Abstract
New all-oral regimens for rifampin-resistant tuberculosis (RR-TB) are being scaled up globally. Measurement of drug concentrations in hair assesses long-term drug exposure. Delamanid (DLM) is likely to be a key component of future RR-TB treatment regimens, but a method to describe its quantification in hair via liquid chromatography-tandem mass spectrometry (LC-MS/MS) has not previously been described. We developed and validated a simple, fast, sensitive, and accurate LC-MS/MS method for quantifying DLM and its metabolite DM-6705 in small hair samples. We pulverized and extracted two milligrams of hair in methanol at 37°C for two hours, and diluted 1:1 with water. A gradient elution method eluted DLM, DM-6705, and the internal standard OPC 14714 within 3 minutes, bringing overall analysis time to 5.5 minutes. The method has limits of detection (LOD) of 0.0003 ng/mg for DLM and 0.003 ng/mg for DM-6705. The established linear dynamic ranges are 0.003–2.1 ng/mg and 0.03–21 ng/mg for DLM and DM-6705, respectively. Eleven of 12 participant hair samples had concentrations within DLM’s linear dynamic range, while all 12 samples had concentrations within the quantifiable range for DM-6705. The ranges of concentrations observed in these clinical samples for DLM and DM-6705 were 0.004–0.264 ng/mg hair and 0.412–12.041 ng/mg hair respectively. We demonstrate that while DLM was detected in hair at very low levels, its primary metabolite DM-6705 had levels approximately 100 times higher. Measuring DM-6705 in hair may accurately reflect long-term adherence to DLM-containing regimens for drug-resistant TB.
Keywords: LC-MS/MS, hair analysis, drug-resistant tuberculosis, objective adherence metrics, delamanid, DM-6705
1. Introduction
Rifampin-resistant tuberculosis (RR-TB) remains a global public health crisis with over 558,000 incident cases per year [1]. Subtherapeutic concentrations of anti-TB drugs (in either drug sensitive or RR-TB) are common [2–4], in particular among people living with HIV [5–6], and have been associated with acquired drug resistance [7–10] and adverse treatment outcomes in drug-sensitive TB [11–12]. Measuring drug levels of anti-TB drugs as objective pharmacologic measures of adherence may critically inform RR-TB management and clinical trials. Unlike HIV regimens where a combination of drugs in a single pill is common, TB medications are taken separately, so adherence monitoring must be performed for every single drug in the regimen. Drug levels in plasma are most commonly measured, but these require phlebotomy, cold chain, and specialized equipment. Hair levels of drugs have been used as a long-term metric of adherence in HIV therapy [13]. Small hair samples are easily collected can be stored at room temperature, and levels in hair are minimally affected by short-term spikes in adherence [14].
Delamanid (DLM, OP 67683, Deltyba), a bicyclic nitroimidazole that exerts activity against M. tuberculosis through generation of intracellular nitric oxide, first demonstrated preclinical efficacy in 2006 [15] and exposure-dependent early bactericidal activity in 2010 [16]. Decreased mortality and increased two-month sputum-culture conversion was noted in a phase IIb trial [17], though a subsequent phase III trial (Trial 213) failed to demonstrate increased treatment success or shorter time to culture conversion when DLM was added to an optimized background regimen [18]. Nevertheless, DLM was approved as an option for RR-TB in 2018 since there was a dire need for RR-TB treatment options. Moreover, DLM has limited drug-drug interactions with antiretroviral medications and may effectively replace or protect other medicines in RR-TB regimens [19]. Therefore, along with bedaquiline and pretomanid, another nitroimidazole, DLM is likely to become an important anti-TB medication for RR-TB globally with scale-up being monitored closely [20].
DLM is a prodrug and is extensively metabolized to at least eight metabolites via a number of pathways [21]. LC-MS/MS methods to measure DLM [15,22,23] and its major metabolite, DM-6705 [15,22], in serum and plasma have been developed. We describe here the development and validation of the first LC-MS/MS method for the quantitative analysis of DLM and its major metabolite DM-6705 in small hair samples. This study extends our previous work developing a multi-analyte panel for other RR-TB drugs in small hair samples for adherence and exposure monitoring [24–27].
2. Materials and Methods
2.1. Chemicals
We purchased reference standards for DLM (>99% chemical purity) from MedKoo Biosciences and the internal standard, OPC 14714 (98% chemical purity), from Toronto Research Chemicals. DM-6705 was donated by Otsuka Pharmaceutical Co. Ltd. (Tokyo, Japan). We purchased HPLC-grade water from Aqua Solutions, Inc. and HPLC-grade acetonitrile and methanol from Honeywell Burdick and Jackson. We purchased > 95% purity formic acid from Sigma-Aldrich.
2.2. Sample Analysis
For analyte extraction, we cut hair strands to 3 cm and weighed out 2 mg on a Sartorius CP124S with 0.1 mg accuracy. We then pulverized the hair using an Omni Bead Ruptor homogenizer. We prepared a solution of 16 ng/mL OPC 14714 in methanol and added 500 μL of that mix to each tube, followed by incubation at 37°C for two hours. After incubation, we added 500 μL water, vortexed, centrifuged, and used the supernatant for LC-MS/MS analysis.
For analysis, we used an LC-MS/MS system (Agilent LC 1260-AB Sciex API 5500, Agilent Technologies, and AB Sciex). We injected 7 μL of sample extract into a Synergi Polar RP column (2.1 × 100 mm, 2.5 μm particle size, Phenomenex) using a gradient elution of water with 0.1% formic acid as mobile phase A (MPA) and acetonitrile as mobile phase B (MPB). The gradient consisted of 65% to 75% MPB from 0–1.68 min, gradient to 100% MPB from 1.68–2.4 min, 100% MPB from 2.4–3.3 min, gradient to 65% MPB from 3.3–3.4 min, and 65% MPB from 3.4–5.5 min. We used electrospray ionization in positive polarity mode and multiple reaction monitoring to scan for masses of the analytes.
We injected quality control (QC) samples at low (0.018 ng/mg DLM, 0.180 ng/mg DM-6705), mid (0.180 ng/mg DLM, 1.8 ng/mg DM-6705), and high (1.8 ng/mg DLM, 18 ng/mg DM-6705) levels and a method blank alongside each calibration curve. These were run at the beginning, middle, and end of each run. QC preparations passed if they were within 15% of the target value and their precision within 15% coefficient of variation (CV).
2.3. Data Analysis
We used AB Sciex Analyst 1.6 and AB Sciex MultiQuant 2.1 for data analysis. We confirmed peak identifications for each analyte using two transitions and retention time. Both DLM and DM-6705 were quantified using area of the drug over area of OPC 14714, which was added to each vial at a total concentration of 8 ng/mL. We used linear regression with 1/x weighting to form the calibration curve.
2.4. Method Validation
We validated the method’s linearity, specificity, sensitivity, precision, accuracy, matrix effect, recovery, injection repeatability, and carryover. To test linearity, we prepared and ran a calibration curve (0.0003–2.1 ng/mg hair for DLM, 0.003–21 ng/mg hair for DM-6705) three separate times across three days and checked for a passing (>95%) coefficient of determination each time. To test specificity, we used hair samples from six RR-TB patients who had not taken DLM. We defined the limit of detection (LOD) as the lowest concentration in our series of calibration standards that had a signal to noise ratio ≥3. We defined the lower limit of quantitation (LLOQ) as the lowest concentration that had a signal to noise ratio ≥10, while keeping the coefficient of determination >95%.
To assess precision and accuracy, we spiked blank hair samples at the LLOQ and with low, mid, and high QC concentrations of the analytes. We ran five replicates of each of the four concentration levels and prepared them separately for three runs over three days. The precision and accuracy pass if the CV and relative error (RE) are ≤ 15% at the QC levels and ≤ 20% at the LLOQ. For testing matrix effect, we spiked five replicates of the three concentrations of the analytes into 1:1 methanol:water and compared it to the average area when spiked into extracted matrix. We subtracted the average area of the solvent replicates from the average area of the matrix replicates and then divided it by the average area of the solvent replicates to determine matrix effect percentage. For testing recovery, we spiked five replicates of the three concentrations of the analytes into extracted matrix and compared it to the average area when analytes were spiked into matrix and then extracted. We divided the average area of the replicates spiked prior to extraction by the average area of the replicates spiked following extraction to determine recovery percentage.
We tested injection repeatability by injecting the analytes at the three concentrations five times in one day. We tested carryover by injecting the analytes at high concentration one time, then two times, then three times, each time preceded and followed by three matrix blanks.
2.5. Patient Hair Testing
Between 1 July 2016 and 1 Jan 2018, small hair samples (20–50 strands) were collected from 12 patients undergoing inpatient, directly observed treatment for RR-TB at the Brooklyn Chest Hospital, Cape Town, South Africa. Each participant provided written informed consent, and ethical approval was obtained from the University of Cape Town Human Research Ethics Committee (187/2016) and the University of California, San Francisco Human Research Protection Program.
Hair samples from the participants were analyzed using our validated method. All participants received a total of 200 mg delamanid daily. We used the 3 cm of participant hair closest to the scalp, which would grow over the course of 90 days on average [28]. If participants had hair ≤ 3 cm, we used the whole strand. However, five of the 12 participants had treatment durations shorter than 90 days. Hence, we corrected for the lack of drug in segments of these hair samples by dividing the measured concentration of DLM and DM-6705 by the ratio of number of treatment days over total days of hair growth (90 days). Correlations between measured DLM and DM-6705 concentrations and duration of treatment were assessed using linear regression and rank correlation analyses. None of the hair samples had visible signs of dye or perm treatment, as all were curly and black.
3. Results
3.1. Method Development
We developed a 5.5-minute method to quantitatively analyze DLM and its metabolite DM-6705 in small (2 mg) hair samples using LC-MS/MS. For the two target analytes and the chosen internal standard (OPC 14714), we analyzed two mass spectral transitions (Figure 1). We used the most abundant transition as the quantifier ion and the other transition as the qualifier ion (Table 1).
Figure 1.
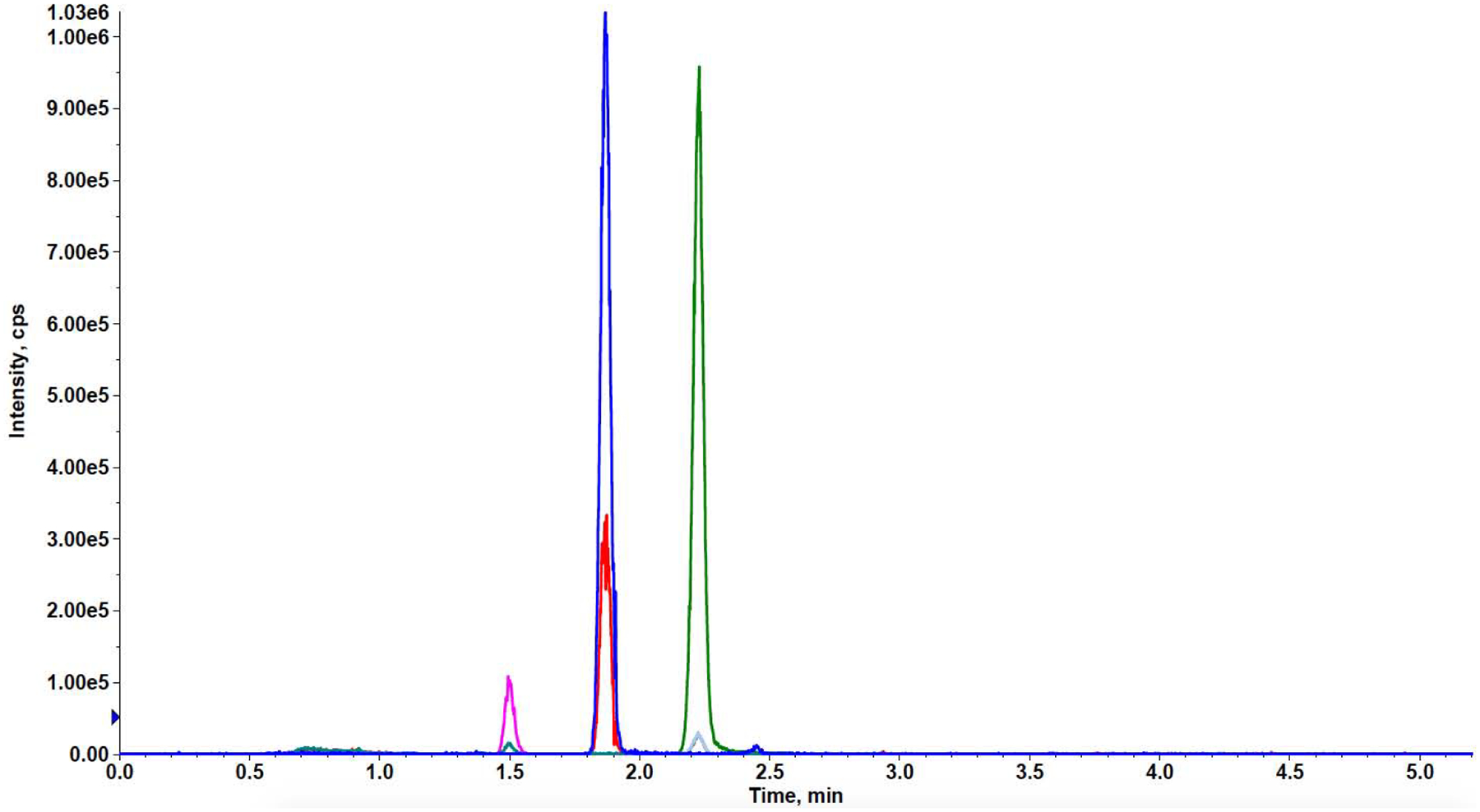
Representative chromatogram of DLM, DM-6705, and OPC 14714 (internal standard). 1.5 ng mg-1 DM-6705, 0.15 ng mg-1 DLM, 16 ng mg-1 OPC-14714. DLM: delamanid.
Table 1.
Mass spectrometer transitions and parameters for DLM and DM-6705
| ID | Q1 Mass (Da) | Q3 Mass (Da) | Time (msec) | DP (volts) | EP (volts) | CE (volts) | CXP (volts) |
|---|---|---|---|---|---|---|---|
| DM-6705-1 | 466.3 | 352.2 | 20 | 151 | 12 | 23 | 30 |
| DM-6705-2 | 466.3 | 200.2 | 20 | 136 | 12 | 23 | 30 |
| DLM-1 | 535.0 | 352.0 | 10 | 36 | 10 | 33 | 22 |
| DLM-2 | 535.0 | 175.0 | 10 | 36 | 10 | 55 | 14 |
| OPC 14714-1 | 459.2 | 296.0 | 2 | 20 | 10 | 33 | 20 |
| OPC 14714-2 | 459.2 | 176.0 | 2 | 20 | 10 | 39 | 14 |
DP: declustering potential, EP: entrance potential, CE: collision energy, CXP: collision cell exit potential, DLM: delamanid
3.2. Method Validation
3.2.1. Specificity
Neither of the analytes were detected in the hair samples of the six control RR-TB patients.
3.2.2. Linearity and Sensitivity
The LOD was 0.0003 ng/mg hair for DLM and 0.003 ng/mg hair for DM-6705. For DLM, the LLOQ was 0.003 ng/mg hair and the upper limit of quantitation (ULOQ) was 2.1 ng/mg hair. For DM-6705, the LLOQ was 0.03 ng/mg hair and the ULOQ was 21 ng/mg hair. The average linearity coefficients of determination for DLM and DM-6705 were 0.997 and 0.995 respectively (Supplementary Table).
3.2.3. Precision and Accuracy
For DLM, the within-run precision at the low, mid, and high QC levels was 4.24%, 2.87%, and 4.06% respectively. At the low, mid, and high QC levels, the between-run precision was 8.64%, 8.40%, and 7.61% CV respectively. At the LLOQ, within-run precision was 2.34% CV and between-run precision was 2.53% CV. For DM-6705, within-run precision at the low, mid, and high QC levels was 2.10%, 1.32%, and 3.12% respectively. At the low, mid, and high QC levels, the between-run precision was 3.30%, 2.11%, and 5.36% CV respectively. At the LLOQ, within-run precision was 3.81% CV and between-run precision was 3.47% CV. (Table 2).
Table 2.
Mean precision and accuracy for DLM and DM-6705 within and between the three runs.
| Within-run | Between-run | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | Parameter | LLOQ | Low | Mid | High | LLOQ | Low | Mid | High |
| DM-6705 | Precision (% CV) | 2.34% | 2.10% | 1.32% | 3.12% | 2.53% | 3.30% | 2.11% | 5.36% |
| Accuracy (% RE) | 9.45% | 5.74% | 11.04% | 4.59% | 8.44% | 9.00% | 12.94% | 5.88% | |
| DLM | Precision (% CV) | 3.81% | 4.24% | 2.87% | 4.06% | 3.47% | 8.64% | 8.40% | 7.61% |
| Accuracy (% RE) | 15.64% | 8.89% | 5.56% | 5.30% | 11.82% | 8.01% | 7.94% | 8.92% | |
DLM: delamanid, RE: relative error
For DLM, the within-run accuracy at the low, mid, and high QC levels was 8.89%, 5.56%, and 5.30% respectively. At the low, mid, and high QC levels, the between-run accuracy was 8.01%, 7.94%, and 8.92% RE respectively. At the LLOQ, within-run accuracy was 15.64% RE and between-run accuracy was 11.82% RE. For DM-6705, the within-run accuracy at the the low, mid, and high QC levels was 5.74%, 11.04%, and 4.59% respectively. At the low, mid, and high QC levels, the between-run accuracy was 9.00%, 12.94%, and 5.88% RE respectively At the LLOQ, within-run accuracy was 9.45% RE and between-run accuracy was 8.44% RE (Table 2).
3.2.4. Matrix Effect and Recovery
The matrix effect of DLM at the low, mid, and high QC levels was −0.03%, −4.02%, and −7.86% respectively. The matrix effect of DM-6705 at the low, mid, and high QC levels was 2.04%, 2.18%, and −5.35% respectively (Table 3).
Table 3.
Matrix effect and recovery for DLM and DM-6705.
| Matrix Effect (%) | ||||||
|---|---|---|---|---|---|---|
| Drug | Low | CV | Mid | CV | High | CV |
| DM-6705 | 2.04% | 7.40% | 2.18% | 5.84% | −5.35% | 2.95% |
| DLM | −0.03% | 5.59% | −4.02% | 6.69% | −7.86% | 4.45% |
| Recovery (%) | ||||||
| Drug | Low | CV | Mid | CV | High | CV |
| DM-6705 | 101.45% | 6.51% | 103.86% | 5.50% | 97.99% | 4.19% |
| DLM | 113.32% | 11.24% | 112.22% | 5.16% | 111.24% | 4.73% |
DLM: delamanid
The recovery of DLM at the low, mid, and high QC levels was 113.32%, 112.22%, and 111.24% respectively. The recovery of DM-6705 at the low, mid, and high QC levels was 101.45%, 103.86%, 97.99% respectively (Table 3).
3.2.5. Injection Repeatability
The CV for each QC level was < 2% for both analytes.
3.2.6. Carryover
No signal above the threshold signal-to-noise ratio of three was detected in any of the matrix blank samples for either analyte.
3.3. Patient Hair Testing
We detected both DLM and DM-6705 in all 12 RR-TB patient samples taking DLM (Table 4). Except for one DLM concentration below the LLOQ, all concentrations were within the quantifiable range for both analytes. For DLM, sample concentrations ranged from 0.004 ng/mg to 0.264 ng/mg, with a median concentration of 0.012 ng/mg. For DM-6705, sample concentrations ranged from 0.412 ng/mg to 12.041 ng/mg, with a median concentration of 2.682 ng/mg.
Table 4.
Hair levels of DLM and DM-6705 in patients taking DLM under directly observed therapy (ng/mg)
| Duration on Treatment (days) | DLM (ng/mg hair) | DM-6705 (ng/mg hair) | |
|---|---|---|---|
| DLM-1 | 55 | 0.007* | 0.684* |
| DLM-2 | 141 | 0.007 | 3.690 |
| DLM-3 | 50 | 0.023* | 0.742* |
| DLM-4 | 48 | 0.169* | 1.707* |
| DLM-5 | 165 | 0.175 | 7.046 |
| DLM-6 | 200 | 0.004 | 3.764 |
| DLM-20 | 70 | 0.013* | 2.410* |
| DLM-22 | 98 | 0.007 | 3.489 |
| DLM-112 | 190 | <LLOQ | 7.158 |
| DLM-113 | 187 | 0.024 | 12.041 |
| DLM-114 | 11 | 0.264 | 1.492 |
| DLM-115 | 62 | 0.053* | 1.854* |
| Median | 105 | 0.012 | 2.682 |
| Geometric Mean | 100 | 0.016 | 2.246 |
sample concentration is corrected for shorter treatment regimen. DLM: delamanid
We observed essentially no correlation (r= −0.05) between the concentrations of DLM and DM-6705 in participant hair samples. We also analyzed the correlation between time-on-drug and drug concentration. For DM-6705, we obtained a Pearson’s correlation coefficient of 0.80 (p=0.003) that was further verified by performing Kendall’s rank correlation (τ= 0.67, p=0.002). For DLM, the Pearson’s and Kendall’s correlations were −0.13 and −0.21 respectively (Figure 2).
Figure 2.
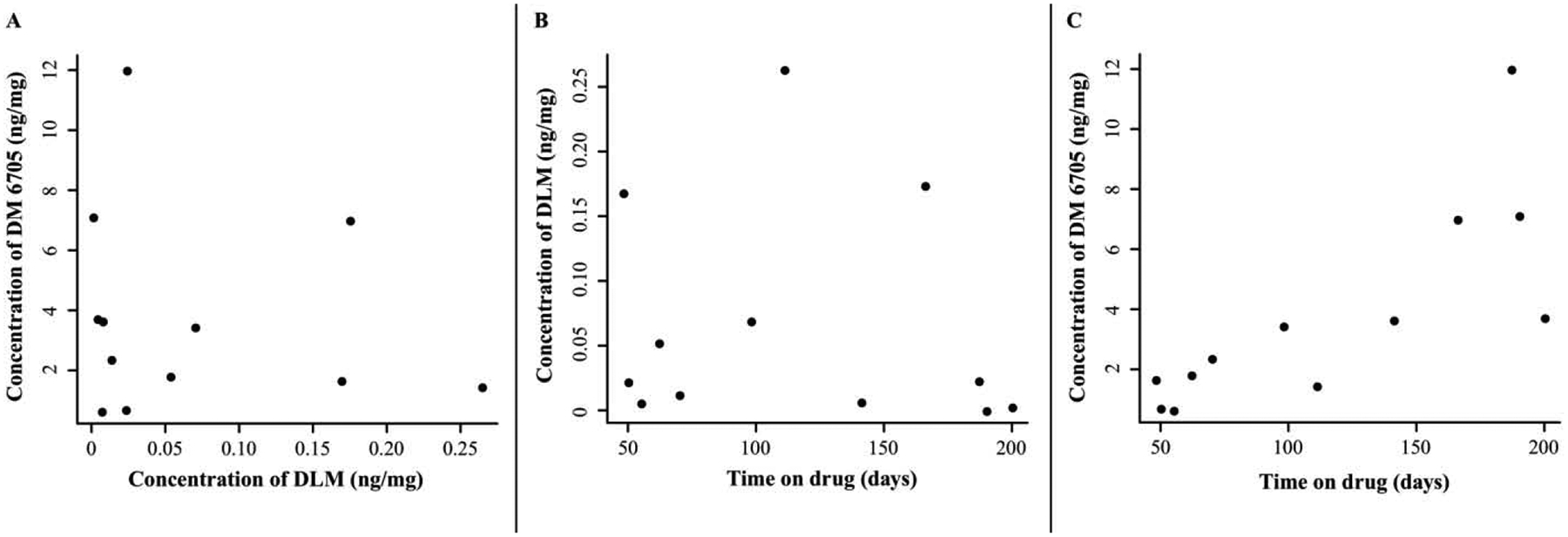
(A) Correlation between hair DLM and DM-6705 levels. (B,C) Correlation between DLM and DM-6705 level respectively and study participant’s time on drug. DLM: delamanid.
4. Discussion
Delamanid will soon play an important role in newer all-oral drug-resistant TB regimens. We describe here the first assay to analyze DLM and its primary metabolite DM-6705 in small hair samples as an objective biomarker of adherence. Medication levels in hair samples can integrate behavior (adherence) and biology (pharmacokinetics), and in the context of HIV treatment and prevention, are predictive of outcomes [29]. Given the need for adherence monitoring of anti-TB drugs during the prolonged duration of treatment required for RR-TB, this hair assay should have utility for an increasingly prevalent infection.
4.1. Method Development
LC-MS/MS methods incorporating the analysis of DLM’s metabolites, especially DM-6705, have been developed for serum and plasma [15,22]. The LLOQs achieved in these assays range between 1–6 ng/mL. The plasma half-life of DLM is 30–38 hours, while its metabolites have half-lives ranging from 121 to 322 hours [30,31]. During sample preparation, DLM is shown to be significantly converted to its metabolites under basic conditions while elevated temperature allows metabolism regardless of pH [22]. Hence, LC-MS/MS methods for plasma and serum extract DLM at low temperature under neutral conditions. Similar LLOQs are achieved for DLM and its metabolite DM-6705. Unlike DLM, however, DM-6705 does not undergo extensive metabolism during sample extraction.
We initially tried to incorporate DLM into a multi-analyte panel of eleven multidrug resistance (MDR)-TB drugs in hair as described in our previous publication [24]. While that method had an LOD for DLM of 0.005 ng/mg, two patients taking DLM in directly observed therapy showed levels in hair barely above this established LOD (0.019 and 0.020 ng/mg hair). The very small separation between the LOD and the observed DLM levels would have limited utility for the multi-analyte panel in monitoring regimens that incorporate DLM. Attempts to improve the method for DLM, however, would be quite challenging considering the LOD is already very low. Metabolites occasionally incorporate into hair to a greater extent than their parent compounds [32]. This served as impetus for incorporating the primary metabolite of DLM, DM-6705, into the multi-analyte panel. Addition of DM-6705 yielded very low, broad peaks for this analyte. Because any adjustment of the method to improve this assay also affected the assay for other drugs in the MDR-TB panel, we moved on to develop a separate method for DLM and DM-6705, described here.
In developing the new method, we adopted the same mass spectrometric parameters established for DLM and DM-6705 in the MDR-TB panel. The transitions we used have been reported in other published methods (Table 1) [15,22,23]. The previous panel provided sharp peaks for DLM so we used the same C18 reversed-phase chromatographic column, Phenomenex Synergi Polar. However, we significantly shortened the chromatographic run from 17 to 5.5 minutes and changed the elution gradient to improve DM-6705’s peak shape. We achieved a slightly improved LLOQ for DLM compared to the MDR-TB panel (0.003 vs 0.010 ng/mg). More importantly, we achieved sharp peaks for DM-6705 and an LLOQ of 0.03 ng/mg hair (Figure 1). Due to the lack of stable isotope-labeled internal standards for DLM and DM-6075, we chose the antipsychotic agent OPC 14714 due to its similar molecular weight and retention time to DLM and DM-6705.
4.2. Method Validation
Validation of any LC-MS/MS-based method requires demonstration of a precise and accurate assay with high specificity, negligible matrix effect, and reproducibly high recovery. Reproducible linear dynamic ranges (700, ULOQ/LLOQ) for both analytes in our hair assay were achieved without any carryover from injection of analytes close to the ULOQ. These validation parameters were achieved after substantial changes to the original extraction protocol for the MDR-TB panel. Although that extraction protocol worked well for 11 drugs in the MDR-TB panel, it suffered from poor precision and recovery rates for DLM and DM-6705. To improve these parameters, the following modifications were made: (1) elimination of evaporation after extraction with methanol; and (2) dilution with water to 50% methanol after a single extraction.
Evaporation after double extraction with methanol in the original method allowed for concentration of the analytes. However, we found that evaporation had a profound negative effect on the precision and recovery of DLM and DM-6705 in the new method. Because of the reported sensitivity of DLM to higher temperature in the plasma assay [15], we also tested lowering the temperature of evaporation, but neither this nor lowering the temperature of extraction improved precision and recovery of the assay. Because the metabolism of DLM during sample extraction in plasma is proposed to be albumin-mediated [33], the lack of albumin in hair may explain the stability of our assay compared to that in plasma. It was only after elimination of evaporation that precision and recovery improved.
Dilution of the extract in 50% methanol is required to facilitate reversed-phase chromatography. Elimination of evaporation did not allow concentration of the extract nor reconstitution into a more aqueous solvent. Despite the lack of concentration, the new method improved the sensitivity of the assay. Concentration also increases the amount of potential interferences in the extract. This may pose signal suppression that overrides the gain in signal from concentration. Elimination of the evaporation step increased the intensity of the peaks for both DLM and DM-6705 and lowered the noise during the chromatography phase, resulting in increased sensitivity.
4.3. Patient Hair Testing
Despite the extremely low LLOQ (0.003 ng/mg), one patient hair sample has DLM concentration <LLOQ and the median concentration observed for 12 patient hair samples was 0.012 ng/mg (Geometric mean = 0.016 ng/mg). In contrast, DM-6705 was quantified in all patient hair samples despite its higher LLOQ (0.03 ng/mg), and its median concentration was 2.682 ng/mg (Geometric mean = 2.246 ng/mg) across patient samples. All concentrations measured in patient hair samples (0.418–12.041 ng/mg hair, Table 4) fall within the linear dynamic range of the assay for DM-6705. In the plasma the typical steady-state concentration of DLM ranges between 340–410 ng/mL at an intake dose of 100 mg twice daily, while that of DM-6705 ranges between 100–220 ng/mL [30]. The reversal of the concentration ratio that we observe in hair cannot be due to a potential loss of DLM to metabolism during sample extraction as both analytes have high recovery rates. This ratio suggests better penetration of DM-6705 than DLM into hair. This reversal has also been observed for other drugs [34] and may be partially explained by higher rates of melanin binding in more basic drugs [35]. DM-6705 has a higher pKa than does DLM, so this same phenomenon may help explain the higher levels of DM-6705 in hair, although there may be various mechanisms driving incorporation into hair [34]. This observation warrants further study that is beyond the scope of developing this assay.
The lack of correlation between DLM and DM-6705 concentrations in participant hair samples is most likely because there are seven other metabolites of DLM reported in plasma that we did not measure using our method [22]. Furthermore, while we observed no correlation between DLM and time on drug, we observed a strong positive correlation between DM-6705 and time on drug, despite having corrected the measurements for the estimated amount of blank hair segments in the samples. This observation along with the fact that the lowest DM-6705 concentration observed for the participant samples is 140X higher than the LOD (0.003 ng/mg) and its median concentration is 894X higher clearly demonstrate that adherence to a regimen incorporating DLM is better monitored via hair levels of its metabolite DM-6705 and justifies its inclusion in our assay. Furthermore, some reports using plasma have indicated the potential role of DM-6705 for QT prolongation, an unwanted side effect of DLM [36]. With the longer timeframe readout afforded by hair levels, this potential association can further be investigated using our assay.
4.4. Limitations
Our study has several limitations. As discussed previously, we only measured one metabolite of DLM, making it difficult to predict if the other metabolites may incorporate at even higher levels than DM-6705. Additionally, our ability to interpret the hair levels we measured is limited because we lack the appropriate references for interpretation. Without careful pharmacokinetic study where hair and blood samples are collected after directly observed therapy, it is hard to correlate hair and plasma levels. One way to use DM-6705 for adherence monitoring would be to establish a threshold level that would serve as a cutoff for judging whether a patient is adherent to the drug regimen. The hair levels we have measured from 12 participants on directly observed therapy offer initial insight into expected levels of DLM and DM-6705 in hair if patients are fully compliant with their regimen, but a much larger study would be required to establish adherence cut-offs.
5. Conclusion
In conclusion, we describe the first method to analyze DLM and its metabolite in hair. This method adds to a growing number of assays we have developed to quantitatively monitor TB drugs in small hair samples [17–20]. There is a wide variety of chemical classes currently being used for drugs in RR-TB treatment, all of which cannot be compatibly incorporated into a single panel. Our current work demonstrates that a separate panel is likely to be required for specific drugs, such as DLM, for effective monitoring. Finally, as with some drugs analyzed in forensics, our strategy of targeting a drug’s metabolite for analysis when its parent structure does not incorporate well into hair has proven effective for DLM monitoring. In the future, we hope to show the clinical utility of this assay to monitor DLM and its metabolite for adherence monitoring in the setting of RR-TB.
Supplementary Material
Highlights.
LC-MS/MS assay for delamanid (DLM) and its metabolite DM-6705 in hair was validated
Delamanid was detected at very low levels in patient hair samples
DM-6705 had levels 100X > DLM and is a better biomarker for long-term adherence
Acknowledgements
The authors would like to thank the contributions of the participants of this study recruited from the University of Cape Town Lung Institute who donated hair samples for the study.
Funding: This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases [R01 AI123024 (P.I. Metcalfe, Gandhi)].
Abbreviations:
- RR-TB
rifampin-resistant tuberculosis
- DLM
delamanid
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LOD
limit of detection
- TB
tuberculosis
- HPLC
high performance liquid chromatography
- MPA
mobile phase A
- MPB
mobile phase B
- QC
quality control
- CV
coefficient of variation
- LLOQ
lower limit of quantitation
- ULOQ
upper limit of quantitation
- MDR-TB
multidrug-resistant tuberculosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare no conflict of interest in the conduct of the work described in the manuscript being submitted.
References
- [1].World Health Organization, Global Tuberculosis Report 2019. www.who.int/tb/publications/global_report/en/,2019. (accessed 30 January 2020).
- [2].Mpagama SG, Ndusilo N, Stroup S, Kumburu H, Peloquin CA, Gratz J, et al. Plasma drug activity in patients on treatment for multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 58 (2014) 782–88. https://doi.org/0.1128/AAC.01549-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barroso EC, Pinheiro VG, Facanha MC, Carvalho MR, Moura ME, Campelo CL, et al. Serum concentrations of rifampin, isoniazid, and intestinal absorption, permeability in patients with multidrug resistant tuberculosis. Am J Trop Med Hyg. 81 (2009) 322–29. 10.4269/ajtmh.2009.81.322. [DOI] [PubMed] [Google Scholar]
- [4].Heysell SK, Moore JL, Peloquin CA, Ashkin D, Houpt ER. Outcomes and use of therapeutic drug monitoring in multidrug-resistant tuberculosis patients treated in Virginia, 2009–2014. Tuberc Respir Dis (Seoul). 78 (2015) 78–84. 10.4046/trd.2015.78.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Daskapan A, de Lange WC, Akkerman OW, Kosterink JG, van der Werf TS, Stienstra Y, Alffenaar JW. The role of therapeutic drug monitoring in individualised drug dosage and exposure measurement in tuberculosis and HIV co-infection. Eur Respir J. 45 (2015) 569–71. 10.1183/09031936.00142614. [DOI] [PubMed] [Google Scholar]
- [6].Gurumurthy P, Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Padmapriyadarsini C, Swaminathan S, et al. Decreased bioavailability of rifampin and other antituberculosis drugs in patients with advanced human immunodeficiency virus disease. Antimicrob Agents Chemother. 48 (2004) 4473–75. 10.1128/AAC.48.11.4473-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Holland DP, Hamilton CD, Weintrob AC, Engemann JJ, Fortenberry ER, Peloquin CA, Stout JE. Therapeutic drug monitoring of antimycobacterial drugs in patients with both tuberculosis and advanced human immunodeficiency virus infection. Pharmacotherapy. 29 (2009) 503–10. 10.1592/phco.29.5.503. [DOI] [PubMed] [Google Scholar]
- [8].Vernon A, Burman W, Benator D, Khan A, Bozeman L. Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Tuberculosis Trials Consortium. Lancet. 353 (1999) 1843–47. 10.1016/s0140-6736(98)11467-8. [DOI] [PubMed] [Google Scholar]
- [9].Benator D, Bhattacharya M, Bozeman L, Burman W, Cantazaro A, Chaisson R, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 360 (2002) 528–34. 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- [10].Van Tongeren L, Nolan S, Cook VJ, FitzGerald JM, Johnston JC. Therapeutic drug monitoring in the treatment of tuberculosis: a retrospective analysis. Int J Tuberc Lung Dis. 17 (2013) 221–24. 10.5588/ijtld.12.0279. [DOI] [PubMed] [Google Scholar]
- [11].Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 48 (2009) 1685–94. 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Prahl JB, Johansen IS, Cohen AS, Frimodt-Moller N, Andersen AB. Clinical significance of 2 h plasma concentrations of first-line anti-tuberculosis drugs: a prospective observational study. J Antimicrob Chemother. 70 (2015) 321–22. https://doi.org/0.1093/jac/dku210. [DOI] [PubMed] [Google Scholar]
- [13].Gandhi M, Greenblatt RM, Bacchetti P, Jin C, Huang Y, Anastos K, et al. A single-nucleotide polymorphism in CYP2B6 leads to >3-fold increases in efavirenz concentrations in plasma and hair among HIV-infected women. J Infect Dis. 206 (2012) 1453–61. 10.1093/infdis/jis508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barroso M, Gallardo E, Vleira DN, Lopez-Rivadulla M, Queiroz JA. Hair: a complementary source of bioanalytical information in forensic toxicology. Bioanalysis. 6133 (2011) 67–79. 10.4155/bio.10.171. [DOI] [PubMed] [Google Scholar]
- [15].Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 3 (2006) e466. 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 15 (2011) 949–54. 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- [17].Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas -Vasquez DE, et al. Delamanid for multi-drug resistant pulmonary tuberculosis. N Engl J Med. 366 (2012) 2151–60. 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- [18].von Groote-Bidlingmaier F, Patientia E, Sanchez E, Balanag V Jr., Ticona E, Segura P, et al. Efficacy and safety of delamanid in combination with an optimised background regimen for treatment of multidrug-resistant tuberculosis: a multicentre, randomised, double-blind, placebo-controlled, parallel group phase 3 trial. Lancet Respir Med. 7 (2019) 249–59. 10.1016/S2213-2600(18)30426-0. [DOI] [PubMed] [Google Scholar]
- [19].World Health Organization, WHO position statement on the use of delamanid for multidrug-resistant tuberculosis. www.who.int/tb/publications/2018/WHOPositionStatementDelamanidUse.pdf?ua=1,2018. (accessed January 2020).
- [20].DR-TB Scale-Up Treatment Action Team (DR-TB STAT), About. http://drtb-stat.org/about/,2015. (accessed August 2019).
- [21].Blair HA, Scott LJ. Delamanid: a review of its use in patients with multidrug-resistant tuberculosis. Drugs. 75 (2015) 91–100. 10.1007/s40265-014-0331-4. [DOI] [PubMed] [Google Scholar]
- [22].Meng M, Smith B, Johnston B, Carter S, Brisson J, Roth S. Simultaneous quantitation of delamanid (OPC-67683) and its eight metabolites in human plasma using UHPLC-MS/MS. Journal of Chromatography B. 1002 (2015) 78–91. 10.1016/j.jchromb.2015.07.058. [DOI] [PubMed] [Google Scholar]
- [23].Hirao Y, Koga T, Koyama N, Shimokawa Y, Umehara K. Liquid Chromatography-Tandem Mass Spectrometry Methods for Determination of Delamanid in Mouse Plasma and Lung. Am J Anal Chem. 6 (2015) 98–105. 10.4236/ajac.2015.62009. [DOI] [Google Scholar]
- [24].Gerona R, Wen A, Aguilar D, Shum J, Reckers A, Bacchetti P, et al. Simultaneous analysis of 11 medications for drug resistant TB in small hair samples to quantify adherence and exposure using a validate LC-MS/MS panel. Journal of Chromatography B. 1125 (2019) 121729. 10.1016/j.jchromb.2019.121729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Metcalfe J, Gerona R, Wen A, Bacchetti P, Gandhi M. An LC-MS/MS-based method to analyze the anti-tuberculosis drug bedaquiline in hair. Int J Tuberc Lung Dis. 21 (2017) 1069–70. 10.5588/ijtld.17.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gerona R, Wen A, Koss C, Bacchetti P, Gandhi M, Metcalfe J. A multi-analyte panel for non-invasive pharmacokinetic monitoring of second-line anti-tuberculosis drugs. Inter J Tuberculosis and Lung Disease. 20 (2016) 991–92. 10.5588/ijtld.16.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gerona R, Wen A, Chin AT, Koss CA, Bacchetti P, Metcalfe J, Gandhi M. Quantifying isoniazid levels in small hair samples: a novel method for assessing adherence during the treatment of latent and active tuberculosis. PLoS One. 11 (2016) e0155887. 10.1371/journal.pone.0155887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Loussouarn G, Lozano I, Panhard S, Callaudin C, El Rawadi C, Genain G. Diversity in human hair growth, diameter, colour and shape. An in vivo study on young adults from 24 different ethnic groups observed in the five continents. Eur J Dermatol. 26 (2016) 144–54. 10.1684/ejd.2015.2726. [DOI] [PubMed] [Google Scholar]
- [29].Gandhi M, Bacchetti P, Ofokotun I, Jin C, Ribaudo HJ, Haas DW, et al. Antiretroviral concentrations in hair strongly predict virologic response in a large Human Immunodeficiency Virus treatment-naive clinical trial. Clin Infect Dis. 68 (2019) 1044–47. 10.1093/cid/ciy764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].European Medicines Agency, Delamanid assessment report. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002552/human_med_001699.jsp&mid=WC0b01ac058001d1242014 (accessed January 2020)
- [31].European Medicines Agency, Delamanid (Deltyba): summary of product characteristics. www.ema.europa.eu/en/documents/product-information/deltyba-epar-product-information_en.pdf,2014. (accessed January 2020).
- [32].Licata M, Rutichelli F, Ferrari A, Baraldi C, Vandelli D, Verri P, et al. Hair testing in clinical setting: simultaneous determination of 50 psychoactive drugs and metabolites in headache patients by LC tandem MS. J Pharm Biomed Anal. 126 (2016) 14–25. 10.1016/j.jpba.2016.04.015. [DOI] [PubMed] [Google Scholar]
- [33].Sasahara K, Shimokawa Y, Hirao Y, Koyama N, Kitano K, Shibata M, Umehara K. Pharmacokinetics and metabolism of delamanid, a novel anti-tuberculosis drug, in animals and humans: importance of albumin metabolism in vivo. Drug Metab Dispos. 43 (2015) 1267–76. 10.1124/dmd.115.064527. [DOI] [PubMed] [Google Scholar]
- [34].Henderson L Mechanisms of drug incorporation into hair. Forensic Sci Intl. 63 (1993) 19–29. 10.1016/0379-0738(93)90256-A. [DOI] [PubMed] [Google Scholar]
- [35].Borges CR, Roberts JC, Wilkins DG, Rollins DE. Cocaine, benzoylecgonine, amphetamine, and N-acetylamphetamine binding to melanin subtypes. J Anal Toxicol. 27 (2003) 125–34. [DOI] [PubMed] [Google Scholar]
- [36].Guglielmetti L, Tiberi S, Burman M, Kunst H, Wejse C, Togonidze T, et al. QT prolongation and cardiac toxicity of new tuberculosis drugs in Europe: a Tuberculosis Network European Trials group (TBnet) study. Eur Respir J. 53 (2018) 1800537. 10.1183/13993003.00537-2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


