Abstract
Background:
Reduced mismatch negativity (MMN) is observed in early psychosis (EP) and correlated with cognition and functioning, but few studies have examined their longitudinal relationships and diagnostic specificity. We examined MMN, neuro- and social-cognition, and functional measures in EP patients with schizophrenia-spectrum (SZ) or bipolar disorder (BD) over a 1-year follow-up.
Methods:
54 EP patients (SZ: n=24; BD: n=30) and 42 healthy controls completed baseline measures: MMN, neuro- and social-cognition, and functional assessments. 30 EP patients completed 12-month follow-up assessments. Patients and controls were compared on MMN at baseline and follow-up, and diagnostic subgroup analyses were performed. Associations amongst MMN, neuro- and social cognition, and clinical measures were examined and predictive models of follow-up outcomes were conducted.
Results:
EP patients showed significantly reduced MMN compared to controls at baseline (p = 0.023). MMN was impaired in SZ patients at baseline (p = 0.017) and follow-up (p = 0.003); BD patients did not differ from controls at either timepoint. MMN was associated with symptom severity and functioning at baseline, and with social cognition and functioning at follow up, but was not predictive of functional outcomes at follow-up.
Conclusions:
MMN abnormalities were evident in EP SZ-spectrum disorders at both timepoints, but not in BD at either timepoint. MMN was associated with functioning cross-sectionally, but did not predict future functional outcomes. However, deficits in MMN were associated with social cognition, which may have downstream effects on community functioning. Implications for targeted interventions to improve social processing and community outcomes are discussed.
Keywords: Early Psychosis, Longitudinal, Mismatch Negativity, Cognition, Functioning
1. Introduction
Mismatch negativity (MMN) is an event-related potential (ERP) that represents an early pre-attentive automatic change detection process occurring in the auditory cortex (see Naatanen et al., 2007; Naatanen et al., 2012 for review). MMN can be elicited using a passive auditory oddball paradigm in response to various types of infrequent deviants (e.g., duration, pitch). Reduced duration-deviant MMN (dMMN) amplitude is a robust observation in patients with chronic schizophrenia spectrum disorders (SZ) with large effect sizes (Cohen’s d ~ 1) (Erickson et al., 2016; Umbricht and Krljes, 2005). In chronic bipolar disorder (BD), studies also find evidence of abnormal MMN, albeit to a lesser degree than in SZ (Hedge’s g ~ 0.40) (Chitty et al., 2013; Erickson et al., 2016; Hermens et al., 2018). Accordingly, MMN has been interpreted as an index of neurobiological phenotype that is shared across psychotic and related disorders (Light and Naatanen, 2013; Javitt et al., 1996) consistent with the Research Domain Criteria (RDoC) framework (Insel et al., 2010). In patients experiencing a first episode of psychosis (FEP), dMMN deficits are also reported, with smaller effect sizes (Cohen’s d ~ 0.4) (Haigh et al., 2017; Hsieh et al., 2019) than in chronic illness, indicating deficits are present early in the disease course and continue worsening over the course of the illness.
In addition to MMN and other neurophysiological abnormalities, FEP patients exhibit deficits across a broad array of domains, including neurocognition, social cognition, and daily functioning. Numerous studies have reported that neurocognition deficits are present early in psychosis (Zabala et al., 2010; Gonzalez-Ortega et al., 2013; Bora and Murray, 2014) and are associated with poorer community functioning (Leeson et al., 2011; Gonzalez-Ortega et al., 2013; Green and Harvey, 2014; Torgalsboen et al., 2015). Social cognition refers broadly to the cognitive functions relevant to perception and processing of social information (Harvey and Penn, 2010) including emotion processing, social perception, theory of mind (TOM)/mental state attribution, and attributional style/bias, as well as more complex concepts such as social metacognition (Pinkham et al., 2016). Although social cognition and neurocognition are often correlated, they appear to exist as at least partially distinct constructs (Sergi et al., 2007), and social cognition may be even more strongly associated with functional outcomes (Fett et al., 2011; Ohmuro et al., 2016). However, longitudinal findings regarding associations amongst neurocognition, social cognition, and outcomes in FEP have been mixed (Gonzalez-Ortega et al., 2019; Stouten et al., 2014).
MMN generation is shown to be sensitive to N-methyl-D-aspartate-type receptor (NMDAR) dysfunction in both primates (8) and in humans (Gunduz-Bruce et al., 2012; Rosburg and Kreitschmann-Andermahr, 2016; Rowland et al., 2016), which is associated with cognition and functioning in both healthy adults and people with psychosis. Accumulating evidence shows that MMN abnormalities in people with SZ and related disorders are tied to downstream functional deficits including measures of social skills, work, independence in daily living, and global ratings of psychosocial functioning (Baldeweg et al., 2004; Light et al., 2007; Naatanen et al., 2011; Light and Braff, 2005; Kawakubo et al., 2007; Wynn et al., 2010; Rasser et al., 2011). These findings suggest that MMN may be associated with other core symptom domains in psychosis such as neurocognition and social cognition, as well as functional outcomes, making it a promising neurophysiological biomarker (Javitt and Freedman, 2015).
Few studies in FEP have examined neurocognition and social cognition in association with electrophysiological biomarkers. Kaur et al. (2011) reported that FEP patients with SZ-spectrum or affective-spectrum disorders exhibited MMN impairments, and that impaired dMMN was correlated with mental control (r=−0.33) and verbal learning (r=−0.34).) Using low resolution brain electromagnetic tomography (LORETA) to examine dMMN deficits in early stage SZ patients and associations with neuropsychological performance, Miyanishi et al. (2013) found that poor working memory was associated with decreased dMMN current density in the frontal lobe. In terms of functioning, associations appear more complex. In the early course of SZ, dMMN amplitude is significantly and negatively correlated with functioning, and working memory is significantly and positively correlated with functioning; however, neurocognition and dMMN were not correlated with one another (Koshiyama et al., 2018), consistent with associations observed in chronic SZ patients (Light and Braff, 2005; Kawakubo and Kasai, 2006; Kiang et al., 2007; Rasser et al., 2011).
MMN studies are typically reported using cross-sectional designs. Only two studies have examined longitudinal changes or diagnostic specificity in early stages of psychosis, with conflicting findings. Koshiyama et al. (2017) found that dMMN deficits were present in FEP patients at baseline and had no progressive reduction over time, whereas Salisbury et al. (2007) reported intact MMN at baseline with progressive reductions over time in people with SZ but not in people with BD. To the best of our knowledge, no study has examined the longitudinal associations among MMN, neurocognition, social cognition, and community functioning in the same cohort of FEP patients. In the present study we report baseline and 12-month follow-up measures from a cohort of early psychosis (EP) patients with SZ-spectrum or BD disorders to examine: 1) MMN differences between controls and EP patients and between diagnostic groups at baseline and follow-up, 2) associations between MMN and neurocognition, social cognition, and functioning at baseline and follow-up, and 3) the predictability of baseline MMN, neurocognition, social cognition, and symptom severity to functional outcomes at follow-up. We employed both interview-based and performance-based measures of functioning in order to assess real-world community functioning (e.g. independence in daily living, role functioning, and social interest/engagement) and performance-based functional capacity. We hypothesized that i) people with EP would exhibit impairments in MMN compared to controls at both timepoints, but that deficits would be more pronounced in patients with SZ than BD, ii) MMN would be significantly associated with neurocognition, social cognition, and community functioning at both timepoints, and iii) baseline MMN, neurocognition, social cognition, and symptom severity would be independently predictive of both community functioning and functioning capacity at follow-up.
2. Methods and Materials
2.1. Subjects
The sample consisted of 54 EP patients and 42 healthy control (HC) subjects. Patients consisted of 24 SZ-spectrum diagnoses and 30 BD with psychotic features, as assessed by the Structured Clinical Interview for DSM-IV (SCID) and review of medical records at initial assessment. Patients were recruited from outpatient clinics, inpatient hospital units, flyers posted at McLean Hospital, and physician referrals. Study inclusion criteria were: 1) ages 18 to 45; 2) fluent in English; 3) IQ>70; 4) diagnosis of SZ, schizoaffective disorder, schizophreniform disorder, psychotic disorder NOS, psychotic depression, or psychotic BD; and 5) EP defined as within the first 3 years of illness onset (Table 2). Exclusion criteria consisted of: 1) diagnosed neurological disorder; 2) brain injury including stroke, loss of consciousness, coma; 3) diagnosed alcohol or drug dependence within 6 months; 4) chronic medical problem that could interfere with study participation, e.g. blindness, deafness, 5) ECT within the past 6 months. The averaged follow-up period for SZ patients was 284.08 days (SD=85.50, range 179–417) and for BD patients was 287.87 days (SD=121.19, range 155–518). HC subjects were recruited from the Partners Research Portal and subject to the same exclusion criteria plus the following: no current or past history of psychotic or affective disorders, no substance abuse or previous chronic dependence, and no first-degree relative with a history of psychosis or BD. The study was approved by McLean Hospital Institutional Review Board. All subjects provided written informed consent after receiving a complete description of the study.
Table 2:
Comparisons of demographic, cognitive, functional and clinical measures by patient diagnostic group at baseline
| SZ Patients | BD Patients | Statistic (df) | p value | |||
|---|---|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | |||
| Age | 22.67 (3.96) | 24 | 23.4 (3.61) | 30 | t = −0.71 (52) | 0.4805 |
| Male (counts and %) | 16 (66.67%) | 24 | 17 (56.67%) | 30 | χ 2 = 0.1346 | 0.714 |
| Education | 14.33 (1.55) | 24 | 15.13 (1.74) | 30 | t = −1.76 (52) | 0.0838 |
| Full Scale IQ (FSIQ) | 116.49 (8.22) | 24 | 116.36 (7.01) | 30 | t = 0.062 (52) | 0.951 |
| Duration of Illness (yr) | 1.45 (1.35) | 24 | 1.17 (1.0) | 29 | t = 0.88 (51) | 0.38 |
| Age of Onset | 21.21 (4.05) | 24 | 22.21 (3.82) | 30 | t = −0.92 (52) | 0.36 |
| MATRICS Social Subscore | 50.14 (9.40) | 21 | 53.14 (10.68) | 29 | t = −1.028 (48) | 0.3091 |
| Composite neurocognition | 42.10 (8.22) | 21 | 46.19 (9.88) | 30 | t = −1.56 (49) | 0.1261 |
| MCAS total score | 42.83 (8.42) | 23 | 48.57 (4.23) | 30 | t = −3.24 (51) | 0.0021 |
| TASIT | 53 (5.70) | 14 | 55.71 (5.09) | 21 | t = −1.47 (33) | 0.1500 |
| UPSA total score | 74.87 (12.50) | 14 | 80.92 (11.03) | 21 | t = −1.51 (33) | 0.1410 |
| CPZ | 205.94 (201.73) | 21 | 154.38 (188.66) | 28 | t = 0.92 (47) | 0.3627 |
| PANSS Positive | 15.13 (6.41) | 23 | 12.03 (6.27) | 30 | t = 1.77 (51) | 0.0835 |
| PANSS Negative | 13.30 (4.51) | 23 | 11.37 (3.75) | 30 | t = 1.71 (51) | 0.0936 |
| PANSS General | 30.87 (5.47) | 23 | 27.07 (7.19) | 30 | t = 2.11 (51) | 0.0398 |
| PANSS Total | 59.30 (12.29) | 23 | 50.47 (14.53) | 30 | t = 2.34 (51) | 0.0231 |
Average baseline demographic, cognitive, and functional measures, represented by mean and standard deviation, were compared between SZ and BD patients. Bolded text represents significant differences between groups.
2.2. Clinical assessments
Clinical measures included the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), the Young Mania Rating Scale (YMRS) (Young, et al., 1978), and the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). Medication information was collected at each assessment timepoint. Antipsychotics included first- and second-generation antipsychotic medications and were converted into chlorpromazine (CPZ) equivalents based on the recommendations of Baldessarini (2013).
2.3. Cognitive assessments
Neurocognition.
Neurocognition was assessed using the MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein et al., 2008). The MCCB consists of 10 tasks across 7 domain scores, including Processing Speed, Attention, Working Memory, Verbal Learning, Visual Learning, Problem Solving, and Social Cognition, and also produces a Composite score. Several studies suggest that the MCCB social cognition composite is less strongly correlated with the neurocognition domain scores (Lewandowski et al., 2014; Van Rheenen et al., 2017) and may even serve as a partial mediator between neurocognitive functioning and community outcomes (Ospina et al., 2018). Based on these findings and our aims of examining specific associations between MMN and neurocognition/MMN and social cognition separately, we calculated a mean MCCB neurocognitive composite score without the social cognition domain score, and report the social cognition domain separately. All scores are reported as age and sex adjusted T scores generated using the MCCB scoring software.
Social Cognition.
Social cognition was assessed using two measures: the Awareness of Social Inference Test (TASIT)-Part Two (McDonald et al., 2003) which measures social inference/Theory of Mind, and the MSCEIT subtest from the MCCB, which measures social and emotional reasoning. The TASIT is comprised of fifteen brief video clips depicting everyday social interactions between two actors. The dialogue is often ambiguous, requiring participants to integrate cues from face, prosody, gesture, and social context (Channon et al., 2005; Leitman et al., 2006; McDonald, 1999). Participants answer 4 questions per video that probe understanding of the intentions, beliefs, and meanings of the speakers and their exchanges. Scores range from 0 to 64. Studies have found deficits in social perception in people with SZ using the TASIT (Kern et al., 2009; Leitman et al., 2006; Sparks et al., 2010. The MSCEIT consists of a series of vignettes read aloud to participants as they follow along in their booklets. Participants are asked to answer questions about effective responses or likely emotional reactions based on the vignettes. Scores are calculated using the MSCEIT scoring algorithm included in the MCCB scoring package, and age and sex adjusted norms are generated in T scores.
2.4. Functional assessments
Functional capacity was assessed using the UCSD Performance-Based Skills Assessment, Brief (UPSA-B) (Mausbach et al., 2007). The UPSA-B is a performance-based measure consisting of two subscales, financial and communication, designed to evaluate ability to perform everyday tasks (Mausbach et al., 2007). Total scores range from 0 to 100; higher scores reflect better performance (McKibbin et al., 2004; Leifker et al., 2009; Patterson and Mausbach, 2010; Green et al., 2011).
Community functioning was evaluated using the Multnomah Community Ability Scale (MCAS) (Barker et al., 1994; Zhou et al., 2018; Monaghan et al., 2019), an interview-based measure developed for use in psychiatric populations that probes several aspects of community functioning including independence in daily living, instrumental role functioning, and social interest/engagement. We used an abbreviated 11-item version to assess community functioning independent of cognitive impairment and symptom severity (Lewandowski et al., 2013). Items are scored 1–5, and higher scores indicate better functioning. We examined the MCAS total and the social and independence factors previously identified by Martin et al. (2015). Studies have demonstrated good predictive validity of MCAS, with poorer scores associated with subsequent hospitalizations (Barker et al., 1994; Zani et al., 1999).
2.5. Electrophysiological recordings and processing
The electroencephalogram (EEG) was recorded continuously using the BioSemi Active Two system (BioSemi Inc, Amsterdam, Netherlands) at a digitization rate of 512 Hz, with a bandpass of DC–104 Hz, and a Common Mode Sense (CMS) as the reference (PO2 site) using a 64-channel electrode cap. EOG electrodes were placed below and at the outer canthi of the left eye. A duration MMN paradigm was used to elicit MMN. Stimuli consisted of 1200 trials presented to the subjects through foam insert earphones. 85% of the stimuli were standard tones (1000Hz, 100ms), and 15% were duration deviant tones (1000Hz, 150ms), with an inter-stimulus interval 200ms and stimulus-onset-asynchrony 300 ms after standards and 350 ms after deviants. Participants were instructed to watch a silent cartoon/video clips (BBC natural program, Charlie Brown) during the stimulus presentation.
Data were processed using BrainVision Analyzer 2 (Brain Products GmbH, Munich, Germany). Data processing was performed offline and blind to group membership using automated procedures. Signals were re-referenced to an average of the mastoids and bandpass filtered between 0.01 to 20 Hz using a zero phase shift Butterworth filter. Data were segmented by stimulus marker from −100 to 400 ms. Segments were baseline corrected using −100 to 0 ms pre-stimulus time and eye-blink corrected using established measures (Gratton et al., 1983). Artifact rejection for individual channels was performed and a given segment was rejected if the voltage gradient exceeded 50 μV/ms, amplitude was +/−100 μV, or the signal was flat (<0.5 μV for >100 ms). MMN waveforms were generated by subtracting ERP waveforms in response to standard tones from the ERPs generated in response to the deviant tones. The MMN amplitude was measured as the peak amplitude between the time window of 100 to 250 milliseconds.
2.6. Statistical Analysis
Statistical analyses were carried out using STATA 15 (StataCorp, College Station, Texas). Duration MMN amplitudes at the Fz electrode were analyzed. Comparisons of control versus patient demographics and clinical features were performed using two-tailed unequal-variance t-tests and Fisher’s exact tests. Linear regression analyses were conducted to examine whether impaired MMN in patients would be observed at each timepoint and diagnostic specificity, controlling for age and sex. Patients’ longitudinal changes were assessed using linear mixed effects models, accounting for interaction between diagnostic group and time.
Relationships between MMN, community functioning (MCAS), functioning capacity (UPSA), neurocognition, social cognition, and symptom severity at baseline and follow-up were assessed using stepwise linear regression analyses (Likelihood Ratio Tests, LRTs) and partial correlational analyses controlling for significant predictors in the regression model to quantify the strength of associations. In the stepwise regression model, MMN was the outcome variable; age, sex, real-world functioning (UPSA, MCAS), social cognition (TASIT, MATRICS-social subscore), symptom severity (PANSS), and composite neurocognition (the average of all six MATRICS subscores except the social subscore) were included as predictors. Two separate analyses were run, one for baseline and one for follow-up. CPZ was initially included as a covariate but was not a significant predictor in the models at either timepoint; this variable was dropped from the regression models. Prediction of real-world functioning at 12-month follow-up (MCAS or UPSA) was assessed using two separate stepwise regression models. In the first model, baseline demographics (age, sex), MMN, composite neurocognition, social cognition, and symptoms (PANSS total) were entered as predictors, and follow-up MCAS, or UPSA, was the outcome variable. In the second model, baseline demographics (age, sex), MMN, composite neurocognition, social cognition, and symptoms (PANSS total) were entered as predictors, and follow-up MCAS Independence subscore, or MCAS Social subscore, was the outcome variable.
3. Results
3.1. Comparison of demographics and clinical variables
Group analyses revealed significant differences between patients and healthy controls in education, composite neurocognition, MCAS, and UPSA at baseline (Table 1A). At follow-up patients had significantly lower MCAS than healthy controls (Table 1B). Patients with SZ had significantly higher PANSS General and PANSS Total scores and lower MCAS functioning than those with BD (Table 2).
Table 1:
Group comparison (patients versus controls) of demographic, cognitive, and functional measures at baseline (A) and follow up (B)
| (A) Baseline | ||||||
| Controls | Patients at Baseline | Statistic (df) | p value | |||
| Mean (Std Errors) | N | Mean (Std Errors) | N | |||
| Age | 22.83 (0.58) | 42 | 23.07 (0.51) | 54 | −0.31 (88.09) | 0.756 |
| Male (counts and %) | 24 (57.14%) | 42 | 33 (61.11%) | 54 | 0.15 | 0.834 |
| Education | 15.5 (.25) | 42 | 14.78 (0.23) | 54 | 2.13 (90.12) | 0.036 |
| Full Scale IQ (FSIQ) | 113.25 (1.076) | 42 | 116.42 (1.021) | 54 | −2.14 (90.99) | 0.035 |
| MATRICS Social Subscore | 54.18 (1.15) | 40 | 51.88 (1.44) | 50 | 1.25 (86.92) | 0.215 |
| Composite neurocognition | 50.90 (0.86) | 40 | 45.90 (0.95) | 49 | 3.91 (87.00) | <0.001 |
| MCAS total score | 54.76 (0.11) | 33 | 46.08 (0.95) | 53 | 9.04 (53.30) | <0.001 |
| TASIT | 56.06 (0.78) | 33 | 54.63 (0.92) | 35 | 1.19 (64.96) | 0.240 |
| UPSA total score | 84.60 (1.45) | 33 | 78.50 (2.00) | 35 | 2.47 (61.15) | 0.017 |
| (B) Follow-up | ||||||
| Controls | Patients at Follow-Up | Statistic (df) | p value | |||
| Mean (Std Errors) | N | Mean (Std Errors) | N | |||
| Age | 22.83 (0.58) | 42 | 24.033 (0.75) | 30 | −1.26 (58.96) | 0.213 |
| Male (counts and %) | 24 (57.14%) | 42 | 20 (66.67%) | 30 | 0.67 | 0.469 |
| Education | 15.5 (.25) | 42 | 14.97 (0.33) | 30 | 1.30 (58.44) | 0.199 |
| MATRICS Social Subscore | 54.18 (1.15) | 40 | 53.64 (2.48) | 25 | 0.20 (34.37) | 0.846 |
| Composite neurocognition | 50.90 (0.86) | 40 | 48.89 (1.52) | 25 | 1.15 (39.17) | 0.257 |
| MCAS total score | 54.76 (0.11) | 33 | 48.69 (1.02) | 29 | 5.90 (28.61) | <0.001 |
| TASIT | 56.06 (0.78) | 33 | 54.25 (1.30) | 24 | 1.19 (38.95) | 0.241 |
| UPSA total score | 84.60 (1.45) | 33 | 83.95 (2.25) | 24 | 0.24 (41.01) | 0.809 |
Table 1A) (top): Average baseline demographic, cognitive, and functional measures, represented by mean and standard errors, were compared between patients and controls. Bolded text represents significant differences between groups. Table 1B) (bottom): Average follow-up demographic, cognitive, and functional measures, represented by mean and standard errors, were compared between patients and controls. Bolded text represents significant differences between groups.
3.2. Comparisons of MMN between groups
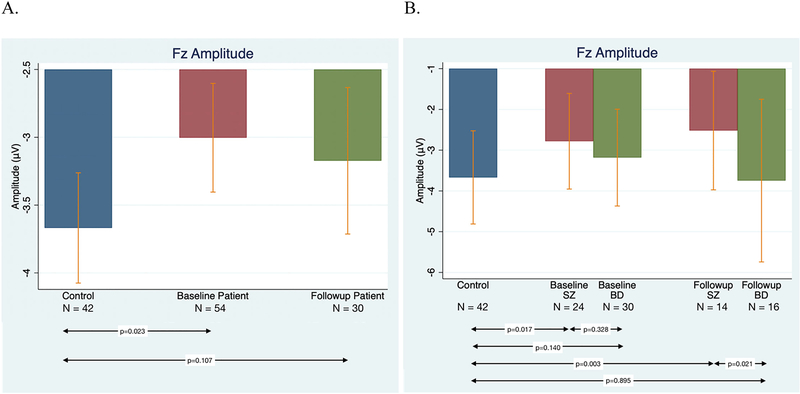
Results of regression analyses including all patients showed that at baseline, MMN of patients differed significantly from controls (β = 0.672, p = 0.023), while at follow-up the group difference was at the trend level (Figure 1A).
Figure 1.
Comparisons of MMN by Group and Diagnosis. Figure 1A) (left): Average Fz amplitudes for different groups. Amplitude for control subjects (blue), early psychosis (EP) patients at baseline (red), and EP patients at 12 months (green). Figure 1B) (right): MMN for control subjects (blue), SZ patients at baseline and 12 months (red), and BD patients at baseline and 12 months (green). (Note: vertical bars represent standard deviations.)
Comparisons by diagnostic group revealed that relative to controls MMN was significantly impaired in the SZ group at both baseline (β = 0.884, p = 0.017) and follow-up (β = 1.283, p = 0.003). Conversely, there were no significant differences between BD patients and controls at either timepoint (baseline: β = 0.502, p = 0.140; follow-up: β = −0.051, p = 0.895) (Figure 1B). Post hoc analyses revealed that SZ and BD patients did not differ significantly from each other at baseline (β = −0.409, p = 0.328), but did differ at follow up (β = −1.291, p = 0.021) with the SZ group showing greater impairment. The time by diagnosis interaction was not significant.
3.3. MMN in relationship with functioning, social cognition, and neurocognition
In two separate analyses (baseline and follow-up), the stepwise regression model included age, sex, UPSA, MCAS, TASIT, MATRICS-social subscore, PANSS, and composite neurocognition as predictors. Patient and control groups differed on full scale IQ (FSIQ), with patients performing slightly better than controls (Table 1). However, FSIQ was not correlated with MMN (r=−0.0001, p=0.9991), and therefore we chose not to include FSIQ as a predictor in the regression models. Results of baseline stepwise regression showed that MMN was significantly correlated with MCAS (p = 0.03; partial correlation = −0.39) and PANSS total (p = 0.02; partial correlation = −0.33). At follow-up stepwise regression, MMN was significantly associated with TASIT (p = 0.024; partial correlation = −0.580), MCAS (p = 0.019; partial correlation = −0.567), and MATRICS social cognition (p = 0.008; partial correlation = 0.528). Post-hoc analyses examining MCAS sub-domains showed that MMN was significantly associated with MCAS social sub-domain (p = 0.027; partial correlation = −0.488) at follow-up. Interrelationships among neurocognition, social cognition, and functioning measures were presented in the supplementary table (Table S1 and S2).
3.4. Predictions of patients’ real-world functioning at follow-up
Baseline demographics (age, sex), MMN, composite neurocognition, social cognition, and symptoms (PANSS total) were included in stepwise regression models to predict follow-up UPSA, MCAS, MCAS Social subscore, or MCAS Independence subscore. None of the baseline clinical variables were found to be significant predictors of follow-up UPSA total score. Baseline composite neurocognition (β = 0.431, p = 0.048) was a significant predictor of follow-up MCAS total score, while diagnosis was a predictor of follow-up MCAS total at a trend level. Analyses examining specific domains of function revealed that baseline diagnosis (β = −2.772, p = 0.023) and baseline composite neurocognition (β = 0.317, p = 0.024) were predictive of follow-up MCAS Social subscore, while baseline composite neurocognition was predictive of follow-up MCAS Independence subscore at a trend level.
4. Discussion
To our knowledge, this study is the first to investigate the relationships among MMN, neurocognition, social cognition, performance-based functional capacity and community functioning in a transdiagnostic cohort of EP patients, as well as the relationships amongst MMN, neurocognition, social cognition, and later functional outcomes. As a group, patients with early psychosis had impaired MMN at study entry compared to controls; however, we found unanticipated differences by diagnosis in which MMN abnormalities were pronounced in people with SZ-spectrum disorders but people with BD did not differ from controls. MMN did not change significantly in either group, remaining stably impaired in people with SZ and no different from controls in people with BD. Across the patient groups MMN was associated with symptom severity and functioning at baseline, and with social cognition and functioning at follow up. Baseline diagnosis and neurocognition were predictive of later functional outcomes, particularly in social and independent functioning domains.
4.1. Comparisons of MMN between groups
The present findings support previous evidence that MMN impairment is a trait biomarker in people with SZ-spectrum illness – present at illness onset and in ultra-high risk populations, and consistently impaired over time – but do not support this conclusion in people with BD with psychosis (Haigh et al., 2017; Hall et al., 2007; Hall et al., 2009; Hsieh et al., 2019; Erickson et al., 2016; Perez et al., 2014). At baseline, symptom severity was associated with MMN across patient groups. However, post-hoc analyses revealed that in SZ patients, healthier MMN amplitude was associated with less PANSS total score (r= 0.21), whereas in BD, higher MMN amplitude was associated with higher PANSS total score (r= −0.41), suggesting that MMN may be a trait marker of illness in SZ, and may also reflect state symptom severity in BD. Supporting this possibility, Kaur et al. (2011) found MMN impairments in both SZ and affective disorders, but reported that the affective disorders group actually exhibited higher symptom severity than the SZ group, potentially driving higher levels of state-related MMN abnormalities.
Previous work has reported that MMN abnormalities may progress with duration of illness (Salisbury et al., 2017). Meta-analyses report that FEP patients already exhibit abnormalities in MMN, but that the magnitude is smaller in FEP than in chronicity, suggesting a progressive nature to MMN impairment, as proposed by Salisbury et al. (2007). Our findings support the presence of MMN abnormalities at the early course of illness in people with SZ-spectrum disorders; however, progressive changes in MMN were not apparent in our sample. Given our relatively short follow-up period of one year, it is possible that worsening MMN may occur more slowly over time, perhaps associated with illness burden, rather than deteriorating precipitously after an initial episode.
4.2. MMN in relationship with functioning, social cognition, and neurocognition
Our results revealed a significant association between MMN and daily functioning in EP. Greater MMN abnormalities were associated with greater functional impairment using interview-based community functioning (MCAS) measures at both timepoints. These findings are consistent with observed MMN-functioning associations in chronic SZ and BD patients (Light and Braff, 2005; Kawakubo and Kasai, 2006; Kiang et al., 2007; Wynn et al., 2010; Rasser et al., 2011; Hermens et al., 2018). Only three previous reports have examined the relationship between MMN and functioning in early psychosis. Hermens et al. (2010) found a significant correlation between MMN amplitude and quality of life in first episode psychosis, and Koshiyama et al. (2018) showed a significant correlation between MMN impairment and lower global functioning (GAF) in early SZ patients. Murphy et al. (2020) reported significant associations between MMN and social functioning using the Global Functioning: Social and Role scales. In the present study we used an interview measure assessing several aspects of community functioning, including independence in daily living, instrumental role functioning, and social interest and engagement. Thus, we were able to extend the literature by demonstrating that MMN was associated with real-world functioning across several domains independent of symptom severity, and that the MMN-community functioning relationship was particularly relevant to the social domain (e.g., social acceptability, social interest, social effectiveness, and social network) consistent with Murphy et al. (2020), similar to findings in people with chronic SZ.
We found that, as expected, healthier MMN was associated with higher social influence ability (TASIT) and better real-world functioning (MCAS) at follow-up. However, we found an unanticipated correlation between MMN and social-t score, showing better MMN was associated with less social emotional ability. Correlations matrix (Table S1b) showed that in the whole sample social-t score was positively correlated with neurocognition, MCAS, and TASIT, as one would expect. The unanticipated correlation between MMN and social-t score was driven by the BD group (r= 0.64), while in SZ MMN was negatively associated with social-t score (r= −0.14). Future studies are warranted to gain better insight of social emotion and MMN in BD patients.
4.3. Predictions of patients’ real-world functioning at follow-up
While we found associations between MMN and functional measures at both timepoints cross-sectionally, contrary to our hypothesis, baseline MMN was not a significant predictor of later functional outcomes. Additionally, a post-hoc mediation analysis failed to find a significant mediation effect of social cognition, although it should be noted that this analysis was likely underpowered. Kaur et al. (2013) also failed to find MMN at frontocentral sites as a predictor of functional outcome; rather, they reported MMN at the mastoids (M1/M2) as predictors. Accordingly, more research is needed before definitive conclusions can be drawn about the utility of MMN in predicting later functioning status. While MMN was not a significant predictor of later functioning, diagnosis and neurocognition at baseline were independently predictive of later functional outcomes, particularly in the social and independent functioning domains, in keeping with previous associations between neurocognition and functioning in FEP (Stouten et al., 2014; Dickerson et al., 2008; Nuechterlein et al., 2011). Of note, while neither MMN nor social cognition were predictive of future functional outcomes, both were associated with functioning at each timepoint suggesting that impairments in MMN may impact functioning cross-sectionally, perhaps via associations with social cognition. These findings have implications for the development of targeted interventions to improve social processing with MMN serving as a potential mechanism of action.
Several limitations should be noted. First, we focused on relatively short-term clinical and functioning outcomes one year after baseline assessment. Thus, the relationships amongst MMN, social cognition, neurocognition, and functional measures occurring after a one-year period are yet to be established. Second, while effects of antipsychotic medications on MMN were not significant in our sample, which is consistent with literature (Korostenskaja et al., 2005; Leung et al., 2007; Pekkonen et al., 2002; Umbricht et al., 1998), a relatively high degree of missingness may have reduced our power to detect significant associations. Also, we were unable to examine potential effects of other medication classes on MMN, and there is a dearth of research assessing the medications effects on MMN. Third, while the UPSA is commonly used to assess functional capacity, some components are outdated and alternative performance-based measures may better capture functioning particularly in young people. Forth, HC were not retested. It is possible HC may have had some increase at re-testing that cannot be ruled out. However, we have reported high and significant reliability for MMN amplitude (ICC=0.67 for peak amplitude) in a prior study (Hall et al., 2006). Lastly, we included the PANSS Total score but not the positive, negative, and general subscales separately due to concerns about overfitting given the relatively modest sample size. A recent study found that positive symptoms specifically were correlated with MMN amplitude in people with SZ (Koshiyama et al., 2020), although an earlier study found no association between symptom severity and MMN (Erikson et al., 2017). Future work in larger samples examining specific associations between MMN abnormalities and PANSS subscales are needed.
In conclusion, we found evidence of early auditory processing deficits by the time of first episode that persisted over time in people with SZ but not with BD, and associations with state clinical symptoms in both SZ and BD, suggesting that MMN may act as a trait-marker of illness in SZ-spectrum disorders and a state marker of symptom severity transdiagnostically. Additionally, our findings suggest that MMN abnormalities are associated with social cognition and may affect community functioning, particularly in the social domain, making it a promising biomarker and a potential treatment target in early psychosis.
Supplementary Material
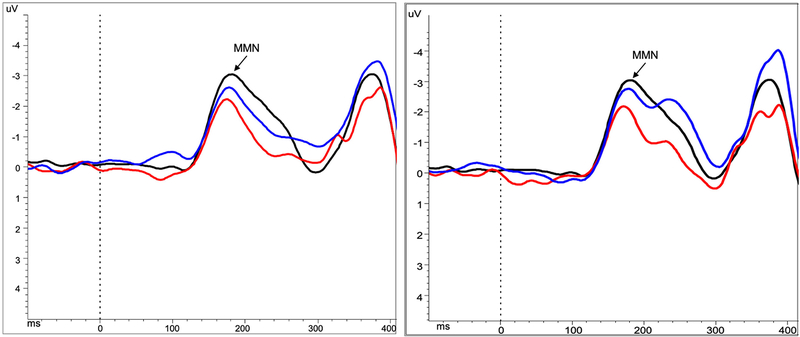
Figure 2.
Grand average MMN waveforms at Fz for control subjects (black), SZ (red) and BP (blue) patients at baseline (left) and follow-up (right)
Note: Peak detection window for MMN was between 120–250ms. HC baseline mean = 185.73 ms, SD = 19.17, range 160–242 ms; Patients baseline mean = 180.52 ms, SD = 19.88, range 139–248 ms; Patients follow-up mean = 179.69 ms, SD = 21.89, range 135–236 ms.
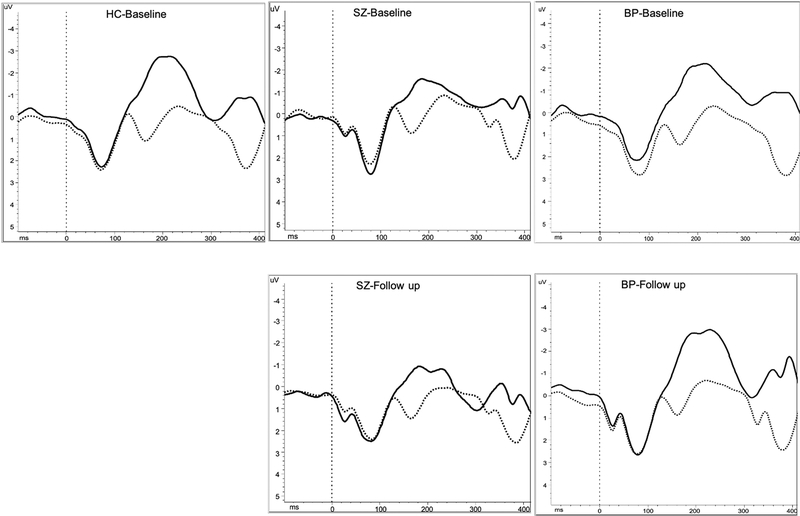
Figure 3.
Grand average waveforms of standard (solid) and target (dotted) tones at Fz for control subjects (left), SZ (middle) and BP (right) patients at baseline (top) and follow-up (bottom)
Acknowledgments
National Institute of Mental Health [R01MH109687]: Mei-Hua Hall, PI
National Institute of Mental Health [R01MH117012]: Kathryn Eve Lewandowski, PI
Role of the Funding Source
Footnotes
Disclosures
Amy Higgins, Kathryn Eve Lewandowski, Saran Liukasemsarn, and Mei-Hua Hall reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldessarini RJ 2013. Chemotherapy in Psychiatry: Pharmacologic Basis of Treatments for Major Mental Illness. Springer. New York, NY. [Google Scholar]
- Baldeweg T, Klugman A, Gruzelier J, Hirsch SR, 2004. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr Res 69(2–3), 203–217. [DOI] [PubMed] [Google Scholar]
- Barker S, Barron N, McFarland BH, Bigelow DA, 1994. A community ability scale for chronically mentally ill consumers: Part I. Reliability and validity. Community mental health journal 30(4), 363–383. [DOI] [PubMed] [Google Scholar]
- Bora E, Murray RM, 2014. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull 40(4), 744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekke J, Kay DD, Lee KS, Green MF, 2005. Biosocial pathways to functional outcome in schizophrenia. Schizophr Res 80(2–3), 213–225. [DOI] [PubMed] [Google Scholar]
- Channon S, Pellijeff A, Rule A, 2005. Social cognition after head injury: sarcasm and theory of mind. Brain Lang 93(2), 123–134. [DOI] [PubMed] [Google Scholar]
- Chitty KM, Lagopoulos J, Lee RS, Hickie IB, Hermens DF, 2013. A systematic review and meta-analysis of proton magnetic resonance spectroscopy and mismatch negativity in bipolar disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 23(11), 1348–1363. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Stallings C, Origoni A, Boronow JJ, Sullens A, Yolken R, 2008. Predictors of occupational status six months after hospitalization in persons with a recent onset of psychosis. Psychiatry Res 160(3), 278–284. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Albrecht M, Ruffle A, Fleming L, Corlett P, Gold J, 2017. No association between symptom severity and MMN impairment in schizophrenia: A meta-analytic approach. Schizophr Res Cogn 9, 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Ruffle A, Gold JM, 2016. A Meta-Analysis of Mismatch Negativity in Schizophrenia: From Clinical Risk to Disease Specificity and Progression. Biol Psychiatry 79(12), 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L, 2011. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience and biobehavioral reviews 35(3), 573–588. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ortega I, de Los Mozos V, Echeburua E, Mezo M, Besga A, Ruiz de Azua S, Gonzalez-Pinto A, Gutierrez M, Zorrilla I, Gonzalez-Pinto A, 2013. Working memory as a predictor of negative symptoms and functional outcome in first episode psychosis. Psychiatry Res 206(1), 8–16. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ortega I, Gonzalez-Pinto A, Alberich S, Echeburua E, Bernardo M, Cabrera B, Amoretti S, Lobo A, Arango C, Corripio I, Vieta E, de la Serna E, Rodriguez-Jimenez R, Segarra R, Lopez-Ilundain JM, Sanchez-Torres AM, Cuesta MJ, Group PE, Zorrilla I, Lopez P, Bioque M, Mezquida G, Barcones F, De-la-Camara C, Parellada M, Espliego A, Alonso-Solis A, Grasa EM, Varo C, Montejo L, Castro-Fornieles J, Baeza I, Dompablo M, Torio I, Zabala A, Eguiluz JI, Moreno-Izco L, Sanjuan J, Guirado R, Caceres I, Garnier P, Contreras F, Bobes J, Al-Halabi S, Usall J, Butjosa A, Sarro S, Landin-Romero R, Ibanez A, Selva G, 2019. Influence of social cognition as a mediator between cognitive reserve and psychosocial functioning in patients with first episode psychosis. Psychol Med, 1–9. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E, 1983. A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology 55(4), 468–484. [DOI] [PubMed] [Google Scholar]
- Green MF, Harvey PD, 2014. Cognition in schizophrenia: Past, present, and future. Schizophr Res Cogn 1(1), e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Schooler NR, Kern RS, Frese FJ, Granberry W, Harvey PD, Karson CN, Peters N, Stewart M, Seidman LJ, Sonnenberg J, Stone WS, Walling D, Stover E, Marder SR, 2011. Evaluation of functionally meaningful measures for clinical trials of cognition enhancement in schizophrenia. Am J Psychiatry 168(4), 400–407. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Reinhart RM, Roach BJ, Gueorguieva R, Oliver S, D’Souza DC, Ford JM, Krystal JH, Mathalon DH, 2012. Glutamatergic modulation of auditory information processing in the human brain. Biol Psychiatry 71(11), 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh SM, Coffman BA, Salisbury DF, 2017. Mismatch Negativity in First-Episode Schizophrenia: A Meta-Analysis. Clin EEG Neurosci 48(1), 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Rijsdijk F, Kalidindi S, Schulze K, Kravariti E, Kane F, Sham P, Bramon E, Murray RM, 2007. Genetic overlap between bipolar illness and event-related potentials. Psychol Med 37(5), 667–678. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Picchioni M, Ettinger U, Bramon E, Freedman R, Murray RM, Sham P, 2006. Heritability and reliability of P300, P50 and duration mismatch negativity. Behavior genetics 36(6), 845–857. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, Murray RM, Sham P, 2009. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med 39(8), 1277–1287. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Penn D, 2010. Social cognition: the key factor predicting social outcome in people with schizophrenia? Psychiatry (Edgmont) 7(2), 41–44. [PMC free article] [PubMed] [Google Scholar]
- Hermens DF, Chitty KM, Kaur M, 2018. Mismatch negativity in bipolar disorder: A neurophysiological biomarker of intermediate effect? Schizophr Res 191, 132–139. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB, 2010. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Progress in neuropsychopharmacology & biological psychiatry 34(6), 822–829. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Lin YT, Chien YL, Hwang TJ, Hwu HG, Liu CM, Liu CC, 2019. Auditory Event-Related Potentials in Antipsychotic-Free Subjects With Ultra-High-Risk State and First-Episode Psychosis. Front Psychiatry 10, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167(7), 748–751. [DOI] [PubMed] [Google Scholar]
- Javitt D, Steinschneider M, Schroeder CE, Arezzo JC, 1996. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A 93, 11962–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Freedman R, 2015. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry 172(1), 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, Hermens DF, 2011. MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophr Res 130(1–3), 203–209.21550211 [Google Scholar]
- Kaur M, Lagopoulos J, Lee RS, Ward PB, Naismith SL, Hickie IB, Hermens DF, 2013. Longitudinal associations between mismatch negativity and disability in early schizophrenia- and affective-spectrum disorders. Progress in neuro-psychopharmacology & biological psychiatry 46, 161–169. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Kamio S, Nose T, Iwanami A, Nakagome K, Fukuda M, Kato N, Rogers MA, Kasai K, 2007. Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res 152(2–3), 261–265. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Kasai K, 2006. Support for an association between mismatch negativity and social functioning in schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry 30(7), 1367–1368. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13(2), 261–276. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Fiske AP, Kee KS, Lee J, Sergi MJ, Horan WP, Subotnik KL, Sugar CA, Nuechterlein KH, 2009. Theory of mind deficits for processing counterfactual information in persons with chronic schizophrenia. Psychol Med 39(4), 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang M, Light GA, Prugh J, Coulson S, Braff DL, Kutas M, 2007. Cognitive, neurophysiological, and functional correlates of proverb interpretation abnormalities in schizophrenia. Journal of the International Neuropsychological Society : JINS 13(4), 653–663. [DOI] [PubMed] [Google Scholar]
- Korostenskaja M, Dapsys K, Siurkute A, Maciulis V, Ruksenas O, Kahkonen S, 2005. Effects of olanzapine on auditory P300 and mismatch negativity (MMN) in schizophrenia spectrum disorders. Progress in neuro-psychopharmacology & biological psychiatry 29(4), 543–548. [DOI] [PubMed] [Google Scholar]
- Koshiyama D, Kirihara K, Tada M, Nagai T, Fujioka M, Koike S, Suga M, Araki T, Kasai K, 2018. Association between mismatch negativity and global functioning is specific to duration deviance in early stages of psychosis. Schizophr Res 195, 378–384. [DOI] [PubMed] [Google Scholar]
- Koshiyama D, Kirihara K, Tada M, Nagai T, Fujioka M, Usui K, Araki T, Kasai K, 2020. Reduced auditory mismatch negativity reflects impaired deviance detection in schizophrenia. Schizophr Bull 46, 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama D, Kirihara K, Tada M, Nagai T, Koike S, Suga M, Araki T, Kasai K, 2017. Duration and frequency mismatch negativity shows no progressive reduction in early stages of psychosis. Schizophr Res 190, 32–38. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Sharma P, Harrison M, Ron MA, Barnes TR, Joyce EM, 2011. IQ trajectory, cognitive reserve, and clinical outcome following a first episode of psychosis: a 3-year longitudinal study. Schizophr Bull 37(4), 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifker FR, Bowie CR, Harvey PD, 2009. Determinants of everyday outcomes in schizophrenia: the influences of cognitive impairment, functional capacity, and symptoms. Schizophr Res 115(1), 82–87. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Ziwich R, Pasternak R, Javitt DC, 2006. Theory of Mind (ToM) and counterfactuality deficits in schizophrenia: misperception or misinterpretation? Psychol Med 36(8), 1075–1083. [DOI] [PubMed] [Google Scholar]
- Leung S, Croft RJ, Baldeweg T, Nathan PJ, 2007. Acute dopamine D(1) and D(2) receptor stimulation does not modulate mismatch negativity (MMN) in healthy human subjects. Psychopharmacology 194(4), 443–451. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Keshavan MS, Sperry SH, Ongur D, 2013. Neuropsychological functioning predicts community outcomes in affective and non-affective psychoses: a 6-month follow-up. Schizophr Res 148(1–3), 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Sperry SH, Cohen BM, Ongur D, 2014. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol Med 44(15), 3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Braff DL, 2005. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry 62(2), 127–136. [DOI] [PubMed] [Google Scholar]
- Light GA, Naatanen R, 2013. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proc Natl Acad Sci U S A 110(38), 15175–15176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL, 2007. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. Journal of cognitive neuroscience 19(10), 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EA, Ongur D, Cohen BM, Lewandowski KE, 2015. Social functioning and age across affective and nonaffective psychoses. J Nerv Ment Dis 203(1), 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL, 2007. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull 33(6), 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S, 1999. Exploring the process of inference generation in sarcasm: a review of normal and clinical studies. Brain Lang 68(3), 486–506. [DOI] [PubMed] [Google Scholar]
- McDonald S, Flanagan S, Rollins J, Kinch J, 2003. TASIT: A new clinical tool for assessing social perception after traumatic brain injury. J Head Trauma Rehabil 18(3), 219–238. [DOI] [PubMed] [Google Scholar]
- McKibbin CL, Brekke JS, Sires D, Jeste DV, Patterson TL, 2004. Direct assessment of functional abilities: relevance to persons with schizophrenia. Schizophr Res 72(1), 53–67. [DOI] [PubMed] [Google Scholar]
- Miyanishi T, Sumiyoshi T, Higuchi Y, Seo T, Suzuki M, 2013. LORETA current source density for duration mismatch negativity and neuropsychological assessment in early schizophrenia. PloS one 8(4), e61152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan CK, Brickman S, Huynh P, Ongur D, Hall MH, 2019. A longitudinal study of event related potentials and correlations with psychosocial functioning and clinical features in first episode psychosis patients. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 145, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. The British journal of psychiatry : the journal of mental science 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Murphy TK, Haigh SM, Coffman BA, Salisbury DF, 2020. Mismatch negativity and impaired social functioning in long-term and in first episode schizophrenia spectrum psychosis. Frontiers in psychiatry, 11, 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R, Kujala T, Escera C, Baldeweg T, Kreegipuu K, Carlson S, Ponton C, 2012. The mismatch negativity (MMN)--a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin Neurophysiol 123(3), 424–458. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Kujala T, Kreegipuu K, Carlson S, Escera C, Baldeweg T, Ponton C, 2011. The mismatch negativity: an index of cognitive decline in neuropsychiatric and neurological diseases and in ageing. Brain : a journal of neurology 134(Pt 12), 3435–3453. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Paavilainen P, Rinne T, Alho K, 2007. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol 118(12), 2544–2590. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 165(2), 203–213. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, Yee CM, Gretchen-Doorly D, Mintz J, 2011. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull 37 Suppl 2, S33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmuro N, Katsura M, Obara C, Kikuchi T, Sakuma A, Iizuka K, Hamaie Y, Ito F, Matsuoka H, Matsumoto K, 2016. Deficits of cognitive theory of mind and its relationship with functioning in individuals with an at-risk mental state and first-episode psychosis. Psychiatry Res 243, 318–325. [DOI] [PubMed] [Google Scholar]
- Ospina LH, Nitzburg GC, Shanahan M, Perez-Rodriguez MM, Larsen E, Latifoglu A, Burdick KE, 2018. Social cognition moderates the relationship between neurocognition and community functioning in bipolar disorder. Journal of affective disorders 235, 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Mausbach BT, 2010. Measurement of functional capacity: a new approach to understanding functional differences and real-world behavioral adaptation in those with mental illness. Annual review of clinical psychology 6, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkonen E, Hirvonen J, Ahveninen J, Kahkonen S, Kaakkola S, Huttunen J, Jaaskelainen IP 2002. Memory-based comparison process not attenuated by haloperidol: a combined MEG and EEG study. Neuroreport 13(1), 177–181. [DOI] [PubMed] [Google Scholar]
- Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, Mathalon DH, 2014. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry 75(6), 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Green MF, Harvey PD, 2016. Social Cognition Psychometric Evaluation: Results of the Initial Psychometric Study. Schizophr Bull 42(2), 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasser PE, Schall U, Todd J, Michie PT, Ward PB, Johnston P, Helmbold K, Case V, Soyland A, Tooney PA, Thompson PM, 2011. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull 37(1), 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissling AJ, Miyakoshi M, Sugar CA, Braff DL, Makeig S, Light GA, 2014. Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. Neuroimage Clin 6, 424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissling AJ, Park SH, Young JW, Rissling MB, Sugar CA, Sprock J, Mathias DJ, Pela M, Sharp RF, Braff DL, Light GA, 2013. Demand and modality of directed attention modulate “pre-attentive” sensory processes in schizophrenia patients and nonpsychiatric controls. Schizophr Res 146(1–3), 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosburg T, Kreitschmann-Andermahr I, 2016. The effects of ketamine on the mismatch negativity (MMN) in humans - A meta-analysis. Clin Neurophysiol 127(2), 1387–1394. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Summerfelt A, Wijtenburg SA, Du X, Chiappelli JJ, Krishna N, West J, Muellerklein F, Kochunov P, Hong LE, 2016. Frontal Glutamate and gamma-Aminobutyric Acid Levels and Their Associations With Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. JAMA Psychiatry 73(2), 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW, 2007. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry 64(5), 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Polizzotto NR, Nestor PG, Haigh SM, Koehler J, McCarley RW, 2017. Pitch and duration mismatch negativity and premorbid intellect in the first hospitalized schizophrenia spectrum. Schizophrenia bulletin, 43(2), 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff DL, Marder SR, Green MF, 2007. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophr Res 90(1–3), 316–324. [DOI] [PubMed] [Google Scholar]
- Sparks A, McDonald S, Lino B, O’Donnell M, Green MJ, 2010. Social cognition, empathy and functional outcome in schizophrenia. Schizophr Res 122(1–3), 172–178. [DOI] [PubMed] [Google Scholar]
- Stouten LH, Veling W, Laan W, van der Helm M, van der Gaag M, 2014. Psychotic symptoms, cognition and affect as predictors of psychosocial problems and functional change in first-episode psychosis. Schizophr Res 158(1–3), 113–119. [DOI] [PubMed] [Google Scholar]
- Torgalsboen AK, Mohn C, Czajkowski N, Rund BR, 2015. Relationship between neurocognition and functional recovery in first-episode schizophrenia: Results from the second year of the Oslo multi-follow-up study. Psychiatry Res 227(2–3), 185–191. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, Kane J, 1998. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry 44(8), 716–725. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Krljes S, 2005. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res 76(1), 1–23. [DOI] [PubMed] [Google Scholar]
- Van Rheenen TE, Lewandowski KE, Tan EJ, Ospina LH, Ongur D, Neill E, Gurvich C, Pantelis C, Malhotra AK, Rossell SL, Burdick KE, 2017. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol Med 47(10), 1848–1864. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Sugar C, Horan WP, Kern R, Green MF, 2010. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol Psychiatry 67(10), 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Zabala A, Rapado M, Arango C, Robles O, de la Serna E, Gonzalez C, Rodriguez-Sanchez JM, Andres P, Mayoral M, Bombin I, 2010. Neuropsychological functioning in early-onset first-episode psychosis: comparison of diagnostic subgroups. European archives of psychiatry and clinical neuroscience 260(3), 225–233. [DOI] [PubMed] [Google Scholar]
- Zani B, McFarland B, Wachal M, Barker S, Barron N, 1999. Statewide replication of predictive validation for the Multnomah Community Ability Scale. Community mental health journal 35(3), 223–229. [DOI] [PubMed] [Google Scholar]
- Zhou TH, Mueller NE, Spencer KM, Mallya SG, Lewandowski KE, Norris LA, Levy DL, Cohen BM, Ongur D, Hall MH, 2018. Auditory steady state response deficits are associated with symptom severity and poor functioning in patients with psychotic disorder. Schizophr Res 201, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.