Abstract
Aging results from intrinsic changes (chronologic) and damage from external exposures (extrinsic) on the human body. The skin is ideal to visually differentiate their unique features. Inherited diseases of DNA repair, such as xeroderma pigmentosum, provide an excellent model for human aging due to the accelerated accumulation of DNA damage. Poikiloderma, atypical lentigines, and skin cancers, the primary cutaneous features of XP, occur in the general population but at a much older age. XP patients also exhibit ocular changes secondary to premature photoaging, including ocular surface tumors and pterygium. Internal manifestations of premature aging, including peripheral neuropathy, progressive sensorineural hearing loss and neurodegeneration, are reported in 25% of XP patients. Internal malignancies, such as lung cancer, CNS tumors, and leukemia/lymphoma occur at a younger age in XP patients, as do thyroid nodules. Premature ovarian failure is overrepresented among XP females, occurring 20 years earlier than in the general population. Taken together, these clinical findings highlight the importance of DNA repair in maintaining genomic integrity. XP is a unique model of human premature aging which is revealing new insights into aging mechanisms.
Keywords: DNA repair, xeroderma pigmentosum, progressive neurologic degeneration, premature menopause, sensorineural deafness, cancer, aging
Introduction:
Xeroderma pigmentosum (XP) is a rare autosomal recessive disorder of DNA repair primarily characterized by photosensitivity and predisposition to skin cancers. XP affects one per million people in the United States and Europe; it is more common in areas of North Africa, Japan, and the Middle East with known founder mutations, with frequencies as high as 1 per 10,000 people (Kleijer et al., 2008; Kraemer et al., 1987).
Originally described in 1874 by Moriz Kaposi, the underlying defect in DNA repair was found 100 years later (Cleaver, 1968; Hebra and Kaposi, 1874), and subsequently identified as nucleotide excision repair (NER), which removes ultraviolet (UV) radiation-induced damage from DNA. (Epstein et al., 1970; Reed et al., 1969). Heterogenicity in the underlying molecular abnormalities (De Weerd-Kastelein et al., 1972) revealed 7 nucleotide excision repair (NER) complementation groups: XPA, XPB/ERCC3, XPC, XPD/ERCC2, XPE/DDB2, XPF/ERCC4, and XPG/ERCC5(DiGiovanna and Kraemer, 2012). The XP variant (XPV) defect is in DNA polymerase eta (POLH)(Burk et al., 1971; Cleaver, 1972; Masutani et al., 1999). Some patients present in childhood with severe burning on minimal sun exposure while others do not, but most develop freckle-like pigmentary lesions in sun exposed areas (Fig 1A) and skin cancer at an early age.
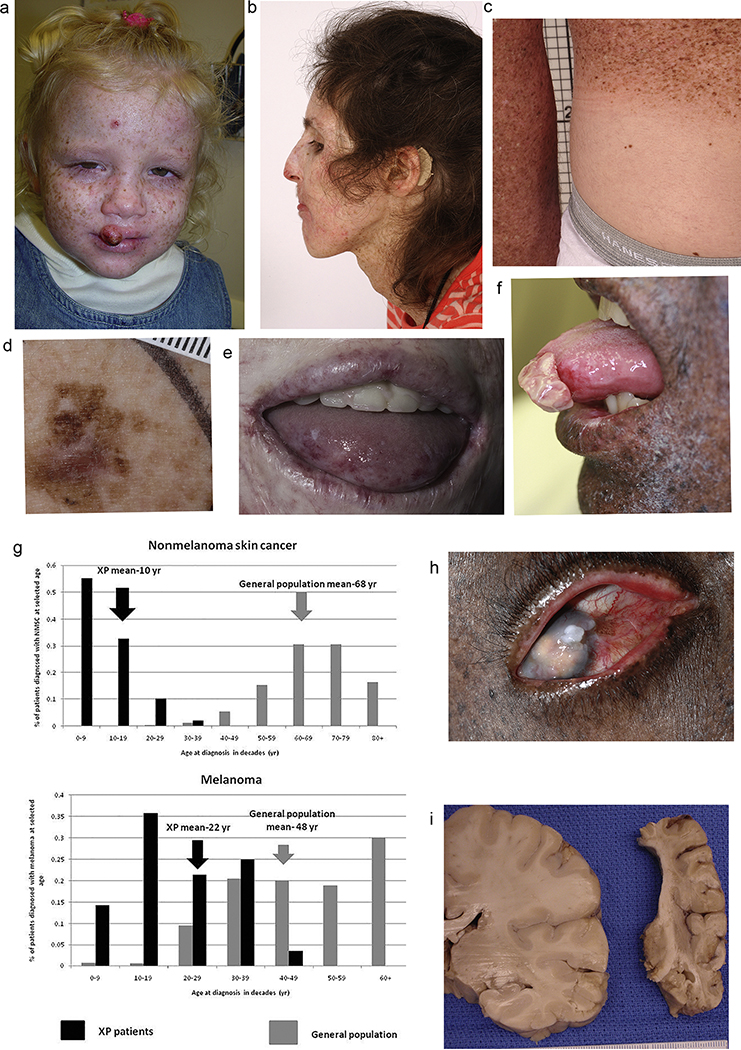
Figure 1.
Clinical features of premature aging in patients with xeroderma pigmentosum. [Patients or their parents or guardians gave permission for publication of their images.]
A. Two-year old girl with XP-C (XP358BE) who did not have acute burning on minimal sun exposure but presented with pigmented skin lesions (lentigos) and a squamous cancer of the lip. [From (Bradford et al., 2011)]
B. Sensorineural deafness can be improved in some patients with hearing aids (XP12BE).
C. Relatively protected buttocks skin (covered by double clothing layers) of 35-year old man shows sparing of the pigmented skin lesions (lentigos) of photodamage (XP19BE).
D. Thirty-five-year old XPC patient (XP24BE) had approximately 200 skin cancers including 11 melanomas in situ and one invasive melanoma. This irregularly pigmented skin lesion is one of her melanoma-in -situ lesions. [From (Lai et al., 2013)]
E. 31-year old woman (XP572BE) with inflammation and scaling of the lips (cheilitis); atrophy and telangiectasias of the tip of tongue.
F. Squamous cell carcinoma tip of the tongue in a 21-year old African man (XP393BE). [From (Mahindra et al., 2008)]
G. Reduced age at skin cancer onset in XP. Xeroderma pigmentosum (XP) skin cancer by age at first skin cancer diagnosis and skin cancer type compared to US general population. Upper panel: Proportion of non-melanoma skin cancer (NMSC) patients diagnosed at selected ages. Lower panel: Proportion of melanoma patients diagnosed at selected ages. Individuals with both NMSC and melanoma were used for both analyses. [From (Bradford et al., 2011)]
H. Seventeen-year old African boy (XP394BE) with reduced vision from corneal clouding and scarring of the UV exposed tissues of the eye. He has a red, vascular, inflammatory corneal mass (pterygium) on the right side of the image and a white ocular surface tumor.
I. Brain of a 36-year old woman with XP-A (XP12BE) at autopsy with severe atrophy weighed equivalent to a 6-month old infant. [From (Lai et al., 2013)]
XP is a multisystem disease with features of premature aging, including early development of premalignant, malignant and inflammatory changes in the skin and mucosa. One quarter of XP patients develop progressive neurologic degeneration, including sensorineural hearing loss (Fig 1B), cognitive impairment, loss of reflexes, peripheral neuropathy, and loss of the abilities to swallow or walk at a young age (DiGiovanna and Kraemer, 2012). In the general population, these are capabilities that commonly diminish with advanced age. In addition to UV-induced and neurologic changes, internal malignancies, ovarian insufficiency, and thyroid abnormalities occur at an early age in XP patients. This wide spectrum of premature aging features signifies the broad role of DNA repair in protection against aging.
Skin and mucous membranes
Skin aging in normal individuals involves both the epidermis (including adnexal structures) and dermal components (collagen, elastin, matrix, vessels). The changes are due to time (chronologic aging) and damage (extrinsic aging). Chronologic aging of the epidermis is associated with thinning, alterations in barrier function, epidermal cell numbers and function, and stem cell function (Branchet et al., 1990). In the dermis, loss of rete ridges and attenuation of dermal papillae result in decreased capacity to resist shearing forces, commonly visualized as senile purpura. Extrinsic skin aging is largely due to UV damage and reflects the depth of penetration of the damaging radiation. UVA and UVB exposure results in increased mutational burden (Martincorena et al., 2015) with subsequent risk of malignancy. UVA penetrates more deeply into the skin than UVB and results in damage to dermal protein, predominantly of elastin and subsequent actinic elastosis and wrinkling.
The skin changes of XP illustrate how the failure to repair UV-induced DNA damage accelerates extrinsic aging, resulting in photoaging and development of cancer (DiGiovanna and Kraemer, 2012; Kraemer et al., 2007; Masaki et al., 2014). Evidence of UV-induced damage is seen in exposed tissues (skin, lips, tongue, and eyes), and by the sparing of double-covered skin (Fig 1C). XP patients develop poikiloderma (hyperpigmentation, hypopigmentation, atrophy and telangiectasia), a feature of advanced photoaging in light skinned individuals in response to UVB, despite minimal sun exposure.
Damage to melanocytes leads to pigmentary changes from mild (freckles) to severe (severe dysplasia/melanoma). Freckling is a common finding in children with XP under the age of 2 years and is rarely seen in children at this age in the general population. As damage accumulates, photoaged skin develops lentigos (Fig 1A, C) - pigmented lesions of varying size, color, and irregular borders that are distributed unevenly across the skin. As damage progresses, these lesions may become premalignant and develop into melanoma both in the general population and more rapidly in XP patients (Fig 1D). Similarly, damage to the epithelial cells of the lips lead to dryness, scaling and inflammation (cheilitis). Sun exposed areas of the tongue may develop abnormal keratinization and vascularization (telangiectasias) [Fig 1E]. All of these exposed tissues accumulate unrepaired DNA damage which can progress across the spectrum from mild functional alteration (xerosis, freckling) to malignancy (Masaki et al., 2014).
Deficient DNA repair in XP is associated with early onset of these changes, decades earlier than in the general population. Basal cell carcinoma, squamous cell carcinoma, and melanoma are common predominantly on UV exposed portions of the skin, eyes and the tip of the tongue (Fig 1F). In young XP patients ( < 20 years), the risk of skin cancer in sun-exposed sites is more than 10,000 times the risk in the general population (Bradford et al., 2011) (Fig 1G). Multiple primary skin cancers are common. The median age of onset of non-melanoma skin cancer in XP patients in the US is 9 years. This is 50 years earlier than in the general population and is thus an indication of the importance of normally functioning DNA repair in the prevention of premature cancers (Fig 1G). There is also a marked reduction in the median age of onset of melanomas of the skin (Fig 1G).
Ophthalmologic
Brooks et al. (2013) studied ocular abnormalities in 87 XP and XP/ trichothiodystrophy (TTD) overlap patients followed longitudinally at the National Institutes of Health (NIH). Dry eye and tear film abnormalities were common, with up to 85% of examined patients showing reduced tear film break up times (TBUT) in at least one eye at a mean patient age of 23 years. In contrast, TBUT in the general population typically shortens after age 45 as a result of meibomian gland dysfunction and age-related lid abnormalities (Ozdemir and Temizdemir, 2010). Eyelid abnormalities resulting from atrophy and scarring also contribute to early onset tear film abnormalities in XP (Brooks et al., 2013).
Multiple types of ocular surface change occur in XP due to UV-induced damage to the anterior eye. Conjunctivitis affects about half of XP patients (Brooks et al., 2013). Conjunctival melanosis, typically in UV-exposed areas, occurs in 20% of XP patients overall, however are overrepresented in patients with a non-burning (38%) as opposed to a burning (17%) phenotype (Brooks et al., 2013). This suggests that strict photoprotection, sometimes implemented earlier in XP patients with burning on minimal sun exposure, may slow the rate of this ocular abnormality.
Pterygia (Fig 1H), UV-induced benign inflammatory conjunctival masses seen in the general population with aging, occur at a younger age in XP patients (Rezvan et al., 2018). Severe keratitis leading to corneal opacification and vascularization can limit vision (Fig 1H). Corneal transplants have restored vision in the general population, however even young XP patients may be poor candidates due to chronic ocular damage with vascularization.
Eyelid and ocular cancers are another XP premature aging feature. While basal and squamous cell carcinoma are the most frequent ocular malignancies, melanoma, conjunctival intraepithelial neoplasia, and atypical fibroxanthoma are also reported (Suarez et al., 2015). In Brooks et al. (2013), 10% of patients had ocular cancers. These occurred at a median age of 16 years, in contrast to a median age of 62 years in the general population (Brooks et al., 2013). Ocular melanoma has been reported in an XP patient as young as 7 years old (Brooks et al., 2013). In addition to their functional and cosmetic morbidity, these cancers can be fatal (Brooks et al., 2013).
Peripheral and Central Nervous Systems
Peripheral neuropathy
Approximately 25% of XP patients develop progressive neurologic degeneration. They have mutations in the XPA, XPB (ERCC3), XPD (ERCC2), XPF (ERCC4) or XPG (ERCC5) genes. (Bradford et al., 2011) Onset may occur early in infancy (de Sanctis–Cacchione syndrome) or be delayed until the second decade of life or later (Anttinen et al., 2008; Robbins et al., 1991; Robbins et al., 1993). Symptoms may include loss of deep tendon reflexes, decreased peripheral sensation (pain, loss of temperature differentiation and kinesthetic sense) and ataxia (Bradford et al., 2011; Robbins, 1988; Robbins et al., 1991; Robbins et al., 1993). Electrodiagnostic testing shows both sensory and motor nerve abnormalities (Bradford et al., 2011; Robbins, 1988). Autopsy examinations of two XP patients (XPA and XPD) with neurologic disease were reported by Lai et al. (2013). The XPA patient had axonal neuropathy and chronic denervation atrophy of skeletal muscle. Interestingly, the autopsy examination of the peripheral nerves of the XPD patient were unremarkable (Lai et al., 2013).
In the general population, peripheral neuropathy is common in older individuals, resulting in ataxia and debilitating falls. The incidence increases with age and may be as high as 50% in adults over age 85 (Lau, 2019).(Lau, 2019) In the elderly the causes include diabetes, drug induced, vascular, nutritional, chronic toxin exposure and idiopathic. Electrodiagnostic studies and nerve biopsies of peripheral neuropathy in the general population and in XP patients were similar (Suzuki, 2013). The occurrence of progressive peripheral neuropathy in XP children or young adults is an example of premature aging in the peripheral nervous system (Robbins, 1988; Suzuki, 2013).
Sensorineural Hearing Loss
Sensorineural hearing loss is a typical early manifestation of XP neurologic degeneration (Fig 1B). Totonchy et al. (2013) reviewed audiology results on 79 XP patients followed at the NIH and found XP-type neurologic degeneration in 17. Of patients with progressive neurologic degeneration, 76% developed sensorineural hearing loss. Four-frequency pure tone average, a standard measure for hearing, correlated with degree of neurodegeneration, with severity of hearing loss paralleling neurological decline. The correlation between sensorineural hearing loss and dementia has also been established in the general population, albeit at a much older age (Gurgel et al., 2014; Lin et al., 2011). Clinically significant hearing loss was detected at a median age of 19 years in the XP cohort, 54 years younger than in the general population (Totonchy et al., 2013). Temporal bone histology in 2 patients with XP-type neurodegeneration and sensorineural hearing loss exhibited significant atrophy of the cochlear neurons when compared with age-matched controls, as well as loss of both peripheral dendrites and central axons (Viana et al., 2013). All 17 patients with neurologic degeneration also exhibited acute burning on minimal sun exposure and were in XP-A or XP-D complementation groups (Bradford et al., 2011; Totonchy et al., 2013).
Progressive neurodegeneration
XP progressive neurodegeneration (PND) involves worsening cognitive impairment, slurred speech, areflexia, ataxia, loss of ambulation and speech, and difficulty swallowing that may necessitate gastric tube placement. MRI studies show cortical thinning of the brain and dilated ventricles consistent with primary neuronal loss. Autopsies of two XP patients with PND in their 40’s (XP-A and XP-D) showed profound cerebral and cerebellar neuronal loss; the brain of one patient was found to be equivalent in weight (660 g) to that of a 6 month old infant (Fig 1 I) (Lai et al., 2013). PND is a major cause of death in XP patients, accounting for 31% of deaths overall. Complications of PND were the predominant cause of death in XP-D patients despite having a large skin cancer burden. XP patients with PND had poorer survival, with a median age at death of 29 years compared to 37 years for those without PND (Bradford et al., 2011). While the neurologic features of XP mirror those of dementia in the general population both clinically and pathologically, the median age at death is substantially older in patients with dementia (Xie et al., 2008). These findings highlight the importance of DNA repair in maintaining the integrity of the nervous system.
Internal malignancies
Central nervous system cancers, typically in XPC, suggests an overall risk of internal neoplasms about 10–20 times that in the general population (Kraemer et al., 1987; Lai et al., 2013). The cause of these early onset CNS neoplasms is not known. Several XP patients who smoked developed lung cancer at an early age. There is experimental evidence that carcinogenic components of cigarette smoke bind to DNA and create lesions that are not repaired in XP cells, thus leading to cancer-causing mutations. A small number of XP patients have had early onset hematologic malignancies, including myelodysplastic syndrome leading to acute myeloid leukemia, diffuse large B-cell lymphoma, and mixed phenotype acute leukemia (Oetjen et al., 2020). Several have a founder mutation in XPC (Sarasin et al., 2019). Additionally, XP patients seen at NIH were recently found to have early onset of thyroid nodules (20 year age reduction) detectable by sonogram and two NIH XP patients were diagnosed with thyroid cancer (Kouatcheu, 2020 (In Press)). This is consistent with prior reports in the literature of thyroid nodules/cancers in XPC patients in the Middle East and North Africa (Ben Rekaya et al., 2013; Hadj-Rabia et al., 2013; Jerbi et al., 2016; Khatri et al., 1992; Messaoud et al., 2013).
Endocrine
Broad age-related changes in endocrine systems have been described in the general population (van den Beld et al., 2018) including the reproductive system. The diagnosis of natural menopause is made after the cessation of regular menses (amenorrhea) for 12 consecutive months, and in United States women occurs at approximately age 50+ 4 years. Premature ovarian insufficiency (POI) or premature menopause is the loss of ovarian function before age 40 years (Gold, 2011; Mishra et al., 2019; Nelson, 2009). The diagnosis of POI is suspected in women who have absent or irregular menses for at least 4 months and demonstrate two elevated blood levels of follicle stimulating hormone at least 1 month apart. Low blood levels of estrogen and anti-mullerian hormone also support the diagnosis of POI (Merideth et al., 2019; Mishra et al., 2019). In the general US population approximately 1% of women experience premature menopause (Nelson, 2009). POI can occur in disorders with defective DNA repair including Fanconi anemia, Bloom syndrome, Werner syndrome and ataxia telangiectasia (Sklavos et al., 2014). Recently, Merideth et al. (2019) reported POI in women with XP. Sixty women (ages 10–61, median 29 years) were examined and 31% of the women age 18 or older had experienced menopause. Premature menopause occurred in 14 of these (78%). The median age of menopause of the XP women (29.5 years) was 20 years earlier than that of the general US population (Merideth et al., 2019) et al., 2019). Premature menopause in XP and other DNA repair diseases highlights the role of genome stability in maintaining normal ovarian function.
Mechanism(s) of Premature Aging in XP patients
Aging is a complex phenomenon that involves dysregulation of cellular processes and age-related reductions in NER, base excision repair (BER), and other repair systems (Lewis et al., 2010; Moriwaki and Takahashi, 2008; Pan et al., 2016; Yamada et al., 2006; Zebian et al., 2019). Specific changes occur at the level of the cells, tissue, and organs. Damage due to intrinsic processes (chronologic aging) or exogenous exposures (extrinsic aging) appear to be mechanistically different. Excessive UVR exposure results in the accumulation of photoproducts, primarily cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts (6–4 PPs) (Apostolou et al., 2019; Marteijn et al., 2014; Tewari et al., 2012). Repair of these DNA lesions by NER prevents cell death, mutagenesis and carcinogenesis. DNA damage accumulates more quickly in XP patients than in the general population leading to clinical features of premature aging.
Nucleotide Excision Repair (NER)
A functional NER pathway is crucial for maintaining genetic integrity as well as for protection against carcinogenesis (Marteijn et al., 2014). This pathway is comprised of two distinct sub-pathways, global genome NER (GG-NER) and transcription-coupled NER (TC-NER), which differ in damage recognition but share the same core mechanism. Most XP patients have defects in either GG-NER or both in GG-NER and TC-NER. GG-NER repairs DNA lesions in the non-transcribed strand of active genes as well as non-transcribing genome (Venter et al., 2001), and the TC-NER repairs transcription-blocking lesions in transcribed DNA strands of active genes (Hanawalt, 2001). The damage recognition proteins XPC and XPE are not required for TC-NER, whereas the other XP proteins (XPA, XPB, XPD, XPF and XPG) are involved in both sub-pathways. RNA polymerase II is the sensor for DNA damage in TC-NER, and when it encounters a lesion, TC-NER-specific factors CSA and CSB play a key role in the activation of the common NER pathway.
Defective Global Genome Nucleotide Excision Repair (XP-C and XP-E)
XP patients have defects in GG-NER, TC-NER, or both. This heterogeneity may help explain some of the distinct clinical features seen within complementation groups. For example, patients with mutations in XP-C and XP-E are predisposed to skin cancer and internal malignancies such as brain tumors, however, do not develop the progressive neurologic degeneration. Because XP-C and XP-E are required for GG-NER but not TC-NER, these patients have defective GG-NER but proficient TC-NER. Defective GG-NER causes the accumulation of DNA lesions which leads to mutagenesis during replication, thus predisposing to skin and some internal cancers. The functional TC-NER, on the other hand, promotes normal cell survival and aging by protecting against DNA damage-mediated disruption of transcription within actively transcribed genes, thus preventing loss of proliferation and cell death(Bohr et al., 1985; Marteijn et al., 2014; Mullenders, 2015; Spivak and Hanawalt, 2015). This preserved functionality is a possible explanation for why XP-C and XP-E patients are not predisposed to the neurologic degeneration often seen in XP-A and XP-D patients. Interestingly, XP-C patients develop skin cancer at an earlier age than XP-E patients. This may be because some XP-E patients, with no neurological defects, possess only partial deficiency in GG-NER (Cleaver and Kraemer, 1989). XP-E cells carrying germline mutations in the p53-regulated DNA repair gene DDB2 are defective in repairing CPDs, but show nearly normal repair of 6–4 PPs (Hwang et al., 1999; Itoh et al., 1999; Moser et al., 2005; Nishi et al., 2009; Oh et al., 2011).
Defective Transcription Coupled Nucleotide Excision Repair (XP-A, XP-B, XP-D, XP-F and XP-G)
In contrast, XP patients with mutations in XP-A, XP-B, XP-D, XP-F and XP-G have defects in both GG-NER and TC-NER, as these genes are required for both sub-pathways. These patients frequently exhibit severe burning on minimal sun exposure, skin cancer predisposition, and neurologic degeneration. These features may be attributable to DNA damage-mediated disruptions of both GG-NER and TC-NER. thus permitting mutagenesis and cell death, respectively (Cleaver and Kraemer, 1989; DiGiovanna and Kraemer, 2012; Kraemer et al., 1987). Additionally, patients with Cockayne Syndrome, another inherited disorder of NER, have defective TC-NER due to mutations in CSA and CSB proteins, have defective TC-NER but normal GG-NER. When this balance between GG-NER and TC-NER is disrupted, cells contain lower numbers of DNA lesions but more transcriptional stress. CS patients develop neurological degeneration but not skin cancer (D’Errico et al., 2007; Kirkali et al., 2009). XP-D and XP-G patients with defects in both GG-NER and TC-NER develop both skin cancers and neurologic degeneration. Based on these clinical observations and supporting studies, it is believed that the defects in GG-NER predispose to cancer development while defects in TC-NER predispose to other features of premature aging such as neurological degeneration.
In addition to their involvement in TC-NER, CSA and CSB proteins play a role in the removal of oxidative DNA damage. The cells from these patients are sensitive to oxidatively-generated DNA damage (Brooks, 2017; D’Errico et al., 2007; Kirkali et al., 2009). CS proteins also play a role in the recognition, signaling and processing of single-strand and double-strand breaks in DNA as well as mitochondrial DNA damage repair (Lindahl, 1993). The features of premature aging in CS patients may be the result of defects in multiple DNA repair pathway, altered mitochondrial function, and redox balance abnormalities in their cells (Cleaver et al., 2014; Terzidis and Chatgilialoglu, 2015; Wang et al., 2012; Yu et al., 2016).
Oxidative Damage
The human genome is constantly exposed to exogenous and endogenous factors that result in DNA damage. UV from sun and carcinogens in tobacco smoke are two major exogenous sources of DNA damage known to be repaired by the NER pathway. Reactive oxygen species (ROS) are a key endogenous source of DNA damage. ROS are known to be generated by UV and ionizing radiation, as well as during normal metabolism secondary to pathophysiological processes. Examples of ROS are hydroxyl radicals, superoxide, singlet oxygen, alpha-oxygen and peroxides. Hydroxyl radicals induce many different base lesions including 8-oxoguanine and thymine glycol in genomic DNA that are removed by BER using different DNA glycosylases (Cadet et al., 1999; Dizdaroglu, 1992; Lindahl, 1993). Hydroxyl radicals also induce a structurally different type of DNA lesion, cyclopurine deoxynucleosides (CPUs). The accumulation of CPUs is thought to be the one of the causes of neurodegeneration in XP patients. CPUs contain the C8−C5′ covalent bond between the sugar and base moieties in the same nucleoside that hinder its repair by BER using glycosylases and are only removed by the NER pathway (Brooks, 2008; Brooks et al., 2000; Kamakura et al., 2012; Kuraoka et al., 2000). The biological effects of these DNA lesions may include blocking of DNA polymerases, inhibition of gene expression and mutagenesis.
Elevated levels of ROS in XP fibroblasts (such as XP-A and XP-D) (Arbault et al., 2004; Hayashi, 2008) and cells from CS patients have been reported (Khobta and Epe, 2013). The defective repair of CPUs in XP-A cells has resulted in the accumulation of CPUs in patient’s cells (Brooks et al., 2000). Elevated levels of CPUs have similarly been reported in CS patients’ cells and CSB gene knockout mice (D’Errico et al., 2007; Kirkali et al., 2009; Kuraoka et al., 2000). Autopsy reports of an XPA and CS patient both suggest that oxidative stress may have played a role in the brain atrophy observed during examination (Hayashi, 2008). In addition to their role in TC-NER, CSA and CSB proteins may play a role in the repair of oxidized DNA bases (Brooks, 2017; D’Errico et al., 2007; Kirkali et al., 2009) by interacting with base excision repair proteins (Le May et al., 2010). Primary fibroblasts from CS patients are defective in the repair of ionizing radiation-induced 8-hydroxyguanine and 8-hydroxyadenine (Tuo et al., 2003). While these findings also suggest that CS cells are more sensitive to oxidative DNA lesions than XP-A cells, further studies on the accumulation of CPUs in neurons of other XP subgroups are warranted. Regardless, this evidence suggests that accelerated premature aging features in CS patients may be the result of defects in multiple DNA repair pathways (Terzidis and Chatgilialoglu, 2015; Wang et al., 2012; Yu et al., 2016), and that neurological abnormalities in XP and CS patients may be associated with the accumulation of CPUs (Brooks, 2008, 2017; Brooks et al., 2000; Kuraoka et al., 2000). Further studies are warranted for a clearer understanding of the role of CPUs in carcinogenesis and neurodegeneration.
R Loop Structures
Transcription may induce DNA damage and genomic instability through R-loop structures, which are formed when a nascent RNA transcript rehybridizes to the template DNA, forcing the nontemplate DNA strand into a single-strand loop (Aguilera and Garcia-Muse, 2012; Aguilera and Gomez-Gonzalez, 2017; Sollier and Cimprich, 2015; Sollier et al., 2014). Some XP-associated proteins, XPF and XPG, have been reported to cleave R-loops in vitro (Tian and Alt, 2000) and in cells (Sollier et al., 2014; Yasuhara et al., 2018). In replicating cells XPF and XPG are critical in the generation of R-loop-dependent double stranded breaks (DSBs)(Sollier et al., 2014). Recently, it has been reported that in non-replicating cells, XPF and XPG endonucleases induce single-strand breaks (SSBs) in R-loops by cleaving one strand; when another SSB is present on the opposing DNA strand, a DSB is then created (Cristini et al., 2019). Findings suggest that the single-stranded DNA in the R-loop must be cleaved at both 3ʹ- and 5ʹ-extremities to induce DSBs and that this dual incision is mediated by XPF/XPG or XPF/FEN1, but the choice between these specific combinations of nucleases are not clearly known. As neurons have reduced DNA repair capacity compared to proliferating cells (Rass et al., 2007), the genomic instability introduced by R-loops may contribute to the development of neurodegeneration in XP patients with defective XPG and XPF genes. Although XP patients with defects in XPF tend to have milder clinical phenotypes, XPF mutations have been associated with another novel disease of accelerated aging, XFE progeroid syndrome (Gregg et al., 2011). Like XP, XFE progeroid syndrome is characterized by neurodegeneration and accelerated aging, without a predisposition to skin cancer. The heterogeneity in clinical features associated with XPF mutations may in some way be linked to the additional function of XPF outside of the NER pathway (Hodskinson et al., 2014; Klein Douwel et al., 2014; Wood, 2010).
DNA Damage Tolerance Defects
Like defects in NER, defects in genes involved in DNA damage tolerance (DDT) are also associated with cancer susceptibility (Pilzecker et al., 2019). The DDT system plays a key role during DNA replication, mainly in response to UV-induced DNA damage. The Y-family TLS POLH gene (Masutani et al., 1999; Pilzecker et al., 2019) can perform error-free trans-lesion synthesis opposite TT CPDs, which ultimately leads to decreased mutagenesis and protection from UV-induced carcinogenesis. Xeroderma pigmentosum variant (XP-V) is the first human disease linked to defective DDT (Masutani et al., 1999). XP-V patients have an increased incidence of skin cancer at an early age, but no neurological abnormalities. This is due to increased mutagenicity from UV-induced DNA damage that occurs with an inherited defect in the POLH gene in cells, despite being NER proficient (Busuttil et al., 2008; Pilzecker et al., 2019; Wang et al., 1993).
Non-DNA Repair Pathways
The mechanisms by which a defective NER pathway contributes to premature menopause, hematological malignancies and thyroid nodules/cancer in XP patients are not fully understood. Interestingly, these features of premature aging are more common in XP-C patients. Recent studies have described the importance of XPC protein in pathways outside of DNA repair. For example, XPC protein has a role in the transcriptional regulation in the cell (Bidon et al., 2018; Le May et al., 2010; Ray et al., 2009; Zebian et al., 2019; Zhu et al., 2009). XPC has a role in maintaining centrosome homeostasis (Wang et al., 2019). XPC deficiency inhibits BRCA1 expression via upregulating its transcriptional repressor, Pit-1. This leads to an accumulation of DSBs and persistent activation of the ATM-Chk1/Chk2 signaling, resulting in prolonged cell cycle arrest and thus centrosome amplification. Centrosome amplification is strongly correlated with aneuploidy and chromosome instability, and is a frequent event observed in many types of cancers (Brown et al., 2010; Chan, 2011; Desai et al., 2018; Dodson et al., 2004; Douthwright and Sluder, 2014; Jiang et al., 2003; Kawamura et al., 2003; Wang et al., 2015). XPC may be crucial for genomic integrity in embryonic stem cells, as it is an important component of the stem cell coactivator complex (SCC), required for the activation of Nanog gene involving Oct4 and Sox2 proteins (Fong et al., 2011). XPC protein undergoes post-translational modification (Rechkunova et al., 2019; Wang et al., 2005), and plays a role in several other DNA repair pathways including BER (Marteijn et al., 2014; Shafirovich et al., 2016; Zebian et al., 2019). The role of DNA repair proteins in non-DNA repair pathways, such as chromatin dynamics, post-translational modification, pluripotency of embryonic stem cells and transcription, should be explored in order to gain additional insight into the mechanisms underlying premature aging phenotypes in XP.
Conclusion
The skin is a unique window showing differences between intrinsic (chronologic) and external aging. Diseases such as XP are a unique model of extrinsic aging from accelerated rate of accumulation of DNA damage. Many of the mucocutaneous and ocular manifestations of XP occur in the general population at a much older age. XP patients also prematurely develop internal manifestations of aging, including peripheral neuropathy, sensorineural hearing loss, neurodegeneration, increased risk of internal malignancies, and premature ovarian failure in females. While the steps of the underlying mechanisms are not well known, these findings establish a solid role for DNA repair and genomic integrity as important factors in prevention of aging and maintaining a fountain of youth. Characterization of the precise mechanisms offers the promise of new insights and opportunities for human quality of life and longevity.
Acknowledgements
This study was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. JDJ was supported by the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, the Colgate-Palmolive Company, alumni of student research programs, and other individual supporters via contributions to the Foundation for the National Institutes of Health. The authors state no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Aguilera A, Garcia-Muse T (2012) R loops: from transcription byproducts to threats to genome stability. Mol Cell 46:115–24. [DOI] [PubMed] [Google Scholar]
- Aguilera A, Gomez-Gonzalez B (2017) DNA-RNA hybrids: the risks of DNA breakage during transcription. Nat Struct Mol Biol 24:439–43. [DOI] [PubMed] [Google Scholar]
- Anttinen A, Koulu L, Nikoskelainen E, Portin R, Kurki T, Erkinjuntti M, et al. (2008) Neurological symptoms and natural course of xeroderma pigmentosum. Brain 131:1979–89. [DOI] [PubMed] [Google Scholar]
- Apostolou Z, Chatzinikolaou G, Stratigi K, Garinis GA (2019) Nucleotide Excision Repair and Transcription-Associated Genome Instability. Bioessays 41:e1800201. [DOI] [PubMed] [Google Scholar]
- Arbault S, Sojic N, Bruce D, Amatore C, Sarasin A, Vuillaume M (2004) Oxidative stress in cancer prone xeroderma pigmentosum fibroblasts. Real-time and single cell monitoring of superoxide and nitric oxide production with microelectrodes. Carcinogenesis 25:509–15. [DOI] [PubMed] [Google Scholar]
- Ben Rekaya M, Jerbi M, Messaoud O, Ben Brick AS, Zghal M, Mbarek C, et al. (2013) Further evidence of mutational heterogeneity of the XPC gene in Tunisian families: a spectrum of private and ethnic specific mutations. Biomed Res Int 2013:316286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidon B, Iltis I, Semer M, Nagy Z, Larnicol A, Cribier A, et al. (2018) XPC is an RNA polymerase II cofactor recruiting ATAC to promoters by interacting with E2F1. Nat Commun 9:2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr VA, Smith CA, Okumoto DS, Hanawalt PC (1985) DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell 40:359–69. [DOI] [PubMed] [Google Scholar]
- Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, et al. (2011) Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet 48:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchet MC, Boisnic S, Frances C, Robert AM (1990) Skin thickness changes in normal aging skin. Gerontology 36:28–35. [DOI] [PubMed] [Google Scholar]
- Brooks BP, Thompson AH, Bishop RJ, Clayton JA, Chan CC, Tsilou ET, et al. (2013) Ocular manifestations of xeroderma pigmentosum: long-term follow-up highlights the role of DNA repair in protection from sun damage. Ophthalmology 120:1324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PJ (2008) The 8,5’-cyclopurine-2’-deoxynucleosides: candidate neurodegenerative DNA lesions in xeroderma pigmentosum, and unique probes of transcription and nucleotide excision repair. DNA Repair (Amst) 7:1168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PJ (2017) The cyclopurine deoxynucleosides: DNA repair, biological effects, mechanistic insights, and unanswered questions. Free Radic Biol Med 107:90–100. [DOI] [PubMed] [Google Scholar]
- Brooks PJ, Wise DS, Berry DA, Kosmoski JV, Smerdon MJ, Somers RL, et al. (2000) The oxidative DNA lesion 8,5’-(S)-cyclo-2’-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells. J Biol Chem 275:22355–62. [DOI] [PubMed] [Google Scholar]
- Brown JA, Bourke E, Liptrot C, Dockery P, Morrison CG (2010) MCPH1/BRIT1 limits ionizing radiation-induced centrosome amplification. Oncogene 29:5537–44. [DOI] [PubMed] [Google Scholar]
- Burk PG, Lutzner MA, Clarke DD, Robbins JH (1971) Ultraviolet-stimulated thymidine incorporation in xeroderma pigmentosum lymphocytes. J Lab Clin Med 77:759–67. [PubMed] [Google Scholar]
- Busuttil RA, Lin Q, Stambrook PJ, Kucherlapati R, Vijg J (2008) Mutation frequencies and spectra in DNA polymerase eta-deficient mice. Cancer Res 68:2081–4. [DOI] [PubMed] [Google Scholar]
- Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, et al. (1999) Hydroxyl radicals and DNA base damage. Mutat Res 424:9–21. [DOI] [PubMed] [Google Scholar]
- Chan JY (2011) A clinical overview of centrosome amplification in human cancers. Int J Biol Sci 7:1122–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver JE (1968) Defective repair replication of DNA in xeroderma pigmentosum. Nature 218:652–6. [DOI] [PubMed] [Google Scholar]
- Cleaver JE (1972) Xeroderma pigmentosum: variants with normal DNA repair and normal sensitivity to ultraviolet light. J Invest Dermatol 58:124–8. [DOI] [PubMed] [Google Scholar]
- Cleaver JE, Brennan-Minnella AM, Swanson RA, Fong KW, Chen J, Chou KM, et al. (2014) Mitochondrial reactive oxygen species are scavenged by Cockayne syndrome B protein in human fibroblasts without nuclear DNA damage. Proc Natl Acad Sci U S A 111:13487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver JE, Kraemer KH (1989) Xeroderma pigmentosum. In: The Metabolic Basis of Inherited Disease (Scriver CR, Beaudet AL, Sly WS, Valle D, eds) 6 ed., New York: McGraw Hill, 2949–71. [Google Scholar]
- Cristini A, Ricci G, Britton S, Salimbeni S, Huang SN, Marinello J, et al. (2019) Dual Processing of R-Loops and Topoisomerase I Induces Transcription-Dependent DNA Double-Strand Breaks. Cell Rep 28:3167–81 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Errico M, Parlanti E, Teson M, Degan P, Lemma T, Calcagnile A, et al. (2007) The role of CSA in the response to oxidative DNA damage in human cells. Oncogene 26:4336–43. [DOI] [PubMed] [Google Scholar]
- De Weerd-Kastelein EA, Keijzer W, Bootsma D (1972) Genetic heterogeneity of xeroderma pigmentosum demonstrated by somatic cell hybridization. Nat New Biol 238:80–3. [DOI] [PubMed] [Google Scholar]
- Desai A, Yan Y, Gerson SL (2018) Advances in therapeutic targeting of the DNA damage response in cancer. DNA Repair (Amst) 66–67:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiovanna JJ, Kraemer KH (2012) Shining a light on xeroderma pigmentosum. J Invest Dermatol 132:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M (1992) Measurement of radiation-induced damage to DNA at the molecular level. Int J Radiat Biol 61:175–83. [DOI] [PubMed] [Google Scholar]
- Dodson H, Bourke E, Jeffers LJ, Vagnarelli P, Sonoda E, Takeda S, et al. (2004) Centrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATM. EMBO J 23:3864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthwright S, Sluder G (2014) Link between DNA damage and centriole disengagement/reduplication in untransformed human cells. J Cell Physiol 229:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JH, Fukuyama K, Reed WB, Epstein WL (1970) Defect in DNA synthesis in skin of patients with xeroderma pigmentosum demonstrated in vivo. Science 168:1477–8. [DOI] [PubMed] [Google Scholar]
- Fong YW, Inouye C, Yamaguchi T, Cattoglio C, Grubisic I, Tjian R (2011) A DNA repair complex functions as an Oct4/Sox2 coactivator in embryonic stem cells. Cell 147:120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB (2011) The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 38:425–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg SQ, Robinson AR, Niedernhofer LJ (2011) Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair (Amst) 10:781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT (2014) Relationship of hearing loss and dementia: a prospective, population-based study. Otol Neurotol 35:775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj-Rabia S, Oriot D, Soufir N, Dufresne H, Bourrat E, Mallet S, et al. (2013) Unexpected extradermatological findings in 31 patients with xeroderma pigmentosum type C. Br J Dermatol 168:1109–13. [DOI] [PubMed] [Google Scholar]
- Hanawalt PC (2001) Controlling the efficiency of excision repair. Mutat Res 485:3–13. [DOI] [PubMed] [Google Scholar]
- Hayashi M (2008) Roles of oxidative stress in xeroderma pigmentosum. Adv Exp Med Biol 637:120–7. [DOI] [PubMed] [Google Scholar]
- Hebra F, Kaposi M (1874) On diseases of the skin including exanthemata, volume III. New Sydenham Society 61:252–8. [Google Scholar]
- Hodskinson MR, Silhan J, Crossan GP, Garaycoechea JI, Mukherjee S, Johnson CM, et al. (2014) Mouse SLX4 is a tumor suppressor that stimulates the activity of the nuclease XPF-ERCC1 in DNA crosslink repair. Mol Cell 54:472–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BJ, Ford JM, Hanawalt PC, Chu G (1999) Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc Natl Acad Sci U S A 96:424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Mori T, Ohkubo H, Yamaizumi M (1999) A newly identified patient with clinical xeroderma pigmentosum phenotype has a non-sense mutation in the DDB2 gene and incomplete repair in (6–4) photoproducts. J Invest Dermatol 113:251–7. [DOI] [PubMed] [Google Scholar]
- Jerbi M, Ben Rekaya M, Naouali C, Jones M, Messaoud O, Tounsi H, et al. (2016) Clinical, genealogical and molecular investigation of the xeroderma pigmentosum type C complementation group in Tunisia. Br J Dermatol 174:439–43. [DOI] [PubMed] [Google Scholar]
- Jiang F, Caraway NP, Sabichi AL, Zhang HZ, Ruitrok A, Grossman HB, et al. (2003) Centrosomal abnormality is common in and a potential biomarker for bladder cancer. Int J Cancer 106:661–5. [DOI] [PubMed] [Google Scholar]
- Kamakura N, Yamamoto J, Brooks PJ, Iwai S, Kuraoka I (2012) Effects of 5’,8-cyclodeoxyadenosine triphosphates on DNA synthesis. Chem Res Toxicol 25:2718–24. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Moriyama M, Shiba N, Ozaki M, Tanaka T, Nojima T, et al. (2003) Centrosome hyperamplification and chromosomal instability in bladder cancer. Eur Urol 43:505–15. [DOI] [PubMed] [Google Scholar]
- Khatri ML, Shafi M, Mashina A (1992) Xeroderma pigmentosum. A clinical study of 24 Libyan cases. J Am Acad Dermatol 26:75–8. [DOI] [PubMed] [Google Scholar]
- Khobta A, Epe B (2013) Repair of oxidatively generated DNA damage in Cockayne syndrome. Mech Ageing Dev 134:253–60. [DOI] [PubMed] [Google Scholar]
- Kirkali G, de Souza-Pinto NC, Jaruga P, Bohr VA, Dizdaroglu M (2009) Accumulation of (5’S)-8,5’-cyclo-2’-deoxyadenosine in organs of Cockayne syndrome complementation group B gene knockout mice. DNA Repair (Amst) 8:274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijer WJ, Laugel V, Berneburg M, Nardo T, Fawcett H, Gratchev A, et al. (2008) Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 7:744–50. [DOI] [PubMed] [Google Scholar]
- Klein Douwel D, Boonen RA, Long DT, Szypowska AA, Raschle M, Walter JC, et al. (2014) XPF-ERCC1 acts in Unhooking DNA interstrand crosslinks in cooperation with FANCD2 and FANCP/SLX4. Mol Cell 54:460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouatcheu SDM J; Tamura D; Khan SG; Lee CR; DiGiovanna JJ; Kraemer KH (2020. (In Press)) Thyroid nodules in xeroderma pigmentosum patients: a feature of premature aging. J Endo Invest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer KH, Lee MM, Scotto J (1987) Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol 123:241–50. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ (2007) Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience 145:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka I, Bender C, Romieu A, Cadet J, Wood RD, Lindahl T (2000) Removal of oxygen free-radical-induced 5’,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc Natl Acad Sci U S A 97:3832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Liu YC, Alimchandani M, Liu Q, Aung PP, Matsuda K, et al. (2013) The influence of DNA repair on neurological degeneration, cachexia, skin cancer and internal neoplasms: autopsy report of four xeroderma pigmentosum patients (XP-A, XP-C and XP-D). Acta Neuropathol Commun 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KHV (2019) Laboratory Evaluation of Peripheral Neuropathy. Semin Neurol 39:531–41. [DOI] [PubMed] [Google Scholar]
- Le May N, Mota-Fernandes D, Velez-Cruz R, Iltis I, Biard D, Egly JM (2010) NER factors are recruited to active promoters and facilitate chromatin modification for transcription in the absence of exogenous genotoxic attack. Mol Cell 38:54–66. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Travers JB, Somani AK, Spandau DF (2010) The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene 29:1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L (2011) Hearing loss and incident dementia. Arch Neurol 68:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T (1993) Instability and decay of the primary structure of DNA. Nature 362:709–15. [DOI] [PubMed] [Google Scholar]
- Mahindra P, DiGiovanna JJ, Tamura D, Brahim JS, Hornyak TJ, Stern JB, et al. (2008) Skin cancers, blindness, and anterior tongue mass in African brothers. J Am Acad Dermatol 59:881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JH (2014) Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol 15:465–81. [DOI] [PubMed] [Google Scholar]
- Martincorena I, Roshan A, Gerstung M, Ellis P, Van LP, McLaren S, et al. (2015) Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T, Wang Y, DiGiovanna JJ, Khan SG, Raffeld M, Beltaifa S, et al. (2014) High frequency of PTEN mutations in nevi and melanomas from xeroderma pigmentosum patients. Pigment Cell Melanoma Res 27:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, et al. (1999) The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 399:700–4. [DOI] [PubMed] [Google Scholar]
- Merideth M, Tamura D, Angra D, Khan SG, Ferrell J, Goldstein AM, et al. (2019) Reproductive Health in Xeroderma Pigmentosum: Features of Premature Aging. Obstet Gynecol 134:814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoud O, Ben Rekaya M, Jerbi M, Ouertani I, Kefi R, Laroussi N, et al. (2013) The experience of a Tunisian referral centre in prenatal diagnosis of Xeroderma pigmentosum. Public Health Genomics 16:251–4. [DOI] [PubMed] [Google Scholar]
- Mishra GD, Chung HF, Cano A, Chedraui P, Goulis DG, Lopes P, et al. (2019) EMAS position statement: Predictors of premature and early natural menopause. Maturitas 123:82–8. [DOI] [PubMed] [Google Scholar]
- Moriwaki S, Takahashi Y (2008) Photoaging and DNA repair. J Dermatol Sci 50:169–76. [DOI] [PubMed] [Google Scholar]
- Moser J, Volker M, Kool H, Alekseev S, Vrieling H, Yasui A, et al. (2005) The UV-damaged DNA binding protein mediates efficient targeting of the nucleotide excision repair complex to UV-induced photo lesions. DNA Repair (Amst) 4:571–82. [DOI] [PubMed] [Google Scholar]
- Mullenders L (2015) DNA damage mediated transcription arrest: Step back to go forward. DNA Repair (Amst) 36:28–35. [DOI] [PubMed] [Google Scholar]
- Nelson LM (2009) Clinical practice. Primary ovarian insufficiency. N Engl J Med 360:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi R, Alekseev S, Dinant C, Hoogstraten D, Houtsmuller AB, Hoeijmakers JH, et al. (2009) UV-DDB-dependent regulation of nucleotide excision repair kinetics in living cells. DNA Repair (Amst) 8:767–76. [DOI] [PubMed] [Google Scholar]
- Oetjen KA, Levoska MA, Tamura D, Ito S, Douglas D, Khan SG, et al. (2020) Predisposition to hematologic malignancies in patients with xeroderma pigmentosum. Haematologica 105:e144–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KS, Imoto K, Emmert S, Tamura D, DiGiovanna JJ, Kraemer KH (2011) Nucleotide excision repair proteins rapidly accumulate but fail to persist in human XP-E (DDB2 mutant) cells. Photochem Photobiol 87:729–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir M, Temizdemir H (2010) Age- and gender-related tear function changes in normal population. Eye (Lond) 24:79–83. [DOI] [PubMed] [Google Scholar]
- Pan MR, Li K, Lin SY, Hung WC (2016) Connecting the Dots: From DNA Damage and Repair to Aging. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilzecker B, Buoninfante OA, Jacobs H (2019) DNA damage tolerance in stem cells, ageing, mutagenesis, disease and cancer therapy. Nucleic Acids Res 47:7163–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass U, Ahel I, West SC (2007) Defective DNA repair and neurodegenerative disease. Cell 130:991–1004. [DOI] [PubMed] [Google Scholar]
- Ray A, Mir SN, Wani G, Zhao Q, Battu A, Zhu Q, et al. (2009) Human SNF5/INI1, a component of the human SWI/SNF chromatin remodeling complex, promotes nucleotide excision repair by influencing ATM recruitment and downstream H2AX phosphorylation. Mol Cell Biol 29:6206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechkunova NI, Maltseva EA, Lavrik OI (2019) Post-translational Modifications of Nucleotide Excision Repair Proteins and Their Role in the DNA Repair. Biochemistry (Mosc) 84:1008–20. [DOI] [PubMed] [Google Scholar]
- Reed WB, Landing B, Sugarman G, Cleaver JE, Melnyk J (1969) Xeroderma pigmentosum. Clinical and laboratory investigation of its basic defect. JAMA 207:2073–9. [DOI] [PubMed] [Google Scholar]
- Rezvan F, Khabazkhoob M, Hooshmand E, Yekta A, Saatchi M, Hashemi H (2018) Prevalence and risk factors of pterygium: a systematic review and meta-analysis. Surv Ophthalmol 63:719–35. [DOI] [PubMed] [Google Scholar]
- Robbins JH (1988) Xeroderma pigmentosum. Defective DNA repair causes skin cancer and neurodegeneration. JAMA 260:384–8. [DOI] [PubMed] [Google Scholar]
- Robbins JH, Brumback RA, Mendiones M, Barrett SF, Carl JR, Cho S, et al. (1991) Neurological disease in xeroderma pigmentosum. Documentation of a late onset type of the juvenile onset form. Brain 114 ( Pt 3):1335–61. [DOI] [PubMed] [Google Scholar]
- Robbins JH, Brumback RA, Moshell AN (1993) Clinically asymptomatic xeroderma pigmentosum neurological disease in an adult: evidence for a neurodegeneration in later life caused by defective DNA repair. Eur Neurol 33:188–90. [DOI] [PubMed] [Google Scholar]
- Sarasin A, Quentin S, Droin N, Sahbatou M, Saada V, Auger N, et al. (2019) Familial predisposition to TP53/complex karyotype MDS and leukemia in DNA repair-deficient xeroderma pigmentosum. Blood 133:2718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafirovich V, Kropachev K, Anderson T, Liu Z, Kolbanovskiy M, Martin BD, et al. (2016) Base and Nucleotide Excision Repair of Oxidatively Generated Guanine Lesions in DNA. J Biol Chem 291:5309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklavos MM, Giri N, Stratton P, Alter BP, Pinto LA (2014) Anti-Mullerian hormone deficiency in females with Fanconi anemia. J Clin Endocrinol Metab 99:1608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollier J, Cimprich KA (2015) Breaking bad: R-loops and genome integrity. Trends Cell Biol 25:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollier J, Stork CT, Garcia-Rubio ML, Paulsen RD, Aguilera A, Cimprich KA (2014) Transcription-coupled nucleotide excision repair factors promote R-loop-induced genome instability. Mol Cell 56:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak G, Hanawalt PC (2015) Photosensitive human syndromes. Mutat Res 776:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez MJ, Rivera-Michlig R, Dubovy S, Rodriguez FJ (2015) Clinicopathological Features of Ophthalmic Neoplasms Arising in the Setting of Xeroderma Pigmentosum. Ocul Oncol Pathol 2:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M (2013) Peripheral neuropathy in the elderly. Handb Clin Neurol 115:803–13. [DOI] [PubMed] [Google Scholar]
- Terzidis MA, Chatgilialoglu C (2015) An ameliorative protocol for the quantification of purine 5’,8-cyclo-2’-deoxynucleosides in oxidized DNA. Front Chem 3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari A, Sarkany RP, Young AR (2012) UVA1 induces cyclobutane pyrimidine dimers but not 6–4 photoproducts in human skin in vivo. J Invest Dermatol 132:394–400. [DOI] [PubMed] [Google Scholar]
- Tian M, Alt FW (2000) Transcription-induced cleavage of immunoglobulin switch regions by nucleotide excision repair nucleases in vitro. J Biol Chem 275:24163–72. [DOI] [PubMed] [Google Scholar]
- Totonchy MB, Tamura D, Pantell MS, Zalewski C, Bradford PT, Merchant SN, et al. (2013) Auditory analysis of xeroderma pigmentosum 1971–2012: hearing function, sun sensitivity and DNA repair predict neurological degeneration. Brain 136:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Jaruga P, Rodriguez H, Bohr VA, Dizdaroglu M (2003) Primary fibroblasts of Cockayne syndrome patients are defective in cellular repair of 8-hydroxyguanine and 8-hydroxyadenine resulting from oxidative stress. FASEB J 17:668–74. [DOI] [PubMed] [Google Scholar]
- van den Beld AW, Kaufman JM, Zillikens MC, Lamberts SWJ, Egan JM, van der Lely AJ (2018) The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol 6:647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. (2001) The sequence of the human genome. Science 291:1304–51. [DOI] [PubMed] [Google Scholar]
- Viana LM, Seyyedi M, Brewer CC, Zalewski C, DiGiovanna JJ, Tamura D, et al. (2013) Histopathology of the inner ear in patients with xeroderma pigmentosum and neurologic degeneration. Otol Neurotol 34:1230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Huang EY, Huang SC, Chung BC (2015) DNA-PK/Chk2 induces centrosome amplification during prolonged replication stress. Oncogene 34:1263–9. [DOI] [PubMed] [Google Scholar]
- Wang H, Huang Y, Shi J, Zhi Y, Yuan F, Yu J, et al. (2019) XPC deficiency leads to centrosome amplification by inhibiting BRCA1 expression upon cisplatin-mediated DNA damage in human bladder cancer. Cancer Lett 444:136–46. [DOI] [PubMed] [Google Scholar]
- Wang J, Clauson CL, Robbins PD, Niedernhofer LJ, Wang Y (2012) The oxidative DNA lesions 8,5’-cyclopurines accumulate with aging in a tissue-specific manner. Aging Cell 11:714–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QE, Zhu Q, Wani G, El-Mahdy MA, Li J, Wani AA (2005) DNA repair factor XPC is modified by SUMO-1 and ubiquitin following UV irradiation. Nucleic Acids Res 33:4023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Maher VM, Mitchell DL, McCormick JJ (1993) Evidence from mutation spectra that the UV hypermutability of xeroderma pigmentosum variant cells reflects abnormal, error-prone replication on a template containing photoproducts. Mol Cell Biol 13:4276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RD (2010) Mammalian nucleotide excision repair proteins and interstrand crosslink repair. Environ Mol Mutagen 51:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Brayne C, Matthews FE, Medical Research Council Cognitive F, Ageing Study c (2008) Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ 336:258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Udono MU, Hori M, Hirose R, Sato S, Mori T, et al. (2006) Aged human skin removes UVB-induced pyrimidine dimers from the epidermis more slowly than younger adult skin in vivo. Arch Dermatol Res 297:294–302. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Kato R, Hagiwara Y, Shiotani B, Yamauchi M, Nakada S, et al. (2018) Human Rad52 Promotes XPG-Mediated R-loop Processing to Initiate Transcription-Associated Homologous Recombination Repair. Cell 175:558–70 e11. [DOI] [PubMed] [Google Scholar]
- Yu Y, Guerrero CR, Liu S, Amato NJ, Sharma Y, Gupta S, et al. (2016) Comprehensive Assessment of Oxidatively Induced Modifications of DNA in a Rat Model of Human Wilson’s Disease. Mol Cell Proteomics 15:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebian A, Shaito A, Mazurier F, Rezvani HR, Zibara K (2019) XPC beyond nucleotide excision repair and skin cancers. Mutat Res 782:108286. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Wani G, Arab HH, El-Mahdy MA, Ray A, Wani AA (2009) Chromatin restoration following nucleotide excision repair involves the incorporation of ubiquitinated H2A at damaged genomic sites. DNA Repair (Amst) 8:262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]