Abstract
BACKGROUND:
Health state utility values (HSUVs) are among the most influential attributes of cost-effectiveness analyses (CEAs). Our objective was to evaluate whether industry-funded studies select systematically different HSUVs compared to studies without industry funding.
METHODS:
Among ten diseases with high disease burden in the United States, we further identified 31 progressive health states. We then searched the Tufts Medical Center’s CEA Registry to identify studies that included HSUVs and were submitted to the registry between 2002 and 2019. Two reviewers mapped the free-text descriptions of health states onto the 31 pre-defined health states. We analyzed the effect of industry funding on the point estimates of these HSUVs with a beta regression. We also analyzed the difference between related health states within studies by funding source with a linear regression.
RESULTS:
After identifying 26,222 HSUVs from 4,198 CEAs, we matched 2,573 HSUVs to the 31 pre-defined health states. We observed large variations within each health state: 12 out of 31 health states included a range of HSUVs greater than 0.5. The point estimate model showed 1 statistically significant difference out of 31 comparisons between studies with any industry funding and those without. The utility difference model found 3 significant difference out of 39 comparisons between CEAs with any industry funding and those without.
LIMITATIONS:
Inclusion of unpublished CEAs may have affected our conclusions about the effect of industry funding on selection of HSUVs. We also relied on free-text descriptions of health states available in the CEA Registry and did not include adjustment for multiple comparisons.
CONCLUSION:
Limited evidence exists that industry-funded studies select different HSUVs compared to non-industry-funded studies for the health states we considered.
Introduction
Health-related quality of life, as measured by health state utility values (HSUVs), is central to the economic evaluation of health interventions. The selection of HSUVs is often a source of variation in value assessments, indicating that they can meaningfully impact policy and reimbursement decisions.1–4 There is little understanding of how HSUVs are chosen, however, and insufficient detail is often reported about this selection process.5,6
Various factors have contributed to substantial uncertainty in HSUVs. Within direct utility elicitation, different methods can provide significantly different HSUVs. The use of indirect assessments, like the EuroQol EQ-5D, can result in different HSUVs for the same health state.7–10 Sourcing HSUVs from patient, general population, or clinical expert samples can also contribute to variation.11,12
Given substantial variability in available HSUVs, analysts could leverage the heterogeneity of HSUV estimates with the implicit goal of improving the estimated value of their product. The HSUVs may be chosen, for example, to create a larger difference between early and advanced disease for a drug that delays disease progression. Alternatively, a drug that offers the possibility of a cure may choose lower HSUVs for disease-related health states in order to maximize the estimated health benefit of returning to full health. Our study aimed to evaluate whether HSUVs selected for industry-funded studies differ from HSUVs in studies without industry funding. While this cannot identify the effect of any difference on the results of these CEAs, it does provide motivation and direction for future work into the interpretation of CEAs.
Methods
Selection of Diseases and Health States for Inclusion
We used the Institute for Health Metrics and Evaluation’s Global Burden of Disease data to identify ten diseases that cause over one million disability-adjusted life-years annually in the U.S.13 We excluded episodic health conditions (e.g., migraine headache) due to challenges in modeling their impact on quality of life. We created a list of health states based on a minimum set of states needed to describe progression within these diseases. This process resulted in a total of 31 predefined health states.
Health State Utility Values in the Tufts Medical Center CEA Registry
The Tufts Medical Center’s CEA Registry contains information pertaining to published CEAs employing quality-adjusted life-years as an outcome measure.14 Information extracted from each CEA includes the HSUVs used, methodological details (e.g., time horizon, perspective), and incremental cost-effectiveness ratios (ICERs) from the results. Among CEAs submitted to the Registry between 2002 and 2019, we extracted all HSUVs used, including the free-text description of each corresponding health state and the study’s funding source by type (e.g., industry, academic). We classified the funding source into categorical variables indicating exclusive, partial, or no industry funding.
Because the descriptions of health states that accompanied HSUVs were not standardized in the CEA Registry, two reviewers (NH and KP) reclassified the HSUVs into one of the 31 pre-designated health states based on the free-text description. HSUVs that did not fit into one of these health states were excluded from the analytic dataset, as were studies that did not mention the funding source. We also excluded HSUVs that contained explicit mention of a certain therapy (e.g., “uncomplicated diabetes with insulin”) and HSUVs related to adverse events (e.g., diabetes due to antipsychotic use), since these were less generalizable. Before independent assessment by two reviewers, a 10% overlapping sample of articles was used to assess inter-rater agreement. We found a Cohen’s kappa of 0.80, which indicates substantial to near perfect agreement.15
Point Estimate Model
In our first of two analyses, we examined the association between HSUV point estimates and funding source across studies. For the point estimate model, we used a beta regression with HSUV as the dependent variable. Included independent variables were health state, funding source, country setting of the CEA, disease area, and year of publication. The regression also included an interaction term between health state and funding source. A more detailed description of the models is in Appendix 1. The models for CEAs with exclusive industry funding and any industry funding were run separately. All analyses were conducted in Stata 16.1 (College Station, Texas).
Utility Difference Model
For our second model, we analyzed the difference between HSUVs within studies by funding source. We began the utility difference model by identifying the differences between HSUVs that reflect increasing levels of severity within the same disease state on a study-by-study basis. We then used an ordinary least squares linear regression with this difference as the dependent variable and the same independent variables as the point estimate model.
Results
Description of health state utility values
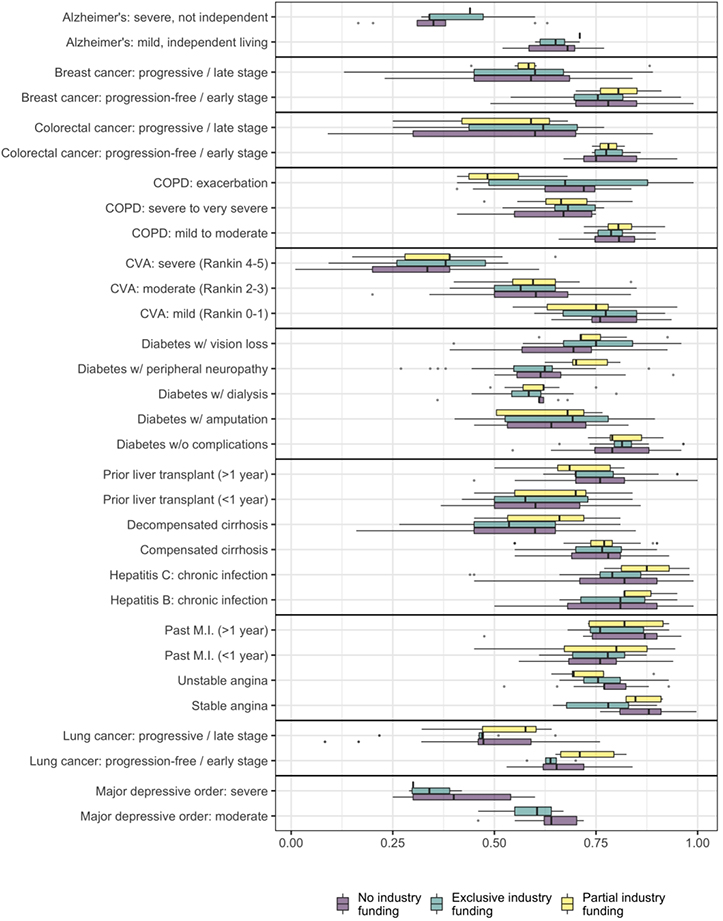
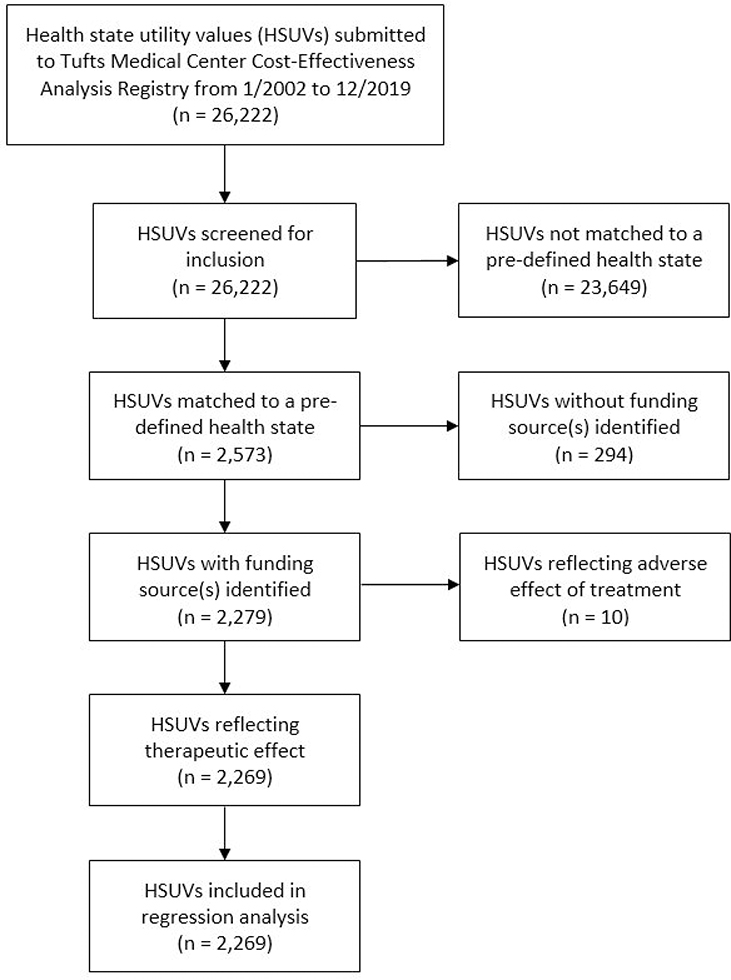
Of 26,222 HSUVs from 4,198 CEAs, we were able to classify 2,573 HSUVs into one of our 31 pre-defined health states. We excluded 294 health states for which the CEA did not indicate the funding source plus an additional ten that were related to adverse effects. This produced a final analytic dataset of 2,269 HSUVs (Appendix 2). These included 873 (38%) HSUVs extracted from CEAs with exclusive industry funding and 92 (4%) HSUVs from studies with partial industry funding (Figure 1). Among these were 1,011 unique health state-specific values, indicating relatively low rates of HSUV reuse between studies.
Figure 1/.
Distribution of cited health state utility values by health state and funding source. CVA: cerebrovascular accident, COPD: chronic obstructive pulmonary disease, MI: myocardial infarction; boxes indicate the area between the 25th and 75th percentiles, while whiskers indicate the largest value up to 1.5 times the distance between the 25th and 75th percentiles.
Point estimate model
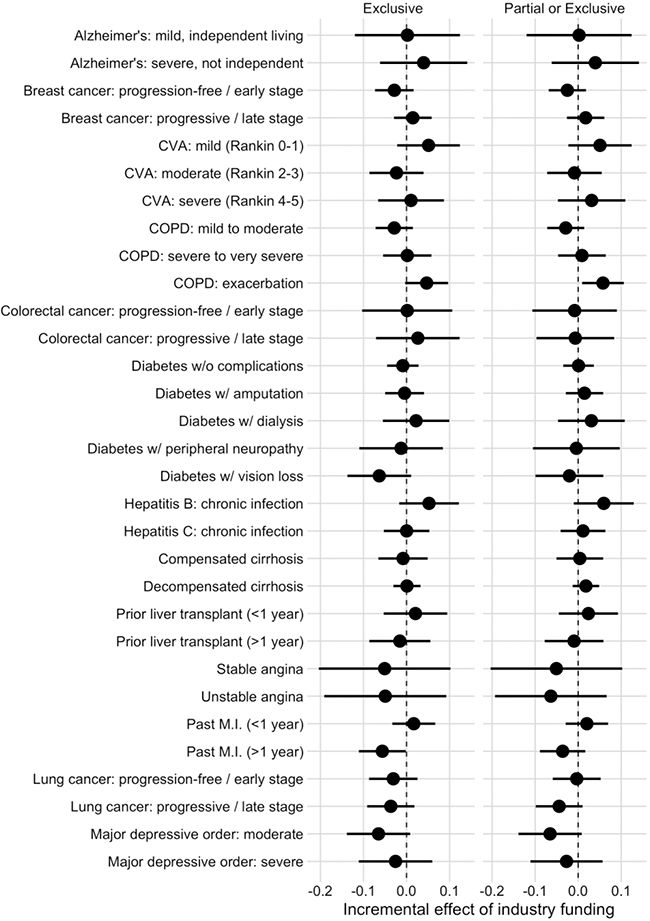
One health state out of 31 showed a significant difference between funding sources in each of the two models (Figure 2). In the model examining the association between funding source and HSUVs, the health state “Past myocardial infarction (>1 year prior)” was 0.056 (95% confidence interval: 0.002 to 0.111) lower in exclusively industry-funded studies. When studies with any industry funding were compared to those without, the health state “Chronic obstructive pulmonary disease: exacerbation” was 0.058 (0.009 to 0.106) higher in industry-funded studies. Full results are in Appendix 3.
Figure 2/.
Incremental effect of industry funding on included health state utility values, with 95% confidence interval
CVA: cerebrovascular accident, COPD: chronic obstructive pulmonary disease, MI: myocardial infarction
Utility difference model
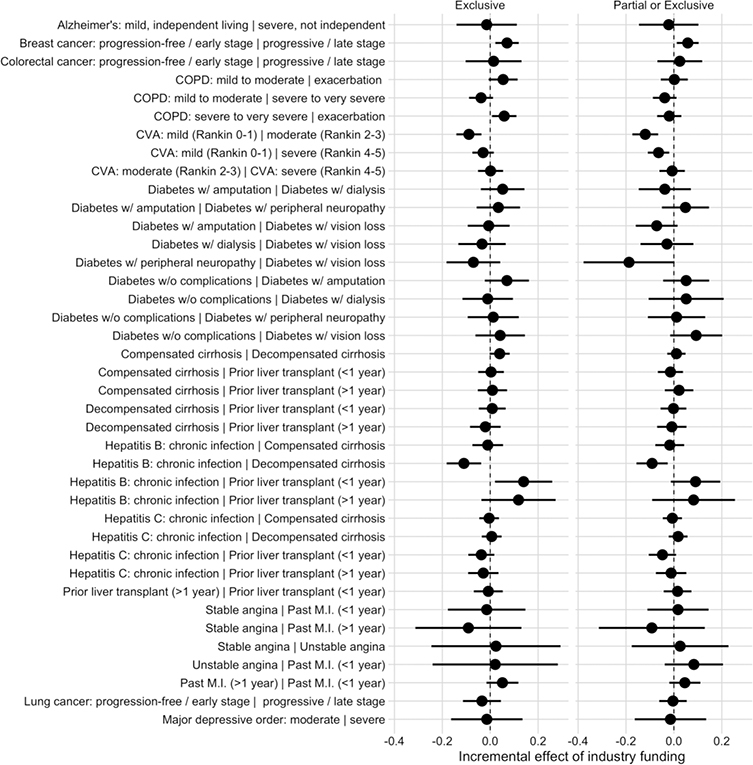
We identified a total of 2,808 HSUV differences from 39 pairs of health states for inclusion in the utility difference model. Five HSUV differences were significantly different between exclusively industry-funded studies and those without exclusive industry funding (Figure 3). The largest of these differences was between the states “Chronic Hepatitis B infection” and “Prior liver transplant (<1 year prior)”, which was 0.139 (0.020 to 0.259) greater in exclusively industry-funded studies compared to studies with other funding sources. Full results are in Appendix 4.
Figure 3/.
Incremental effect of industry funding on the difference between health state utility values within diseases, with 95% confidence interval
CVA: cerebrovascular accident, COPD: chronic obstructive pulmonary disease, MI: myocardial infarction
Three of these five HSUV differences remained significant when we compared health state pairs from studies with any industry funding to those without any industry funding. Of these three, the largest was the difference between mild and moderate cerebrovascular accident, which was 0.120 (0.066 to 0.174) smaller in industry-funded versus non-industry-funded studies.
Discussion
Using regression analysis on a sample of HSUVs collected from CEAs published over 18 years, we found limited support for the hypothesis that industry funding affects the selection of HSUVs. Point estimates of HSUVs are largely not significantly different between CEAs with and without industry funding. However, the difference between HSUVs for related health states within studies was significantly different more often between industry-funded and non-industry-funded studies.
There was substantial variation in the HSUVs assigned to each state, as well as relatively low rates of unmodified reuse for HSUVs across studies, suggesting that the possibility of manipulating the selection of HSUVs was not limited by a lack of choice: for each health state we examined there was an ample range of possible values from which to select.
While several studies have shown that industry-funded CEAs produce more favorable ICERs, little has been published on sponsorship bias in the selection of HSUVs.16–19 Bilcke et al. found no statistically significant difference between the utility decrements assigned to herpes zoster disease in industry-funded and non-industry-funded CEAs.20 Other studies have focused on how industry sponsorship is associated with choosing the most favorable from several available outcome measures and with lower assumed effectiveness of comparator products.21,22
Our study is limited in several ways. We would have liked to have included the source population for each HSUV (e.g., patients, clinical experts) in the regression analysis, but these data were not collected in the CEA Registry. Despite good levels of inter-rater agreement, errors in the standardization of HSUVs into health states, such as misclassifying health states or misinterpreting disutility as utility, may have occurred. We see little reason to suspect that these errors would have correlated with funding source, though. We also faced the issue of publication bias. It is possible that unpublished CEAs used for internal purposes by payers or industry may select HSUVs based on different criteria. No database that includes these CEAs exists, however. Finally, it is possible that our few seemingly significant results stemmed from multiple comparisons. However, given the complex correlation structure of our data and the hypothesis generating nature of this study, we did not attempt to correct for this issue.
Future research in this area should concentrate on identifying other possible sources of unexplained variation between CEAs. Work also remains on standardizing the reporting and selection of HSUVs which, despite the existence of best practice guidelines, continues to be heterogeneous in methodology and quality.23
Conclusion
As drug prices and the stakes of coverage decisions increase, it has become important to improve awareness among decision-makers about how CEAs can be used to advantage some products over others. Our study, though, finds little evidence that industry-sponsored studies select different HSUVs versus non-industry-sponsored studies. However, future studies are needed to determine whether this also translates to a lack of sponsorship bias in the results of published CEAs. Because HSUVs are an important source of variation in CEAs, scenario and one-way sensitivity analyses – as well as transparency about how and why certain HSUVs were used – will continue to be valuable in understanding the effects of selecting one set of HSUVs over another, regardless of the study’s funding source.
Acknowledgments
NH was supported during this work by a training grant from the Agency for Healthcare Research and Quality (T32 HS013853) and from the National Cancer Institute at the National Institutes of Health (T32 CA09168). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ or NIH.
Appendix 1: Model details
Point estimate model
We used a beta regression with health state utility value (HSUV) as the independent variable. To improve the performance of the beta regression near the extremes (i.e., 0 and 1), we used the transformation described in Hunger, et al.1, which slightly shrinks extreme values toward the midpoint of 0.5:
where Y is the unmodified utility value and N is the total number of values included in the analytic dataset.
The independent variables were health state (categorical), a binary indicator for industry funding, country in which the published cost-effectiveness study was set (categorical), disease (categorical), and year of publication (categorical). There was an interaction term between health state and the funding indicator. The setting variable was transformed from a country-level indicator to a continent-level indicator for countries that had fewer than ten included HSUVs. Year of publication was binned into four categories: 2002–2005, 2006–2010, 2011–2015, and 2015–2019.
The regression used was:
where:
y : utility value of the health state
β0 : intercept coefficient
β1/x1 : (coefficient for) categorical indicator for health state
β2/x2 : (coefficient for) binary indicator for industry funding
β3/x3 : (coefficient for) categorical indicator for setting of published study, either country or continent (the latter for countries with fewer than ten HSUVs included)
β4/x4 : (coefficient for) categorical indicator for disease area containing the health state
β5/x5 : (coefficient for) categorical indicator for year of publication, binned as described above
β6 : interaction term for health state and funding
We performed this regression twice: once where only exclusively-funded studies were considered to be industry-funded and once for studies with any industry funding.
The Stata commands used were:
betareg utility exclusive#state setting disease year margins r.exclusive, over(state)
and
betareg utility partial#state setting disease year margins r.partial, over(state)
Note that the partial variable above includes both partially and exclusively industry-funded studies.
Utility difference model
We used an ordinary least squares regression (OLS) to model the differences between health states within a given disease area on a study-by-study basis. Our motivation was to determine whether industry-funded studies had notable differences between health states, since these differences are more likely to impact the overall utility of an intervention. Because we examined differences, we did not apply the transformation of Hunger, et al. that we applied to the point estimate model.
The dependent variable for the OLS regression was the difference between two health states within the same disease area. The independent variables were the pair of health states (categorical), a binary indicator for industry funding, country in which the published cost-effectiveness study was set (categorical), disease (categorical), and year of publication (categorical). There was an interaction between the pair of health states and industry funding. We applied the same transformations to the setting and year of publication as described in the point estimate model.
The regression used was:
where:
y : difference in utility values of the health states
β0 : intercept coefficient
β1/x1 : (coefficient for) categorical indicator for pair of health states
β2/x2 : (coefficient for) binary indicator for industry funding
β3/x3 : (coefficient for) categorical indicator for setting of published study, either country or continent (the latter for countries with fewer than ten HSUVs included)
β4/x4 : (coefficient for) categorical indicator for disease area containing the health state
β5/x5 : (coefficient for) categorical indicator for year of publication, binned as described above
β6 : interaction term for the pair of health states and funding
The Stata commands used were:
regress utility exclusive#state setting disease year margins r.exclusive, over(state)
and
regress utility partial#state setting disease year margins r.partial, over(state)
As above, the partial variable above includes both partially and exclusively industry-funded studies.
Appendix 2: PRISMA diagram for included health states
Appendix 3: Detailed results for point estimate model
Incremental effect of industry funding on estimated health state utility value (with 95% confidence interval). Ranges that do not include 0 are italicized.
| Exclusive industry funding | Partial or exclusive industry funding | |||
|---|---|---|---|---|
| State | n / vs. total | Incremental effect | n / vs. total | Incremental effect |
| Alzheimer’s: mild, independent living | 6 / 32 | 0.002 (−0.12 to 0.124) | 13 / 32 | 0.002 (−0.12 to 0.124) |
| Alzheimer’s: severe, not independent | 16 / 61 | 0.04 (−0.062 to 0.141) | 33 / 61 | 0.04 (−0.062 to 0.141) |
| Breast cancer: progression-free / early stage | 30 / 197 | −0.028 (−0.073 to 0.017) | 67 / 197 | −0.025 (−0.069 to 0.018) |
| Breast cancer: progressive / late stage | 65 / 292 | 0.015 (−0.029 to 0.059) | 136 / 292 | 0.017 (−0.026 to 0.061) |
| CVA: mild (Rankin 0–1) | 41 / 205 | 0.051 (−0.022 to 0.124) | 97 / 205 | 0.051 (−0.023 to 0.124) |
| CVA: moderate (Rankin 2–3) | 28 / 163 | −0.023 (−0.086 to 0.04) | 68 / 163 | −0.009 (−0.072 to 0.055) |
| CVA: severe (Rankin 4–5) | 34 / 188 | 0.01 (−0.066 to 0.087) | 81 /188 | 0.031 (−0.047 to 0.11) |
| COPD: mild to moderate | 23 / 97 | −0.029 (−0.072 to 0.015) | 50 / 97 | −0.029 (−0.072 to 0.014) |
| COPD: severe to very severe | 30 / 121 | 0.002 (−0.054 to 0.058) | 68 / 121 | 0.009 (−0.047 to 0.064) |
| COPD: exacerbation | 24 / 99 | 0.047 (−0.003 to 0.097) | 52 / 99 | 0.058 (0.009 to 0.106) |
| Colorectal cancer: progression-free / early stage | 4 / 53 | 0.002 (−0.103 to 0.106) | 10 / 53 | −0.008 (−0.107 to 0.09) |
| Colorectal cancer: progressive / late stage | 8 / 79 | 0.026 (−0.071 to 0.124) | 19 / 79 | −0.007 (−0.097 to 0.084) |
| Diabetes w/o complications | 16 / 109 | −0.009 (−0.045 to 0.028) | 45 / 109 | 0.001 (−0.035 to 0.036) |
| Diabetes w/ amputation | 18 / 87 | −0.005 (−0.05 to 0.041) | 43 / 87 | 0.015 (−0.029 to 0.058) |
| Diabetes w/ dialysis | 20 / 91 | 0.022 (−0.055 to 0.099) | 51 / 91 | 0.031 (−0.047 to 0.108) |
| Diabetes w/ peripheral neuropathy | 24 / 108 | −0.013 (−0.11 to 0.085) | 58 / 108 | −0.004 (−0.105 to 0.097) |
| Diabetes w/ vision loss | 13 / 85 | −0.063 (−0.137 to 0.011) | 38 / 85 | −0.02 (−0.099 to 0.058) |
| Hepatitis B: chronic infection | 18 / 101 | 0.052 (−0.017 to 0.122) | 39 / 101 | 0.059 (−0.01 to 0.129) |
| Hepatitis C: chronic infection | 53 / 293 | 0 (−0.053 to 0.053) | 124 / 293 | 0.011 (−0.041 to 0.063) |
| Compensated cirrhosis | 48 / 293 | −0.008 (−0.065 to 0.049) | 121 / 293 | 0.004 (−0.05 to 0.058) |
| Decompensated cirrhosis | 39 / 262 | 0.001 (−0.031 to 0.033) | 100 / 262 | 0.018 (−0.013 to 0.049) |
| Prior liver transplant (<1 year) | 28 / 159 | 0.021 (−0.053 to 0.094) | 71 /159 | 0.024 (−0.045 to 0.093) |
| Prior liver transplant (>1 year) | 22 / 124 | −0.016 (−0.087 to 0.055) | 54 / 124 | −0.01 (−0.078 to 0.059) |
| Stable angina | 6 / 47 | −0.051 (−0.204 to 0.102) | 17 / 47 | −0.051 (−0.204 to 0.102) |
| Unstable angina | 4 / 47 | −0.049 (−0.191 to 0.092) | 16 / 47 | −0.063 (−0.193 to 0.066) |
| Past M.I. (<1 year) | 23 / 146 | 0.017 (−0.033 to 0.067) | 61 / 146 | 0.02 (−0.029 to 0.07) |
| Past M.I. (>1 year) | 21 / 106 | −0.056 (−0.111 to −0.002) | 49 / 106 | −0.036 (−0.089 to 0.017) |
| Lung cancer: progression-free / early stage | 6 / 95 | −0.031 (−0.087 to 0.026) | 23 / 95 | −0.003 (−0.059 to 0.052) |
| Lung cancer: progressive / late stage | 10 / 123 | −0.037 (−0.092 to 0.018) | 30 / 123 | −0.044 (−0.099 to 0.01) |
| Major depressive order: moderate | 4 / 24 | −0.065 (−0.139 to 0.008) | 8 / 24 | −0.065 (−0.139 to 0.008) |
| Major depressive order: severe | 4 / 33 | −0.026 (−0.111 to 0.06) | 9 / 33 | −0.027 (−0.111 to 0.057) |
Appendix 4: Detailed results for utility difference model
Incremental effect of industry funding on the difference between health state utility values within diseases (with 95% confidence interval). Ranges that do not include 0 are italicized.
| Exclusive industry funding | Partial or exclusive industry funding | |||
|---|---|---|---|---|
| State comparison | n / vs. total | Incremental effect | n / vs. total | Incremental effect |
| Alzheimer’s: mild, independent living | severe, not independent | 7 / 17 | −0.015 (−0.141 to 0.111) | 8 / 17 | −0.022 (−0.146 to 0.102) |
| Breast cancer: progression-free / early stage | progressive / late stage | 36 / 152 | 0.07 (0.021 to 0.119) | 44 / 152 | 0.058 (0.012 to 0.104) |
| Colorectal cancer: progression-free / early stage | progressive / late stage | 5 / 120 | 0.014 (−0.103 to 0.131) | 8 / 120 | 0.025 (−0.069 to 0.118) |
| COPD: mild to moderate | exacerbation | 24 / 93 | 0.054 (−0.007 to 0.115) | 31 / 93 | 0.002 (−0.055 to 0.058) |
| COPD: mild to moderate | severe to very severe | 44 / 108 | −0.038 (−0.089 to 0.012) | 54 / 108 | −0.038 (−0.088 to 0.011) |
| COPD: severe to very severe | exacerbation | 42 / 105 | 0.058 (0.007 to 0.11) | 55 / 105 | −0.019 (−0.07 to 0.031) |
| CVA: mild (Rankin 0–1) | moderate (Rankin 2–3) | 51 / 96 | −0.089 (−0.142 to −0.037) | 60 / 96 | −0.12 (−0.174 to −0.066) |
| CVA: mild (Rankin 0–1) | severe (Rankin 4–5) | 59 /132 | −0.03 (−0.075 to 0.015) | 73 / 132 | −0.064 (−0.109 to −0.019) |
| CVA: moderate (Rankin 2–3) | CVA: severe (Rankin 4–5) | 49 / 96 | 0.001 (−0.051 to 0.054) | 57 / 96 | −0.007 (−0.061 to 0.046) |
| Diabetes w/ amputation | Diabetes w/ dialysis | 20 / 33 | 0.052 (−0.039 to 0.143) | 26 / 33 | −0.038 (−0.147 to 0.071) |
| Diabetes w/ amputation | Diabetes w/ peripheral neuropathy | 24 / 36 | 0.034 (−0.057 to 0.125) | 27 / 36 | 0.048 (−0.05 to 0.147) |
| Diabetes w/ amputation | Diabetes w/ vision loss | 14 / 36 | −0.006 (−0.094 to 0.081) | 22 / 36 | −0.072 (−0.159 to 0.016) |
| Diabetes w/ dialysis | Diabetes w/ vision loss | 10 / 30 | −0.034 (−0.133 to 0.065) | 23 / 30 | −0.029 (−0.14 to 0.082) |
| Diabetes w/ peripheral neuropathy | Diabetes w/ vision loss | 11 / 21 | −0.07 (−0.183 to 0.042) | 19 / 21 | −0.187 (−0.377 to 0.003) |
| Diabetes w/o complications | Diabetes w/ amputation | 15 / 31 | 0.07 (−0.023 to 0.162) | 20 / 31 | 0.051 (−0.045 to 0.147) |
| Diabetes w/o complications | Diabetes w/ dialysis | 17 / 26 | −0.011 (−0.116 to 0.095) | 23 / 26 | 0.051 (−0.106 to 0.208) |
| Diabetes w/o complications | Diabetes w/ peripheral neuropathy | 16 / 25 | 0.013 (−0.094 to 0.119) | 19 / 25 | 0.011 (−0.109 to 0.131) |
| Diabetes w/o complications | Diabetes w/ vision loss | 10 / 26 | 0.041 (−0.062 to 0.145) | 18 / 26 | 0.093 (−0.016 to 0.202) |
| Compensated cirrhosis | Decompensated cirrhosis | 52 / 185 | 0.039 (−0.003 to 0.081) | 75 / 185 | 0.01 (−0.028 to 0.049) |
| Compensated cirrhosis | Prior liver transplant (<1 year) | 37 / 96 | 0.003 (−0.05 to 0.057) | 55 / 96 | −0.014 (−0.067 to 0.038) |
| Compensated cirrhosis | Prior liver transplant (>1 year) | 29 / 74 | 0.009 (−0.052 to 0.07) | 40 / 74 | 0.022 (−0.038 to 0.081) |
| Decompensated cirrhosis | Prior liver transplant (<1 year) | 33 / 90 | 0.008 (−0.048 to 0.065) | 48 / 90 | −0.002 (−0.056 to 0.052) |
| Decompensated cirrhosis | Prior liver transplant (>1 year) | 25 / 69 | −0.02 (−0.085 to 0.044) | 35 / 69 | −0.009 (−0.07 to 0.053) |
| Hepatitis B: chronic infection | Compensated cirrhosis | 22 / 81 | −0.01 (−0.075 to 0.054) | 27 / 81 | −0.017 (−0.078 to 0.043) |
| Hepatitis B: chronic infection | Decompensated cirrhosis | 16 / 78 | −0.11 (−0.182 to −0.038) | 21 / 78 | −0.091 (−0.156 to −0.026) |
| Hepatitis B: chronic infection | Prior liver transplant (<1 year) | 6 / 26 | 0.139 (0.02 to 0.259) | 10 / 26 | 0.09 (−0.013 to 0.194) |
| Hepatitis B: chronic infection | Prior liver transplant (>1 year) | 6 / 11 | 0.118 (−0.037 to 0.273) | 8 / 11 | 0.082 (−0.091 to 0.255) |
| Hepatitis C: chronic infection | Compensated cirrhosis | 63 / 163 | −0.005 (−0.046 to 0.037) | 79 / 163 | −0.006 (−0.046 to 0.034) |
| Hepatitis C: chronic infection | Decompensated cirrhosis | 56 / 175 | 0.006 (−0.035 to 0.048) | 70 / 175 | 0.018 (−0.022 to 0.057) |
| Hepatitis C: chronic infection | Prior liver transplant (<1 year) | 51 / 90 | −0.037 (−0.092 to 0.017) | 60 / 90 | −0.048 (−0.105 to 0.009) |
| Hepatitis C: chronic infection | Prior liver transplant (>1 year) | 31 / 65 | −0.029 (−0.093 to 0.035) | 36 / 65 | −0.012 (−0.075 to 0.052) |
| Prior liver transplant (>1 year) | Prior liver transplant (<1 year) | 28 / 78 | −0.008 (−0.069 to 0.053) | 38 / 78 | 0.015 (−0.043 to 0.073) |
| Past M.I. (>1 year) | Past M.I. (<1 year) | 24 / 62 | 0.051 (−0.016 to 0.118) | 31 / 62 | 0.046 (−0.019 to 0.111) |
| Stable angina | Past M.I. (<1 year) | 3 / 18 | −0.015 (−0.177 to 0.147) | 6 / 18 | 0.017 (−0.111 to 0.145) |
| Stable angina | Past M.I. (>1 year) | 2 / 6 | −0.091 (−0.313 to 0.13) | 2 / 6 | −0.092 (−0.314 to 0.129) |
| Stable angina | Unstable angina | 1 / 10 | 0.024 (−0.246 to 0.294) | 2 / 10 | 0.026 (−0.176 to 0.228) |
| Unstable angina | Past M.I. (<1 year) | 1 / 23 | 0.021 (−0.241 to 0.282) | 6 / 23 | 0.083 (−0.038 to 0.205) |
| Lung cancer: progression-free / early stage | progressive / late stage | 11 /210 | −0.035 (−0.114 to 0.045) | 22 / 210 | −0.004 (−0.062 to 0.054) |
| Major depressive order: moderate | severe | 4 / 15 | −0.014 (−0.164 to 0.135) | 4 / 15 | −0.014 (−0.164 to 0.135) |
Footnotes
NH declares no conflicts of interest.
DDK is an employee of the Center for the Evaluation of Value and Risk in Health at Tufts Medical Center, maintaining the CEA Registry, which is used as a data source for this study. The CEA Registry is supported by subscription revenue from pharmaceutical and device companies, academic institutions, and government agencies.
KSP declares no conflicts of interest.
BD declares no conflicts of interest.
Hunger M, Döring A, Holle R. Longitudinal beta regression models for analyzing health-related quality of life scores over time. BMC medical research methodology. 2012 Dec 1;12(1):144.
References
- 1.Jeong K, Cairns J. Systematic review of health state utility values for economic evaluation of colorectal cancer. Health economics review. 2016;6(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorat T, Neumann PJ, Chambers JD. Hemophilia burden of disease: a systematic review of the cost-utility literature for hemophilia. Journal of managed care & specialty pharmacy. 2018;24(7):632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han R, François C, Toumi M. Systematic review of health state utility values used in European pharmacoeconomic evaluations for chronic hepatitis C: impact on cost-effectiveness results. Applied Health Economics and Health Policy. Published online 2020:1–16. [DOI] [PubMed] [Google Scholar]
- 4.Ara R, Hill H, Lloyd A, Woods HB, Brazier J. Are Current Reporting Standards Used to Describe Health State Utilities in Cost-Effectiveness Models Satisfactory? Value in Health. 2020;23(3):397–405. [DOI] [PubMed] [Google Scholar]
- 5.Papaioannou D, Brazier J, Paisley S. Systematic searching and selection of health state utility values from the literature. Value in health. 2013;16(4):686–695. [DOI] [PubMed] [Google Scholar]
- 6.Ara R, Brazier J, Peasgood T, Paisley S. The identification, review and synthesis of health state utility values from the literature. Pharmacoeconomics. 2017;35(1):43–55. [DOI] [PubMed] [Google Scholar]
- 7.Arnold D, Girling A, Stevens A, Lilford R. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. Bmj. 2009;339:b2688. [DOI] [PubMed] [Google Scholar]
- 8.Doctor JN, Bleichrodt H, Lin HJ. Health utility bias: a systematic review and meta-analytic evaluation. Medical Decision Making. 2010;30(1):58–67. [DOI] [PubMed] [Google Scholar]
- 9.McDonough CM, Tosteson TD, Tosteson AN, Jette AM, Grove MR, Weinstein JN. A longitudinal comparison of 5 preference-weighted health state classification systems in persons with intervertebral disk herniation. Medical Decision Making. 2011;31(2):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briggs AH, Belozeroff V, Feeny D. Comparison of health state utility estimates from instrument-based and vignette-based methods: a case study in kidney disease. BMC research notes. 2019;12(1):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Expert review of pharmacoeconomics & outcomes research. 2010;10(5):553–566. [DOI] [PubMed] [Google Scholar]
- 12.Meregaglia M, Cairns J. A systematic literature review of health state utility values in head and neck cancer. Health and quality of life outcomes. 2017;15(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorsey ER, Elbaz A, Nichols E, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology. 2018;17(11):939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Center for the Evaluation of Value and Risk in Health. The Cost-Effectiveness Analysis Registry [Internet]. (Boston: ), Institute for Clinical Research and Health Policy Studies, Tufts Medical Center. Available from: www.cearegistry.org Accessed on August 6, 2020. [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. biometrics. Published online 1977:159–174. [PubMed] [Google Scholar]
- 16.Baker CB, Johnsrud MT, Crismon ML, Rosenheck RA, Woods SW. Quantitative analysis of sponsorship bias in economic studies of antidepressants. The British Journal of Psychiatry. 2003;183(6):498–506. [DOI] [PubMed] [Google Scholar]
- 17.Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. Bmj. 2006;332(7543):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catala-Lopez F, Sanfelix-Gimeno G, Ridao M, Peiro S. When are statins cost-effective in cardiovascular prevention? A systematic review of sponsorship bias and conclusions in economic evaluations of statins. PloS one. 2013;8(7):e69462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DD, Silver MC, Kunst N, Cohen JT, Ollendorf DA, Neumann PJ. Perspective and Costing in Cost-Effectiveness Analysis, 1974–2018. PharmacoEconomics. Published online 2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilcke J, Verelst F, Beutels P. Sponsorship bias in base-case values and uncertainty bounds of health economic evaluations? A systematic review of herpes zoster vaccination. Medical Decision Making. 2018;38(6):730–745. [DOI] [PubMed] [Google Scholar]
- 21.Polyzos NP, Valachis A, Mauri D, Ioannidis JP. Industry involvement and baseline assumptions of cost-effectiveness analyses: diagnostic accuracy of the Papanicolaou test. CMAJ. 2011;183(6):E337–E343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peura PK, Martikainen JA, Purmonen TT, Turunen JH. Sponsorship-related outcome selection bias in published economic studies of triptans: systematic review. Medical Decision Making. 2012;32(2):237–245. [DOI] [PubMed] [Google Scholar]
- 23.Brazier J, Ara R, Azzabi I, et al. Identification, review, and use of health state utilities in cost-effectiveness models: an ISPOR good practices for outcomes research task force report. Value in Health. 2019;22(3):267–275. [DOI] [PubMed] [Google Scholar]