Introduction
Mahaim fiber is a general term that has become synonymous with antegrade-only conducting accessory pathways (APs) with decremental (atrioventricular [AV] node–like) properties that are typically right-sided. These include atrisofascicular pathways, short AV pathways with decremental properties, and nodofascicular and nodoventricular pathways. Mapping of atriofascicular pathways is challenging, in part because the accessory pathway potentials recorded at their atrial origin are typically small and multiple distal insertions have been reported.1,2 We report the case of a patient with atriofascicular antidromic tachycardia whose AP was clearly identified using a high-resolution mapping catheter combined with a 3-dimensional electroanatomic mapping system.
Case report
A 64-year-old woman with recurrent symptomatic wide complex tachycardia of the left bundle branch block morphology with a cycle length of 292 ms (Figure 1A) was referred to our institution for catheter ablation. Twelve-lead electrocardiography during sinus rhythm was normal without any overt preexcitation (Figure 1B). Programmed atrial stimulation resulted in increasing preexcitation following an increase in the AV interval along with shortening of the His-ventricular interval at shorter pacing cycle lengths. The right bundle branch (RBB) electrogram preceded His-bundle activation with preexcitation during atrial pacing and tachycardia (Figure 1C). An atrial extrastimulus delivered during tachycardia at the timing of AV nodal refractoriness advanced preexcited ventricular, retrograde His, and atrial electrograms without affecting the retrograde activation sequence (Figure 1C). The tachycardia was diagnosed as an atriofascicular antidromic tachycardia over an AP inserting into the RBB.
Figure 1.
A: Twelve-lead electrocardiogram during tachycardia with a left bundle branch block morphology with a cycle length of 292 ms. B: Twelve-lead electrocardiogram during sinus rhythm. No overt preexcitation was seen. C: Intracardiac electrograms during atriofascicular antidromic tachycardia over an accessory pathway inserting into the right bundle branch (RBB). The RBB electrogram preceded His bundle activation during tachycardia. Note that an extra atrial stimulation delivered during tachycardia at the timing of atrioventricular nodal refractoriness advanced preexcited ventricular, retrograde His, and atrial electrograms without affecting the retrograde activation sequence.
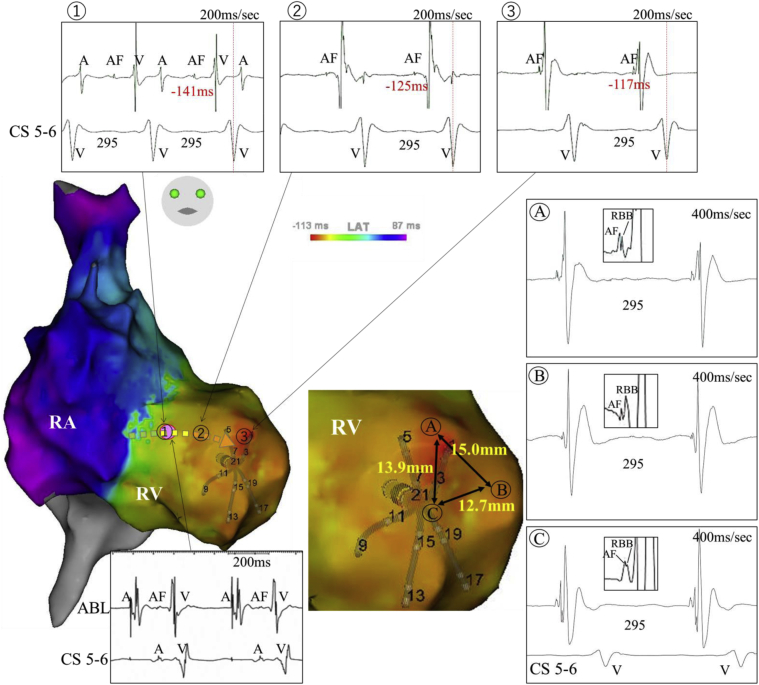
The right atrium (RA), tricuspid annulus (TA), and right ventricle (RV) were mapped during tachycardia using a 5-armed star-shaped 20-polar high-resolution mapping catheter with 2-6-2-mm spacing (PentaRay™; Biosense Webster, Diamond Bar, CA) combined with a 3-dimensional electroanatomic mapping system (CARTO3TM; Biosense Webster). A propagation map was generated, demonstrating the entire tachycardia circuit. Ventricular activation started from the apical anterolateral RV and propagated along the septal RV toward the atrioventricular groove. The impulse traveled to the septal RA via the atrioventricular node and descended to the lateral RA along with the TA; then atriofascicular pathway conduction was sequentially identified from the lateral TA to the apical anterolateral RV, from which the RV myocardial activation initiated (Figure 2). With manual annotation of the atriofascicular potentials and the RBB potentials, the conduction of an atriofascicular fiber was clearly visualized (Supplementary Movie). Of note, fused atriofascicular fiber and RBB potentials were seen at multiple sites in a surrounding area about 1 cm2 wide adjacent to the earliest ventricular activation site, demonstrating an arborized connection between the atriofascicular fiber and the RBB (Figure 2). Radiofrequency (RF) energy application at the proximal atrial insertion site along the TA terminated the tachycardia. The conduction over the AP was no longer seen and the tachycardia was rendered noninducible. The patient has been free from tachycardia recurrence for the subsequent 2 years.
Figure 2.
Activation maps during atriofascicular antidromic tachycardia over an atriofascicular fiber. Atriofascicular potentials are excluded from the annotation. Serial atriofascicular fiber potentials were recorded, extending from the right lateral tricuspid annulus to the apical anterolateral right ventricle adjacent to the earliest myocardial activation site (1–3). A dotted arrow shows the direction of the atriofascicular fiber conduction and the pink dot indicates the site of successful ablation. Fused atriofascicular fiber and distal right bundle branch (RBB) potentials were seen at multiple sites in a surrounding area over 1 cm2 wide adjacent to the earliest ventricular activation site, demonstrating an arborized connection between atriofascicular fibers and the distal RBB (A–C). A = atrial potential; AF = atriofascicular potential; CS = coronary sinus; V = ventricular myocardial potential.
Discussion
Atriofascicular fibers exhibit unique properties such as unidirectional, anterograde conduction with decremental properties and connect the lateral RA to the distal RBB.1,2 In a study using a conventional mapping catheter, Haissaguerre and colleagues1 suggest that distal insertion of atriofascicular fibers may be arborized and located in an area 0.5 to 2 cm in diameter.1 In a later study, the anatomical course of atriofascicular fibers was partially shown with the use of a 3-dimensional mapping system.3 Nevertheless, to the best of our knowledge, the entire atriofascicular fiber conduction and the connection between the atriofascicular fibers and the RBB at the ventricular insertion has never been fully visualized owing to its tiny potentials.
Recently, the advantage of the high-resolution mapping catheter has been recognized in finding very tiny but crucial potentials within the tachycardia circuit,4 as exemplified by our patient’s case. Activation of all atriofascicular fibers during atriofascicular antidromic tachycardia was spatially visualized on 3-dimensional geometry, and the connection between the atriofascicular fibers and the RBB was clearly identified. A previous report suggests that RF ablation of atriofascicular pathways is more efficient at the proximal atrial insertion site along the TA or along the course of the pathway at a site where a discrete potential is identified rather than at the distal insertion.2 In the present case, the distal insertion of the atriofascicular fiber was arborized over 1 cm2, suggesting that multiple applications of RF energies may be required to completely disconnect the distal insertion. In accordance with previous reports,1,2 catheter ablation was successful in targeting the atriofascicular fiber potential close to the TA.
Footnotes
Funding Sources: The authors have no funding sources to disclose.
Disclosures: The authors have no conflicts to disclose.
KEY TEACHING POINTS.
-
•A propagation map demonstrates the entire circuit of atriofascicular antidromic tachycardia.
-
•A high-resolution mapping catheter is useful in identifying sequential atriofascicular fiber conduction from the lateral tricuspid annulus to the apical anterolateral right ventricle.
-
•Fused atriofascicular potentials and distal right bundle branch potentials were seen at multiple sites in a surrounding area about 1 cm2 wide adjacent to the earliest ventricular activation site, demonstrating the arborized connection between atriofascicular fibers and the distal right bundle branch.
Appendix. Supplementary data
A propagation map with annotation of the atriofascicular potentials and the right bundle branch (RBB) potentials demonstrating the entire circuit of atriofascicular antidromic tachycardia over an accessory pathway. The atriofascicular fiber activation starts from the right lateral tricuspid annulus (TA) to the apical anterolateral right ventricle (RV) adjacent to the earliest myocardial activation site, then ventricular activation breaks out and propagates along the septal RV toward the atrioventricular groove. The impulse travels to the septal right atrium (RA) via the atrioventricular node, descends to the lateral RA along with the tricuspid annulus (TA), then activates the atriofascicular fiber. The atriofascicular potentials are shown in Figure 2.
References
- 1.Haissaguerre M., Cauchemez B., Marcus F. Characteristics of the ventricular insertion sites of accessory pathways with antegrade decremental conduction properties. Circulation. 1995;91:1077–1085. doi: 10.1161/01.cir.91.4.1077. [DOI] [PubMed] [Google Scholar]
- 2.Kothari S., Gupta A.K., Lokhandwala Y.Y. Atriofascicular pathways: Where to ablate? Pacing Clin Electrophysiol. 2006;29:1226–1233. doi: 10.1111/j.1540-8159.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 3.Morita N., Kobayashi Y., Katoh T., Takano T. Anatomic and electrophysiologic evaluation of a right lateral atrioventricular Mahaim fiber. Pacing Clin Electrophysiol. 2005;28:1138–1141. doi: 10.1111/j.1540-8159.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 4.Tschabrunn C.M., Roujol S., Dorman N.C., Nezafat R., Josephson M.E., Anter E. High-resolution mapping of ventricular scar: Comparison between single and multielectrode catheters. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A propagation map with annotation of the atriofascicular potentials and the right bundle branch (RBB) potentials demonstrating the entire circuit of atriofascicular antidromic tachycardia over an accessory pathway. The atriofascicular fiber activation starts from the right lateral tricuspid annulus (TA) to the apical anterolateral right ventricle (RV) adjacent to the earliest myocardial activation site, then ventricular activation breaks out and propagates along the septal RV toward the atrioventricular groove. The impulse travels to the septal right atrium (RA) via the atrioventricular node, descends to the lateral RA along with the tricuspid annulus (TA), then activates the atriofascicular fiber. The atriofascicular potentials are shown in Figure 2.