Introduction
The magnet-activated switch in implanted cardiac devices, including cardioverter-defibrillators and permanent pacemakers (PPM), is susceptible to interference from consumer devices with built-in magnets. For example, a recent case report showed that a patient’s implanted cardioverter-defibrillator (ICD) magnet mode became activated owing to interaction with his e-cigarette.1 While testing has shown that cellular phones and smart watches themselves have minimal interference with cardiac devices,2 this has failed to account for the magnets used in the wristband of smart watches and fitness trackers. Fitness trackers are capable of tracking heart rate, step count, and other variables of interest to fitness-minded consumers. In 2019, the company Fitbit, manufacturer of several different devices, sold nearly 16 million units.3 The popular Apple Watch also features these capabilities, with Garmin and Samsung producing models as well. These products are lightweight and low-profile and sometimes sold with a built-in magnet-clasped wristband. Though this magnet is small, most manufacturers have adopted the magnetic element neodymium, which is known to interfere with device functions.4 We investigated whether these magnetic wristbands had clinically relevant effects on ICD function through ex vivo testing.
Key Teaching Points.
-
•
Magnets used in the wristbands of fitness trackers and smart watches can interfere with implanted cardiac devices; however, this is not widely appreciated by manufacturers of the watches or the cardiac devices.
-
•
Possible complications include implanted cardioverter-defibrillator deactivation and permanent pacemaker mode switch.
-
•
Patients should be counseled on this risk and advised to keep their wristbands at least 6 inches away from their cardiac devices, and not to wear them to sleep.
Case report
A 55-year-old woman with history of sustained ventricular tachycardia secondary to arrhythmogenic right ventricular dysplasia was treated with a dual-chamber ICD for secondary prevention. She was recommended to avoid strenuous exercise to reduce arrhythmic risk. She subsequently purchased an Apple Watch with fitness tracking capabilities to monitor her heart rate, which she wore sometimes overnight. While asleep one night, she was awoken by several beeps emanating from her implanted cardiac device. Subsequent interrogation of the device showed no alerts or abnormal parameters. Upon further investigation, it was found that the device had reverted to magnet mode, owing to magnetic interference from the fitness watch’s wristband. No other possible sources of interference were identified. This was replicated in the office, where interrogation of the ICD confirmed magnetic reversion when placed in proximity to the wristband. The watch itself did not have magnetic interference.
Methods and materials
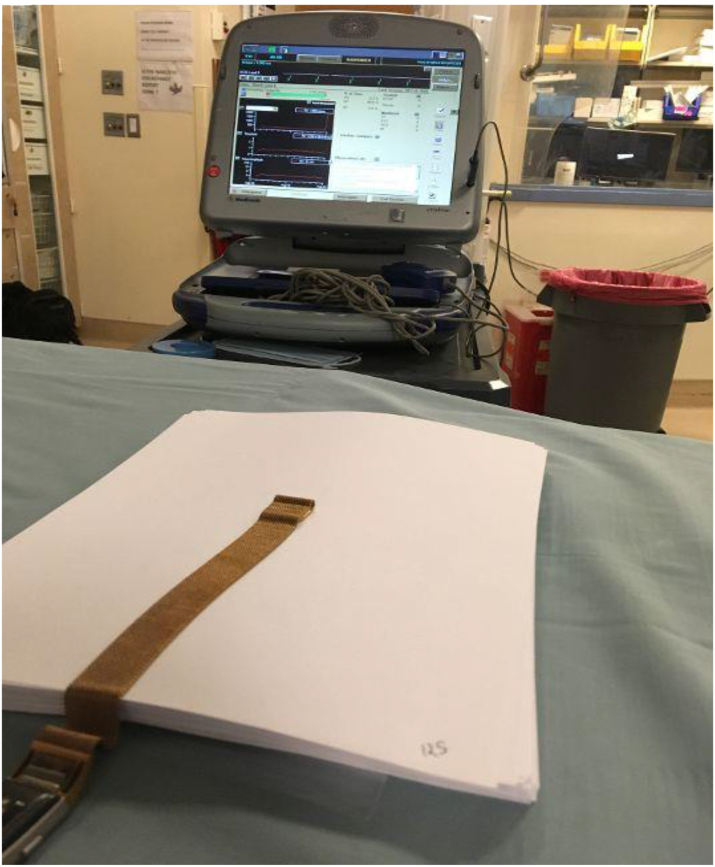
The Medtronic Visia AF MRI S DF-1 single-chamber ICD (Medtronic, Minneapolis, MN) was used for testing. In the electrophysiology lab, sheets of commercial printer paper were stacked to create distance on top of the device that could be easily quantified, and the maximum distance where different magnets could deactivate the ICD was measured. This distance was then confirmed without any paper stacked on the device (Figure 1). Paper and air were chosen as the contact media as they are very weakly diamagnetic and thus do not significantly attenuate the magnetic field of the tested wristbands. This is similar to human tissue itself.5
Figure 1.
Fitbit wristband magnet deactivating implanted cardioverter-defibrillator at depth of 2.4 cm.
Fitbit and Apple Watch wristbands (similar to the patient’s in the described case) were used for testing. The results were compared against 2 clinical magnets: a donut magnet and a Medtronic programming head (Medtronic, Minneapolis, MN). The fitness trackers were oriented in the fashion in which they would come up against the ICD in real life, as if the volar aspect of a patient’s wrist with the magnet clasped came into contact with their chest.
Results
The Fitbit and Apple Watch wristband magnets could deactivate the ICD up to distances of 2.4 and 2.0 centimeters, respectively. Meanwhile, the clinical magnets in the donut and programming head could deactivate up to 8.0 and 7.0 centimeters, respectively (Table 1).
Table 1.
Maximum distances, in centimeters, at which various magnets could deactivate the implanted cardioverter-defibrillator
| Magnet type | Distance at which ICD deactivates (cm) |
|---|---|
| Fitbit wristband | 2.4 |
| Apple Watch wristband | 2.0 |
| Donut magnet | 8.0 |
| Programming head | 7.0 |
ICD = implanted cardioverter-defibrillator.
Discussion
The fitness tracker wristband magnets were considerably weaker than clinical-grade magnets; however, the magnetic field strength was still capable of deactivating the ICD at clinically relevant distances, as most devices are implanted subcutaneously. The implantable devices produced by all major manufacturers are potentially susceptible to this interference, as most of the devices utilize a magnet sensor. Older devices relied on a Reed switch, whereas newer devices may be equipped with alternative sensors including Hall-effect sensors or magnetosensitive resistors. Subcutaneous ICD devices may be also vulnerable, as their location of implantation allows closer interaction with the left wrist of the patient.
While magnets could deactivate the ICD tachy-therapies, resulting in untreated sustained ventricular arrhythmia, the most feared complication on PPM devices is a switch to asynchronous pacing modes (DOO/VOO).6 Such a switch could cause an R-on-T event, especially for those with intrinsic native rhythms, triggering a malignant arrhythmia.
The manufacturers of these wristbands as well as the cardiac devices should include appropriate warnings in their user manuals and online resources. Some fitness tracker creators have issued appropriate warnings alongside their products. The Apple Watch user guide acknowledges that the wristband as well as charging apparatus are capable of interfering with ICDs and PPMs.7
Conclusion
Wearable fitness tracker accessories can contain powerful magnets capable of interfering with ICD or PPM functions. Patients should be counseled on this and recommended to take appropriate precautions, such as choosing a nonmagnetic wristband, keeping magnetic wristbands at least 6 inches from the implanted device, and removing magnetic wristbands before going to bed.
Footnotes
Funding Sources: The authors have no funding sources to disclose.
Conflicts of Interest: The authors have no conflicts to disclose.
References
- 1.Shea J.B., Aguilar M., Sauer W., Tedrow U. Unintentional magnet reversion of an implanted cardiac defibrillator by an electronic cigarette. HeartRhythm Case Rep. 2020;6:121–123. doi: 10.1016/j.hrcr.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacour P., Parwani A.S., Schuessler F. Are contemporary smartwatches and mobile phones safe for patients with cardiovascular implantable electronic devices? JACC Clin Electrophysiol. 2020;6:1158–1166. doi: 10.1016/j.jacep.2020.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Tankovska H. Fitbit device unit sales worldwide 2010-2019. https://www.statista.com/statistics/472591/fitbit-devices-sold/
- 4.Wolber T., Ryf S., Binggeli C. Potential interference of small neodymium magnets with cardiac pacemakers and implantable cardioverter-defibrillators. Heart Rhythm. 2007;4:1–4. doi: 10.1016/j.hrthm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Di Luzio S., Obletter G., Comani S., Del Gratta C., Romani G.L. Magnetic mapping of DC fields related to tissue susceptibility in the human body. In: Williamson S.J., Hoke M., Stroink G., Kotani M., editors. Advances in Biomagnetism. Springer; Boston, MA: 1989. [Google Scholar]
- 6.Jacob S., Panaich S.S., Maheshwari R., Hadded J.W., Padanilam B.J., John S.K. Clinical applications of magnets on cardiac rhythm management devices. Europace. 2011;14:1222–1230. doi: 10.1093/europace/eur137. [DOI] [PubMed] [Google Scholar]
- 7.Apple Apple Watch: User Guide. 2020. https://support.apple.com/guide/watch/important-safety-information-apdcf2ff54e9/watchos