEDD4 | Known | N888K/WT | rs759199057 | missense | 0.0000119 | M | 59 | 0.01 | Survived |
| Known | G451A/WT | rs60811367 | missense | 0.0017470 | M | 54 | 0.0013 | Survived | |
| M | 39 | Deceased | |||||||
| Known | I1237T/WT | rs373718024 | missense | 0.0003550 | M | 47 | 0.0159 | Deceased | |
| Known | R877Q/WT | rs201295772 | missense | 0.0000958 | M | 83 | 0.03176 | Deceased | |
| Known | I843R/WT | rs375088434 | missense | 0.0000199 | F | 77 | 0.0091 | Deceased | |
| Known | T727I/WT | rs61754989 | missense | 0.0036830 | F | 72 | 1 | Deceased | |
| Known | D129N/WT | rs150886795 | missense | 0.0002875 | M | 61 | 0.1233 | Deceased | |
| Known | S29R/WT | rs115484917 | missense | 0.0026250 | M | 39 | 0.1555 | Deceased | |
| NEDD4L | Known | c.698C>T/WT | rs202231187 | missense | 0.0039100 | M | 73 | 1 | Survived |
| Known | c.1258-5A>C/WT | rs768158353 | splicing | 0.0000853 | M | 64 | 0.0141 | Deceased | |
| Known | c.698C>T/WT | rs202231187 | missense | 0.0039100 | M | 54 | 1 | Deceased | |
| SMURF1 | Known | R564Q/WT | rs182340234 | missense | 0.0000199 | F | 52 | 0.0068 | Survived |
| Known | T223M/WT | rs371859465 | missense | 0.0000805 | F | 80 | 0.022 | Survived | |
| SMURF2 | Known | G10E/WT | rs866321574 | missense | 0.0061730 | F | 36 | 0.1166 | Survived |
| M | 73 | Deceased | |||||||
| Known | I142V/WT | rs145845053 | missense | 0.0005564 | M | 14 | 0.1281 | Survived | |
| WWP1 | Known | c.2395-4C>T/WT | rs188228045 | splicing | 0.0000040 | F | 89 | 1 | Deceased |
| Known | c.540-5T>C/WT | rs187132881 | splicing | 0.0023640 | F | 83 | 0.5073 | Deceased | |
| Known | c.1836G>A/WT | rs150841032 | splicing | 0.0002012 | M | 76 | 0.1019 | Deceased | |
| WWP2 | Known | R803C/WT | rs747018644 | missense | 0.0000043 | M | 54 | 0.0049 | Survived |
| New | M114I/WT | rs377573067 | splicing | / | F | 83 | / | Deceased |
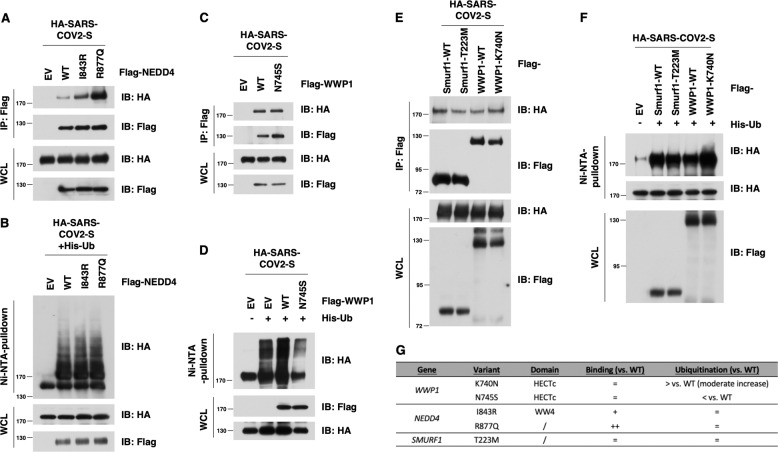
In a second step, we extended the genetic study to an independent cohort of 710 unrelated COVID-19 critical patients and 483 controls with asymptomatic or mild SARS-CoV-2 infection belonging to the international CHGE Consortium data29,30, and we performed a PCA-adjusted burden test in order to evaluate a possible difference in the number of variants with MAF < 0.01. The analysis did not reveal an enrichment of pLOF/missense/inframe variants for any of the examined genes in severely affected patients when compared to the asymptomatic and paucisymptomatic infected controls (Supplementary Table 1). As those tests involved a large number of variants, it is likely that most of them are neutral and strongly decreased the power of this analysis by diluting the signal. Therefore, we performed a more detailed investigation of the variants that were present in at least two critical cases and absent in infected controls. We identified 13 variants among which, three of them emerged as deleterious in all in silico prediction tools (Supplementary Table 2). Two of the three identified deleterious variants were in NEDD4 (I843R and R877G), and one in WWP1 (N745S). Each of the three variants was present in two patients, and 3 out of the six patients carrying any of these variants died (Table 1, Supplementary Table 1). Interestingly, WWP1 has a known binding activity with the protein S of the virus, and the N745S missense variant previously characterized by Lee et al.31, leads to aberrant WWP1 enzymatic activation with subsequent PTEN inactivation, thereby triggering hyperactive growth-promoting PI3K signaling in cellular and murine models.
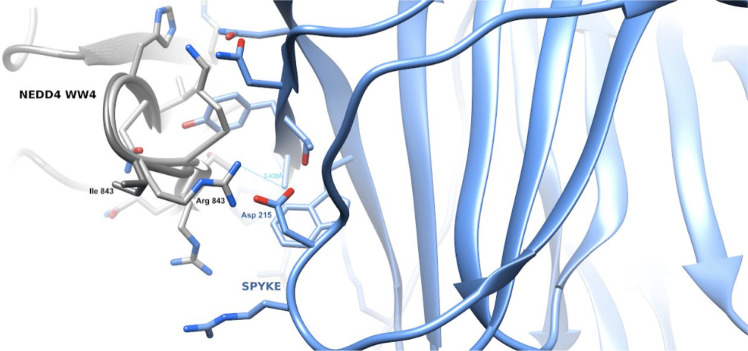
Next, we performed a more in depth in silico analysis of these three identified variants (I843R and R877G in NEDD4; N745S in WWP1). The I843R variant, mapping into the NEDD4 WW4 (in isoform 3), has a potential impact on the protein ability to interact with its substrates. Specifically, the 3D model of the variant WW4 domain in complex with the SARS-CoV-2 Spike (S) shows that the PPAY S residues interacting with the WW domain place the Asp215 S residue in close proximity with the Arg843 side-chain (Fig. 4), suggesting a stronger interaction between the two proteins compared to the WW4 domain wild type. The R877G variant maps between the WW4 and the HECTs domain of NEDD4 and most in silico methods for the evaluation of the impact of the variant (see Methods). The N745S variant in WWP1 maps inside its HECTc domain and seems not to affect the stability or the pathogenicity of the protein. Its position shows that the variation can influence the interaction between the HECT-type E3 ubiquitin transferase and its substrates. Furthermore, Lee et al.31 have demonstrated that this mutation can lead to an open and enzymatically active conformation of WWP1.
Fig. 4. The Figure shows the 3D model of the WW domain of NEDD4 in complex with the SARS- CoV-2 Spike protein.
The WW domain is displayed as a ribbon model, with the interface residue side-chains in gray. The Spike protein is displayed as a ribbon model, and the side-chains of its interface residues are shown in light blue. The Arg843–Asp215 residues are in close proximity and favor a stronger interaction between the variant WW domain and Spike with respect to the wt domain.
To corroborate the in silico analysis, we next explored how these identified putative gain of function (GOF) mutations might impact the ability to interact or ubiquitinate the S protein. Notably, the two NEDD4 variants derived from COVID-19 patients were able to more avidly bind with the SARS-CoV-2 Spike (S) protein compared to wt-NEDD4 (Fig. 5A, B). We also observed a slight increase in ubiquitination of the SARS-CoV-2 S protein in cells by the NEDD4 mutants (Fig. 5A, B), indicating that the GOF of NEDD4 might exert its regulatory function via direct interaction with the SARS-CoV-2 S protein and other S associated proteins. Similarly, we tested the K740N-WWP1 mutant, a gain of function mutant, implicated in cancer susceptibility as a positive control31. Once again, we observed increased binding and ubiquitination of the mutants over the WT control (Fig. 5E, F). However, despite the marked increased binding with Spike protein for the R877Q-NEDD4 mutant, we observed relatively comparable ability for WT-NEDD4 and R877Q-NEDD4 in promoting ubiquitination of the SARS-COV2 S protein in cells under this experimental setting (Fig. 5B). These results indicate that the NEDD4 hotspot mutations might exert its COVID-19 regulatory function via direct physiological interaction with the SARS-COV2 S protein and other S associated proteins, and the putative role of NEDD4-mediated ubiquitination of spike protein in COVID-19 biology awaits further in-depth studies. We also compared WWP1-WT versus WWP1 K740N and N745S, two germline variants that were implicated in cancer susceptibility and demonstrated to be gain-of-function mutation towards the tumor suppressor PTEN31. We utilized the WWP1 K740N mutant as a control for the WWP1 N745S COVID-19 associated mutant. We found that both WWP1 K750N and N745S mutants displayed comparable binding ability with SARS-CoV-2 S protein compared to WT-WWP1 (Fig. 5C–F). However, we observed a slight increase for K740N-mediated ubiquitination of the SARS-COV2 S protein, but a moderate decrease for N745S-mediated ubiquitylation of the SARS-COV2 S protein. These results argue that different WWP1 mutations might utilize different mechanisms to impact COVID-19 biology, which requires additional in-depth studies in the future. On the other hand, in comparison with the WT counterpart, the Smurf-1-T223M mutant exhibited comparable binding or ubiquitination ability on SARS-CoV-2 S protein (Fig. 5E, F), at least in this experimental setting. Given the close similarity between their biochemical features and the reported functional redundancies among NEDD4 family members, these data suggest that several NEDD4 family E3 ligases might participate in regulating COVID-19 egression via direct interaction with and ubiquitination of the SARS-CoV-2 S protein and associated proteins (Fig. 5G), but their potential complementary roles, as well as their biological and functional impacts in COVID-19 biology await further investigation.
Fig. 5. Gain-of-Function mutants in NEDD4, but not SMURF1 and WWP1, display elevated interaction with, but comparable ubiquitination of the SARS-CoV-2 S protein.
A, C IB of flag-immunoprecipates and WCL derived from HEK293T cells that were transfected with HA-SARS-CoV-2 S and NEDD4/WWP1 WT and mutants. B, D IB of Ni-NTA pulldown and WCL derived from HEK293T cells that were transfected with SARS-CoV-2 S and NEDD4/WWP1 WT and mutants. E IB of flag-immunoprecipates and WCL derived from HEK293T cells that were transfected with HA-SARS-CoV-2 S and WT and mutants for Smurf1 or WWP1. F IB of Ni-NTA pulldown and WCL derived from HEK293T cells that were transfected with SARS-CoV-2 S and WT and mutants for Smurf1 or WWP1. G Binding and ubiquitination activity in WWP1/NEDD4/SMURF1 mutants vs. wild-type (WT).
The HECT inhibitor I3C is effective in mediating SARS-CoV-2 antiviral effect in in vitro cellular models
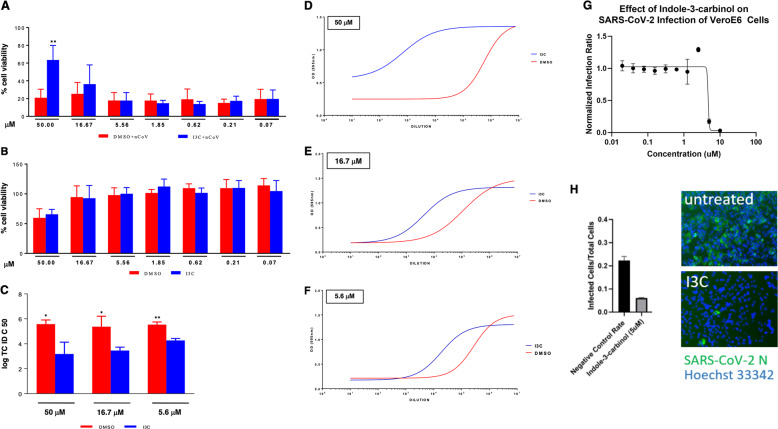
We next hypothesized that if HECT-E3 ligases are indeed functionally relevant for the viral life cycle of COVID-19, Indole-3-carbinol (I3C), a natural NEDD4 and WWP1 inhibitor from Brassicaceae, might display a direct antiviral effect. We evaluated at first the impact of I3C on the cytopathic effect (CPE) induced by SARS-CoV-2 infection in Vero E6 cells. We treated the cells with I3C using a 3-fold concentration scale ranging between 50 and 0.069 μM. The drug was added at different time points, before (1 h) and after (1, 24, and 48 h) SARS-Cov-2 multiplicity of infection (MOI = 0.001). CPE was evaluated 72 h post-infection, when culture media were collected for viral titer measurement. We found that I3C reduced by about 60% the SARS-CoV-2-induced CPE in Vero E6 cells at 50 μM, when compared to DMSO-treated cells (Fig. 6A), while it was not effective at lower concentrations. Similar results were obtained using a 10-fold increased MOI at 48 h post-infection. To note that the concentration of 50 μM or 0.05% of I3C and DMSO, respectively, is partially toxic for the cells when treated for 72 h (Fig. 6B).
Fig. 6. I3C inhibited SARS-CoV-2-induced CPE and viral production in Vero E6 cells.
Cells were treated with different doses of I3C (from 50 to 0.069 μM; 1:3 serial dilutions) or DMSO (from 0.5 to 6.9 × 10−4 v/v percentage) 1 h before SARS-CoV-2 infection (MOI = 0.001) in four replicates. Absorption of the virus was allowed for 1 h at 37 °C in presence of I3C or DMSO treatments. The unabsorbed virus was removed and replaced by fresh medium with I3C or DMSO as above. Cells were then treated with either I3C or DMSO every 24 h and incubated at 37 °C with 5% CO2 for 72 h when the survival of infected (A) or not infected (B) cells was measured by crystal violet staining assay. The results were evaluated setting the uninfected control cells as 100% and the remaining values represented as a relative value. Experiments (n = 4) were performed in triplicate and data are expressed as mean +/−SD. Back-titration of virus progeny released by SARS-CoV-2-infected cells, treated as above, was performed on Vero E6 cells. Survival of the cells was measured by crystal violet staining assay. Results were analyzed using Graph Pad (GraphPad Prism 8 XML ProjecT) with nonlinear regression curve fit (Inhibitor vs. response-Variable slope (four parameters)) ((D–F) and data expressed as log TCID50/100 μl (C)). Statistically significant differences between DMSO and I3C are represented as *P < 0.05 or **P < 0.002 determined using the paired t-test. Vero E6 cells were challenged with SARS-CoV-2. After 1.5 d, cells were fixed, stained with an N specific antibody and Hoechst 33342 for cell nuclei. Infected cells and total cell nuclei were counted by image analysis. The infection efficiency was normalized to infection seen in vehicle (DMSO) treated cells (G, H).
Importantly, however, a much greater effect was observed when assessing the impact of I3C treatment on the in vitro viral production. To this end, we measured the amount of infectious SARS-CoV-2 released by the infected cells treated with either I3C (50, 16.67, and 5.56 μM) or DMSO (0.5, 0.167 and 0.056% (v/v)). Notably, I3C significantly reduced the SARS-CoV-2 production at all the concentrations tested (Fig. 6C–F), with a virus yield reduction ranging from 2 to 4 log at the various I3C concentrations. The I3C-mediated decreased of viral production was also evident when cells were infected at higher MOI, although with lower efficacy (Supplementary Fig. 2). Since I3C reduced the viral production not only at 50 μM, when the CPE inhibition is clearly appreciated (Fig. 6D), but also at 16.67 and 5.56 μM, when SARS-CoV-2-induced CPE was not affected by I3C, it is likely that I3C reduced the viral release rather than a viral entry and/or replication leading to cell damage. Overall, these data demonstrated that I3C exerts a direct anti-SARS-CoV-2 replication activity.
We further tested the potential efficacy of I3C utilizing a inhibition assay in Vero cells (see “Methods”: “Virus infection inhibition assay”). Vero cells were grown to 70–90% confluency. After incubation with the virus cells were fixed in formalin and then stained with SARS-CoV-2 antibody against the N protein and a fluorescently tagged secondary antibody. Cell nuclei were stained with Hoechst 33342 dye. The cells were imaged using a Cytation (Biotek) automated imaging system to visualize the blue fluorescent nuclei and the green fluorescent infected cells expressing virus N protein. Images were analyzed by CellProfiler software using a customized analysis pipeline to count the nuclei and the infected cells. Infection efficiency is expressed as a function of infected cells/cell nuclei counted. Once again I3C was effective at inhibiting COVID-19 in this assay with an IC50 of 4.7 μm (Fig. 6G, H).
Discussion
Several studies have shown that HECT proteins act as a functional interface between viral or cellular proteins containing PPxY motifs and the E-class vacuolar protein-sorting pathway (VPS)32. HECT domains can participate in specific protein-protein interactions33 and hence ubiquitination of substrate proteins, which appears necessary for PPxY-dependent viral budding. Among the HECT family members, WWP1 and NEDD4 have been the most implicated in PPxY motif-dependent viral budding, and their HECT ubiquitin ligase activity is required for this activity34. Critically, these two HECT ubiquitin ligases can physically and functionally interact forming heterodimeric complexes, and are druggable by a well-tolerated natural compound from Cruciferous vegetables35. Additionally, gain of function germ line mutations of WWP1 have been identified in cancer susceptibility syndromes and in cancer patients31.
We do not yet know the molecular mechanisms that govern several aspects of SARS-CoV-2 life cycle such as its entry, replication, assembly, budding, and particularly the egression of the virus. Recently, however, recently, Ghosh et al.36, using virus-specific imaging and reporter methodologies, demonstrated that ß-Coronaviruses utilize lysosomal trafficking for exit, rather than biosynthetic secretory pathway most commonly used by other enveloped viruses. The biochemical and molecular characterization of these steps and above all the identification of the proteins involved in these processes, is therefore crucial to develop drugs that could interfere and block fundamental processes in the biology of the virus and pave the way for new therapeutic approaches.
Based on in silico analysis, it was recently proposed that because SARS-CoV-2 encodes PPxY late domain motifs it might be capable of recruiting HECT family members and, therefore, the ESCRT complex to improve virus budding and release, favoring cellular reinfections. Interestingly, the PPxY motif is not present in SARS-CoV proteins. The presence of the motif PPxY might contribute to explain why SARS-CoV-2 is more contagious compared to SARS-CoV22.
Here we demonstrated that WWP1, WWP2, and NEDD4 are overexpressed during SARS-CoV-2 infection and that their expression co-localizes with areas of infection in lung tissue both in mice and humans. In addition, we also demonstrated that NEDD4 and WWP1, physically interact with and ubiquitylate the SARS-CoV-2 S protein. This demonstrates a direct involvement of the HECT family proteins and, in particular, of NEDD4 and WWP1 in the virus life cycle. It is therefore conceivable that a greater production or an increased enzymatic activity of members of these members of the HECT family could favor the exacerbation of the infection. The sole fact that they are overexpressed in concomitance with the SARS-CoV-2 infection suggests that the virus may take advantage from this pathway, as it has been shown for other RNA viruses.
Additionally, and in line with this notion, we identified three variants that bind more avidly to the SARS-CoV-2 S protein: two rare NEDD4 variants (I843R and R877G) and the N745S germinal variant in WWP1, which was already characterized in cancer studies31. The increased binding affinity could favor the ubiquitination of viral and cellular proteins thus implementing vesicular packaging and virions release. These variants suggest the existence of a particular genetic constraint against loss of function or gain of function given the multifunctionality of HECT proteins31. It is worth noting that a recent paper reports that, in vitro, WWP1 K740 and 745S mutants displayed comparable ability as WT-WWP1 in largely monoubiquitinating PTEN. These mutants, therefore, do not appear to act as gain-of-function mutation in this in vitro setting37. On one hand, this report is consistent with what was previously reported in vivo where NEDD4 was found to mono-ubiquitinate PTEN and also cooperate with WWP1 in promoting K27-polyubiquitination of PTEN in cells, through heterodimeric interactions likely at plasma membrane38. On the other hand, the observed differences in WT-WWP1 vs. WWP1 mutants might stem from the monoubiquitination of PTEN observed in vitro ub assay vs. K27-polyubiquitination of PTEN detected in cells. It is possible that some key cellular factors, likely WWP1 interacting proteins, such as NEDD4, might be required for polyubiquitination of PTEN, both in cells and in vitro. Nonetheless, we also observed different ability for K740N and N745S WWP1 mutants in comparison with WT-WWP1 to promote ubiquitination of the SARS-CoV-2-S protein in cells (Fig. 5D, F). These results are in keeping with the Cole group37 to demonstrate that different WWP1 mutation might utilize different mechanism to control its downstream pathways, including PTEN and SARS-CoV-2 S protein, which warrants additional in-depth investigation to reveal the underlying complicated mechanism that is likely to be context dependent or ever unique to each individual mutation of WWP1. Further studies are warranted to analyze from a biochemical and functional point of view all the variants identified in these genes (Supplementary Table 1) in order to access the genetic enrichment found in a complete and unbiased way. It is also worth noting that several of the variants identified in these genes have been observed in both asymptomatic and critical subjects. Interestingly, we extended the genetic study to a second independent cohort of about 30,000 participants in the Healthy Nevada Project (HNP, Renown Health, Reno, Nevada, USA)39, to further corroborate our results. This analysis led to results comparable to the ones previously described. Moreover, we identified 9 additional rare variants never detected before in 9 COVID-19 patients, which may affect splicing (Supplementary Table 3).
HECT family members also play pivotal roles in the regulation of the innate immune response, and although the pathogenesis of COVID-19 is still under investigation, it is clear that the innate immunity plays a crucial role in protective or destructive responses upon SARS-CoV-2 infection40.
It is therefore conceivable that HEC family members may affect the outcome and natural history of the COVID-19 infection also impacting non-cell autonomous anti-viral defense mechanisms.
For instance, ITCH controls the stability of critical immune system proteins41 and acts upstream of B-cell lymphoma 6 (Bcl-6), the main transcription factor involved in coordinating follicular helper T-cell differentiation and immunoglobulin G (IgG) in response to acute viral infections42; WWP2 negatively regulates Toll-like receptor 3 (TLR3)-mediated innate immune response by targeting TIR-domain containing adapter-inducing interferon-β (TRIF) for ubiquitination and degradation43. The innate immune system acts as first responder for the detection and clearance of viral infections. Innate immune cells secrete proinflammatory cytokines that inhibit viral replication, stimulate the adaptive immune response, and recruit other immune cells to the site of infection40. In this respect, in an international cohort29, we recently showed that about 3% of COVID-19 critical patients carried loss-of-function variants in genes coding for proteins involved in type I IFN innate immunity, thus representing an important target for a deeper investigation of their role in the pathogenesis30. Collectively our results indicate that the risk of susceptibility to severe COVID-19 is unlikely to be influenced predominantly by rare variants of HECT genes in the MAF range <0,01. Rather, it is possible that rare (or private) variants may contribute substantially to the severity of the COVID-19 phenotype, suggesting that both very large sample sizes and gene-based association tests will be needed to carefully identify risk genetic factors.
While further studies will clarify the role and molecular mechanisms whereby HECT family members control the viral life cycle and the susceptibility and severity of COVID-19, our findings have immediate therapeutic implications for the treatment of infection and the prevention of the most severe outcomes triggered by the virus. The fact that I3C is effective in reducing SARS-CoV-2 production in vitro prompts the immediate assessment of its efficacy in clinical trials (Supplementary Fig. 3). I3C is, in fact, well-tolerated in both animal models and phase I trials in humans at doses effective in the in vitro cell models35,44. It is therefore conceivable to rapidly reposition I3C in Phase II clinical trials in humans to test its ability to prevent the clinical severity of COVID-19.
Materials and methods
International CHGE Consortium database
Between March and April 2020, 130 patients with COVID-19 diagnosis were enrolled on Protocol no. 50/20 (Tor Vergata University Hospital). Informed consent was obtained from each patient.
To further improve our cohort, other 710 cases and 483 controls were enrolled from the COVID Human Genetic Effort, International CHGE Consortium (Casanova J.L. and Su H., https://www.covidhge.com), as described in Zhang et al.30.
The institutional review boards of each participating Institution approved the protocol prior to patient enrollment. The study was conducted in agreement with the principles of Declaration of Helsinki.
Whole exome sequencing and data pre-processing
Genomic DNA was extracted from peripheral blood samples using standard procedures and Qiagen blood DNA mini Kit (Qiagen, Hilden, Germany). Library preparation and whole exome capture were performed by using the Twist Human Core Exome Kit (Twist Bioscience, South San Francisco, CA, USA) according to the manufacture’s protocol and sequenced on the Illumina NovaSeq 6000 platform. The BaseSpace pipeline (Illumina, Inc., San Diego, CA, USA) and the TGex software (LifeMap Sciences, Inc., Alameda, CA, USA) were used for the variant calling and annotating variants, respectively. Sequencing data were aligned to the hg19 human reference genome. A minimum depth coverage of 30X was considered suitable for analysis, based on the guidelines of the American College of Medical Genetics and Genomics. All variants were examined for coverage and Qscore (minimum threshold of 30) and visualized by the Integrative Genome Viewer (IGV).
Gene expression
Patients’ recruitment and sample collection for HECT3 ligase family expression study
During the months of March and April 2020, we collected the nasopharyngeal and oropharyngeal swabs of 62 subjects with acute respiratory symptoms or contacts with COVID-19 confirmed cases, arrived at the attention of the Emergency Room (ER) of Policlinico Tor Vergata, PTV (Rome, Italy). As widely described by Amati et al.45, patients’ swabs were referred to the Virology Unit of PTV for the molecular diagnostic test detecting the presence of SARS-CoV-2 nucleic acids using used the Allplex™ 2019-nCoV Assay (Seegene Inc, http://www.seegene.com/upload/product/Allplex_2019_nCoV_performance_data.pdf).
SARS-CoV-2 positive (n = 37) and negative (n = 25) samples were used for RNA expression analysis.
Real-time PCR and statistical analysis
The total RNA extracted from nasopharyngeal and oropharyngeal swabs was evaluated by NanoDrop DS-11 (DeNovix) and 100 ng of total RNA was been reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). We analyzed the expression of the 9 members of the HECT3 ligase family: WWP1, WWP2, NEDD4, NEDD4L, ITCH, SMURF1, SMURF2, HECW1, and HECW2 genes; GAPDH, ACTB, and RPLP0 genes were used for data normalization. Real-time PCRs (qRT-PCRs) have been performed using ABI7500 Fast Real-time PCR System (Life Technologies) with Sybr Green Assay (Power Sybr Green PCR Master Mix, Life Technologies) and specific primer pairs (Table 2).
The qRT-PCR expression analyses were performed in triplicate. Data analysis was performed using the comparative threshold cycle (Ct) method quantification (2−ΔCt method) (as described by Rizzacasa et al. at https://www.protocols.io/view/comparative-ct-method-quantification-2-ct-method-zp7f5rn).
Statistical analysis was performed using GraphPad Prism 7.0 (GraphPad Software, USA). D’Agostino & Pearson, Shapiro–Wilk, and Kolmogorov–Smirnov normality tests were used to assess the distribution of gene expression data derived from qRT-PCR assays. Since gene expression data did not pass the normality test (p ≤ 0.05), Mann–Whitney test was used for data comparison between SARS-CoV-2 positive and negative groups. In graphs, gene expression is represented as median with range. Significance was set at a minimum of p ≤ 0.05.
Histology and Immunohistochemistry
Autopsy tissue was sourced from patients (n = 3) at the Harris County Institute of Forensic Sciences and Memorial Herman Hospital, Texas Medical Center, who tested positive for SARS-CoV-2 by nasopharyngeal swab RT-PCR. Autopsy lungs were removed and fixed in 10% neutral buffered formalin for 24 h. BALB/c mice (n = 3) were transduced with 2.5e8 PFUs of AdV-hACE2. Then, 5 days later, mice were infected with SARS-CoV-2 and tissues were collected from day 4 post infection. Murine lungs were fixed in 10% neutral buffered formalin for 48 h. All samples were processed, paraffin embedded and sectioned at 4 μm at HistoWiz (histowiz.com).
Immunohistochemistry was performed by HistoWiz using Bond Polymer Refine Detection Kit (Leica Biosystems) and Leica Bond Rx automated stainer (Leica Biosystems) with the following antibodies: rabbit polyclonal anti-SARS-CoV-2 Nucleocapsid (N) protein (Novus Biologicals 100-56576, 0.5 mg/ml), rabbit polyclonal anti-human NEDD4 (EMD Millipore 07-049, 1 mg/ml), and mouse monoclonal (1A7) anti-human WWP1 (Abnova H00011059-M01, 0.32 mg/ml). Slides were coverslipped using a TissueTek-Prisma film coverslipper (Sakura). Whole slide scanning (40x) was performed on an Aperio AT2 (Leica Biosystems).
Animal work was conducted adhering to the institution’s guidelines for animal use, and followed the guidelines and basic principles in the United States Public Health Service Policy on Humane Care and for Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals by certified staff in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International accredited facility (protocol n. IACUC-2014-0255).
Image analysis and statistical analysis
Whole slide svs images of the three different stains were aligned to one another using the automated image alignment QuPath software (https://qupath.github.io), to permit the identification of similar regions within the tissue section between separate stains. Digital image analysis was performed using Definiens TissueStudio 4.0 software (AstraZeneca, Munich Germany), in which nucleus detection was performed on the hematoxylin counterstain, and positive cells were identified using a threshold for DAB staining to identify positive stained images for each biomarker (NEDD4, WWP1, and SARS-CoV2 Nucleocapsid protein).
Following quantification, expression (as % positive cells) of NEDD4 and WWP1 was plotted against SARS-CoV2 expression using Prism software. Six random regions of high SARS-CoV2 expression (>20% positive cells) and 6 random regions low SARS-CoV-2 expressions (<20% positive cells) were identified, and expression of NEDD4 and WWP1 within these regions were quantified. Two-tailed t-tests were performed to compare expression of NEDD4 and WWP1 between the high and low SARS-CoV-2 expression groups.
In silico analysis
For the NEDD4 and WWP1 proteins the evaluation of the probability that the selected variants can have an impact on the protein function has been extracted from the Ensembl genome browser46, relying on the SIFT, PolyPhen, CADD, REVEL, MetalR, and Mutation Assessor methods46–51. For the evaluation of the Ile843Arg NEDD4 variant, we built the 3D model of the fourth WW domain of isoform 3 using the 1I5H PDB structure as template (displaying 87% sequence identity with our query52). The chosen template corresponds to the fourth WW domain of rat NEDD4 protein. The reconstruction of the possible complex between the human WW4 domain of NEDD4 and the Spike protein of SARS-CoV-2 was performed using the 4N7H PDB complex formed by the WW3 of human NEDD4 protein in complex with its bound peptide, characterized by the typical PPxY WW binding sequence53. The 3D model of the WW4 domain of NEDD4 was superposed on the Cα and Cβ atoms of the complex interface, while residues 24–28 of the Spike protein (PDB: 6XR8 were superposed on the corresponding PPxY sequence of the 4N7H peptide54. The complex model was subsequently assessed with 100 cycles of steepest descent minimization and evaluated with the Chimera v1.14 software55.
Functional experiments
Immunoblots and co-immunoprecipitation experiments
Immunoblots and co-immunoprecipitation analysis of COVID-19 Spike and HECT-family members (wt vs. mutants) were performed in HEK293T cells as previously described30,31, and as described in the legends to Figs. 1 and 5.
HECT inhibitor I3C: cells
Vero E6 cells are kidney epithelial cells originally extracted from an African green monkey (Chlorocebus sp.; formerly called Cercopithecus aethiops). Cells were maintained in Minimum Essential Medium (MEM), supplemented with heat inactivated 10% fetal bovine serum (FBS), 2 mM L-glutamine and 1% penicillin/streptomycin solution (Sigma-Aldrich, Cat.No. R0883; F7524; G7513; P0781, respectively) and maintained at 5% CO2, 37 °C.
I3C antiviral test
The antiviral activity of I3C has been tested by a cytopathic effect (CPE) inhibition assay using Vero E6 cells infected with the SARS-CoV-2 strain isolated at INMI L. Spallanzani IRCCS (2019-nCoV/Italy-INMI1; GenBank MT06615656). The extent of in vitro inhibition of SARS-CoV-2-driven cell damage (CPE) by I3C is expressed as percentage of surviving cells.
Briefly, cell monolayers growing in 96-well plates (3 × 104 cells/well) were treated for 1 h with 1:3 serial dilutions of I3C before SARS-CoV-2 infection. DMSO was used as uninfected control since I3C is solubilized in this compound. Cells were infected at MOI = 0.001 using MEM supplemented with heat inactivated 2% FBS and 2 mM L-glutamine in the presence of I3C/DMSO treatments. After 1 h of incubation the viral input was replaced by fresh medium containing either I3C or DMSO. Cells were then treated with either I3C or DMSO every 24 h and incubated at 37 °C with 5% CO2 for 72 h, when cell viability was measured by a standard crystal violet staining assay, measuring the optical density (OD) at 595 nM. Results were analyzed using Graph Pad (GraphPad Prism 8 XML ProjecT) and reported as the percentage of survived cells respect to the not-infected cells.
SARS-CoV-2 yield reduction assay
SARS-CoV-2 progeny released in culture medium during the antiviral assays was back-titrated by CPE assay on Vero E6 cells. The media of I3C- or DMSO-treated SARS-CoV-2-infected cultures were serially diluted in four replicates using MEM supplemented with 2% FCS, 2 mM L-glutamine, loaded on Vero E6-containing 96-well plates (3 × 104 cells/well), and incubated at 37 °C for 72 h; CPE was measured by a standard crystal violet staining as described above. Results were analyzed using Graph Pad (GraphPad Prism 8 XML ProjecT) with nonlinear regression curve fit (Inhibitor vs. response-variable slope (four parameters)) and virus titres expressed as log tissue culture infectious dose (TCIC)50/100 μl.
Virus Infection inhibition assay
Vero E6 cells were obtained from ATCC (Manassas, VA, USA) and grown in DMEM with 10% fetal bovine serum (FBS) at 37 °C. The virus strain utilized was isolated from a traveler returning to Washington State, USA from Wuhan, China (USA-WA1/2020) and was obtained from BEI resources (Manassas, VA, USA). The virus stock was passaged twice on Vero E6 cells by challenging at an MOI of less than 0.01 and incubating until cytopathology was seen (after about 3 days). A sample of the culture supernatant was sequenced by NGS and was consistent with the original isolate without evidence of contaminants. The virus stock was stored at −80 °C until used.
For evaluation of indole-3-carbinol against infection with wild type SARS-CoV-2, the compound was dissolved to 10 mM in DMSO and then diluted in culture medium before addition to cells. The compound was added to VeroE6 cells incubated for a minimum of 1 hour, then challenged with virus at an MOI of less than 0.2. Dosing ranged from a final concentration of 10 µM down to 0.02 µM in a two-fold dilution series. As a positive control, 5 µM E-64 was used as it was previously reported to inhibit SARS-CoV-2 infection57. Negative controls were <0.5% DMSO. After a ~1.5 day incubation, cells were treated with 10% buffered formalin for at least 6 h, washed in PBS and virus antigen stained with SARS-CoV-2 specific antibody (Sino Biologicals, MM05) together with Hoechst 33342 dye to stain cell nuclei. Plates were imaged by a Biotek Cytation 1 microscope and automated image analysis was used to count total number of infected cells and total cell nuclei. CellProfiler software (Broad Institute, MA, USA) was used for image analysis using a customized processing pipeline (available upon request to RAD). Infection efficiency was calculated as the ratio of infected cells to total cell nuclei, and treatment conditions were normalized to the average of the negative controls. Loss of cell nuclei was used to flag treatments suggestive of toxicity. IC50 value was calculated using dose-response models fitted by GraphPad Prism software. The assay was performed in duplicate in 384 well plates.
Supplementary information
Acknowledgements
We thanks the patients and their families for placing their trust in us. We thank also Professor Manuela Helmer Citterich from Department of Biology, Tor Vergata University of Rome, for her essential contribute to the in silico experiments. We are indebted to Francesca Pisanu for her assistance to the work.
Appendix I: COVID Human Genetic Effort
Laurent Abel1, Alessandro Aiuti2, Saleh Al-Muhsen3, Fahd Al-Mulla4, Mark S. Anderson5, Andrés Augusto Arias6, Hagit Baris Feldman7, Dusan Bogunovic8, Alexandre Bolze9, Anastasiia Bondarenko10, Ahmed A. Bousfiha11, Petter Brodin12, Yenan Bryceson13, Carlos D. Bustamante14, Manish J. Butte15, Giorgio Casari16, Samya Chakravorty17, John Christodoulou18, Antonio Condino-Neto19, Stefan M. Constantinescu20, Megan A. Cooper21, Clifton L. Dalgard22, Murkesh Desai23, Beth A. Drolet24, Sara Espinosa-Padilla25, Jacques Fellay26, Carlos Flores27, José Luis Franco6, Antoine Froidure28, Peter K. Gregersen29, Filomeen Haerynck30, David Hagin31, Rabih Halwani32, Lennart Hammarström33, Jim Heath34, Sarah E. Henrickson35, Elena Hsieh36, Kohsuke Imai37, Yuval Itan38, Timokratis Karamitros39, Kai Kisand40, Cheng-Lung Ku41, Yu-Lung Lau42, Yun Ling43, Carrie L. Lucas44, Tom Maniatis45, Davoud Mansouri46, László Maródi47, Isabelle Meyts48, Joshua D. Milner49, Kristina Mironska50, Trine H. Mogensen51, Tomohiro Morio52, Lisa F.P. Ng53, Luigi D. Notarangelo54, Antonio Novelli55, Giuseppe Novelli56, Satoshi Okada57, Tayfun Ozcelik58, Jana M. Pachlopnik59, Qiang Pan-Hammarström60, Rebeca Perez de Diaz61, Anna M. Planas62, Carolina Prando63, Aurora Pujol64, Lluis Quintana-Murci65, Laurent Renia53, Carlos Rodríguez-Gallego66, Vanessa Sancho-Shimizu67, Vijay Sankaran68, Mohammed Shahrooei69, Andrew L. Snow22, Pere Soler-Palacín70, András N. Spaan71, Stuart G. Tangye72, Stuart Turvey73, Furkan Uddin74, Mohammed J. Uddin75, Diederik van de Beek76, Donald C. Vinh77, Horst von Bernuth78, Pawel Zawadzki79, Helen C. Su54*, Jean-Laurent Casanova80*
1INSERM U1163, University of Paris, Imagine Institute, Paris, France. 2San Raffaele Telethon Institute for Gene Therapy, IRCCS Ospedale San Raffaele, and Vita Salute San Raffaele University, Milan, Italy. 3Immunology Research Laboratory, Department of Pediatrics, College of Medicine and King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia. 4Dasman Diabetes Institute, Department of Genetics and Bioinformatics, Dasman, Kuwait. 5Diabetes Center, University of California, San Francisco, San Francisco, CA, USA. 6Universidad de Antioquia, Group of Primary Immunodeficiencies, Antioquia, Colombia. 7The Genetics Institute, Tel Aviv Sourasky Medical Center and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. 8Icahn School of Medicine at Mount Sinai, New York, NY, USA. 9Helix, San Mateo, CA, USA. 10Shupyk National Medical Academy for Postgraduate Education, Kiev, Ukraine. 11Clinical Immunology Unit, Department of Pediatric Infectious Disease, CHU Ibn Rushd and LICIA, Laboratoire d’Immunologie Clinique, Inflammation et Allergie, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco. 12SciLifeLab, Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden. 13Department of Medicine, Center for Hematology and Regenerative Medicine, Karolinska Institutet, Stockholm, Sweden. 14Stanford University, Stanford, CA, USA. 15Division of Immunology, Allergy, and Rheumatology, Department of Pediatrics, UCLA, Los Angeles, CA, USA; Department of Microbiology, Immunology, and Molecular Genetics, UCLA, Los Angeles, CA, USA. 16Medical Genetics, IRCCS Ospedale San Raffaele, Milan, Italy. 17Department of Pediatrics and Children’s Healthcare of Atlanta, Emory University, Atlanta, GA, USA. 18Murdoch Children’s Research Institute, Victoria, Australia. 19Department of Immunology - Institute of Biomedical Sciences - University of São Paulo, São, Brazil. 20Endocrinology Department, Cliniques Universitaires Saint Luc, Brussels, Belgium. 21Washington University School of Medicine, St. Louis, MO, USA. 22Department of Anatomy, Physiology & Genetics, Uniformed Services University of the Health Sciences, Bethesda, MD, USA. 23Bai Jerbai Wadia Hospital for Children, Mumbai, India. 24School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA. 25Instituto Nacional de Pediatria (National Institute of Pediatrics), Mexico City, Mexico. 26School of Life Sciences, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland; Precision Medicine Unit, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland. 27Genomics Division, Instituto Tecnológico y de Energías Renovables (ITER), Santa Cruz de Tenerife, Spain; Research Unit, Hospital Universitario N.S. de Candelaria, Santa Cruz de Tenerife, Spain; Instituto de Tecnologías Biomédicas (ITB), Universidad de La Laguna, San Cristóbal de La Laguna, Spain; CIBER de Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain. 28Institut de Recherche Expérimentale et Clinique, Pôle de Pneumologie, Université catholique de Louvain, Belgium Service de pneumologie, Cliniques Universitaires Saint-Luc, Brussels, Belgium. 29Feinstein Institute for Medical Research, Northwell Health USA, Manhasset, NY, USA. 30Department of Paediatric Immunology and Pulmonology, Centre for Primary Immunodeficiency Ghent (CPIG), PID Research Laboratory, Jeffrey Modell Diagnosis and Research Centre, Ghent University Hospital, Edegem, Belgium. 31The Genetics Institute Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. 32Sharjah Institute of Medical Research, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates. 33Department of Laboratory Medicine, Karolinska Institutet, Stockholm, Sweden. 34Institute for Systems Biology, Seattle, WA, USA. 35Perelman School of Medicine, University of Pennsylvania Children’s Hospital of Philadelphia, Division of Allergy-Immunology, Philadelphia, PA, USA. 36Department of Immunology and Microbiology, Department of Pediatrics, Division of Allergy and Immunology, University of Colorado, School of Medicine Children’s Hospital Colorado, Aurora, CO, USA. 37Riken, Tokyo, Japan. 38Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA. 39Bioinformatics and Applied Genomics Unit, Department of Microbiology, Hellenic Pasteur Institute, Athens, Greece. 40Molecular Pathology, Department of Biomedicine, Institute of Biomedicine and Translational Medicine, University of Tartu, Tartu Estonia. 41Chang Gung University, Taoyuan County, Taiwan. 42Department of Paediatrics & Adolescent Medicine, The University of Hong Kong, Hong Kong, China. 43Shanghai Public Health Clinical Center, Fudan University, Shanghai, China. 44Department of Immunobiology, Yale University School of Medicine, New Haven, CT, USA. 45Zukerman Mind Brain Behavior Institute, Columbia University, New York, NY, USA; New York Genome Center, New York, NY, USA. 46Department of Clinical Immunology and Infectious Diseases, National Research Institute of Tuberculosis and Lung Diseases, The Clinical Tuberculosis and Epidemiology Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Masih Daneshvari Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 47PID Clinical Unit and Laboratory, Department of Dermatology, Venereology and Dermato-oncology. 48Department of Pediatrics, University Hospitals Leuven, Leuven, Belgium; Laboratory for Inborn Errors of Immunity, KU Leuven, Leuven, Belgium. 49Department of Pediatrics, Columbia University Irving Medical Center. New York, NY, USA. 50University Clinic for Children’s Diseases, Department of Pediatric Immunology, Medical Faculty, University “St.Cyril and Methodij” Skopje, North Macedonia. 51Department of Biomedicine, Aarhus University, Aarhus, Denmark; Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark. 52Tokyo Medical & Dental University Hospital, Tokyo, Japan. 53A*STAR ID labs, Agency for Science, Technology and Research (A*STAR), Singapore; Singapore Immunology Network, Agency for Science, Technology and Research (A*STAR), Singapore. 54National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA. 55Laboratory of Medical Genetics, IRCCS Bambino Gesù Children’s Hospital, Rome, Italy. 56Department of Biomedicine and Prevention, Tor Vergata University of Rome, Rome, Italy. 57Department of Pediatrics, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan. 58Department of Molecular Biology and Genetics, Bilkent University, Bilkent - Ankara, Turkey. 59Department of Immunology, University Children’s Hospital Zurich, Zurich, Switzerland. 60Department of Biosciences and Nutrition, Karolinska Institutet, Stockholm, Sweden. 61Laboratory of Immunogenetics of Human Diseases, Innate Immunity Group, IdiPAZ Institute for Health Research, La Paz Hospital, Madrid, Spain. 62IIBB-CSIC, IDIBAPS, Barcelona, Spain. 63Faculdades Pequeno Príncipe, Instituto de Pesquisa Pelé Pequeno Príncipe, Curitiba, Brazil. 64Neurometabolic Diseases Laboratory, Bellvitge Biomedical Research Institute (IDIBELL), L’Hospitalet de Llobregat, Barcelona, Spain; Catalan Institution of Research and Advanced Studies (ICREA), Barcelona, Spain; Center for Biomedical Research on Rare Diseases (CIBERER), ISCIII, Barcelona, Spain. 65Human Evolutionary Genetics Unit, CNRS UMR2000, Institut Pasteur, Paris, France; Human Genomics and Evolution, Collège de France, Paris, France. 66Department of Immunology, Hospital Universitario de Gran Canaria Dr. Negrín, Canarian Health System, Las Palmas de Gran Canaria, Spain; Department of Clinical Sciences, University Fernando Pessoa Canarias, Las Palmas de Gran Canaria, Spain. 67Imperial College London, London, UK. 68Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA. 69Saeed Pathobiology and Genetics Lab, Tehran, Iran; Department of Microbiology and Immunology, Clinical and Diagnostic Immunology, KU Leuven, Leuven, Belgium. 70Pediatric Infectious Diseases and Immunodeficiencies Unit. Vall d’Hebron Barcelona Hospital Campus. Barcelona, Spain. 71St. Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, NY, USA; University Medical Center Utrecht, Amsterdam, Netherlands. 72Garvan Institute of Medical Research, Darlinghurst, NSW, Australia; St. Vincent’s Clinical School, Faculty of Medicine, UNSW Sydney, NSW, Australia. 73Department of Pediatrics, British Columbia Children’s Hospital, The University of British Columbia, Vancouver, BC, Canada. 74Holy Family Red Crescent Medical College; Centre for Precision Therapeutics, NeuroGen Children’s Healthcare; Genetics and Genomic Medicine Centre, NeuroGen Children’s Healthcare, Dhaka, Bangladesh. 75Mohammed Bin Rashid University of Medicine and Health Sciences, College of Medicine, Dubai, United Arab Emirates; The Centre for Applied Genomics, Department of Genetics and Genome Biology, The Hospital for Sick Children, Toronto, Ontario, Canada. 76Amsterdam UMC, University of Amsterdam, Department of Neurology, Amsterdam Neuroscience, Amsterdam, Netherlands. 77Department of Medicine, Division of Infectious Diseases, McGill University Health Centre, Montréal, Québec, Canada; Infectious Disease Susceptibility Program, Research Institute, McGill University Health Centre, Montréal, Québec, Canada. 78Department of Pediatric Pneumology, Immunology and Intensive Care, Charité Universitätsmedizin, Berlin University Hospital Center, Berlin, Germany; Labor Berlin GmbH, Department of Immunology, Berlin, Germany; Berlin Institutes of Health (BIH), Berlin-Brandenburg Center for Regenerative Therapies, Berlin, Germany. 79Molecular Biophysics Division, Faculty of Physics, A. Mickiewicz University, Poznań, Poland. 80The Rockefeller University, Howard Hughes Medical Institute, Necker Hospital, New York, NY, USA.
*Leaders of the COVID Human Genetic Effort.
Appendix II: French COVID Cohort Study Group
Laurent Abel1, Claire Andrejak2, François Angoulvant3, Delphine Bachelet4, Romain Basmaci5, Sylvie Behillil6, Marine Beluze7, Dehbia Benkerrou8, Krishna Bhavsar4, François Bompart9, Lila Bouadma4, Maude Bouscambert10, Mireille Caralp11, Minerva Cervantes-Gonzalez12, Anissa Chair4, Alexandra Coelho13, Camille Couffignal4, Sandrine Couffin-Cadiergues14, Eric D’Ortenzio12, Charlene Da Silveira4, Marie-Pierre Debray4, Dominique Deplanque15, Diane Descamps16, Mathilde Desvallées17, Alpha Diallo18, Alphonsine Diouf13, Céline Dorival8, François Dubos19, Xavier Duval4, Philippine Eloy4, Vincent VE Enouf20, Hélène Esperou21, Marina Esposito-Farese4, Manuel Etienne22, Nadia Ettalhaoui4, Nathalie Gault4, Alexandre Gaymard10, Jade Ghosn4, Tristan Gigante23, Isabelle Gorenne4, Jérémie Guedj24, Alexandre Hoctin13, Isabelle Hoffmann4, Salma Jaafoura21, Ouifiya Kafif4, Florentia Kaguelidou25, Sabina Kali4, Antoine Khalil4, Coralie Khan17, Cédric Laouénan4, Samira Laribi4, Minh Le4, Quentin Le Hingrat4, Soizic Le Mestre18, Hervé Le Nagard24, François-Xavier Lescure4, Yves Lévy26, Claire Levy-Marchal27, Bruno Lina10, Guillaume Lingas24, Jean Christophe Lucet4, Denis Malvy28, Marina Mambert13, France Mentré4, Noémie Mercier18, Amina Meziane8, Hugo Mouquet20, Jimmy Mullaert4, Nadège Neant24, Marion Noret29, Justine Pages30, Aurélie Papadopoulos21, Christelle Paul18, Nathan Peiffer-Smadja4, Ventzislava Petrov-Sanchez18, Gilles Peytavin4, Olivier Picone31, Oriane Puéchal12, Manuel Rosa-Calatrava10, Bénédicte Rossignol23, Patrick Rossignol32, Carine Roy4, Marion Schneider4, Caroline Semaille12, Nassima Si Mohammed4, Lysa Tagherset4, Coralie Tardivon4, Marie-Capucine Tellier4, François Téoulé8, Olivier Terrier10, Jean-François Timsit4, Théo Trioux4, Christelle Tual33, Sarah Tubiana4, Sylvie van der Werf34, Noémie Vanel35, Aurélie Veislinger33, Benoit Visseaux16, Aurélie Wiedemann26, Yazdan Yazdanpanah36
1Inserm UMR 1163, Paris, France. 2CHU Amiens, France. 3Hôpital Necker, Paris, France. 4Hôpital Bichat, Paris, France. 5Hôpital Louis Mourrier, Colombes, France. 6Institut Pasteur, Paris, France. 7F-CRIN Partners Platform, AP-HP, Université de Paris, Paris, France. 8Inserm UMR 1136, Paris, France. 9Drugs for Neglected Diseases Initiative, Geneva, Switzerland. 10Inserm UMR 1111, Lyon, France. 11Inserm Transfert, Paris, France. 12REACTing, Paris, France. 13Inserm UMR 1018, Paris, France. 14Inserm, Pôle Recherche Clinique, Paris, France. 15CIC 1403 Inserm-CHU Lille, Paris, France. 16Université de Paris, IAME, INSERM UMR 1137, AP-HP, University Hospital Bichat Claude Bernard, Virology, Paris, France. 17Inserm UMR 1219, Bordeaux, France. 18ANRS, Paris, France. 19CHU Lille, Lille, France. 20Pasteur Institute, Paris, France. 21Inserm sponsor, Paris, France. 22CHU Rouen–SMIT, Rouen, France. 23FCRIN INI-CRCT, Nancy, France. 24Inserm UMR 1137, Paris, France. 25Centre d’Investigation Clinique, Inserm CIC1426, Hôpital Robert Debré, Paris, France. 26Inserm UMR 955, Créteil, France; Vaccine Research Instiute (VRI), Paris, France. 27F-CRIN INI-CRCT, Paris, France. 28CHU de Bordeaux–SMIT, Bordeaux, France. 29RENARCI, Annecy, France. 30Hôpital Robert Debré, Paris, France. 31Hôpital Louis Mourier–Gynécologie, Colombes, France. 32University of Lorraine, Plurithematic Clinical Investigation Centre Inserm CIC-P; 1433, Inserm U1116, CHRU Nancy Hopitaux de Brabois, F-CRIN INI-CRCT (Cardiovascular and Renal Clinical Trialists), Nancy, France. 33Inserm CIC-1414, Rennes, France. 34Institut Pasteur, UMR 3569 CNRS, Université de Paris, Paris, France. 35Hôpital la Timone, Marseille, France. 36Bichat–SMIT, Paris, France.
Appendix III: CoV-Contact Cohort
Loubna Alavoine1, Karine K. A. Amat2, Sylvie Behillil3, Julia Bielicki4, Patricia Bruijning5, Charles Burdet6, Eric Caumes7, Charlotte Charpentier8, Bruno Coignard9, Yolande Costa1, Sandrine Couffin-Cadiergues10, Florence Damond8, Aline Dechanet11, Christelle Delmas10, Diane Descamps8, Xavier Duval1, Jean-Luc Ecobichon1, Vincent Enouf3, Hélène Espérou10, Wahiba Frezouls1, Nadhira Houhou11, Emila Ilic-Habensus1, Ouifiya Kafif11, John Kikoine11, Quentin Le Hingrat8, David Lebeaux12, Anne Leclercq1, Jonathan Lehacaut1, Sophie Letrou1, Bruno Lina13, Jean-Christophe Lucet14, Denis Malvy15, Pauline Manchon11, Milica Mandic1, Mohamed Meghadecha16, Justina Motiejunaite17, Mariama Nouroudine1, Valentine Piquard11, Andreea Postolache11, Caroline Quintin1, Jade Rexach1, Layidé Roufai10, Zaven Terzian11, Michael Thy18, Sarah Tubiana1, Sylvie van der Werf3, Valérie Vignali1, Benoit Visseaux8, Yazdan Yazdanpanah14
1Centre d’Investigation Clinique, Inserm CIC 1425, Hôpital Bichat Claude Bernard, APHP, Paris, France. 2IMEA Fondation Léon M’Ba, Paris, France. 3Institut Pasteur, UMR 3569 CNRS, Université de Paris, Paris, France. 4University of Basel Children’s Hospital. 5Julius Center for Health Sciences and Primary Care, Utrecht, Netherlands. 6Université de Paris, IAME, Inserm UMR 1137, F-75018, Paris, France; Hôpital Bichat Claude Bernard, APHP, Paris, France. 7Hôpital Pitiè Salpétriere, APHP, Paris. 8Université de Paris, IAME, INSERM UMR 1137, AP-HP, University Hospital Bichat Claude Bernard, Virology, Paris, France. 9Santé Publique France, Saint Maurice, France. 10Pole Recherche Clinique, Inserm, Paris, France. 11Hôpital Bichat Claude Bernard, APHP, Paris, France. 12APHP, Paris, France. 13Virpath Laboratory, International Center of Research in Infectiology, Lyon University, INSERM U1111, CNRS UMR 5308, ENS, UCBL, Lyon, France. 14IAME Inserm UMR 1138, Hôpital Bichat Claude Bernard, APHP, Paris, France. 15Service des Maladies Infectieuses et Tropicales; Groupe Pellegrin-Place Amélie-Raba-Léon, Bordeaux, France. 16Hôpital Hotel Dieu, APHP, Paris, France. 17Service des Explorations Fonctionnelles, Hôpital Bichat–Claude Bernard, APHP, Paris, France. 18Center for Clinical Investigation, Assistance Publique-Hôpitaux de Paris, Bichat-Claude Bernard University Hospital, Paris, France.
Author contributions
G.N. and P.P.P. performed study concept and design, wrote the draft of the paper, and supervised the project; J.L. and W.W. performed functional studies using HECT proteins; M.B., and B.R. performed RNA expression analysis and statistical tests; T.A., D.G., M.R.C., J.J.P., and R.A.D. provided in vivo SARS-CoV-2 assays and inhibition tests; A.N., D.C., and E.A. contributed to carry out the molecular genetic data and performed the analysis; J.-L.C., A.C., L.A., B.B., V.L.C., A.L., R.G., G.E., L.D.N., H.C.S., and J.J.G. provided acquisition, analysis and interpretation of genetic data, and wrote the paper; S.G. performed RNA expression experiments, contributed with reagents, materials and analysis tools; K.C., T.M., F.A., F.K., and J.M. performed histological analysis in human lung tissue and in mouse models; A.G. and G.P. provided in silico analysis; A.C. and A.L. evaluated and recruited patients to COVID and/or control cohorts; Y.T.L., Y.U., J.G., and V.L.C. contributed to provide clinical phenotype; J.M., C.T., L.D.N., H.C.S., and S.S. performed review and revision of the paper. All authors read and approved the final version of the manuscript.
Fundings
The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, the Rockefeller University, the St. Giles Foundation, the NIH (R01AI088364), the National Center for Advancing Translational Sciences (NCATS), the NIH Clinical and Translational Science Award (CTSA) program (UL1 TR001866), a Fast Grant from Emergent Ventures, Mercatus Center at George Mason University, the Yale Center for Mendelian Genomics and the GSP Coordinating Center funded by the National Human Genome Research Institute (NHGRI) (UM1HG006504 and U24HG008956), the French National Research Agency (ANR) under the “Investments for the Future” program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), the FRM and ANR GENCOVID project, ANRS-COV05, the Square Foundation, Grandir–Fonds de Solidarité pour l’Enfance, the SCOR Corporate Foundation for Science, Institut National de la Santé et de la Recherche Médicale (INSERM), the University of Paris. The French COVID Cohort study group was sponsored by Inserm and supported by the REACTing consortium and by a grant from the French Ministry of Health (PHRC 20-0424). L.D.H. and H.C.S. are supported by the Division of Intramural Research, National Institute of Allergy and Infectious Disease, National Institutes of Health. This study was also supported in part by a grant of Rome Foundation (Italy, Prot 317 A/I) to G.N.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Additional supporting information may be found in the online version of this article at the publisher’s web site.
Ethics approval and consent to participate
See “Materials and methods” section.
Conflict of interest
The authors declare no competing interests.
Footnotes
Leaders of the COVID Human Genetic Effort: Helen C. Su, Jean-Laurent Casanova
Edited by R.A. Knight
COVID Human Genetic Effort consortium members and their affiliations appears in Appendix I
French COVID Cohort Study Group consortium members and their affiliations appears in Appendix II
CoV-Contact Cohort consortium members and their affiliations appears in Appendix III
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lists of collaborators and their affiliations are listed at the end of the paper.
Contributor Information
Giuseppe Novelli, Email: novelli@med.uniroma2.it.
Pier Paolo Pandolfi, Email: pierpaolo.pandolfiderinaldis@renown.org.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-021-03513-1.
References
- 1.Zhou P, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaliaperumal P, Kole T, Chugh N. Application of healthcare networking in COVID 19—a brief report. Disaster Med. Public Health Prep. 2020;12:1–10. doi: 10.1017/dmp.2020.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keech, C. et al. Phase 1–2 Trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Eng. J. Med.383, 2320–2332 (2020). [DOI] [PMC free article] [PubMed]
- 4.Wang Y, Xing M, Zhou D. Coronavirus disease-19 vaccine development utilizing promising technology. Curr. Opin. HIV AIDS. 2020;15:351–358. doi: 10.1097/COH.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 5.Walsh, E. E. et al. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. medRxiv. Aug 20,2020.08.17.20176651. Preprint at 10.1101/2020.08.17.20176651 (2020).
- 6.Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel JH, et al. Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajendran K, et al. Convalescent plasma transfusion for the treatment of COVID-19, systematic review. J. Med. Virol. 2020;92:1475–1483. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19, a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo P, et al. Metformin treatment was associated with decreased mortality In COVID-19 patients with diabetes in retrospective analysis. Am. J. Trop. Med. Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovato, E. C. W. et al. Repurposing drugs for the management of patients with confirmed coronavirus disease 2019 (COVID-19). Curr. Pharm. Des. 27, 115–126 (2020). [DOI] [PubMed]
- 12.Cantini F, et al. Immune therapy, or antiviral therapy, or both for COVID-19, a systematic review. Drugs. 2020;80:1929–1946. doi: 10.1007/s40265-020-01421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantini F, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia multicentre study. J. Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon DE, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheffner M, Kumar S. Mammalian HECT ubiquitin-protein ligases, biological and pathophysiological aspects. Biochim. Biophys. Acta. 2014;1843:61–74. doi: 10.1016/j.bbamcr.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt O, Teis D. The ESCRT machinery. Curr. Biol. 2012;22:116–120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vietri M, Radulovic M, Stenmark H. The many functions of ESCRTs. Nat. Rev. Mol. Cell. Biol. 2020;21:25–42. doi: 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 18.Bernassola F, Chillemi G, Melino G. HECT-type E3 ubiquitin ligases in cancer. Trends Biochem. Sci. 2019;44:1057–1075. doi: 10.1016/j.tibs.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases, functional diversity within a common modular architecture. Oncogene. 2004;23:1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 20.Ingham RJ, et al. WW domains provide a platform for the assembly of multiprotein networks. Mol. Cell. Biol. 2005;25:7092–7106. doi: 10.1128/MCB.25.16.7092-7106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bieniasz PD. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344:55–63. doi: 10.1016/j.virol.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Maaroufi, H. SARS-CoV-2 encodes a PPxY late domain motif that is known to enhance budding and spread in enveloped RNA viruses. bioRxiv 2020.04.20.052217. Preprint at 10.1101/2020.04.20.052217 (2020).
- 23.Han Z, et al. Ubiquitin ligase WWP1 interacts with Ebola virus VP40 To regulate egress. J. Virol. 2017;91:e00812–e00817. doi: 10.1128/JVI.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih SC, Sloper-Mould KE, Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J. 2000;19:187–198. doi: 10.1093/emboj/19.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sternberg A, Naujokat C. Structural features of coronavirus SARS-CoV-2 spike protein, Targets for vaccination. Life Sci. 2020;257:118056. doi: 10.1016/j.lfs.2020.118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor, molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novelli A, et al. Analysis of ACE2 genetic variants in 131 Italian SARS-CoV-2-positive patients. Hum. Genomics. 2020;14:29. doi: 10.1186/s40246-020-00279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latini A, et al. COVID-19 and genetic variants of protein involved in the SARS-CoV-2 entry into the host cells. Genes. 2020;11:1010. doi: 10.3390/genes11091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casanova JL, Su HC, Effort CHG. A global effort to define the human genetics of protective immunity to SARS-CoV-2 infection. Cell. 2020;181:1194–1199. doi: 10.1016/j.cell.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd45. doi: 10.1126/science.abe1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YR, et al. WWP1 Gain-of-Function Inactivation of PTEN in Cancer Predisposition. N. Engl. J. Med. 2020;382:2103–2116. doi: 10.1056/NEJMoa1914919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Serrano J, Eastman SW, Chung W, Bieniasz PD. HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J. Cell. Biol. 2005;168:89–101. doi: 10.1083/jcb.200408155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang L, et al. Structure of an E6AP-UbcH7 complex, insights into ubiquitination by the E2-E3 enzyme cascade. Science. 1999;286:1321–1326. doi: 10.1126/science.286.5443.1321. [DOI] [PubMed] [Google Scholar]
- 34.Zhadina M, Bieniasz PD. Functional interchangeability of late domains, late domain cofactors and ubiquitin in viral budding. PLoS Pathog. 2010;6:e1001153. doi: 10.1371/journal.ppat.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quirit JG, et al. Indole-3-carbinol (I3C) analogues are potent small molecule inhibitors of NEDD4-1 ubiquitin ligase activity that disrupt proliferation of human melanoma cells. Biochem. Pharmacol. 2017;127:13–27. doi: 10.1016/j.bcp.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh S, et al. Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell. 2020;S0092-8674:31446-X. doi: 10.1016/j.cell.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jian, H., Dempsey, D. R. & Cole, P. A. Ubiquitin ligase activities of WWP1 germline variants K740N and N745S. Biochemistry 60, 357–364 (2021). [DOI] [PMC free article] [PubMed]
- 38.Lee YR, et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science. 2019;17:364. doi: 10.1126/science.aau0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grzymski JJ, et al. Population genetic screening efficiently identifies carriers of autosomal dominant disease. Nat. Med. 2020;26:1235–1239. doi: 10.1038/s41591-020-0982-5. [DOI] [PubMed] [Google Scholar]
- 40.Petrone L, et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin. Microbiol. Infect. 2020;S1198-743:30605–4. doi: 10.1016/j.cmi.2020.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aki D, Li Q, Li H, Liu YC, Lee JH. Immune regulation by protein ubiquitination, roles of the E3 ligases VHL and Itch. Protein Cell. 2019;10:395–404. doi: 10.1007/s13238-018-0586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma S, et al. Marginal zone lymphoma of palatine tonsil with prominent plasmacytic differentiation, A CARE-compliant article and review of literature. Medicine. 2018;97:e9648. doi: 10.1097/MD.0000000000009648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, et al. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc. Natl Acad. Sci. USA. 2013;110:5115–5120. doi: 10.1073/pnas.1220271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed GA, et al. Single -dose and multiple-dose administration of indole-3-carbinol to women, pharmacokinetics based on 3,3’-diindolylmethane. Cancer Epidemiol. Biomark. Prev. 2006;15(12):2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 45.Amati F, et al. Expression profiles of the SARS-CoV-2 host invasion genes in nasopharyngeal and oropharyngeal swabs of COVID-19 patients. Heliyon. 2020;6:e05143. doi: 10.1016/j.heliyon.2020.e05143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howe, K. L. et al. Ensembl 2021. Nucleic Acids Res. 10.1093/nar/gkaa942 (2020).
- 47.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;4:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaser R, Adusumalli S, Leng SN, Sikic M, Ng PC. SIFT missense predictions for genomes. Nat. Protoc. 2016;11:1–9. doi: 10.1038/nprot.2015.123. [DOI] [PubMed] [Google Scholar]
- 49.Rentzsch, P., Witten, D., Cooper, G. M., Shendure, J. & Kircher, M. CADD predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 47, D886–D894 (2018). [DOI] [PMC free article] [PubMed]
- 50.Ioannidis NM, et al. REVEL, an ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 2016;99:877–885. doi: 10.1016/j.ajhg.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations, application to cancer genomics. Nucleic Acids Res. 2011;39:e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanelis V, Rotin D, Forman-Kay JD. Solution structure of a Nedd4 WW domain-ENaC peptide complex. Nat. Struct. Biol. 2008;8:407–412. doi: 10.1038/87562. [DOI] [PubMed] [Google Scholar]
- 53.Qi S, O’Hayre M, Gutkind JS, Hurley JH. Structural and biochemical basis for ubiquitin ligase recruitment by arrestin-related domain-containing protein-3 (ARRDC3) J. Biol. Chem. 2014;289:4743–4752. doi: 10.1074/jbc.M113.527473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai Y, et al. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369:1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pettersen EF, et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 56.Colavita F, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann. Intern. Med. 2020;173:242–24. doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Additional supporting information may be found in the online version of this article at the publisher’s web site.