Sepsis is the leading cause of death in noncardiac intensive care units (ICU) and accounts for approximately 40% of ICU expenditures.1 Currently, there are no drugs approved for the treatment of sepsis.2,3 Patients that survive sepsis suffer long-term physical and cognitive disabilities and the 1 year mortality rate for sepsis patients remains high.4-6 Secondary infections are a common cause of morbidity and mortality among sepsis patients, both in the hospital and post-discharge settings.6 It is generally accepted that sepsis-induced immunosuppression is an important contributing factor to the increased susceptibility of septic patients to opportunistic and nosocomial infections.6 The mechanisms that mediate sepsis-induced immunosuppression are complex and multifactorial; however, it is known that sepsis results in a significant depletion and dysfunction of CD4 and CD8 T cells, which correlates with decreased immune responsiveness and impaired antimicrobial functions.
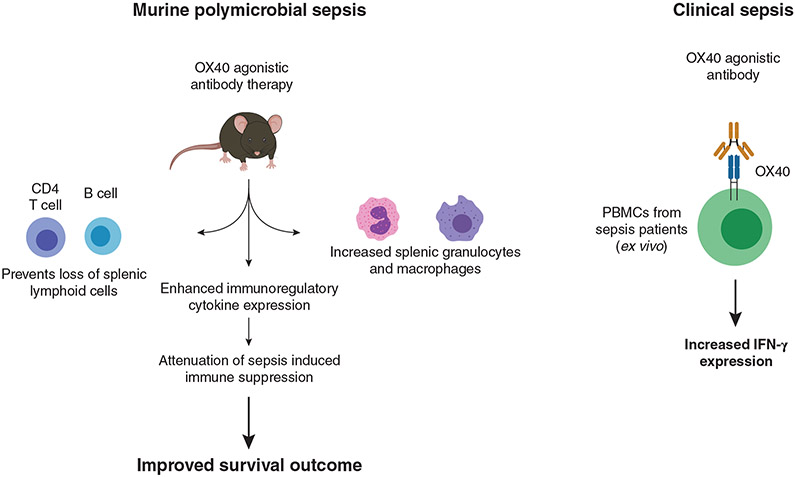
The paper by Unsinger and colleagues in this issue of the Journal of Leukocyte Biology examines the effect of OX40 stimulation on sepsis-induced immunosuppression. The authors report that therapeutic administration of an OX40 agonistic antibody in a murine model of polymicrobial sepsis reverses T cell dysfunction, increases lymphoid cell numbers in the spleen, and, more importantly, OX40 antibody therapy increased survival outcome in a murine model of polymicrobial sepsis (Fig. 1).
FIGURE 1. OX40 activation with an agonistic antibody attenuated sepsis-induced immunosuppression and improved survival outcome in a mouse model of sepsis.
In addition, the OX40 agonistic antibody increased IFN-γ expression in leukocytes from septic patients
OX40 is a transmembrane glycoprotein receptor that is part of the TNF receptor family.7 It is primarily expressed on activated T cells7 and is activated by engagement of the OX40 ligand, expressed by dendritic cells, macrophages, and activated B cells, to serve a costimulatory function.7 OX40 activation induces lymphocyte proliferation and stimulates antiapoptotic signaling pathways that promote lymphocyte survival during periods of inflammation. At present, there is considerable interest in the immunotherapeutic potential of OX40 as an anticancer therapy. However, there are few studies of OX40 in sepsis. In the current study, an agonistic antibody, directed against OX40, was employed. A significant strength of this study is that the agonistic antibody was administered in a therapeutic regimen, that is, 6 and 48 h after the induction of sepsis. This more closely mimics the clinical scenario in which OX40 agonist therapy would be administered to patients during the early phases of sepsis.
In addition to improved survival outcome and improved T cell function in septic mice treated with OX40 antibody, a number of other significant observations emerged from this study. By way of example, OX40 expression was increased on CD4, but not CD8, T cells in response to sepsis and this enhancement persisted for up to 5 days. OX40 therapy also increased the number of CD4 T cells in the spleen at 5 days after the induction of sepsis. However, CD8 T cell numbers were not increased following OX40 antibody therapy. This suggests that the effect of OX40 antibody therapy in sepsis is biased toward CD4 T cell responses. It is well known that circulating lymphocytes are decreased in sepsis.8 Interestingly, OX40 agonistic therapy resulted in an additional 50% decrement in circulating lymphocytes in the presence of sepsis. The authors speculate that this may be due to trafficking of the lymphocytes from the circulation to sites of infection via up-regulation of adhesion molecules; however, adhesion molecule expression was not assessed in this study, nor was recruitment of lymphocytes to the site of infection, that is, the peritoneal cavity. Interestingly, both macrophages and neutrophils were significantly increased in the spleen in response to OX40 agonism. Although OX40 is primarily expressed on lymphocytes, it can also be expressed to a limited extent on some myeloid cells, such as granulocytes.7 It is interesting to speculate that OX40 agonism not only impacts CD4 T cell trafficking and tissue localization, but also may impact to some degree the tissue content of myeloid cells.
As with all studies, there are some potential limitations to the Unsinger study. The authors point out that an important mechanism of sepsis-induced immunosuppression is “impaired lymphocyte IFN-γ production.” However, using 2 different assay methods, they failed to see a significant decrement in IFN-γ production in control septic mice, when compared with naïve (nonseptic) controls. This result could be interpreted to mean that the septic mice were not significantly immunosuppressed at the time of study. However, the data do clearly show that therapy with the OX40 antibody potently induced IFN-γ expression in septic mice and prevented or reversed the loss of splenic CD4 T cells and B cells. It is also important to point out that questions have been raised regarding the relevance of the murine cecal ligation and puncture (CLP) model of sepsis with respect to its usefulness as a paradigm for the evaluation of antisepsis therapeutics. To address this issue, Unsinger and colleagues examined the effect of OX40 agonism in peripheral blood mononuclear cells derived from sepsis patients. They found that the OX40 antibody increased IFN-γ production in peripheral blood mononuclear cells isolated from septic patients (Fig. 1). This is a significant strength of the study because it suggests that OX40 stimulation with an agonistic antibody may be useful in preventing and/or treating sepsis-induced immunosuppression in humans. Another potentially important finding was that OX40 agonism increased IFN-γ expression in PBMCs derived from critically ill surgery, trauma, or myocardial ischemia patients that did not have sepsis. Critically ill patients can exhibit significant immunosuppression even in the absence of sepsis. The observation that OX40 immunotherapy significantly enhances immune function in leukocytes derived from these patients may extend the potential utility of this therapeutic approach to nonseptic patients in the ICU that may be prone to deficits in immune function.
One concern about the application of OX40 agonist therapy during inflammatory syndromes, such as sepsis, is that it could worsen the “cytokine storm” and induce significant physiologic dysfunction and organ injury. OX40 has been implicated as a factor promoting T cell-mediated immunopathology in the lung during viral infections.9 As pointed out by the authors in the discussion, Karulf et al.10 previously reported that OX40-deficient mice and mice treated with an OX40 blocking antibody showed improved survival, decreased cytokine production, and lessened organ injury in a severe model of CLP-induced sepsis. These findings indicate that investigators should tread carefully when applying OX40 agonism in the clinical setting.
When considered as a whole, the study of Unsinger and colleagues provide important insights into OX40-mediated immunotherapy as a potential approach for the treatment of sepsis-induced immunosuppression. However, the implications of this study may extend far beyond modulation of OX40 activity. Indeed, studies of this type may increase interest in immune adjuvants as effective prophylactics and therapeutics for a variety of immunosuppressive disorders.
ACKNOWLEDGMENTS
This work was supported, in part, by NIH GM119197 (Sherwood and Williams), AI151210 (Sherwood), GM083016 (Williams), and C06RR0306551 (ETSU).
Footnotes
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JC. Why have clinical trials in sepsis failed?. Trends Mol Med. 2014;20(4):195–203. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306(23):2614–2615. [DOI] [PubMed] [Google Scholar]
- 4.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Opal S. Immunosuppression and secondary infection in sepsis: part, not all, of the story. JAMA. 2016;315(14):1457–1459. [DOI] [PubMed] [Google Scholar]
- 6.Stortz JA, Murphy TJ, Raymond SL, et al. Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock. 2018;49(3):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willoughby J, Griffiths J, Tews I, Cragg MS. OX40: structure and function - What questions remain? Mol Immunol. 2017;83:13–22. [DOI] [PubMed] [Google Scholar]
- 8.Unsinger J, Kazama H, McDonough JS, Griffith TS, Hotchkiss RS, Ferguson TA. Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. J Immunol. 2010;184:6766–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys IR, Walzl G, Edwards L, Rae A, Hill S, Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med. 2003;198(8):1237–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karulf M, Kelly A, Weinberg AD, Gold JA. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol. 2010;185(8):4856–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]