Abstract
Introduction
Patients with Alzheimer's disease and related dementias (ADRD) face substantial challenges in selecting, and remaining enrolled in, health insurance. Little is known about how patients with ADRD experience the Medicare Advantage (MA) program.
Methods
We used, hospital, outpatient, and post‐acute care data to identify MA beneficiaries with and without ADRD in 2014. Multinomial logit models estimated the percentage of people who disenrolled to traditional Medicare (TM) or switched to a different MA plan in 2015.
Results
Among non‐dually eligible beneficiaries, 9.0% (95% confidence interval [CI]: 8.0, 9.1) with ADRD disenrolled while 19.7% (95% CI: 19.6, 19.9) switched plans within MA compared to a disenrollment rate of 4.2% (95% CI: 4.2, 4.2) and switching rate of 22.8% (95% CI: 22.9, 22.8) for persons without ADRD.
Discussion
MA enrollees with ADRD tend to disenroll at substantially higher rates than those without ADRD. This may be indicative of their care needs not being met in the program.
Keywords: health insurance, Medicare, Medicare Advantage
1. INTRODUCTON
Medicare Advantage (MA) is the privately run and capitated segment of the Medicare program and now enrolls 34% of all Medicare beneficiaries. 1 MA insurers may each offer dozens of different plans that compete in an open market. While past work has found that MA may be successful in improving enrollee outcomes, 1 recent studies have found that beneficiaries with greater needs disenroll at substantial rates. 2 , 3 , 4 , 5 These disenrollment patterns may be a result of poor satisfaction with an enrollee's plan, which could lead to disruptions in a beneficiary's continuity of care. 6
It is well established that patients with Alzheimer's disease and related dementias (ADRD) face substantial out‐of‐pocket cost burdens. 7 , 8 , 9 , 10 , 11 Unlike in traditional Medicare (TM), MA plans place limits on out‐of‐pocket spending, and provide additional care management services, both of which may be of use to patients with ADRD. 12 However, other work has found that beneficiaries with ADRD may face additional challenges in choosing the right insurance plan. 13 , 14 Plans vary in their cost sharing, benefit coverage, and other requirements such as prior authorization. One recent study found that MA enrollees with ADRD may have less access to services than their counterparts in TM. 15 With high variation in health need among those with ADRD, it may be challenging for patients and their caregivers to select optimal plans. 16 Given these challenges, beneficiaries with ADRD may also disenroll at higher rates.
In this paper, we characterize MA enrollment, disenrollment, and plan switching among beneficiaries with ADRD using national Medicare data.
2. METHODS
2.1. Data sources
Our primary source of data was the 2014–2015 Medicare Master Beneficiary Summary File (MBSF), which includes demographic and enrollment characteristics of 100% of the Medicare population. We use these data to identify enrollment in MA in each month during the study, and to assess disenrollment to TM or switching to another MA plan.
We include several sources to classify patients who have ADRD. First, we use the Medicare Provider and Analysis Review File (MedPAR), which includes hospitalization records for all TM and more than 90% of MA beneficiaries. 17 The MedPAR records include up to 25 diagnosis fields, which we use to find records of ADRD diagnoses. Next, for patients who were admitted to a nursing home, we use the Minimum Data Set 3.0 (MDS), which is a mandatory assessment of all nursing home stays nationally, including those enrolled in MA. Next, we use the Outcome and Assessment Information Set (OASIS), which is an assessment collected on all enrollees who use home health services. The OASIS file contains diagnoses fields that we use to flag for ADRD. Finally, we use the Medicare Risk Score File, a newly available file that contains for each MA and Part D enrollee a variety of diagnoses codes reported by plans to the Centers for Medicare and Medicaid Services (CMS) for the purposes of risk adjustment. The risk score file contains checkbox fields indicating whether an enrollee has ADRD and is available for all MA enrollees. Outside of these individual‐level data, we also use publicly available MA plan characteristic files that are released annually by CMS.
2.2. Study population
We include all MA beneficiaries enrolled at any point in 2014 who survive at least until the start of 2015. We exclude beneficiaries who died in 2014 as they would not have the ability to choose a new plan in 2015. We also exclude beneficiaries who moved their zip code of residence between 2014 and 2015 as moving residences may prompt beneficiaries to change their plan enrollment. We stratify all our analyses by whether someone is dually eligible with the Medicaid program, as there are substantial differences in disenrollment patterns between those in Medicaid and those who are not, in part because CMS permits dual eligible enrollees to switch plans or disenroll at any time during the year while other beneficiaries are locked into their plan selections. 2
2.3. Classification of ADRD
RESEARCH IN CONTEXT
Systematic review: Prior work has found that Medicare Advantage (MA) enrollees with greater health needs tend to switch to traditional Medicare (TM) at substantially higher rates. While there has been some recent research on the experiences of MA enrollees with Alzheimer's disease (AD) among specific plans, there have been no national studies of the experience patients with AD in the program.
Interpretation: Our findings indicate that a large proportion of patients with AD disenroll from the MA program. Our results were robust to several sensitivity specifications.
Future directions: Plan disenrollment is an important measure of patient satisfaction with MA. To expand on this research a combination of quantitative surveys and qualitative interviews may enhance our understanding of the factors that lead to high disenrollment of AD and related dementias patients from the MA program.
Prior research has found that traditional insurance claims sources are limited in their ability to successfully classify beneficiaries as having ADRD. 18 , 19 While we do not have detailed clinical data on beneficiaries in this study, we are able to use some measures of functional status from available assessment data to assess if disenrollment varies by cognitive status. In our primary analysis, we considered someone to have ADRD if they had at least one ADRD diagnosis from across any of the included datasets (MDS, MedPAR, OASIS, and Risk Score File). Even with the inclusion of each source of data, it is likely we are undercounting the true extent of ADRD in this population. We used the diagnosis codes used in the Chronic Conditions Warehouse definition to classify ADRD.
2.4. Outcome variables
Our primary outcome of interest for this study is the change in enrollment status between 2014 and 2015. An enrollee in MA at the end of the year or in certain other circumstances may choose to remain in their same plan, remain in the MA program but switch to a different plan, or disenroll to TM. We consider this a multinomial outcome in all of our analyses.
2.5. Other variables
From the MBSF, we identify beneficiaries who are dually enrolled in Medicare and Medicaid. We also include enrollee age, sex, and race/ethnicity (classified as non‐Hispanic White, non‐Hispanic Black, non‐Hispanic Asian, non‐Hispanic Native American/American, and Hispanic) as additional adjustors. From our claims and assessment data, we created flags indicating an enrollee had any hospital use, any nursing home use, or any home health use as utilization is often an important driver of disenrollment. 4
To adjust for the role that MA plan characteristics may play in enrollment decisions, we also included plan characteristics used in past work 2 , 20 including plan quality measured by star ratings (unrated, 2–2.5 stars, 3–3.5 stars, 4–4.5 stars, 5 stars); plan monthly premium; plan max out‐of‐pocket maximum; number of plans in enrollee's county; and indicators for increases in plans’ premiums, star ratings, or out‐of‐pocket maximums to capture changes in plan characteristics from 1 year to the next that may prompt a disenrollment, and indicators that the enrollee was in the highest‐rated, lowest premium, or lowest out‐of‐pocket maximum available in their county of residence.
2.6. Statistical analysis
First, we compare demographics and the 2014 plan characteristics of beneficiaries with and without ADRD. We then fit multinomial logit models for all MA beneficiaries with an outcome of remaining in their plan, switching plans or contracts within MA, or disenrolling to TM. We include all enrollee demographic and plan characteristics as adjustors in the model and use robust standard errors. We stratify all our models by ADRD status and by dual eligibility status, as those dually eligible with Medicaid may switch plans at any time within a year. From each model we calculate the adjusted mean disenrollment rates.
As prior work has found that use of different services is related to MA disenrollment, 4 we next compare disenrollment and switching rates for MA enrollees with and without ADRD diagnoses across use categories. We compared those who had no major use events to those who had hospital, nursing home, or home health use during 2014.
To assess whether enrollees with different levels of ADRD severity had different disenrollment patterns, among those who had any nursing home stay, we compared disenrollment and switching rates across different levels of the Cognitive Function Scale (CFS). We calculated the CFS based on the first recorded MDS record we had for each enrollee in the year. Across each use type and CFS level, we calculate adjusted switching and disenrollment rates using similar multinomial logit models as with our primary analysis.
In sensitivity checks, we also compared disenrollment rates across different levels of the MA star ratings. We also assessed how frequently diagnoses of ADRD appeared across each of the different data sources combined for this analysis. All analysis was conducted using Stata 15 and an alpha of 0.05.
3. RESULTS
From an initial sample of 15,874,796 MA enrollees we excluded 625,545 enrollees who died before having the opportunity to select a new plan, and 70,517 who moved from one year to the next. Our final sample for this analysis included 15,179,172 MA beneficiaries without a diagnosis of ADRD and 943,772 beneficiaries that had any ADRD diagnosis. In Appendix A in supporting information we include a table comparing diagnoses across different data sources for the MA enrollees.
In Table 1 we compare the descriptive characteristics of beneficiaries across each payment type and across ADRD status. Beneficiaries with ADRD tended to be older (80.0 vs. 70.6 years); more often dually eligible (33.5% vs. 17.6%); more often female (62.6% vs. 56.3%); and have higher rates of nursing home, home health, and hospital use. Comparing MA plan characteristics, MA enrollees with ADRD were more often enrolled in a special needs plan (24.4% vs. 15.5%); however, most other plan characteristics were similar. All comparisons between these characteristics were statistically significant at the P < .001 level.
TABLE 1.
Demographics of Medicare enrollees, by Alzheimer's disease and related dementia diagnoses
| Non ADRD | ADRD | |
|---|---|---|
| N | 15,179,172 | 943,772 |
| Mean age | 70.6 (10.2) | 79.9622 (9.) |
| Race/ethnicity | ||
| White | 11994338 (79.0%) | 731719 (77.5%) |
| Black | 1752405 (11.5%) | 122671 (13.0%) |
| Other/unknown | 484411 (3.2%) | 18905 (2.0%) |
| Asian | 375473 (2.5%) | 20071 (2.1%) |
| Hispanic | 540425 (3.6%) | 48609 (5.2%) |
| NA/AI | 32120 (0.2%) | 1797 (0.2%) |
| Female | 8545238 (56.3%) | 600186 (63.6%) |
| Dual eligible | 2674249 (17.6%) | 316171 (33.5%) |
| Any home health use | 1519914 (10.0%) | 343077 (36.4%) |
| Any nursing home use | 902295 (5.9%) | 400943 (42.5%) |
| Any hospital use | 2267134 (14.9%) | 405538 (43.0%) |
| Plan characteristics | ||
| Enrolled in SNP | 1421912 (15.5%) | 120829 (24.4%) |
| Star rating category | ||
| Unrated | 5552115 (36.6%) | 367604 (39.0%) |
| 2 to 2.5 | 120876 (0.8%) | 6720 (0.7%) |
| 3 to 3.5 | 4845378 (31.9%) | 290188 (30.7%) |
| 4 to 4.5 | 4275079 (28.2%) | 252942 (26.8%) |
| 5 stars | 385724 (2.5%) | 26318 (2.8%) |
| Premium ($) | 30.3 (46.3) | 32.9 (49.4) |
| Out‐of‐pocket maximum ($) | 4617.9 (1615.8) | 4514.7 (1600.0) |
| Plan HCC score | 1.0 (.2) | 1.1 (.3) |
| Enrolled in highest rated plan | 2822656 (28.9%) | 167035 (28.6%) |
| Enrolled in lowest premium plan | 5183764 (53.1%) | 280498 (48.0%) |
| Enrolled in lowest max OOP | 556854 (5.7%) | 31148 (5.3%) |
| Number of plans in market | 40.5 (25.9) | 43.0 (26.1) |
Notes: Includes all enrollees in 2014 who survived until the end of the year. The variables under Plan Characteristics are only applicable to the study beneficiaries that are in MA. Plan characteristics are from the 2014 plan year. Dual eligibility includes full and partial dual at any point during 2014.
Abbreviations: ADRD, Alzheimer's disease and related dementias; HCC, hierarchical condition category; MA, Medicare Advantage; OOP, out of pocket; SNP, Special Needs Plans.
In Figure 1 we present the primary results of our disenrollment and switching analysis stratifying by ADRD status and dual eligibility. After adjusting for demographic, plan, and market characteristics, among non‐dual enrollees, those with ADRD had significantly higher disenrollment rates (9.0%, 95% confidence interval [CI]: 8.9%, 9.1%), and significantly lower plan switching rates (19.8%, 95% CI: 19.6%, 19.9%) than enrollees without ADRD (4.2%, 95% CI: 4.2%, 4.2%, and 22.8%, 95% CI: 22.8%, 22.9%, respectively). Among dually eligible MA enrollees, the disenrollment rate for those with ADRD was 19.9% (95% CI: 13.8%, 14.1%) compared to 5.3% (95% CI: 5.2%, 5.3%) among those without any diagnosis of ADRD. We include full regression output for our models in the supporting information.
FIGURE 1.
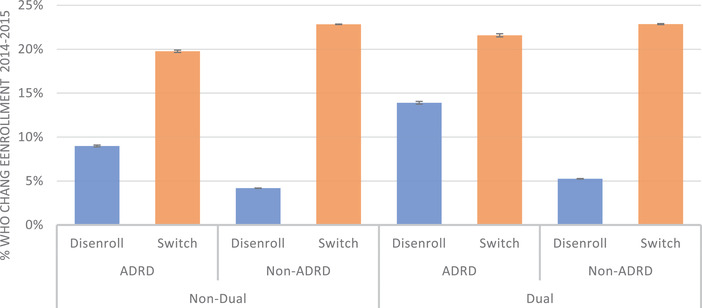
Adjusted disenrollment and switch rates, by Alzheimer's disease or related dementia (ADRD) diagnosis and dual eligibility status. Adjusted estimates for Medicare Advantage (MA) come from a multinomial logit model with a three‐category outcome of (1) disenroll from MA to traditional Medicare (TM), (2) switch plans within MA, and (3) stay in the same plan (not shown). Models, stratified by ADRD and dual‐eligibility status, adjust for sex; age; race/ethnicity; star rating category; plan premium; plan max out‐of‐pocket payment; plan hierarchical conditions risk score; number of plans in enrollee's county; indicators for increases in plans’ premiums, ratings, or out‐of‐pocket maximums; and indicators that the enrollee was in the highest‐rates, lowest premium, or lowest out‐of‐pocket maximum available in their county of residence. All models used robust standard errors. Beneficiaries who died or moved during the study period are excluded
In Figure 2, we compare disenrollment for non‐dual MA enrollees by whether they had a given use type in a year, adjusted for demographic and plan characteristics. Among enrollees without major use, 4.8% (95% CI: 4.6, 4.9) with ADRD disenrolled compared to 3.7% (95% CI: 3.7%, 3.7%) without an ADRD diagnosis. Comparatively, among those with ADRD, 13.4% (95% CI: 13.3%, 13.6%) of those with any hospital use, 15.3% (95% CI:15.1%, 15.5%) with any nursing home use and 13.2% (95% CI: 13%, 13.4%) with any home health use disenrolled. Across all use types, those with ADRD disenrolled at greater rates than those without ADRD. In sensitivity models, we found interactions between ADRD and use were statistically significant (P < .001). We present a version of this figure including those who are dually eligible in Appendix B in supporting information.
FIGURE 2.
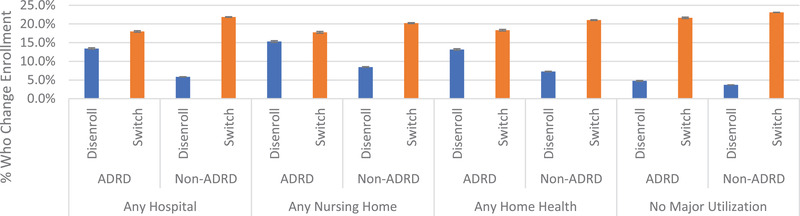
Adjusted disenrollment from Medicare Advantage (MA)to traditional Medicare and plan switching within MA for persons with and without Alzheimer's disease and related dementia (ADRD), by type of use. Adjusted estimates for disenrollment and plan switching comes from a multinomial logit model stratified by ADRD and use type, adjusting for sex; age; race/ethnicity; star rating category; plan premium; plan max out‐of‐pocket payment; plan hierarchical conditions risk score; number of plans in enrollee's county; indicators for increases in plans’ premiums, ratings, or out‐of‐pocket maximums; and indicators that the enrollee was in the highest‐rates, lowest premium, or lowest out‐of‐pocket maximum available in their county of residence. This figure includes only non‐dual beneficiaries. No major use indicates that the enrollee did not have any hospital, nursing home, or home health use; however, they may have had outpatient use
In Figure 3 we present disenrollment and switching rates among those with any MDS assessment by the enrollee's CFS score. After adjusting for demographic and plan characteristics, 7.2% (95% CI: 7.1%, 7.3%) of enrollees with a CFS score of 1 disenrolled and 20.8% (95% CI: 20.6%, 21%) switched plans while among those with a CFS score of 4, 22.4% (95% CI: 21.7%, 23.0%) disenrolled while 15.8% (95% CI: 15.3%, 16.4%) switched. These trends were similar for those who were dually enrolled with Medicaid in Appendix C in supporting information.
FIGURE 3.
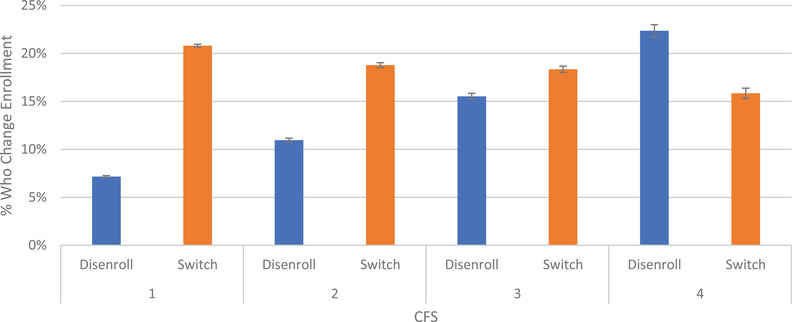
Adjusted disenrollment from Medicare Advantage (MA) to traditional Medicare and plan switching within MA for persons with and without Alzheimer's disease and related dementia, by cognitive function score. This figure only includes beneficiaries who had a nursing home stay, which is necessary for calculating the Cognitive Function Scale (CFS). CFS ranges from 1 to 4, where 4 represents the most cognitively impaired and 1 represents the least. Results are for all non‐dual enrollees adjusted from a multinomial logit model
In Appendix D in supporting information, we also find that as MA star rating increases, disenrollment tended to decrease among those with and without ADRD.
4. DISCUSSION
In this study of disenrollment and switching rates between MA and TM, we found that enrollees with ADRD were substantially more likely to disenroll from MA to TM compared to those without ADRD. Disenrollment rates were significantly higher among those who had costly use such as nursing home stays or hospitalizations. These results were modified depending upon the star rating of the MA plan in which beneficiaries were enrolled, suggesting that better plans were able to alter the otherwise problematic experience of health care use for their members.
Taken together, our findings indicate that enrollees with ADRD may not currently have their needs met by the MA program. This finding aligns with prior work that has found those with advanced care needs are more likely to disenroll from MA; however, the rates of disenrollment we found among those with ADRD were substantially higher compared to other conditions. 2 , 4 , 5 Our finding that plan switching rates are lower among ADRD patients also aligns with that of past work that finds those with cognitive impairments may have challenges in selecting optimal plans and may get “stuck” in their current plans despite having options that could reduce their out‐of‐pocket costs. 13 This difficulty in changing plans has important implications for the design of health insurance markets. The markets for insurance coverage in MA and the Affordable Care Act are predicated on beneficiaries identifying plans in their best interest. 21 If beneficiaries with ADRD or their caregivers have difficulty in selecting plans, then these markets may not be in their best interest.
This study builds off of one previous study that characterized ADRD in the MA population. 22 While the previous study also found high rates of disenrollment, our study has several additional key contributions. First, we use data from nearly all MA plans in the United States, while the previous study was limited to enrollees in one MA plan. Second, the assessment data we used allows us to compare the role that use of hospital and nursing home services plays in disenrollment. Third, we are able to distinguish between those who disenroll to TM, those who switch plans within MA, and those who die. We are also able to compare disenrollment rates between those who are dually enrolled with Medicaid and those who are not, which is an important distinction as each group has different underlying disenrollment rates due to CMS policies around open enrollment periods.
There are several reasons why disenrollment may be particularly high among the Medicare ADRD population. MA plans have the option to create narrow provider networks, 23 , 24 , 25 and prior work has found that beneficiaries in MA have less access to high‐quality nursing homes, hospitals, and home health agencies than TM beneficiaries. 26 , 27 , 28 Having high‐quality nursing homes available may be particularly important for beneficiaries with ADRD and their caregivers for long‐term care planning. Additionally, MA plans may require prior authorizations to receive treatment, which may also present barriers for beneficiaries with ADRD. Disenrollment from plans may lead to disruptions in the continuity of care for beneficiaries and may subject them to higher out‐of‐pocket costs in TM. 3
In an effort to improve the services available in MA for beneficiaries with more complex health needs, CMS has allowed for the creation of Chronic Disease Special Needs Plans (SNPs). Companies that offer MA plans may create SNPs for specific chronic conditions such as ADRD and are given more flexibility by CMS in designing their benefits to serve the unique needs of each population. During our study period only one ADRD SNP was offered so we are unable to evaluate yet if these SNP plans may be associated with different enrollment outcomes. Furthermore, as of 2019, CMS has given plans new flexibility in offering supplemental benefits in their benefits packages. Many of these new benefits such as adult day care and caregiver supports may be particularly valuable for beneficiaries with ADRD; however, uptake of these new services appears to be limited. 29
This study has several limitations. First, we use a cross‐sectional design so our results cannot be interpreted as causal in nature. That being said, the associations we present are highly indicative of a different experience in MA for patients with ADRD than those without. Second, we are limited by the variables available in administrative and assessment data. We likely undercount the number of beneficiaries with ADRD, particularly those who in earlier stages who do not require extensive medical care. Third, to use the plan risk score file in our analysis, we were limited to comparisons of switching between 2014 and 2015. It is unlikely, however, that these trends have changed in most recent years.
The proliferation of SNPs and additional benefits for those with ADRD may be valuable for controlling costs and improving outcomes; however, any beneficial impact these programs might have is predicated on those with ADRD remaining enrolled. As of now, the high levels of disenrollment we measure may be indicative of MA plans not currently meeting the complex needs of this patient population.
CONFLICTS OF INTEREST
Vincent Mor is chair of the scientific advisory board and a consultant at NaviHealth, Inc., as well as former director of PointRight, Inc., where he holds < 1% equity. All other authors have no interests to declare.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
The authors thank Kali Thomas and Emmanuelle Belanger for providing feedback on an earlier draft. This work was supported by the National Institute on Aging (5P01AG027296).
Meyers DJ, Rahman M, Rivera‐Hernandez M, Trivedi AN, Mor V. Plan switching among Medicare Advantage beneficiaries with Alzheimer's disease and other dementias. Alzheimer's Dement. 2021;7:e12150. 10.1002/trc2.12150
REFERENCES
- 1. Neuman P, Jacobson GA. Medicare advantage checkup. N Engl J Med. 2018;379:2163‐2172. [DOI] [PubMed] [Google Scholar]
- 2. Meyers DJ, Belanger E, Joyce N, McHugh J, Rahman M, Mor V. Analysis of drivers of disenrollment and plan switching among medicare advantage beneficiaries. JAMA Intern Med. 2019;179:524‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyers DJ, Trivedi AN, Mor V. Limited medigap consumer protections are associated with higher reenrollment in medicare advantage plans. Health Aff (Millwood). 2019;38:782‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahman M, Keohane L, Trivedi AN, Mor V. High‐cost patients had substantial rates of leaving medicare advantage and joining traditional medicare. Health Aff (Millwood). 2015;34:1675‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Q, Trivedi AN, Galarraga O, Chernew ME, Weiner DE, Mor V. Medicare advantage ratings and voluntary disenrollment among patients with end‐stage renal disease. Health Aff (Millwood). 2018;37:70‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005;3:159‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Black CM, Mehta V, Dubin B, Khandker RK, Ambegaonkar BM, Marsico M. Health care use among newly diagnosed Alzheimer's (AD) patients in a U.S. Commercial medicare advantage insurance plan. Alzheimers Dement J Alzheimers Assoc. 2017;13:P859. [Google Scholar]
- 8. Joyce AT, Zhao Y, Bowman L, Flynn JA, Carter CT, Ollendorf DA. Burden of illness among commercially insured patients with Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc. 2007;3:204‐210. [DOI] [PubMed] [Google Scholar]
- 9. Weiner M, Powe NR, Weller WE, Shaffer TJ, Anderson GF. Alzheimer's disease under managed care: implications from medicare utilization and expenditure patterns. J Am Geriatr Soc. 1998;46:762‐770. [DOI] [PubMed] [Google Scholar]
- 10. Dwibedi N, Findley PA, Wiener CR, Shen C, Sambamoorthi U. Alzheimer disease and related disorders and out‐of‐pocket health care spending and burden among elderly medicare beneficiaries. Med Care. 2018;56:240‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suehs BT, Davis CD, Alvir J, et al. The clinical and economic burden of newly diagnosed Alzheimer's disease in a medicare advantage population. Am J Alzheimers Dis Dement. 2013;28:384‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kester R, Unützer J, Hogan D, Huang H. Antipsychotic prescribing patterns in a medicare advantage population of older individuals with dementia. J Ment Health. 2017;26:167‐171. [DOI] [PubMed] [Google Scholar]
- 13. McWilliams JM, Afendulis CC, McGuire TG, Landon BE. Complex medicare advantage choices may overwhelm seniors—especially those with impaired decision making. Health Aff (Millwood). 2011;30:1786‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan S, Elbel B. Low cognitive ability and poor skill with numbers may prevent many from enrolling in medicare supplemental coverage. Health Aff (Millwood). 2012;31:1847‐1854. [DOI] [PubMed] [Google Scholar]
- 15. Park S, White L, Fishman P, Larson EB, Coe NB. Health care utilization, care satisfaction, and health status for medicare advantage and traditional medicare beneficiaries with and without Alzheimer disease and related dementias. JAMA Netw Open. 2020;3:e201809‐e201809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rivera‐Hernandez M, Blackwood KL, Moody KA, Trivedi AN. Plan switching and stickiness in medicare advantage: a qualitative interview with medicare advantage beneficiaries. Med Care Res Rev. 2020:1077558720944284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panagiotou OA, Kumar A, Gutman R, et al. Hospital readmission rates in medicare advantage and traditional medicare: a retrospective population‐based analysis. Ann Intern Med. 2019;171:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin P‐J, Kaufer DI, Maciejewski ML, Ganguly R, Paul JE, Biddle AK. An examination of Alzheimer's disease case definitions using medicare claims and survey data. Alzheimers Dement J Alzheimers Assoc. 2010;6:334‐341. [DOI] [PubMed] [Google Scholar]
- 19. Newcomer R, Clay T, Luxenberg JS, Miller RH. Misclassification and selection bias when identifying alzheimer's disease solely from medicare claims records. J Am Geriatr Soc. 1999;47:215‐219. [DOI] [PubMed] [Google Scholar]
- 20. Reid RO, Deb P, Howell BL, Shrank WH. Association between medicare advantage plan star ratings and enrollment. JAMA. 2013;309:267‐274. [DOI] [PubMed] [Google Scholar]
- 21. Abraham JM, Feldman R, Carlin C, Christianson J. The effect of quality information on consumer health plan switching: evidence from the buyers health care action group. J Health Econ. 2006;25:762‐781. [DOI] [PubMed] [Google Scholar]
- 22. Jutkowitz E, Bynum JPW, Mitchell SL, et al. Diagnosed prevalence of Alzheimer's disease and related dementias in medicare advantage plans. Alzheimers Dement Diagn Assess Dis Monit. 2020;12:e12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feyman Y, Figueroa JF, Polsky DE, Adelberg M, Frakt A. Primary care physician networks in medicare advantage. Health Aff (Millwood). 2019;38:537‐544. [DOI] [PubMed] [Google Scholar]
- 24. Jaconson G, Trilling A, Neuman T, Damico A, Gold M. Medicare Advantage Hospital Networks: How much do they vary? Kaiser Family Foundation. https://www.kff.org/medicare/report/medicare‐advantage‐hospital‐networks‐how‐much‐do‐they‐vary/ June 20th, 2016.
- 25. Meyers, David J , Rahman, Momotazur , & Trivedi, AN . Narrow Primary Care Networks in Medicare Advantage. J Gen Intern Med. 2021. https://link.springer.com/article/10.1007%2Fs11606‐020‐06534‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyers DJ, Mor V, Rahman M. Medicare advantage enrollees more likely to enter lower‐quality nursing homes compared to fee‐for‐service enrollees. Health Aff (Millwood). 2018;37:78‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meyers DJ, Trivedi AN, Mor V, Rahman M. Comparison of the quality of hospitals that admit medicare advantage patients vs traditional medicare patients. JAMA Netw Open. 2020;3:e1919310‐e1919310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwartz ML, Kosar CM, Mroz TM, Kumar A, Rahman M. Quality of home health agencies serving traditional medicare vs medicare advantage beneficiaries. JAMA Netw Open. 2019;2:e1910622‐e1910622.31483472 [Google Scholar]
- 29. Meyers DJ, Durfey SNM, Gadbois EA, Thomas KS. Early adoption of new supplemental benefits by medicare advantage plans. JAMA. 2019;321:2238‐2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


