Abstract
Background:
Intensive lifestyle interventions (ILI) are the first-line approach to effectively treat obesity and manage associated cardiometabolic risk factors. Because few people have access to ILI in academic health centers, primary care must implement similar approaches for a meaningful impact on obesity and cardiometabolic disease prevalence. To date, however, effective lifestyle-based obesity treatment in primary care is limited. We examined the effectiveness of a pragmatic ILI for weight loss delivered in primary care among a racially diverse, low-income population with obesity for improving cardiometabolic risk factors over 24 months.
Methods:
The PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial randomly allocated 18 clinics equally to usual care (UC) or an ILI and subsequently enrolled 803 (351 UC, 452 ILI) adults (67% African American; 84% female) with obesity from participating clinics. The UC group continued to receive their normal primary care. The ILI group received a 24-month high-intensity lifestyle-based obesity treatment program, embedded in the clinic setting and delivered by health coaches in weekly sessions initially and monthly sessions in months 7 through 24.
Results:
As recently demonstrated, ILI lost significantly more weight over 24 months than UC (mean difference −4.51% [95% CI: −5.93, −3.10; P<0.01]). Fasting glucose decreased more in ILI compared to UC at 12 months (mean difference −7.1 mg/dL [95% CI: −12.0, −2.1; P<0.01]) but not 24 months (mean difference −0.8 mg/dL [95% CI: −6.2, 4.6; P=0.76]). Increases in HDL cholesterol were greater in ILI than UC at both timepoints (mean difference at 24 months: 4.6 mg/dL [95% CI: 2.9, 6.3; P<0.01]). Total:HDL cholesterol ratio and metabolic syndrome severity (z-score) decreased more in ILI than UC at both timepoints with significant mean differences of the change of −0.31 (95% CI: −0.47, −0.14; P<0.01) and −0.21 (95% CI: −0.36, −0.06; P=0.01) at 24 months, respectively. Changes in total cholesterol, LDL cholesterol, triglycerides, and blood pressure did not differ significantly between groups at any timepoint.
Conclusions:
A pragmatic ILI consistent with national guidelines and delivered by trained health coaches in primary care produced clinically relevant improvements in cardiometabolic health in an underserved population over 24 months.
Keywords: lifestyle, obesity, cardiovascular disease prevention, cardiovascular outcomes, primary care
INTRODUCTION
In 2017–2018, the prevalence of obesity among U.S. adults was an estimated 42.4%, with severe, Class III obesity being 9.2%.1 Obesity is associated with serious chronic health risks such as cardiovascular disease (CVD), type 2 diabetes, several cancers, depression, and premature mortality,2 presenting substantial public health and economic burden in many countries.3 Certain sociodemographic groups are particularly affected by the obesity epidemic and consequently are at the highest risk for deleterious health effects; African American adults had an estimated prevalence of obesity as high as 49.6% in 2017–2018, and among all adults, those aged 40–59 years had the highest prevalence of severe obesity (body mass index [BMI] ≥40 kg/m2; 11.5%).1 Along with race/ethnicity, additional social determinants of health, such as lower levels of education, income, and food security, intersect to increase risk of obesity and related comorbidities.4–6 Importantly, such inequities are further sustained by policies (e.g., healthcare access and affordability), systems (e.g., racism, discrimination, segregation), and environments (e.g., community, neighborhood) that also lead to higher obesity levels in racial/ethnic minorities.7,8 Such health disparities are particularly apparent in states like Louisiana, where the median household income and food security rates are among the lowest in the U.S.9,10
Intensive lifestyle interventions (ILI) are the first-line approach to promote weight loss (WL) and effectively treat obesity and manage associated health risks, as outlined in the 2013 American Heart Association (AHA)/American College of Cardiology (ACC)/The Obesity Society (TOS) Guidelines for the Management of Overweight and Obesity in Adults.11,12 Large trials, such as the Diabetes Prevention Program (DPP)13 and Look AHEAD,14 have shown that high-intensity lifestyle interventions conducted in academic health centers can induce WL of 5.8% and 6.4% over 2 years, respectively, and that these weight reductions are accompanied by health-beneficial changes in blood pressure (BP), glucose control, and dyslipidemia.15–17
However, only a small proportion of the population has access to ILI in academic health centers. Therefore, uptake of similar approaches by primary care, the cornerstone of medical care in the U.S., is imperative for a meaningful impact on the global obesity prevalence and for achieving public health goals to reduce health inequities.18 Despite recommendations by the U.S. Preventive Service Task Force that physicians offer intensive multi-component behavioral interventions to individuals with obesity,19 to date, effective lifestyle-based obesity treatment in primary care is often lacking and long-term success for WL and improvement in cardiometabolic risk factors is consequently limited.20 This is in part due to infrequent treatment contacts and time constraints during visits and, importantly, because of primary care practitioners’ lack of training in behavior therapy and nutrition education.20,21 WL interventions based in primary care have produced a range of WL (1–2 kg in low-intensity interventions to 4–7 kg in higher-intensity interventions).20,22 For example, in the Practice-based Opportunities for Weight Reduction (POWER) trials, there was greater WL experienced in the Baltimore (5.2%) and Philadelphia (4.7%) trials compared to the Boston trial (1.7%).23–25 The Boston sample consisted predominantly of African Americans with low annual income, and the intervention consisted of monthly counseling telephone calls in the first 12 months, followed by bimonthly calls in the remaining 12 months (18 total calls), in addition to self-monitoring via a website or via an interactive voice response system.25
We conducted a 24-month cluster-randomized trial in which a high-intensity lifestyle intervention was delivered face-to-face by trained health coaches embedded within primary care clinics among an underserved population with obesity.26 The primary outcome measure of the trial was percent change in body weight from baseline to month 24. The ILI group lost significantly more weight than the usual care (UC) group, with a mean difference of −4.51% (95% confidence interval [C]I: −5.93, −3.10) between the groups (P<0.01).27 Similar to the trial’s demonstrated effectiveness for WL, we hypothesized that participants receiving the ILI would show improvements in cardiometabolic risk factors relative to those receiving UC.
METHODS
Design and Participants
The PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial was a 24-month high-intensity lifestyle intervention delivered within primary care clinics among a racially diverse, low-income population with obesity. The PROPEL trial was conducted between April 2016 and September 2019, and the study protocol and all procedures were approved by Pennington Biomedical Research Center’s Institutional Review Board. The data that support the findings of this study are available from the corresponding author upon reasonable request. Study materials and the statistical analysis plan are available as supplementary material associated with the PROPEL primary outcome paper.27 The trial’s design and rationale have been published in detail.26 In brief, 18 primary care clinics across Louisiana were randomly allocated in equal numbers to either an ILI group or a UC group. Participants were recruited from the participating clinics and deemed eligible if they were 20–75 years old, had a BMI of 30–50 kg/m2, and were patients at a participating clinic. Participants were excluded if they were currently participating in a WL program, used WL medication, had undergone bariatric surgery within the last two years, or had lost >10 lbs of weight within the last six months. The complete list of eligibility criteria is described elsewhere.26 All participants provided written informed consent before enrollment in the study.
Intervention
Participants at clinics allocated to the ILI group received a comprehensive, high-intensity lifestyle intervention program,26 based on the DPP,28 Look AHEAD,29 and CALERIE30 studies and consistent with the 2013 AHA/ACC/TOS Guidelines.12 The regimen was adapted to be literacy and culturally appropriate for a low-income target population; as reported previously, 31% of PROPEL participants scored 6 or lower on the Rapid Estimate of Adult Literacy in Medicine Short Form (REALM-SF) health literacy assessment at baseline, corresponding to less than a 9th-grade education and indicating limited health literacy.26 The ILI program was embedded in the primary care clinics and consisted of weekly sessions with trained health coaches (16 face-to-face and 6 via phone) during the first six months and at least monthly sessions for the remaining 18 months, alternating between face-to-face and phone sessions. All health coaches had higher education degrees in nutrition, physical activity (PA), or behavioral medicine and received further training in the management of obesity and related comorbidities, fundamentals of health literacy, and patient communication and education before the start of the intervention. During the intervention, the health coaches worked with participants to meet the predefined individual goal of 10% WL by coaching them to develop and adhere to personalized action plans focusing on changes in eating, diet, and PA behavior. To monitor WL progress and promote intervention fidelity, participants were encouraged to measure their weight daily with the provided BodyTrace© scale (BodyTrace Inc., Palo Alto, CA), which transmitted weight data wirelessly and in real time to a computer tracking system. This system plotted the weight data onto a personalized weight graph, which was available via a website at any time, and allowed participants and health coaches to detect deviations from the intended WL progress quickly.31,32 If deviations occurred, the personalized action plans were adjusted, utilizing additional components of the ‘toolbox’ approach (i.e., tailored behavioral, nutritional, and PA strategies) that has been shown to improve intervention efficacy in previous clinical trials.28–30 Primary care providers (PCP) in ILI clinics received a series of webinars on lifestyle weight management practices, lipid management, and ways to improve communication with patients with low health literacy and/or obesity throughout the intervention period.
Participants at clinics allocated to the UC group continued to receive their normal care from their PCP during the 24-month intervention period. In addition, they received several newsletters on topics such as the importance of sleep for health, tips for limiting sitting time, brain and memory health, and smoking cessation. PCP in UC clinics received information on the Centers for Medicare and Medicaid Services (CMS) approach for intensive behavioral therapy for obesity33 via a presentation by PROPEL staff at baseline and an informational brochure on the same topic annually.
Outcome Measures
Fasting Blood Glucose and Lipids
Fasting blood glucose (FBG) and lipids (total cholesterol, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], and triglycerides) were measured at baseline, month 12, and month 24 using fingerstick blood samples and the Cholestech LDX® Analyzer (Alere Inc., Waltham, MA). Participants were instructed to arrive at each study visit after an overnight fast (≥10 h), and the Cholestech Analyzer was calibrated daily before analyzing participant blood samples using standard controls. Further, we calculated non-HDL cholesterol (non-HDL-C; total cholesterol – HDL-C) levels and the total:HDL-C ratio, with the former representing a good surrogate measure of apolipoprotein B as it includes all atherogenic lipoproteins such as LDL, very low-density lipoprotein, and intermediate-density lipoproteins.34 Non-HDL-C and the total:HDL-C ratio have both previously been shown to be strongly associated with long-term risk of atherosclerotic CVD.35,36
Blood Pressure
Resting systolic BP (SBP) and diastolic BP (DBP) were measured using a validated automated BP monitor (Model HEM-907XL, OMRON Corporation, Kyoto, Japan) at baseline and all follow-up visits following five minutes of seated rest. At each timepoint, two measurements were taken with one minute in between measurements. If the two measurements differed by more than 20 mmHg (SBP) or 10 mmHg (DBP), a third measurement was obtained and the mean of the two closest measurements was used for analysis. In addition, mean arterial pressure (MAP) was calculated as
Metabolic Syndrome Severity Z-Score
In addition to the prespecified outcome measures, we calculated metabolic syndrome severity z-score (MetS-Z) values for all assessment visits, as described previously.37 In contrast to the traditional binary metabolic syndrome (MetS) classification, a continuous MetS severity score allows better detection of a worsening or improving condition over time.37 In brief, the MetS-Z was derived from the five traditional components of the MetS (waist circumference, SBP, FBG, fasting HDL-C, and fasting triglycerides) utilizing the 1999–2010 National Health and Nutrition Examination Survey (NHANES) data for adults aged 20–64 years via a factor analysis approach.37 To take differences in MetS criteria by race/ethnicity into account, different equations for computing the MetS-Z were generated for each of six subgroups (males and females of non-Hispanic whites, non-Hispanic blacks, and Hispanics).37 The resulting MetS-Z acts as a continuous biomarker of MetS severity38 that correlates with other established MetS risk markers, such as insulin and adiponectin.39 In addition, MetS-Z is associated with long-term type 2 diabetes39–41 and CVD risk,39,42,43 even when including the individual components of MetS in the model.40,43
Statistical Analyses
The PROPEL trial was powered for the primary outcome (mean percent WL from baseline to month 24), and the total sample size provided at least 97% power to detect a mean difference of 3.5% in the primary outcome between the ILI group and the UC group at 24 months.26 The cardiometabolic risk factors reported herein are secondary outcomes. All outcomes were analyzed at all available timepoints in the context of repeated measures linear mixed-effects multi-level models, which included random cluster (clinic) effects. Covariates in the models included study arm, assessment timepoint, and their interaction, as well as age, sex, and race. Further, binary medication use variables for hypertension, diabetes, and high cholesterol (use vs. no use) at each timepoint were entered into the respective models as additional covariates. Clinic-level covariates (total number of patients, percentage of participants who were African Americans, percentage of patients on Medicaid) were nonsignificant and results of analyses that included these additional covariates did not differ meaningfully; therefore, the models without these clinic-level covariates are reported. We conducted intention-to-treat analyses that included all participants as randomized, regardless of the number of assessments obtained, and employed restricted maximum likelihood using all available data. Missing values were assumed to be missing at random. To examine the heterogeneity of treatment effects, we conducted three prespecified subgroup analyses (African Americans vs. other races, women vs. men, and younger [21–42 years] vs. middle [43–56 years] and older [57–74 years] adults), as well as two explorative sub-group analyses (participants with diabetes vs. without diabetes and with hypertension vs. without hypertension).
In additional analyses, including only participants allocated to the ILI group, we used mixed linear regression models to estimate the effect of percent weight change (the primary outcome of the PROPEL trial26,27) on change in cardiometabolic risk factors. The random clustering effects of clinics were taken into account. Change in weight and cardiometabolic risk factors at all available timepoints were entered into the models allowing for different slopes at each timepoint. The regression model of predicted cardiometabolic risk factors for the ith ILI participant in clinic j at time k can be expressed as . Further, we analyzed change in cardiometabolic outcomes in the ILI group by categories of WL (<5%, 5% – <10%, ≥10%).
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) for Windows with the significance level set to 0.05 (two-sided).
RESULTS
Participants
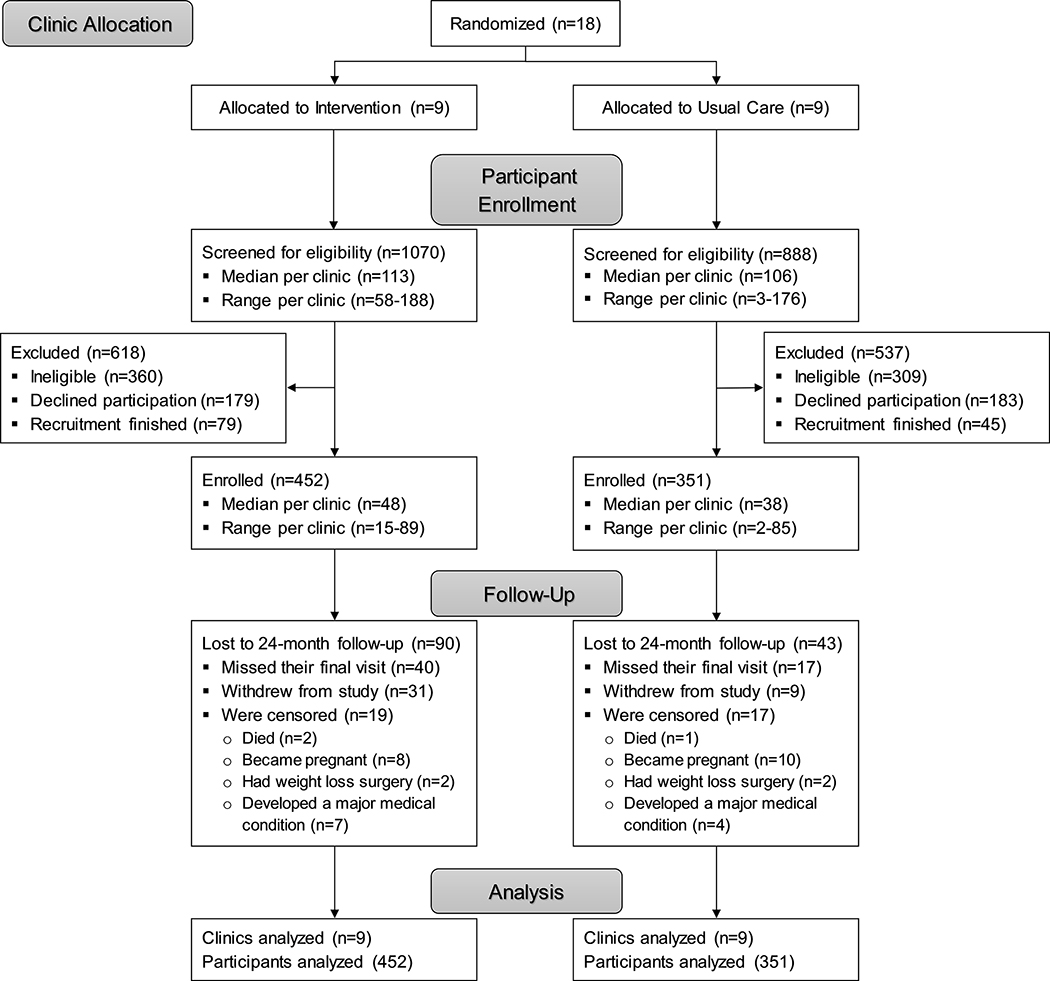
The PROPEL trial enrolled a total of 803 (67% African American; 84% female) adults with a mean age of 49.4 years (standard deviation [SD]: 13.1) and a mean BMI of 37.2 kg/m2 (SD: 4.7) into the ILI (n=452) and the UC group (n=351). Baseline characteristics are displayed in Table 1. There was a greater proportion of African Americans, women, and participants with diabetes in the ILI group, and participants in the ILI group had significantly lower FBG, total:HDL-C, and MetS-Z values and higher HDL-C values at baseline compared to those in UC (all P≤0.03). One hundred and thirty-three participants (16.6%) were lost to follow-up at 24 months due to various reasons. Eighteen clinics (9 in each group) and 803 participants (452 in ILI and 351 in UC) were included in the primary analysis (Figure 1).
Table 1.
Participant Characteristics at Baseline.
| UC | ILI | All | |
| Participants, n (%) | 351 (43.7) | 452 (56.3) | 803 |
| Race, n (%) | |||
| African American | 208 (59.3) | 332 (73.5)* | 540 (67.3) |
| White | 113 (32.2) | 95 (21.0)* | 208 (25.9) |
| Other | 30 (8.5) | 25 (5.5) | 55 (6.8) |
| Sex, n (%) | |||
| Male | 71 (20.2) | 54 (11.9)* | 125 (15.6) |
| Female | 280 (79.8) | 398 (88.1)* | 678 (84.4) |
| Diabetes, n (%) | 104 (29.6) | 103 (22.8)* | 207 (25.8) |
| Hypertension, n (%) | 196 (55.8) | 237 (52.4) | 433 (53.9) |
| mean ± SD | mean ± SD | mean ± SD | |
| Age (years) | 50.2 ± 13.6 | 48.8 ± 12.7 | 49.4 ± 13.1 |
| Body Weight (kg) | 102.7±17.0 | 101.6±16.4 | 102.1±16.7 |
| Body Mass Index (kg/m2) | 37.2±4.8 | 37.3±4.6 | 37.2±4.7 |
| Waist Circumference (cm)† | 113.9 ± 12.6 | 113.1 ± 12.4 | 113.4 ± 12.5 |
| Fasting Glucose (mg/dL)‡ | 112.3 ± 40.2 | 106.4 ± 31.9* | 109.0 ± 35.8 |
| Total Cholesterol, mg/dL) § | 180.0 ± 36.7 | 179.6 ± 37.5 | 179.8 ± 37.1 |
| LDL Cholesterol (mg/dL)|| | 106.7 ± 31.5 | 105.7 ± 32.8 | 106.2 ± 32.2 |
| HDL Cholesterol (mg/dL)# | 47.8 ± 14.4 | 50.5 ± 14.4* | 49.3 ± 14.4 |
| Non-HDL Cholesterol (mg/dL)†† | 132.0 ± 35.6 | 128.5 ± 37.1 | 130.1 ± 36.4 |
| Total:HDL Cholesterol Ratio‡‡ | 4.04 ± 1.40 | 3.80 ± 1.38* | 3.91 ± 1.39 |
| Triglycerides (mg/dL)§§ | 131.6 ± 69.4 | 125.2 ± 72.8 | 128.0 ± 71.3 |
| Systolic Blood Pressure (mmHg) | 122.6 ± 16.5 | 123.1 ± 16.3 | 122.9 ± 16.4 |
| Diastolic Blood Pressure (mmHg) | 78.4 ± 10.6 | 79.7 ± 10.6 | 79.1 ± 10.6 |
| Mean Arterial Pressure (mmHg) | 93.1 ± 11.4 | 94.2 ± 11.4 | 93.7 ± 11.4 |
| MetS Severity Z-Score|||| | 1.05 ± 1.18 | 0.87 ± 0.96* | 0.95 ± 1.06 |
Significantly different from UC (P<0.05). Chi-square tests were used to test for differences among the groups on categorical variables, and t-test was used to test for differences between ILI and UC on baseline values of continuous variables.
Data available for 349 of 351 participants (UC), 451 of 452 participants (ILI), and 800 of 803 total.
Data available for 344 of 351 participants (UC), 439 of 452 participants (ILI), and 783 of 803 total.
Data available for 340 of 351 participants (UC), 434 of 452 participants (ILI), and 774 of 803 total.
Data available for 328 of 351 participants (UC), 402 of 452 participants (ILI), and 730 of 803 total.
Data available for 343 of 351 participants (UC), 438 of 452 participants (ILI), and 781 of 803 total.
Data available for 339 of 351 participants (UC), 432 of 452 participants (ILI), and 771 of 803 total.
Data available for 339 of 351 participants (UC), 432 of 452 participants (ILI), and 771 of 803 total.
Data available for 333 of 351 participants (UC), 411 of 452 participants (ILI), and 744 of 803 total.
Data available for 306 of 351 participants (UC), 393 of 452 participants (ILI), and 699 of 803 total.
Abbreviations: ILI, intensive lifestyle intervention; MetS, metabolic syndrome; UC, usual care.
Figure 1.
Participant flow through the PROPEL trial.
Change in Outcome Measures
As recently demonstrated,27 the ILI group (−4.99% [95% CI: −6.02, −3.96]) lost more weight than the UC group (−0.48% [95% CI: −1.57, 0.61]), with a mean difference of −4.51% (95% CI: −5.93, −3.10) between the groups (P<0.01). Table 2 describes the change in cardiometabolic risk factors over two years. FBG decreased from baseline to 12 months (−4.5 mg/dL [standard error (SE): 2.1; P=0.04]) but not 24 months (−0.8 mg/dL [SE: 2.1; P=0.70]) in the ILI group. In the UC group, FBG did not change significantly at either timepoint (all P>0.20) leading to a significant mean difference of −7.1 mg/dL (SE: 2.4; P<0.01) at 12 months and −0.8 mg/dL (SE: 2.5; P=0.76) at 24 months in favor of the ILI group. HDL-C increased in the ILI group by 4.7 mg/dL (SE: 0.6; P<0.01) at 12 months and by 4.3 mg/dL (SE: 0.7; P<0.01) at 24 months, whereas they did not change in the UC group at either timepoint. The mean difference in HDL-C between the two groups was 4.1 mg/dL (SE: 0.8; P<0.01) at 12 months and 4.6 mg/dL (SE: 0.8; P<0.01) at 24 months, both in favor of the ILI group. Similarly, we found a significant mean difference in total:HDL-C ratio between the two groups at both timepoints with a reduction in the ILI group relative to the UC group of −0.29 (SE: 0.07; P<0.01) at 12 months and of −0.31 (SE: 0.08; P<0.01) at 24 months. Further, MetS-Z values were decreased in the ILI group at 12 months (−0.35 [SE: 0.06; P<0.01]) and 24 months (−0.20 [SE: 0.06; P<0.01]), whereas they did not change in the UC group at either timepoint. The mean difference of the change in MetS-Z between the groups was −0.40 (SE: 0.07; P<0.01) at 12 months and −0.21 (SE: 0.07; P=0.01) at 24 months, both in favor of the ILI group. There were no significant differences in total cholesterol, LDL-C, non-HDL-C, triglycerides, SBP, DBP, or MAP between the two groups at any timepoint (all P≥0.11). We conducted two sensitivity analyses: (a) excluding all data for timepoints at which participants reported not having taken their BP or diabetes medication before the study visit (Table I in the Supplement) and (b) excluding all outliers (±3 SD of the change in the respective outcome) from the analysis (Table II in the Supplement). For BP and FBG, only those outliers who also reported not having taken their respective medication before the study visit were excluded. In general, the results for the two sensitivity analyses did not differ meaningfully from the main analysis (Table 2); however, in contrast to the main analysis, sensitivity analysis b yielded a significant increase in total cholesterol in the ILI group relative to the UC group with a mean difference of 6.6 mg/dL (SE: 2.0; P<0.01) at 24 months.
Table 2.
Change in cardiometabolic risk factors over two years.
| UC | ILI | Difference | P | |
|---|---|---|---|---|
| Fasting Glucose (mg/dL) | ||||
| At 12 months | 2.6 (−1.5, 6.7) | −4.5 (−8.9, −0.1) | −7.1 (−12.0, −2.1) | <0.01 |
| At 24 months | 0.0 (−4.4, 4.3 | −0.8 (−5.4, 3.7) | −0.8 (−6.2, 4.6) | 0.76 |
| Total Cholesterol (mg/dL) | ||||
| At 12 months | 0.8 (−3.2, 4.9) | 2.9 (−1.2, 7.0) | 2.0 (−3.0, 7.0) | 0.40 |
| At 24 months | 0.8 (−3.6, 5.1) | 5.2 (0.8, 9.5) | 4.4 (−1.1, 9.8) | 0.11 |
| LDL Cholesterol (mg/dL) | ||||
| At 12 months | 1.3 (−2.2, 4.7) | 1.2 (−2.5, 4.9) | −0.1 (−4.4, 4.2) | 0.97 |
| At 24 months | 1.9 (−1.9, 5.7) | 3.5 (−0.4, 7.4) | 1.6 (−3.2, 6.4) | 0.49 |
| HDL Cholesterol (mg/dL) | ||||
| At 12 months | 0.6 (−0.7, 1.9) | 4.7 (3.3, 6.0) | 4.1 (2.4, 5.7) | <0.01 |
| At 24 months | −0.3 (−1.7, 1.1) | 4.3 (2.9, 5.7) | 4.6 (2.9, 6.3) | <0.01 |
| Non-HDL Cholesterol (mg/dL) | ||||
| At 12 months | 1.0 (−3.0, 5.0) | −0.5 (−4.5, 3.6) | −1.4 (−6.4, 3.6) | 0.55 |
| At 24 months | 1.7 (−2.7, 6.1) | 1.9 (−2.5, 6.2) | 0.2 (−5.4, 5.8) | 0.95 |
| Total:HDL Cholesterol Ratio | ||||
| At 12 months | 0.01 (−0.11, 0.13) | −0.28 (−0.41, −0.16) | −0.29 (−0.44, −0.14) | <0.01 |
| At 24 months | 0.11 (−0.03, 0.24) | −0.20 (−0.34, −0.06) | −0.31 (−0.47, −0.14) | <0.01 |
| Triglycerides (mg/dL) | ||||
| At 12 months | −0.2 (−11.2, 10.8) | −7.8 (−18.9, 3.3) | −7.6 (−21.4, 6.3) | 0.26 |
| At 24 months | −3.6 (−14.5, 7.4) | −9.3 (−20.2, 1.7) | −5.7 (−19.4, 8.0) | 0.39 |
| Systolic Blood Pressure (mmHg) | ||||
| At 6 months | 1.2 (−1.5, 4.0) | −0.2 (−2.8, 2.4) | −1.4 (−4.1, 2.2) | 0.42 |
| At 12 months | 2.1 (−0.7, 4.9) | 0.4 (−2.3, 3.0) | −1.8 (−4.4, 2.0) | 0.33 |
| At 18 months | 1.1 (−1.8, 4.0) | −0.2 (−2.9, 2.5) | −1.3 (−4.1, 2.5) | 0.48 |
| At 24 months | 0.4 (−2.5, 3.3) | 1.9 (−0.8, 4.7) | 1.6 (−1.3, 5.3) | 0.41 |
| Diastolic Blood Pressure (mmHg) | ||||
| At 6 months | 0.2 (−1.6, 2.1) | −0.9 (−2.7, 0.8) | −1.2 (−3.5, 1.2) | 0.32 |
| At 12 months | 0.2 (−1.7, 2.1) | −1.3 (−3.1, 0.4) | −1.5 (−3.9, 0.9) | 0.21 |
| At 18 months | −0.7 (−2.6, 1.1) | −1.8 (−3.6, 0.0) | −1.1 (−3.5, 1.4) | 0.37 |
| At 24 months | −0.6 (−2.5, 1.3) | −0.6 (−2.4, 1.2) | 0.0 (−2.4, 2.5) | 0.97 |
| Mean Arterial Pressure (mmHg) | ||||
| At 6 months | 0.6 (−1.5, 2.6) | −0.7 (−2.6, 1.3) | −1.2 (−3.9, 1.4) | 0.35 |
| At 12 months | 0.8 (−1.3, 2.9) | −0.8 (−2.8, 1.2) | −1.6(−4.3, 1.1) | 0.24 |
| At 18 months | −0.1 (−2.3, 2.0) | −1.3 (−3.3, 0.7) | −1.2 (−3.9, 1.6) | 0.40 |
| At 24 months | −0.3 (−2.5, 1.9) | 0.3 (−1.8, 2.3) | 0.6 (−2.2, 3.4) | 0.69 |
| Metabolic Syndrome Severity Z-Score | ||||
| At 12 months | 0.05 (−0.07, 0.17) | −0.35 (−0.48, −0.23) | −0.40 (−0.54, −0.26) | <0.01 |
| At 24 months | 0.01 (−0.12, 0.13) | −0.20 (−0.33, −0.07) | −0.21 (−0.36, −0.06) | 0.01 |
Values are mean (95% confidence intervals). Bold font indicates statistical significance (P<0.05)
Abbreviations: ILI, intensive lifestyle intervention; UC, usual care.
Subgroup Analyses
A potential race effect was found for FBG at 12 months, where a significant difference between UC and ILI was shown for African Americans but not for other races (Table III in the Supplement). Women and men responded overall similarly to the intervention; however, for HDL-C, the difference between UC and ILI was nearly twice as large in men compared to women (both timepoints) and for FBG at 12 months, the difference between groups was five times larger in men than in women (Table IV in the Supplement). For total:HDL-C ratio and Mets-Z (both at 24 months), a significant difference between groups was found for women but not for men. When interpreting these sex-based subgroup analyses, the markedly smaller sample of men (20.2% men in UC, 11.9% men in ILI, 15.6% men overall) should be noted, which likely contributed to the substantial variability in the data for men for all outcomes. Further, there was a potential age effect for FBG with a significant reduction in ILI compared to UC only in older adults (Table V in the Supplement), noting that younger participants in both groups increased FBG substantially by approximately 20 mg/dL (P<0.01 for all) at both timepoints (data not shown). Similarly, only older adults in ILI decreased their total:HDL-C ratio and MetS-Z significantly at both timepoints compared to UC, showing a substantial reduction in MetS-Z compared to the other age groups. A potential diabetes-dependent treatment effect was found as only participants with diabetes showed a significant difference in FBG between UC and ILI at 12 months (Table VI in the Supplement), which was likely driven by a substantial decrease of 15.6 mg/dL (SE: 7.5; P=0.05; data not shown) in the ILI group. At 24 months, the mean difference in FBG between UC and ILI among those with diabetes was no longer significant. Further, significant reductions in total:HDL-C ratio and MetS-Z in the ILI group compared to the UC group at both timepoints were only found for individuals without diabetes. A significant difference in SBP, DBP, and MAP between UC and ILI was only found for individuals without hypertension and only for some but not all of the timepoints (Table VII in the Supplement).
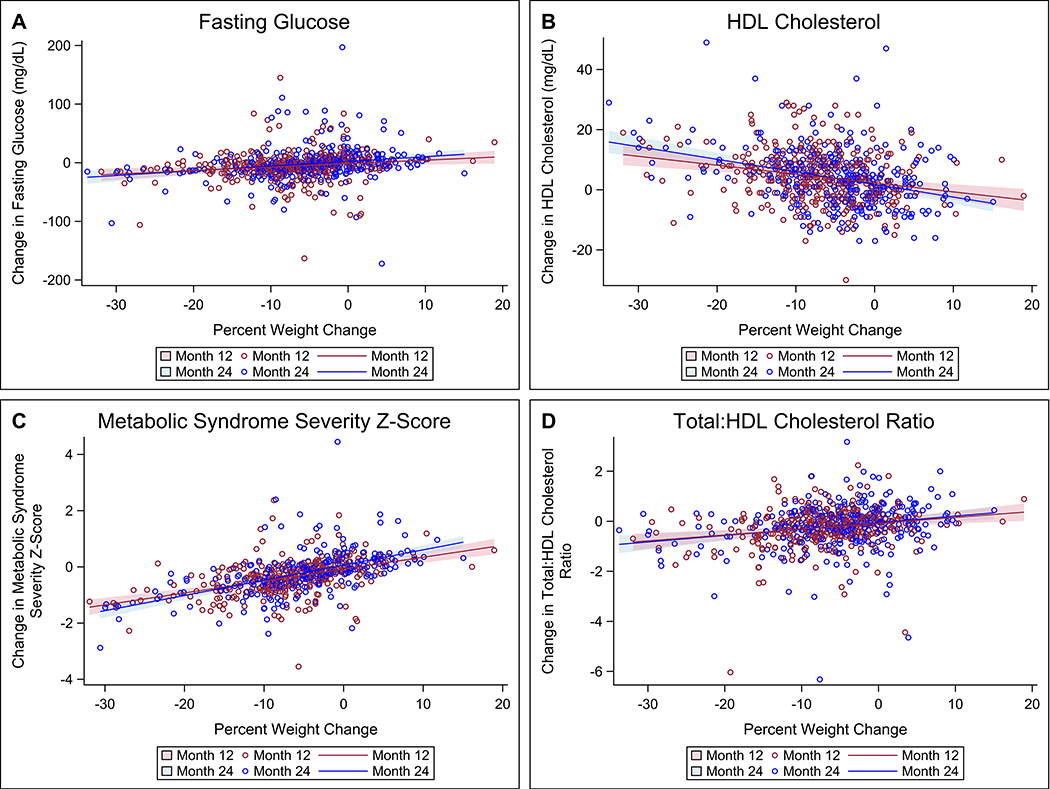
The regression models revealed significant direct associations between percent weight change and change in FBG (Figure 2A: Month 12: B=0.59, SE=0.20, P<0.01; Month 24: B=0.80, SE=0.19, P<0.01), MetS-Z (Figure 2C: Month 12: B=0.04, SE=0.01, P<0.01; Month 24: B=0.05, SE=0.01, P<0.01), and total:HDL-C ratio (Figure 2D: Month 12: B=0.02, SE=0.01, P<0.01; Month 24: B=0.03, SE=0.01, P<0.01). Further, we found a significant inverse association between percent weight change and the change in HDL-C (Figure 2B: Month 12: B=−0.30, SE=0.07, P<0.01; Month 24: B=−0.42, SE=0.06, P<0.01). The significant associations were consistent across time. To improve readability, the random clustering effects of the clinics, yielding nine regression lines (one for each clinic) for each timepoint, are not incorporated in Figure 2. For completeness, Figure I in the Supplement illustrates the associations presented in Figure 2 with the inclusion of the random clustering effects of the clinics. Associations between percent weight change and changes in DBP, MAP, and triglycerides were not consistent across time, and changes in SBP, total cholesterol, LDL-C, and non-HDL-C were not predicted by percent weight change at any timepoint. Table 3 additionally shows the change in cardiometabolic risk factors in the ILI group over two years by categories of WL. While participants with <5% WL showed no significant changes in any of the outcomes at any timepoint (except HDL-C at 12 months), WL ≥5% and particularly ≥10% was associated with significant improvements in FBG, HDL-C, total:HDL-C ratio, triglycerides, and Mets-Z, with changes in outcomes seen at ≥10% WL generally exceeding those at ≥5% − <10% WL. Effects for other outcomes were not consistent across time.
Figure 2. Association between percent weight change and change in fasting glucose levels (A), HDL cholesterol (B), Metabolic Syndrome Severity Z-Score (C), and total:HDL cholesterol ratio (D).
The mixed linear regression models included weight change and change in the respective cardiometabolic risk factor for all available timepoints, producing different slopes and 95% confidence intervals (shaded area) of the regression lines at each timepoint. To improve readability, the random clustering effects of the clinics are not incorporated in the graphs as this would yield nine regression lines (one for each clinic) for each timepoint.
Table 3.
Change in cardiometabolic risk factors in the ILI group over two years by categories of weight loss.
| <5% Weight Loss | 5% − <10% Weight Loss | ≥10% Weight Loss | |
|---|---|---|---|
| Fasting Glucose (mg/dL) | |||
| At 12 months | −0.5 (−6.5, 5.5) | −10.5 (−16.0, −5.1) | −7.2 (−11.3, −3.0) |
| At 24 months | 3.4 (−2.1, 8.9) | −5.1 (−11.2, 1.0) | −10.9 (−15.7, −6.0) |
| Total Cholesterol (mg/dL) | |||
| At 12 months | 1.0 (−4.4, 6.4) | 5.6 (−0.4, 11.6) | 3.7 (−2.3, 9.6) |
| At 24 months | 3.7 (−1.1, 8.5) | 4.7 (−1.9, 11.4) | 8.1 (1.1, 15.0) |
| LDL Cholesterol (mg/dL) | |||
| At 12 months | −0.3 (−5.3, 4.7) | 4.6 (−0.5, 9.7) | −0.5 (−7.4, 6.4) |
| At 24 months | 3.7 (−0.7, 8.1) | 1.9 (−3.7, 7.6) | 1.0 (−6.8, 8.9) |
| HDL Cholesterol (mg/dL) | |||
| At 12 months | 2.1 (0.2, 3.9) | 4.4 (2.7, 6.2) | 7.5 (5.7, 9.4) |
| At 24 months | 1.6 (−0.1, 3.3) | 4.3 (2.4, 6.3) | 9.4 (7.2, 11.6) |
| Non-HDL Cholesterol (mg/dL) | |||
| At 12 months | −0.7 (−5.6, 4.3) | 1.9 (−3.3, 7.0) | −4.1 (−9.9, 1.8) |
| At 24 months | 3.0 (−1.5, 7.5) | 0.0 (−5.8, 5.7) | −4.0 (−11.1, 3.0) |
| Total:HDL Cholesterol Ratio | |||
| At 12 months | −0.13 (−0.30, 0.05) | −0.18 (−0.33, −0.03) | −0.48 (−0.65, −0.31) |
| At 24 months | −0.02 (−0.18, 0.14) | −0.25 (−0.42, −0.08) | −0.52 (−0.72, −0.31) |
| Triglycerides (mg/dL) | |||
| At 12 months | −1.6 (−19.6, 16.5) | −7.8 (−18.3, 2.7) | −19.5 (−33.5, −5.6) |
| At 24 months | −0.7 (−17.4, 16.0) | −12.0 (−23.4, −0.5) | −25.3 (−41.0, −9.7) |
| Systolic Blood Pressure (mmHg) | |||
| At 6 months | 2.0 (−1.2, 5.1) | −0.5 (−3.2, 2.2) | −2.1 (−6.2, 1.9) |
| At 12 months | 1.0 (−2.1, 4.2) | 0.4 (−2.4, 3.2) | −0.6 (−4.8, 3.5) |
| At 18 months | 0.2 (−2.8, 3.2) | 0.2 (−2.7, 3.1) | −2.3 (−6.6, 2.0) |
| At 24 months | 2.9 (0.0, 5.7) | −1.1 (−4.2, 1.9) | 1.6 (−2.9, 6.1) |
| Diastolic Blood Pressure (mmHg) | |||
| At 6 months | 1.2 (−0.8, 3.2) | −1.5 (−3.5, 0.6) | −3.0 (−5.4, −0.6) |
| At 12 months | −0.5 (−2.5, 1.6) | −1.5 (−3.6, 0.6) | −2.3 (−4.8, 0.2) |
| At 18 months | −1.2 (−3.1, 0.8) | −1.2 (−3.4, 1.0) | −3.9 (−6.5, −1.3) |
| At 24 months | 0.6 (−1.2, 2.5) | −2.9 (−5.2, −0.6) | −1.4 (−4.1, 1.3) |
| Mean Arterial Pressure (mmHg) | |||
| At 6 months | 1.4 (−0.8, 3.7) | −1.1 (−3.3, 1.0) | −2.7 (−5.6, 0.1) |
| At 12 months | 0.0 (−2.3, 2.3) | −0.9 (−3.1, 1.3) | −1.8 (−4.7, 1.2) |
| At 18 months | −0.7 (−2.9, 1.5) | −0.8 (−3.0, 1.5) | −3.4 (−6.5, −0.4) |
| At 24 months | 1.4 (−0.7, 3.5) | −2.3 (−4.7, 0.0) | −0.4 (−3.6, 2.7) |
| Metabolic Syndrome Severity Z-Score | |||
| At 12 months | −0.07 (−0.20, 0.06) | −0.49 (−0.63, −0.34) | −0.67 (−0.80, −0.54) |
| At 24 months | 0.11(−0.01, 0.23) | −0.41 (−0.57, −0.24) | −0.79 (−0.94, −0.64) |
Values are mean (95% confidence intervals). Bold font indicates a significant (P<0.05) change from baseline.
Abbreviations: ILI, intensive lifestyle intervention.
DISCUSSION
The present results show that a high-intensity lifestyle-based obesity treatment program, consistent with the 2013 AHA/ACC/TOS Guidelines and delivered in primary care among an underserved population, elicits significant improvements in several cardiometabolic outcomes, highlighting the clinical relevance of the PROPEL intervention. Specifically, the PROPEL intervention led to reductions in FBG over 12 months, which were, however, not sustained over 24 months. Further, the ILI group showed beneficial increases in HDL-C at 12 and 24 months compared to the UC group which remained relatively unchanged at both timepoints. This increase in HDL-C likely drove the significant beneficial decrease in total:HDL-C ratio in the ILI group compared to the UC group at both timepoints, particularly since total cholesterol, LDL-C, and non-HDL-C did not change during the intervention in either group. Importantly, the PROPEL intervention yielded improvements in specific disease risk over time, as demonstrated by the significant decrease in MetS-Z in the ILI group compared to the UC group at 12 and 24 months. Participants in the ILI group with more WL during the 24-month intervention period showed greater improvements in FBG, HDL-C, total:HDL-C ratio, triglycerides and MetS-Z than those with less WL, as shown by the regression model and the analysis of change in cardiometabolic outcomes by WL category.
Similar to our results, the primary care-based behavioral lifestyle intervention of the POWER-UP trial, which showed a mean WL of 4.7% at 24 months (PROPEL 5.0% at 24 months27), found a significant difference in FBG between the enhanced lifestyle intervention group and the control group at 12 but not 24 months.23 However, POWER-UP found a decrease in FBG in the intervention group at both timepoints, while we saw significant reductions in the ILI group at 12 months only. FBG in the control groups of both trials remained relatively stable throughout the entire study.
The lack of change in FBG from baseline to 24 months as observed for the ILI group in PROPEL is comparable to the DPP (mean WL of 5.8% at 24 months13), which was conducted in academic health centers. DPP likewise did not find changes in FBG from baseline to 24 months in the lifestyle intervention group (baseline values of ~106 mg/dL, comparable to PROPEL); however, the DPP found a significant mean difference of approximately 5 mg/dL between the groups at 24 months.15 Look AHEAD, also conducted in academic health centers (mean WL of 6.4% at 24 months14), found decreases in FBG at 12 months in both the lifestyle intervention group and control group, with a significantly greater decrease in the intervention group (mean difference approximately 14 mg/dL), however.44 It is noteworthy that Look AHEAD specifically enrolled individuals with type 2 diabetes who were approximately 10 years older than PROPEL participants on average. Further, improvements in HDL-C in the ILI group relative to the UC group in PROPEL were approximately twice as large as compared to the observed increases in Look AHEAD at 12 and 24 months (approximately +2 mg/dL for both timepoints);17,44 the DPP and POWER-UP trials did not find a significant difference between the intervention and respective control group at any timepoint.16,23 The improvements in HDL-C are of particular interest, as HDL-C is less affected by statins and non-statin cholesterol-lowering drugs than LDL-C and all apolipoprotein B-containing lipoproteins,45,46 and changes in HDL-C are consequently more likely to be attributable to the ILI program.47 Although these results are undeniably positive, it has to be emphasized that HDL-C is more a marker than a mediator of cardiovascular risk. While higher HDL-C is generally strongly associated with a lower CVD risk in epidemiological studies, in intervention trials, increases in HDL-C via pharmacological means or due to WL and/or exercise do not consistently lead to improvements in hard CVD endpoints.48,49 It has been suggested that the total:HDL-C ratio may be a superior predictor of CVD event risk compared to classic lipid parameters,50,51 and the beneficial decreases in this important cumulative index of the atherogenic risk in our study therefore underline the clinical relevance of the present findings. The significant reductions in MetS-Z at both timepoints in the ILI group compared to the UC group additionally demonstrate the cardiometabolic risk reduction that was achieved by PROPEL’s high-intensity lifestyle intervention. The effect was comparable, albeit slightly attenuated, compared to DPP’s lifestyle intervention group which showed decreases of 0.40 (PROPEL: 0.35) and 0.31 (PROPEL: 0.20) at 12 and 24 months, respectively.38 Decreases of the magnitude as reported for DPP’s lifestyle intervention group are associated with a significantly reduced 5-year risk of diabetes (hazard ratio 0.57),38 and a similar or slightly blunted protective effect can consequently be assumed for PROPEL’s ILI group. While the demonstrated improvements in cardiometabolic risk factors speak to the success of PROPEL’s ILI program, and sustained improvements in these risk factors are likely beneficial for long-term CVD risk, it has to be acknowledged that to date, de-facto reductions in CVD events through ILIs have not yet been shown.16,17
It is further noteworthy that despite the clinically relevant WL and accompanying improvements in metabolic parameters in PROPEL, there were no significant improvements in BP (SBP, DPB, or MAP) at any timepoint. This is different from large trials conducted in academic health centers such as Look AHEAD (average baseline BP of 129/70)17,44 and DPP (average baseline BP of 124/78),16 which both showed significant reductions in SBP and DBP between the lifestyle intervention group and control group at 12 and 24 months. Similar to our results, POWER-UP (average baseline BP of 121/76), which likewise was conducted in primary care clinics, did not find significant changes in BP between the intervention group and the respective control group. The specific reason for this lack of effect in PROPEL is of course conjecture; however, over 50% of participants were prescribed antihypertensive medication at baseline and throughout the trial. The concurrent drug treatment along with the relatively normal mean BP values at baseline (123/80 in ILI, 123/78 in UC), indicating a predominantly well-controlled BP, possibly masked any BP-lowering effect achieved by the PROPEL intervention. It is further surprising that we did not find any improvements in LDL-C and non-HDL-C following the 24-month intervention especially since ~70% of participants had hypercholesterolemia at baseline. Akin to BP, however, a substantial proportion of participants (~30%) was prescribed cholesterol-lowering medication at baseline and throughout the trial. This likely contributed to relatively stable values in all apolipoprotein B-containing lipoproteins that may have overshadowed any potentially cholesterol-lowering effect elicited by the PROPEL intervention.
There were no race differences regarding the change in cardiometabolic risk factors over 24 months. This is interesting as the main outcomes paper showed less WL in African Americans compared to other races,27 and an attenuated response in cardiometabolic risk factors would consequently be conceivable. Similarly, in this pragmatic trial, there were no notable differences in outcomes between women and men, participants with diabetes vs without diabetes, or participants with hypertension vs. without hypertension over 24 months.
We did, however, find an age effect for FBG and Mets-Z with significant improvements in the ILI group compared to the UC group at both timepoints only in older adults. This is congruent with findings from DPP52 and suggests that the effectiveness of the trial’s ILI in preventing the deterioration of glucose tolerance is enhanced in older adults (60+ years).
There is a gap between obesity management guidelines and what is currently implemented in primary care. Treatment models based on the AHA/ACC/TOS Guidelines adapted to real-life settings and which add effective delivery methods for obesity treatment in primary care are needed. Results from our trial demonstrate that significant WL in primary care is possible by the addition of a health coach to the collaborative care team. The PROPEL approach is scalable and likely achievable in most primary care settings. However, for broad implementation of such approaches and to allow underserved populations in particular to receive effective obesity and concomitant disease risk treatment more easily, the CMS reimbursement regulations need to be extended to include coverage of health coach-delivered obesity treatment in primary care, as used in PROPEL. To date, CMS reimburses intensive behavioral therapy for obesity only if delivered by a primary care practitioner in the context of a hospital, clinic, or physician’s office,33 making the implementation of approaches to reduce health inequities unnecessarily difficult.
Strengths and Limitations
A major strength of PROPEL is its sample, consisting of a racially diverse, low-income population that typically lacks access to effective WL treatment in clinical research or primary care. The minimal inclusion/exclusion criteria of the trial allow for broad generalizability to other underserved populations across the U.S. Further, the present results underline the effectiveness of a comprehensive and scalable WL and cardiometabolic risk factor treatment model that applies to many primary care settings. Finally, the trial’s cluster-randomized design minimized contamination effects between the two study arms. A limitation of the trial, as is often the case in lifestyle interventions,53 is that the sample was mostly women, which limits the generalizability of the present results for both sexes. A further limitation is that while the Cholestech LDX® Analyzer measures glucose, total cholesterol, HDL-C, and triglycerides, the LDL-C levels are calculated from the total cholesterol, HDL-C, and triglyceride tests result. To address this limitation, we calculated non-HDL-C which represents the cholesterol concentration of all atherogenic lipoproteins.36 Finally, while we accounted for BP, glucose, and cholesterol medication use (use vs no use) at each timepoint, we were unable to measure changes in dosage over time as well as medication adherence. These shortcomings may have influenced our results.
Conclusions
A pragmatic high-intensity lifestyle-based obesity treatment program, consistent with the 2013 AHA/ACC/TOS Guidelines and delivered by trained health coaches in primary care, yielded significant improvements in several cardiometabolic risk markers in an underserved population over 24 months, which could translate into long-term reduction in CVD risk.
Supplementary Material
CLINICAL PERSPECTIVE.
What Is New?
The PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial examined the effectiveness of an intensive lifestyle intervention (ILI) for weight loss delivered by trained health coaches in primary care among a racially diverse, low-income population with obesity for improving cardiometabolic risk factors over 24 months.
While pragmatic, PROPEL’s ILI is consistent with national guidelines and demonstrated clinically relevant improvements in HDL cholesterol, total:HDL cholesterol ratio, metabolic syndrome severity (12 and 24 months), and fasting glucose (12 months only).
The PROPEL model is a viable option to deliver effective obesity and cardiometabolic risk factor treatment in primary care.
What Are the Clinical Implications
The present results underline the cardiometabolic effectiveness of a comprehensive weight loss intervention model delivered by trained health coaches in a primary care setting.
The collaborative care approach of the PROPEL model likely offers more successful primary care-based obesity treatment than the existing Centers for Medicare and Medicaid Services (CMS)-supported model which solely relies on primary care practitioners for obesity treatment with, to date, limited success.
Broader implementation of the PROPEL model would particularly allow underserved populations to receive effective obesity and concomitant cardiometabolic disease risk treatment and thereby potentially contribute to reducing health inequities.
ACKNOWLEDGMENTS
The authors would like to acknowledge the patients and stakeholders who are members of the PROPEL Patient Advisory Boards, Community Monitoring Board, and the Project Management Committee (Chris Lodge and Ava Zebrick, MSHCM), who significantly impacted the trial’s design and conduct. The authors are also indebted to the PROPEL patients, assessment technicians and health coaches, without whom this study would not have been possible. The authors gratefully acknowledge the contributions of Willie C. White III, MPH and the David Raines Community Health Centers, Dr. Gary Wiltz and the Teche Action Clinic sites, Michael G. Griffin and Dr. Robert Post and the Daughters of Charity Services of New Orleans, and the Ochsner Health System and Access Health Louisiana clinic sites. The authors also thank our Data and Safety Monitoring Board for monitoring patient safety and the overall conduct of the trial: Robert Ross, PhD (Chair), John Lefante, PhD, Michael Rolfsen, MD, and Chris Lodge.
The PROPEL Research Group includes: Pennington Biomedical Research Center: Peter T. Katzmarzyk, PhD (PI), Robert L. Newton, Jr., PhD (Outcomes Assessment Director), Corby K. Martin, PhD (Intervention Director), John W. Apolzan, PhD (Intervention Co-Director), William Johnson, PhD (Biostatistician), Kara D. Denstel, MPH (Project Manager), Emily F. Mire, MS (Data Manager), Robert K. Singletary, Jr., MHS, Cheryl Lewis, MPH, Phillip Brantley, PhD, Ronald Horswell, PhD, Betty Kennedy, PhD, Dachuan Zhang, MAppStats, Stephanie Authement, RD, LDN, MS, Shiquita Brooks, RDN, LDN, Danielle S. Burrell, M.Ed., MCHES, Leslie Forest-Everage, MA, Angelle Graham Ullmer, RDN, LDN, MS, Laurie Murphy, RDN, LDN, Cristalyn Reynolds, MA, Kevin Sanders, MS, RDN, LDN, Stephen Bower, MS, Daishaun Gabriel, MHA, Hillary Gahagan, MPH, Tabitha K. Gray, MA, Jill Hancock, MPH, Marsha Herrera, Brittany Molinere, Georgia Morgan, MA, Brittany Neyland, Stephanie Rincones, Deanna Robertson, MA, Ekambi Shelton, MPH, Russell J. Tassin, MS, Kaili Williams; Louisiana State University Health Sciences Center at New Orleans: Benjamin F. Springgate, MD; Louisiana State University Health Sciences Center at Shreveport: Terry C. Davis, PhD, Connie L. Arnold, PhD; Ochsner Health System: Eboni Price-Haywood, MD, Carl J. Lavie, MD, Jewel Harden-Barrios, MEd; Tulane University Medical School: Vivian A. Fonseca, MD, Tina K. Thethi, MD (Medical Monitor), Jonathan Gugel, MD; Xavier University: Kathleen B. Kennedy, PhD, Daniel F. Sarpong, PhD, Amina D. Massey.
Study data were collected and managed using REDCap electronic data capture tools hosted at Pennington Biomedical. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
SOURCES OF FUNDING
Research reported in this article was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (OB–1402–10977). The statements in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee. Additional support was provided by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health that funds the Louisiana Clinical and Translational Science Center, and NORC Grant # P30DK072476 entitled “Nutrition and Metabolic Health Through the Lifespan” sponsored by National Institute of Diabetes and Digestive and Kidney Diseases. CH receives funding via an NIH NIDDK National Research Service Award (T32DK064584); JLD is funded by the American Heart Association (Grant # 20POST35210907). Our thanks also go to Health and Nutrition Technology (Carmel, CA) for providing the HealthOne formula and to Nutrisystem (Fort Washington, PA) for providing us with the meal replacements used in the study.
Non-standard Abbreviations and Acronyms
- CMS
Centers for Medicare and Medicaid Services
- ACC
American College of Cardiology
- AHA
American Heart Association
- BMI
body mass index
- BP
blood pressure
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- DPP
Diabetes Prevention Program
- FBG
fasting blood glucose
- HDL-C
high-density lipoprotein cholesterol
- ILI
intensive lifestyle intervention
- LDL-C
low-density lipoprotein cholesterol
- MAP
mean arterial pressure
- MetS
metabolic syndrome
- MetS-Z
metabolic syndrome severity z‑score
- NHANES
National Health and Nutrition Examination Survey
- PA
physical activity
- PCP
primary care provider
- PROPEL
PROmoting Successful Weight Loss in Primary CarE in Louisiana
- REALM‑SF
Rapid Estimate of Adult Literacy in Medicine Short Form
- SBP
systolic blood pressure
- TOS
The Obesity Society
- UC
usual care
- WL
weight loss
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Louisiana State University (LSU), Pennington Biomedical Research Center, and Montclair State University have interest in the intellectual property surrounding the weight graph that was used in the intervention and CKM, among others, is an inventor of the technology. Licensing of that technology results in financial benefits to LSU, Pennington Biomedical Research Center, Montclair State University and the inventors. CKM further reports grants from WW, Egg Board, Academy of Nutrition and Dietetics, and ABGIL, personal fees from NaturallySlim and ACAP, Health WW, Zafgen, Florida Hospital, Metagenic, Gila Therapeutics, Academy of Nutrition and Dietetics, ABGIL, Adaptic, EHE health, and OpenFit outside the submitted work. VF reports grants from Bayer, Boehringer Ingelheim, and Gilead, personal fees from Takeda, Novo Nordisk, Sanofi-Aventis, Eli Lilly, Abbott, AstraZeneca, and Intarcia, and stock options from Microbiome Technologies, Insulin Algorithms, BRAVO4Health, and Amgen outside the submitted work. CJL reports personal fees from Amgen, AstraZeneca, Esperion, Sanofi, and Regeneron outside the submitted work. The work related to this manuscript occurred prior to Dr. Eboni Price-Haywood’s appointment to the PCORI Board of Governors. Other authors have no conflicts of interest.
Clinical Trial Registration: ClinicalTrials.gov Identifier NCT02561221 (https://clinicaltrials.gov/ct2/show/NCT02561221)
REFERENCES
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no. 360. Hyattsville, MD: National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 2.Kopelman P Health risks associated with overweight and obesity. Obes Rev. 2007;8:13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 3.Tremmel M, Gerdtham U-G, Nilsson PM, Saha S. Economic Burden of Obesity: A Systematic Literature Review. Int J Environ Res Public Health. 2017;14:435. doi: 10.3390/ijerph14040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL. Prevalence of Obesity Among Adults, by Household Income and Education — United States, 2011–2014. MMWR Morb Mortal Wkly Rep. 2017;66:1369–1373. doi: 10.15585/mmwr.mm6650a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140:304–310. doi: 10.3945/jn.109.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 7.Kumanyika SK. A Framework for Increasing Equity Impact in Obesity Prevention. Am J Public Health. 2019;109:1350–1357. doi: 10.2105/AJPH.2019.305221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muncan B Cardiovascular disease in racial/ethnic minority populations: illness burden and overview of community-based interventions. Public Health Rev. 2018;39:32. doi: 10.1186/s40985-018-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman G Household Income: 2018. American Community Survey Briefs. Washington, DC: U.S. Census Bureau; 2019. https://www.census.gov/content/dam/Census/library/publications/2019/acs/acsbr18-01.pdf [Google Scholar]
- 10.Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household Food Security in the United States in 2017. ERR-256, U.S. Department of Agriculture; 2018. https://www.ers.usda.gov/webdocs/publications/90023/err-256.pdf [Google Scholar]
- 11.Jensen MD, Ryan DH. New Obesity Guidelines: Promise and Potential. JAMA. 2014;311:23–24. doi: 10.1001/jama.2013.282546. [DOI] [PubMed] [Google Scholar]
- 12.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-sunyer X et al. Effect of Weight Loss With Lifestyle Intervention on Risk of Diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, Krakoff J, Otto A, Ryan DH, Vitolins MZ. Four-Year Weight Losses in the Look AHEAD Study: Factors Associated with Long-Term Success. Obesity (Silver Spring). 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The DPP Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orchard TJ, Temprosa M, Barrett-Connor E, Fowler S, Goldberg R, Mather K, Marcovina S, Montez M, Ratner R, Saudek C et al. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med. 2013;30:46–55.doi: 10.1111/j.1464-5491.2012.03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services, Healthy People 2020 Framework: The Vision, Mission, and Goals of Healthy People 2020 [Internet]. 2011. [cited 2019 May 3]. Available from: www.healthypeople.gov
- 19.Moyer VA, U.S. Preventive Services Task Force. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 20.Carvajal R, Wadden TA, Tsai AG, Peck K, Moran CH. Managing obesity in primary care practice: a narrative review. Ann N Y Acad Sci. 2013;1281:191–206. doi: 10.1111/nyas.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagoto SL, Pbert L, Emmons K. The Society of Behavioral Medicine position statement on the CMS decision memo on intensive behavior therapy for obesity. Transl Behav Med. 2012;2:381–383. doi: 10.1007/s13142-012-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tronieri JS, Wadden TA, Chao AM, Tsai AG. Primary Care Interventions for Obesity: Review of the Evidence. Curr Obes Rep. 2019;8:128–136. doi: 10.1007/s13679-019-00341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, Kumanyika S, Schmitz KH, Diewald LK, Barg R et al. A Two-Year Randomized Trial of Obesity Treatment in Primary Care Practice. N Engl J Med. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appel LJ, Clark JM, Yeh H-C, Wang N-Y, Coughlin JW, Daumit G, Miller ER, Dalcin A, Jerome GJ, Geller S et al. Comparative Effectiveness of Weight-Loss Interventions in Clinical Practice. N Engl J Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, Emmons KM, Rosner BA, Colditz GA. Obesity Treatment for Socioeconomically Disadvantaged Patients in Primary Care Practice. Arch Intern Med. 2012;172:565–574. doi:10/1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katzmarzyk PT, Martin CK, Newton RL, Apolzan JW, Arnold CL, Davis TC, Denstel KD, Mire EF, Thethi TK, Brantley PJ et al. Promoting Successful Weight Loss in Primary Care in Louisiana (PROPEL): Rationale, design and baseline characteristics. Contemp Clin Trials. 2018;67:1–10. doi: 10.1016/j.cct.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katzmarzyk PT, Martin CK, Newton RL, Apolzan JW, Arnold CL, Davis TC, Price-Haywood EG, Denstel KD, Mire EF, Thethi TK, et al. Weight Loss in Underserved Patients — A Cluster-Randomized Trial. N Engl J Med. 2020;383:909–918. doi: 10.1056/NEJMoa2007448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Look AHEAD Research Group, Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, Roberts S, Das SK. The CALERIE Study: design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials. 2011;32:874–881. doi: 10.1016/j.cct.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin CK, Miller AC, Thomas DM, Champagne CM, Han H, Church T. Efficacy of SmartLossSM, a smartphone-based weight loss intervention: Results from a randomized controlled trial. Obesity (Silver Spring). 2015;23:935–942. doi: 10.1002/oby.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin CK, Gilmore LA, Apolzan JW, Myers CA, Thomas DM, Redman LM. Smartloss: A Personalized Mobile Health Intervention for Weight Management and Health Promotion. JMIR MHealth UHealth. 2016;4:e18. doi: 10.2196/mhealth.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Medicare & Medicaid Services. Decision Memo for Intensive Behavioral Therapy for Obesity (CAG-00423N) [Internet]. [cited 2020 Mar 26]. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253&
- 34.Harper CR, Jacobson TA. Using Apolipoprotein B to Manage Dyslipidemic Patients: Time for a Change? Mayo Clin Proc. 2010;85:440–445. doi: 10.4065/mcp.2009.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, Dagenais GR, Després JP. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med. 2001;161:2685–2692. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 36.Brunner FJ, Waldeyer C, Ojeda F, Salomaa V, Kee F, Sans S, Thorand B, Giampaoli S, Brambilla P, Tunstall-Pedoe H et al. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. Lancet. 2019;394:2173–2183. doi: 10.1016/S0140-6736(19)32519-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63:218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeBoer MD, Filipp SL, Gurka MJ. Use of a Metabolic Syndrome Severity Z Score to Track Risk During Treatment of Prediabetes: An Analysis of the Diabetes Prevention Program. Diabetes Care. 2018;41:2421–2430. doi: 10.2337/dc18-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeBoer MD, Gurka MJ, Morrison JA, Woo JG. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond). 2016;40:1353–1359. doi: 10.1038/ijo.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, Guo Y, Cardel M, Pearson TA, DeBoer MD. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: the Atherosclerosis Risk In Communities Study and Jackson Heart Study. Diabetologia. 2017;60:1261–1270. doi: 10.1007/s00125-017-4267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58:2745–2752. doi: 10.1007/s00125-015-3759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J Am Coll Cardiol. 2015;66:755–757. doi: 10.1016/j.jacc.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeBoer MD, Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, Guo Y, Pearson TA. Independent Associations Between Metabolic Syndrome Severity and Future Coronary Heart Disease by Sex and Race. J Am Coll Cardiol. 2017;69:1204–1205. doi: 10.1016/j.jacc.2016.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Look AHEAD Research Group, Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blaha MJ, Martin SS. How Do Statins Work?: Changing Paradigms With Implications for Statin Allocation. J Am Coll Cardiol. 2013;62:2392–2394. doi: 10.1016/j.jacc.2013.08.1626. [DOI] [PubMed] [Google Scholar]
- 46.Chapman MJ. Are the effects of statins on HDL-cholesterol clinically relevant? Eur Heart J Suppl. 2004;6:C58–C63. doi: 10.1016/j.ehjsup.2004.04.002. [DOI] [Google Scholar]
- 47.Escolà-Gil JC, Julve J, Griffin BA, Freeman D, Blanco-Vaca F. HDL and lifestyle interventions. Handb Exp Pharmacol. 2015;224:569–592. doi: 10.1007/978-3-319-09665-0_18. [DOI] [PubMed] [Google Scholar]
- 48.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–625. doi:10.1016.S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 49.Rader DJ. Mediterranean Approach to Improving High-Density Lipoprotein Function. Circulation. 2017;135:644–647. doi: 10.1161/CIRCULATIONAHA.117.026278. [DOI] [PubMed] [Google Scholar]
- 50.Shai I, Rimm EB, Hankinson SE, Curhan G, Manson JE, Rifai N, Stampfer MJ, Ma J. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004;110:2824–2830. doi: 10.1161/01.CIR.0000146339.57154.9B. [DOI] [PubMed] [Google Scholar]
- 51.Millán J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, Pallardo LF, Masana L, Mangas A, Hernández-Mijares A, González-Santos P et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. doi: 10.2147/VHRM.S6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, Fowler S, Dagogo-Jack S, Andres R. The Influence of Age on the Effects of Lifestyle Modification and Metformin in Prevention of Diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075–1081. doi: 10.1093/Gerona/61.10.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity (Silver Spring). 2012;20:1234–1239. doi: 10.1038/oby.2011.140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.