Introduction
Neuroimmunity in chronic musculoskeletal pain has received considerable attention [44; 56; 75]. The innate immune system utilizes humoral components such as cytokines to affect other systems involved in chronic pain [77]. Maladaptive function of innate monocytes and cytokines contributes to chronic inflammation and pain [41; 60; 80; 81; 83]. Monocytes are heterogeneous cells that demonstrate notable plasticity. They serve as precursors of tissue-resident macrophages, present antigens, and have a prominent role in tissue repair [32; 33; 48]. In humans and animals, three subtypes of circulating monocytes have been described [27; 86]: classical, intermediate and non-classical. Differential expression of surface markers and secretory profiles for pro- and anti-inflammatory cytokines [27; 86] are related to monocyte phenotype, are mutable, and are subject to phenotypic changes [27]. Classical monocytes are considered pro-inflammatory and comprise ~80% of the cell population [75] whereas non-classical monocytes are anti-inflammatory and intermediate monocytes have both pro- and anti-inflammatory profiles [87]. Changes in monocyte phenotype are associated with inflammatory conditions such as rheumatoid arthritis [39] and systemic lupus erythematosus [48]. Thus, monocyte phenotype is relevant to understanding pain modulation in chronic pain conditions.
It is posited that monocyte dysregulation contributes to pain and other somatic symptoms in fibromyalgia (FM) [4]. FM is also marked by fatigue, reduced physical function, anxiety, depression, pain catastrophizing, and fear of movement [12; 28; 65; 67]. While prior studies show alterations in circulating cytokine levels of individuals with FM, these findings are inconsistent [1; 22; 26; 80; 81]. Some studies have reported elevated levels of IL- β, IL-6, TNF-α and IL-10 in the supernatants of stimulated PBMCs in culture [13; 81], whereas others reported equivalent levels of cytokine production to healthy controls [2; 80]. Moreover, previous studies have not controlled for body mass index (BMI), which is higher in individuals with FM and is associated with increased cytokine synthesis and secretion [71; 80; 81]. In animal models, intramuscular injection of IL-6 and IL-1β, produce hyperalgesia [1; 22; 26], and blockade of IL-1β reduces hyperalgesia [30], suggesting that increases in pro-inflammatory cytokines contribute to muscle pain. Our prior work and others have shown lower levels of IL-5 in tissue and plasma in animal models and individuals with FM [6; 71]. However, it is uncertain if myeloid cells, specifically monocytes, are involved in the IL-5 response. Also, the relationships between monocyte phenotype and FM symptomology remain unclear.
As such, the primary aims of this study were to characterize the relationships between monocyte phenotype and pain-related symptoms in women with FM. We hypothesized that women with FM would have a greater percentage of classical monocytes with higher levels of secreted pro-inflammatory cytokines compared to women without pain. Additionally, we hypothesized that classical monocytes would be associated with pain-related symptoms in women with FM. Based on observed atypical expression of IL-5 from human monocytes, our secondary exploratory aim was to examine the role of IL-5 in an animal model of widespread muscle pain. Finally, we examined relationships between IL-5 and pain outcomes in women with FM.
Methods
Human Studies
Study Design
The current study is an ancillary analysis of baseline data from a subset of participants enrolled in a phase II dual-site randomized, controlled trial testing the efficacy of long-term transcutaneous electrical nerve stimulation (TENS) in women with FM (Fibromyalgia Activity Study with TENS, FAST; ClinicalTrials.gov identifier NCT01888640; registered on June 28, 2013). The study was approved by the institutional review boards of both study sites. A detailed study protocol has been previously published [51].
Study Participants
Participants were women recruited from communities surrounding the University of Iowa Hospitals and Clinics site using a variety of recruitment strategies. Fibromyalgia (FM) Group. Inclusion criteria for participants with FM were 1) English-speaking, 2) between 18 and 70 years old, 3) diagnosed with FM based on the 1990 and 2011 American College of Rheumatology criteria, and 4) reported an average pain rating of 4 or higher on the Numeric Rating Scale (NRS) over the last seven days. The clinical trial design and participant recruitment for the FAST clinical trial was conducted prior to the 2016 updates to the FM diagnostic criteria. Participants with FM were excluded if they reported an average pain intensity of less than 4 out 10 over the last seven days, reported previous TENS use within the last five years, had neuropathy or an autoimmune disorder, or were pregnant [51]. Cytokine secretion and flow cytometry experiments were conducted using technical replicates from different cryovials. For the cytokine secretion experiments, 19 women with the full complement of blood and clinical outcome data were included in the analyses. For flow cytometry experiments, 32 women with the full complement of blood and clinical outcome data were included in the analyses. There were no differences in the demographic characteristics of these cohorts. No Pain (NP) Control Group. Thirty-nine women without pain were matched for age and BMI to women with FM were included in the study. The cohort of women in the NP group had the full complement of blood and clinical outcome data. For cytokine secretion and flow cytometry experiments, 36 women with the full complement of blood and cytokine data were included in the analyses. In addition to exclusion criteria for FM participants, NP control group participants were excluded if they had an acute or chronic pain condition such as FM, osteoarthritis, mechanical or non-specific spinal pain, or reported an average pain intensity 1 or higher out 10 over the last seven days using the NRS.
Sample Collection and Analysis
Human Blood Sample Collection:
Blood draws were performed in the Central Research Unit by nurses trained in clinical research methods. Blood samples were obtained via sterile venipuncture and collected in two 8 mL Ficoll-Hypaque cell preparation tubes with sodium citrate (BD Vacutainer™,Thermo Fisher Scientific, Waltham, MA, USA).
Human PBMC isolation and cryopreservation:
Buffy coat separation from whole blood was done by density gradient centrifugation at 2800 rpm for 30 minutes at room temperature within two hours of sample collection. PBMCs were isolated and washed twice at room temperature in Dulbecco’s phosphate-buffered saline (DPBS) via centrifugation at 1500 rpm for 15 minutes using sterile methods. Cell quantity and viability were determined using the 0.4% Trypan Blue exclusion test [70]. To maintain consistency and reduce assay variability between flow cytometry and multiplex assay experiments for the FM and NP cohorts, PBMCs were resuspended in cryopreservation media containing Roswell Park Memorial Institute (RPMI) – 1640 medium supplemented with 12.5% human serum albumin (HSA) and 10% dimethyl sulfoxide (DMSO). Cryopreservation media was added in a dropwise fashion to the PBMC suspension in HSA + RPMI – 1640 prior to being immediately aliquoted into 1 mL cryovials (3–7 per participant) at concentrations between 6–10 x 106 cells/mL. Cryovials were initially placed in a controlled cooling container (Nalgene Mr. Frosty, Millipore Sigma, St. Louis, MO USA) with isopropanol at -80ºC for 24 hours. After 24 hours, cryovials were transferred directly to the vaporized phase of liquid nitrogen for sample storage prior to analyses. Cryopreserved samples remained in storage for up to 2 months prior to thawing and analyses.
Thawing.
Cryovials containing PBMCs were retrieved from vaporized liquid nitrogen, and were rapidly thawed via immersion with light agitation in a 37 ºC water bath per protocol for FM [71]. Cryopreserved samples were individually thawed until small ice crystals were visible. Immediately after thawing, warmed (37°C) 1 mL RPMI – 1640 supplemented with 10% sterile, heat inactivated FBS and 1% penicillin-streptomycin was added dropwise to the cryovial for a final volume of 2 mL. The 2 mL suspension was added dropwise to 8 mL to supplemented RPMI -1640 culture media in a 15 mL polypropylene conical tube and centrifuged at 1500 rpm for 15 minutes. The supernatant was aspirated, and cells were resuspended and washed in 1:1 DPBS/Accumax prior to centrifugation. PBMCs were thawed in the presence of DMSO for less than 5 minutes to minimize cytotoxic effects of prolonged exposure to DMSO, thus, reducing the risk for low cell viability and functionality for experiments [59]. Although the cells were subject to one freeze/thaw cycle, these thawing methods have been shown to yield 95% PBMC cell viability for flow cytometry [31] and PBMC cell culture [3; 35] in individuals with FM [13; 52; 53].
Monocyte Stimulation:
Nineteen participants with FM and 36 NP participants with complete cytokine and outcome measures data were included in the analyses. Isolated monocyte suspension volumes were adjusted to 1 x 106 cells/mL in Isocove’s Modified Dulbecco’s medium (IMDM, Thermo Fisher Scientific, Waltham, MA) supplemented with 10% heat-inactivated fetal bovine serum, 5000 U/ml penicillin, 5mg/mL streptomycin, and 1 mM L-glutamine (all from Sigma-Aldrich, St. Louis, MO). Isolated monocytes were plated at 250 μL per well in 48-well flat-bottom, tissue-treated plates for 24 hours prior to treatment to achieve a minimum of 90% confluence and to promote recovery from the stress of the isolation and purification procedures. Monocytes were treated with LPS from Escherichia coli (serotype O111:B4, Sigma-Aldrich, St. Louis, MO) with 20 μL LPS for a final concentration of 50 μg/mL [77] or in 20 μL DPBS (saline) to evaluate spontaneous cytokine secretion at 37 °C and 5% CO2. LPS is a potent activator of a short-term inflammatory response in monocytes and macrophages, and induces synthesis and secretion of inflammatory mediators through TLR4 signaling [50; 74] involving LPS binding protein to a glycosylphosphatidylinositol (GPi) tether on the CD14 monocyte cell surface marker [57; 79]. After 24 hours, supernatants were aliquoted into Eppendorf tubes and stored in −80 °C until analysis. Cytokine levels were measured using the Invitrogen Cytokine Human Magnetic 10-Plex Panel (Thermo Fisher Scientific, Waltham, MA, USA), a commercially available immunoassay kit, for IL-5, IL- β, IL- 6, IL- 10, IL- 4, TNF- α, IL- 2, IL- 8, GM-CSF, and IFN- Ɣ as per the manufacturer's instructions. Quality control for immunoassays included blank samples (media) and nine standard samples containing serial dilutions of 1:1 assay diluent/media within each processing run. Immunoassay analysis was performed on the Luminex® 200TM analyzer (Luminex, Austin, TX, USA).
Flow Cytometry:
Isolation and quantitation of human monocyte subpopulations were completed using flow cytometry. 32 participants with FM and 36 NP participants with complete flow cytometry and outcome measures data were included in the analyses. Flow cytometry experiments were performed in a separate cohort of FM participants but in the same cohort of NP participants as the isolated monocyte culture experiments. Aliquots of cryopreserved PBMCs were thawed in 37 °C warm water bath prior to analysis per protocol [23] prior to washing and resuspension in 1:1 ratio of DPBS and cell dissociation solution (Accumax, Innovative Cell Technologies, Inc, San Diego, CA). Following multiple washes and incubation with Fc Receptor Blocker (Innovex, Richmond, CA) to minimize non-specific antibody binding, the cells were incubated with a cocktail of fluorescently-tagged monoclonal antibodies for 30 minutes at 4 °C comprised of: anti-human CD14-phycoerythrin/cyanine (PE/Cy7;clone HCD14), anti-human CD16-Alexa Fluor 488 (clone 3G8), anti-human CD64-Brilliant Violet 421 (BV421; clone 10.1), anti-human HLA-DR-phycoerythrin (PE; clone L243), and anti-human CD163-allophycocyanin (APC; clone GHI/61). All monoclonal antibodies were from BioLegend (San Diego, CA), and volumes were titrated to determine appropriate concentrations per manufacturer instructions. Cells were also stained with propidium Iodide (PI) to determine cell viability. Data from flow cytometry experiments was acquired on the Becton Dickinson LSR II and analyzed using FACSDiva software (BD Biosciences, San Jose, CA). A more detailed description of flow cytometry methods are provided in Supplemental Materials.
Patient-Reported Measures
Pain Intensity and Interference:
Pain intensity at rest (resting pain) and during movement (movement-evoked pain) was assessed using the 11-point NRS at baseline. Movement-evoked pain was assessed at the 5-minute mark for the six-minute walk test (6MWT). Overall pain severity and interference were also assessed using the Brief Pain Inventory (BPI) short form [19].
Pain Sensitivity:
Pressure pain threshold (PPT) was measured at three sites (cervical, lumbar, lower leg) to assess primary and secondary hyperalgesia using a digital pressure algometer (Somedic AB, Farsta, Sweden). Mechanical pressure from a 1cm2 algometer probe was applied at a rate of 40 kPa/sec. Lower PPTs indicate higher pain sensitivity, and thus, the presence of primary or secondary hyperalgesia [21]. Conditioned pain modulation (CPM) was also measured using cold water immersion as the conditioning stimulus and PPT as the test stimulus. An increase in PPT after cold water immersion (ratio of post/pre-PPT values > 1) indicates intact descending pain inhibition whereas no change or a reduction in PPT (ratio of post/pre-PPT values < 1) indicates a lack of descending pain inhibition [21; 78].
Disease Impact:
Fibromyalgia disease impact was measured using the Revised Fibromyalgia Impact Questionnaire (FIQR) at Visits 1 and 2 [9]. Summative scores of the weighted domain scores comprise the total score (range 0-100) and were averaged from both visits. Higher scores are indicative of greater disease severity and impact.
Fatigue:
Fatigue intensity at rest (resting fatigue) and during movement (movement-evoked fatigue) was assessed using the 11-point NRS at baseline visits. Maximum movement-evoked fatigue was assessed at the 5-minute mark during the six-minute walk test (6MWT). Global fatigue was assessed using the Multidimensional Assessment of Fatigue - Global Fatigue Index (MAF - GFI). Item anchors range from 1 = ‘no fatigue’ to 50 = ‘severe fatigue’ [7; 45].
Physical function:
All participants performed the six-minute walk test (6MWT), an objective measure of physical endurance. Participants walked for six minutes on a 100 foot walkway, and the total distance was measured. The 6MWT has been validated in chronic pain populations, and is frequently used as an objective measure of physical function in clinical practice [34; 54].
Pain-Related Psychological Assessments:
Pain catastrophizing was assessed using the Pain Catastrophizing Scale (PCS). Scores range from 0 to 52, with higher scores reflecting greater pain catastrophizing [72; 73]. Fear of movement was measured using the Tampa Scale of Kinesiophobia (TSK). Scores range from 17 to 68, with higher scores indicate greater fear of movement or reinjury [61]. Anxiety and depression were assessed using the Patient-Reported Outcomes Measurement Information System (PROMIS) Short Forms 8a and 8b, respectively (healthmeasures.net). Both Short Forms demonstrate high internal consistency (Cronbach’s alpha <0.93) and moderate convergent validity with the Revised Fibromyalgia Impact Questionnaire [46].
Animal Studies
Animals:
All experiments were carried out in accordance with protocols approved by the local ethical committee for animal experiments at the University of Texas at Dallas (UT-Dallas) Richardson, US. Adult C57BL/6 female mice (9-12 weeks, 20-25g) were used. Animals were purchased from Jackson Lab and bred at the UT-Dallas Animal Facility with standard temperature, and 12-hour light/dark cycle (lights on at 6 AM and lights off 6 PM). Mice were housed in standard cages with 4 to 5 animals per cage, and food and water were provided ad libitum. All behavioral experiments and associated data analyses were carried out during the light cycle period in between 6 AM to 6 PM.
Experimental model of widespread chronic musculoskeletal pain:
An experimental model for widespread musculoskeletal pain was developed and validated to recapitulate the clinical signs of FM [66]. As previously described, animals were deeply anesthetized with isoflurane, then injected in the ipsilateral gastrocnemius muscle with 20 l of acidic saline (pH 4.0) or normal saline (pH 7.2) as an experimental control on day 0. Five days later, a second injection of acidic or normal saline was injected in the respective mice.
Behavioral experiments.
Von Frey:
Animals were tested for mechanical hypersensitivity using the previously described von Frey assay [16]. Animals were habituated to a von Frey station made of acrylic and mesh and test environment prior to the assessment of baseline measurements. After 2 baseline recordings performed 2-3 days apart, the animals were randomly assigned to different experimental groups. Mechanical hypersensitivity was determined by assessment of paw withdrawal threshold in response to the application of calibrated von Frey hair filaments using the up-down method [16]. A series of filaments with logarithmically incremental stiffness of 2.83, 3.22, 3.61, 3.84, 4.08, 4.17 (converted to the 0.07, 0.16, 0.4, 0.6, 1, 1.4 g, respectively) were applied to the plantar surface of the hind paw and held for 2-3 seconds. To avoid tissue damage or unintentional agitation, a cut off of 2g was applied. A brisk withdrawal of paw, paw licking, or paw shaking was noted as a positive response. The withdrawal of the ipsilateral and contralateral paws were measured and recorded separately. All behavior experiments were done with the experimenter blinded to group assignment.
Drug and drug delivery.
Pharmacological experiments were performed to understand the role of IL-5 in nociception. IL-5 dose response experiments: Two intravenous injections of three doses of IL-5, 0.1 pg/mice (4 pg/kg), n = 6 per group , 0.1 ng/mice (4ng/kg), n = 6 per group and 0.1 μg/mice (4 μg/kg) , n = 8 in IL-5 treatment and in vehicle treatment group (n = 7), were used 24 hours apart (starting at day 10 post acidic or control saline local injections). Mice were held in a tail veil injector restrainer (Plas-Labs, VWR, US) and their tail dipped in 30-35°C warm water for 2-4 minutes prior to the injection to dilate the tail vein. The pharmacological effect of IL-5 on mechanical hypersensitivity was assessed with measuring a von Frey at different time points. Experimenters were blinded for all experiments. Once an adequate dose was chosen we also determined peripheral monocyte phenotypes from IL-5 treated animals 10 days post acidic or control saline local injections using flow cytometry.
Flow Cytometry.
For all flow experiments, monocytes were taken from peripheral blood mononuclear cell (PBMC) isolates using a previously described Ficoll protocol [5; 49]. Isolated cells were washed twice with 1× PBS (pH 7.4), centrifuged at 400 × g followed with resuspension in flow buffer (0.5% BSA + 0.02% glucose in 1× PBS (pH 7.4)). PBMCs were then incubated in blocking buffer (flow buffer + CD16/32 purified antibody)(eBioscience-16-0161-85), to block non-specific binding via the Fc receptor for 10 minutes. In all experiments, to identify the monocyte population, primary conjugated antibody cocktails were prepared using anti-mouse CD11b-APC-cy7 (Life Technology, A15390) and anti-mouse CD45 (Tonbo, 500451). For IL-5-induced polarization experiments anti-mouse CD86-eFlour450 (eBioscience, 48-0862-82) antibody was incubated for 30 min blocking buffer at 4°C in the dark. Cells were then washed twice with flow buffer and subjected to fixation and permeabilization (BD Biosciences) for 20 min at 4°C in the dark. Cells were then washed twice with perm/wash buffer followed with intracellular staining of CD206 (anti-mouse CD206-APC; eBioscience, 17206180). For monocyte phenotyping and IL-5 production experiments, anti-mouse/human IL-5-PE (eBioscience, 12705282) for 30 min at 4°C, in the dark. Data were acquired and analyzed using BD LSR Fortessa cell analyzer (BD Bioscience, San Diego, CA) and FlowJo software (San Carlos, CA), n=6 in pH4 injection group and n=6 in pH 7.2 injection group for IL-5 expression in monocytes study. n= 7, pH4 + IL5 treatment; n= 5, pH4 + vehicle; n= 5, pH7.2 + IL5; n= 4, pH4 + vehicle, mice per group for polarization-flow cytometry study.
Statistics
Human Data.
Descriptive statistics for demographic data and patient-reported outcome variables were calculated for both cohorts. Data were assessed for normality using a Kolmogrov-Smirnoff test and are presented in original units [42]. Differences in cytokine secretion concentrations from monocyte populations were determined using the Wilcoxon Rank-Sum Test. The Benjamini-Hochberg procedure was used to adjust for multiple comparisons [8]. Relationships between cytokines, monocyte subpopulations, and patient-reported outcome measures were determined using Spearman’s Rho correlation coefficients as appropriate. Additionally, a LASSO regression analysis was performed to examine predictors of resting pain, movement-evoked pain, resting fatigue, and movement-evoked fatigue. Four models were created for resting and movement-evoked pain and for resting and movement-evoked fatigue outcome variables. The independent variables for each of the models are cytokine concentrations for the LPS-evoked treatment condition. The LASSO regression was used to select variables to include in the respective models, and the tuning parameter for the model is chosen via cross-validation methods [25]. All analyses were performed using R statistical software (version 3.5.2). Statistical significance was set at p<0.05 (two-tailed), and were adjusted appropriately for multiple comparisons.
Animal Data.
Data were analyzed using GraphPad Prism software v8 (San Diego, US) and expressed as mean ± SEM. Behavioral data were analyzed with two-way ANOVA with Sidak’s multiple comparisons test to compare with different group. IL-5 pharmacological experiment was analyzed using two-way ANOVA with Dunnett’s post-test to assess the anti-nociceptive effect of IL-5 at different timepoints. A one-way ANOVA with Sidak’s multiple comparison test was applied to analyze flow cytometry data.
Study approval
Human study.
Participants were a subset of participants who participated in a phase II dual-site randomized, controlled trial testing the efficacy of transcutaneous electrical nerve stimulation (TENS) in women with FM (Fibromyalgia Activity Study, FAST; ClinicalTrials.gov identifier NCT01888640; registered on June 28, 2013). The study was approved by the institutional review boards of both study sites. Participants for this study were recruited from communities surrounding the University of Iowa Hospitals and Clinics site using a variety of recruitment strategies. Animal studies. All experiments were carried out in accordance with protocols approved by the local ethical committee for animal experiments at the University of Texas at Dallas (UT-Dallas) Richardson, US.
Results
Participant characteristics.
As expected, women with FM reported higher pain intensity (resting, movement-evoked), more pain interference, greater disease impact (FIQR), greater fatigue (resting, movement-evoked), and reduced physical function (6MWT) than age and BMI-matched women without pain (No Pain (NP))(Table 1). Moreover, the FM group demonstrated greater evoked pain sensitivity (PPT) and diminished central pain inhibition (CPM) compared with women without pain (Supplemental Table 1) as well as higher levels of pain catastrophizing, movement-related fear, anxiety, and depression (Table 1).
Table 1.
Demographic characteristics for women with fibromyalgia (FM) and women without pain (NP) (N = 69)
| Mean (SD) Range | FM N = 32 | NP N = 37 | p |
|---|---|---|---|
| Age, years | 50.7 (12.1) 23 – 66 | 51.4 (11.7) 20 – 67 | 0.53 |
| BMI (kg/m2) | 34.7 (9.0) 20 - 61 | 31.3 (8.4) 18 - 46 | 0.29 |
| Resting pain (0-10) | 7 [IQR: 2] | 0 [IQR: 1] | <0.01** |
| Movement Pain (0-10) | 7 [IQR: 3] | 0 [IQR: 0] | <0.01** |
| BPI Severity (0-10) | 7 [IQR: 2] | 0 [IQR: 1] | <0.01** |
| BPI Interference (0-10) | 6 [IQR: 2] | 0 [IQR: 0] | <0.01** |
| FIQ-R (0 – 100) | 60.4 (14.2) 28 – 92 | 4.0 (4.1) 0 - 17 | <0.01** |
| Resting Fatigue (0-10) | 7 [IQR: 3] | 1 [IQR: 0] | <0.01** |
| Movement Fatigue (0-10) | 7 [IQR: 3] | 1 [IQR: 0] | <0.01** |
| 6MWT distance (feet) | 1311 (265) 600 - 1740 | 1696 (203) 1235 - 2105 | <0.01** |
| PCS (0 –52) | 21.3 (12.7) 3 - 51 | 2.0 (4.1) 0 - 18 | <0.01** |
| TSK (17 - 68) | 38.2 (7.4) 22 - 55 | 27.5 (5.3) 18 - 42 | <0.01** |
| PROMIS – Anxiety (T-score) | 60.7 (7.5) 46 - 80 | 45.2 (6.1) 37 - 56 | <0.01** |
| PROMIS – Depression (T-score) | 57.6 (8.7) 37 - 81 | 44.1 (5.9) 37 - 57 | <0.01** |
Resting pain intensity measured using the Numeric Rating Scale (0-10); Movement Pain measured using the Numeric Rating Scale (0-10) during the Six-Minute Walk Test; Pain Interference measured using the Brief Pain Inventory Severity and Interference Subscales (BPI); Disease impact measured using the Fibromyalgia Impact Questionnaire (FIQ-R); Resting fatigue intensity measured using the Numeric Rating Scale (0-10); Movement fatigue measured using the Numeric Rating Scale (0-10) during the Six-Minute Walk Test; The Six-Minute Walk Test (6MWT) physical function measure; Pain catastrophizing measured using the Pain Catastrophizing Scale (PCS); Fear of Movement measured using the Tampa Scale of Kinesiophobia (TSK); Anxiety measured using the PROMIS Anxiety Short Form 8a; Depression measured using the PROMIS Depression Short Form 8b.
Women with FM have higher levels of IL-5 synthesis and secretion from monocytes compared with the No Pain (NP) group.
We examined LPS-evoked and spontaneous synthesis and secretion of 10 cytokines from monocytes using a multiplex analysis. FM participants had greater LPS-evoked synthesis and secretion of IL-5 (Figure 1A) from monocytes than participants in the NP group. Interestingly, there were no statistically significant differences in LPS-evoked secretion of IL-1β (Figure 1B) or IFN-γ (Figure 1C). There was less LPS-evoked synthesis and secretion of GM-CSF (Figure 1D). In addition, FM participants had greater LPS-evoked synthesis and secretion of IL-2 (Figure 1E) and IL-4 (Figure 1F) from monocytes than participants in the NP group. Interestingly, FM participants had less LPS-evoked synthesis and secretion of TNF-α (Figure 1G). Surprisingly, there were no differences in LPS-evoked synthesis and secretion of IL-6 (Figure 1H), IL-8 (Figure 1I), or IL-10 (Figure 1J) from monocytes after correction for multiple comparisons. Spontaneous synthesis and secretion of GM-CSF, IFN- γ, IL-10, IL-1β, IL-2, IL-4, IL-5, and TNF-α from monocytes was greater in FM participants compared with NP participants after accounting for multiple comparisons (Supplementary Figure 1A–J).
Figure 1. LPS-stimulated human peripheral monocytes from participants with fibromyalgia (FM) and the No Pain (NP) group.
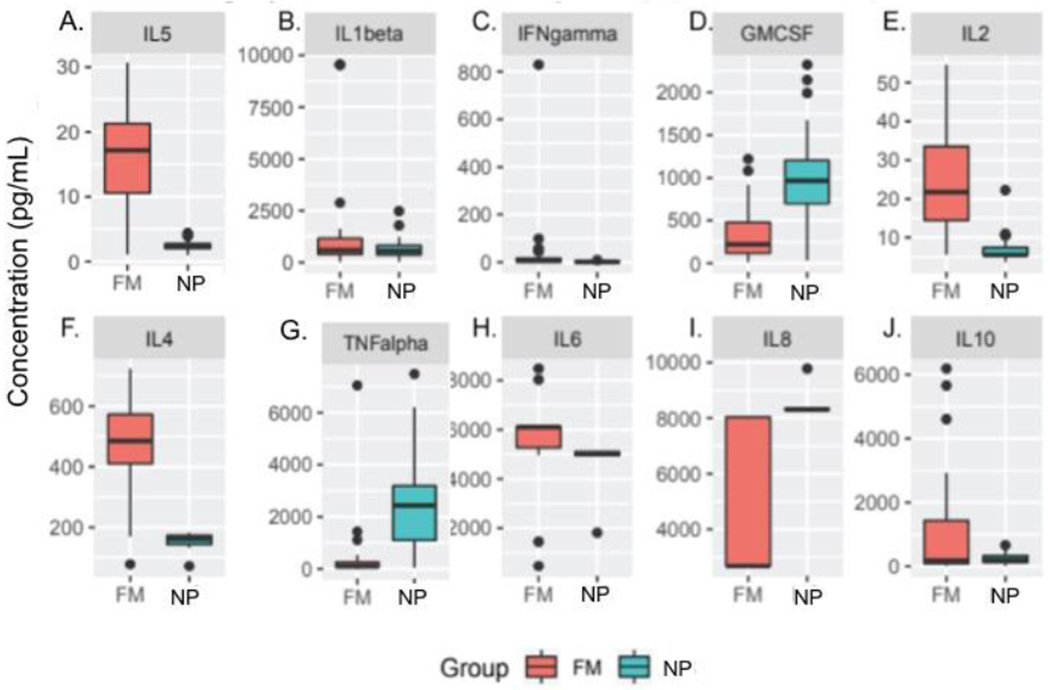
Graphs illustrate cytokine synthesis and secretion by isolated peripheral monocytes from participants with FM (red bars, n = 19) and NP participants (blue bars, n = 33). Cytokine Human Magnetic 10-Plex cytokine assay showed increased LPS-evoked synthesis and secretion of IL-5 (A), IL-2 (E), and -IL-4 (F) in FM participants compared with NP participants. There was less LPS-evoked synthesis and secretion of GM-CSF (D) and TNF-α (G). Data are presented as mean ± SEM. The Wilcoxon Rank-Sum Test with corrections for multiple comparisons was used to compare respective groups. * p<0.05 versus NP group.
Women with FM have a lower percentage of intermediate monocytes and a higher percentage of non-classical monocytes than the NP control group.
We used flow cytometry to determine monocyte phenotype based on cell surface marker expression: classical (CD14++/CD16−), intermediate (CD14++/CD16+), and non-classical (CD14+/CD16++). The gating strategy is shown in Figure 2A. FM participants had a significantly higher percentage of non-classical monocytes (CD14+/CD16++) and a lower percentage of intermediate monocytes (CD14++/CD16+) compared with the NP group (Figure 2B). Interestingly, there were no differences in the percentage of classical monocytes (CD14++/CD16) between FM participants and NP control participants.
Figure 2. Difference in monocyte subpopulations in between fibromyalgia (FM) and No Pain (NP) participants.
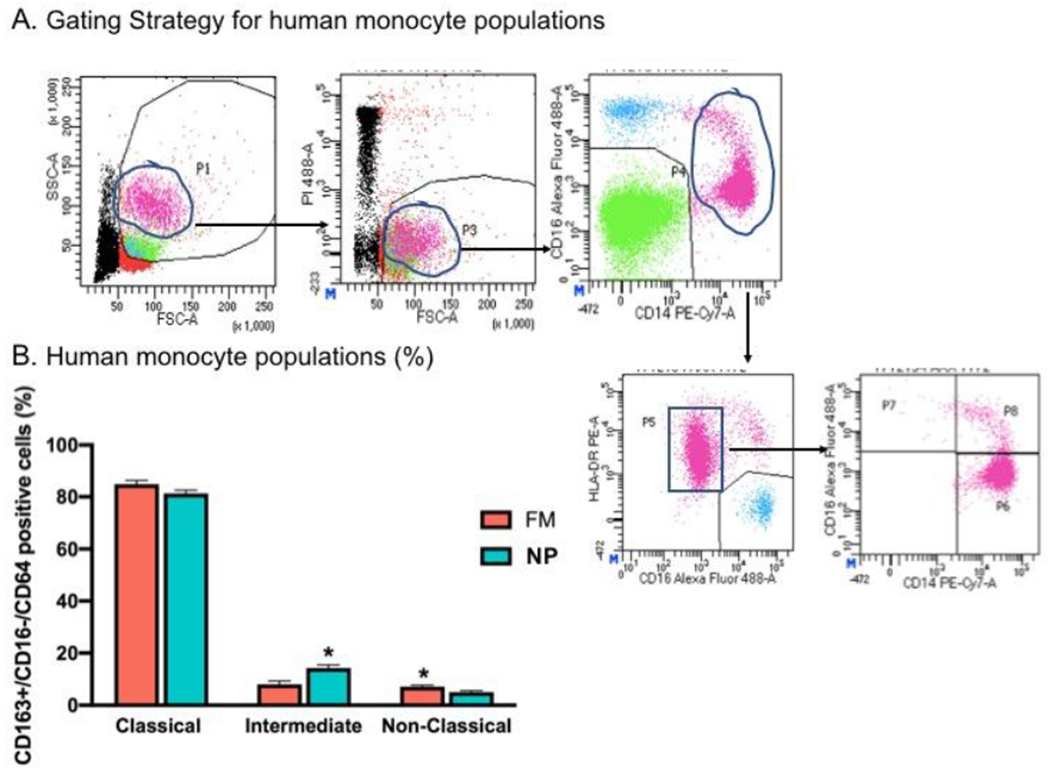
A) Gating strategy to isolate the monocyte population. B) Bar graphs indicate monocyte subpopulations (Classical, Intermediate and Non-Classical from (FM) participants (red bars, n = 32) and NP (blue bars, n = 37). Flow cytometry data analysis shows that women with FM had a higher percentage of Intermediate monocytes (CD14++/CD16+) and lower percentage of Non-Classical monocytes (CD14+/CD16++) compared with women without pain (NP). Data are represented as mean ± SEM. The Wilcoxon Rank-Sum Test with corrections for multiple comparisons was used to compare respective groups. * p<0.05 versus NP group.
LPS-evoked and spontaneous (PBS) synthesis and secretion of IL-5 and other cytokines from monocytes are significantly associated with pain-related outcomes in women with FM.
We examined the relationship between cytokine synthesis and secretion from human monocytes and pain-specific outcome measures in individuals with FM using Spearman’s Rho correlation coefficients and LASSO regression analysis as appropriate.
IL-5.
Greater LPS-evoked and spontaneous secretion of IL-5 was strongly associated with higher levels of resting pain intensity and movement-evoked pain, more pain interference, greater disease impact (FIQR), and higher levels of fatigue (resting, movement-evoked, global) (Table 2). Additionally, greater synthesis and secretion of IL-5 (LPS-evoked and spontaneous) was associated with lower PPTs, a measure of evoked pain sensitivity, at all sites. Further, greater synthesis and secretion of IL-5 (LPS-evoked and spontaneous) was associated with reduced physical function (6MWT) and more pain-related psychological disturbances (pain catastrophizing, fear of movement, anxiety, and depression) (Supplemental Table 2). Lastly, IL-5 was the strongest predictor of resting pain intensity, movement-evoked pain, resting fatigue intensity, and movement-evoked fatigue when accounting for collinearity with other cytokines in the LASSO regression models (Table 3A–D).
Table 2.
Correlations between evoked and spontaneous cytokine release from peripheral monocytes and pain-related outcomes from women with and without fibromyalgia.
| IL-5 | IL-1β | IL-6 | IL-10 | IL-4 | TNF- α | IL-2 | GM-CSF | IFN- Ɣ | |
|---|---|---|---|---|---|---|---|---|---|
| Pain Intensity and Pain Interference | |||||||||
| Resting Pain | |||||||||
| LPS | 0.73* | 0.24 | 0.66* | 0.17 | 0.74* | −0.53* | 0.74* | −0.40* | 0.58* |
| PBS | 0.73* | 0.59* | 0.54* | 0.49* | 0.73* | 0.40* | 0.79* | 0.34 | 0.70* |
| Movement Pain | |||||||||
| LPS | 0.69* | 0.18* | 0.61 | 0.11 | 0.71* | −0.55* | 0.63* | −0.41 | 0.50* |
| PBS | 0.74* | 0.54* | 0.52* | 0.45* | 0.71* | 0.37 | 0.72* | 0.34 | 0.65* |
| Pain Interference | |||||||||
| LPS | 0.86* | 0.33 | 0.39* | 0.48* | 0.88* | −0.41* | 0.74* | −0.41* | 0.24 |
| PBS | 0.74* | 0.33 | 0.45* | 0.37 | 0.75* | 0.28 | 0.64* | 0.14 | 0.20 |
| Disease Impact | |||||||||
| FIQR | |||||||||
| LPS | 0.79* | 0.31 | 0.35* | 0.41* | 0.88* | −0.42* | 0.74* | −0.42* | 0.21 |
| PBS | 0.74* | 0.31 | 0.44* | 0.36 | 0.77* | 0.24 | 0.64* | 0.13 | 0.17 |
| Fatigue | |||||||||
| Resting Fatigue | |||||||||
| LPS | 0.73* | 0.24 | 0.66* | 0.17 | 0.74* | −0.53* | 0.74* | −0.40* | 0.58* |
| PBS | 0.78 | 0.59* | 0.54* | 0.49* | 0.73* | 0.40* | 0.79* | 0.34 | 0.70* |
| Movement Fatigue | |||||||||
| LPS | 0.61* | 0.11 | 0.59* | 0.06 | 0.50* | −0.55* | 0.64* | −0.41* | 0.47* |
| PBS | 0.73* | 0.53* | 0.42* | 0.39* | 0.57* | 0.37 | 0.63* | 0.35 | 0.66* |
| Global Fatigue | |||||||||
| LPS | 0.78* | 0.27 | 0.33* | 0.41* | 0.82* | −0.43* | 0.70* | −0.44* | 0.21 |
| PBS | 0.68* | 0.29 | 0.40* | 0.33 | 0.71* | 0.28 | 0.63* | 0.12 | 0.17 |
Resting pain intensity measured using the Numeric Rating Scale (0-10); Movement Pain measured using the Numeric Rating Scale (0-10) during the Six-Minute Walk Test; Pain Interference measured using the Brief Pain Inventory Interference Subscale (0-10); Disease impact measured using the Fibromyalgia Impact Questionnaire (FIQ-R); Resting fatigue intensity measured using the Numeric Rating Scale (0-10); Movement fatigue measured using the Numeric Rating Scale (0-10) during the Six-Minute Walk Test;
Bold font indicates significance; no asterisk: p < 0.05;
p ≤ 0.001
Table 3.
LASSO Regression Models
| A) Resting Pain | |
|---|---|
| Term | Estimate |
| (Intercept) | 2.56 |
| IL-5 | 0.83 |
| IL-4 | 0.81 |
| GM-CSF | −0.04 |
| IL- β | - |
| IL-6 | - |
| IL-10 | - |
| IL-4 | - |
| TNF- α | - |
| IL-2 | - |
| B) Movement Pain | |
| Term | Estimate |
| (Intercept) | 2.87 |
| IL-5 | 1.41 |
| IL-4 | 0.10 |
| GM-CSF | - |
| IL- β | - |
| IL-6 | - |
| IL-10 | - |
| IL-4 | - |
| TNF- α | - |
| IL-2 | - |
| C) Resting Fatigue | |
| Term | Estimate |
| (Intercept) | 2.88 |
| IL-5 | 1.10 |
| IL-4 | 0.81 |
| GM-CSF | - |
| IL- β | - |
| IL-6 | - |
| IL-10 | - |
| IL-4 | - |
| TNF- α | −0.13 |
| IL-2 | - |
| D) Movement Fatigue | |
| Term | Estimate |
| (Intercept) | 2.84 |
| IL-5 | 0.49 |
| IL-4 | 0.27 |
| GM-CSF | - |
| IL- β | - |
| IL-6 | - |
| IL-10 | - |
| IL-4 | - |
| TNF- α | −0.13 |
| IL-2 | - |
IL-1β.
Greater LPS-evoked synthesis and secretion of IL-1β was moderately associated with higher levels of pain interference (ρ=0.39), greater disease impact (ρ=0.31) (Table 2), and higher levels of self-reported depression (ρ=0.32) (Supplemental Table 2). There were no significant associations between LPS-evoked synthesis and secretion of IL-1β and any measures of pain or fatigue. Greater spontaneous synthesis and secretion (PBS) of IL-1β from monocytes was moderately associated with higher resting pain intensity (ρ=0.59), more movement-evoked pain (ρ=0.54), more pain interference (ρ=0.33), greater disease impact (ρ=0.31), and higher levels of fatigue (ρ>0.29). There were no significant associations between spontaneous synthesis and secretion of IL-1β and PPTs at any site, physical function, or pain-related psychological variables.
IL-6.
Greater LPS-evoked and spontaneous synthesis and secretion of IL-6 was moderately to strongly associated with higher resting pain intensity and movement-evoked pain, more pain interference, greater disease impact, higher resting fatigue intensity, and higher levels of fatigue (resting, movement-evoked, global) (Table 2). Further, greater synthesis and secretion of IL-6 (LPS-evoked and spontaneous) was moderately associated with more pain catastrophizing, greater fear of movement, and higher levels of self-reported anxiety (Supplemental Table 2). Greater spontaneous synthesis and secretion of IL-6 was moderately associated with lower PPTs at all sites (Supplemental Table 2). There were no significant associations between LPS-evoked synthesis and secretion of IL-6 and PPTs at any site, physical function, or self-reported depression.
IL-10.
Greater LPS-evoked synthesis and secretion of IL-10 was moderately associated with more pain interference (ρ=0.48), greater disease impact (ρ=0.41), and higher levels of global fatigue (ρ=0.41) (Table 2). Further, greater synthesis and secretion of IL-10 was moderately associated with lower PPTs at all sites, more pain catastrophizing (ρ=0.36), greater fear of movement (ρ=0.31), and higher levels of self-reported anxiety, and depression. There were no significant correlations between LPS-evoked synthesis and secretion of IL-10 from monocytes and levels of pain or fatigue (Table 2). Greater spontaneous synthesis and secretion of IL-10 from monocytes was moderately associated with higher resting pain intensity (ρ=0.49) and more movement-evoked pain (ρ=0.45), more pain interference (ρ=0.37), greater disease impact (ρ=0.36), higher levels of fatigue (resting, movement-evoked, global), and lower PPT at the lumbar site (Table 2, Supplemental Table 2). There were no significant associations between spontaneous synthesis and secretion of IL-6 and PPTs at the cervical and leg sites, physical function, pain catastrophizing, or fear of movement.
IL-4.
Surprisingly, greater LPS-evoked and spontaneous secretion of IL-4 was strongly associated with higher levels of resting pain intensity and movement-evoked pain, more pain interference, greater disease impact (FIQR), and higher levels of fatigue (resting, movement-evoked, global)(Table 2). Additionally, greater synthesis and secretion of IL-4 (LPS-evoked and spontaneous) was moderately associated with lower PPTs at all sites, reduced physical function (6MWT), and more pain-related psychological disturbances (pain catastrophizing, fear of movement, anxiety, and depression) (Supplemental Table 2). Finally, IL-4 was a strong predictor of resting pain intensity and resting fatigue, and a moderate predictor of movement-evoked pain and movement-evoked fatigue when accounting for collinearity with other cytokines in the LASSO regression models (Table 3A–D).
TNF-α.
Interestingly, greater LPS-evoked synthesis and secretion of TNF-α was moderately associated with lower resting pain intensity (ρ=−0.53), less movement-evoked pain (ρ=−0.55), less pain interference (ρ=−0.41), less disease impact (ρ=−0.42), and lower levels of fatigue (resting, movement-evoked, global) (Table 2). Additionally, LPS-evoked greater synthesis and secretion of TNF-α was moderately associated with higher PPT at the cervical site (ρ=0.36), increased physical function (ρ=0.31), and less pain-related psychological disturbances (pain catastrophizing, fear of movement, anxiety, and depression) (Supplemental Table 2). As expected, greater spontaneous synthesis and secretion of TNF-α was moderately associated with higher levels of resting pain intensity and movement-evoked pain, pain interference, greater disease impact (FIQR), and higher levels of fatigue (resting, movement-evoked, global) (Table 2). Further, greater spontaneous synthesis and secretion of TNF-α from monocytes was not significantly associated with PPTs at any site, physical function, or any pain-related psychological variables (Supplemental Table 2). Finally, TNF-α was a weak negative predictor of resting and movement-evoked fatigue when accounting for collinearity with other cytokines in the LASSO regression models (Table 3C and 4D).
Table 4.
Correlations between monocyte phenotype and pain-related outcomes from women with and without fibromyalgia (FM).
| Classical (CD14++/CD16+) | Intermediate (CD14+/CD16+) | Non-classical (CD14+/CD16++) | |
|---|---|---|---|
| Pain Intensity and Interference | |||
| Resting Pain | 0.20 | −0.45* | 0.27 |
| Movement Pain | 0.22 | −0.47* | 0.26 |
| Pain Interference | 0.23 | −0.41* | 0.26 |
| Pain Sensitivity | |||
| PPT Cervical | −0.15 | −0.27 | −0.19 |
| PPT Lumbar | −0.19 | −0.32 | −0.19 |
| PPT Leg | −0.12 | −0.32 | −0.33 |
| Disease Impact | |||
| FIQR | 0.23 | −0.42* | 0.28 |
| Fatigue | |||
| Resting Fatigue | 0.19 | −0.36* | 0.17 |
| Movement Fatigue | 0.26 | −0.42* | 0.17 |
| Global Fatigue (MAF) | 0.14 | −0.33 | 0.29 |
| Physical Function | |||
| 6MWT (ft) | −0.18 | 0.32* | −0.20 |
| Pain-Related Psychological Variables | |||
| Pain Catastrophizing | 0.36* | −0.38* | −0.02 |
| Fear of Movement | 0.21 | −0.27 | 0.06 |
| Anxiety | 0.40* | −0.46 | 0.02 |
| Depression | 0.30 | −0.40 | 0.10 |
Resting pain intensity, Numeric Rating Scale (0-10);Movement Pain, Numeric Rating Scale (0-10) during the Six-Minute Walk Test; Pain Interference, Brief Pain Inventory Interference Subscale (0-10); Pain sensitivity, Pressure Pain Threshold (PPT) at the cervical, lumbar, and lower leg regions (kPA); Disease impact, Fibromyalgia Impact Questionnaire (FIQ-R); Resting fatigue intensity, Numeric Rating Scale (0-10); Movement fatigue, Numeric Rating Scale (0-10) during the Six-Minute Walk Test; Global fatigue, Multidimensional Assessment of Fatigue Global Fatigue Index (1-50); The Six-Minute Walk Test (6MWT); Pain catastrophizing, Pain Catastrophizing Scale (PCS); Fear of Movement, Tampa Scale of Kinesiophobia; Anxiety, PROMIS Anxiety Short Form 8a; Depression, PROMIS Depression Short Form 8b. Bold font indicates significance; no asterisk: p < 0.05;
p ≤ 0.001
IL-2.
Greater LPS-evoked and spontaneous synthesis and secretion of IL-2 was strongly associated with higher levels of resting pain intensity and movement-evoked pain, more pain interference, greater disease impact, and higher levels of fatigue (resting, movement-evoked, global) (Table 2). Additionally, greater synthesis and secretion of IL-2 (LPS-evoked and spontaneous) was moderately associated with lower PPT at all sites, reduced physical function (6MWT), and more pain-related psychological disturbances (pain catastrophizing, fear of movement, anxiety, and depression) (Supplemental Table 2).
GM-CSF.
Greater LPS-evoked synthesis and secretion of GM-CSF was moderately associated with lower resting pain intensity (ρ=−0.40) and movement-evoked pain (ρ=−0.41), less pain interference (ρ=−0.41), less disease impact (ρ=−0.42), and lower levels of fatigue (resting, movement-evoked, global) (Table 2). Additionally, greater LPS-evoked synthesis and secretion of GM-CSF was moderately associated with higher PPT at the cervical site (ρ=0.37), less pain catastrophizing (ρ=−0.32), and lower levels of self-reported anxiety and depression (Supplemental Table 2). Greater spontaneous synthesis and secretion of GM-CSF was moderately associated with higher levels of resting pain intensity (ρ=0.34) and movement-evoked pain (ρ=0.34), higher resting fatigue (ρ=0.34) and movement-evoked fatigue (ρ=0.35) (Table 2). Greater spontaneous synthesis and secretion of GM-CSF from monocytes was not significantly associated with pain interference, disease impact, global fatigue, PPT at the lumbar and leg sites, physical function, or pain-related psychological variables. Lastly, synthesis and secretion of GM-CSF was a weak negative predictor of resting pain intensity when accounting for collinearity with other cytokines in the LASSO regression models (Table 3A).
IFN-Ɣ.
Greater synthesis and secretion (LPS-evoked and spontaneous) of IFN-Ɣ was moderately to strongly associated with higher levels of resting pain intensity and movement-evoked pain, and with lower levels of resting and movement-evoked fatigue (Table 2). There were no significant correlative relationships between the LPS-evoked or spontaneous synthesis and secretion of IFN-Ɣ and pain interference, disease impact, levels of global fatigue, PPT, physical function, or pain-related psychological variables (Table 2, Supplemental Table 2).
Intermediate and non-classical human monocytes are significantly associated with pain and pain-related outcomes.
The percentages of monocyte subsets were significantly correlated with variables across all FM symptom domains (Table 4).
Classical monocytes.
A higher percentage of classical monocytes (CD14++/CD16−) was moderately associated with higher levels of movement-evoked fatigue (ρ=0.26), more pain catastrophizing (ρ=0.36), and higher levels of anxiety (ρ=0.40) and depression (ρ=0.30). Intriguingly, there were no significant correlative relationships between the percentage of classical monocytes and pain intensity (resting, movement-evoked), pain interference, PPTs at any site, disease impact, resting or global fatigue, physical function, or fear of movement (Table 4).
Intermediate monocytes.
A higher percentage of intermediate monocytes (CD14++/CD16+) was moderately associated with lower levels of resting pain intensity (ρ=−0.45) and movement-evoked pain (ρ=−0.47). Additionally, a higher percentage of intermediate monocytes was moderately associated with less pain interference, higher PPTs at all sites, less disease impact, lower levels of fatigue, higher levels of physical function, and less pain-related psychological disturbances (Table 4).
Non-classical monocytes.
Interestingly, a higher percentage of non-classical monocytes (CD14+/CD16++) was moderately associated with higher levels of resting pain intensity (ρ=0.27), movement-evoked pain (ρ=0.26), more pain interference (ρ=0.26), greater disease impact (ρ=0.28), and higher levels of global fatigue (ρ=0.29). Further, a higher percentage of non-classical monocytes was associated with lower PPT (thus, more evoked pain sensitivity) at the leg site (ρ=−0.33). There were no significant correlative relationships between the percentage of non-classical monocytes and PPTs at the cervical and lumbar sites, resting or movement-evoked fatigue, physical function, or pain-related psychological variables (Table 4).
Intravenous injection of IL-5 reverses mechanical hyperalgesia in female mice with widespread muscle pain with accompanying polarization of peripheral monocytes.
Since IL-5 had strong correlations with nearly all pain measures in individuals with FM, we tested the function of IL-5 in a preclinical model of widespread muscle pain, a prominent symptom in FM [66] This model utilizes two unilateral intramuscular injections of acidic saline (pH 4) into the gastrocnemius muscle of female mice 5 days apart (Figure 3A). As previously shown [66], there was a reduction in mechanical withdrawal thresholds of the paw bilaterally that lasted for weeks (Figure 3B). The related area under the curve was significantly reduced in acidic saline compared to normal saline n=8/group)(Figure 4). Systemic injection of IL-5 24 hours after induction of the widespread muscle pain murine model produced a dose-dependent increase in mechanical withdrawal thresholds (Figure 4A and Supplementary Figure 3A–G).
Figure 3. Assessment of pain like behavior and IL-5 expression in established experimental model of widespread muscle pain.
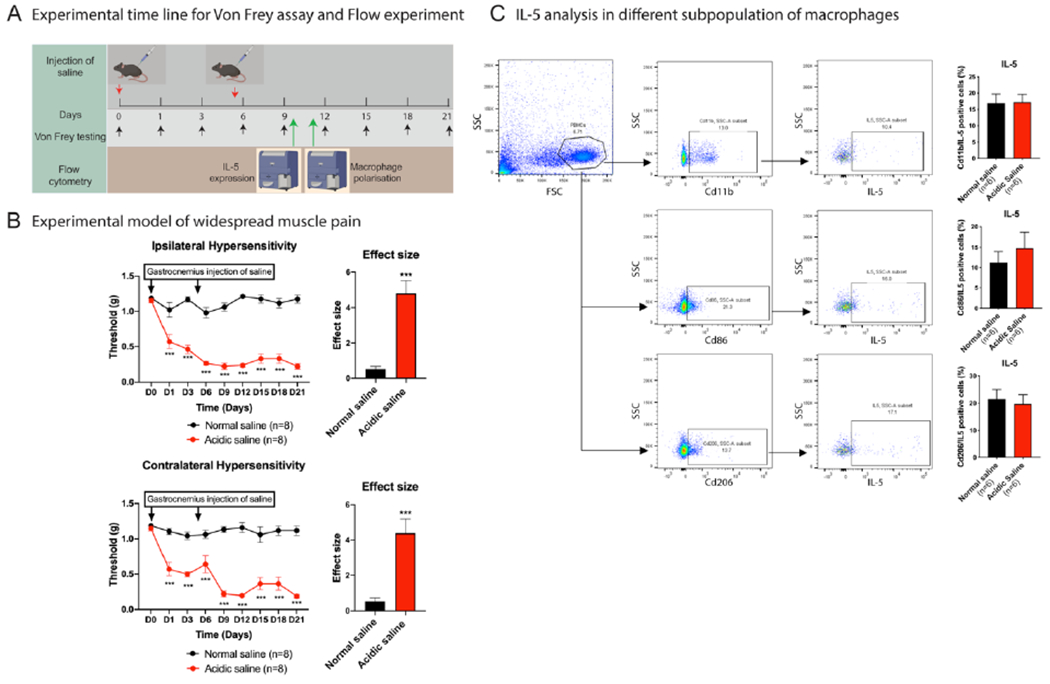
A) Represents the experimental timeline for gastrocnemius injection of normal and acidic saline on Day 0 (D0) and Day 5 (D5) to induce pain in the animal model of widespread muscle pain, and time point for the Von Frey testing to assess pain like behavior and flow cytometry analysis on Day 10 (D10) for IL5 expression. B) Line graph represents gastrocnemius injection of acidic saline on D0 and D5 on ipsilateral side induces the bilateral persistent pain line behavior (mechanical hypersensitivity) C. Flow cytometry analysis of peripheral monocytes isolated from the widespread muscle pain and control mice on D10. Gating strategy was applied to examine expression of IL-5 in different subpopulation of monocytes. Data are represented as mean ± SEM. n = 8 mice per group for hypersensitivity test (Von Frey testing) and n = 6 mice per group for flow cytometry experiment. Two-way AVOVA with Sidak’s multiple comparisons test was applied to compare two different groups. ***p < 0.001 versus normal saline injected group.
Figure 4. Effect of IL-5 on widespread muscle pain behavior and monocyte polarization.
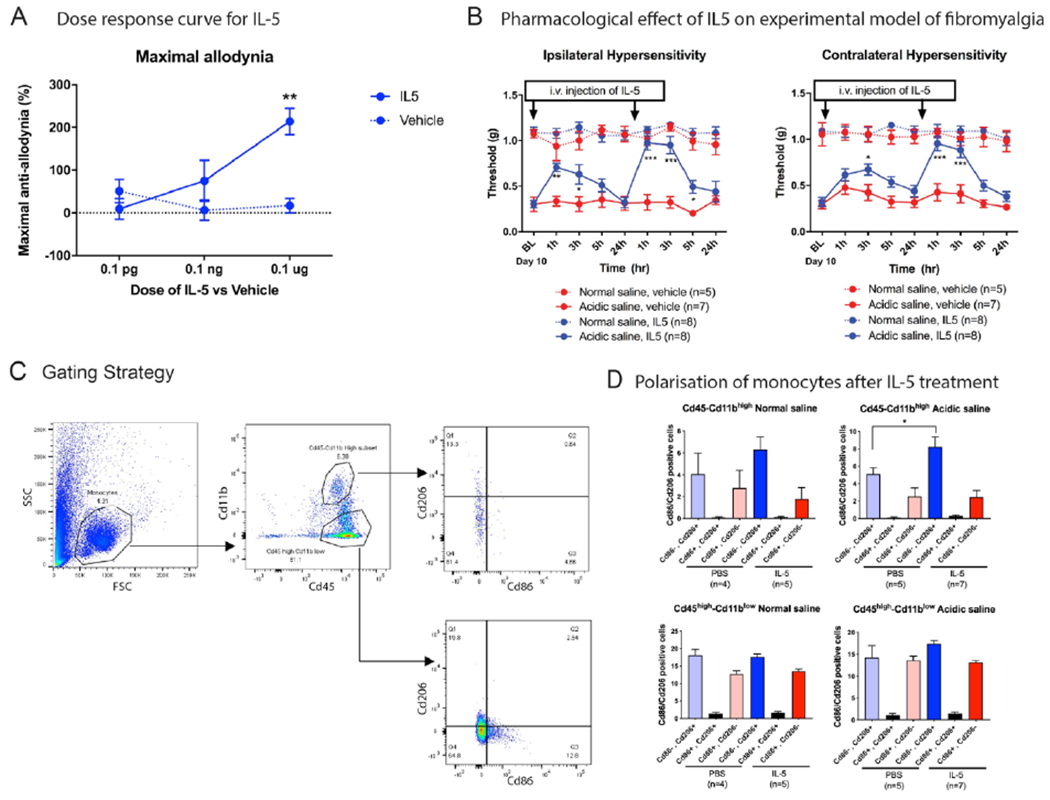
A) Maximal anti-allodynia was measured using three different doses of IL-5 (0.1 pg/mouse, 0.1 ng/mouse, n=6 and 0.1 μg/mouse, n=6). B) Intravenous injection of IL-5 (0.1 μg/mouse) reverses widespread musculoskeletal induced pain behavior in ipsilateral and contralateral hind paw. C) Gating strategy to isolate the monocyte population. D) Flow cytometry data analysis shows the intravenous injection of IL-5 polarize the peripheral monocytes into more non-classical (M2). Data are represented as mean ± SEM. n= 5-8 mice per group for IL-5 pharmacological study in normal and acidic saline injected mice. n=4-7 mice per group in normal and acidic saline injected mice after treatment of IL-5 for polarization-flow cytometry study. Two-way ANOVA with Dunnett’s post-test was applied to assess the anti-nociceptive effect of IL-5. Ordinary one-way ANOVA with Sidak’s multiple comparison test was applied to assess the flow cytometry data. *p<0.05 verses vehicle treatment from normal saline and acidic saline injected group.
Since we observed that participants with FM have higher levels of IL-5 secretion from monocytes as well as differences in the percentages of intermediate and non-classical monocytes compared with NP, we then tested if i.v. IL-5 altered polarization of circulating monocytes. Interestingly, we found a significant increase in the percentage of non-classical (CD206+/M2) monocytes from pH 4 injected mice injected with IL-5 compared to vehicle (8.23 ± 1.12 %, n=7, pH 4 + IL-5 treatment; 5.14 ± 0.69 %, n= 5, pH 4 + Vehicle treatment, p<0.01) (Figure 4D). There was no change in classical (CD86+/M1) monocytes in pH 4 injected mice injected with IL-5 compared to vehicle (2.47 ± 0.79 %, n=7, pH 4 + IL-5 treatment; 2.55 ± 0.97 %, n=5, pH 4 + Vehicle treatment; p>0.05 )(Figure 4D). Similarly, IL-5 treatment in the control mice (normal saline group) resulted in a greater percentage of non-classical (CD206+) monocytes (6.30 ± 1.15 %, n=5, pH 7.2 + IL+5 treatment; 4.07 ± 1.89 %, n=5, pH 7.2 + Vehicle treatment, p<0.01) but do not show differences for Classical (CD86+) monocytes (1.77 ± 1.03 %, n=5, pH 7.2 + IL+5 treatment; 2.79 ± 1.58 %, n=5, pH 7.2 + Vehicle treatment , p<0.01)(Figure 4D). Finally, we examined IL-5 production from monocytes from control mice injected with pH 7.2 compared with mice injected with pH 4.0. Flow cytometry on isolated monocytes showed no change in IL-5 level between groups for any of the cell types (n=6 mice per group, Figure 3C).
Discussion
The primary aims of this study were to characterize relationships between monocyte phenotype and FM symptoms, and to determine the role of IL-5 in pain modulation. The current study showed that women with FM exhibit differences in immune phenotype as evidenced by altered levels of pro-and anti-inflammatory cytokines, a higher percentage of non-classical monocytes, and a lower percentage of intermediate monocytes compared with women without pain (NP group). Moreover, LPS-evoked and spontaneous secretion of IL-5 and other select cytokines (IL-1β, IL-6, IL-10, IL-4, TNF–α, IL-2, GM-CSF, and IFN-Ɣ) from isolated monocytes are associated with several pain-related outcomes. Further, we show for the first time that increases in IL-5 systemically reduce hyperalgesia in an animal model of widespread muscle pain while also polarizing monocytes to the non-classical phenotype, which parallels our observations in women with FM. Taken together, our combined preclinical and clinical data reveal a previously unrecognized role of IL-5 in regulating monocyte phenotype and function, resolution of widespread hyperalgesia, and modulation of other somatic and psychological symptoms in FM. We believe that evoked and spontaneous production of IL-5 by monocytes is a novel finding, and suggests a previously undetermined mechanism of action in anti-nociception from myeloid cells. To our knowledge, the production of IL-5 by monocytes, macrophages, or other myeloid cells and the anti-nociceptive effect of IL-5 in people with FM and in an animal model of widespread muscle pain has not been previously shown.
Myeloid cells have potent signaling functions that include the synthesis and secretion of cytokines and chemokines [37; 38; 69]. Monocytes are significant producers of IL-β [40] and IL-10 [58], but not of IL-5. Additionally, although the function of IL-5 in nociception has been investigated, its role in nociception and pain in chronic musculoskeletal pain has not been extensively studied. In the current study, monocytes from women with FM had greater evoked and spontaneous secretion of IL-5 and other select cytokines previously implicated in pain (IL-β, IL-6, IL- 10, IL-4, TNF-α, IL- 2, GM-CSF, and IFN-Ɣ), confirming previous data obtained using stimulated PBMCs [53; 60; 81]. Although other studies have shown changes in IL-5 levels in FM patients, these studies were conducted on serum or plasma samples and were therefore not focused on the production of IL-5 from a specific cell type [80]. Moreover, in an animal model of widespread muscle pain, IL-5 injection produces analgesia in a dose-dependent manner, and polarizes monocytes toward an anti-inflammatory phenotype. Talbot et al reported that IL-5 activated nodose ganglia, and contributed to cytokine production in lymphoid cells in an animal model of allergic airway inflammation. Further, our previous study in animals with neuropathic pain identified a decrease in IL-5 at the site of injury and in the spinal cord [11]. However, the role of IL-5 in analgesia in an animal model of neuropathic pain was not investigated. Moreover, findings from complementary human data was not reported. Since IL-5 has anti-nociceptive properties in a validated animal model of widespread muscle pain, the human data might suggest a compensatory change in the phenotype and function of myeloid cells to modulate nociception and pain in the periphery. Though the specific function of IL-5 was not investigated, Capossela et al and others reported paradoxically higher levels of IL-4 and IL-10 in plasma and secreted from PBMCs in adults with chronic musculoskeletal pain [13; 14; 81]. Lastly, we have extended prior findings by comprehensively characterizing relationships between IL-5 secreted from monocytes and distinct clinical features of FM. Importantly, since pain-related psychological symptoms significantly influence pain, fatigue, function, and other symptoms of FM [11], IL-5 could be a promising candidate biomarker of several prominent core symptoms associated with FM [10]. It is important to note, however, that further analysis of the cell types involved in cytokine production and mechanisms of action in a model of widespread musculoskeletal pain is essential. We hope that the current study will help to illuminate the important anti-nociceptive role of IL-5 produced by myeloid cells in FM and other debilitating chronic widespread pain conditions.
Previous studies have shown changes in cytokine secretion from PBMCs and their associations with select FM symptoms, though these findings are inconsistent [2; 13; 60; 80; 81]. Researchers have reported higher secretion of IL-1β, IL-6, TNF-α and IL-10 from stimulated monocytes and other PBMCs in culture[13; 81], whereas others reported no difference in cytokine production to healthy controls [2; 80]. Some potential reasons for these inconsistencies are small sample sizes and methodological differences between studies. In addition, lack of consideration for confounders such as anxiety and depression [48], higher BMI, and physical function [20] may also contribute to the variability in study findings. The current study features a comprehensive phenotyping of women with FM that specifically measures these confounding variables. Further, women in the NP group were matched by age and BMI to address the potential influence of these confounders. We show that higher levels of IL-5 and other anti-inflammatory cytokines (IL-10, IL-4, and GM–CSF) are associated with more pain, greater disease impact, more fatigue, and greater impact of pain-related psychological variables, consistent with previous studies [12]. Interestingly, IL-4 and IL-5 have similar correlative relationships to pain, disease impact, fatigue, physical function, and pain-related psychological variables in the current study. Very few groups have comprehensively examined or described the interaction between IL-4 and IL-5 cytokines. IL-4 plays a role in activating B cells in plasma and decreases Th1 responses [62]. High levels of IL-4 can lead to the development of allergies [63]. In animal models of neuropathic pain, IL-4 induces polarization of unstimulated macrophages to an M2 phenotype [55] and promotes prolonged analgesia through localized production of opioid peptides [15]. Moreover, IL-4 enhances production of anti-inflammatory cytokines, including IL-5, in Th1 cells [47], and may induce a shift toward a Th2 phenotype in the context of chronic stress [43]. On the other hand, it is established that IL-5 is a key activator of eosinophils and plays a role in allergic rhinitis and asthma [29; 36; 84]. In keeping with this, therapeutic targeting of IL-5 cytokines has been used to dampen allergies and asthma [24; 64; 82]. Yet, the anti-nociceptive role of IL-5 has not been well characterized mechanistically in human or animal models of chronic pain. Our results show that injection of IL-5 has a significant anti-nociceptive effect in an animal model of widespread muscle pain (Figure 3B). Further, in women with FM, the concentrations of IL-4 and IL-5 are highly collinear (Supplementary Figure 2). It is, therefore, possible that the anti-nociceptive effect of IL-5 occurs by promoting a Th2 response to counteract the Th1 response [18; 68] similar to the effect of IL-4 [47].
The current study showed a decrease in intermediate monocytes in individuals with FM, which is consistent with previous findings in individuals with FM (though not statistically significant [76]) and SLE [48]. We extended these findings by showing moderate associations of monocyte phonotype with pain, disease impact, fatigue, physical function, and pain-related psychological variables. Others have found that overexpression of CD64 on classical and intermediate monocytes is associated with increased disease activity in adults with rheumatoid arthritis [39]. In addition, there is a greater percentage of non-classical monocytes in individuals with SLE compared with healthy controls [48]. The current study suggests that an intermediate monocyte phenotype may have an anti-nociceptive function in FM. Furthermore, IL-5 may contribute to monocyte polarization toward an anti-inflammatory phenotype since systemic IL-5 in mice induced a phenotypic switch toward non-classical monocyte subpopulations. Thus, we conclude that the anti-nociceptive effect of IL-5 is an overall anti-inflammatory effect across the peripheral nervous and immune systems.
There are some limitations that could affect the generalizability of conclusions drawn from these findings. First, the human experiments featured a cross-sectional study design, thus, we cannot determine causation. Additionally, participants in the parent clinical trial included only women with FM having reported pain of at least 4/10 on the Numeric Rating Scale (NRS). Thus, the results may not be generalizable to men with FM or to women with FM having pain levels less than 4/10. One experimental limitation of the human participants’ data is that we analyzed monocyte phenotype and cytokine secretion profiles in separate experiments with relatively small sample size. Further, the authors acknowledge that cell viability and gene expression along cytokine pathways may have been affected by the cryopreservation and post-thaw stimulation of PBMCs [17; 85]. Thus, evoked and spontaneous secretion of select cytokines from the isolated monocytes could have been blunted. Future studies should include a replication of these findings using fresh PBMCs.
Taken together, our human and animal data suggest that induction of a phenotypic switch in monocytes is associated with the evoked and spontaneous secretion of pro- and anti-nociceptive cytokines that may contribute to gain or loss of function in sensory neurons and contribute to the progression of chronic widespread pain associated somatic symptoms. Future studies could investigate IL-5 therapy as a novel strategy to manage FM symptomology.
Supplementary Material
Acknowledgments
This work is supported by NIH UM1 AR06338 and NIH UM1 AR06338-S1. Human study data was collected and managed using REDCap electronic data capture tools hosted at the University of Iowa (supported by NIH 54TR001013). FAST data collection was completed at the CTSA at University of Iowa (supported by NIH U54TR001356) and Vanderbilt University (supported by NIH UL1TR000445). Animal work was supported by NIH grant NS096030 to MDB. The authors would like to acknowledge Jessica Danielson for her contributions to data collection for the human study. The authors would also like to acknowledge the University of Iowa Flow Cytometry Facility for flow cytometry experiments conducted for the human study. Conflict of interest. The authors report grants from National Institutes of Health during the conduct of the study. The authors have declared that no other conflicts of interest exist.
REFERENCES
- [1].Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. The Journal of neuroscience : the official journal of the Society for Neuroscience 2000;20(12):4680–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amel Kashipaz MR, Swinden D, Todd I, Powell RJ. Normal production of inflammatory cytokines in chronic fatigue and fibromyalgia syndromes determined by intracellular cytokine staining in short-term cultured blood mononuclear cells. Clinical and experimental immunology 2003;132(2):360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Angel S, von Briesen H, Oh YJ, Baller MK, Zimmermann H, Germann A. Toward Optimal Cryopreservation and Storage for Achievement of High Cell Recovery and Maintenance of Cell Viability and T Cell Functionality. Biopreservation and biobanking 2016;14(6):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anisman H, Baines MG, Berczi I, Bernstein CN, Blennerhassett MG, Gorczynski RM, Greenberg AH, Kisil FT, Mathison RD, Nagy E, Nance DM, Perdue MH, Pomerantz DK, Sabbadini ER, Stanisz A, Warrington RJ. Neuroimmune mechanisms in health and disease: 2. Disease. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 1996;155(8):1075–1082. [PMC free article] [PubMed] [Google Scholar]
- [5].Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schürmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nature medicine 2000;6(5):583–588. [DOI] [PubMed] [Google Scholar]
- [6].Behm FG, Gavin IM, Karpenko O, Lindgren V, Gaitonde S, Gashkoff PA, Gillis BS. Unique immunologic patterns in fibromyalgia. BMC clinical pathology 2012;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Belza BL, Henke CJ, Yelin EH, Epstein WV, Gilliss CL. Correlates of fatigue in older adults with rheumatoid arthritis. Nursing research 1993;42(2):93–99. [PubMed] [Google Scholar]
- [8].Benjamini YHY. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J R Statist Soc B 1995;57(1):289–300. [Google Scholar]
- [9].Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis research & therapy 2009;11(4):R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bobinski F, Ferreira TA, Cordova MM, Dombrowski PA, da Cunha C, Santo CC, Poli A, Pires RG, Martins-Silva C, Sluka KA, Santos AR. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain 2015;156(12):2595–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bobinski F, Teixeira JM, Sluka KA, Santos ARS. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018;159(3):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Borchers AT, Gershwin ME. Fibromyalgia: A Critical and Comprehensive Review. Clinical reviews in allergy & immunology 2015;49(2):100–151. [DOI] [PubMed] [Google Scholar]
- [13].Bote ME, Garcia JJ, Hinchado MD, Ortega E. Fibromyalgia: anti-inflammatory and stress responses after acute moderate exercise. PloS one 2013;8(9):e74524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Capossela S, Pavlicek D, Bertolo A, Landmann G, Stoyanov JV. Unexpectedly decreased plasma cytokines in patients with chronic back pain. Journal of pain research 2018;11:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Celik MO, Labuz D, Keye J, Glauben R, Machelska H. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI insight 2020;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [17].Chen J, Bruns AH, Donnelly HK, Wunderink RG. Comparative in vitro stimulation with lipopolysaccharide to study TNFalpha gene expression in fresh whole blood, fresh and frozen peripheral blood mononuclear cells. Journal of immunological methods 2010;357(1-2):33–37. [DOI] [PubMed] [Google Scholar]
- [18].Chitnis T, Salama AD, Grusby MJ, Sayegh MH, Khoury SJ. Defining Th1 and Th2 immune responses in a reciprocal cytokine environment in vivo. Journal of immunology (Baltimore, Md : 1950) 2004;172(7):4260–4265. [DOI] [PubMed] [Google Scholar]
- [19].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore: 1994;23(2):129–138. [PubMed] [Google Scholar]
- [20].Dailey DL, Frey Law LA, Vance CG, Rakel BA, Merriwether EN, Darghosian L, Golchha M, Geasland KM, Spitz R, Crofford LJ, Sluka KA. Perceived function and physical performance are associated with pain and fatigue in women with fibromyalgia. Arthritis research & therapy 2015;18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dailey DL, Rakel BA, Vance CG, Liebano RE, Amrit AS, Bush HM, Lee KS, Lee JE, Sluka KA. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain 2013;154(11):2554–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dina OA, Khasar SG, Gear RW, Levine JD. Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience 2009;160(2):501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Disis ML, dela Rosa C, Goodell V, Kuan LY, Chang JC, Kuus-Reichel K, Clay TM, Kim Lyerly H, Bhatia S, Ghanekar SA, Maino VC, Maecker HT. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. Journal of immunological methods 2006;308(1-2):13–18. [DOI] [PubMed] [Google Scholar]
- [24].Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. The Cochrane database of systematic reviews 2017;9:Cd010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- [26].Gong WY, Abdelhamid RE, Carvalho CS, Sluka KA. Resident Macrophages in Muscle Contribute to Development of Hyperalgesia in a Mouse Model of Noninflammatory Muscle Pain. The journal of pain : official journal of the American Pain Society 2016;17(10):1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews Immunology 2005;5(12):953–964. [DOI] [PubMed] [Google Scholar]
- [28].Gracely RH, Ceko M, Bushnell MC. Fibromyalgia and depression. Pain research and treatment 2012;2012:486590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Greenfeder S, Umland SP, Cuss FM, Chapman RW, Egan RW. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respiratory research 2001;2(2):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gregory NS, Brito RG, Fusaro M, Sluka KA. ASIC3 Is Required for Development of Fatigue-Induced Hyperalgesia. Molecular neurobiology 2016;53(2):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hønge BL, Petersen MS, Olesen R, Møller BK, Erikstrup C. Optimizing recovery of frozen human peripheral blood mononuclear cells for flow cytometry. PloS one 2017;12(11):e0187440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Italiani P, Boraschi D. Development and Functional Differentiation of Tissue-Resident Versus Monocyte-Derived Macrophages in Inflammatory Reactions. Results and problems in cell differentiation 2017;62:23–43. [DOI] [PubMed] [Google Scholar]
- [33].Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nature reviews Immunology 2017;17(6):349–362. [DOI] [PubMed] [Google Scholar]
- [34].Kaleth AS, Slaven JE, Ang DC. Determining the Minimal Clinically Important Difference for 6-Minute Walk Distance in Fibromyalgia. American journal of physical medicine & rehabilitation 2016;95(10):738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kleeberger CA, Lyles RH, Margolick JB, Rinaldo CR, Phair JP, Giorgi JV. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clinical and diagnostic laboratory immunology 1999;6(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. International immunology 2009;21(12):1303–1309. [DOI] [PubMed] [Google Scholar]
- [37].Lacy P Editorial: secretion of cytokines and chemokines by innate immune cells. Frontiers in immunology 2015;6:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood 2011;118(1):9–18. [DOI] [PubMed] [Google Scholar]
- [39].Luo Q, Xiao P, Li X, Deng Z, Qing C, Su R, Xu J, Guo Y, Huang Z, Li J. Overexpression of CD64 on CD14(++)CD16(−) and CD14(++)CD16(+) monocytes of rheumatoid arthritis patients correlates with disease activity. Experimental and therapeutic medicine 2018;16(3):2703–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Madej MP, Töpfer E, Boraschi D, Italiani P. Different Regulation of Interleukin-1 Production and Activity in Monocytes and Macrophages: Innate Memory as an Endogenous Mechanism of IL-1 Inhibition. Frontiers in pharmacology 2017;8:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nature reviews Neuroscience 2005;6(7):521–532. [DOI] [PubMed] [Google Scholar]
- [42].Marsaglia GTW, Wang J. . Evaluating Kolmogrov’s Distribution. Journal of Statistical Software 2003;8(18):1–4. [Google Scholar]
- [43].Marshall GD Jr The adverse effects of psychological stress on immunoregulatory balance: applications to human inflammatory diseases. Immunology and allergy clinics of North America 2011;31(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McMahon SB, La Russa F, Bennett DL. Crosstalk between the nociceptive and immune systems in host defence and disease. Nature reviews Neuroscience 2015;16(7):389–402. [DOI] [PubMed] [Google Scholar]
- [45].Mease PJ, Clauw DJ, Arnold LM, Goldenberg DL, Witter J, Williams DA, Simon LS, Strand CV, Bramson C, Martin S, Wright TM, Littman B, Wernicke JF, Gendreau RM, Crofford LJ. Fibromyalgia syndrome. The Journal of rheumatology 2005;32(11):2270–2277. [PubMed] [Google Scholar]
- [46].Merriwether EN, Rakel BA, Zimmerman MB, Dailey DL, Vance CGT, Darghosian L, Golchha M, Geasland KM, Chimenti R, Crofford LJ, Sluka KA. Reliability and Construct Validity of the Patient-Reported Outcomes Measurement Information System (PROMIS) Instruments in Women with Fibromyalgia. Pain medicine (Malden, Mass) 2017;18(8):1485–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mitchell RE, Hassan M, Burton BR, Britton G, Hill EV, Verhagen J, Wraith DC. IL-4 enhances IL-10 production in Th1 cells: implications for Th1 and Th2 regulation. Scientific reports 2017;7(1):11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Scientific reports 2015;5:13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].N S. Faster Isolation of PBMC Using Ficoll-Paque® Plus in the Eppendorf Multipurpose Benchtop Centrifuges 5920 R and 5910 R. [Google Scholar]
- [50].Ngkelo A, Meja K, Yeadon M, Adcock I, Kirkham PA. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signalling. Journal of inflammation (London, England) 2012;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Noehren B, Dailey DL, Rakel BA, Vance CG, Zimmerman MB, Crofford LJ, Sluka KA. Effect of transcutaneous electrical nerve stimulation on pain, function, and quality of life in fibromyalgia: a double-blind randomized clinical trial. Physical therapy 2015;95(1):129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ortega E, Bote ME, Giraldo E, Garcia JJ. Aquatic exercise improves the monocyte pro- and anti-inflammatory cytokine production balance in fibromyalgia patients. Scandinavian journal of medicine & science in sports 2012;22(1):104–112. [DOI] [PubMed] [Google Scholar]
- [53].Ortega E, Garcia JJ, Bote ME, Martin-Cordero L, Escalante Y, Saavedra JM, Northoff H, Giraldo E. Exercise in fibromyalgia and related inflammatory disorders: known effects and unknown chances. Exercise immunology review 2009;15:42–65. [PubMed] [Google Scholar]
- [54].Pankoff BA, Overend TJ, Lucy SD, White KP. Reliability of the six-minute walk test in people with fibromyalgia. Arthritis care and research : the official journal of the Arthritis Health Professions Association 2000;13(5):291–295. [DOI] [PubMed] [Google Scholar]
- [55].Pannell M, Labuz D, Celik MO, Keye J, Batra A, Siegmund B, Machelska H. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. Journal of neuroinflammation 2016;13(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pinho-Ribeiro FA, Verri WA Jr., Chiu IM. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends in immunology 2017;38(1):5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Plevin RE, Knoll M, McKay M, Arbabi S, Cuschieri J. The Role of Lipopolysaccharide Structure in Monocyte Activation and Cytokine Secretion. Shock (Augusta, Ga) 2016;45(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Prasse A, Germann M, Pechkovsky DV, Markert A, Verres T, Stahl M, Melchers I, Luttmann W, Müller-Quernheim J, Zissel G. IL-10-producing monocytes differentiate to alternatively activated macrophages and are increased in atopic patients. The Journal of allergy and clinical immunology 2007;119(2):464–471. [DOI] [PubMed] [Google Scholar]
- [59].Ramachandran H, Laux J, Moldovan I, Caspell R, Lehmann PV, Subbramanian RA. Optimal thawing of cryopreserved peripheral blood mononuclear cells for use in high-throughput human immune monitoring studies. Cells 2012;1(3):313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rodriguez-Pinto I, Agmon-Levin N, Howard A, Shoenfeld Y. Fibromyalgia and cytokines. Immunology letters 2014;161(2):200–203. [DOI] [PubMed] [Google Scholar]
- [61].Roelofs J, Goubert L, Peters ML, Vlaeyen JW, Crombez G. The Tampa Scale for Kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. European journal of pain (London, England) 2004;8(5):495–502. [DOI] [PubMed] [Google Scholar]
- [62].Rush JS, Hodgkin PD. B cells activated via CD40 and IL-4 undergo a division burst but require continued stimulation to maintain division, survival and differentiation. European journal of immunology 2001;31(4):1150–1159. [DOI] [PubMed] [Google Scholar]
- [63].Schmidt-Weber CB. Anti-IL-4 as a new strategy in allergy. Chemical immunology and allergy 2012;96:120–125. [DOI] [PubMed] [Google Scholar]
- [64].Shrimanker R, Pavord ID. Interleukin-5 Inhibitors for Severe Asthma: Rationale and Future Outlook. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy 2017;31(2):93–103. [DOI] [PubMed] [Google Scholar]
- [65].Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016;338:114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle & nerve 2001;24(1):37–46. [DOI] [PubMed] [Google Scholar]
- [67].Smith HS, Barkin RL. Fibromyalgia syndrome: a discussion of the syndrome and pharmacotherapy. American journal of therapeutics 2010;17(4):418–439. [DOI] [PubMed] [Google Scholar]
- [68].Smith KM, Pottage L, Thomas ER, Leishman AJ, Doig TN, Xu D, Liew FY, Garside P. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. Journal of immunology (Baltimore, Md : 1950) 2000;165(6):3136–3144. [DOI] [PubMed] [Google Scholar]
- [69].Striz I, Brabcova E, Kolesar L, Sekerkova A. Cytokine networking of innate immunity cells: a potential target of therapy. Clinical science (London, England : 1979) 2014;126(9):593–612. [DOI] [PubMed] [Google Scholar]
- [70].Strober W Trypan blue exclusion test of cell viability. Current protocols in immunology 2001;Appendix 3:Appendix 3B. [DOI] [PubMed] [Google Scholar]
- [71].Sturgill J, McGee E, Menzies V. Unique cytokine signature in the plasma of patients with fibromyalgia. Journal of immunology research 2014;2014:938576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sullivan M, Karlsson J, Ware JE Jr. The Swedish SF-36 Health Survey--I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Social science & medicine (1982) 1995;41(10):1349–1358. [DOI] [PubMed] [Google Scholar]
- [73].Sullivan MJL BS, Pivik J. The pain catastrophizing scale: development and validation. Psychological Assessment 1995;7:432–524. [Google Scholar]
- [74].Takashiba S, Van Dyke TE, Amar S, Murayama Y, Soskolne AW, Shapira L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappaB. Infection and immunity 1999;67(11):5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Talbot S, Foster SL, Woolf CJ. Neuroimmunity: Physiology and Pathology. Annual review of immunology 2016;34:421–447. [DOI] [PubMed] [Google Scholar]
- [76].Taylor AG, Fischer-White TG, Anderson JG, Adelstein KE, Murugesan M, Lewis JE, Scott MM, Gaykema RP, Goehler LE. Stress, Inflammation and Pain: A Potential Role for Monocytes in Fibromyalgia-related Symptom Severity. Stress and health : journal of the International Society for the Investigation of Stress 2016;32(5):503–513. [DOI] [PubMed] [Google Scholar]
- [77].Totsch SK, Sorge RE. Immune System Involvement in Specific Pain Conditions. Molecular pain 2017;13:1744806917724559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tousignant-Laflamme Y, Page S, Goffaux P, Marchand S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain research 2008;1230:73–79. [DOI] [PubMed] [Google Scholar]
- [79].Tucureanu MM, Rebleanu D, Constantinescu CA, Deleanu M, Voicu G, Butoi E, Calin M, Manduteanu I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of G(i)-protein inhibitor. International journal of nanomedicine 2018;13:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Uceyler N, Hauser W, Sommer C. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC musculoskeletal disorders 2011;12:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology (Oxford, England) 2001;40(7):743–749. [DOI] [PubMed] [Google Scholar]
- [82].Wang FP, Liu T, Lan Z, Li SY, Mao H. Efficacy and Safety of Anti-Interleukin-5 Therapy in Patients with Asthma: A Systematic Review and Meta-Analysis. PloS one 2016;11(11):e0166833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A. Monocytes/Macrophages control resolution of transient inflammatory pain. The journal of pain : official journal of the American Pain Society 2014;15(5):496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yanagibashi T, Satoh M, Nagai Y, Koike M, Takatsu K. Allergic diseases: From bench to clinic - Contribution of the discovery of interleukin-5. Cytokine 2017;98:59–70. [DOI] [PubMed] [Google Scholar]
- [85].Yang J, Diaz N, Adelsberger J, Zhou X, Stevens R, Rupert A, Metcalf JA, Baseler M, Barbon C, Imamichi T, Lempicki R, Cosentino LM. The effects of storage temperature on PBMC gene expression. BMC immunology 2016;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, Schutt C, Passlick B, Pforte A. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. European journal of immunology 1993;23(9):2053–2058. [DOI] [PubMed] [Google Scholar]
- [87].Ziegler-Heitbrock L The CD14+ CD16+ blood monocytes: their role in infection and inflammation. Journal of leukocyte biology 2007;81(3):584–592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


