Abstract
Background:
Despite the well-established association between T cell-mediated inflammation and non-ischemic heart failure (HF), the specific mechanisms triggering T cell activation during the progression of HF and the antigens involved are poorly understood. We hypothesized that myocardial oxidative stress induces the formation of isolevuglandin (IsoLG)-modified proteins that function as cardiac neoantigens to elicit CD4+ T cell receptor (TCR) activation and promote HF.
Methods:
We used transverse aortic constriction (TAC) in mice to trigger myocardial oxidative stress and T cell infiltration. We profiled the TCR repertoire by mRNA sequencing of intramyocardial activated CD4+ T cells in Nur77GFP reporter mice, which transiently express GFP upon TCR engagement. We assessed the role of antigen presentation and TCR specificity in the development of cardiac dysfunction using antigen presentation-deficient MhcII−/− mice, and TCR transgenic OTII mice that lack specificity for endogenous antigens. We detected IsoLG-protein adducts in failing human hearts. We also evaluated the role of reactive oxygen species (ROS) and IsoLGs in eliciting T cell immune responses in vivo by treating mice with the antioxidant TEMPOL, and the IsoLG scavenger 2-hydroxybenzylamine (2-HOBA) during TAC, and ex-vivo in mechanistic studies of CD4+ T cell proliferation in response to IsoLG-modified cardiac proteins.
Results:
We discovered that TCR antigen recognition increases in the left ventricle (LV) as cardiac dysfunction progresses, and identified a limited repertoire of activated CD4+ T cell clonotypes in the LV. Antigen presentation of endogenous antigens was required to develop cardiac dysfunction since MhcII−/− mice reconstituted with CD4+ T cells, and OTII mice immunized with their cognate antigen were protected from TAC-induced cardiac dysfunction despite the presence of LV-infiltrated CD4+ T cells. Scavenging IsoLGs with 2-HOBA reduced TCR activation and prevented cardiac dysfunction. Mechanistically, cardiac pressure overload resulted in ROS dependent dendritic cell accumulation of IsoLG-protein adducts which induced robust CD4+ T cell proliferation.
Conclusions:
Collectively, our study demonstrates an important role of ROS-induced formation of IsoLG-modified cardiac neoantigens that lead to TCR-dependent CD4+ T cell activation within the heart.
Keywords: Inflammation, Heart Failure, Isolevuglandins, Oxidative Stress
Introduction
Recent evidence has implicated innate and adaptive immune mechanisms in maladaptive cardiac remodeling that develops in response to cardiac pressure overload.1 Several studies utilizing transverse aortic constriction (TAC) have demonstrated that CD4+ T cells, infiltrate the left ventricle (LV) and promote adverse cardiac remodeling.2–4 CD4+ T cell deficient mice, including Tcra−/−, MhcII−/− and Rag2−/−, retain normal cardiac function in the onset of TAC and do not develop adverse LV remodeling.2, 5 However, the exact trigger of CD4+ T cell activation remains poorly understood. Classic naïve CD4+ T cell clonal expansion and proliferation occur when the T cell receptor (TCR) encounters its cognate antigen presented by major histocompatibility complex class II (MHCII) on the surface of dendritic cells (DCs) in draining lymph nodes. Macrophages and B cells can further activate CD4+ T cells to sustain immune responses at sites of inflammation.6 Depletion of CD11c+ DCs and blockade of T-cell costimulatory molecules significantly attenuates LV fibrosis and hypertrophy due to prolonged exposure to TAC,7, 8 suggesting that CD4+ T cell activation is likely dependent on this classic activation pathway. However, the nature and location of the antigens initiating and sustaining CD4+ T cell activation during the progression of HF remains unknown. Growing evidence highlights a role for myocardial reactive oxygen species (ROS) in the development of adverse cardiac remodeling.9, 10, 11 ROS can induce non-enzymatic peroxidation of arachidonic acid and the formation of highly reactive Isolevuglandins (IsoLGs), which covalently adduct to lysine residues and alter protein structure and function.12 IsoLG-protein adducts have been reported to be presented by DCs to elicit T cell activation in angiotensin II-induced hypertension, yet their role in cardiac remodeling and function is unknown.13, 14
We hypothesized that ROS-induced IsoLGs modify cardiac proteins, which trigger TCR-activation and clonal expansion of CD4+ T cells that progressively induce cardiac dysfunction. We identify a limited repertoire of activated LV infiltrated CD4+ T cells in response to cardiac pressure overload and elucidate the mechanism for CD4+ T cell activation, which involves the formation of IsoLG-cardiac protein neontigens presented by DCs to elicit CD4+ T cell activation. Additionally, we demonstrate the benefit of scavenging IsoLGs in preventing CD4+ T cell activation and cardiac dysfunction.
Methods
A detailed description of methods can be found in the Supplemental Materials. Raw and processed sequencing data have been deposited in GEO (Gene Expression Omnibus) repository under accession number GSE161172. All other data, methods, and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Mice
All animal studies were approved by the Tufts University Institutional Animal Care and Use Committee. Mice were bred and maintained under pathogen-free conditions at Tufts University animal facilities and treated in compliance with the Guide for the Care and Use of Laboratory Animals (National Academy of Science). C57BL/6 wild-type (wt), Nur77GFP, OTII, MhcII−/− mice (all purchased from Jackson Laboratories and bred and maintained in house) were euthanized at 9–18 weeks of age for tissue collection.
Human subjects
Human studies were approved by Tufts University IRB. Subjects gave informed consent and de-identified viable LV free wall tissue from control subjects (n = 2) was obtained from the National Disease Research Interchange (NDRI), and from end-stage HF subjects after LV Assist Device (LVAD) support (n = 3).
Statistics
A priori power calculation using G* Power software assuming α of 0.05 to obtain 80% power was used in our studies to determine sample size. Data are presented as the mean ± SD. Statistical analyses were done by nonparametric Mann-Whitney test (2-tailed) to adjust for nonequal Gaussian distribution when comparing two groups. Multiple group comparisons were performed by one way ANOVA with Bonferroni posttest or two-way ANOVA with two categorical grouping variables where indicated using GraphPad Prism software (Graphpad). Differences were considered statistically significant at *p≤ 0.05, **p≤ 0.01, ***p≤ 0.001.
Results
A limited repertoire of CD4+ T cell clonotypes respond to endogenous cardiac antigens in response to cardiac pressure overload
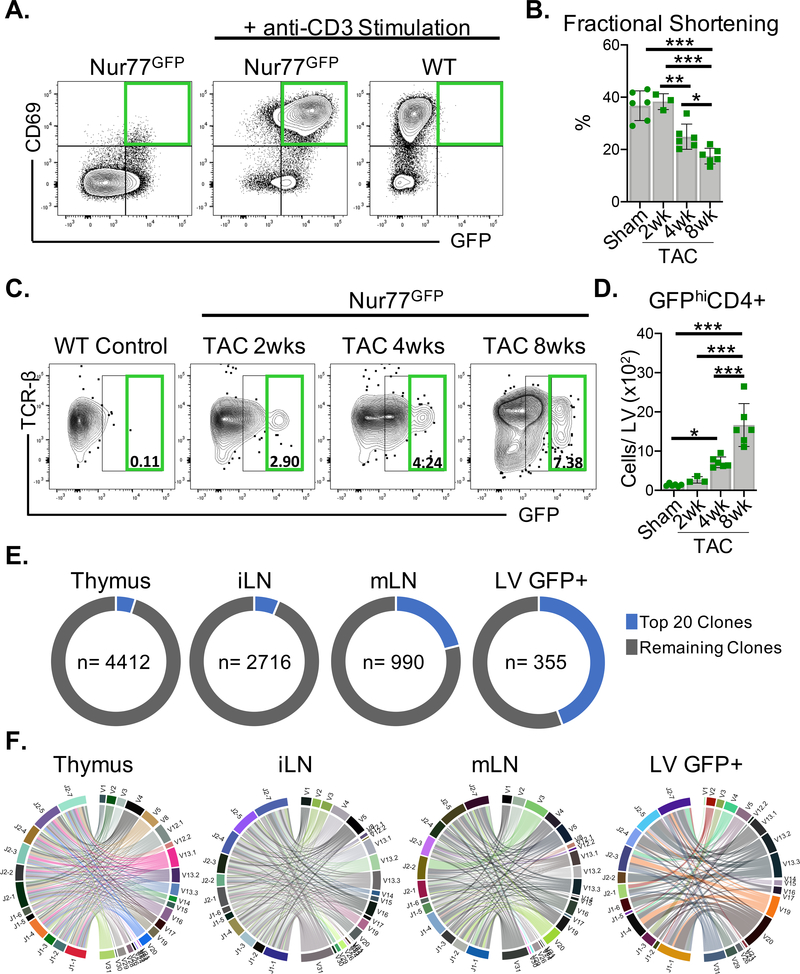
We utilized T cell activation reporter Nur77GFP mice, which transiently express GFP exclusively downstream of TCR signaling, with the intensity of the GFP signal indicating the strength of TCR stimulation to map T cell activation in the left ventricle (LV).15 We validated this reporter system in vitro by anti-CD3 stimulation of CD4+ T cells (Figure. 1A). Remarkably, GFPhiCD4+ T cells were present in the LV as early as 2 weeks after TAC and increased in number as fractional shortening progressively declined over 8 weeks (Figure. 1, B–D). The TCR alpha and beta chains are encoded by multiple V, D, and J gene segments that undergo somatic recombination to produce a diverse repertoire of T cells with unique TCRs capable of recognizing a vast range of antigens. In response to cognate antigen, CD4+ T cells clonally expand and skew the diverse TCR repertoire towards antigen-specific clonotypes. We performed TCR clonal analysis of 5000 TCR-activated GFP+CD4+ T cells sorted from the LV, and 5000 CD4+ T cells sorted from the thymus, the inguinal lymph nodes (iLNs) and the heart-draining mediastinal lymph nodes (mLNs) by bulk RNA sequencing of the TCR β chain 8wks after TAC. As expected, the greatest number of unique TCR clonotypes (4412 clones) – and therefore the highest TCR diversity – was identified in the thymus. The mLNs showed a much lower degree of TCR clonal diversity relative to the peripheral iLNs, suggesting cardiac antigen-driven clonal expansion (Figure. 1E). Strikingly, TCR-activated GFP+CD4+ T cells in the LV represented the lowest TCR diversity relative to other sites, with the frequency of the top 20 most abundant unique TCR clonotypes accounting for 44% of this CD4+ T cell population (Figure. 1E, Table 1). Chord diagrams depict the distribution of V-J gene segment combinations for each organ, with each colored chord connecting unique V-J pairs representing a set of clonotypes, with the thickness proportionately scaled to the relative abundance of clones using these gene segment pairs (Figure. 1F). Taken together, these data demonstrate that a limited repertoire of CD4+ T cells increasingly engage antigens in the heart as systolic function decays.
Figure 1. CD4+ T cells are activated via the TCR in the LV in response to TAC.
(A) CD4+ T cells isolated from wt or Nur77GFP mice were stimulated with immobilized anti-CD3 (5μg/mL) in vitro for 72 hours where indicated. GFP expression and the control T cell activation marker CD69 were evaluated by FACS. (B-F) Nur77GFP mice underwent Sham surgery for 8wks and TAC surgery for 2–8wks. (B) Transthoracic echocardiography was used to measure LV fractional shortening. (C-D) Leukocytes were isolated from the LV and the total number of GFPhiCD4+ T cells was quantified by FACS. (E-F) Bulk TCR clonal analysis after 8wks of TAC surgery (n=3 TAC Nur77GFP mice pooled) was performed on 5000 CD4+ T cells sorted from the thymus, inguinal lymph nodes (iLN), mediastinal lymph nodes (mLN) and 5000 GFP+CD4+ T cells sorted from the LV by next generation RNA sequencing. (E) The frequency of the most abundant 20 clones from each site are represented in the pie graph along with total number (n) of unique clones identified. (F) CD4+ T cell clonotypes are represented in chord diagrams, with ribbons proportionately scaled by V-J pair frequency connecting segment pairs. Error bars represent mean ± SD. (* p<0.05, ** p<0.01, *** p<0.001; one-way ANOVA test).
Antigen presentation to CD4+ T cells by Major histocompatibility complex class II (MHCII) is required for cardiac dysfunction to develop in response to cardiac pressure overload.
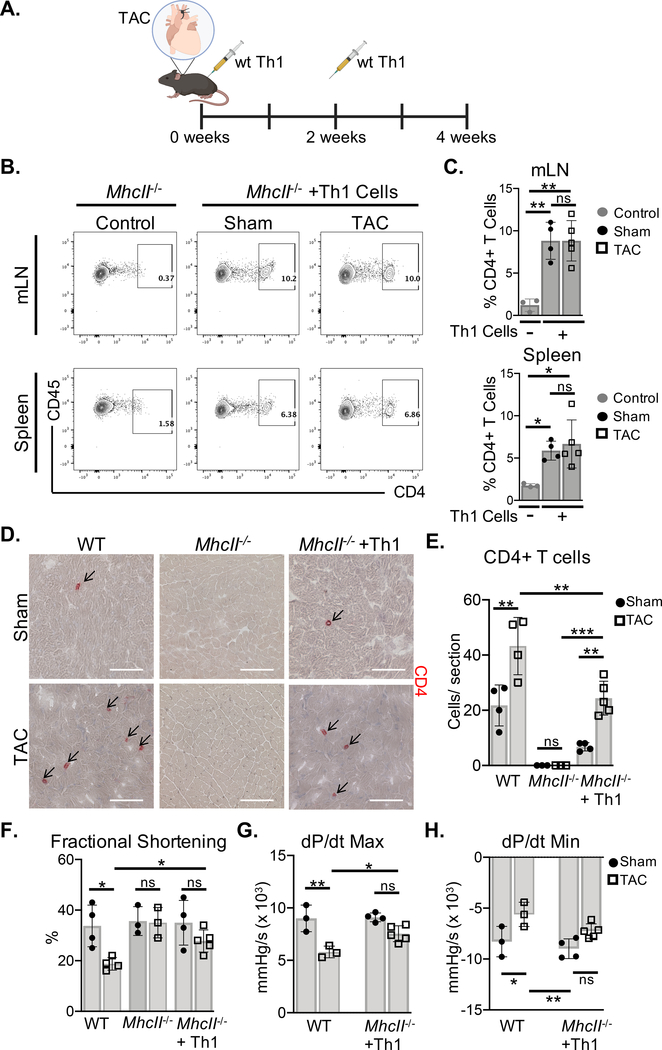
We next evaluated whether CD4+ Th1 cells, known to infiltrate the LV and induce adverse cardiac remodeling in response to cardiac pressure overload,3 promote cardiac dysfunction in the absence of MHCII antigen presentation. MhcII−/− mice lack both CD4+ T cells and the ability to present antigen to CD4+ T cells, and are protected from pressure overload-induced cardiac dysfunction.2, 5 We adoptively transferred wt Th1 cells to MhcII−/− mice in the onset of TAC (Figure. 2A). The reconstitution of MhcII−/− mice was confirmed by identifying donor CD4+ T cells in the recipient mediastinal lymph nodes (mLNs) and spleen, demonstrating similar CD4+ T cell frequencies in Sham and TAC mice (Figure. 2B–C). However, CD4+ T cell infiltration into the LV was increased in TAC mice as compared to Sham mice, which is also observed in wt (MhcII sufficient) mice (Figure. 2D–E). However, while LV CD4+ T cell infiltration resulted in a decline in % fractional shortening in wt mice, LV CD4+ T cell infiltration in MhcII−/− mice was not sufficient to significantly impair cardiac contractile function (Figure. 2F, Table I in the Supplement). As expected, MhcII−/− mice without donor Th1 cells showed no evidence of LV CD4+ T cell infiltration and preservation of fractional shortening in response to TAC (Figure. 2D–F, Table I in the Supplement). Furthermore, the cardiac contractility (dP/dt max) and relaxation (dP/dt min) indicies were not significantly altered in Th1-recipient MhcII−/− mice with TAC, but were significantly reduced in wt mice with TAC (Figure. 2G–H, Table II in the Supplement). Thus, MHCII-mediated antigen presentation to CD4+ T cells is required for the development of cardiac dysfunction induced by TAC.
Figure 2. MhcII−/− mice reconstituted with Th1 cells do not develop cardiac dysfunction in response to TAC.
(A) Wt Th1 cells were differentiated in vitro and transferred to MhcII−/− mice via intraperitoneal injection, 2 days and 2 weeks after Sham and TAC. surgery. (B-C) The reconstitution with CD4+ T cells was evaluated in the mediastinal lymph nodes (mLNs) and spleen by FACS, and compared to control MhcII−/− mice that did not receive Th1 cells. (D-E) Frozen LV tissue sections isolated from wt, MhcII−/−, and MhcII−/− mice reconstituted with Th1 cells, 4 weeks after Sham or TAC surgery were used to determine CD4+ T cell LV infiltration. (F) Transthoracic echocardiography was used to measure LV fractional shortening from wt, MhcII−/−, and MhcII−/− mice reconstituted with Th1 cells. (G-H) LV hemodynamic measurements were acquired to determine dP/dt max and dP/dt min as parameters of cardiac contractility and relaxation respectively in MhcII−/− mice reconstituted with Th1 cells compared to untreated wt mice. Scale bars: 100μm. Error bars represent mean ± SD. (* p<0.05, ** p<0.01, *** p<0.001; one-way ANOVA test, two-way ANOVA with two categorical grouping variables).
OTII CD4+ T cells in the LV do not promote cardiac dysfunction in response to cardiac pressure overload.
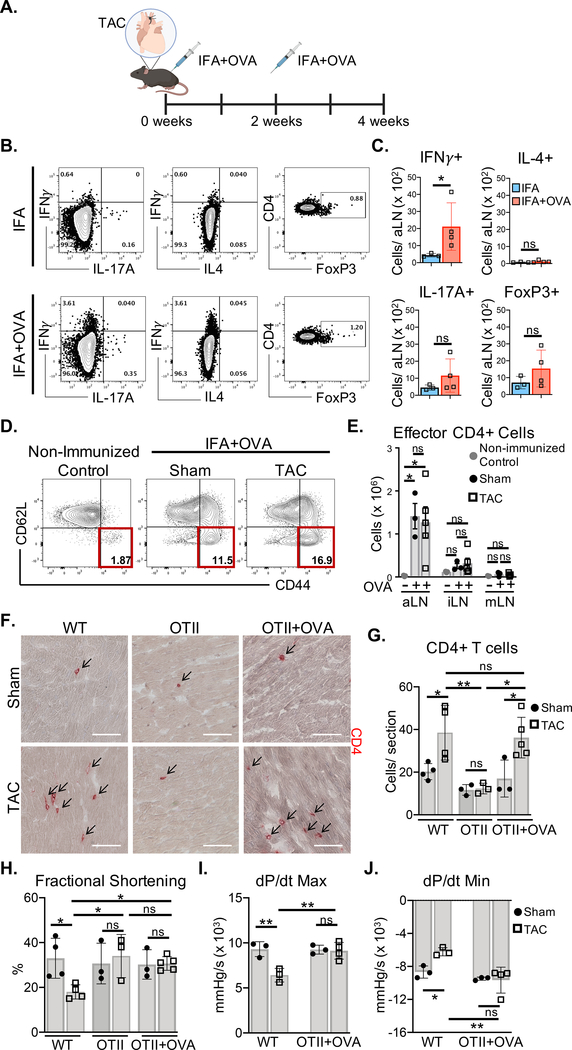
We next investigated whether the presentation of endogenous cardiac antigens to CD4+ T cells is necessary to induce cardiac dysfunction using TCR transgenic OTII mice, with a CD4+ T cell repertoire restricted to specificity for exogenous chicken ovalbumin peptide (OVA). OVA immunization of OTII mice induced IFNγ+ CD4+ T cell expansion in the axillary lymph nodes (aLN) draining the OVA immunization site, but not IL-4+ or IL-17A+ CD4+ T cells, or regulatory T cells (Tregs) (Figure. 3A–C). Additionally, CD62LloCD44hi effector CD4+ T cells, were equally generated in Sham and TAC aLNs (Figure. 3D–E). Interestingly, CD4+ T cell activation was not detected in the mLN in spite of cardiac pressure overload, validating the TCR specificity in OTII mice for OVA, and the lack of TCR specificity for endogenous antigens induced by TAC (Figure. 3E). Non-immunized OTII mice lacked CD4+ T cell infiltration into the LV in response to TAC, and showed preserved fractional shortening (Figure. 3F–H, Table I in the Supplement). Remarkably, LV CD4+ T cell recruitment was observed in OVA-immunized OTII mice in response to TAC and was comparable to wt TAC mice (Figure. 3F–G). However, fractional shortening, dP/dt max and dP/dt min were preserved in contrast to wt TAC mice (Figure. 3H–J, Tables I and II in the Supplement). These data demonstrate that although TCR-stimulated OTII CD4+ T cells have tropism for the heart in TAC mice, a TCR response to endogenous cardiac antigens is required to promote cardiac dysfunction.
Figure 3. Activated OVA-specific CD4+ T cells recruited to the LV of OTII mice do not mediate cardiac dysfunction in response to TAC.
(A) OTII mice were immunized with ovalbumin peptide (OVA) emulsified in Incomplete Freund’s adjuvant (IFA), 2 days and 2 weeks after Sham and TAC surgery. (B-C) Cytokine production and FoxP3 expression was evaluated in CD4+ T cells from the draining axillary lymph nodes (aLNs) of immunized OTII mice by intracellular staining after 3–5hrs of PMA/ionomycin stimulation. (D-E) 4 weeks after surgery CD4+ T cell activation was evaluated by quantifying the number of effector (CD62Llo CD44hi) CD4+ T cells in the draining axillary lymph nodes (aLN) as well as the inguinal lymph nodes (iLN) and mediastinal lymph nodes (mLNs) by FACS. (F-G) IHC in frozen LV tissue sections was used to determine CD4+ T cell infiltration in wt mice, OTII mice, and OTII mice immunized with OVA, 4 weeks after Sham and TAC surgeries. (H) Transthoracic echocardiography was used to measure LV fractional shortening (I-J) LV hemodynamic measurements were acquired to determine dP/dt max and dP/dt min in OTII mice immunized with OVA and wt mice, 4 weeks after Sham and TAC surgeries. Scale bars: 100 μm. Error bars represent mean ± SD. (* p<0.05, ** p<0.01; one-way ANOVA test, two-way ANOVA with two categorical grouping variables).
Cardiac pressure overload promotes LV oxidative stress and Isolevuglandin (IsoLG)-protein adduct formation.
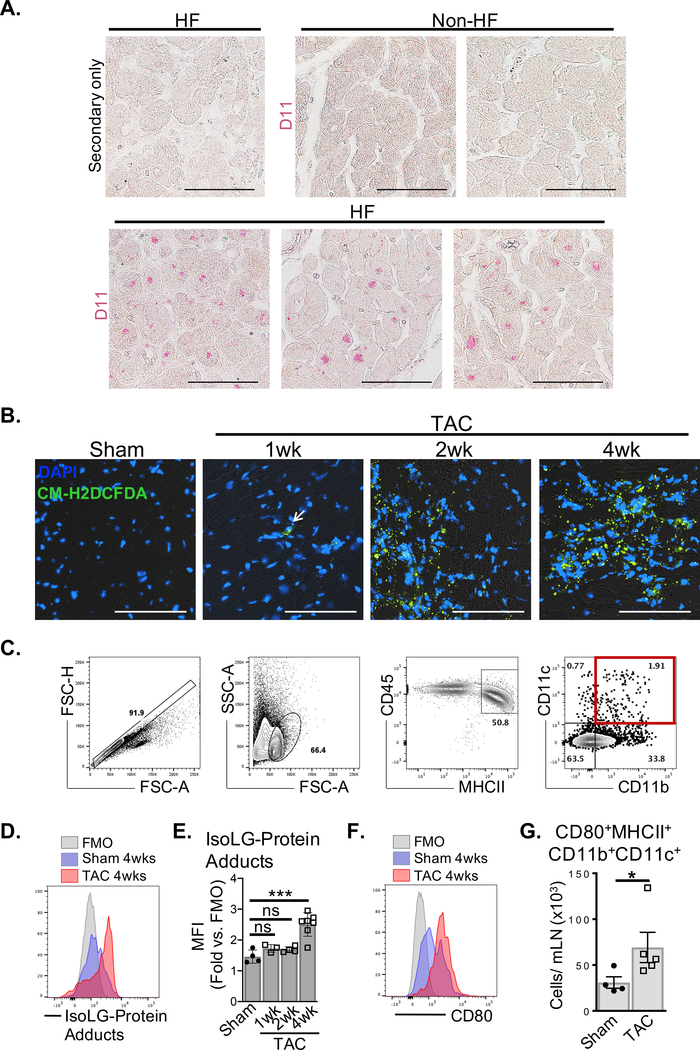
We next focused on Isolevuglandins (IsoLGs) formed by reactive oxygen species (ROS) peroxidation of arachidonic acid, which modify proteins by covalently binding to lysine,13 potentially forming cardiac neoantigens responsible for TCR activation of CD4+ T cells. Using D11, a single chain fragment variable (scfv) recombinant antibody against IsoLG-protein adducts, we identified IsoLG-protein adduct accumulation in the LV of end-stage nonischemic HF patients, but not non-HF controls (Figure 4A). We next tested whether cardiac pressure overload progressively induces myocardial ROS and promotes DC presentation of IsoLG-modified proteins to CD4+ T cells in the mLNs of wt mice. Intracellular ROS was undetectable in murine Sham LV tissue sections but was detected as early as 1 week after TAC by immunofluorescence, with stronger ROS signals detected after 2 and 4 weeks (Figure 4B). Moreover, myocardial ROS was associated with increased accumulation of IsoLG-protein adducts in CD45+MHCII+CD11b+CD11c+ DCs in the mLN after 4 weeks of TAC (Figure 4C–E), and these expressed significantly increased CD80 required for T cell costimulation compared to Sham mice (Figure. 4F–G). These data demonstrate that cardiac pressure overload promotes oxidative stress and the formation of IsoLG-protein adducts in the heart, and migration of DCs loaded with IsoLG-protein adducts to the mLN.
Figure 4. HF is associated with increased accumulation of Isolevuglandin (IsoLG)-protein adducts.
(A) Fixed LV tissue sections from non-HF (n=2) and end-stage nonischemic HF patients (n=3) were probed for the presence of IsoLG-protein adducts with D11 scfv recombinant antibody. (B) Frozen LV tissue sections from wt Sham (4wks post-surgery) and TAC (1wk, 2wk and 4wk post-surgery) mice were probed for intracellular ROS by CM-H2DCFDA. (Images representative of n≥3 mice each) (C) CD45+MHCII+CD11b+CD11c+ DCs isolated from the mLNs of wt Sham and TAC mice were identified by FACS. (D-E) DCs mice were then probed for intracellular IsoLG-protein adducts with D11 scfv recombinant antibody and quantified. (F-G) The total number of mature DCs expressing CD80 in wt Sham and TAC mLNs was quantified by FACS. Scale bars: 100μm. Error bars represent mean ± SD. (* p<0.05, *** p<0.001; Mann-Whitney test, one-way ANOVA test for comparison of 3 or more groups).
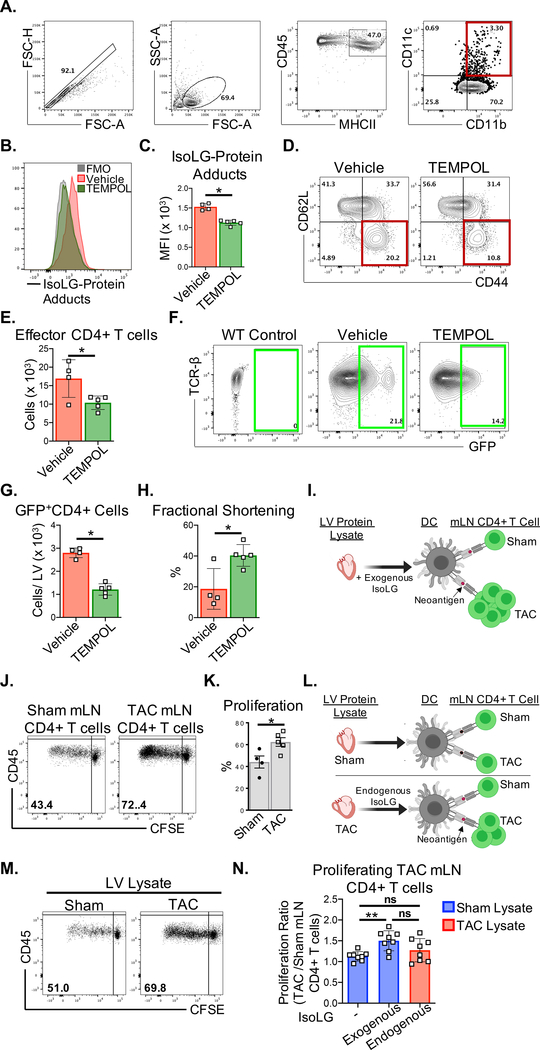
Cardiac oxidative stress promotes CD4+ T cell responses to IsoLG-modified proteins and cardiac dysfunction
To assess the role of ROS in CD4+ T cell activation in the LV and mLN, we treated Nur77GFP mice with the antioxidant TEMPOL (4-Hydroxy-TEMPO), a membrane-permeable radical scavenger and superoxide dismutase-mimetic. CD45+MHCII+CD11b+CD11c+ DCs in the mLN of TEMPOL treated mice showed decreased accumulation of IsoLG-protein adducts in response to TAC, compared to vehicle treatment (Figure 5A–C), as well as decreased CD62LloCD44hi effector CD4+ cells in the mLN (Figure. 5D–E). Moreover, GFP+CD4+ cells in the LV of TEMPOL treated Nur77GFP mice were significantly decreased, indicating reduced TCR engagement by cardiac antigens (Figure. 5F–G). Importantly, TEMPOL treatment resulted in preserved cardiac contractile function, in contrast to the significant decline in fractional shortening observed in the vehicle treatment group (Figure. 5H, Table I in the Supplement). To determine if IsoLG-modified cardiac proteins presented by DCs elicit CD4+ T cell responses in the mLN during TAC, we exposed mLN CD4+ T cells from wt Sham and TAC mice to DCs loaded with murine LV protein lysate treated with exogenous IsoLGs (Figure 5I). CD4+ T cells derived from TAC mLNs showed significantly higher proliferation in response to IsoLG-modified LV protein lysate, compared to Sham derived mLN CD4+ T cells (Figure 5J–K). We further evaluated mLN CD4+ T cell proliferation in response to endogenous IsoLG modified LV protein lysate from TAC mice (Figure 5L). Although there was a trend towards increased proliferation of TAC mLN CD4+ T cells in response to DCs loaded with TAC LV protein lysate compared to Sham LV protein lysate (Figure 5M), there was significantly higher proliferation when these cells were exposed to DCs loaded with LV proteins treated with exogenous IsoLGs (Figure 5N). This difference may reflect the lower concentrations of endogenous IsoLG-protein adducts in the TAC LV relative to the exogenous IsoLG (100μM) treatment of LV protein lysate in vitro. These data combined demonstrate that ROS-induced IsoLG-modified proteins function as neoantigens that elicit CD4+ T cell responses in the onset of cardiac pressure overload.
Figure 5. Oxidative stress during TAC promotes LV CD4+ T cell activation in response to IsoLG-modified proteins.
(A-H) Nur77GFP mice were treated orally with the antioxidant 4-hydroxy-TEMPO (TEMPOL) administered ad libitum in the drinking water (1mM) for 4 weeks post TAC surgery. (A-C) CD45+MHCII+CD11b+CD11c+ DCs were isolated from the heart-draining mLNs of vehicle and TEMPOL treated mice and probed for intracellular IsoLG-protein adducts by FACS using D11 scfv recombinant antibody. (D-E) CD62LloCD44hi effector CD4+ T cells in the mLNs were quantified by FACS. (F-G) Total GFP+CD4+ T cells from the whole LV of vehicle and TEMPOL treated Nur77GFP mice were quantified by FACS (H) Transthoracic echocardiography was used to measure LV fractional shortening. (I) CD4+ T cells were purified from the mLN of wt Sham and TAC surgery mice, and cocultured with BMDCs pulsed with 100μg/mL of Sham LV protein lysate pretreated with IsoLGs (100μM). (J-K) CD4+ T cell proliferation was quantified after 72hrs based on the CFSE dilution in mLN CD4+ T cells. (L-N) BMDCs were pulsed with 100μg/mL of Sham or TAC LV protein lysate, or Sham LV lysate pretreated with 100μM of IsoLG and co-cultured with CFSE-labeled mLN CD4+ T cells from Sham and TAC surgery mice. Normalized data are represented as the fold change in proliferation of TAC mLN CD4+ T cells relative to Sham mLN CD4+ T cells in response to the indicated LV protein lysate conditions. Error bars represent mean ± SD. (* p<0.05, ** p<0.01; Mann-Whitney test, One-way ANOVA test for comparison of 3 or more groups).
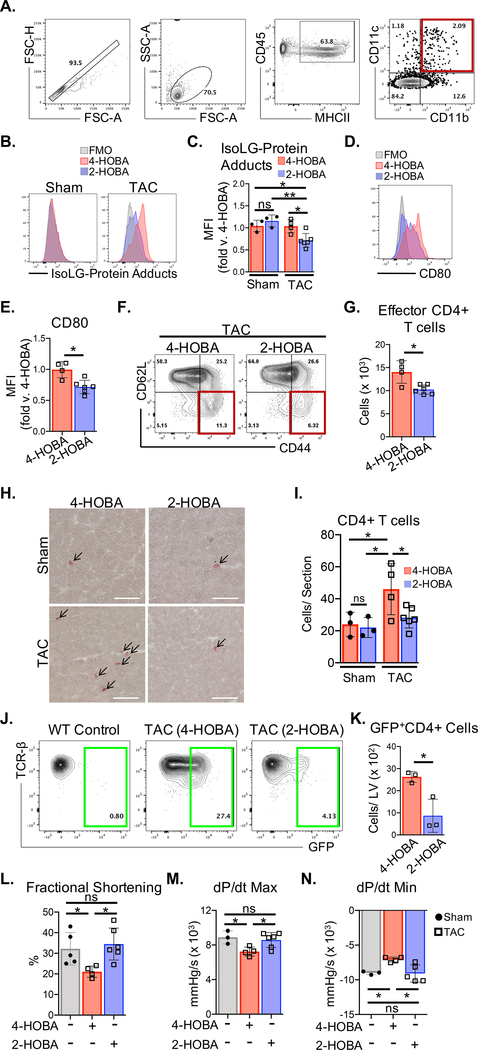
Scavenging IsoLGs inhibits CD4+ T cell activation in the mLN and TCR engagement in the LV and prevents cardiac dysfunction.
The formation of IsoLG-protein adducts can be prevented by IsoLG scavengers such as 2-hydroxybenzylamine (2-HOBA),13 which preferentially reacts with IsoLGs 1000 times faster than lysine. 4-hydroxybenzylamine (4-HOBA), an isomer of 2-HOBA with much less reactivity towards IsoLGs was used as a control. 2-HOBA treatment significantly decreased the intracellular accumulation of IsoLG-protein adducts in CD45+MHCII+CD11b+CD11c+ mLN DCs from wt TAC mice, while not significantly altering baseline levels in Sham mice (Figure. 6A–C). DCs from 2-HOBA treated TAC mice also showed decreased CD80 expression compared to 4-HOBA treated control mice (Figure 6, D–E). Importantly, neither 2-HOBA nor 4-HOBA directly impaired DC maturation (CD80 expression) in vitro (Figure I in the Supplement). 2-HOBA treatment significantly decreased CD62LloCD44hi CD4+T cells in the mLN (Figure. 6F–G), and reduced CD4+ T cell infiltration into the LV (Figure. 6H–I). Remarkably, 2-HOBA treatment of Nur77GFP mice resulted in significantly fewer GFP+CD4+ T cells in the LV, indicating that not only is CD4+ T cell infiltration into the LV less frequent, but active engagement of antigens within the LV is also diminished (Figure. 6J–K). Fractional shortening, dP/dt max, and dP/dt min were preserved in 2-HOBA treated wt mice after 4 weeks of TAC, in contrast to the 4-HOBA control treatment group (Figure. 6J–N, Tables I and II in the Supplement). These results demonstrate that CD4+ T cell TCR recognition of IsoLG-modified cardiac neoantigens is critical for the development of cardiac dysfunction in response to TAC and can effectively be targeted by the IsoLG scavenger 2HOBA.
Figure 6. The IsoLG scavenger 2-HOBA reduces CD4+ T cell activation and ameliorates cardiac dysfunction due to TAC.
Mice were treated orally with the IsoLG scavenger 2-HOBA and the control drug 4-HOBA administered ad libitum in the drinking water (1g/L) for 4 weeks after TAC surgery. (A) CD45+MHCII+CD11b+CD11c+ DCs were isolated from the mLNs of wt mice and (B-C) intracellular IsoLG-protein adduct accumulation was measured using D11 scfv recombinant antibody, and (D-E) CD80 surface expression was quantified by FACS. (F-G) Total CD62LloCD44hi effector CD4+ T cells in the mLNs of wt TAC mice were quantified by FACS. (H-I) IHC was used to determine CD4+ T cell infiltration in frozen LV tissue sections from wt mice. (J-K) Total GFP+CD4+ T cells from LV tissues of Nur77GFP mice treated with 2-HOBA and 4-HOBA were quantified by FACS, 4 weeks after TAC surgery. (L) Transthoracic echocardiography was used to measure LV fractional shortening and (M-N) LV hemodynamic measurements were acquired to determine dP/dt max and dP/dt min, comparing untreated wt Sham mice to wt TAC mice treated with 4-HOBA and 2-HOBA. Scale bars: 100μm. Error bars represent mean ± SEM. (* p<0.05, ** p<0.01; Mann-Whitney test, One-way ANOVA test for comparison of 3 or more groups).
Discussion
Several recent studies have demonstrated a critical role of CD4+ T cells in the initiation and progression of pressure overload-induced cardiac dysfunction.2, 3, 5, 16 However, the mechanisms triggering this adaptive immune response and the significance of CD4+ T cell antigen specificity had not been delineated. The results of this study demonstrate for the first time that TCR recognition of antigens by CD4+ T cells occurs in the LV, by a limited repertoire of CD4+ T cells during pressure overload-induced cardiac dysfunction. We further demonstrate the requirement of CD4+ T cell TCR specificity for endogenous cardiac neoantigens generated by highly reactive, ROS-induced IsoLGs, which are present in the human failing heart. In mice, IsoLG-modified cardiac proteins induce CD4+ T cell activation in response to cardiac pressure overload. Antioxidant treatment and scavenging IsoLGs in vivo both prevent cardiac dysfunction by inhibition of CD4+ T cell activation in the mLNs and TCR engagement in the heart. Hence, this ROS/IsoLG/CD4+ T cell axis might hold promise for translational applications.
Pressure overload in the heart is associated with the release of immunogenic damage associated molecular patterns (DAMPS) that when recognized by pattern recognition receptors (PRRs) in DCs, lead to their activation and migration to the draining lymph nodes, where they elicit T cell immune responses.4, 17, 18 While in this report we do not identify the specific alarmin signal mediating DC activation in the heart, our results demonstrate that DCs are activated in the mLNs, and thus highly express the co-stimulatory molecule CD80. We also find that DCs take up IsoLG-modified cardiac antigens in response to TAC and can functionally induce CD4+ T cell proliferation and activation. While previous studies have demonstrated that specific depletion of bone marrow-derived CD11c+ cells, which include DCs and other innate cells, or pharmacological inhibition of the co-stimulatory molecules CD80 and CD86 attenuates adverse cardiac remodeling due to TAC;8, 19 our results uniquely highlight the ability of activated DCs to uptake cardiac antigens and induce CD4+ T cell activation in response to cardiac pressure overload. Our data demonstrating that MhcII−/− mice reconstituted with effector CD4+ T cells exhibit LV infiltration of CD4+ T cell, but do not develop cardiac dysfunction further supports this critical role of antigen presenting cells (APCs) in the development of cardiac dysfunction. Moreover, the increasing presence of GFPhiCD4+ T cells in the hearts of Nur77GFP mice as systolic function declines, demonstrate for the first time that MHCII-TCR engagement occurs in the LV.
We utilize the OTII mouse strain, with an encoded CD4+ T cell specificity for exogenous ovalbumin (OVA), to control for antigen specificity and address the requirement for CD4+ T cell recognition of endogenous antigens in the LV in order for cardiac dysfunction to develop in the onset of pressure overload. OVA activated CD4+ T cells are efficiently recruited to the LV; however, our studies support the necessity for TCR recognition of cardiac-specific antigens for the development of cardiac dysfunction. First, OVA induced a similar degree of CD4+ T cell activation in the peripheral LNs of the Sham and TAC groups, with the greatest activation occurring at the site of immunization. However, the mLNs draining the heart did not show increased CD4+ T cell activation in response to TAC, demonstrating that a cardiac antigen-specific response is excluded in this OVA-restricted experimental model. Second, OVA-stimulated CD4+ T cells, although able to traffic to the heart in response to pressure overload, are not sufficient to induce cardiac dysfunction once in the heart; and third, in the absence of OVA immunization, OTII mice do not develop signs of CD4+ T cell inflammation or cardiac dysfunction in response to TAC, as previously described.5 Putting these data together with previously published work indicating that cardiomyocyte-specific OVA expressing (c-MyOVA) OTII mice develop adverse cardiac remodeling in response to TAC,20 we conclude that although CD4+ T cell priming in the lymphoid organs is sufficient for recruitment to the myocardium, additional TCR stimulation by cardiac antigens in the LV tissue is required to fully promote cardiac dysfunction. We previously reported that Th1 cells generated in a non-antigen specific manner were sufficient to traffic to the heart and modulate some aspects of cardiac dysfunction in a MHCII sufficient host that had the ability to present antigen, and proposed that endogenous cardiac antigens were needed to fully develop cardiac pathology.3 Our new results indicating that Th1 cells in MhcII−/− mice are unable to induce systolic dysfunction and the identification of cardiac neoantigens supports this premise.
Our TCR profiling of heart and mLN CD4+ T cells in the context of cardiac pressure overload demonstrates an enrichment of CD4+ T cell clones at these sites, relative to peripheral lymphoid tissues further from the heart. The enrichment of specific TCRβ chains suggests that clonal expansion occurs in response to specific antigens. Our data indicating that IsoLG-modified cardiac proteins induced robust CD4+ T cell proliferation and expansion of TAC, but not Sham mLN derived cells suggest that these CD4+ T cell clonotypes activated in the heart in response to TAC are responsive to cardiac neoantigens induced by pathologically high LV pressures for which T cells have not been selected against. This is further supported by data demonstrating that scavenging IsoLGs results in decreased TCR stimulation in Nur77GFP mice. Whether minor autoreactive CD4+ T cell clonotypes that evaded thymic selection are present will require further investigation.
Our findings demonstrating that CD4+ T cells respond to specific neoantigens, generated by highly reactive IsoLGs formed under conditions of oxidative stress are new in the context of heart failure. We identify early LV ROS production, which precedes significant increases in IsoLG-protein adduct accumulation in mLN DCs (Figure 4B). This aligns with previously published work similarly demonstrating that LV ROS is induced as early as 1 week post TAC.21 This supports a model in which LV ROS progressively generate IsoLGs, which ultimately produce neoantigens that are presented to CD4+ T cells in the mLNs. Primed CD4+ T cells then reencounter IsoLG-protein adducts in the LV and mediate cardiac dysfunction. Antioxidants have been shown to have therapeutic benefit in experimental models of HF.9–11, 21 However, several clinical trials assessing long-term antioxidant therapy, did not demonstrate any benefit in preventing cardiovascular disease.22–24 Excessive ROS may have adverse effects, however ROS also represent important intracellular mediators in physiological functions like vasodilation, angiogenesis, cell growth, and redox signaling pathways25. Our results using TEMPOL demonstrate that ROS is critical for LV IsoLG formation and TCR engagement. Specifically targeting these deleterious IsoLGs downstream of ROS, may represent a more effective therapeutic approach to dampen CD4+ T cell activation in response to IsoLG modified cardiac neoantigens. Bi-directional signaling between T cells and DCs occurs within the immune synapse and simultaneously regulates CD4+ T cell activation and DC maturation.26 Our results support this bi-directionality with 2-HOBA impairing both CD4+ T cell activation, and DC CD80 expression in vivo, similarly to what we reported in the context of hypertension.13 ROS promote the formation of IsoLGs via rearrangement of H2-isoprostane intermediates in the F2-isoprostane pathway of free radical-mediated lipid peroxidation.27 IsoLGs adduct to lysine residues on proteins forming immunogenic neoantigens that have been described for a number of diseases associated with oxidative stress, including alcohol-induced liver damage, atherosclerosis, Alzheimer’s disease, asthma and hypertension.13,27 In particular in experimental models of hypertension, phagocyte-derived NADPH oxidase ROS production have been implicated in promoting T cell activation by DCs as a mechanism that leads to organ damage.13 Moreover, mice lacking components of the NADPH oxidase complex, including p47phox, Nox1, and Nox4, are protected from hypertension whereas overexpression of either p22phox or Nox1 has the opposite effect.28 Our data suggest that ROS are predominantly expressed in leukocyte rich areas in the LV of TAC mice. Given that proinflammatory monocytes and macrophages, which highly express the subunits of NADPH oxidase, precede T cell infiltration in response to TAC,29 we propose that these are a major source of the oxidative stress resulting in IsoLG generation and downstream CD4+ T cell activation. It is also possible that LV oxidative stress derived from increased mitochondrial ROS production in cardiomyocytes or cardiac fibroblasts trigger IsoLG modified proteins that can be scavenged by phagocytes and then presented to CD4+ T cells. This, together with nitric oxide synthase uncoupling, and xanthine oxidase could contribute to the observed increase in IsoLG accumulation. And indeed, attenuating cardiac oxidative stress by inhibiting nitric oxide synthase uncoupling or xanthine oxidase, or overexpressing various antioxidants have all been effective in preventing HF development in mice.9, 30, 31 Furthermore, targeting oxidative stress with ROS scavengers, including the antioxidant N-acetyl-cysteine, has been associated with reduced LV hypertrophy and preserved cardiac function.10, 11 Our data demonstrate these strategies are likely beneficial through inhibition of CD4+ T cell activation.
There are limitations and future directions in this study that should be noted, but to the best of our knowledge, we provide the first experimental evidence that active TCR engagement of CD4+ T cells occurs in the heart, and identify specific endogenous cardiac antigens that induce CD4+ T cell activation that have direct consequences on cardiac function. Although our TCR sequencing studies point towards clonal expansion of a limited repertoire of CD4+ T cells, single cell TCR sequencing of the TCR alpha and beta chains will be required to fully characterize the antigen-specific clonotypes. This limitation does not detract from the finding of immunodominant clones in the heart in response to cardiac pressure overload, which holds promise to identify pathogenic CD4+ T cell clones with specificity towards cardiac neoantigens based on the single cell TCR profiling. Due to the relative scarcity of GFPhiCD4+ T cells in the heart, the TCR clonal analysis was performed in all GFP+CD4+ T cells in the heart. The GFP signal induced in Nur77GFP CD4+ T cells is both transient and sensitive to the strength TCR stimulation.15 Therefore, GFPlowCD4+ T cells may represent a dynamic population of TCR activated cells that are either gaining of losing GFP signal, as well as CD4+ T cells receiving weak TCR stimulation. Future studies focused on enrichment and sequencing of GFPhiCD4+ T cells may offer a better strategy to identify pathogenic CD4+ T cell clones. The Th1 cells transferred to MhcII−/− recipient mice that successfully migrate to the pressure-overloaded myocardium (Figure 2) are broadly stimulated in vitro with anti-CD3 and anti-CD28. As such, the CD4+ T cell clonotypes likely differ from those clones found highly enriched in the LV of TAC mice (Figure 1). While these studies have a small sample size, which may report small effects as non-statistically significant between Sham and TAC surgery MhcII−/− mice which received Th1 cells, we conclude that the inability of MhcII−/− recipient mice to induce TCR stimulation determines that despite TAC induced CD4+ T cell infiltration in the LV in this setting, systolic function is still preserved.
While we demonstrate that IsoLG cardiac protein-adducts are taken up by DCs and induce CD4+ T cell proliferation and expansion, there are additional cardiac antigen presenting cells, such as macrophages and B cells, that can also contribute to CD4+ T cell activation in the mLNs and heart. We demonstrate the beneficial effects of 2-HOBA in preventing antigen-driven CD4+ T cell activation, and 2-HOBA may also limit IsoLG-protein adduct accumulation in other cells and modulate cardiac function this way. Additional studies will also be required to determine the therapeutic value of 2-HOBA treatment in mice which have already developed systolic dysfunction. Moreover, we identify IsoLG-modified cardiac proteins as important neoantigens, but additional immunogenic proteins may function as antigens to induce the expansion of distinct CD4+ T cell clones, which remain to be characterized.
In conclusion, our study supports the relevance of cardiac neoantigens induced by oxidative stress, which trigger classic TCR activation of CD4+ T cells and provides novel mechanistic insight to therapeutically ameliorate cardiac dysfunction by dampening CD4+ T cell responses.
Supplementary Material
Clinical Perspective.
What is new?
We report that T cell receptor (TCR) recognition of antigens occurs within the heart as cardiac dysfunction progresses and identify a limited repertoire of activated CD4+ T cell clonotypes in the heart.
We identify isolevuglandin (IsoLG)-modified cardiac proteins as neoantigens that elicit TCR activation and promote HF, and demonstrate that the antioxidant TEMPOL and IsoLG scavengers prevent CD4+ T cell mediated cardiac dysfunction in response to cardiac pressure overload.
What are the clinical implications?
Anti-inflammatory and antioxidant therapies have not successfully translated into clinical practice. CD4+ T cell activation within the heart may underlie the persistent and chronic inflammation seen in heart failure.
Specifically targeting deleterious IsoLGs downstream of ROS may represent a more effective therapeutic approach to decrease CD4+ T cell activation and therapeutically ameliorate cardiac dysfunction.
Acknowledgments
Funding Sources
These studies were supported by NIH R01 HL123658 and R01 HL144477 (PA), NIH T32 HL 69770, NIH T32AI007077-34, NIH F31HL140883 (NN), NIH K01HL130497 (AK) and the American Heart Association 18PRE34020084 (NN), 19POST34430075 (FC).
Non-standard Abbreviations and Acronyms
- 2-HOBA
2-hydroxybenzylamine
- 4-HOBA
4-hydroxybenzylamine
- aLN
axillary lymph node
- APC
antigen presenting cell
- DAMPS
damage associated molecular patterns
- DC
dendritic cell
- iLN
inguinal lymph node
- IsoLGs
Isolevuglandins
- MHCII
major histocompatibility complex class II
- mLN
mediastinal lymph node
- OVA
ovalbumin peptide
- PRR
pattern recognition receptor
- ROS
reactive oxygen species
- scfv
single chain fragment variable
- TAC
transverse aortic constriction
- TCR
T cell receptor
- TEMPOL
4-Hydroxy-TEMPO
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Carrillo-Salinas FJ, Ngwenyama N, Anastasiou M, Kaur K and Alcaide P. Heart Inflammation: Immune Cell Roles and Roads to the Heart. Am J Pathol. 2019;189:1482–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velazquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM and Alcaide P. Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure. Circ Heart Fail. 2015;8:776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, Blanton RM and Alcaide P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. The Journal of Experimental Medicine. 2017;214:3311–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvador AM, Nevers T, Velazquez F, Aronovitz M, Wang B, Abadia Molina A, Jaffe IZ, Karas RH, Blanton RM and Alcaide P. Intercellular Adhesion Molecule 1 Regulates Left Ventricular Leukocyte Infiltration, Cardiac Remodeling, and Function in Pressure Overload-Induced Heart Failure. J Am Heart Assoc. 2016;5:e003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, Delage C, Calise D, Dutaur M, Parini A and Pizzinat N. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–24. [DOI] [PubMed] [Google Scholar]
- 6.Epelman S, Liu PP and Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Kwak D, Fassett J, Liu X, Yao W, Weng X, Xu X, Xu Y, Bache RJ, Mueller DL and Chen Y. Role of bone marrow-derived CD11c+ dendritic cells in systolic overload-induced left ventricular inflammation, fibrosis and hypertrophy. Basic Res Cardiol. 2017;112:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Kwak D, Fassett J, Hou L, Xu X, Burbach BJ, Thenappan T, Xu Y, Ge JB, Shimizu Y, Bache RJ and Chen Y. CD28/B7 Deficiency Attenuates Systolic Overload-Induced Congestive Heart Failure, Myocardial and Pulmonary Inflammation, and Activated T Cell Accumulation in the Heart and Lungs. Hypertension. 2016;68:688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takimoto E and Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–8. [DOI] [PubMed] [Google Scholar]
- 10.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC and Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–5. [DOI] [PubMed] [Google Scholar]
- 11.Goh KY, He L, Song J, Jinno M, Rogers AJ, Sethu P, Halade GV, Rajasekaran NS, Liu X, Prabhu SD, Darley-Usmar V, Wende AR and Zhou L. Mitoquinone ameliorates pressure overload-induced cardiac fibrosis and left ventricular dysfunction in mice. Redox Biol. 2019;21:101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies SS. Modulation of protein function by isoketals and levuglandins. Subcell Biochem. 2008;49:49–70. [DOI] [PubMed] [Google Scholar]
- 13.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, 2nd and Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao L, Patrick DM, Aden LA and Kirabo A. Mechanisms of isolevuglandin-protein adduct formation in inflammation and hypertension. Prostaglandins Other Lipid Mediat. 2018;139:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J and Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngwenyama N, Salvador AM, Velazquez F, Nevers T, Levy A, Aronovitz M, Luster AD, Huggins GS and Alcaide P. CXCR3 regulates CD4+ T cell cardiotropism in pressure overload-induced cardiac dysfunction. JCI Insight. 2019;4:125527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin BN, Wang C, Zhang CJ, Kang Z, Gulen MF, Zepp JA, Zhao J, Bian G, Do JS, Min B, Pavicic PG Jr., El-Sanadi C, Fox PL, Akitsu A, Iwakura Y, Sarkar A, Wewers MD, Kaiser WJ, Mocarski ES, Rothenberg ME, Hise AG, Dubyak GR, Ransohoff RM and Li X. T cell-intrinsic ASC critically promotes T(H)17-mediated experimental autoimmune encephalomyelitis. Nat Immunol. 2016;17:583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higashikuni Y, Tanaka K, Kato M, Nureki O, Hirata Y, Nagai R, Komuro I and Sata M. Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through interleukin-1beta upregulation via nuclear factor kappaB activation. J Am Heart Assoc. 2013;2:e000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Kwak D, Fassett J, Liu X, Yao W, Weng X, Xu X, Xu Y, Bache RJ, Mueller DL and Chen Y. Role of bone marrow-derived CD11c(+) dendritic cells in systolic overload-induced left ventricular inflammation, fibrosis and hypertrophy. Basic Res Cardiol. 2017;112:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groschel C, Sasse A, Rohrborn C, Monecke S, Didie M, Elsner L, Kruse V, Bunt G, Lichtman AH, Toischer K, Zimmermann WH, Hasenfuss G and Dressel R. T helper cells with specificity for an antigen in cardiomyocytes promote pressure overload-induced progression from hypertrophy to heart failure. Sci Rep. 2017;7:15998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Date MO, Morita T, Yamashita N, Nishida K, Yamaguchi O, Higuchi Y, Hirotani S, Matsumura Y, Hori M, Tada M and Otsu K. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J Am Coll Cardiol. 2002;39:907–12. [DOI] [PubMed] [Google Scholar]
- 22.Chae CU, Albert CM, Moorthy MV, Lee IM and Buring JE. Vitamin E supplementation and the risk of heart failure in women. Circ Heart Fail. 2012;5:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivekananthan DP, Penn MS, Sapp SK, Hsu A and Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–23. [DOI] [PubMed] [Google Scholar]
- 24.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR, Hope and Investigators H-TT. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–47. [DOI] [PubMed] [Google Scholar]
- 25.Di Meo S, Reed TT, Venditti P and Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev. 2016;2016:1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, Romani L and Puccetti P. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–42. [DOI] [PubMed] [Google Scholar]
- 27.Shang L, Weng X, Wang D, Yue W, Mernaugh R, Amarnath V, Weir EK, Dudley SC, Xu Y, Hou M and Chen Y. Isolevuglandin scavenger attenuates pressure overload-induced cardiac oxidative stress, cardiac hypertrophy, heart failure and lung remodeling. Free Radic Biol Med. 2019;141:291–298. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ 2nd, Madhur MS and Harrison DG. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M and Prabhu SD. CCR2(+) Monocyte-Derived Infiltrating Macrophages Are Required for Adverse Cardiac Remodeling During Pressure Overload. JACC Basic Transl Sci. 2018;3:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maytin M, Siwik DA, Ito M, Xiao L, Sawyer DB, Liao R and Colucci WS. Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation. 2004;109:1168–71. [DOI] [PubMed] [Google Scholar]
- 31.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y and Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards DA, Aronovitz MJ, Calamaras TD, Tam K, Martin GL, Liu P, Bowditch HK, Zhang P, Huggins GS and Blanton RM. Distinct Phenotypes Induced by Three Degrees of Transverse Aortic Constriction in Mice. Sci Rep. 2019;9:5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.