Abstract
Auditory hallucinations are a debilitating symptom of schizophrenia. Effective treatment is limited because the underlying neural mechanisms remain unknown. Our study investigates how local and long-range functional connectivity is associated with auditory perceptual disturbances (APD) in schizophrenia. APD was assessed using the Auditory Perceptual Trait and State Scale. Resting state fMRI data were collected for N=99 patients with schizophrenia. Local functional connectivity was estimated using regional homogeneity (ReHo) analysis; long-range connectivity was estimated using resting state functional connectivity (rsFC) analysis. Mediation analyses tested whether local (ReHo) connectivity significantly mediated associations between long-distance rsFC and APD. Severity of APD was significantly associated with reduced ReHo in left and right putamen, left temporoparietal junction (TPJ), and right hippocampus-pallidum. Higher APD was also associated with reduced rsFC between the right putamen and the contralateral putamen and auditory cortex. Local and long-distance connectivity measures together explained 40.3% of variance in APD (P < 0.001), with the strongest predictor being the left TPJ ReHo (P < 0.001). Additionally, TPJ ReHo significantly mediated the relationship between right putamen – left putamen rsFC and APD (Sobel test, P = 0.001). Our findings suggest that both local and long-range functional connectivity deficits contribute to APD, emphasizing the role of striatum and auditory cortex. Considering the translational impact of these circuit-based findings within the context of prior clinical trials to treat auditory hallucinations, we propose a model in which correction of both local and long-distance functional connectivity deficits may be necessary to treat auditory hallucinations.
Keywords: Schizophrenia, hallucinations, resting fMRI, regional homogeneity (ReHo)
1. Introduction
Auditory hallucinations are debilitating perception-like experiences that are common symptoms in schizophrenia (American Psychiatric Association, 2013); roughly 60–80% of individuals with schizophrenia report auditory hallucinations at some point during the course of illness (McCarthy-Jones, 2012; Sartorius et al., 1986; Waters et al., 2014). Despite focused efforts to elucidate the underlying neurobiology (Alderson-Day et al., 2016; Thomas et al., 2015), the brain circuit abnormalities that cause this symptom remain unknown. Prior task-based and symptom-capture fMRI studies showed aberrant activity levels in brain regions involved in processing of auditory information including: superior temporal gyrus (STG) (Horga et al., 2014; Jardri et al., 2011; Powers et al., 2017); inferior frontal gyrus (Jardri et al., 2011; Powers et al., 2017), anterior insula (Jardri et al., 2011; Powers et al., 2017), striatum (Cassidy et al., 2018; Powers et al., 2017), and hippocampus (Jardri et al., 2011). Likewise, resting-state functional connectivity (rsFC) analyses that probe coherence of resting fMRI activity between different brain regions report patterns of aberrant activity in similar circuits: STG (Clos et al., 2014; Gavrilescu et al., 2010; Hoffman, R et al., 2012; Shinn et al., 2013; Sommer et al., 2012), inferior frontal gyrus (Hoffman, R et al., 2012; Shinn et al., 2013; Vercammen et al., 2010), anterior insula (Clos et al., 2014; Sommer et al., 2012), temporoparietal junction (TPJ) (Clos et al., 2014; Vercammen et al., 2010), striatum (Hoffman et al., 2012), and hippocampus (Clos et al., 2014; Sommer et al., 2012). While these long-distance rsFC deficits of auditory hallucinations are well described in schizophrenia (Clos et al., 2014; Gavrilescu et al., 2010; Hoffman et al., 2012; Shinn et al., 2013; Sommer et al., 2012; Vercammen et al., 2010), less is known regarding the contribution of altered functional communication within local circuits. We hypothesized that auditory perceptual disturbance (APD) severity is underwritten by both altered long-distance connectivity between nodes of the network described above and local connectivity strength within nodes of the network. To test this hypothesis, we combined use of quantitative assessment of APD severity across a large sample of schizophrenia patients (n=99) with a novel regional homogeneity (ReHo) approach to calculate the coherence in activity in neighboring voxels to study local connectivity with whole-brain rsFC analysis. A mediation model tested the hypothesis that local connectivity strength within particular nodes of the AH network mediates associations between long-distance connectivity and APD.
A major challenge for clinical researchers seeking to identify a core neural network underlying auditory hallucinations concerns the pervasive heterogeneity of these experiences. The frequency and duration of auditory hallucinations is highly variable across individuals with schizophrenia: some patients may hear occasional distortions in everyday speech while others report hearing threatening voices and shouting multiple times per day. Moreover, severity of auditory hallucinations can be exacerbated by stressful circumstances and be reduced by treatment with antipsychotic medications. No stand-alone clinical assessment currently exists that probes such heterogeneity of APD capturing symptoms from subtle experiences of hearing murmurs to overt verbal hallucinations, in addition to their persistence in frequency and duration over time. An Auditory Perceptual Trait and State Scale was developed to better capture these aspects of APD heterogeneity (Puvvada et al., 2018). We used this new approach to capture APD in the past week and combined local and long-distance functional connectivity analyses to produce a mediation model that tested the influence of local (within-node) connectivity strength on associations between long-distance (between-node) connectivity and APD.
Experimental Materials and Methods
2.1. Participants
N=99 patients with schizophrenia spectrum disorders (SSD), including N=85 patients with schizophrenia and n=14 patients with schizoaffective disorder, were recruited from outpatient clinics of the Maryland Psychiatric Research Center and neighboring outpatient clinics. All SSD patients were taking antipsychotic medications with exception of five who were unmedicated at time of study. Total daily dose was calculated as chlorpromazine equivalent dose.(Woods, 2003) Control participants (N=111) were recruited using local media advertisements and were frequency-matched on age, sex and current smoking status with SSD participants (Table 1). The Structured Clinical Interview for DSM-IV was performed on all participants.(First et al., 2002) Control participants with a current Axis I diagnosis were excluded; controls were also required to have no family history of psychosis in two generations. Exclusion criteria for both groups included history of head trauma with clinical sequelae, major neurological conditions, intellectual disability, and substance abuse or dependence within the past 6 months (except nicotine). Written informed consent was obtained from all participants as approved by the University of Maryland IRB.
Table 1.
Demographic and Clinical Information.
| Schizophrenia (n=99) | Healthy control (n=111) | t or χ2 statistic | p-value | |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | 36.2±13.3 | 36.7±13.1 | −0.27 | 0.79 |
| Sex (M/F) | 71/28 | 75/36 | 0.43 | 0.55 |
| Smoker/Nonsmoker | 61/38 | 69/42 | 0.01 | 1.00 |
| Subject Motion | ||||
| Mean FD | 0.097±0.05 | 0.101±0.05 | −0.69 | 0.49 |
| Clinical & Cognitive | ||||
| APD score | 9.5±11.2 | n/a | ||
| BPRS score | 39.3±12.0 | n/a | ||
| Processing speeda | 59.7±21.1 | 74.4±16.8 | −5.4 | < 0.001 |
| Working memorya | 17.5±5.6 | 20.8±4.3 | −4.6 | < 0.001 |
| UPSA-2b | 84.3±22.9 | 99.9±21.3 | −4.5 | < 0.001 |
FD=Framewise Displacement; APSS=Auditory Perception State Scale; BPRS = Brief Psychiatric Rating Scale; UPSA-2 = UCSD Performance-Based Skills Assessment 2;
calculation based on data from n = 92 SSD patients, n=99 controls;
calculation based on data from n = 81 SSD patients, n=85 controls
2.2. Clinical and Cognitive Assessments
To assess APD in the past week, we calculated total score for the auditory-state subscale of the Auditory Perceptual Trait and State Scale. This self-report scale measures heterogeneous presentation of APD ranging from subtle distortions of sounds as subclinical auditory phenomena to overt psychotic symptoms of hearing voices. Self-reported experiences are rated using 12 items: items 1–8 assess subclinical auditory phenomena and items 9–12 assess overt clinical auditory hallucinations. Each item is rated on a 5-point Likert scale assessing severity by frequency of occurrences in the past week, with “0” indicating no occurrence and “4” indicating high frequency. The total score for the past week was used to evaluate recent APD state. The full scale is available at http://www.mdbrain.org/APTS.pdf and the initial validation studies are now published (Puvvada et al., 2018). The full scale also attempted to capture the “trait” or longitudinally experienced symptoms over one’s lifetime (excluding the past two weeks) by using the same 12 items, although that measure was not used in the current study.
Overall psychiatric symptoms were assessed with the 20-item Brief Psychiatric Rating Scale (BPRS) total score (Overall and Gorham, 1962; Woerner et al., 1988). The Wechsler Abbreviated Scale of Intelligence digit-sequencing and digit-symbol-coding subscales were used to assess working memory and processing speed, respectively (Wechsler, 1999); these tasks were selected as they are among the most robust tasks separating those with SSD vs. controls in meta-analysis across all cognitive domains (Dickinson et al., 2007). Overall function was evaluated using the UCSD Performance-Based Skills Assessment 2 (UPSA-2) (Mausbach et al., 2008).
2.3. Imaging
Resting-state fMRI data were collected at the University of Maryland Center for Brain Imaging Research using a Siemens 3T TRIO MRI (Erlangen, Germany) system equipped with a 32-channel phase array head coil. Resting-state functional T2*-weighted images were obtained (TR=2s, TE=30ms, flip angle=90°, FOV=248mm, 128×128 matrix, 1.94×1.94 in-plane resolution, 4mm slice thickness, 37 axial slices, 444 volumes per run; 2 runs*444 volumes = 888 total volumes). During the scan, participants were asked to keep their eyes closed and relax.
Resting-state fMRI data preprocessing was performed using the Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) group’s resting-state analysis pipeline (Adhikari et al., 2018a) implemented in AFNI software (Cox, 1996). Preprocessing included PCA-based denoising to improve signal-to-noise ratio, despiking, slice-timing correction, motion correction, registration of the base volume to the ENIGMA EPI template (derived from 1,100 datasets collected across 22 sites) (Adhikari et al., 2018b), and smoothing using a 4 mm full-width-half-maximum Gaussian kernel. As a part of the pipeline, time points with excessive motion (>0.2 mm estimated displacement from one time point to the next), including neighboring time points and outlier voxels fraction (>0.1) are censored from statistical analysis. For a detailed flowchart of preprocessing procedures for replication purposes, please reference (Adhikari et al., 2018a) and (Supplemental Figure 1).
Framewise displacement (FD) was calculated for each image; FD differentiates head realignment parameters across frames and generates a 6-dimensional times series that represents instantaneous head motion (Power et al., 2012). All individuals in the current analysis had mean FD ≤ 0.25 to better control for potential confounding effects of motion and motion artifacts on the rsfMRI signal.
2.4. ReHo Calculation
ReHo was calculated at each voxel in the brain, defined as the Kendall’s coefficient of concordance score with the BOLD time series of neighboring voxels (Zang et al., 2004). By estimating synchronization of BOLD activity of a voxel to its neighboring voxels, ReHo provides an estimate of local functional connectivity. The number of time series within a given cluster was defined as 27 to account for face-wise, edge-wise, and node-wise neighbors per recommendations to cover all directions in 3D space (Jiang and Zuo, 2016). ReHo was calculated as the Kendall’s coefficient score at each voxel with its 26 nearest-neighboring voxels using the MATLAB function “y_reho.m” available in the DPABI_V3.0_171210 package. For each subject, a ReHo map was generated consisting of the collection of all voxels’ Kendall’s coefficient scores. Fisher’s z transformation was applied to ReHo maps for further statistical analyses.
2.5. Statistical analysis
We first performed a voxelwise regression analysis of ReHo in the SSD patients with total APD score as our primary regressor of interest to identify regions where local resting connectivity was associated with APD. To explore how long-range connectivity with these identified regions might be related to APD in the SSD patient sample, significant clusters were saved as binary masks, and we performed whole-brain rsFC analysis between voxels in the mask and all other voxels in the brain, including APD score as a regressor. To determine whether observed effects on brain activity were predominately driven by overt hallucinations vs. subclinical phenomena, we performed two additional voxelwise regression analyses of ReHo in SSD patients using overt-hallucination score (total score of Auditory Perceptual State Scale items 9–12) and subclinical score (total score of Auditory Perceptual State Scale items 1–8) as separate regressors.
Our primary aim was to examine resting-state functional biomarkers of APD across a large clinical sample with SSD. We lacked APD data to perform similar voxelwise ReHo and rsFC analyses in the control sample. To contextualize the results in the patient sample, we examined group (SSD patient versus control) differences in voxelwise regression analysis. Conjunction analysis compared overlap of regions in the whole-brain (alpha-corrected) maps of (1) significant patient-control differences, (2) ReHo significantly associated with APD in patients, and (3) long-distance rsFC significantly associated with APD in patients. For all ReHo and rsFC voxelwise analyses, significant results were thresholded using Monte Carlo simulations by AFNI’s 3dClustSim with autocorrelation function option to yield corrected (whole-brain) results thresholded at alpha<0.05, corresponding to cluster-size threshold of 49 voxels at voxelwise p=0.001.
Subject-specific estimates of ReHo and rsFC in significant clusters were extracted to examine their contributions to APD, controlling for confounding effects of age, gender, mean FD and general psychiatric symptoms without hallucination (using total BPRS score with item 12 for hallucinatory behavior removed) on ReHo measures. Finally, mediation analyses were conducted using the SPSS Process macro (model four selected for simple mediation analysis). These analyses explored whether and how ReHo measures found to be significantly associated with APD in primary analyses might mediate the relationship between longer-range functional connectivity deficits and APD score using Sobel test statistics. Based on the significant findings from our primary analysis, eight simple mediation models were tested (4 local × 2 long-distance) (see Table 3). For post-hoc analyses, Bonferroni-corrections were applied to correct for the number of tests to yield corrected p<0.05 (For mediation analyses, p = 0.05/8 = 0.00625). Bivariate Pearson correlation analyses explored associations between extracted rsFC and clinical measures including UPSA-2, cognitive measures, symptom severity, and chlorpromazine-equivalent dose.
Table 3.
Results of Mediation Analyses.
| Mediation Model | Test Statistics | ||||
|---|---|---|---|---|---|
| Independent Predictor (X) | Dependent Outcome (Y) | Mediator (M) | Sobel test statistic | SE | p-value |
| Rt putamen - left TPJ rsFC | Aud. perceptual disturbance | ReHo: right putamen | −2.36 | 5.11 | 0.018 |
| Rt putamen - left TPJ rsFC | Aud. perceptual disturbance | ReHo: left putamen | −2.48 | 5.56 | 0.013 |
| Rt putamen - left TPJ rsFC | Aud. perceptual disturbance | ReHo: left TPJ | −1.67 | 5.02 | 0.094 |
| Rt putamen - left TPJ rsFC | Aud. perceptual disturbance | ReHo: right hippocampus | −2.70 | 5.77 | 0.007 |
| Rt putamen - left putamen rsFC | Aud. perceptual disturbance | ReHo: right putamen | −1.36 | 12.21 | 0.174 |
| Rt putamen - left putamen rsFC | Aud. perceptual disturbance | ReHo: left putamen | −2.31 | 6.97 | 0.021 |
| Rt putamen - left putamen rsFC | Aud. perceptual disturbance | ReHo: left TPJ | −3.18 | 3.59 | 0.001* |
| Rt putamen - left putamen rsFC | Aud. perceptual disturbance | ReHo: right hippocampus | −2.71 | 5.21 | 0.007 |
Significant after Bonferroni correction for analyzing eight models.
3. Results
3.1. Clinical Characteristics
Controls and SSD patients were frequency-matched in age, sex, and current smoking status; patients and controls also did not differ on overall motion as calculated by mean FD (Table 1). SSD had significantly impaired processing speed, working memory, and global function compared with controls (Table 1).
3.2. APD Score Validation
In SSD patients, total APD scores were significantly correlated with BPRS hallucinatory behavior item scores (r = 0.47, P < 0.001) and also total BPRS score without hallucination item (r = 0.28, P = 0.006); APD correlation with BPRS hallucination item was significantly greater than APD correlation with total BPRS without hallucination item (z = 1.85, P = 0.03).
3.3. Regional Homogeneity and APD in SSD
In SSD patients, higher APD scores were significantly associated with reduced ReHo in left putamen, right putamen, left TPJ, and right hippocampus extending into pallidum (Figure 1). The four ReHo measures for these nodes were not significantly correlated with age, sex, current smoking status nor working memory or processing speed scores for cognition, overall function by UPSA-2, nor total non-hallucination psychiatric symptoms (total BPRS score without hallucination item) in the patients. Chlorpromazine dose was nominally associated with ReHo in right putamen (r = −0.23, P = 0.02) but not with ReHo in left putamen (r = −0.11, P = 0.30), left TPJ (r = −0.04, P 0.68), or hippocampus (r = 0.05, P = 0.58).
Figure 1. Decreased ReHo Associated with Auditory Perceptual Disturbance in Patients with Schizophrenia.
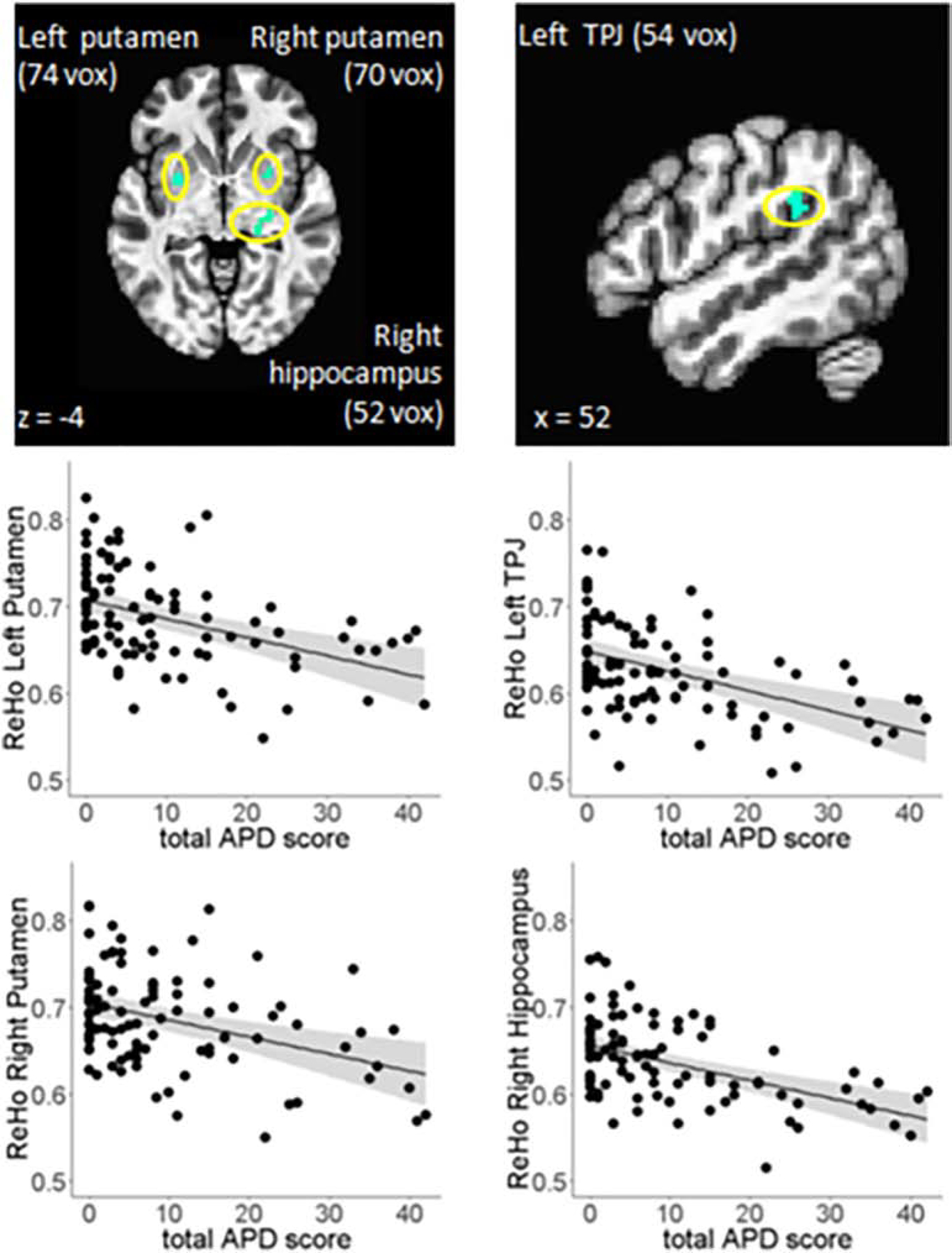
Voxelwise regression analysis revealed total auditory perceptual state score was associated with decreased ReHo in four regions: left putamen, right putamen, left TPJ, and right hippocampus-pallidum. Bottom panel: Partial regression plots showing significant associations between auditory perception and ReHo after covarying for effects of age and gender.
To confirm that these ReHo measures were specifically associated with APD (and not overall symptoms in patients) and the observed associations were not driven by effects of confounding variables, regression analyses controlling for effects of age, gender, chlorpromazine dose, mean FD, and total BPRS score (minus hallucinatory behavior item 12) confirmed that APD scores significantly predicted ReHo in left putamen (t = −4.4, P < 0.001), right putamen (t = −4.1, P < 0.001), left TPJ (t = −5.1, P < 0.001), and right hippocampus-pallidum (t = −5.0, P < 0.001) (Figure 1).
There were no significant associations between subclinical auditory perceptual disturbance score (total score for items 1–8) and ReHo; however, scores for overt hallucinations (total score for items 9–12) were significantly associated with reduced ReHo of a number of regions most prominently in subcortical (basal ganglia and thalamic) regions (Supplemental Table 1).
3.4. Resting State Functional Connectivity and APD in SSD
Each of the four significant regions was used as a seed for whole brain rsFC analysis to understand how local connectivity effects on APD may be related to rsFC across distant regions in SSD. Higher APD scores were significantly associated with lower rsFC only at the right putamen seed, where the right putamen - left putamen and right putamen - left STG rsFC were significantly and negatively associated with APD score (Figure 2).
Figure 2. Local Connectivity Mediating Effects of Long-Distance Connectivity on Auditory Perceptual Disturbance.
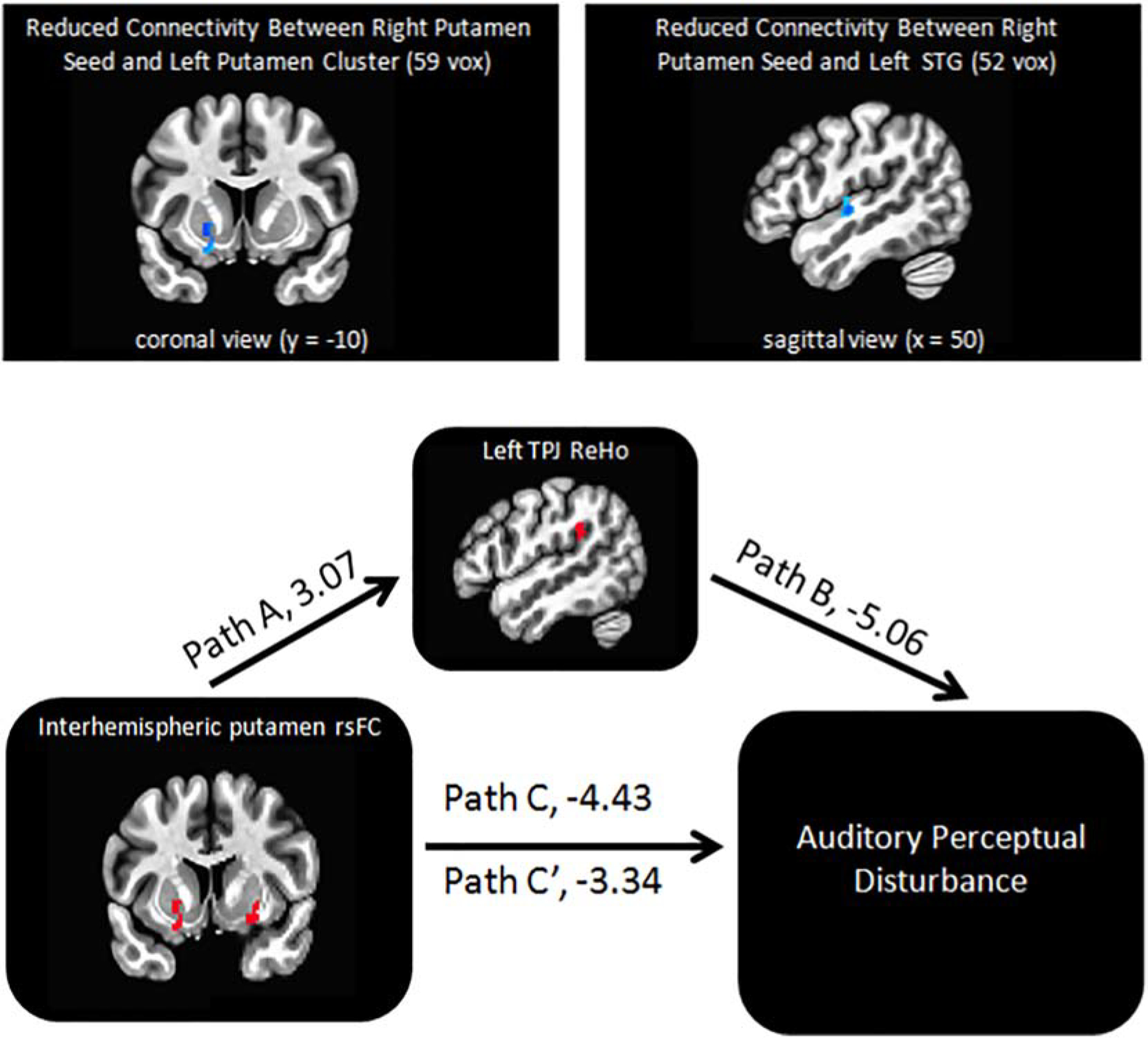
Voxelwise regression analyses of resting-state functional connectivity revealed total auditory perceptual state score was significantly associated with reduced connectivity between right putamen seed and (1) left putamen and (2) left STG (top panel). The significant mediation model from our exploratory mediation analyses of APD (4 local × 2 long-distance): Left TPJ ReHo strongly mediated the relationship between right putamen – left putamen rsFC and auditory disturbance (bottom panel); t-values for each path are presented.
The two rsFC measures were not significantly correlated with mean FD, smoking status, scores for cognition, overall function, or BPRS score (without the hallucination item); chlorpromazine dose was nominally correlated with rsFC between right and left putamen (r = −0.23, P = 0.027), but not with putamen-STG rsFC (r = 0.04, P = 0.71). Regression analyses controlling for effects of age, sex, mean FD, chlorpromazine dose and total adjusted BPRS score (with hallucinatory behavior item removed) confirmed that APS scores significantly predicted putamen-STG rsFC (t = −4.1, P < 0.001) and putamen-putamen rsFC (t = −3.8, P < 0.001).
3.5. Relative Contributions to APD
Next, we modeled the relative contributions of the six rsfMRI measures (4 ReHo + 2 rsFC) on APD in patients. Together, the predictors explained 40.3% of variance in APD (F = 10.4, P < 0.001). The strongest predictors were left TPJ ReHo (t = −3.9, P < 0.001) and STG-putamen rsFC (t = −2.5, P = 0.01), together accounting for 33% of the variance in APD; APD was also significantly associated with putamen-putamen rsFC (t = −2.2, P = 0.03), and ReHo in the right hippocampal-pallidal region (t = −2.4, P = 0.02) (Table 2). No significant collinearity was detected among these rsfMRI predictors (Tolerance > 0.2; variance inflation factor < 5).
Table 2.
Results of Full Connectivity Model of Auditory Perceptual Disturbance (APD ~ 4 ReHo + 2 rsFC)
| Model Statistics | |||
|---|---|---|---|
| R2 | F-value | p-value | |
| 0.403 | 10.4 | < 0.001 | |
| Individual Regression Coefficients | |||
| Std. Beta | t-value | p-value | |
| Left TPJ ReHo | −0.36 | −3.9 | < 0.001 |
| STG-putamen rsFC | −0.23 | −2.5 | 0.01 |
| Rt Hippocampal ReHo | −0.26 | −2.4 | 0.02 |
| Putamen-putamen rsFC | −0.29 | −2.2 | 0.03 |
| Left Putamen ReHo | 0.03 | 0.23 | 0.82 |
| Rt Putamen ReHo | 0.20 | 1.2 | 0.24 |
To explore whether any of the local connectivity ReHo measures might mediate observed relationships between APD and long-distance, interhemispheric rsFC (right putamen – left putamen; right putamen – left STG) in patients, we performed mediation analysis in eight mediation model (4 local × 2 long-distance), with statistical significance thresholded at p = 0.05/8 = 0.00625 using Bonferroni correction. Only left TPJ ReHo significantly partially mediated the relationship between right putamen – left putamen rsFC and APD (Sobel test statistic = 3.19, P = 0.001) (Figure 2; Results from all eight models are in Table 3).
3.6. ReHo: SSD Patients vs. Controls
Relative to controls, patients had significantly increased ReHo in left middle frontal, right middle frontal and left cerebellum, but decreased ReHo in many regions including bilateral STG, bilateral postcentral gyri, bilateral occipital cortex, and bilateral middle temporal, and paracentral lobule (Full list of regions and anatomical locations reported in Supplemental Table 2). Conjunction analysis was performed to compare overlap of regions in the whole-brain (alpha-corrected) maps of (1) significant patient-control differences, (2) ReHo significantly associated with APD in patients, and (3) long-distance rsFC significantly associated with APD in patients. We found no overlap of voxels in the regions significantly associated with APD in SSD patients and voxels identified in the analysis of significant patient-control differences, although some of the findings are found in similar (but non-overlapping) anatomical locations (Supplemental Figure 2 shows the proximity of the three non-overlapping superior temporal regions identified in the ReHo, rsFC, and patient-control comparison analyses). Independent sample t-tests tested whether SSD patients had nominally-significant changes in the six rsfMRI measures described above relative to controls; SSD patients had nominally-significant (uncorrected) reductions in left TPJ ReHo (t = 2.40, P = 0.02), right hippocampal ReHo (t= 2.66, P = 0.008), and putamen-STG rsFC (t = 2.34, P = 0.02) (Full results reported in Supplemental Table 3).
4. Discussion
We tested a regression model that combined distant and local functional connectivity deficits to explain the severity of APD in schizophrenia. This model explained 40% of the variance in APD severity and identified a network of altered connectivity within nodes involved in auditory processing including bilateral putamen, left TPJ, and right hippocampus-pallidum. In addition, APD was associated with reduced long-distance rsFC between the right putamen node and nodes in the contralateral putamen and auditory cortex. Finally, a mediation model tested the influence of local (within-node) connectivity strength on associations between rsFC between nodes (putamen-putamen; putamen-STG) and severity of APD. Intriguingly, local TPJ connectivity partially mediated the relation between interhemispheric putamen-putamen rsFC and APD severity.
The human TPJ – situated at the intersection of the posterior end of the superior temporal sulcus, the inferior parietal lobule, and the lateral occipital cortex – performs many cognitive functions including attention, self-perception, social perception and language processing (Igelström and Graziano, 2017). Much of language processing appears to be lateralized to the left hemisphere as the left TPJ has been shown to be active during speech perception (Fiez et al., 1996; Oberhuber et al., 2016), verbal working memory (Oberhuber et al., 2016; Ravizza et al., 2011, 2004), and imagining speech (Aleman et al., 2005; Shergill et al., 2001). The left TPJ is the most commonly stimulated site in prior transcranial magnetic stimulation (TMS) trials for the treatment of auditory hallucinations, but results were mixed especially in trials with sufficient sham controls (Chibbaro et al., 2005; Fitzgerald et al., 2005; Hoffman et al., 2005, 2003, 1999; Lee et al., 2005; McIntosh et al., 2004; Poulet et al., 2005; Saba et al., 2006), raising some questions on whether exclusively focusing on TPJ is the right strategy (see (Slotema et al., 2014) for full review). Importantly, the directionality of our finding – reduced TPJ ReHo associated with APD – might suggest that the prevailing “TPJ hyperactivation” account of auditory hallucinations should be reconsidered as this theory has encouraged an inhibitory (1 Hz) TMS strategy in many trials (Chibbaro et al., 2005; Fitzgerald et al., 2005; Hoffman et al., 2005, 2003, 1999; Lee et al., 2005; McIntosh et al., 2004; Poulet et al., 2005; Saba et al., 2006). Evidence supporting the “hyperactive TPJ” theory that fueled early TMS trials came from two small studies that found TPJ activation during active auditory hallucinations in a subset of patients.(Lennox et al.; Silbersweig et al., 1995) Consistent with this account, a meta-analysis of auditory-hallucination-activation studies found that the left inferior parietal cortex was consistently activated during auditory hallucinations in schizophrenia patients (Jardri et al., 2011).
While prior evidence from hallucination-activation studies lends support favoring a “hyperactive TPJ” account of auditory hallucinations, the findings from resting fMRI studies support the notion that this symptom is related to reduced rsFC with temporoparietal regions (Clos et al., 2014; Vercammen et al., 2010). Relative to controls, schizophrenia patients with auditory hallucinations had reduced rsFC between left TPJ and right inferior frontal gyrus (Vercammen et al., 2010) and also between left angular gyrus and left inferior temporal cortex (Clos et al., 2014). Intriguingly, patients with auditory hallucinations also had reduced rsFC between left angular gyrus and surrounding inferior parietal cortex (Clos et al., 2014), which is consistent with our finding using ReHo analysis that is specialized to detect changes in BOLD signal coherence at the local scale. To our knowledge, no previous imaging studies have investigated the neural mechanisms of APD in schizophrenia using ReHo and showed a relationship of abnormal TPJ ReHo and APD. Using a different imaging analytical tool, our finding of decreased left TPJ ReHo further underscores the importance of this region in the generation of auditory disturbances in schizophrenia, and raises the possibility for development of alternative TMS protocols exploring neural target engagement by local and long distance rsfMRI approaches to evaluate the efficacy of different TMS protocols.
We found that local TPJ connectivity as indexed by ReHo significantly mediated long-range connectivity between right and left putamen. In anatomical tracing studies of non-human primates, multimodal sensory association cortex in the inferior parietal lobe projects to the putamen via fiber pathways of the external capsule (Schmahmann and Pandya, 2006). Temporoparietal cortex was also shown to be anatomically connected to the ipsilateral putamen in prior diffusion weighted imaging analyses of humans (Cacciola et al., 2017; Kucyi et al., 2012). The only prior ReHo analysis exploring auditory hallucinations to date reported abnormal ReHo in bilateral putamen in medication naïve first-episode schizophrenia patients with auditory hallucinations (Cui et al., 2016), which is consistent with our finding of associations between APD and both local and long-distance (interhemispheric) putamen functional connectivity in the present analysis. Moreover, we found that only total score for severity of overt hallucinations, and not subclinical auditory phenomena, were associated with reduced ReHo in multiple clusters in the putamen (Supplemental Table 1). Together, these findings suggest that the effects of bilateral rsFC on APD – and auditory hallucinations in particular – may occur partially through local TPJ connectivity effects, raising the interesting hypothesis that the TPJ could be used as a cortical window to modulate dysregulated functional connectivity of the putamen.
Our finding of a significant association between APD and putamen-STG rsFC further highlights the role of striatum in formation of APD, and is consistent with prior reports establishing links between APD and altered dorsal striatal functional communication in schizophrenia (Cui et al., 2016; Hoffman, R et al., 2012). While past research has focused on the role that the dorsal striatum plays in motor control, reinforcement learning, and reward learning (Lanciego et al., 2012; Schultz, 2016), emerging research suggests that the putamen plays a functional role in auditory perception and language: putamen-STG functional communication was shown to play a role in processing the timing of auditory signals (Geiser et al., 2012; Nenadic et al., 2003; Pastor et al., 2006), and right and left putamen were consistently coactivated with STG and other key nodes of language networks in a recent meta-analysis of language studies (Viñas-Guasch and Wu, 2017). Anatomical tracing analyses also show that these two regions are directly connected by white matter pathways (Reale and Imig, 1983; Schmahmann and Pandya, 2006). In rats, striatal neurons were shown to be responsive to presentation of auditory stimuli (Bordi et al., 1993; Bordi and LeDoux, 1992), and stimulation of auditory cortico-striatal axons was shown to bias decision-making in an auditory discrimination task (Znamenskiy and Zador, 2013). These studies provide some basic evidence supporting a potential role of auditory-striatal signaling in auditory perception and decision-making. Relatively little is known about how this circuit drives auditory decision-making in healthy human subjects, but there is growing evidence that the basal ganglia contribute to speech perception (Lim et al., 2014). Schizophrenia patients with auditory hallucinations showed deficient prediction error signals in both putamen and auditory cortex during a speech discrimination task (Horga et al., 2014), which raises the interesting hypothesis that abnormal activity in this circuit could bias the striatum towards falsely detecting speech (Horga and Abi-Dargham, 2019). Our current findings add to this growing literature and can fuel development of more robust clinical models of APD and novel therapies for treating the complex causes of APD.
Finally, our observation of a significant association between APD and reduced hippocampal ReHo is highly consistent with prior rsfMRI findings implicating its involvement in the generation of hallucinations (Clos et al., 2014; Hare et al., 2018, 2017; Sommer et al., 2012). In a prior meta-analysis, the hippocampus showed the highest likelihood of activation (Jardri et al., 2011) during active auditory hallucinations, further underscoring its importance in the generation of core symptoms of schizophrenia. Our finding of a significant association between reduced resting hippocampal ReHo and APD is consistent with a prior report of a significant association between reduced hippocampal resting activity and AH severity in schizophrenia (Hare et al., 2017). Importantly dysregulated hippocampal signaling may lead to perceptual disturbances in auditory but also other sensory modalities (Ford et al., 2015; Hare et al., 2017).
Our analysis of patient versus control differences in ReHo are consistent with prior studies implicating increased ReHo in frontal regions (Cui et al., 2016; Wang et al., n.d.; Xiao et al., 2017; Yu et al., 2013) but decreased ReHo in STG (Liu et al., 2006; Wang et al., n.d.; Xiao et al., 2017) and parietal/occipital cortex.(Liu et al., 2006; Wang et al., n.d.; Yu et al., 2013). Intriguingly, the areas significantly associated with APD in the patient sample did not directly overlap with the areas showing the most robust patient-control differences. Significant reductions in ReHo in the patient versus control analysis localized to a large cluster in left STG containing primary auditory cortex (BA 41), but functional connectivity of adjacent clusters in auditory association cortex were strongly associated with APD in SSD patients (Supplemental Figure 2). Based on these data, we speculate that targeted subregions within left temporal and parietal cortex may serve as “hotspots” for the generation of APD, perhaps due to their targeted patterns of connectivity with subcortical regions such as the putamen.
There are several limitations of the current analysis. All but five patients (5% of sample) were medicated at the time of study. This prevented us from completely ruling out effects of antipsychotic medication, although we controlled for potentially confounding medication effects by including chlorpromazine dose as a nuisance covariate in our primary regression analyses. We tested eight simple mediation models, but one additional limitation is that these mediation models did not adjust for potential confounding effects of antipsychotic (chlorpromazine-equivalent) dose. Finally, we only had cross-sectional data from this large sample, limiting our ability to make directional causal claims regarding observed correlations between brain and clinical measures.
In summary, the current study may provide new insight into the formation of APD in schizophrenia where both local and long-distance functional dysconnectivity between auditory and striatal hubs may jointly contribute to this symptom. More studies are needed to replicate these findings, but our data raise the possibility that modifying these patterns of local and long-distance connectivity through brain stimulation and other therapeutics may improve the effectiveness of the ongoing effort to treat auditory hallucinations in schizophrenia.
Supplementary Material
Acknowledgments
Support was received from the National Institutes of Health grants: R01MH111671, R01MH112180, R01MH116948, R01EB01561, R01DC014085, U01MH108148, UG3DA047685, P50MH103222, T32MH067533, 5T32MH073526, S10OD023696, and a State of Maryland contract (M00B6400091), and a University of Maryland Seed Grant (14-103).
Abbreviations:
- APD
auditory perceptual disturbance(s)
- BPRS
Brief Psychiatric Rating Scale
- ENIGMA
Enhancing Neuroimaging Genetics through Meta-Analysis
- FD
framewise displacement
- ReHo
regional homogeneity
- rsFC
resting state functional connectivity
- SSD
schizophrenia spectrum disorder
- STG
superior temporal gyrus
- TPJ
temporoparietal junction
- UPSA-2
UCSD Performance-Based Skills Assessment 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures of Conflicts of Interest
Dr. Hong has received or is planning to receive research funding or consulting fees from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Luye Pharma, Sound Pharma, Takeda, and Regeneron. All other authors declare no financial interests that could represent a conflict of interest.
References
- Adhikari BM, Jahanshad N, Shukla D, Glahn DC, Blangero J, Fox PT, Reynolds RC, Cox RW, Fieremans E, Veraart J, Novikov DS, Nichols TE, Hong LE, Thompson PM, Kochunov P, 2018a. Comparison of heritability estimates on resting state fMRI connectivity phenotypes using the ENIGMA analysis pipeline. Hum. Brain Mapp 39, 4893–4902. 10.1002/hbm.24331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari BM, Jahanshad N, Shukla D, Turner J, Grotegerd D, Dannlowski U, Kugel H, Engelen J, Dietsche B, Krug A, Kircher T, Fieremans E, Veraart J, Novikov DS, Boedhoe PSW, van der Werf YD, van den Heuvel OA, Ipser J, Uhlmann A, Stein DJ, Dickie E, Voineskos AN, Malhotra AK, Pizzagalli F, Calhoun VD, Waller L, Veer IM, Walter H, Buchanan RW, Glahn DC, Hong LE, Thompson PM, Kochunov P, 2018b. A resting state fMRI analysis pipeline for pooling inference across diverse cohorts: an ENIGMA rs-fMRI protocol. Brain Imaging Behav. 10.1007/s11682-018-9941-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B, Diederen K, Fernyhough C, Ford JM, Horga G, Margulies DS, McCarthy-Jones S, Northoff G, Shine JM, Turner J, van de Ven V, van Lutterveld R, Waters F, Jardri R, 2016. Auditory Hallucinations and the Brain’s Resting-State Networks: Findings and Methodological Observations. Schizophr. Bull 42, 1110–1123. 10.1093/schbul/sbw078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Formisano E, Koppenhagen H, Hagoort P, de Haan EHF, Kahn RS, 2005. The Functional Neuroanatomy of Metrical Stress Evaluation of Perceived and Imagined Spoken Words. Cereb. Cortex 15, 221–228. 10.1093/cercor/bhh124 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition.
- Bordi F, LeDoux J, 1992. Sensory tuning beyond the sensory system: an initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J. Neurosci. Off. J. Soc. Neurosci 12, 2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux J, Clugnet MC, Pavlides C, 1993. Single-unit activity in the lateral nucleus of the amygdala and overlying areas of the striatum in freely behaving rats: rates, discharge patterns, and responses to acoustic stimuli. Behav. Neurosci 107, 757–769. 10.1037/0735-7044.107.5.757 [DOI] [PubMed] [Google Scholar]
- Cacciola A, Calamuneri A, Milardi D, Mormina E, Chillemi G, Marino S, Naro A, Rizzo G, Anastasi G, Quartarone A, 2017. A Connectomic Analysis of the Human Basal Ganglia Network. Front. Neuroanat 11, 85. 10.3389/fnana.2017.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy CM, Balsam PD, Weinstein JJ, Rosengard RJ, Slifstein M, Daw ND, Abi-Dargham A, Horga G, 2018. A Perceptual Inference Mechanism for Hallucinations Linked to Striatal Dopamine. Curr. Biol. CB 28, 503–514.e4. 10.1016/j.cub.2017.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibbaro G, Daniele M, Alagona G, Di Pasquale C, Cannavò M, Rapisarda V, Bella R, Pennisi G, 2005. Repetitive transcranial magnetic stimulation in schizophrenic patients reporting auditory hallucinations. Neurosci. Lett 383, 54–57. 10.1016/j.neulet.2005.03.052 [DOI] [PubMed] [Google Scholar]
- Clos M, Diederen KMJ, Meijering AL, Sommer IE, Eickhoff SB, 2014. Aberrant connectivity of areas for decoding degraded speech in patients with auditory verbal hallucinations. Brain Struct. Funct 219, 581–594. 10.1007/s00429-013-0519-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. Int. J 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cui L-B, Liu K, Li C, Wang L-X, Guo F, Tian P, Wu Y-J, Guo L, Liu W-M, Xi Y-B, Wang H-N, Yin H, 2016. Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr. Res 173, 13–22. 10.1016/j.schres.2016.02.039 [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM, 2007. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch. Gen. Psychiatry 64, 532–542. 10.1001/archpsyc.64.5.532 [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME, Balota DA, Tallal P, Petersen SE, 1996. PET Activation of Posterior Temporal Regions during Auditory Word Presentation and Verb Generation. Cereb. Cortex 6, 1–10. 10.1093/cercor/6.1.1 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MG, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders — Patient Edition (SCID-I/P, 11/2002 revision).
- Fitzgerald PB, Benitez J, Daskalakis JZ, Brown TL, Marston NAU, de Castella A, Kulkarni J, 2005. A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J. Clin. Psychopharmacol 25, 358–362. 10.1097/01.jcp.0000168487.22140.7f [DOI] [PubMed] [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, Potkin SG, Van Erp TGM, Turner JA, Mueller BA, Calhoun VD, Voyvodic J, Belger A, Bustillo J, Vaidya JG, Preda A, McEwen SC, Mathalon DH, 2015. Visual hallucinations are associated with hyperconnectivity between the amygdala and visual cortex in people with a diagnosis of schizophrenia. Schizophr. Bull 41, 223–232. 10.1093/schbul/sbu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilescu M, Rossell S, Stuart GW, Shea TL, Innes-Brown H, Henshall K, McKay C, Sergejew AA, Copolov D, Egan GF, 2010. Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol. Med 40, 1149–1158. 10.1017/S0033291709991632 [DOI] [PubMed] [Google Scholar]
- Geiser E, Notter M, Gabrieli JDE, 2012. A corticostriatal neural system enhances auditory perception through temporal context processing. J. Neurosci. Off. J. Soc. Neurosci 32, 6177–6182. 10.1523/JNEUROSCI.5153-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare SM, Ford JM, Ahmadi A, Damaraju E, Belger A, Bustillo J, Lee HJ, Mathalon DH, Mueller BA, Preda A, Erp V,M,TG, Potkin SG, Calhoun VD, Turner JA, 2017. Modality-dependent impact of hallucinations on low-frequency fluctuations in schizophrenia. Schizophr. Bull 43, 389–396. 10.1093/schbul/sbw093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare SM, Law AS, Ford JM, Mathalon DH, Ahmadi A, Damaraju E, Bustillo J, Belger A, Lee HJ, Mueller BA, Lim KO, Brown GG, Preda A, van Erp TGM, Potkin SG, Calhoun VD, Turner JA, 2018. Disrupted network cross talk, hippocampal dysfunction and hallucinations in schizophrenia. Schizophr. Res 199, 226–234. 10.1016/j.schres.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R, Fernandez T, Pittman B, Hampson M, 2012. Elevated functional connectivity along the corticostriatal loop and the mechanism of auditory verbal hallucinations in patients with schizophrenia. Biol. Psychiatry 69, 407–414. 10.1016/j.biopsych.2010.09.050.ELEVATED [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, Charney DS, 1999. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices.” Biol. Psychiatry 46, 130–132. 10.1016/S0006-3223(98)00358-8 [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Gueorguieva R, Hawkins KA, Varanko M, Boutros NN, Wu Y, Carroll K, Krystal JH, 2005. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol. Psychiatry 58, 97–104. 10.1016/j.biopsych.2005.03.041 [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, Krystal JH, 2003. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch. Gen. Psychiatry 60, 49–56. [DOI] [PubMed] [Google Scholar]
- Horga G, Abi-Dargham A, 2019. An integrative framework for perceptual disturbances in psychosis. Nat. Rev. Neurosci 20, 763–778. 10.1038/s41583-019-0234-1 [DOI] [PubMed] [Google Scholar]
- Horga Guillermo, Fernández-Egea E, Mané A, Font M, Schatz KC, Falcon C, Lomeña F, Bernardo M, Parellada E, 2014. Brain metabolism during hallucination-like auditory stimulation in schizophrenia. PloS One 9, e84987. 10.1371/journal.pone.0084987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga G, Schatz KC, Abi-Dargham A, Peterson BS, 2014. Deficits in predictive coding underlie hallucinations in schizophrenia. J. Neurosci 34, 8072–8082. 10.1523/JNEUROSCI.0200-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelström KM, Graziano MSA, 2017. The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia 105, 70–83. 10.1016/j.neuropsychologia.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Jardri R, Pouchet A, Pins D, Thomas P, 2011. Cortical activations during auditory verbal hallucinations in schizophrenia : A coordinate-based meta-analysis. Am. J. Psychiatry 168, 73–81. [DOI] [PubMed] [Google Scholar]
- Jiang L, Zuo X-N, 2016. Regional Homogeneity. The Neuroscientist 22, 486–505. 10.1177/1073858415595004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman-Fogel I, Hodaie M, Davis KD, 2012. Hemispheric Asymmetry in White Matter Connectivity of the Temporoparietal Junction with the Insula and Prefrontal Cortex. PLoS ONE 7. 10.1371/journal.pone.0035589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Luquin N, Obeso JA, 2012. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harb. Perspect. Med 2. 10.1101/cshperspect.a009621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Kim W, Chung Y-C, Jung K-H, Bahk W-M, Jun T-Y, Kim K-S, George MS, Chae J-H, 2005. A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci. Lett 376, 177–181. 10.1016/j.neulet.2004.11.048 [DOI] [PubMed] [Google Scholar]
- Lennox B, park S, Medley I, Morris P, Jones P, n.d. The functional anatomy of auditory hallucinations in schizophrenia - ScienceDirect. Psychiatry Res. Neuroimaging 100, 13–20. [DOI] [PubMed] [Google Scholar]
- Lim S-J, Fiez JA, Holt LL, 2014. How may the basal ganglia contribute to auditory categorization and speech perception? Front. Neurosci 8. 10.3389/fnins.2014.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T, 2006. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport 17, 19–22. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Bowie CR, Harvey PD, Twamley EW, Goldman SR, Jeste DV, Patterson TL, 2008. Usefulness of the UCSD performance-based skills assessment (UPSA) for predicting residential independence in patients with chronic schizophrenia. J. Psychiatr. Res 42, 320–327. 10.1016/j.jpsychires.2006.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy-Jones S, 2012. Hearing Voices: The Histories, Causes and Meanings of Auditory Verbal Hallucinations. Cambridge University Press, New York. [Google Scholar]
- McIntosh AM, Semple D, Tasker K, Harrison LK, Owens DGC, Johnstone EC, Ebmeier KP, 2004. Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Res. 127, 9–17. 10.1016/j.psychres.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Nenadic I, Gaser C, Volz H-P, Rammsayer T, Häger F, Sauer H, 2003. Processing of temporal information and the basal ganglia: new evidence from fMRI. Exp. Brain Res 148, 238–246. 10.1007/s00221-002-1188-4 [DOI] [PubMed] [Google Scholar]
- Oberhuber M, Hope TMH, Seghier ML, Parker Jones O, Prejawa S, Green DW, Price CJ, 2016. Four Functionally Distinct Regions in the Left Supramarginal Gyrus Support Word Processing. Cereb. Cortex N. Y. NY 26, 4212–4226. 10.1093/cercor/bhw251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR, 1962. The Brief Psychiatric Rating Scale. Psychol. Rep 10, 799–812. 10.2466/pr0.1962.10.3.799 [DOI] [Google Scholar]
- Pastor MA, Macaluso E, Day BL, Frackowiak RSJ, 2006. The neural basis of temporal auditory discrimination. NeuroImage 30, 512–520. 10.1016/j.neuroimage.2005.09.053 [DOI] [PubMed] [Google Scholar]
- Poulet E, Brunelin J, Bediou B, Bation R, Forgeard L, Dalery J, d’Amato T, Saoud M, 2005. Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol. Psychiatry 57, 188–191. 10.1016/j.biopsych.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes K. a., Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59, 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AR, Mathys C, Corlett PR, 2017. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 357, 596–600. 10.1126/science.aan3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvvada KC, Summerfelt A, Du X, Krishna N, Kochunov P, Rowland LM, Simon JZ, Hong LE, 2018. Delta Vs Gamma Auditory Steady State Synchrony in Schizophrenia. Schizophr. Bull 44, 378–387. 10.1093/schbul/sbx078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA, 2004. Functional dissociations within the inferior parietal cortex in verbal working memory. NeuroImage. 10.1016/j.neuroimage.2004.01.039 [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Hazeltine E, Ruiz S, Zhu DC, 2011. Left TPJ activity in verbal working memory: Implications for storage- and sensory-specific models of short term memory. NeuroImage 55, 1836–1846. 10.1016/j.neuroimage.2010.12.021 [DOI] [PubMed] [Google Scholar]
- Reale RA, Imig TJ, 1983. Auditory cortical field projections to the basal ganglia of the cat. Neuroscience 8, 67–86. 10.1016/0306-4522(83)90026-x [DOI] [PubMed] [Google Scholar]
- Saba G, Verdon CM, Kalalou K, Rocamora JF, Dumortier G, Benadhira R, Stamatiadis L, Vicaut E, Lipski H, Januel D, 2006. Transcranial magnetic stimulation in the treatment of schizophrenic symptoms: a double blind sham controlled study. J. Psychiatr. Res 40, 147–152. 10.1016/j.jpsychires.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Sartorius N, Jablensky A, Korten A, Ernberg G, Anker M, Cooper JE, Day R, 1986. Early manifestations and first-contact incidence of schizophrenia in different cultures. A preliminary report on the initial evaluation phase of the WHO Collaborative Study on determinants of outcome of severe mental disorders. Psychol. Med 16, 909–928. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Pandya D, 2006. Fiber Pathways of the Brain. Oxford University Press, New York. [Google Scholar]
- Schultz W, 2016. Reward functions of the basal ganglia. J. Neural Transm 123, 679–693. 10.1007/s00702-016-1510-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Bullmore ET, Brammer MJ, Williams SCR, Murray RM, McGUIRE PK, 2001. A functional study of auditory verbal imagery. Psychol. Med 31, 241–253. 10.1017/S003329170100335X [DOI] [PubMed] [Google Scholar]
- Shinn AK, Baker JT, Cohen BM, Ongur D, 2013. Functional connectivity of left Heschl’s gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr. Res 143, 260–268. 10.1016/j.schres.2012.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, 1995. A functional neuroanatomy of hallucinations in schizophrenia. Nature 378, 176–179. 10.1038/378176a0 [DOI] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, van Lutterveld R, Hoek HW, Sommer IEC, 2014. Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol. Psychiatry, Schizophrenia and Bipolar Disorder: New Insights into Novel Mechanisms 76, 101–110. 10.1016/j.biopsych.2013.09.038 [DOI] [PubMed] [Google Scholar]
- Sommer IE, Clos M, Meijering AL, Diederen KMJ, Eickhoff SB, 2012. Resting state functional connectivity in patients with chronic hallucinations. PLoS ONE 7, 3–10. 10.1371/journal.pone.0043516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N, Rossell SL, Waters F, 2015. The Changing Face of Hallucination Research: The International Consortium on Hallucination Research (ICHR) 2015 Meeting Report: Table 1. Schizophr. Bull sbv183–sbv183. 10.1093/schbul/sbv183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, den Boer J. a., Liemburg EJ, Aleman A, 2010. Auditory Hallucinations in Schizophrenia Are Associated with Reduced Functional Connectivity of the Temporo-Parietal Area. Biol. Psychiatry 67, 912–918. 10.1016/j.biopsych.2009.11.017 [DOI] [PubMed] [Google Scholar]
- Viñas-Guasch N, Wu YJ, 2017. The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct. Funct 222, 3991–4004. 10.1007/s00429-017-1450-y [DOI] [PubMed] [Google Scholar]
- Wang S, Wang G, LV H, Wu R, Zhao J, Guo W, n.d. Abnormal regional homogeneity as a potential imaging biomarker for adolescent-onset schizophrenia: A resting-state fMRI study and support vector ma… - PubMed - NCBI [WWW Document] URL https://www.ncbi.nlm.nih.gov/pubmed/28587813 (accessed 12.10.19). [DOI] [PubMed]
- Waters F, Collerton D, Ffytche DH, Jardri R, Pins D, Dudley R, Blom JD, Mosimann UP, Eperjesi F, Ford S, Larøi F, 2014. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr. Bull 40 Suppl 4, S233–45. 10.1093/schbul/sbu036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1999. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation, New York, NY. [Google Scholar]
- Woerner MG, Mannuzza S, Kane JM, 1988. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol. Bull 24, 112–117. [PubMed] [Google Scholar]
- Woods SW, 2003. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry 64, 663–667. [DOI] [PubMed] [Google Scholar]
- Xiao B, Wang S, Liu J, Meng T, He Y, Luo X, 2017. Abnormalities of localized connectivity in schizophrenia patients and their unaffected relatives: a meta-analysis of resting-state functional magnetic resonance imaging studies. Neuropsychiatr. Dis. Treat 13, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Hsieh M, Wang H-L, Liu C-M, Liu C-C, Huang T-J, Chien Y-L, Hwu H-G, Tseng W-Y, 2013. Frequency dependent alterations in regional homogeneity of baseline brain activity in schizophrenia. - PubMed - NCBI [WWW Document] URL https://www.ncbi.nlm.nih.gov/pubmed/23483911 (accessed 12.10.19). [DOI] [PMC free article] [PubMed]
- Zang Y, Jiang T, Lu Y, He Y, Tian L, 2004. Regional homogeneity approach to fMRI data analysis. NeuroImage 22, 394–400. 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- Znamenskiy P, Zador AM, 2013. Corticostriatal neurones in auditory cortex drive decisions during auditory discrimination. Nature 497, 482–485. 10.1038/nature12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


