Abstract
Platelets play an essential role in maintaining vascular integrity after injury. In addition, platelets contribute to the immune response to pathogens. For instance, they express receptors that mediate binding of viruses, and toll-like receptors that activate the cell in response to pathogen-associated molecular patterns. Platelets can be beneficial and/or detrimental during viral infections. They reduce blood-borne viruses by engulfing the free virus and presenting the virus to neutrophils. However, platelets can also enhance inflammation and tissue injury during viral infections. Here, we will discuss the roles of platelets in viral infection.
1. Introduction
Platelets are released by megakaryocytes in large numbers into the blood.1 The main function of these small anucleate cells is to maintain vascular integrity after injury of the vasculature. Uncontrolled platelet activation can also lead to intravascular thrombosis. Platelets contain receptors that mediate adhesion to the damaged vessel wall, such as glycoprotein (GP) Ib-V-IX, GPVI and α2β1, and receptors that respond to soluble agonists, such as P2Y12, thromboxane receptor and protease-activated receptor (PAR) 1 and 4.2 Platelets contain three different types of granule. Dense (δ) granules contain mediators that regulate vascular tone, such as nucleotides (e.g. ADP and GTP), bioactive amides (e.g. histamine and serotonin) and bioactive ions (e.g. Ca2+ and PO3−). Alpha (α) granules contain proteins that can be classified into five groups: adhesion molecules, platelet microbicidal proteins and kinocidins, mitogenic factors, coagulation factors and protease inhibitors. Finally, lysosomal (λ) granules contain enzymes, such as proteases and glycosidases, that can modulate platelet-fibrin retraction needed for wound healing.3 A variety of platelet inhibitors, including acetylsalicylic acid (ASA) and P2Y12 receptor antagonists, are used to prevent arterial thrombosis.2
1.1. Immune functions of platelets
Platelets play a role in the immune response to pathogens.4 They express a variety of receptors, including lectins, integrins, and toll-like receptors (TLRs), that mediate pathogen binding and cellular activation (Figure).5–9 In addition, human platelets express Fc receptor FcγRIIA, which recognize immune complexes.10 Infections are often associated with thrombocytopenia due to increased platelet activation.8, 9 Activated platelets release extracellular vesicles (EVs) that express CD62P (P-selectin) and contain an array of chemokines, such as CCL5 (RANTES), and cytokines, such as IL-1β.11 Activated platelets also bind to leukocytes, including monocytes, neutrophils, eosinophils and lymphocytes. This binding is mediated by various receptor-receptor pairs, such as platelet CD62P and leukocyte P-selectin glycoprotein ligand 1 (PSGL-1) and platelet CD40 and leukocyte CD154 (CD40 ligand, CD40L) and platelet GPIbα and leukocyte MAC-1 (CD11b/CD18).11 Platelet binding induces gene expression and cellular response in the leukocytes.11, 12 Platelet-leukocyte aggregates are used as a marker of platelet activation in infectious diseases. Moreover, platelet binding to neutrophils and eosinophils can induce the formation of extracellular traps, such as neutrophil extracellular traps (NETs).11–16 This extracellular DNA immobilizes pathogens but can also contribute to thrombosis and tissue damage.17, 18 Platelets modulate natural killer (NK) cell activity by either direct interaction or indirectly by releasing mediators, including TGFβ and EVs.19, 20 Platelets also facilitate the recruitment of a variety of inflammatory cells, including neutrophils, cytotoxic T cells and dendritic cells, to sites of inflammation.21, 22 Platelet-derived CXCL4 (platelet factor 4, PF4), CCL5 and serotonin activate T cells.23 Platelets bind to and activate dendritic cells through JAM C-Mac1, CD62P-PSGL-1 and CD40-CD154 interactions.11, 24 Finally, platelets contribute to adaptive immunity by increasing IgG1, IgG2 and IgG3 production by B cells.6, 11
Figure. Platelet receptors which mediate virus-platelet interaction and initiate intracellular signaling events.
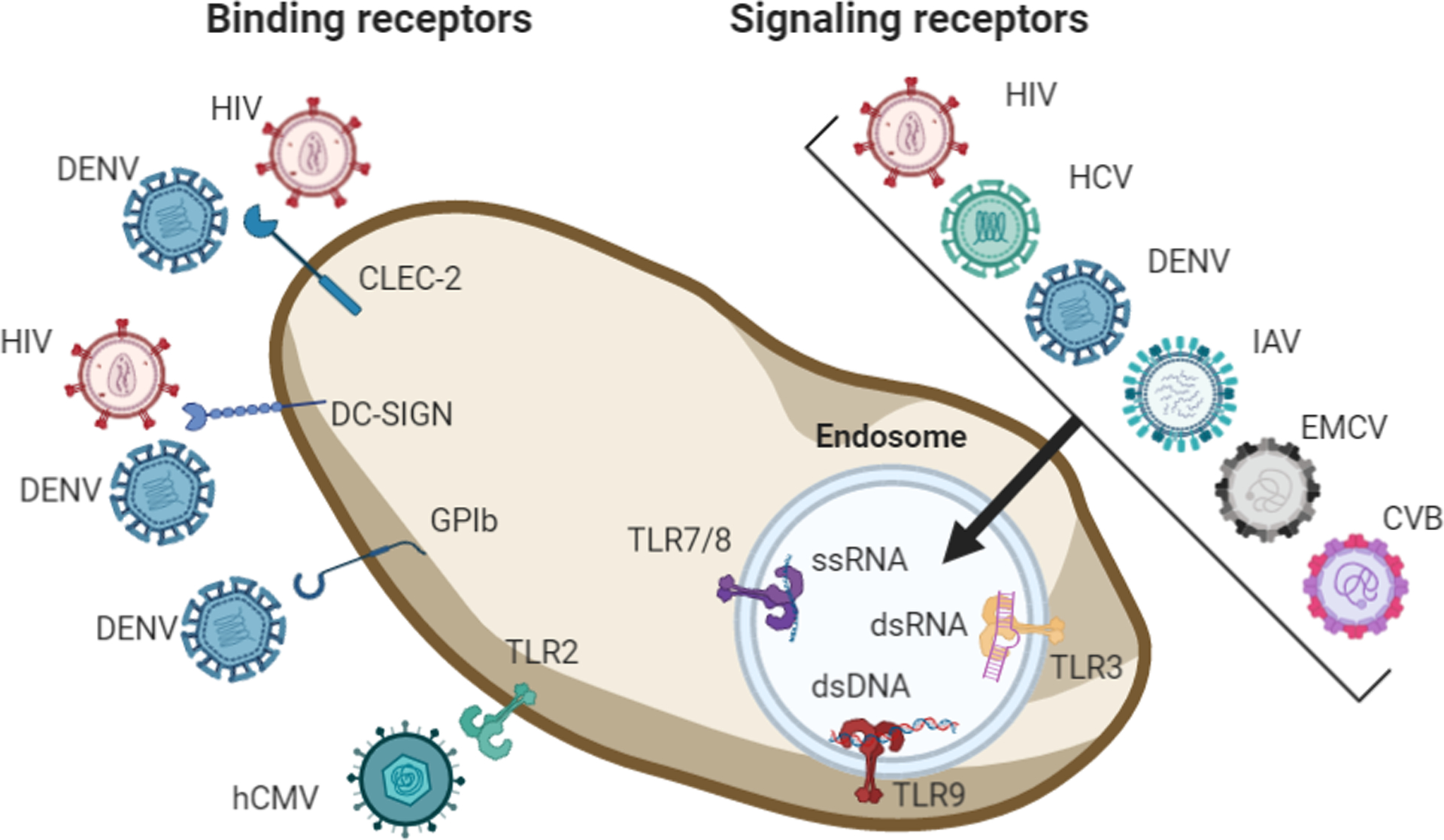
HIV and DENV bind to CLEC-2 and DC-SIGN. DENV binds to GPIb and human CMV (hCMV) interacts with TLR2. Depending on the virus, HIV, HCV, DENV, IAV, EMCV and CVB3 can be detected depending on their genomic state as ssRNA, dsDNA or as dsRNA directly or as genomic intermediate during replication of ssRNA viruses by endosomal expressed TLRs. TLR7/8 detect ssRNA, TLR3 dsRNA and TLR9 dsDNA. Figure created in BioRender.com.
1.2. Platelets and viruses
A variety of viruses, including enteroviruses [Coxsackievirus, CVB; encephalomyocarditis virus, EMCV], influenza A viruses (IAV), coronaviruses (CoV), dengue virus (DENV), human immunodeficiency virus type-1 (HIV-1), hepatitis C virus (HCV), herpes [herpes simplex virus type 1, HSV-1; cytomegalovirus, CMV; vaccinia virus], and adenoviruses, have been shown to bind to and activate platelets (Figure).9, 11, 25 Larger DNA viruses from the herpes viral family, such as HSV-1, bind to platelets but are not internalized.26–28 Binding of human CMV to TLR2 on platelets triggers platelet binding to neutrophils as well as to T cells, B cells and dendritic cells.28 In contrast, smaller RNA viruses, such as CVB, EMCV, DENV, IAV, HIV-1, and HCV, bind to platelets and are internalized.6, 29–33 HIV-1 and DENV bind to two C-type lectins called dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) and C-type lectin-like receptor 2 (CLEC-2) (Figure).34, 35 In addition, DENV binds to GPIb.36 Interestingly, DENV replicates in platelets.34 A study found that DENV and H5N1 IAV activation of CLEC-2 increases release of EVs.13 Immune complexes formed during infection, such as IAV, can activate human platelets via platelet FcγRIIA signaling.10
Platelets express all 10 TLRs.37, 38 They recognize viral single-stranded (ss) RNA or double-stranded (ds) RNA via the intracellular receptors TLR7/8 or TLR3, respectively (Figure). DsRNA is a by-product of ssRNA virus replication. Stimulation of platelet TLR7 leads to cell surface expression of CD62P and formation of platelet-neutrophil aggregates whereas TLR3 stimulation does not induce CD62P surface expression but increases platelet responsiveness to thromboxane, ADP or collagen stimulation.11, 30, 39 In addition, platelets express TLR9 which detects dsDNA from DNA viruses.40
Infection with RNA viruses often induces thrombocytopenia. For instance, DENV infection in humans lead to thrombocytopenia and hemorrhagic fever. DENV infection of rhesus macaques led to the formation of platelet-monocyte aggregates at 24 hours and platelet-neutrophils aggregates 3 days post infection.41 Platelet-monocyte aggregates are also increased during HIV and IAV infection.42, 43 H1N1 IAV binding to platelets also induces release of EVs.10 Parvovirus B19 infection in humans is associated with increased levels of circulating platelet-derived EVs.44 However, parvovirus B19 infection in mice did not increase platelet EV levels in vivo.44 EMCV infection of mice leads to the formation of platelet-neutrophil aggregates 1 hour post infection and thrombocytopenia 24 hours post infection.30 In a mouse model of CVB3 infection, platelets internalize CVB3 and express phosphatidylserine and CD62P on their surface and bind to neutrophils.6 However, CVB3 does not replicate in platelets.6
Platelets can be beneficial or detrimental to the host during virus infection.6, 45, 46 In this review we will focus on the role of platelets in virus infection of the heart and lung.
2. Platelets and virus infection of the heart
Viral infections can result in myocarditis, which is a leading cause of sudden cardiac death in children and young adults.47 Indeed, several different viruses, including enteroviruses, adenoviruses, herpesviruses, HIV, parvovirus B19, and IAV, have been detected in endomyocardial biopsies. CVB3 and EMCV are known to induce viral myocarditis in humans and animals (Table 1). Clinical observations and animal studies showed that enteroviral virus infection causes thrombocytopenia.6, 30, 48, 49 One study showed that either injection of a TLR7 agonist into mice or EMCV infection of mice led to thrombocytopenia that was abolished in TLR7 knockout mice.30 However, TLR7 stimulation did not initiate a prothrombotic phenotype in platelets.30 In a mouse model of CVB3 infection, depletion of platelets led to increased levels of CVB3 and a reduced adaptive immune response.6 In a mouse model of EMCV infection, depletion of platelets increased mortality.30 These studies indicate that platelets are protective by limiting viremia and contributing to long lasting immunity. Interestingly, we found that CD62P deficiency resulted in increased CVB3 myocarditis in mice, which may be due to decreased platelet-leukocyte binding and removal of virus infected platelets.50
Table 1:
Role of platelets in viral infection of the heart
| Pathogen | Observation | References |
|---|---|---|
| CVB3 | Platelets engulf CVB3 and limit viremia | 6 |
| CVB3 | Infection leads to platelet activation | 6 |
| EMCV | Platelet TLR7 recognizes EMCV which leads to platelet activation | 30 |
| CVB3, EMCV | Infection associated with thrombocytopenia | 6, 30, 48, 49 |
| CVB3, EMCV | Platelets limit overall virus infection | 6, 30 |
| CVB3, EMCV | Platelet-neutrophil aggregates enhance viral clearance | 6, 30 |
| CVB3 | Platelet depletion affects adaptive immune responses | 6 |
| CVB3 | Platelet depletion results in more severe myocarditis and increased mortality | 6 |
Severe acute respiratory syndrome CoV-2 (SARS-CoV-2) infection and Coronavirus disease 2019 (COVID-19) is associated with cardiac injury suggesting that the virus directly affects the heart.51 Indeed, viral genomes have been detected in the hearts of COVID-19 patients with myocarditis and heart failure.52–54 At present, the role of platelets in the cardiac pathology associated with COVID-19 is not known.
3. Platelets and respiratory virus infection
There are a variety of viruses that infect the lung (Table 2). For instance, seasonal flu is a recurring public health threat causing serious morbidity and mortality in the young and elderly populations.55 Infection with respiratory viruses, such as IAV or respiratory syncytial virus (RSV) infections, is associated with an increased incidence of myocardial infarction.56 IAV infection is also associated with venous thromboembolism.57 Thrombocytopenia was shown to be a risk factor for mortality in acute IAV infection.58 Platelets are activated during IAV infection directly by locally generated agonists or damage signals.10, 59 In addition, H1N1 IAV immune complexes directly activates platelets through FcγRIIA.10 Furthermore, H1N1 IAV-platelet interaction increases GPIIb/IIIa activation, platelet thromboxane signaling and EV release.10
Table 2:
Role of platelets in respiratory virus infection
| Pathogen | Observation | References |
|---|---|---|
| IAV, RSV, SARS-CoV-2 | Increased risk of cardiovascular events including myocardial infarction or venous thrombosis | 56, 57, 63–65 |
| IAV, SARS-CoV-2 | Platelets can engulf viruses and limit viremia | 15, 68, 69 |
| IAV, SARS-CoV-2 | Formation of platelet-monocyte, platelet-neutrophil and platelet-T cell aggregates | 16, 42, 67, 68 |
| IAV | Platelets bind to endothelial cells | 60 |
| IAV, SARS-CoV-2 | Platelets enhance NET formation | 13, 15, 17, 18, 66 |
| H1N1 | Directly activates platelets via FcγRIIA | 10 |
| H1N1, SARS-CoV-2 | Virus-platelet interaction increases platelet activation and EV release | 10, 59, 67, 68 |
| H5N1 | Binding to CLEC-2 enhances platelet EV release | 13 |
| H1N1 | Accumulation of platelets in lung correlates with CD62P expression and disease severity | 16, 45, 61 |
| IAV | Thrombocytopenia during IAV infection predicts increased mortality | 58 |
| H1N1 | Anti-platelet drugs (e.g. ASA or clopidogrel) improve outcome in infected mice | 16, 45, 46, 60 |
| SARS-CoV-2 | Megakaryocytes in capillaries of lungs and hearts | 70, 71 |
Human platelets can engulf IAV in vitro and patients infected with IAV have viral particles within platelets.15 In mouse models, accumulation of platelets within the lung correlated with IAV disease progression.16, 45 Early after IAV infection platelets form heterotypic aggregates with monocytes.42 Platelet-neutrophil aggregates were shown to transmigrate from the circulation into the airspace of IAV infected mouse lungs.16 In vitro studies showed that platelets attach to IAV infected endothelial cells via integrin mediated mechanisms which can be reduced by anti-platelet drugs.60 Importantly, administration of the anti-platelet drugs ASA and clopidogrel reduced IAV-induced lung injury in mice.16, 45, 60 We confirmed that ASA improved the survival of mice infected with H1N1 IAV.46 Levels of CD62P increased in the lungs of IAV infected mice, which correlated with increased platelet numbers in the lung.45, 61 In addition, clopidogrel was shown to reduce levels of CD62P but did not IL-6 or CXCL1 in the lungs of IAV infected mice.16 We observed that CD62P deficiency in mice was associated with increased survival after IAV infection compared to wild-type controls.50 These studies indicate that platelets contribute to lung injury in a mouse model of IAV infection possible by increasing inflammation, neutrophil recruitment and NET formation.17, 18 One study showed that IAV infection of human platelets led to engulfment of the virus, activation of TLR7 and expression of C3, which trigger neutrophils to release NETs.15
CoVs, including HCoVs 229E, NL63, OC43 and HKU1, are among the causative agents of the common cold that usually infect the nose and upper respiratory tract.62 However, more severe disease is linked to emerging CoV strains: SARS-CoV, Middle East respiratory syndrome (MERS) CoV and SARS-CoV-2. SARS, MERS and COVID-19 are associated with thrombocytopenia in humans and mice.63 Reduced platelet count is associated with increased morbidity and mortality in pandemic CoV infections.63 COVID-19 patients also have an increased risk of thrombosis.64, 65
The potential role of platelets in COVID-19 was recently discussed.66 As expected, two studies recently reported platelet activation in COVID-19 patients.67, 68 Platelets from COVID-19 patients had increased levels of CD62P and platelet-neutrophil, platelet-leukocyte and platelet-T cell aggregates numbers were increased.68 Similarly, a second study found increased levels of platelet-monocyte aggregates in severe COVID-19 patients.67 Two studies have also shown platelets from COVID-19 patients contain SARS-CoV-2 mRNA, which indicates that platelets can take up viral mRNA.68, 69 Interestingly, postmortem studies observed megakaryocytes in capillaries of lungs and hearts of COVID-19 patients.70, 71 Importantly, megakaryocytes are present in the lung and have immunomodulatory activity.1, 72, 73 In addition, neutrophil-mediated thrombo-inflammation is a feature of COVID-19 and associated with disease severity with increased levels of NETs in pulmonary vessels and in the circulation.4, 74–82
One study showed that SARS-CoV-2 infection leads to changes in platelet gene expression.68 These changes were similar to the pattern observed in platelets from pandemic H1N1 IAV infected patients, suggesting a common anti-viral response in platelets after viral infection.68 Platelets from COVID-19 patients expressed increased levels of the antiviral protein interferon induced transmembrane protein 3 (IFITM3).68 Expression of a nonfunctional IFITM3 variant correlates with COVID-19 severity.83 In mouse studies, IFITM3 was shown to protect against IAV infection.84, 85
The available studies indicate that COVID-19 patients have hyperactive platelets that may contribute to the COVID-19 pathophysiology. Ongoing clinical trials are evaluating the effect of different platelet inhibitors in COVID-19 patients, such as NCT04368377, NCT04445623 and NCT04409834. We eagerly await the results of these trials.
Conclusion
Platelets play a role in the host immune response to viruses. They can engulf viral particles to reduce viremia. However, they can also enhance inflammation and tissue injury during respiratory viral infections. At present, we do not know if platelet inhibition will be beneficial in COVID-19 patients. More work is needed to determine the role of platelets in modulating the innate immune response to viral infections.
Acknowledgement
We want to thank Drs. Yohei M. Hisada, Steven P. Grover and Robert H. Lee for providing critical comments to the manuscript. This work was supported by grants from the NIH to S.A. (HL142799) and to N.M. (HL119523).
Footnotes
Declaration of Interest
All authors contributed significantly to the study presented. All authors have read and approved the article. This manuscript has not been published in any language or has been submitted to any other journal at the same time. There are no conflicts of interest with regard to the authors. The authors declare that they have no competing financial interests.
References
- 1.Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegue E and Looney MR. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackman N, Bergmeier W, Stouffer GA and Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nature reviews Drug discovery. 2020;19:333–352. [DOI] [PubMed] [Google Scholar]
- 3.Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12:426–37. [DOI] [PubMed] [Google Scholar]
- 4.Jenne CN, Urrutia R and Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. International journal of laboratory hematology. 2013;35:254–61. [DOI] [PubMed] [Google Scholar]
- 5.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH and Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nature medicine. 2007;13:463–9. [DOI] [PubMed] [Google Scholar]
- 6.Negrotto S, Jaquenod de Giusti C, Rivadeneyra L, Ure AE, Mena HA, Schattner M and Gomez RM. Platelets interact with Coxsackieviruses B and have a critical role in the pathogenesis of virus-induced myocarditis. Journal of thrombosis and haemostasis : JTH. 2015;13:271–82. [DOI] [PubMed] [Google Scholar]
- 7.Chabert A, Hamzeh-Cognasse H, Pozzetto B, Cognasse F, Schattner M, Gomez RM and Garraud O. Human platelets and their capacity of binding viruses: meaning and challenges? BMC immunology. 2015;16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaujac C, Boukour S and Cramer-Borde E. Platelets and viruses: an ambivalent relationship. Cellular and molecular life sciences : CMLS. 2010;67:545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assinger A Platelets and infection - an emerging role of platelets in viral infection. Frontiers in immunology. 2014;5:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boilard E, Pare G, Rousseau M, Cloutier N, Dubuc I, Levesque T, Borgeat P and Flamand L. Influenza virus H1N1 activates platelets through FcgammaRIIA signaling and thrombin generation. Blood. 2014;123:2854–63. [DOI] [PubMed] [Google Scholar]
- 11.Koupenova M, Clancy L, Corkrey HA and Freedman JE. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circulation research. 2018;122:337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marx C, Novotny J, Salbeck D, Zellner KR, Nicolai L, Pekayvaz K, Kilani B, Stockhausen S, Burgener N, Kupka D, Stocker TJ, Weckbach LT, Pircher J, Moser M, Joner M, Desmet W, Adriaenssens T, Neumann FJ, Gerschlick AH, Ten Berg JM, Lorenz M and Stark K. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. 2019;134:1859–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung PS and Hsieh SL. CLEC2 and CLEC5A: Pathogenic Host Factors in Acute Viral Infections. Frontiers in immunology. 2019;10:2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M and Wagner DD. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koupenova M, Corkrey HA, Vitseva O, Manni G, Pang CJ, Clancy L, Yao C, Rade J, Levy D, Wang JP, Finberg RW, Kurt-Jones EA and Freedman JE. The role of platelets in mediating a response to human influenza infection. Nature communications. 2019;10:1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulavendran S, Rudd JM, Maram P, Thomas PG, Akhilesh R, Malayer JR, Chow VTK and Teluguakula N. Combination Therapy Targeting Platelet Activation and Virus Replication Protects Mice against Lethal Influenza Pneumonia. American journal of respiratory cell and molecular biology. 2019;61:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashar HK, Mueller NC, Rudd JM, Snider TA, Achanta M, Prasanthi M, Pulavendran S, Thomas PG, Ramachandran A, Malayer JR, Ritchey JW, Rajasekhar R, Chow VTK, Esmon CT and Teluguakula N. The Role of Extracellular Histones in Influenza Virus Pathogenesis. The American journal of pathology. 2018;188:135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, Forsyth PA, McFadden G and Kubes P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–80. [DOI] [PubMed] [Google Scholar]
- 19.Placke T, Kopp HG and Salih HR. Modulation of natural killer cell anti-tumor reactivity by platelets. Journal of innate immunity. 2011;3:374–82. [DOI] [PubMed] [Google Scholar]
- 20.Sadallah S, Schmied L, Eken C, Charoudeh HN, Amicarella F and Schifferli JA. Platelet-Derived Ectosomes Reduce NK Cell Function. J Immunol. 2016;197:1663–71. [DOI] [PubMed] [Google Scholar]
- 21.Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M, Idzko M, Bode C and Wagner DD. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood. 2013;121:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM and Guidotti LG. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nature medicine. 2005;11:1167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi G, Field DJ, Ko KA, Ture S, Srivastava K, Levy S, Kowalska MA, Poncz M, Fowell DJ and Morrell CN. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. The Journal of clinical investigation. 2014;124:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maitre B, Mangin PH, Eckly A, Heim V, Cazenave JP, Lanza F, Hanau D and Gachet C. Immature myeloid dendritic cells capture and remove activated platelets from preformed aggregates. Journal of thrombosis and haemostasis : JTH. 2010;8:2262–72. [DOI] [PubMed] [Google Scholar]
- 25.Portier I and Campbell RA. Role of Platelets in Detection and Regulation of Infection. Arteriosclerosis, thrombosis, and vascular biology. 2021;41:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forghani B and Schmidt NJ. Association of herpes simplex virus with platelets of experimentally infected mice. Archives of virology. 1983;76:269–74. [DOI] [PubMed] [Google Scholar]
- 27.Bik T, Sarov I and Livne A. Interaction between vaccinia virus and human blood platelets. Blood. 1982;59:482–7. [PubMed] [Google Scholar]
- 28.Assinger A, Kral JB, Yaiw KC, Schrottmaier WC, Kurzejamska E, Wang Y, Mohammad AA, Religa P, Rahbar A, Schabbauer G, Butler LM and Soderberg-Naucler C. Human cytomegalovirus-platelet interaction triggers toll-like receptor 2-dependent proinflammatory and proangiogenic responses. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:801–9. [DOI] [PubMed] [Google Scholar]
- 29.Danon D, Jerushalmy Z and De Vries A. Incorporation of influenza virus in human blood platelets in vitro. Electron microscopical observation. Virology. 1959;9:719–22. [DOI] [PubMed] [Google Scholar]
- 30.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K and Freedman JE. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Almeida AJ, Campos-de-Magalhaes M, Antonietti CL, Brandao-Mello CE, da Silva ML, de Oliveira RV, do Espirito-Santo MP, Yoshida CF and Lampe E. Autoimmune thrombocytopenia related to chronic hepatitis C virus infection. Hematology. 2009;14:49–58. [DOI] [PubMed] [Google Scholar]
- 32.Youssefian T, Drouin A, Masse JM, Guichard J and Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–9. [DOI] [PubMed] [Google Scholar]
- 33.Noisakran S, Gibbons RV, Songprakhon P, Jairungsri A, Ajariyakhajorn C, Nisalak A, Jarman RG, Malasit P, Chokephaibulkit K and Perng GC. Detection of dengue virus in platelets isolated from dengue patients. The Southeast Asian journal of tropical medicine and public health. 2009;40:253–62. [PubMed] [Google Scholar]
- 34.Simon AY, Sutherland MR and Pryzdial EL. Dengue virus binding and replication by platelets. Blood. 2015;126:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaipan C, Soilleux EJ, Simpson P, Hofmann H, Gramberg T, Marzi A, Geier M, Stewart EA, Eisemann J, Steinkasserer A, Suzuki-Inoue K, Fuller GL, Pearce AC, Watson SP, Hoxie JA, Baribaud F and Pohlmann S. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. Journal of virology. 2006;80:8951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Attatippaholkun N, Kosaisawe N, Y UP, Supraditaporn P, Lorthongpanich C, Pattanapanyasat K and Issaragrisil S. Publisher Correction: Selective Tropism of Dengue Virus for Human Glycoprotein Ib. Sci Rep. 2018;8:6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koupenova M, Mick E, Mikhalev E, Benjamin EJ, Tanriverdi K and Freedman JE. Sex differences in platelet toll-like receptors and their association with cardiovascular risk factors. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee M, Huang Y, Joshi S, Popa GJ, Mendenhall MD, Wang QJ, Garvy BA, Myint T and Whiteheart SW. Platelets Endocytose Viral Particles and Are Activated via TLR (Toll-Like Receptor) Signaling. Arteriosclerosis, thrombosis, and vascular biology. 2020;40:1635–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anabel AS, Eduardo PC, Pedro Antonio HC, Carlos SM, Juana NM, Honorio TA, Nicolas VS and Sergio Roberto AR. Human platelets express Toll-like receptor 3 and respond to poly I:C. Human immunology. 2014;75:1244–51. [DOI] [PubMed] [Google Scholar]
- 40.Thon JN, Peters CG, Machlus KR, Aslam R, Rowley J, Macleod H, Devine MT, Fuchs TA, Weyrich AS, Semple JW, Flaumenhaft R and Italiano JE Jr. T granules in human platelets function in TLR9 organization and signaling. The Journal of cell biology. 2012;198:561–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onlamoon N, Noisakran S, Hsiao HM, Duncan A, Villinger F, Ansari AA and Perng GC. Dengue virus-induced hemorrhage in a nonhuman primate model. Blood. 2010;115:1823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rondina MT, Brewster B, Grissom CK, Zimmerman GA, Kastendieck DH, Harris ES and Weyrich AS. In vivo platelet activation in critically ill patients with primary 2009 influenza A(H1N1). Chest. 2012;141:1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh MV, Davidson DC, Kiebala M and Maggirwar SB. Detection of circulating platelet-monocyte complexes in persons infected with human immunodeficiency virus type-1. Journal of virological methods. 2012;181:170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachelier K, Biehl S, Schwarz V, Kindermann I, Kandolf R, Sauter M, Ukena C, Yilmaz A, Sliwa K, Bock CT, Klingel K and Bohm M. Parvovirus B19-induced vascular damage in the heart is associated with elevated circulating endothelial microparticles. PloS one. 2017;12:e0176311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le VB, Schneider JG, Boergeling Y, Berri F, Ducatez M, Guerin JL, Adrian I, Errazuriz-Cerda E, Frasquilho S, Antunes L, Lina B, Bordet JC, Jandrot-Perrus M, Ludwig S and Riteau B. Platelet activation and aggregation promote lung inflammation and influenza virus pathogenesis. American journal of respiratory and critical care medicine. 2015;191:804–19. [DOI] [PubMed] [Google Scholar]
- 46.Tatsumi K, Schmedes CM, Houston ER, Butler E, Mackman N and Antoniak S. Protease-activated receptor 4 protects mice from Coxsackievirus B3 and H1N1 influenza A virus infection. Cellular immunology. 2019;344:103949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoniak S and Mackman N. Coagulation, protease-activated receptors, and viral myocarditis. Journal of cardiovascular translational research. 2014;7:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaga A, Katata Y, Suzuki A, Otani K, Watanabe H, Kitaoka S and Kumaki S. Perinatal Coxsackievirus B3 Infection with Transient Thrombocytopenia. Tohoku J Exp Med. 2016;239:135–8. [DOI] [PubMed] [Google Scholar]
- 49.Bryant PA, Tingay D, Dargaville PA, Starr M and Curtis N. Neonatal coxsackie B virus infection-a treatable disease? European journal of pediatrics. 2004;163:223–8. [DOI] [PubMed] [Google Scholar]
- 50.Egnatz G, Baharathi V, Tatsumi K, Mackman N and Antoniak S. P-selectin (CD62P) Deficiency Is Associated with Increased Coxsackievirus B3 Myocarditis But Not Influenza A Virus Infection in Mice [abstract]. Res Pract Thromb Haemost 2020;4. [Google Scholar]
- 51.Kadosh BS, Garshick MS, Gaztanaga J, Moore KJ, Newman JD, Pillinger M, Ramasamy R, Reynolds HR, Shah B, Hochman J, Fishman GI and Katz SD. COVID-19 and the Heart and Vasculature: Novel Approaches to Reduce Virus-Induced Inflammation in Patients With Cardiovascular Disease. Arteriosclerosis, thrombosis, and vascular biology. 2020;40:2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindner D, Fitzek A, Brauninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, Blankenberg S, Puschel K and Westermann D. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, Wenzel P, Hamm C, Westenfeld R, Schultheiss M, Gross U, Morawietz L and Schultheiss HP. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wenzel P, Kopp S, Gobel S, Jansen T, Geyer M, Hahn F, Kreitner KF, Escher F, Schultheiss HP and Munzel T. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovascular research. 2020;116:1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keilman LJ. Seasonal Influenza (Flu). Nurs Clin North Am. 2019;54:227–243. [DOI] [PubMed] [Google Scholar]
- 56.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, Katz K, Ko DT, McGeer AJ, McNally D, Richardson DC, Rosella LC, Simor A, Smieja M, Zahariadis G and Gubbay JB. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. The New England journal of medicine. 2018;378:345–353. [DOI] [PubMed] [Google Scholar]
- 57.Obi AT, Tignanelli CJ, Jacobs BN, Arya S, Park PK, Wakefield TW, Henke PK and Napolitano LM. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord. 2019;7:317–324. [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Delgado JC, Rovira A, Esteve F, Rico N, Manez Mendiluce R, Ballus Noguera J and Berrade J. Thrombocytopenia as a mortality risk factor in acute respiratory failure in H1N1 influenza. Swiss medical weekly. 2013;143:w13788. [DOI] [PubMed] [Google Scholar]
- 59.Hottz ED, Bozza FA and Bozza PT. Platelets in Immune Response to Virus and Immunopathology of Viral Infections. Front Med (Lausanne). 2018;5:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugiyama MG, Gamage A, Zyla R, Armstrong SM, Advani S, Advani A, Wang C and Lee WL. Influenza Virus Infection Induces Platelet-Endothelial Adhesion Which Contributes to Lung Injury. Journal of virology. 2016;90:1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tinoco R, Carrette F, Henriquez ML, Fujita Y and Bradley LM. Fucosyltransferase Induction during Influenza Virus Infection Is Required for the Generation of Functional Memory CD4(+) T Cells. J Immunol. 2018;200:2690–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paules CI, Marston HD and Fauci AS. Coronavirus Infections-More Than Just the Common Cold. JAMA : the journal of the American Medical Association. 2020. [DOI] [PubMed] [Google Scholar]
- 63.Mackman N, Antoniak S, Wolberg AS, Kasthuri R and Key NS. Coagulation Abnormalities and Thrombosis in Patients Infected With SARS-CoV-2 and Other Pandemic Viruses. Arteriosclerosis, thrombosis, and vascular biology. 2020:ATVBAHA120314514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mackman N, Antoniak S, Wolberg AS, Kasthuri R and Key NS. Coagulation Abnormalities and Thrombosis in Patients Infected With SARS-CoV-2 and Other Pandemic Viruses. Arteriosclerosis, thrombosis, and vascular biology. 2020;40:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J and Berger JS. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA : the journal of the American Medical Association. 2020;324:799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koupenova M Potential role of platelets in COVID-19: Implications for thrombosis. Res Pract Thromb Haemost. 2020;4:737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pao CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA and Bozza PT. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, Petrey AC, Tolley ND, Guo L, Cody M, Weyrich AS, Yost CC, Rondina MT and Campbell RA. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, Khalki L, Limami Y, Zaid N, Sadki K, Ben El Haj R, Mahir W, Belayachi L, Belefquih B, Benouda A, Cheikh A, Langlois MA, Cherrah Y, Flamand L, Guessous F and Boilard E. Platelets Can Associate with SARS-Cov-2 RNA and Are Hyperactivated in COVID-19. Circulation research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J and Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS and Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pariser DN, Hilt ZT, Ture SK, Blick-Nitko SK, Looney MR, Cleary SJ, Roman-Pagan E, Saunders J 2nd, Georas SN, Veazey J, Madere F, Santos LT, Arne A, Huynh NP, Livada AC, Guerrero-Martin SM, Lyons C, Metcalf-Pate KA, McGrath KE, Palis J and Morrell CN. Lung megakaryocytes are immune modulatory cells. The Journal of clinical investigation. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrell CN, Pariser DN, Hilt ZT and Vega Ocasio D. The Platelet Napoleon Complex-Small Cells, but Big Immune Regulatory Functions. Annual review of immunology. 2019;37:125–144. [DOI] [PubMed] [Google Scholar]
- 74.Shi H, Zuo Y, Yalavarthi S, Gockman K, Zuo M, Madison JA, Blair C, Woodward W, Lezak SP, Lugogo NL, Woods RJ, Lood C, Knight JS and Kanthi Y. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. Journal of leukocyte biology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ng H, Havervall S, Rosell A, Aguilera K, Parv K, von Meijenfeldt FA, Lisman T, Mackman N, Thalin C and Phillipson M. Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients With COVID-19. Arteriosclerosis, thrombosis, and vascular biology. 2020:ATVBAHA120315267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, Cody MJ, Manne BK, Portier I, Harris ES, Petrey AC, Beswick EJ, Caulin AF, Iovino A, Abegglen LM, Weyrich AS, Rondina MT, Egeblad M, Schiffman JD and Yost CC. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, Vanwinge C, Cataldo D, Oury C, Delvenne P and Marichal T. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. The Journal of experimental medicine. 2020;217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, Schneider AH, Caetite D, Tavares LA, Paiva IM, Rosales R, Colon D, Martins R, Castro IA, Almeida GM, Lopes MIF, Benatti MN, Bonjorno LP, Giannini MC, Luppino-Assad R, Almeida SL, Vilar F, Santana R, Bollela VR, Auxiliadora-Martins M, Borges M, Miranda CH, Pazin-Filho A, da Silva LLP, Cunha LD, Zamboni DS, Dal-Pizzol F, Leiria LO, Siyuan L, Batah S, Fabro A, Mauad T, Dolhnikoff M, Duarte-Neto A, Saldiva P, Cunha TM, Alves-Filho JC, Arruda E, Louzada-Junior P, Oliveira RD and Cunha FQ. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. The Journal of experimental medicine. 2020;217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, Scherer C, Rudelius M, Zoller M, Hochter D, Keppler O, Teupser D, Zwissler B, von Bergwelt-Baildon M, Kaab S, Massberg S, Pekayvaz K and Stark K. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 2020;142:1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou E, Tsironidou V, Tsigalou C, Tektonidou M, Konstantinidis T, Papagoras C, Mitroulis I, Germanidis G, Lambris JD and Ritis K. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. The Journal of clinical investigation. 2020;130:6151–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y and Knight JS. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, Sturzl M, Staats L, Mahajan A, Schauer C, Kremer AN, Volkl S, Amann K, Evert K, Falkeis C, Wehrfritz A, Rieker RJ, Hartmann A, Kremer AE, Neurath MF, Munoz LE, Schett G and Herrmann M. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Qin L, Zhao Y, Zhang P, Xu B, Li K, Liang L, Zhang C, Dai Y, Feng Y, Sun J, Hu Z, Xiang H, Knight JC, Dong T and Jin R. Interferon-Induced Transmembrane Protein 3 Genetic Variant rs12252-C Associated With Disease Severity in Coronavirus Disease 2019. The Journal of infectious diseases. 2020;222:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Everitt AR, Clare S, Pertel T, John SP, Wash RS, Smith SE, Chin CR, Feeley EM, Sims JS, Adams DJ, Wise HM, Kane L, Goulding D, Digard P, Anttila V, Baillie JK, Walsh TS, Hume DA, Palotie A, Xue Y, Colonna V, Tyler-Smith C, Dunning J, Gordon SB, Gen II, Investigators M, Smyth RL, Openshaw PJ, Dougan G, Brass AL and Kellam P. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484:519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kenney AD, McMichael TM, Imas A, Chesarino NM, Zhang L, Dorn LE, Wu Q, Alfaour O, Amari F, Chen M, Zani A, Chemudupati M, Accornero F, Coppola V, Rajaram MVS and Yount JS. IFITM3 protects the heart during influenza virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:18607–18612. [DOI] [PMC free article] [PubMed] [Google Scholar]


